Abstract
Background:
Prolactin (PRL) is involved in the regulation of glucose metabolism since high PRL serum levels are associated with low incidence of type 2 diabetes mellitus (T2DM). Therefore, the aim of the present study was to assess the metabolic effects of PRL on glucose homeostasis in men with T2DM.
Methods:
Eighty male patients with T2DM compared with 25 male healthy controls matched with patients for age and weight were divided into four groups: Group (A): patients with T2DM on metformin (n = 29), Group (B): patients with T2DM on glyburide (n = 30), Group (C): patients with T2DM on glyburide plus metformin (n = 21), and Group (D): healthy male subjects as control (n = 25). Body mass index (BMI) and blood pressure measurements were determined. Fasting blood glucose (FBG), glycated hemoglobin, total cholesterol, triglyceride (TG), high-density lipoprotein, low-density lipoprotein, atherogenic index, fasting serum insulin, insulin resistance (IR), and β-cell function of the pancreas were determined by homeostatic model assessment-2 (HOMA-IR). Furthermore, C-reactive protein and PRL serum level were determined in patients with T2DM and healthy control men.
Results:
BMI of T2DM patients was higher as compared with control (P = 0.003). Combination therapy (glyburide plus metformin) in patients with T2DM showed better effect on most of glycemic indices and lipid profile than glyburide or metformin monotherapy (P < 0.05). PRL serum level was higher in patients with T2DM as compared with control (P = 0.001). PRL serum level was high in glyburide-treated patients as compared with metformin-treated patients (P = 0.002).
Conclusion:
This study concludes that elevated PRL serum level in patients with T2DM is associated with diabetic complications. Diabetic pharmacotherapy mainly metformin reduced PRL serum level in patients with T2DM through amelioration of IR.
Keywords: Glyburide, metformin, prolactin, type 2 diabetes mellitus
INTRODUCTION
Prolactin (PRL) is a pituitary hormone essential for various physiological functions in the human body. It is not only important for the initiation and maintenance of lactation, but seems to be also involved in reproduction, growth and development, and metabolism. These different functions of PRL can only be fulfilled due to the fact that the PRL receptor is expressed in different tissues and cells such as lymphoid cells, endometrium, prostate, adipocytes, and β-cell of the pancreas.[1,2] Into the aspect, PRL is involved in the regulation of glucose metabolism through regulation the function of pancreatic β-cell.[3]
Formerly, PRL was regarded as a diabetogenic factor since; pathological hyperprolactinemia led to insulin resistance (IR) and impairment of pancreatic β-cell function.[4]
In recent times, PRL has an important task in the regulation of glucose metabolism; PRL through activation of pancreatic β-cell PRL receptors stimulates insulin secretion and reduces of glucose threshold for insulin secretion.[5] Similarly, PRL through activation of peroxisome proliferative-activated receptor gamma (PPAR-γ) inhibits lipolysis and activates adipocyte differentiation.[6]
Marshania found that high PRL in type 2 diabetes mellitus (T2DM) is regarded as a compensatory mechanism against T2DM-induced glucotoxicity and inflammatory changes.[7] As well, diabetic pharmacotherapy may affect PRL levels in patients with T2DM. Metformin reduces PRL whereas glyburide increases PRL due to variability in the hypothalamic dopaminergic activity, which plays an important role in the regulation of PRL from anterior pituitary.[8,9]
Even though previous investigations about the potential effects of PRL in T2DM and its complications are scarce, existing experimental studies suggest an influence of PRL on T2DM through its metabolic effects on adipose tissue; development and growth of pancreatic β-cells; and IR and lipid metabolism.[10] The ability of PRL to stimulate insulin and suppress adipocytokines release further suggests a potential role in the manifestation of IR.[11] Although these studies support the view that PRL promotes the growth and survival of pancreatic β-cells and supports insulin secretion, other studies were not able to detect any correlation between PRL and metabolic profile in patients with T2DM.[12]
Therefore, the rational of the present study depends on the hypothesis, which is does high PRL hormone in T2DM is a causative or protective factor?
