Abstract
Background:
Indoor fine particulate air pollution (PM2.5) is linked to asthma morbidity; however, whether vitamin D status influences individual susceptibility to airborne exposures is unclear.
Objective:
We aimed to determine if vitamin D modifies effects of indoor PM2.5 upon asthma symptoms in urban children.
Methods:
120 children aged 5–12 years with physician-diagnosed asthma were evaluated at baseline and every 3 months for 9 months. Indoor PM2.5, serum 25-hydroxy vitamin D (25-OH D) levels and asthma symptoms were simultaneously assessed at each time point. Adjusting for confounders, generalized estimating equations assessed 3-way interaction effects of 25-OH D, obesity and PM upon asthma symptoms.
Results:
Children were of mean (SD) age 9.7 (2.2) years, 36% were obese, and 95% self-reported black race. Mean (SD) PM2.5 indoor exposure was 38.2 (42.9) μg/m3 and 25-OH D was 19.1 (7.5) ng/ml. Three-way interaction models demonstrated significantly greater PM2.5-associated effects on daytime asthma symptoms only among obese children with low 25-OH D levels (ORPM2.5=1.26,p =0.049 at vitamin D=15.5 ng/ml, increasingly stronger PM effects at levels<15.5 ng/ml). In homes with increased PM2.5, higher 25-OH D was associated with decreased symptom odds (e.g., ORVitamin D = 0.87; p=0.049 at PM2.5 = 52.5 μg/m3, increasingly protective effects >52.5 μg/m3) among obese children.
Conclusion:
Among obese urban children with asthma, low individual 25-OH vitamin D enhanced adverse respiratory effects associated with indoor PM2.5. In high PM2.5 environments, 25-OH D was protective against asthma symptoms. Optimizing vitamin D status in children may help reduce asthma morbidity driven by indoor air pollution.
Keywords: Vitamin D, asthma, particulate matter, obesity
Introduction
Asthma is the most common chronic disease of childhood in the United States,1 with the burden of disease disproportionately affecting urban minority populations such as blacks.2–4 While a variety of environmental factors may explain high asthma morbidity in urban populations,5,6 there is strong evidence demonstrating that indoor fine particulate matter (PM2.5) within urban homes of children with asthma contributes to increased respiratory morbidity, including greater symptoms and medication use.7,8 Conversely, dietary factors, including intake of food components thought to have anti-oxidant, anti-allergic, or anti-inflammatory properties, have independently been shown to be protective against asthma outcomes.9–12 Despite these published relationships, whether personal nutritional status influences the susceptibility to the respiratory effects of indoor air pollution in children is unknown. Determining individual protective factors against urban indoor pollution in vulnerable populations may inform public health strategies aimed at mitigating its adverse effects.
Specifically, growing evidence for vitamin D’s role in asthma has suggested a link between low 25-hydroxy (OH) vitamin D serum levels and worse asthma health in pediatric populations,13 leading to increased respiratory symptoms and exacerbations,14,15 impaired lung function,16–20 and hospitalizations.21 Furthermore, mechanistic studies have suggested protective effects of vitamin D against both allergic and pollutant-induced airway disease through anti-oxidant and immune-modulatory pathways.22–30 This is especially relevant to black pediatric populations who have the highest rates of vitamin D deficiency in the country, with increasing rates of insufficiency and deficiency over the last 2 decades,31 likely due to a combination of reduced UV-mediated dermal production and poor dietary intake.32 Furthermore, urban minority populations, such as blacks, are disproportionately overweight and obese,33 which not only independently contributes to asthma-related morbidity,34–36 but may also enhance the respiratory effects of indoor PM.37,38 Taken together, a coincidence of high levels of exposure to urban indoor air pollution, known risks for suboptimal vitamin D status,32,39,40 and greater obesity among blacks, raises the question of whether inadequate vitamin D status may render children in such populations more vulnerable to the adverse respiratory effects of indoor pollutant exposures, and may help to explain the disproportionate asthma morbidity within this population.
Therefore, the aim of this analysis was to investigate if personal vitamin D status in a predominantly black urban cohort of children with asthma modifies the response to indoor airborne particulate exposures, and if these effects differ by obesity status. We hypothesized that low serum 25-OH vitamin D levels are associated with an increased susceptibility to the effects of indoor PM2.5 on asthma symptoms, and that these effects may be more pronounced in obese children.
Methods
Study Population
Participants were recruited and enrolled from the Domestic Indoor Particulate Matter and Childhood Asthma Morbidity (DISCOVER) study, a longitudinal cohort study of school-aged children aged 5–12 years with physician-diagnosed asthma. Children were recruited from Johns Hopkins pediatric outpatient clinic and emergency room encounters, as well as from participant lists from prior asthma studies, and enrolled between January 2009 and January 2015. Children were included in the study if they reported symptoms of asthma and/or reliever medication use in the last 6 months and lived in their current residence in East Baltimore for at least 6 months, and were excluded if they had a current diagnosis of another pulmonary disease or were planning to move to a different residence within the study period. The Johns Hopkins Institutional Review Board (IRB) approved the study. Each participant had demographic, medication, and asthma severity data, anthropomorphic measurements (to calculate body mass index (BMI) percentile),41 and spirometric lung function, assessed at a baseline visit. Participants were subsequently followed over one week every 3 months over a 9-month period, extending across all 4 seasons.
