Abstract
Background:
Debates over whether climate change could lead to the amplification of Lyme disease (LD) risk in the future have received much attention. Although recent large-scale disease mapping studies project an overall increase in Lyme disease risk as the climate warms, such conclusions are based on climate-driven models in which other drivers of change, such as land-use/cover and host population distribution, are less considered.
Objectives:
The main objectives were to project the likely future ecological risk patterns of LD in Europe under different assumptions about future socioeconomic and climate conditions and to explore similarity and uncertainty in the projected risks.
Methods:
An integrative, spatially explicit modeling study of the ecological risk patterns of LD in Europe was conducted by applying recent advances in process-based modeling of tick-borne diseases, species distribution mapping, and scenarios of land-use/cover change. We drove the model with stakeholder-driven, integrated scenarios of plausible future socioeconomic and climate change [the Shared Socioeconomic Pathway (SSPs) combined with the Representative Concentration Pathways (RCPs)].
Results:
The model projections suggest that future temperature increases may not always amplify LD risk: Low emissions scenarios (RCP2.6) combined with a sustainability socioeconomic scenario (SSP1) resulted in reduced LD risk. The greatest increase in risk was projected under intermediate (RCP4.5) rather than high-end (RCP8.5) climate change scenarios. Climate and land-use change were projected to have different roles in shaping the future regional dynamics of risk, with climate warming being likely to cause risk expansion in northern Europe and conversion of forest to agriculture being likely to limit risk in southern Europe.
Conclusions:
Projected regional differences in LD risk resulted from mixed effects of temperature, land use, and host distributions, suggesting region-specific and cross-sectoral foci for LD risk management policy. The integrated model provides an improved explanatory tool for the system mechanisms of LD pathogen transmission and how pathogen transmission could respond to combined socioeconomic and climate changes. https://doi.org/10.1289/EHP4615
Introduction
The demand for plausible future estimates and patterns of disease risks under global environmental change is widespread given that they are a useful aid in setting priorities for health research, policy, and training (Altizer et al. 2013; Kraemer et al. 2016; Murray and Lopez 1997). Lyme disease (LD) is the most prevalent vector-borne disease in Europe and which is transmitted mainly by the sheep tick Ixodes ricinus. The risk of LD for human populations is linked to both tick abundance and infection prevalence (Mysterud et al. 2016). Ticks are reported to have recently experienced an increase in abundance (Gray et al. 2009) and a shift in their geographical range toward higher elevations and latitudes in northern Europe (Jore et al. 2014). With no vaccines on the market, the prevention of LD relies heavily on risk communication, rapid diagnosis, and personal protection measures, ideally targeted at high-risk areas and effective intervention points (Quine et al. 2011). Effective targeting and prioritization (e.g., of LD over other public health threats) requires a good understanding of the processes driving the spatial and temporal changes in risk. This relies on our competency in integrating multidisciplinary resources and data in projecting future risk dynamics.
Transmission of LD pathogens between animals is the result of a complex and multifactorial system. The spatial and temporal heterogeneous nature of the environmental parameters affects the biological processes of a broad range of host and pathogen species involved (Hartemink et al. 2015; Randolph 2001). In addition, human activities could alter the functions of ecosystems and thereby the abundance and distribution of ticks and their hosts (Lambin et al. 2010; Kilpatrick et al. 2017). The effect of changes in these factors on tick abundance, distribution, and pathogen transmission has been studied empirically and quantitatively, in a varying degree of detail (Estrada-Peña et al. 2014; Kurtenbach et al. 2006; Pfäffle et al. 2013), providing increasingly firm empirical grounds on which models can be elaborated. Yet model-based studies for LD remain small in number, with those focusing on future risk projections being solely temperature-driven and predicting that the range of LD risk expands as the climate warms (McPherson et al. 2017; Ostfeld and Brunner 2015). At present, no comprehensive studies exist that incorporate changes in land cover and host distribution with climate change when projecting future risk patterns. It thus seems that advances in empirical investigations have far outpaced our ability to integrate these new findings into a more complete modeling framework for LD transmission. This has resulted in a bottleneck wherein tick biologists and health professionals await development of the modeling tools needed to better target awareness campaigns and mitigation measures to where they will be most needed (Parham et al. 2015).
In this study, we conducted an integrative modeling exercise to project potential future patterns of the ecological risk of LD in Europe. By linking LD risk to the larger context of global environmental change, our main objective was to explore similarity and uncertainty in future disease risks emerging from comparing projections under different assumptions about future socioeconomic and climate conditions.
Methods
Our approach applied agent-based modeling of LD dynamics (the LYMERISK model) (Li et al. 2014, 2016) and ensemble modeling of host species distributions [using the BIOMOD platform (R Development Core Team)] (Thuiller et al. 2009) to European scenarios of land-use/cover change [using the Impacts and Risks from High-end Scenarios: Strategies for Innovative Solutions (IMPRESSIONS) Integrated Assessment Platform2 (IAP2)] (Harrison et al. 2019) under combined scenarios of plausible socioeconomic and climate change (Harrison et al. 2019). Four parameters or inputs of the LYMERISK model (i.e., temperature, forest land cover, deer population distribution, and pathogen transmission hosts) were selected as key drivers of future LD risk (see theoretical framework in Figure 1; integrative modeling strategies in Figure 2), based on expert knowledge, literature-driven evidence (Medlock et al. 2013; Ostfeld and Brunner 2015; Pfäffle et al. 2013; Kilpatrick et al. 2017), previous analyses on model sensitivity (Li et al. 2012a, 2016), and data availability. These four parameters were chosen because a) temperature influences activity, diapause, interstadial development, fertility, and mortality of ticks as well as habitat suitability for their hosts; b) forest land cover serves as the major habitat type for both ticks and host animals, c) deer are considered to be the most important tick reproduction hosts in Europe, making the distribution of the deer population an important consideration; and d) pathogen transmission hosts can harbor the Borrelia burgdorferi sensu lato complex of spirochete bacteria, the pathogenic agents of LD and transmit them to ticks.
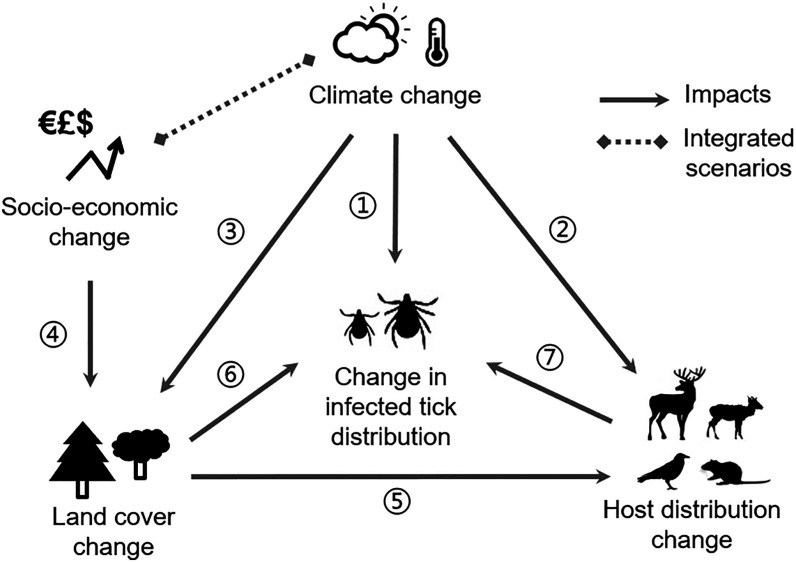
Figure 1.
Theoretical framework and key drivers of Lyme disease risk dynamics. Climate change: (1) Climate influences tick phenology and distribution of ticks; (2) climate suitability influences distribution of tick hosts (e.g., deer, rodents, and birds); (3) climate influences growth of plant species and profitability of land, driving land-use/cover change. Socioeconomic change: (4) Socioeconomics influence the demand and preferences for how land is used, which affects conversion between land cover types. Land cover change: (5) Land cover influences host type and abundance as well as microclimate and, hence, (6) distribution of ticks. Host distribution change: (7) Availability of hosts influences tick survival and pathogen transmission.
Figure 2.
The integrative modeling strategy for Lyme disease risk projection. The LYMERISK model is an agent-based model for the ecological risk of Lyme disease, with three interactive agent layers representing ticks, reproduction and transmission host animals, and habitats. BIOMOD is a platform for the ensemble prediction of species’ distributions and is used to project future distribution patterns of key tick host species. The Integrated Assessment Platform for climate change impact assessment is a product of the IMPRESSIONS project and provides projections in habitats and climate conditions.
The LYMERISK Model for the Spatiotemporal Dynamics of Lyme Disease Risk
The LYMERISK model is an upscaled and modified version of a recent spatially explicit, agent-based model of the regional ecological risks of LD (Li et al. 2012a, 2014, 2016). It has three interactive agent layers (i.e., ticks, hosts, and habitat) that are programed to evolve in discrete time steps (monthly) following a set of behavior rules. Behaviors are predefined rules that agents use to update their attributes in the model, and they represent the ecological processes and interactions of tick and host populations and habitats. To ease the computational burden and to allow the fundamental spatial dynamics and seasonality to be represented at the pan-European level, a cell size of 10′ (arc minutes) and a time step of 1 month were adopted in the present model (which also followed the resolution of input climate data produced by the IMPRESSIONS project (Holman et al. 2015).