As a result, the objective of the present study was to assess PRL serum levels in patients with T2DM in relation to the effect of metformin and/or glyburide.
METHODS
This study involved 80 male patients with T2DM compared with 25 male healthy controls matched for age; they recruited from Iraqi International Endocrine Center. All recruited patients and healthy controls were subdivided into the following four groups:
Group (A): T2DM patients on metformin therapy 1500 mg/day (n = 29)
Group (B): T2DM patients on glyburide therapy 10 mg/day (n = 30)
Group (C): T2DM patients on combination therapy (n = 21)
Group (D): healthy male individuals (n = 25).
All patients and enrolled individuals gave informed verbal consent for their participation in this study. This study was permitted by Scientific Ethical Committee in College of Medicine, Al-Mustansiriyia University Baghdad, Iraq, under ethical permission number RVN199NM 8/12/2017 in accordance with the Declaration of Helsinki.[13]
Full medical history and physical examinations were done by internist and endocrinologist to evaluate the healthy status of recruited patients and determining the inclusion and exclusion criteria of recruited patients.
Inclusion criteria included male patients with T2DM with the age range of 40–55 years on metformin and/or glyburide therapy for at least 5 years of duration.
Exclusion criteria included psychological diseases, neurological diseases, hypothyroidism, end-stage kidney disease; chronic kidney disease, hepatic dysfunctions, connective tissue disorders, history of intake of dopamine receptor agonist or antagonist agents or verapamil, malignant disorders, and sexual dysfunctions.
Anthropometric and biochemical measurements
Body mass index (BMI), BMI = body weight (kg)/height (cm2) was measured according to the previous study.[14] Blood pressure was measured at the supine position by digital automated blood pressure monitoring 2 h apart. Pulse pressure = systolic blood pressure (SBP)-diastolic blood pressure (DBP) and mean arterial pressure (MAP), MAP = SBP + 2DBP/3.[15]
A volume of 10 mL of venous blood was taken after overnight fasting from recruited patients and individuals. The blood samples were centrifugated at 3000/rpm and stored at −20 Ċ for later analysis. Fasting blood glucose (FBG) was determined by the glucose oxidase method.[16] Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) were measured by auto-analyzer (ERBE diagnostic Manheim, Germany). Low-density lipoprotein (LDL) was estimated by the Friedewald equation.[17] VLDL = TG/5 and atherogenic index (AI) = log (TG/HDL) were estimated by specific equations.[18]
Fasting serum insulin (FSI) was measured by ELISA kit method (insulin human ELISA kit, Catalog number: KAQ1251 Abcam, USA). IR and β-cell function of the pancreas were determined by homeostatic model assessment (HOMA-2).[19] Glycated hemoglobin (HbA1c) was measured by ELISA kit method (human HbA1c AIi1c, GHbA1c, MBS702379, Abcam, USA). Human C-reactive protein (CRP) was measured by ELISA kit method (CRP ab99995, Abcam, USA). PRL serum level ng/ml was estimated by ELISA kit method (human PRL quantikine, solid-phase sandwich ELISA 5617, Abcam, USA). All kit procedures were completed according to the kit instructions of manufacturing company.
Statistical analysis
The data presented as mean ± standard deviation and unpaired Student's t-test was used to determine the significance of differences. Analysis of variance followed by Bonferroni's post hoc test. Data analysis was done using SPSS (IBM SPSS Statistics for Windows version 20.0, 2014 Armonk, NY, USA, IBM, Corp). P < 0.05 was regarded as statistically significant.