Environmental assessments: Indoor particulate matter
Personal Environmental Monitors (PEM) (SKC, Inc., Eighty Four, Pennsylvania, USA) designed to collect particles ≤2.5 μm (PM2.5) were placed within participant homes in the room identified by caregivers where the child spent the most time. PEMs were loaded with 37-mm filters (2.0 μm pore-size polytetrafluoroethylene (Teflo) Pall Laboratory), deployed for one-week at each time, and operated at a flow rate of 4 lpm to gravimetrically assess weeklong mean PM2.5 levels at three-month intervals. Constant airflow was maintained for integrated sampling using portable sampling pumps (BGI Inc. Waltham, MA)). Flow rate was calibrated before and at the end of sampling using an electronic flow calibrator (Defender, Mesa labs). PM gravimetric analysis was conducted on a microbalance (XP2U, Mettler Toledo, Inc., Columbus, OH) after filters were equilibrated for 24 hours at constant temperature and humidity.
Quantification of Serum 25-hydroxy vitamin D
Participants had blood drawn at baseline and every 3 months thereafter during home visits. Batched archived serum vitamin D concentrations were measured at the CLIA-certified Immunology Laboratory at the Johns Hopkins Hospital using the chemiluminescence immunoassay (DiaSorin, Stillwater, MN), which measures both vitamin D2 and D3 forms and is reported as a total serum 25(OH) vitamin D concentration. Within-person mean values and corresponding standard deviations were calculated for each individual. In descriptive analyses, Vitamin D sufficiency was defined as values >30 ng/mL, 20 to 30 ng/mL as insufficient, and below 20 ng/mL as deficient to be consistent with prior studies of children,42,43, especially with asthma,18,19 however, given lack of prior information on levels of 25-OH which may modify PM effects on asthma outcomes, continuous values of 25-OH D were used in main analyses.
Asthma assessment
Baseline asthma severity was classified using the National Asthma Education and Prevention Program guidelines (NAEPP)44 and comprised of caregiver-provided data regarding respiratory symptoms, medication use, and limitations in activity, as well as spirometric measurements. Lung function parameters were assessed using a portable spirometer (KOKO spirometer, SX model, nSpire Health, Longmont, CO), in accordance with American Thoracic Society guidelines using established predicted values for forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).45,46 Daytime asthma symptoms (trouble breathing, being bothered, having limitation in activities), night time symptoms, and any need for rescue medication (i.e. albuterol) use were assessed using a pediatric asthma diary,47 a validated daily questionnaire completed by the caregiver twice daily over the one-week period of each study visit as previously described.48 Daytime symptoms were measured on a 6-point Likert scale and were dichotomized into being absent or present, with composite daytime symptoms representing none vs. any positive response across the three daily symptoms. Nighttime symptoms included an assessment of the number of awakenings caused by asthma symptoms on a 4-point Likert scale, dichotomized into being absent or present. Allergic sensitization was performed by either allergic skin testing (ALK, Round Rock, Texas) or with serum allergen-specific IgE quantification corresponding to 14 aeroallergens (RAST, Pharmacia Diagnostics AB, Uppsala, Sweden), with atopy defined as a positive sensitization to at least one individual allergen (IgE≥0.35). Peripheral venipuncture was performed during each week of monitoring and samples were also analyzed for complete blood count (CBC) and differential.
Statistical Analysis
Participants from the DISCOVER cohort were included into the analytic population if they had complete serum 25-OH vitamin D and indoor PM measurements. Descriptive analyses were performed to generate standard distributions of means and proportions. Multivariate ordered logistic and linear regression were used to detect cross-sectional associations between within-person mean 25-OH vitamin D levels and asthma outcomes (e.g. severity, lung function). Associations between individual average 25-OH vitamin D and baseline obese status, as well as seasonal differences across all samples, were tested using one-way ANOVA. To assess associations between repeated measures of individual vitamin D status and asthma symptoms or neutrophil percentage, generalized estimating equation regression analysis was used to account for intra-participant correlation of the observations.49 Three-way interaction regression models were constructed to explore the effect modification of continuous values of 25-OH vitamin D on the association between weekly indoor PM exposure, obesity, and daily respiratory symptoms and adjusted for age, sex, caregiver education, inhaled corticosteroid (ICS) use, and season. Subsequently, the modifying effects of 25-OH vitamin D levels upon the response to PM2.5 were examined across obesity status (obese vs. non-obese). P values <0.05 were considered to be statistically significant. Analyses were performed using STATA statistical software (version 15, College Station, Texas).
Results
Participant characteristics and indoor PM exposure.
One hundred and twenty school-aged children had serum vitamin D levels assessed on at least one occasion during the study follow-up period and had complete environmental and respiratory symptom data to comprise the analytic population. Mean (standard deviation (SD)) age of participants was 9.7 (2.2) years with 55% boys. Body mass index (BMI) across all children was, on average, in the 71% percentile, with 36% qualifying as obese. Participants were classified across a range of asthma severity and almost half of children reported taking inhaled corticosteroids. Average individual indoor PM2.5 exposure over the 7-day monitoring periods across visits had a mean (SD) of 38.2 (42.9) μg/m3 (Table 1). There were no significant differences between the analytic group of 120 children and the overall cohort with respect to age, sex, race, household income, BMI percentile, asthma severity, lung function, or PM exposure (Table S1).
Table 1.