Tick population ecology and phenology. Four continuous I. ricinus tick life stages were considered: egg, larval, nymph, and adult. Tick population dynamics was driven by monthly, life stage–specific interstadial developmental rates (temperature-dependent), mortality rates (density- and habitat-dependent), and feeding rates (density-dependent), which were recalculated at every time step (Table 1). Developmental diapause was considered by delaying the development of ticks who feed in late summer or early fall (e.g., after August) until early summer of the next year. Seasonal behavioral change was based on evidence that ticks that molt in late summer may become reluctant to become active and feed (Campbell 1948), which could be due to either behavioral diapause or a form of temperature-controlled quiescence. Similar patterns in questing tick phenology (early summer peak of tick population, in particular, nymphs) have also been reported in many European studies, including those in Belgium (Li et al. 2012b), the UK (Randolph 2004), Switzerland (Pérez et al. 2012), Hungary (Egyed et al. 2012), Norway (Qviller et al. 2014), and Spain (Barandika et al. 2010). To represent this seasonal behavioral change in the model, a suppression of tick questing activity from mid-summer to winter (e.g., July–December) was considered and assumed to affect nymphs and adults only, according to existing evidence (Gray et al. 2016). A similar suppression was also applied by Dobson et al. (2011) to model tick phenology in the UK and Spain.
Table 1.
Model parameters related to the population ecology and phenology of the Ixodes ricinus tick.
| Symbol | Parameter | Value/equation |
|---|---|---|
| Average eggs per adult () | 2,000 | |
| m | Mortality rates in forests (per month) | |
| Questing larvae, nymphs and adults | 0.12, 0.12, 0.08 | |
| Developing from | ||
| Engorged larvae into questing nymphs | 0.12 | |
| Engorged nymphs into questing adults | 0.04 | |
| Engorged adults into eggs | 0.08 | |
| Eggs into questing larvae | 0.08 | |
| Sf | Scaling factors for m in forests, shrubs, and grassland | 1, 1.5, 3 |
| Temperature-dependent proportion of questing ticks | See “Impacts of temperature on ticks” section. | |
| Basal feeding mortality rates (per capita) | ||
| Larvae, nymphs, and adults on transmission hosts | 0.65, 0.65, 0.55 | |
| Larvae, nymphs, and adults on reproduction hosts | 0.6, 0.6, 0.5 | |
| Interstadial development rate functions | ||
| Eggs developing into larvae | () | |
| Larvae developing into nymphs | () | |
| Nymphs developing into adults | () | |
| Adults producing eggs | () | |
| dp | Proportion of ticks undergo diapause | |
| Developmental diapause after August | 0.9 | |
| Behavioral diapause after June for nymphs and adults | 0.9, 0.6 | |
| Host-finding probability (per month) | ||
| Transmission host for questing larvae and nymphs | ||
| Transmission host for questing adults | ||
| Reproduction host for questing larvae and nymphs | ||
| Reproduction host for questing adults | ||
| C | Maximum tick attachments on one host (per month) | |
| Larvae, nymphs, and adults on one transmission host | 400, 25, 0.4 | |
| Larvae, nymphs, and adults on one reproduction host | 800, 800, 400 |
Note: D, reproduction host density per hectare; R, transmission hosts density (per hectare); T, temperature (°C).
Impacts of temperature on ticks. Temperature was assumed to influence the interstadial development and questing activity of ticks. In addition to the temperature-dependent functions used by Li et al. (2016), a few more temperature regulations were introduced to regulate the ticks’ activity and survival at the northern and southern range of the simulated tick distribution across Europe. First, we assumed the onset of questing activity of nymphs and adult ticks to occur at (monthly average daily mean temperature) and that of larvae to occur at based on a number of previous findings: Qviller et al. (2014) found ticks in Norway were active at low densities at temperatures ; Perret et al. (2000) reported that ticks were not sampled in Switzerland when daily average temperatures were , occasionally sampled at daily average temperatures of between 1.9°C and 3.8°C, and always sampled when daily average temperatures were ; and Randolph (2004) mentioned that questing activity of larvae usually requires warmer conditions compared with the questing activity of nymphs and adult ticks. Second, it has been reported that extreme temperatures influence the population dynamics of ticks, with cold temperatures being more harmful than warm temperatures (Ostfeld and Brunner 2015). A short exposure to can be lethal to ticks (MacLeod 1935), whereas if temperatures are , then a relative humidity of is required for tick survival (Macleod 1936; Milne 1949a, 1949b, 1950). However, these studies also indicated that ticks are capable of seeking favorable microclimatic conditions by, for example, moving into the forest litter when the air becomes too warm or dry. Therefore, in this model, only the negative influence of cold temperatures on tick survival was considered, and the effect was assumed to be rational: survival rate would be decreased by 0–90% when the average temperature dropped from to . Third, the climate of origin can affect tick host-seeking behavior in Europe (Gilbert et al. 2014). To account for such an influence, we assumed that the effect of temperature on the rate of active questing by ticks could be approximated by a logistic function of monthly temperature , where e is the natural logarithm base, L is the maximum value of the rate (assuming maximum 95% of the questing ticks are active in natural environments), T0 is the temperature where the rate’s midpoint (47.5%) is achieved, and k is the curve’s steepness. When initializing the model with the monthly temperature grids, T0 and k were estimated based on two known points on the curve: a) at 5°C, an active rate of 0.05 was assumed for all ticks, that is, ; and b) at 9°C, a linear regressive function was used to estimate the active rate for each cell, that is, (, built based on the empirical data presented by Gilbert et al. (2014), where is the mean temperature in May of the cell. As a result, a logistic function was built for every cell and was used at each time step to estimate the rate based on its monthly mean temperature.
Pathogen transmission. The systemic transmission and transstadial transmission of B. burgdorferi s.l. were considered in this model. Although other transmission routes have been reported as possible (e.g., between co-feeding ticks or from females to eggs), they were not modeled because recent debates have suggested that their significance on the transmission of B. burgdorferi s.l. remains unclear [see the review and discussion in the study by Li et al. (2016)]. The susceptible–infectious functions developed previously (Li et al. 2012a, 2016) were used for the systemic pathogen transmission between the tick and transmission populations (with and being the host-to-tick and tick-to-host transmission efficiencies, respectively). It was also assumed that a low infection rate in questing larval ticks () was due to interrupted contacts between larval ticks and hosts. Moreover, the efficiency of transstadial transmission was assumed to be 100%.
Host population and distribution. Populations of two host types (transmission and tick reproduction hosts, which include a range of common tick host species) were represented as host agents in the model. Transmission hosts are those capable of harboring a pathogen and transmitting it to ticks. Common European transmission hosts of B. burgdorferi include rodents, birds, and lagomorphs, with rodent species being the most abundant. Tick reproduction hosts do not transmit a pathogen to ticks but are important in maintaining tick populations by feeding large numbers of adult ticks, which then reproduce. Deer are the most important reproduction host for ticks in Europe and, hence, were considered to represent all reproduction host species in the model. Habitat suitability maps were used to approximate host population distributions. Each cell was given a value between 0 and 1 to indicate the capability of the cell to support the full carrying capacity, which was estimated as a multiplication of species presence, climate suitability, and extent of suitable habitats, allowing more hosts to be present in places with a greater extent of habitat and better climate suitability. A number of species that are regarded as major tick hosts in Europe were considered, and their common habitat types are summarized in Table 2. Climate suitability for each selected species was modeled using the BIOMOD platform, which uses an ensemble of different species distribution modeling approaches (Thuiller et al. 2009), with a set of climate variables selected for non-bird species distribution modeling (Harrison et al. 2006), including growing degree days , absolute minimum temperature expected over a 20-y period, annual maximum temperature, accumulated annual soil water deficit, and accumulated annual soil water surplus. These climate variables were calculated based on the IMPRESSIONS climate indices, following the methods used previously (Harrison et al. 2006; Pearson et al. 2002). Parameters related to host dynamics and movements are summarized in Table 3. The rodent population was assumed to follow a seasonal pattern modeled by the functions used by Li et al. (2016), which considered a 1-y periodic birth rate (), a 3-y periodic carrying capacity (), and a combined mortality (). These functions were initially used to approximate rodent population dynamics in hantavirus investigations (Amirpour Haredasht et al. 2011; Sauvage et al. 2003, 2007). In this model, they were modified to fit the monthly time step and to take into account the potential impacts of habitat suitability by applying it as a multiplier [] to the carrying capacity (). Common bird host species—including, blackbird (Turdus merula), song thrush (Turdus philomelos), dunnock (Prunella modularis), European robin (Erithacus rubecula) and great tit (Cyanistes caeruleus)—are widely distributed across Europe. However, although birds are less abundant than rodents and their temporal population dynamics are similar to that of rodents in that they breed from spring onward, their population size increases over the summer and then is re-set to the breeding population again in the next spring. As a result, the population dynamics of birds was not modeled explicitly, and birds were assumed to be present in forests throughout the study region.
Table 2.
Host species and their common habitat types considered in modeling Lyme disease risk.
| Type | Species | Name | Use of land cover as habitat | ||
|---|---|---|---|---|---|
| Forests | Shrubs | Grass | |||
| Rodent | Apodemus sylvaticus | Wood mouse | ✓ | ✓ | ✓ |
| Rodent | Apodemus flavicollis | Yellow-necked field mouse | ✓ | — | — |
| Rodent | Apodemus agrarius | Black striped field mouse | ✓ | — | ✓ |
| Rodent | Clethrionomys glareolus / Myodes glareolus | Bank vole | ✓ | — | — |
| Rodent | Microtus arvalis | Common vole | — | — | ✓ |
| Rodent | Microtus agrestis | Meadow vole/field vole | ✓ | ✓ | ✓ |
| Deer | Capreolus capreolus | Roe deer | ✓ | ✓ | — |
| Deer | Cervus elaphus | Red deer | ✓ | ✓ | — |
| Deer | Dama dama | Fallow deer | ✓ | ✓ | — |
| Deer | Alces alces | Moose | ✓ | — | — |
Note: The species listed in this table are the most common host species of Ixodes ricinus ticks in Europe. Rodent species are capable of harboring and transmitting Borrelia burgdorferi sensu lato, the pathogenic agents of Lyme disease. Deer species do not transmit pathogens to ticks, but they can feed large numbers of adult ticks, which then reproduce. Habitats influence tick survival and host animal population and movement. They are classified into three groups (forests, shrubs, and grass). Possible extents of these habitats by the 2050s were projected and used to estimate future Lyme disease risks. —, not applicable.
Table 3.
Model parameters related to host population dynamics and movement patterns.
| Symbol | Parameter | Value/equation |
|---|---|---|
| Birth rate of transmission hosts per month (t) | ||
| Carrying capacity of transmission hosts per month (t) | ||
| Mortality rate of transmission hosts per month (t) | ||
| Carrying capacity of reproduction hosts (per kilometer squared) | ||
| MC | Movement capacity () | |
| Reproduction hosts in home ranging phase | 2.2 (winter/spring); 1.5 (summer/fall) | |
| Reproduction hosts in migration phases | 8.5 | |
| pT | Time spent in different habitat types (% of time step) | |
| Home ranging transmission hosts in forests, shrubs, and grassland | 60, 30, 10 | |
| Home ranging reproduction hosts in forests and shrubs | 85, 15 | |
| Grazing reproduction hosts in grassland | 35 | |
| Proportion of reproduction hosts venture for grazing (%) | See “Host movement” section | |
| Proportion of reproduction hosts in seasonal migration phase (%) | 20 (summer, uphill); 10 (winter, downhill) |
Note: , habitat suitability for reproduction hosts; , habitat suitability for transmission hosts.
Host movement. Behavioral rules for host movement followed the assumptions developed in previous studies (Li et al. 2014, 2016) but were modified to include the within-cell movements of rodents between habitat types. Two movement phases were considered, namely, the home-ranging and dispersal phases. In practice, animals spend different proportions of time in different habitat types and constantly migrate between habitat types to establish home ranges. This home-ranging movement pattern was modeled for both reproduction and transmission hosts and was assumed to complete within each cell, given that the size of the 10′ cell () is far larger than the common home range sizes of both host types, for example, for rodents (Kikkawa 1964) and for deer (Morellet et al. 2013). Although reproduction hosts were assumed to inhabit forests and shrubs, they may venture for grazing onto grassland, especially when food resources are limited. In the model, the rate of reproduction host grazing () in a cell was estimated by , which is a function of the numbers of reproduction hosts (D), the extent of suitable reproduction host habitats [forest () and open habitats ()], the extent of grassland (), and a parameter indicating the maximum overlapping rate of reproduction host home ranges (). Thus, grazing on grassland was triggered when deer populations were so concentrated that the existing deer habitats were unable to cover the minimum required home range for the population. A relatively greater extent of grassland could also increase the chance of grazing. Moreover, the home range of a reproduction host has been found to be associated with a number of environmental variables, among which the influence of elevation, annual temperature, and latitude were included in the model. It was assumed in the model that the reproduction host home range size () was enlarged by by each increase in elevation (Mysterud 1999). The increase in a cell’s annual temperature and latitude was assumed to lead to changes of and 1.6% in deer home range size, respectively (Morellet et al. 2013). In the dispersal phase, animals move over longer distances to new habitats. For example, reproduction hosts were assumed to perform seasonal uphill/downhill migration. Transmission host dispersal was not modeled explicitly given the relatively low movement capability of rodents at the spatial scale considered. The transition rules for displacement of reproduction hosts used previously (Li et al. 2014, 2016) were integrated in the present study.
Habitat. Habitat agents represent the composition of the habitat types in a cell; thus the percentages of different types were stored as states for each cell. Three generalized habitat types were considered in the model, namely, forests, shrubs, and grassland. As described previously, in different habitat types, ticks were assumed to have different survival rates and hosts were assumed to perform different movement patterns within and between cells. Tick reproduction hosts were assumed to inhabit forests and shrubs and to occasionally venture into grassland for grazing. Transmission hosts were assumed to inhabit all three habitat types; however, their densities differed between habitat classes, that is, they were more numerous in the forest and least numerous in the grassland. Habitat agents were assumed to be static throughout each simulation; thus no behaviors were specified for this agent type in this model, that is, habitats did not move between cells or change in spatial distribution over time.
Projecting Baseline Risk Patterns
Climate condition. Climate data were retrieved from IMPRESSIONS data products for both baseline (1981–2010, the WATCH-WFDEI data) and future projections (Harrison et al. 2019).
Habitat distribution. Baseline habitat data were generated by categorizing the CORINE 2012 land cover data [provided by the Copernicus Land Monitoring Service (CLC)] into forests (CLC311, broad-leaved forest; CLC312, coniferous forest; CLC313, mixed forest), shrubs (CLC322, moors and heathland; CLC323, sclerophyllous vegetation; CLC324, transitional woodland-shrub), and grassland (CLC321, natural grasslands). The three habitats were further associated with the IMPRESSIONS IAP2’s land-use outputs of forests (both managed and unmanaged), unmanaged land (with no woodland and productive purpose), and extensive grassland (for sheep and rough grazing), respectively.
Host distribution. Climate suitability maps were produced for each species using the BIOMOD platform. Observed species distributions were downloaded from the International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species™ (https://www.iucnredlist.org/). The model was first trained with a covering Europe and North Africa taken from previous studies (Harrison et al. 2006; Pearson et al. 2002) for each selected species and then applied for Europe only for the climate suitability of the species on the grid data used in the model. Baseline deer (cervidae) distributions (see Table S1) were generated using a dasymetric approach developed previously (Li et al. 2016) to disaggregate the species-specific deer population estimates (Apollonio et al. 2010) into the grid based on the observed range and habitat suitability (a function of species presence, climate suitability, and extent of suitable habitats). This involved calculating the generalized deer habitat suitability for a cell of deer presence as the sum of habitat suitability of the four deer species weighted by their country-level populations and re-scaled to [0,1]. Then, the cell-level deer population was estimated as total deer population in the country × cell suitability/suitability summed across all cells in the country. The generalized baseline habitat suitability for transmission hosts was estimated by summing up the suitability of the selected species, then dividing by six (the number of rodent species included in the study). Transmission host population was then simulated using the dynamic functions described in the “Host movement” section of this paper.
Projecting Future Changes in Key Disease Risk Drivers
Climate change. Climate change projections produced in the IMPRESSIONS project are based on the Intergovernmental Panel on Climate Change (IPCC) representative concentration pathway (RCP) emissions scenarios (RCP2.6, RCP4.5, and RCP8.5), covering a range of radiative forcing values by 2,100 relative to preindustrial levels (2.6, 4.5, and , respectively). Coupled general circulation–regional climate (GCM-RCM) models were used and projections were bias-adjusted (Madsen et al. 2016). A number of climate indices (10 temperature-based and 10 precipitation-based) were calculated on the grid for the whole of Europe, using the bias-adjusted daily projections. Climate projections were made available for the three future time slices (the 2020s, 2050s, and 2080s) for the integrated assessment of climate change impacts within the IMPRESSIONS project.
Combined scenarios of climate and socioeconomic change. The three RCPs considered were combined with four shared socioeconomic pathways (SSPs) representing trends in economics, demographics, lifestyles, technological development, governance, and other societal factors. The four SSPs are a) SSP1 (which describes a sustainable future with less inequality); b) SSP3 (which depicts a fragmented future with a low-level of economic growth and a seriously degraded environment); c) SSP4 (which portrays an unequal future of increased social, economic, and political disparities); and d) SSP5 (which refers to an economically driven future that is highly industrialized and fossil-fuel based). Land manager decisions are heavily influenced by socioeconomic factors, leading to potentially distinctive land-use patterns under different SSPs. The following combinations of RCPs and SSPs were considered in the IMPRESSIONS project, according to consistency in their underlying assumptions about energy use and associated emissions as explained in Madsen et al. (2016): SSP1 × RCP4.5, SSP3 × RCP4.5, SSP4 × RCP4.5, SSP3 × RCP8.5, and SSP5 × RCP8.5. In addition, we added the combination of SSP1 × RCP2.6 to cover lower-end climate change consistent with the Paris Agreement. Moreover, under each RCP, a number of coupled GCM-RCM models (see Table S2) were selected and used (Harrison et al. 2019; Madsen et al. 2016), leading to a total number of 20 model simulations to project future risk patterns under the six combined scenarios (see Table S3).