Participant characteristics (N=120)
| Variables | Distribution | |
|---|---|---|
| Age (yr.; mean, SD) | 9.7 | 2.2 |
| Child sex (n, %) | ||
| Girls | 54.0 | 45.0 |
| Boys | 66.0 | 55.0 |
| Race/Ethnicity (n, %) | ||
| Black | 114.0 | 95.0 |
| Non-Black | 6.0 | 5.0 |
| Body mass Index (percentile; mean, SD) | 71.3 | 29.7 |
| Caregiver’s education (n, %) | ||
| Not high school graduate | 38.0 | 31.9 |
| High school graduate | 52.0 | 43.7 |
| Some college or beyond | 29.0 | 24.4 |
| Household income (annual) (n, %) | ||
| < $25,000 | 52.0 | 43.3 |
| $25,000-$50,000 | 17.0 | 14.2 |
| >$50,000 | 4.0 | 3.3 |
| Not reported | 47.0 | 39.2 |
| Health insurance (n,%) | ||
| Private | 9.0 | 7.5 |
| Public | 109.0 | 90.8 |
| Other | 2.0 | 1.7 |
| Atopic status (n, %) | ||
| Atopic | 80.0 | 67.8 |
| Asthma severity (n, %) | ||
| Mild intermittent | 26.0 | 21.7 |
| Mild persistent | 11.0 | 9.2 |
| Moderate persistent | 40.0 | 33.3 |
| Severe persistent | 43.0 | 35.8 |
| Inhaled corticosteroid use (n, %) | ||
| Yes | 55.0 | 47.8 |
| Vitamin D (n; %) | ||
| <20 | 76.0 | 63.3 |
| 20–30 | 32.0 | 26.7 |
| >30 | 12.0 | 10.0 |
| Lung Function (mean, SD) | ||
| FEV1 % predicted | 92.1 | 17.0 |
| FVC % predicted | 97.5 | 17.2 |
| FEF 25–75% predicted | 79.0 | 33.6 |
| PM2.5 (μg/m3; mean, SD) | 38.2 | 42.9 |
25-OH Vitamin D levels.
Within-person averages of 25-OH vitamin D across all visits in this cohort were distributed around a mean (SD) of 19.1 (7.5) ng/mL; within-person variability was 5.2 ng/mL (Table R1). Serum levels did not differ significantly between non-obese vs. obese groups (one-way ANOVA between groups p=0.98). Almost two thirds of children (63%) had a within-person average of serum 25-OH vitamin D below 20 ng/mL, the threshold traditionally regarded as deficient. One-third of participants had 25-OH vitamin D levels drawn across all 4 seasons, and across all individual samples (n=311), there was significant seasonal variation (p=0.0001), with the summer season with the highest levels (mean (SD) 23.3 (10.0) ng/mL) compared to the winter, which had the lowest levels (mean (SD) 16.2 (8.1) ng/mL).
Independent association of serum 25-OH vitamin D and asthma health.
The relationship between 25-OH vitamin D within-person mean levels and baseline asthma severity and lung function is illustrated in Table 2. There was no significant association between vitamin D status and severity classification, however, significant positive associations were found between personal 25-OH D levels and baseline lung function (coefficient 0.519; p=0.029 for FVC % predicted), translating to approximately a 5% increase in FVC % predicted for a 10 ng/ml increase in 25-OH vitamin D. There was also suggestion of a significant relationship between increasing serum 25-OH D and lower FEV1/FVC, possibly due to the effect of vitamin D on higher FVC. Sensitivity analysis with models including ICS use and/or season did not significantly change these results (data not shown). In contrast, in adjusted models including data across all repeated visits, there was no statistically significant association between serum 25-OH vitamin D levels and recorded asthma symptoms (Table S2). Adjusting for lung function (FEV1) in sensitivity analysis illustrated similar non-significant relationships.
Table 2.
Association between mean serum 25-OH vitamin D levels and baseline asthma severity and lung function1
| Coef. | 95 CI | p | ||
|---|---|---|---|---|
| Asthma Severity (OR)2 | 1.015 | 0.965 | 1.067 | 0.564 |
| FEV1% Pred3 | 0.169 | −0.292 | 0.630 | 0.468 |
| FVC% Pred3 | 0.519 | 0.053 | 0.985 | 0.029 |
| FEV1/FVC3 | −0.002 | −0.005 | 0.000 | 0.078 |
| FEF25–75% Pred3 | −0.526 | −1.407 | 0.355 | 0.239 |
Models include age, sex, BMI, and caregiver’s education. Multivariate ordered logistic and linear regression were used. For ordered logistic regression, the parallel regression assumption was tested and confirmed by Brant test.
For asthma severity, ordered logistic regression was used. Asthma severity is a four-level ordinal variable, and the coefficient represents the odds ratio of worse severity per one ng/mL increase in 25-OH vitamin D.
For FEV1 % Pred, FVC % Pred, FEV1/FVC, and FEF25–75% Pred, linear regression was used. The coefficient represents the change in lung function per one ng/mL increase in 25-OH vitamin D.
Interaction of 25-OH vitamin D levels on PM2.5 and asthma symptoms.
Given the literature supporting the independent effects of vitamin D status, obesity, and air pollution individually upon asthma symptoms, we constructed 3-way interaction models for the regression of symptoms on serum 25-OH vitamin D, obesity, and fine PM and found statistically significant interaction effects for all individual asthma symptoms, nighttime symptoms, and rescue medication use (data not shown). Based on these fully-adjusted 3-way interaction models and holding obesity status constant, 2-way interactions demonstrated that lower serum 25-OH vitamin D levels augmented the adverse relationship between PM2.5 and limited activity (pint =0.003), trouble breathing (pint =0.054), feeling bothered by asthma (pint =0.030), having any daytime symptoms (pint = 0.006), nighttime symptoms (pint =0.034), and needing rescue medication (pint =0.032) among obese children. Among non-obese children, there were no significant 2-way interactions. (Table S3).
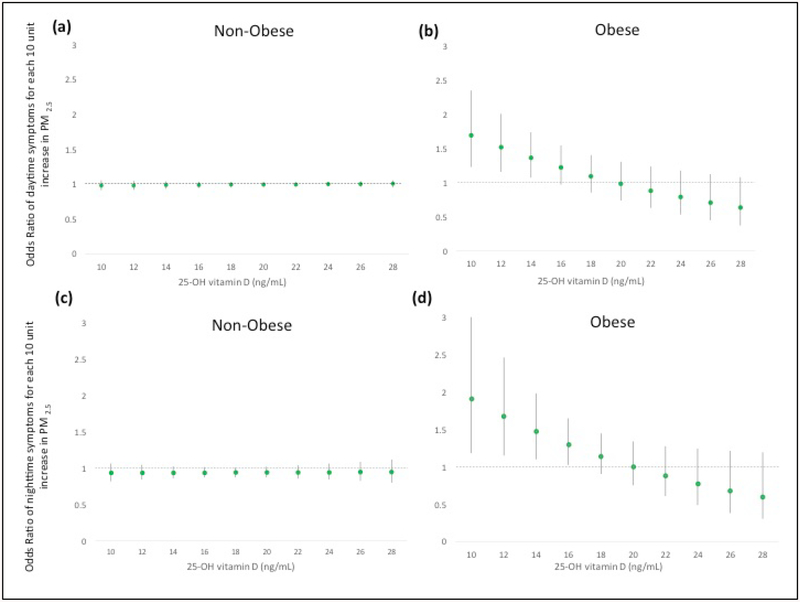
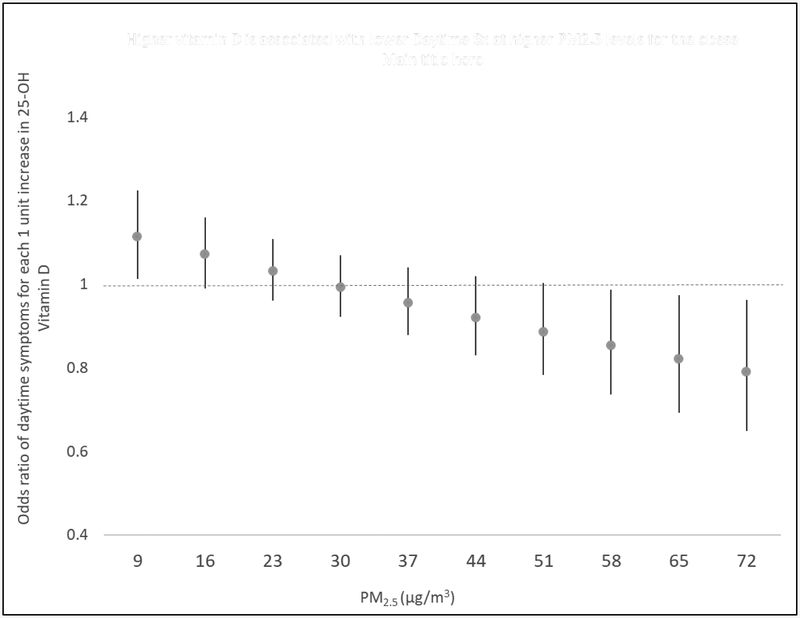
Notably, among obese children, adverse effects of PM2.5 on daytime asthma symptoms were increased and statistically significant at low 25-OH vitamin D levels (ORPM2.5=1.26, p=0.049 for vitamin D=15.5 ng/ml). PM2.5 had increasingly stronger effect on daytime asthma symptoms at decreasing levels of Vitamin D below 15.5 ng/ml) (Figure 1a). Among non-obese children, vitamin D status did not modify PM’s effect upon risk of daytime asthma symptoms (Figure 1b). Similarly, adverse effects of PM2.5 on nighttime asthma symptoms were observed at low 25-OH vitamin D levels (<16.4 ng/ml) among obese, but not non-obese, children (Figure 1c, d). Consistent with these results, at extreme levels of indoor air particulate pollution, higher vitamin D levels were found to be significantly protective against increased odds of daytime asthma symptoms associated with PM2.5 (e.g., ORVitamin D = 0.87; p=0.049 at PM2.5 = 52.5 μg/m3, increasingly stronger effects>52.5 μg/m3) among obese children (Figure 2).
Figure 1. Effect of PM2.5 on daytime and nighttime asthma symptoms varies by serum 25-OH Vitamin D levels for children with asthma.