Land-use/cover change. Land-use/cover change is an important component of the IAP2 developed by the IMPRESSIONS project (Harrison et al. 2019; Holman et al. 2016) and which is based on the CLIMSAVE IAP (Harrison et al. 2015, 2016). The IMPRESSIONS IAP2 integrates a range of meta-models (reduced-form models that emulate the performance of more complex sectoral models, including agriculture, forests, biodiversity, urban development, water resources, and flooding) to simulate the cross-sectoral effects of climate and socioeconomic scenarios across Europe. The core function is provided by the Silsoe Whole Farm Model (SFARMOD) meta-model (Audsley et al. 2015), which allocates available rural land based on profitability and constraints from urban land use, irrigation availability, flood frequency, and food and timber demand. The productivity of different types of forests and crop/grassland systems under different management regimes and climate/soil characteristics are estimated by meta-models of the Growth Of Trees Is Limited by WAter model () (Gracia et al. 1999) and an agro-climatic simulation model (ROIMPEL) (Audsley et al. 2006), respectively. Urban land-use change is modeled by the Regional Urban Growth (RUG) meta-model (Reginster and Rounsevell 2006), which explores trends in urbanization as a function of population and gross domestic product, residential preferences (proximity to amenities, attractiveness of the coast), and strictness of the planning regulations to limit sprawl. Water availability is simulated by the WaterGAP (WGMM) meta-model (Wimmer et al. 2015) based on average river discharge. Food and timber demand constraints are associated with the socioeconomic scenarios. The two main factors affecting the extent of forest land are a) expansion/shrinkage of agricultural land to meet demand for agricultural products, such as cereals, white or red meat and bio-energy, taking into account changes in agricultural productivity, agricultural land management, food imports, population, and lifestyles (e.g., changes in diets); and b) the general awareness toward the environment, for example, the need for setting aside land for conservation. The change in forest land is further modified by the concentration assumed in the RCPs through a fertilization effect on forest growth. For future projections, change rates in the three habitat types estimated from the IMPRESSIONS IAP2 were applied to the baseline habitat layer.
Host distribution change. The generalized suitability of deer and transmission hosts was calculated using the same approach that was applied for the baseline. Future changes in deer population (generalized across the four species) were projected at the cell-level by a linear regressive function of habitat suitability developed based on the baseline deer population distribution (see Figure S1). The population of transmission host (generalized across the six species) was simulated using the functions introduced previously.
Disease Risk Mapping Exercises
Risk of LD was taken as the density of active infected nymphal ticks (DIN) at the cell level. As under each combined scenario, three to four coupled GCM-RCM models were selected and used; the final cell-level DIN was calculated by taking an average of the DIN values projected under different GCM-RCM models. To facilitate comparison of results between different combined scenarios, a fixed set of five risk levels were defined for comparing the changes in the extent of risk areas. The five levels were distinguished with the four quartiles of projected mean annual value of baseline DIN (per ha) at the cell level: null (); negligible (); low (); moderate (); and high (). Following the previous study (Li et al. 2016), all simulations were initialized with assumed initial densities of and for the total and infected active and host-seeking (or questing) nymphs, respectively, in forests. All results were recorded after 600 time-steps (50 y) to ensure potential dispersal of ticks to other suitable habitats had been completed and stabilized yearly cycles of ticks had been reached. The high-risk range during the peak season (PHR) was mapped because it represents the most risky areas and time for humans. The winter low-risk range (WLR) was of interest because public awareness of disease risks over winter is limited and a mild winter was found to be associated with greater tick infection prevalence in Europe (Estrada-Peña et al. 2011).
Evaluation of the Modeling Approach and Projections
Evaluations were conducted from two perspectives. Quantitatively, the projected baseline (2010) spatial patterns were compared with observational or estimated data from various sources to assess whether the model could correctly reproduce the patterns. Data used for comparison included tick distribution data and a B. burgdorferi s.l. prevalence map at the European level (Estrada-Peña et al. 2013, 2011), tick distribution maps in northern Europe (Jaenson et al. 2012; Jore et al. 2014; Laaksonen et al. 2017) and data on country-specific infection prevalence of ticks (Rauter and Hartung 2005). Qualitatively, a) the soundness of the structure of LYMERISK model in explaining the LD transmission system and b) the usefulness of our projections in representing the current risk distributions and stimulating discussions on adapting to future climate change were evaluated through discussion and questionnaires at several stakeholder workshops conducted between 2014 and 2016 in Hungary and Scotland, for which finer-scale disease risk modeling exercises were designed (Clarke et al. 2017).
Results
Model Uncertainty and Baseline Performances
In this study, the primary source of uncertainty arose from the scenario settings due to the inherent uncertainty in future socioeconomic and climate conditions. These scenarios derived from the IMPRESSIONS project, which has extensively explored their effects on the outputs of the IAP2 model as discussed by Harrison et al. (2019). A secondary source of uncertainty was related to the LYMERISK model, which depends on the extent to which model parameters influence the model outcomes. The results of a sensitivity analysis of the LYMERISK model were found to be identical to previous model sensitivity investigations (Li et al. 2014, 2016), with developmental mortality rate from engorged larvae into questing nymphs, efficiencies of systemic transmission, mortality rate of questing nymphs, basal mortality rate of feeding larvae, maximum attachment for larvae on transmission hosts, and transmission host-finding probabilities for larvae and nymphs being the most sensitive model parameters.
The model evaluation results suggest that model outputs are within the bounds of biological plausibility and an overall acceptance of the modeling approach and its results by stakeholders. Projected baseline (2010) seasonal variation of DIN (see Figure S2) showed two distinctive activity periods of infected ticks: low activity over the winter from December to January of the next year and peak activity between May and June (see Figure S3). Geographical limits are mainly driven by a) temperature (see Figure S4) in the north, with low temperature reducing the survival and shortening the questing period, and b) suitable habitat (forests) distribution (see Figure S5) in the south, which consequently affected host distribution (see Figure S6 for deer and Figure S7 for transmission host distribution). In the model, these result from temperature-driven activity rates and the season-dependent developmental and behavioral diapause. Such projected seasonal characteristics correspond well with existing knowledge (Gray et al. 2016; Kurtenbach et al. 2006).
Compared with the I. ricinus tick occurrence data set produced in Estrada-Peña et al. (2013), our model projected a wider distribution of I. ricinus nymphs in the north (see Figure S8). It should be noted that the area-without-occurrence records of Estrada-Peña et al. (2013) may be understood as unknown rather than tick absence due to the sparsity of biological records for this taxa. Furthermore, our projections are in good agreement with a number of other tick maps in Europe, including the tick distribution map of the European Centre for Disease Prevention and Control (ECDC 2019) and the national distribution maps produced for north European countries, that is, Norway (Jore et al. 2011), Sweden (Jaenson et al. 2012), and Finland (Laaksonen et al. 2017). Overall, the baseline model projection of tick distribution agrees with existing evidence.
A risk map of the B. burgdorferi s.l. distribution in Europe is available in the paper by Estrada-Peña et al. (2011). Our model projected a smaller range of infected ticks in the UK and Ireland, but a greater range in Spain (see Figure S9). For the UK, the projected distribution of DIN in Scotland is in good agreement with the LD risk map produced previously (Li et al. 2016). For Spain, our model may have overestimated the situation, or Estrada-Peña et al. (2011) may have underestimated it given that when training their model, they used only a limited data set from Spain (from Barral et al. 2002) based on ticks in the Basque Country, Spain). Moreover, the distribution of LD incidence in 2009 (Bonet Alavés et al. 2016) suggested that there may be a wider distribution of B. burgdorferi s.l. in Spain than Estrada-Peña et al. (2011) projected, assuming a certain amount of tick bites took place locally and close to the residence (Mulder et al. 2013). We further compared the results with data provided in a literature review of the Borrelia infection prevalence of nymphal and adult ticks in European countries (Rauter and Hartung 2005) using violin charts (see Figure S10). The observed infection prevalence for each country in general falls within the projected range, except for Portugal, where a study reported an infection prevalence of in adults.
Projected Changes in Risk Drivers by the 2050s under Combined Climate and Socioeconomic Scenarios
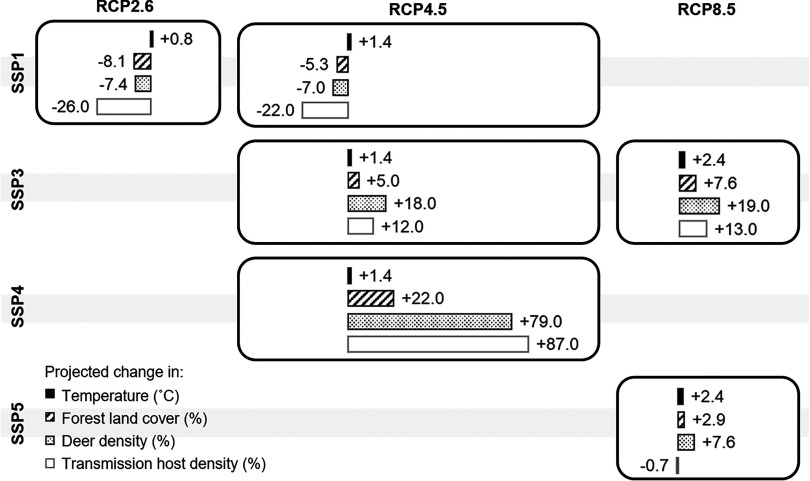
Taking averaged cell-level projections for each RCP, the projected overall increases in temperature by the 2050s across the European countries considered were 0.8, 1.4, and 2.4°C under RCP2.6, RCP4.5, and RCP8.5, respectively (Figure 3). Northern Europe was projected to experience relatively greater increases in temperature than other regions (see Table S4).