Graphs demonstrate the marginal effects of PM2.5 on daytime (panels a, b) and nighttime (panels c, d) asthma symptoms by vitamin D level, while holding obesity status constant. GEE with AR1 correlation structure and robust standard error estimator was used. Models are fully adjusted by age, sex, caregiver education, season, and ICS use and include the necessary lower-order interaction and main effect terms. The Y-axis represents the odds ratio (OR) for symptoms for each 10 μg/m3 in PM2.5. The x-axis ranges from the 10th percentile to 90th percentile level of 25-OH vitamin D. At lower 25-OH vitamin D levels (<15.5 ng/mL and <16.4 ng/mL), higher PM2.5 exposures are increasingly associated with significantly greater risk of daytime and nighttime symptoms, respectively, among obese children (panel b, d). No effect modification was seen among non-obese children (panel a, c).
Figure 2. Effect of serum 25-OH vitamin D levels on daytime asthma symptoms in obese children with asthma in children exposed to high indoor levels of PM2.5.
Graphs demonstrate the effect of incremental changes in 25-OH D level (y axis) at each level of PM2.5 exposure (x-axis). GEE with AR1 correlation structure and robust standard error estimator were used. The model was fully adjusted for age, sex, caregiver education, season, and ICS use, and includes the necessary lower-order interaction and main effect terms. The Y-axis represents the odds ratio (OR) for daytime symptoms for each 1 ng/ml change in 25-OH vitamin D. The x-axis ranges from the 10th percentile to 90th percentile level of PM2.5. At PM2.5 concentrations greater than 52.5 μg/ml, higher 25-OH vitamin D levels are associated with a decreased odds ratio of daytime asthma symptoms in obese children.
Modifying effect of 25-OH vitamin D levels on the association of PM2.5 and systemic inflammation
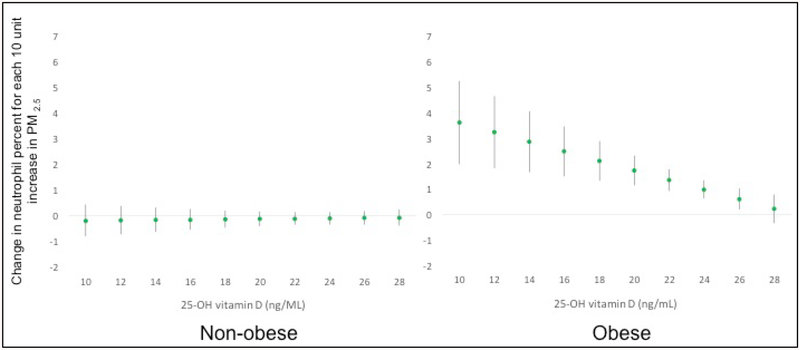
To investigate whether serum 25-OH vitamin D levels might influence the association between PM2.5 and peripheral leukocytes and their subtypes, as a reflection of systemic inflammation, we constructed 3-way interaction adjusted models for the regression of peripheral cell counts on serum 25-OH vitamin D, obesity, and fine PM. There was no statistically significant adjusted interaction of 25-OH vitamin D upon PM2.5 and total white blood cell (WBC) or absolute neutrophil counts (data not shown). However, a 3-way interaction effect was noted for peripheral neutrophil percentage, and the 2-way interaction suggested as significant for obese children only (pint 0.056). Among the obese, PM2.5 was associated with a significantly greater increase in neutrophil percentage as Vitamin D level decreased (e.g. beta coefficient = 0.47 for each 10 μg/m3 increase in PM2.5, p=0.049 at 25-OH vitamin D=26.8 ng/ml, with increasingly stronger effects <26.8 ng/ml) (Figure 3). For example, at median 25-OH D levels of 18 ng/ml in this subgroup, neutrophil percentages were increased by 2.12 (p<0.001) for every 10 μg/m3 increase in fine PM, and at even lower levels (e.g. 25th percentile), the predicted change in neutrophil percentage was 2.78 (p<0.001). There was no significant effect of lower levels of 25-OH vitamin D upon PM2.5-associated changes in peripheral neutrophil percentage among non-obese children.
Figure 3. Effect of PM2.5 on peripheral neutrophil percentage varies by serum 25-OH Vitamin D levels for children with asthma.
Graphs are based on the 3-way interaction model between PM2.5, vitamin D, and obesity, while holding obesity status constant and observing the marginal effect of a change in 25-OH vitamin D level. GEE with exchange correlation structure and robust standard error estimator was used. Models are fully adjusted for age, sex, caregiver education, season, and ICS use, and all models with interaction terms include the necessary lower-order interaction and main effect terms. The Y-axis represents the change in neutrophil percentage for each 10 μg/m3 in PM2.5. The x-axis ranges from the 10th percentile to 90th percentile level of 25-OH vitamin D. At lower 25-OH vitamin D levels (<26.8 ng/mL), higher PM2.5 exposures are increasingly associated with significantly higher peripheral neutrophil percentage (βPM2.5 = 0.47) among obese children. No effect modification was seen among non-obese children.
Discussion
This is the first population study to demonstrate that personal vitamin D status in urban children with asthma modifies the risk of respiratory symptoms associated with indoor air pollution. Specifically, we found that serum 25-OH vitamin D levels below 15.5 ng/mL increased individual susceptibility to the respiratory effects of indoor air pollution, particularly in obese children. Conversely, higher levels of serum 25-OH vitamin D conferred a protective effect against pollutant-associated symptoms in this subgroup. Our findings have direct public health implications for potential strategies to improve the resilience of vulnerable populations against adverse effects of urban indoor air pollution.
Vitamin D has been described in several studies to have independent effects upon inflammatory processes responsible for asthma morbidity,24,28,50,51 however, an immune-modulatory role in response to air pollution has been underexplored. Inflammatory responses to ambient particulate air pollution exposure alone have been shown to generate a systemic reaction through mechanisms that enhance bone-marrow release of polymorphonuclear leukocytes into the peripheral circulation.52–54 Analysis within our study, performed to elucidate potential mechanisms through which vitamin D could be influencing such pollutant-induced asthmatic responses, revealed that low 25-OH vitamin D levels significantly enhanced the marginal effects of PM2.5 upon the neutrophilic composition of peripheral blood. Such preliminary findings suggest that suboptimal vitamin D status might increase the odds of environmental asthma symptoms through inflammatory pathways. In fact, in vitro studies examining the effects of organic dust inhalation upon neutrophil chemokine induction in both human and murine cells have demonstrated that the pro-inflammatory response is attenuated in cells pretreated with vitamin D, as well as in mice fed a high vitamin D diet compared to those fed a low vitamin D diet.29 These results suggest a role of vitamin D in mediating air pollutant-induced airway inflammatory response through the modulation of neutrophil chemoattractants. Therefore, our study represents an epidemiologic correlate highlighting similar modifying effects of varying vitamin D status within a population of high-risk, exposed children with asthma.
In our study population, a significant proportion of children had serum 25-OH vitamin D levels that would be categorized as deficient, with 89% of children demonstrating suboptimal vitamin D status, according to thresholds prescribed for bone health. This degree of deficiency is consistent with published national high rates of deficiency among blacks.31 As there are no established thresholds for deficiency and insufficiency specifically relevant for allergic/asthmatic disease, we employed continuous values in our analyses and showed that marginal effects of PM2.5 upon asthma symptoms became statistically significant below traditionally defined deficiency of 20 ng/mL (15.5), with dose-dependent effects below this cut-off. Similarly, approximate ranges of 25-OH vitamin D for which marginal effects of PM2.5 on neutrophil percentage were found to be significant fell below the traditional threshold of sufficiency of 30 ng/mL (26.8). Accordingly, in our cohort composed predominantly of black children demographically at risk to have serum levels below these thresholds, lower vitamin D status was not only associated with lower lung function, as published by others,18,20,55 but more importantly, enhanced the clinical and inflammatory asthmatic responses to indoor particulate pollution. These novel findings support the concept that a prescription for a global optimal serum vitamin D levels may be a misguided goal, and thresholds of sufficiency may instead need to be tailored according to particular subpopulations of children56 and/or with regards to specific health outcomes.
Notably, the interaction relationships observed in our cohort were only identified for obese children. The reasons for this are unclear. Similar to our findings, Lautenbacher et al. reported that within their cohort of African-American and Hispanic children, lung function parameters were found to be lower in the subset of children with both vitamin D deficiency and obesity, but not among normal-weight children with asthma,55 thereby implying a synergistic role for obesity and vitamin D for asthma morbidity. Our results validate and extend this literature further to asthma symptoms within a similar urban minority subpopulation. Current literature supports relationships that triangulate obesity, inadequate vitamin D, and ambient pollutants together, though the directionality of each association is not well understood.57 Several hypotheses have been put forth as possible underlying mechanisms for these complex interrelationships, including obesity-mediated vitamin D bioavailability (possibly due to fat sequestration of this vitamin),58 microbiome alterations influencing vitamin D-regulated gut immune function,59,60 and inflammation or metabolic dysregulation found in vitamin D deficient individuals,61 all which may reflect vitamin D’s direct and indirect immune-protective roles in asthma-related outcomes. Further studies are needed to disentangle these co-existent factors among minority populations and gain a better understanding on how they may jointly contribute to asthma-related morbidity in susceptible children.
Our study has several strengths. Our particular cohort of children in Baltimore represents a demographic that uniquely has high risks for obesity, vitamin D deficiency, and indoor air pollution, and manifests a heavy burden of asthma. In addition, repeated measures of serum 25-OH vitamin D in each child allowed for a robust appreciation of interactions observed with time-varying concurrent changes in pollutant exposures and asthma control. Our findings warrant replication in future larger cohorts in order to provide further definitive evidence that vitamin D status and obesity can influence the impact of air pollutants on childhood asthma morbidity. Limitations in our work include the inability to generalize our findings to other urban populations, for example Hispanic children who also share a heavy burden of asthma. Our findings are hypothesis generating, but we did not do correction for multiple testing and findings should be confirmed in larger group of subjects. Furthermore, though we have used traditional measures of vitamin D status that measure serum levels of 25-OH vitamin D, we were unable to test for other related markers such as blood levels of vitamin D receptor binding protein or other vitamin D metabolites that may have independent effects upon the asthmatic response to inhaled toxins.62 Consideration of the entire vitamin D pathway involved in the protective relationships illustrated by our findings is critical to identify important targets for future therapeutic interventions aimed at reducing the burden of asthma symptoms.
In conclusion, this is the first report to demonstrate that vitamin D increases the susceptibility to the adverse effects of indoor air pollution among obese children with asthma. Protective effects of increasing vitamin D towards asthma symptoms within this population were observed in highly polluted urban homes. These results offer hope for potential future strategies to combat the hazards of environmental pollutants faced by vulnerable populations.
Supplementary Material
Highlight box:
What is already known about this topic? Indoor fine particulate air pollution (PM2.5) is associated with higher asthma morbidity in urban children; factors modifying susceptibility to PM’s respiratory effects are unknown. Growing epidemiologic and mechanistic evidence links vitamin D status and asthma.