Figure 3.
Projected changes in disease risk drivers by the 2050s in Europe under the combined scenarios of plausible shared socioeconomic pathway (SSP) and representative concentration pathway (RCP) changes. Projected changes in temperature, forest land cover, and densities of deer and transmission hosts were summarized for each combined scenario by taking averaged values derived from different climate models under different combined scenarios.
On average, projected increases in forest land were greatest under SSP4 × RCP4.5 (by 22%) and decreases were projected under SSP1 × RCP2.6 (by 7.4%) and SSP1 × RCP4.5 (by 7.0%) (Figure 3). The increase in forest land under SSP4 was simulated because this scenario favors further intensification of agricultural production due to improvements in crop breeding and other agricultural technologies. This means that more food can be produced per hectare. This, combined with a lower population and increased food imports, would result in less land area being needed for food production, which would release land for forestry to expand. The decrease in forest land under the two SSP1 scenarios relates to a projected increase in extensively managed agriculture combined with decreases in food imports and increases in bioenergy and set-aside. Overall, this leads to more forest land being converted to agricultural areas to meet food demand.
The projected changes in the overall host density in general followed the changes in forest land. The greatest overall increase in deer and transmission host densities was projected under SSP4 × RCP4.5 (by 79% and 87%) and decreases were projected under SSP1 × RCP2.6 (by 7.4% and 26%) and SSP1 × RCP4.5 (by 7% and 22%). However, because climate suitability varied spatially, decreases in host densities were projected for southern Europe under all scenarios, except for SSP4 × RCP4.5, under which an increase in transmission host density (by 9.7%) was projected.
Projected Differences in Lyme Disease Risk between European Countries by the 2050s
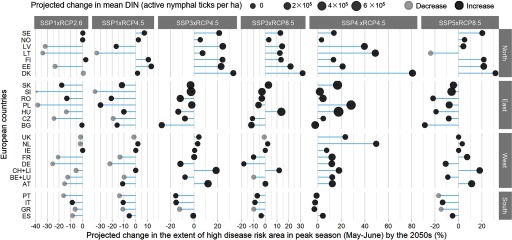
Projected country-level changes (by the 2050s) in the mean DIN and extent of PHR (peak season high-risk range, representing the time periods and areas of highest risk) across Europe (Figure 4) differed considerably between regions and between combined socioeconomic and climate scenarios. Even though all scenarios assumed increases in temperature, other drivers of disease risk such as forest land cover and host distribution were projected to change along different trajectories, resulting in highly heterogeneous and uncertain future risk patterns.
Figure 4.
Projected changes in Lyme disease risk by the 2050s in European countries. The extent of high disease risk area in peak season (%, lines in the figure) and mean density of infected nymphal ticks (per ha, closed circles in the figure, with light/dark gray indicating projected decrease/increase) are summarized across combined scenarios of the Shared Socioeconomic Pathway (SSP) and the Representative Concentration pathway (RCP) changes. The countries are classified into four geographical divisions (north, east, west, and south). Country abbreviations: AT, Austria; BE, Belgium; BG, Bulgaria; CH, Switzerland; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; GR, Greece; HU, Hungary; IE, Ireland; IT, Italy; LI, Liechtenstein; LT, Lithuania; LU, Luxembourg; LV, Latvia; NL, Netherlands; NO, Norway; PL, Poland; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia; SK, Slovakia; UK, United Kingdom.
Northern European countries, including Norway, Finland, and Sweden, were projected to have increased infected tick density and an extended high-risk range in the peak season under all scenarios. Although the distribution of forest land cover and host animals were projected to be relatively stable in these northern areas, the change in disease risks resulted from a projected overall increase in temperature, amplifying tick questing activity and prolonging the duration of the tick activity season, thereby leading to an expanded risk area toward higher altitudes and latitudes. In contrast, southern European countries, including Italy, Portugal, and Greece, were projected to have reduced areas of high risk during the peak season under all scenarios. In some scenarios, for example SSP1 × RCP2.6, this can be directly explained by a projected decrease in forested lands. In other scenarios with projected forest expansion, such as SSP4 × RCP4.5, this was due to a projected decline in climate suitability for hosts, leading to reduced host distributions and, hence, a reduced tick distribution.
Mapping the Risk Patterns: The Least and Most Risky Scenarios
The least risky scenario combination was SSP1 × RCP2.6, under which reductions in both infected tick density (mean DIN, Figure 4) and distribution (WLR, Figure 5C; PHR, Figure 5D) were projected in comparison with the baseline projection. The projected shrinkage in forest land cover (by ) played a major role because it restricted the distribution of ticks and was projected to lead to reductions in host density (by and for both deer and transmission hosts. Examples of projected changes in risk drivers using the MPI Earth System Model running on low resolution grid (MPI-ESM-LR); the regional climate model (REMO) (GCM/RCM) model are provided in panel B of Figures S4–S7. In addition, in the SSP1 × RCP2.6 scenario combination, the projected temperature increase was low () and so its effect on tick activity and the climate suitability of hosts was relatively minor. Even though a warmer climate allowed more ticks to be active in winter, the WLR was projected to reduce in general, owing to reduced forest land cover and host populations. Bulgaria was projected to have an increase in forest (by 5%) and hence an increase in WLR (by 11.8%). Northern Europe and the majority of the British Isles were projected to remain clear from risk during winter. The greatest decreases in WLR were projected for Portugal, France, and Spain by , , and of their territories, respectively. Moreover, at the country level, almost all countries were projected to have a reduced WLR, except for three northern countries, Norway, Finland, and Sweden, which had marginal increases (by 0.03–2.7%) and slight expansions of WLR toward the north. This was due to the relatively greater increases in temperature (by 1.0–1.4°C) projected for these three countries. The greatest size reductions in WLR were projected for Slovenia, Poland, and Lithuania, with , , and of their territories, respectively.
Figure 5.
Projected baseline and future Lyme disease risk ranges under SSP1 × RCP2.6 and SSP4 × RCP4.5 scenarios. The projected distribution of risk indicator, DIN (infected nymphal ticks per ha), at the baseline of 2010 in (A) winter and (B) peak season are classified into five levels: null (), negligible (), low (), moderate (), and high (). Among the six combined scenarios considered, the SSP1 × RCP2.6 represents the least risky future in which the low emissions scenario (RCP2.6) is combined with a sustainability socioeconomic scenario (SSP1). Both the (C) winter low-risk range and (D) peak season high-risk range were projected to likely decrease by the 2050s. The blue/red color gradients indicate agreement among projections under different climate models, with darker meaning higher agreement. The patterned blue/red area refers to the baseline projections for comparison. The SSP4 × RCP4.5 is the most risky future in which the intermediate emissions scenario (RCP4.5) is combined with an unequal future of increased socioeconomic disparities (SSP4), under which an overall increase and geographical changes in both (E) winter low-risk range and (F) peak season high-risk range by the 2050s were projected.
The most risky scenario combination was under SSP4 × RCP4.5, with projected increases in all mean DIN (Figure 3), WLR (Figure 4E), and PHR (Figure 4F), resulting from the projected increase in forest land (by 21.7%) and temperature (by 1.4°C). In addition, host densities were projected to increase dramatically (by 79% and 87% for deer and transmission hosts, respectively), as a result of increased forest land, which outweighed the effect of a projected decrease in climate suitability in some regions (especially in the south) under this intermediate climate change scenario. Projections of risk driver changes using the Hadley Global Environment Model 2 - Earth System (HadGEM2-ES); the Rossby Centre regional atmospheric model (RAC4) (GCM/RCM) model are provided in panel C of Figures S4–S7. Because more habitats and hosts were projected to be available, and a warmer climate was projected to allow more ticks to be active during winter, the risky area during winter (i.e., WLR) was projected to expand in general. Portugal was the only exception; it had a decrease of 7.1% in the size of WLR due to projected declines in climate suitability and losses of deer density by 21.4%. Northern Europe and the majority of the British Isles were projected to face a dramatic expansion of risk during winter, with the greatest increases occurring in the Netherlands, Hungary, and Denmark (by 51.4%, 50.3%, and 38.1% of their territories, respectively). Furthermore, almost all countries considered were projected to have expanded PHR except for three southern countries (Greece, Italy, and Portugal) and one eastern country (Bulgaria), which had only marginal shrinkages in PHR of between 1% and 3% due to more uneven distributions of host animals in response to reduced climate suitability in some areas. However, because the mean DIN was projected to increase in all countries, people who frequently use forest habitats in these countries may face greater risks of being in contact with infected ticks. The greatest increases in PHR were projected for Denmark (by 80.5% of its territory), the Netherlands (by 49.6%), and Lithuania (by 48.9%).
Discussion
This study used an integrated modeling approach to explore possible future patterns of the DIN of I. ricinus ticks, the key ecological risk indicator of LD. The approach was applied to several scenarios of combined changes in climate, socioeconomics, and land use that were codeveloped with stakeholders. The modeling approach integrated multidisciplinary knowledge and was accepted by the stakeholders engaged in the study for its usefulness in explaining changing LD risk patterns and stimulating discussions on climate change adaptation strategies.