What does this article add to our knowledge? Our report demonstrates the influence of higher serum 25-OH vitamin D levels to mitigate the adverse respiratory response associated with indoor PM2.5 exposure among obese urban children with asthma.
How does this study impact current management guidelines? These results raise awareness of the potential protective effects of serum 25-OH vitamin D levels among exposed obese urban children, relevant for designing future targeted therapies to reduce the burden of asthma in this population.
Acknowledgements/Funding:
This study was funded by Environmental Protection Agency: (Grant Numbers RD-83615201, RD-83451001, RD83213901); National Institute of Health: (Grant Numbers F32 PA-09-210, 1P50ES015903,P01ES018176, P50ES018176, 4KL2TR001077-04, 1P50ES015903-01)
Abbreviations:
- PM
particulate matter
- 25-OH
25-hydroxy
- BMI
body mass index
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- ICS
inhaled corticosteroid
- PEM
Personal Environmental Monitor
- CBC
complete blood count
- WBC
white blood cell
- OR
odds ratio
- SD
standard deviation
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
References
- (1).National Survey of Children’s Health, Data query from the Child and Adolescent Health Measurement Initiative. 2011/12; Available at: http://childhealthdata.org/browse/survey.
- (2).Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012. May;(94)(94):1–8. [PubMed] [Google Scholar]
- (3).Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol 2017. September;140(3):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Louisias M, Phipatanakul W. Managing Asthma in Low-Income, Underrepresented Minority, and Other Disadvantaged Pediatric Populations: Closing the Gap. Curr Allergy Asthma Rep 2017. September 15;17(10):68-017-0734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol 2010. March;125(3):540–544. [DOI] [PubMed] [Google Scholar]
- (6).Matsui EC. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy 2014. May;69(5):553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).McCormack MC, Breysse PN, Matsui EC, Hansel NN, Peng RD, Curtin-Brosnan J, et al. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol 2011. April;106(4):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect 2009. February;117(2):294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Garcia-Larsen V, Del Giacco SR, Moreira A, Bonini M, Charles D, Reeves T, et al. Asthma and dietary intake: an overview of systematic reviews. Allergy 2016. April;71(4):433–442. [DOI] [PubMed] [Google Scholar]
- (10).Saadeh D, Salameh P, Caillaud D, Charpin D, De Blay F, Kopferschmitt C, et al. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: data from the french six cities study. BMC Public Health 2015. September 30;15:993-015-2320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Han YY, Forno E, Alvarez M, Colon-Semidey A, Acosta-Perez E, Canino G, et al. Diet, Lung Function, and Asthma Exacerbations in Puerto Rican Children. Pediatr Allergy Immunol Pulmonol 2017. December 1;30(4):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP, ISAAC Phase Two Study Group. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax 2010. June;65(6):516–522. [DOI] [PubMed] [Google Scholar]
- (13).Han YY, Forno E, Celedon JC. Vitamin D Insufficiency and Asthma in a US Nationwide Study. J Allergy Clin Immunol Pract 2017. May-Jun;5(3):790–796.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012. July 15;186(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: An emerging public health problem. J Family Community Med 2014. September;21(3):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Batmaz SB, Arikoglu T, Tamer L, Eskandari G, Kuyucu S. Seasonal variation of asthma control, lung function tests and allergic inflammation in relation to vitamin D levels: a prospective annual study. Postepy Dermatol Alergol 2018. February;35(1):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bai YJ, Dai RJ. Serum levels of vitamin A and 25-hydroxyvitamin D3 (25OHD3) as reflectors of pulmonary function and quality of life (QOL) in children with stable asthma: A case-control study. Medicine (Baltimore) 2018. February;97(7):e9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Alyasin S, Momen T, Kashef S, Alipour A, Amin R. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res 2011. October;3(4):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pollard SL, Lima JJ, Mougey E, Romero K, Tarazona-Meza C, Tomaino K, et al. Free 25(OH)D concentrations are associated with atopy and lung function in children with asthma. Ann Allergy Asthma Immunol 2017. July;119(1):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yao TC, Tu YL, Chang SW, Tsai HJ, Gu PW, Ning HC, et al. Serum 25-hydroxyvitamin D levels in relation to lung function and exhaled nitric oxide in children. J Pediatr 2014. December;165(6):1098–1103.e1. [DOI] [PubMed] [Google Scholar]
- (21).Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009. May 1;179(9):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Igde M, Baran P, Oksuz BG, Topcuoglu S, Karatekin G. Association between the oxidative status, Vitamin D levels and respiratory function in asthmatic children. Niger J Clin Pract 2018. January;21(1):63–68. [DOI] [PubMed] [Google Scholar]
- (23).Sypniewska G, Krintus M, Fulgheri G, Siodmiak J, Kuligowska-Prusinska M, Stepien-Jaszowska B, et al. 25-Hydroxyvitamin D, biomarkers of eosinophilic inflammation, and airway remodeling in children with newly diagnosed untreated asthma. Allergy Asthma Proc 2017. May 1;38(3):29–36. [DOI] [PubMed] [Google Scholar]
- (24).Sommer A, Fabri M. Vitamin D regulates cytokine patterns secreted by dendritic cells to promote differentiation of IL-22-producing T cells. PLoS One 2015. June 24;10(6):e0130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Szentpetery SE, Han YY, Brehm JM, Acosta-Perez E, Forno E, Boutaoui N, et al. Vitamin D insufficiency, plasma cytokines, and severe asthma exacerbations in school-aged children. J Allergy Clin Immunol Pract 2017. August 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang R, Zhao H, Dong H, Zou F, Cai S. 1alpha,25-dihydroxyvitamin D(3) counteracts the effects of cigarette smoke in airway epithelial cells. Cell Immunol 2015. June;295(2):137–143. [DOI] [PubMed] [Google Scholar]