Unlike other vector-borne diseases such as dengue, for which most empirical studies have found that temperature plays a dominating role in disease transmission (Messina et al. 2015), transmission of tick-borne diseases in the natural environment is often associated with a much broader range of factors than only temperature, including, for instance, host populations (Estrada-Peña and de la Fuente 2017) and habitats (Lambin et al. 2010). However, existing studies on future LD risk patterns over Europe have mostly adopted an associative approach by relating the ecological risk (density of ticks) to climate variables only (Alkishe et al. 2017; Williams et al. 2015). Similarly, studies projecting the basic reproduction number () of tick populations in Canada were also temperature-driven (McPherson et al. 2017; Ogden et al. 2014). By taking advantage of recent developments in agent-based modeling of pathogen dynamics, continental-level projections of land-use/cover change and climate suitability modeling of host distributions, we were able to project complex interactions between pathogens and ticks and their host animals over heterogeneous landscapes.
The nymphal life stage was targeted because it causes the majority of human LD infections (LoGiudice et al. 2003). We investigated how risk may be influenced by changes in a few key disease drivers including temperature, forest land cover, and host distribution. Even though tick questing activity and pathogen transmission were projected to increase as the climate warms, socioeconomic drivers that restricted the extent of habitats were projected to play a regulating role in shaping the spatial patterns of DIN. Because these disease risk drivers were projected to be evolving toward different cardinal directions under different scenarios, the overall future geographical pattern of DIN in Europe (up to the 2050s) was projected to be rather uncertain and may be either reduced or extended, depending on the region of Europe and the scenario combination in question.
Different roles of the disease drivers on DIN pattern were projected for different regions, implying different targets for regional policies for health risk reduction. The northern limit of tick distribution in Scandinavia was projected to expand under all scenarios given the projected temperature rises and relatively stable patterns of forests and host distributions. These findings are in line with a number of empirical studies (Jaenson et al. 2012; Jore et al. 2014), emphasizing the direct impacts of climate change on tick distribution in this region. In the Baltic region, where climate change alone was not found to be able to offer a sufficient explanation for the observed patterns of tick-borne encephalitis (Sumilo et al. 2007), our projections suggest a highly uncertain spatiotemporal pattern for LD risk due to large projected variations in land use under the different socioeconomic scenarios. The projected reduction in DIN in many Mediterranean areas was due to a projected decrease in forest land driven by the extensification of agriculture in southern Europe under most scenarios. Such extensification results from a loss in agricultural profitability due to reduced crop productivity as the climate changes (associated with heat and drought stress and irrigation water availability), based on both empirical evidence and model simulations (Holman et al. 2017).
Although similarities were found between the results of our study and relevant climate niche modeling studies (Alkishe et al. 2017; Williams et al. 2015)—such as an expansion of LD risk in northern Europe and gradually decreased suitability in southern Europe—there were several clear differences. First, the greatest spatial expansion in risk was not found under the most high-end climate scenario (RCP8.5) but, instead, under the intermediate climate scenario (RCP4.5) when combined with a socioeconomic scenario (SSP4) describing an unequal future of increased social, economic, and political disparities. Second, LD risks were not always projected to be amplified alongside a temperature increase (i.e., RCPs 2.6, 4.5, and 8.5), and the risk could even be reduced under the low-emission climate change scenario when associated with a sustainable socioeconomic scenario (i.e., SSP1 × RCPs 2.6). Third, the reduction in LD risk in southern Europe was projected to be less uncertain in our study because of the likely decrease in forest land as a consequence of agricultural extensification, as explained earlier in this section.
Tackling climate change could offer significant global health benefits because many mitigation and adaptation responses to climate change are directly linked to reductions of disease burden (Watts et al. 2015). Hence, it has been recognized that an improved understanding of the health risks of climate change and projections of future health risk changes are important for policy makers to take proactive actions to protect populations and communities from the adverse effects of climate change (Hosking and Campbell-Lendrum 2012; Tong et al. 2016). It is within this context that our study provides a useful and comprehensive example of how the health risks associated with socioeconomic and climate change could be assessed using scenario analysis. Model projections further pinpoint the importance of understanding ecosystem service trade-offs (Shackleton et al. 2016) to reduce maladaptation, especially for the Mediterranean areas where forest land were projected to decrease and lead to LD risk reduction. Future forest-based adaptation strategies should be carefully implemented based on a full evaluation across all the costs and benefits provided by forest ecosystems.
Uncertainties and potential errors in our integrated modeling approach arose from several aspects. For the LYMERISK model, a number of simplifying assumptions, such as the generalization of host and pathogen species and habitat types, were applied in order to balance model complexity and payoff, following the principles of the pattern-oriented modeling approach (Grimm et al. 2005). The sensitivity and shortcomings of the model components on pathogen transmission and strategies for host distribution mapping have already been discussed in detail by Li et al. (2016). Future research is needed to improve understanding of the key factors determining the success of pathogen transmission (e.g., efficiencies of pathogen transmission, host-finding probabilities) in natural environments as well as a more realistic representation of host population dynamics because these are highly sensitive parameters where empirical information is the least reliable. In particular, an improved knowledge and representation of habitat and host diversity and their relationships to B. burgdorferi s.l. transmission is required to account for the possible spatial impacts of forest fragmentation (and connectivity) and poor habitat and reservoir-incompetent host species on disease risk at large scales. Given the limited quantitative data on the effect of cold temperature on tick mortality, we had to rely on the experimental data (MacLeod 1935), and so the model assumed that I. ricinus survival falls from almost 100% at to zero at . In reality, it is likely that some ticks in their natural environment may survive in refuges where they are more protected from very low air temperatures. Field evidence on the direct role that climate plays on the desiccation effects on tick survival at the northern and southern edges of existing I. ricinus distribution may help improve the current projections and aid further application of the LYMERISK model in North Africa. Moreover, studies that focus on the inclusion of human behavior in LD risk modeling approaches are also important because this determines exposure in the ecosystem. Exposure is more sensitive to socioeconomic changes because it is related to population distribution, migration, and forest visitation patterns at various scales (Li et al. 2015, 2017). Addressing exposure risk over short time horizons with finer time steps can therefore help in targeting people at risk and raising awareness in a timely manner.
Because human exposure is an important determinant of the public health outcome of B. burgdorferi s.l. circulation, details of future land planning under various socioeconomic scenarios, such as the SSPs used in this study, will be relevant to examine. Previous research has highlighted the role of loose building in fragmented forests (Linard et al. 2007), and more extensive knowledge of the effect of various forms of land planning will be useful but is largely lacking at this point. Furthermore, given the high complexity of landscape decision-making and the multiple demands on landscapes it is essential that any planning and policy not be undertaken in silos but, rather, planning and policy should take into account the interdependencies between sectors and how they are affected by multiple pressures. The IMPRESSIONS IAP2 used here to project habitat change attempts to do this. Work with stakeholders revealed three key ways in which people can help to move toward a sustainable future: a) using integrated resource management that takes account of the multifunctional nature of our landscapes and aims to ensure both resource security and environmental protection while moving toward self-sufficiency; b) shifting toward sustainable lifestyles through education and awareness raising; and c) using good governance with longer-term visions based on sustainability, transparency, and participation. These recommendations consider the need to minimize human exposure to LD as just one of many demands on policy design.
In conclusion, this study projected several potential risk scenarios for LD in Europe by taking into account the potential impacts of socioeconomic and climate changes. The models projected that the greatest increase in LD risk might not necessarily always be under the most high-end climate change scenario and, in the best case scenario, the risk could decrease. Projected regional differences in LD risk resulted from mixed effects of temperature, land use, and host distributions, suggesting region-specific and cross-sectoral foci for LD risk management policy.
Supplementary Material
Acknowledgments
The research leading to these results was partially funded by the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement number 603416 (the IMPRESSIONS Project – Impacts and Risks from High-End Scenarios: Strategies for Innovative Solutions; http://www.impressions-project.eu). L.G. was supported by the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS). B.V.P. was supported by the Natural Environment Research Council under the Sustainable Use of Natural Resources to Improve Human Health and Support Economic Development (SUNRISE) project (grant NE/R000131/1).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4615).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alkishe AA, Peterson AT, Samy AM. 2017. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One 12(12):e0189092, PMID: 29206879, 10.1371/journal.pone.0189092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341(6145):514–519, PMID: 23908230, 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Amirpour Haredasht S, Barrios JM, Maes P, Verstraeten WW, Clement J, Ducoffre G, et al. . 2011. A dynamic data-based model describing nephropathia epidemica in Belgium. Biosyst Eng 109(1):77–89, 10.1016/j.biosystemseng.2011.02.004. [DOI] [Google Scholar]
- Apollonio M, Andersen R, Putman R. 2010. European Ungulates and Their Management in the 21st Century. Cambridge, UK:Cambridge University Press. [Google Scholar]
- Audsley E, Pearn KR, Simota C, Cojocaru G, Koutsidou E, Rounsevell MDA, et al. . 2006. What can scenario modelling tell us about future European scale agricultural land use, and what not? Environ Sci Policy 9(2):148–162, 10.1016/j.envsci.2005.11.008. [DOI] [Google Scholar]
- Audsley E, Trnka M, Sabaté S, Maspons J, Sanchez A, Sandars D, et al. . 2015. Interactively modelling land profitability to estimate European agricultural and forest land use under future scenarios of climate, socio-economics and adaptation. Clim Change 128(3–4):215–227, 10.1007/s10584-014-1164-6. [DOI] [Google Scholar]
- Barandika JF, Hurtado A, Juste RA, García-Pérez AL. 2010. Seasonal dynamics of Ixodes ricinus in a 3-year period in northern Spain: first survey on the presence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis 10(10):1027–1035, PMID: 20455780, 10.1089/vbz.2009.0148. [DOI] [PubMed] [Google Scholar]
- Barral M, García-Pérez AL, Juste RA, Hurtado A, Escudero R, Sellek RE, et al. . 2002. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus (Acari: Ixodidae) ticks from the Basque Country, Spain. J Med Entomol 39(1):177–184, PMID: 11931254, 10.1603/0022-2585-39.1.177. [DOI] [PubMed] [Google Scholar]
- Bonet Alavés E, Guerrero Espejo A, Cuenca Torres M, Gimeno Vilarrasa F. 2016. Incidencia de la enfermedad de Lyme en España [in Spanish]. Med Clin (Barc) 147(2):88–89, PMID: 26971976, 10.1016/j.medcli.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Campbell JA. 1948. Life History and Development of the Sheep Tick Ixodes Ricinus Linnaeus in Scotland, under Natural and Controlled Conditions [Dissertation]. Edinburgh, UK:University of Edinburgh. [Google Scholar]
- Clarke L, Rounsevell M, Dunn M, Lourenço TC, Tàbara D, Pinter L, et al. . 2017. Climate Change Impacts, Adaptation and Vulnerability Model Applications in Three Regional to Local Scale Case Studies in Europe, EU FP7 IMPRESSIONS Project Deliverable D3C.2. Prepared under contract from the European Commission by the Natural Environment Research Council Centre for Ecology and Hydrology (Grant agreement No 603416).