- (27).Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax 2014. June;69(6):508–515. [DOI] [PubMed] [Google Scholar]
- (28).Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1alpha,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol 2013. August;132(2):297–304.e3. [DOI] [PubMed] [Google Scholar]
- (29).Golden GA, Wyatt TA, Romberger DJ, Reiff D, McCaskill M, Bauer C, et al. Vitamin D treatment modulates organic dust-induced cellular and airway inflammatory consequences. J Biochem Mol Toxicol 2013. January;27(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol 2003. September;112(3):585–592. [DOI] [PubMed] [Google Scholar]
- (31).Ginde AA, Liu MC, Camargo CA,Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009. March 23;169(6):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Harris SS. Vitamin D and African Americans. J Nutr 2006. April;136(4):1126–1129. [DOI] [PubMed] [Google Scholar]
- (33).Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006. April 5;295(13):1549–1555. [DOI] [PubMed] [Google Scholar]
- (34).Akinbami LJ, Rossen LM, Fakhouri THI, Simon AE, Kit BK. Contribution of weight status to asthma prevalence racial disparities, 2–19 year olds, 1988–2014. Ann Epidemiol 2017. August;27(8):472–478.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am J Epidemiol 2013. October 1;178(7):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Grammer LC, Weiss KB, Pedicano JB, Kimmel LG, Curtis LS, Catrambone CD, et al. Obesity and asthma morbidity in a community-based adult cohort in a large urban area: the Chicago Initiative to Raise Asthma Health Equity (CHIRAH). J Asthma 2010. June;47(5):491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).McCormack MC, Belli AJ, Kaji DA, Matsui EC, Brigham EP, Peng RD, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J 2015. January 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams DL, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol 2013. April;131(4):1017–23, 1023.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 2007. July;86(1):150–158. [DOI] [PubMed] [Google Scholar]
- (40).Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982. January 9;1(8263):74–76. [DOI] [PubMed] [Google Scholar]
- (41).BMI Percentile Calculator for Child and Teen. Available at: https://nccd.cdc.gov/dnpabmi/calculator.aspx, 2018.
- (42).Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005. July;16(7):713–716. [DOI] [PubMed] [Google Scholar]
- (43).Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 2009. November;124(5):1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007. November;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- (45).Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999. January;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- (46).Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005. August;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- (47).Santanello NC, Davies G, Galant SP, Pedinoff A, Sveum R, Seltzer J, et al. Validation of an asthma symptom diary for interventional studies. Arch Dis Child 1999. May;80(5):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nnodum BN, McCormack MC, Putcha N, Hwang S, Paulin LM, Brigham EP, et al. Impact of Physical Activity on Reporting of Childhood Asthma Symptoms. Lung 2017. December;195(6):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data. 2nd ed Oxford University: Oxford University Press; 2002. [Google Scholar]
- (50).Janeva-Jovanovska E, Dokic D, Jovkovska-Kaeva B, Breskovska G, Goseva Z, Minov J, et al. Relationship between Vitamin D, Inflammation and Lung Function In Patients with Severe Uncontrolled Asthma. Open Access Maced J Med Sci 2017. November 19;5(7):899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Pfeffer PE, Hawrylowicz CM. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest 2017. September 18. [DOI] [PubMed] [Google Scholar]
- (52).van Eeden SF, Hogg JC. Systemic inflammatory response induced by particulate matter air pollution: the importance of bone-marrow stimulation. J Toxicol Environ Health A 2002. October 25;65(20):1597–1613. [DOI] [PubMed] [Google Scholar]
- (53).Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, et al. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol 2002. July;27(1):34–41. [DOI] [PubMed] [Google Scholar]
- (54).van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2(1):61–67. [DOI] [PubMed] [Google Scholar]
- (55).Lautenbacher LA, Jariwala SP, Markowitz ME, Rastogi D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr Pulmonol 2016. December;51(12):1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Greer FR. Defining vitamin D deficiency in children: beyond 25-OH vitamin D serum concentrations. Pediatrics 2009. November;124(5):1471–1473. [DOI] [PubMed] [Google Scholar]
- (57).Barrea L, Savastano S, Di Somma C, Savanelli MC, Nappi F, Albanese L, et al. Low serum vitamin D-status, air pollution and obesity: A dangerous liaison. Rev Endocr Metab Disord 2017. June;18(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000. September;72(3):690–693. [DOI] [PubMed] [Google Scholar]
- (59).Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol 2011. May;127(5):1128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol 2011. May;127(5):1087–94; quiz 1095–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Bellia A, Garcovich C, D’Adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med 2013. February;8(1):33–40. [DOI] [PubMed] [Google Scholar]
- (62).Bratke K, Wendt A, Garbe K, Kuepper M, Julius P, Lommatzsch M, et al. Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clin Exp Immunol 2014. July;177(1):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.