- Dobson ADM, Finnie TJR, Randolph SE. 2011. A modified matrix model to describe the seasonal population ecology of the European tick Ixodes ricinus. J Appl Ecol 48(4):1017–1028, 10.1111/j.1365-2664.2011.02003.x. [DOI] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control). 2019. Tick maps [internet]. https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps [accessed 10 June 2019].
- Egyed L, Elő P, Sréter-Lancz Z, Széll Z, Balogh Z, Sréter T. 2012. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis 3(2):90–94, PMID: 22445929, 10.1016/j.ttbdis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, de la Fuente J. 2017. Host distribution does not limit the range of the tick Ixodes ricinus but impacts the circulation of transmitted pathogens. Front Cell Infect Microbiol 7:405, PMID: 29085806, 10.3389/fcimb.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Farkas R, Jaenson TGT, Koenen F, Madder M, Pascucci I, et al. . 2013. Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the western Palearctic. A digital data set. Exp Appl Acarol 59(3):351–366, PMID: 22843316, 10.1007/s10493-012-9600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Ortega C, Sánchez N, DeSimone L, Sudre B, Suk JE, et al. . 2011. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl Environ Microbiol 77(11):3838–3845, PMID: 21498767, 10.1128/AEM.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Ostfeld RS, Peterson AT, Poulin R, de la Fuente J. 2014. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol 30(4):205–214, PMID: 24636356, 10.1016/j.pt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Aungier J, Tomkins JL. 2014. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: implications for resilience to climate change? Ecol Evol 4(7):1186–1198, PMID: 24772293, 10.1002/ece3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CA, Tello E, Sabaté S, Bellot J. 1999. GOTILWA: an integrated model of water dynamics and forest growth. In: Ecology of Mediterranean Evergreen Oak Forests. Berlin, Germany:Springer, 163–179. [Google Scholar]
- Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. 2009. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis 2009:593232, PMID: 19277106, 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Kahl O, Lane RS, Levin ML, Tsao JI. 2016. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis 7(5):992–1003, PMID: 27263092, 10.1016/j.ttbdis.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm V, Revilla E, Berger U, Jeltsch F, Mooij WM, Railsback SF, et al. . 2005. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science 310(5750):987–991, PMID: 16284171, 10.1126/science.1116681. [DOI] [PubMed] [Google Scholar]
- Harrison PA, Berry PM, Butt N, New M. 2006. Modelling climate change impacts on species’ distributions at the European scale: implications for conservation policy. Environ Sci Policy 9(2):116–128, 10.1016/j.envsci.2005.11.003. [DOI] [Google Scholar]
- Harrison PA, Dunford RW, Holman IP, Cojocaru G, Madsen MS, Chen PY, et al. . 2019. Differences between low-end and high-end climate change impacts in Europe across multiple sectors. Reg Environ Change 19(3):695–709, 10.1007/s10113-018-1352-4. [DOI] [Google Scholar]
- Harrison PA, Dunford RW, Holman IP, Rounsevell M. 2016. Climate change impact modelling needs to include cross-sectoral interactions. Nat Clim Change 6(9):885–890, 10.1038/nclimate3039. [DOI] [Google Scholar]
- Harrison PA, Holman IP, Berry PM. 2015. Assessing cross-sectoral climate change impacts, vulnerability and adaptation: an introduction to the CLIMSAVE project. Clim Change 128(3–4):153–167, 10.1007/s10584-015-1324-3. [DOI] [Google Scholar]
- Hartemink N, Vanwambeke SO, Purse BV, Gilbert M, Van Dyck H. 2015. Towards a resource-based habitat approach for spatial modelling of vector-borne disease risks. Biol Rev Camb Philos Soc 90(4):1151–1162, PMID: 25335785, 10.1111/brv.12149. [DOI] [PubMed] [Google Scholar]
- Holman I, Audsley E, Berry P, Brown C, Bugmann H, Clarke L, et al. . 2015. Specification for European model improvement and development. IMPRESSIONS Deliverable D3B.1. Prepared under contract from the European Commission by the Natural Environment Research Council Centre for Ecology and Hydrology (Grant agreement No 603416).
- Holman IP, Brown C, Janes V, Sandars D. 2017. Can we be certain about future land use change in Europe? A multi-scenario, integrated-assessment analysis. Agric Syst 151:126–135, PMID: 28163353, 10.1016/j.agsy.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman IP, Harrison PA, Metzger MJ. 2016. Cross-sectoral impacts of climate and socio-economic change in Scotland: implications for adaptation policy. Reg Environ Change 16(1):97–109, 10.1007/s10113-014-0679-8. [DOI] [Google Scholar]
- Hosking J, Campbell-Lendrum D. 2012. How well does climate change and human health research match the demands of policymakers? A scoping review. Environ Health Perspect 120(8):1076–1082, PMID: 22504669, 10.1289/ehp.1104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. 2012. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors 5:8–22, PMID: 22233771, 10.1186/1756-3305-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore S, Vanwambeke SO, Viljugrein H, Isaksen K, Kristoffersen AB, Woldehiwet Z, et al. . 2014. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit Vectors 7:11, PMID: 24401487, 10.1186/1756-3305-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore S, Viljugrein H, Hofshagen M, Brun-Hansen H, Kristoffersen AB, Nygård K, et al. . 2011. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasit Vectors 4:84:, PMID: 21595949, 10.1186/1756-3305-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa J. 1964. Movement, activity and distribution of the small rodents Clethrionomys glareolus and Apodemus sylvaticus in woodland. J Anim Ecol 33(2):259–299, 10.2307/2631. [DOI] [Google Scholar]
- Kilpatrick AM, Dobson ADM, Levi T, Salkeld DJ, Swei A, Ginsberg HS, et al. . 2017. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc Lond B Biol Sci 372(1722):20160117, PMID: 28438910, 10.1098/rstb.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Hay SI, Pigott DM, Smith DL, Wint GRW, Golding N. 2016. Progress and challenges in infectious disease cartography. Trends Parasitol 32(1):19–29, PMID: 26604163, 10.1016/j.pt.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4(9):660–669, PMID: 16894341, 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- Laaksonen M, Sajanti E, Sormunen JJ, Penttinen R, Hänninen J, Ruohomäki K, et al. . 2017. Crowdsourcing-based nationwide tick collection reveals the distribution of Ixodes ricinus and I. persulcatus and associated pathogens in Finland. Emerg Microbes Infect 6(5):1–7, PMID: 28487561, 10.1038/emi.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. 2010. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr 9:54, PMID: 20979609, 10.1186/1476-072X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Colson V, Lejeune P, Speybroeck N, Vanwambeke SO. 2015. Agent-based modelling of the spatial pattern of leisure visitation in forests: a case study in Wallonia, south Belgium. Environ Model Softw 71:111–125, 10.1016/j.envsoft.2015.06.001. [DOI] [Google Scholar]
- Li S, Gilbert L, Harrison PA, Rounsevell M. 2016. Modelling the seasonality of Lyme disease risk and the potential impacts of a warming climate within the heterogeneous landscapes of Scotland. J R Soc Interface 13(116):20160140, PMID: 27030039, 10.1098/rsif.2016.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hartemink N, Speybroeck N, Vanwambeke SO. 2012a. Consequences of landscape fragmentation on Lyme disease risk: a cellular automata approach. PLoS One 7(6):e39612, PMID: 22761842, 10.1371/journal.pone.0039612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Heyman P, Cochez C, Simons L, Vanwambeke SO. 2012b. A multi-level analysis of the relationship between environmental factors and questing Ixodes ricinus dynamics in Belgium. Parasit Vectors 5:149, PMID: 22830528, 10.1186/1756-3305-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Juhász-Horváth L, Pedde S, Pintér L, Rounsevell MDA, Harrison PA. 2017. Integrated modelling of urban spatial development under uncertain climate futures: a case study in Hungary. Environ Model Softw 96:251–264, 10.1016/j.envsoft.2017.07.005. [DOI] [Google Scholar]
- Li S, Vanwambeke SO, Licoppe AM, Speybroeck N. 2014. Impacts of deer management practices on the spatial dynamics of the tick Ixodes ricinus: a scenario analysis. Ecol Modell 276:1–13, 10.1016/j.ecolmodel.2013.12.023. [DOI] [Google Scholar]
- Linard C, Lamarque P, Heyman P, Ducoffre G, Luyasu V, Tersago K, et al. . 2007. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. Int J Health Geogr 6:15, PMID: 17474974, 10.1186/1476-072X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A 100(2):567–571, PMID: 12525705, 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod J. 1935. Ixodes ricinus in relation to its physical environment: II. The factors governing survival and activity. Parasitology 27(1):123–144, 10.1017/S0031182000015006. [DOI] [Google Scholar]
- Macleod J. 1936. Ixodes ricinus in relation to its physical environment: IV. An analysis of the ecological complexes controlling distribution and activities. Parasitology 28(3):295–319, 10.1017/S0031182000022502. [DOI] [Google Scholar]
- Madsen MS, Maule CF, Christensen JH, Fronzek S, Carter T. 2016. IMPRESSIONS Climate Scenarios, EU FP7 IMPRESSIONS Project Deliverable D2.3. Prepared under contract from the European Commission by the Natural Environment Research Council Centre for Ecology and Hydrology (Grant agreement No 603416).
- McPherson M, García-García A, Cuesta-Valero FJ, Beltrami H, Hansen-Ketchum P, Macdougall D, et al. . 2017. Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environ Health Perspect 125(5):057008, PMID: 28599266, 10.1289/EHP57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, et al. . 2013. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6:1, PMID: 23281838, 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP, Brady OJ, Pigott DM, Golding N, Kraemer MUG, Scott TW, et al. . 2015. The many projected futures of dengue. Nat Rev Microbiol 13(4):230–239, PMID: 25730702, 10.1038/nrmicro3430. [DOI] [PubMed] [Google Scholar]
- Milne A. 1949a. The ecology of the sheep tick, Ixodes ricinus L.: host relationships of the tick: part 1. Review of previous work in Britain. Parasitology 39(3–4):167–172, PMID: 18112000, 10.1017/S0031182000083724. [DOI] [PubMed] [Google Scholar]
- Milne A. 1949b. The ecology of the sheep tick, Ixodes ricinus L.: host relationships of the tick: part 2. Observations on hill and moorland grazings in northern England. Parasitology 39(3–4):173–197, PMID: 18124151, 10.1017/S0031182000083736. [DOI] [PubMed] [Google Scholar]
- Milne A. 1950. The ecology of the sheep tick, Ixodes ricinus L.: microhabitat economy of the adult tick. Parasitology 40(1–2):14–34, PMID: 15401167, 10.1017/S0031182000017820. [DOI] [PubMed] [Google Scholar]
- Morellet N, Bonenfant C, Börger L, Ossi F, Cagnacci F, Heurich M, et al. . 2013. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. J Anim Ecol 82(6):1326–1339, PMID: 23855883, 10.1111/1365-2656.12105. [DOI] [PubMed] [Google Scholar]
- Mulder S, van Vliet AJH, Bron WA, Gassner F, Takken W. 2013. High risk of tick bites in Dutch gardens. Vector Borne Zoonotic Dis 13(12):865–871, PMID: 24107214, 10.1089/vbz.2012.1194. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. 1997. Alternative projections of mortality and disability by cause 1998–2020: Global Burden of Disease Study. Lancet 349(9064):1498–1504, PMID: 9167458, 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Mysterud A. 1999. Seasonal migration pattern and home range of roe deer (Capreolus capreolus) in an altitudinal gradient in southern Norway. J Zoology 247(4):479–486, 10.1017/S0952836999004070. [DOI] [Google Scholar]
- Mysterud A, Easterday WR, Stigum VM, Aas AB, Meisingset EL, Viljugrein H. 2016. Contrasting emergence of Lyme disease across ecosystems. Nat Commun 7:11882, PMID: 27306947, 10.1038/ncomms11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Radojević M, Wu X, Duvvuri VR, Leighton PA, Wu J. 2014. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect 122(6):631–638, PMID: 24627295, 10.1289/ehp.1307799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Brunner JL. 2015. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci 370(1665):20140051, PMID: 25688022, 10.1098/rstb.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, et al. . 2015. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos Trans R Soc Lond B Biol Sci 370(1665):20130551, PMID: 25688012, 10.1098/rstb.2013.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RG, Dawson TP, Berry PM, Harrison PA. 2002. SPECIES: a Spatial Evaluation of Climate Impact on the Envelope of Species. Ecol Modell 154(3):289–300, 10.1016/S0304-3800(02)00056-X. [DOI] [Google Scholar]
- Pérez D, Kneubühler Y, Rais O, Gern L. 2012. Seasonality of Ixodes ricinus ticks on vegetation and on rodents and Borrelia burgdorferi sensu lato genospecies diversity in two Lyme Borreliosis–endemic areas in Switzerland. Vector Borne Zoonotic Dis 12(8):633–644, PMID: 22607074, 10.1089/vbz.2011.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret J-L, Guigoz E, Rais O, Gern L. 2000. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol Res 86(7):554–557, PMID: 10935905, 10.1007/s004360000209. [DOI] [PubMed] [Google Scholar]
- Pfäffle M, Littwin N, Muders SV, Petney TN. 2013. The ecology of tick-borne diseases. Int J Parasitol 43(12–13):1059–1077, PMID: 23911308, 10.1016/j.ijpara.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Quine CP, Barnett J, Dobson ADM, Marcu A, Marzano M, Moseley D, et al. . 2011. Frameworks for risk communication and disease management: the case of Lyme disease and countryside users. Philos Trans R Soc Lond B Biol Sci 366(1573):2010–2022, PMID: 21624921, 10.1098/rstb.2010.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qviller L, Grøva L, Viljugrein H, Klingen I, Mysterud A. 2014. Temporal pattern of questing tick Ixodes ricinus density at differing elevations in the coastal region of western Norway. Parasit Vectors 7:179, PMID: 24725997, 10.1186/1756-3305-7-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE. 2001. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Philos Trans R Soc Lond B Biol Sci 356(1411):1045–1056, PMID: 11516382, 10.1098/rstb.2001.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by Ixodid ticks as vectors. Parasitology 129(Suppl):S37–S65, PMID: 15938504, 10.1017/S0031182004004925. [DOI] [PubMed] [Google Scholar]
- Rauter C, Hartung T. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 71(11):7203–7216, PMID: 16269760, 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster I, Rounsevell M. 2006. Scenarios of future urban land use in Europe. Environ Plan B Urban Anal City Sci 33(4):619–636, 10.1068/b31079. [DOI] [Google Scholar]
- Sauvage F, Langlais M, Pontier D. 2007. Predicting the emergence of human hantavirus disease using a combination of viral dynamics and rodent demographic patterns. Epidemiol Infect 135(1):46–56, PMID: 16753079, 10.1017/S0950268806006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage F, Langlais M, Yoccoz NG, Pontier D. 2003. Modelling hantavirus in fluctuating populations of bank voles: the role of indirect transmission on virus persistence. J Anim Ecol 72(1):1–13, 10.1046/j.1365-2656.2003.00675.x. [DOI] [Google Scholar]
- Shackleton CM, Ruwanza S, Sinasson Sanni GK, Bennett S, De Lacy P, Modipa R, et al. . 2016. Unpacking Pandora’s box: understanding and categorising ecosystem disservices for environmental management and human wellbeing. Ecosystems 19(4):587–600, 10.1007/s10021-015-9952-z. [DOI] [Google Scholar]
- Sumilo D, Asokliene L, Bormane A, Vasilenko V, Golovljova I, Randolph SE. 2007. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS One 2(6):e500, PMID: 17551580, 10.1371/journal.pone.0000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Lafourcade B, Engler R, Araújo MB. 2009. BIOMOD – a platform for ensemble forecasting of species distributions. Ecography 32(3):369–373, 10.1111/j.1600-0587.2008.05742.x. [DOI] [Google Scholar]
- Tong S, Confalonieri U, Ebi K, Olsen J. 2016. Managing and mitigating the health risks of climate change: calling for evidence-informed policy and action. Environ Health Perspect 124(10):A176–A179, PMID: 27689449, 10.1289/EHP555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, et al. . 2015. Health and climate change: policy responses to protect public health. Lancet 386(10006):1861–1914, PMID: 26111439, 10.1016/S0140-6736(15)60854-6. [DOI] [PubMed] [Google Scholar]
- Williams HW, Cross DE, Crump HL, Drost CJ, Thomas CJ. 2015. Climate suitability for European ticks: assessing species distribution models against null models and projection under AR5 climate. Parasit Vectors 8:440, PMID: 26310856, 10.1186/s13071-015-1046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer F, Audsley E, Malsy M, Savin C, Dunford R, Harrison PA, et al. . 2015. Modelling the effects of cross-sectoral water allocation schemes in Europe. Clim Change 128(3–4):229–244, 10.1007/s10584-014-1161-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.