RESULTS
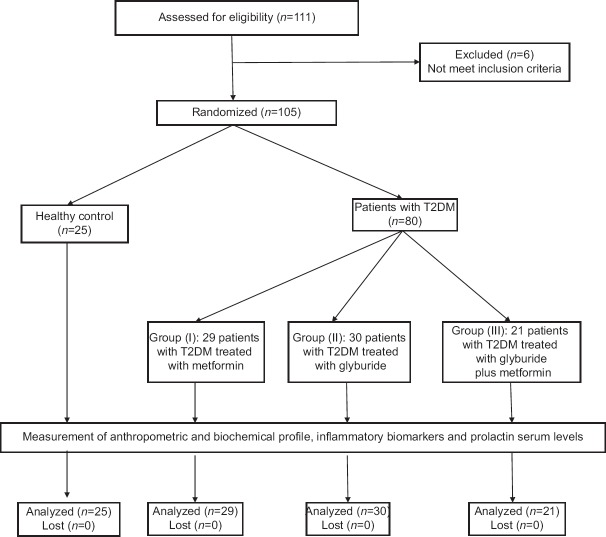
Consort flow-chart of the present study demonstrated that out of 111 recruited patients and enrolled individuals only 105 were randomized due to the exclusion of six patients that were not met inclusion criteria. Therefore, 111 male individuals and patients were randomized into 80 patients with T2DM and 25 healthy controls. Patients with T2DM were subdivided into three groups according to the current pharmacotherapy into metformin group (n = 29), glyburide group (n = 30), and their combination (n = 21). All patients with T2DM and healthy control were analyzed without discontinuation, as shown in Figure 1.
Figure 1.
Consort flow diagram of the present study
Findings of the present study illustrated that patients with T2DM illustrated insignificant differences regarding age (P = 0.33). Bodyweight of patients with T2DM was high compared with control (P = 0.0001). As well, most of the T2DM patients were associated with other attendant illnesses such as 86.25% and 96.25% of them were hypertensive and dyslipidemic, respectively. About 36.25% of T2DM patients were on metformin therapy, 37.5% on glyburide, and 26.25% on their combination. Besides, other drugs were used such as statins 97.5%, omega-3 fatty acid 91.25%, antiplatelets 92.5%, and angiotensin-converting enzyme inhibitors 55%. All control subjects were healthy without a history of any medications except for tonic agents which were used by 7.5%, [Table 1].
Table 1.
Characteristics of the present study
| Variables | Control (n=25) | T2DM (n=80) | P |
|---|---|---|---|
| Age (years) | 43.98±3.21 | 44.71±3.63 | 0.33 |
| Body weight (kg) | 81.77±8.83 | 99.85±9.53 | 0.0001 |
| Height (m) | 179.77±3.88 | 179.34±3.21 | 0.57 |
| Concurrent diseases | |||
| Hypertension | 0 | 69 (86.25) | |
| Dyslipidemia | 0 | 77 (96.25) | |
| Peripheral vascular diseases | 0 | 11 (13.75) | |
| Asthma | 0 | 5 (6.25) | |
| COPD | 0 | 9 (11.25) | |
| IHD | 0 | 75 (93.75) | |
| Current therapy | |||
| Metformin | 0 | 29 (36.25) | |
| Glyburide | 0 | 30 (37.5) | |
| Metformin + glyburide | 0 | 21 (26.25) | |
| Other drugs | |||
| Statins | 0 | 78 (97.5) | |
| Omega-3 fatty acid | 0 | 73 (91.25) | |
| Antiplatelets | 0 | 74 (92.5) | |
| Anticoagulants | 0 | 5 (6.25) | |
| Theophylline | 0 | 9 (11.25) | |
| ACEI | 0 | 44 (55) | |
| CCBs | 0 | 12 (15) | |
| Trimetazidine | 0 | 39 (48.75) | |
| Tonics | 6 (7.5) | 72 (90) | 0.0001 |
Data are expressed as mean±SD, n (%), unpaired t-test was used. BMI: Body mass index, WHR: Waist-hip ratio, COPD: Chronic obstructive pulmonary disease, IHD: Ischemic heart disease, ACEI: Angiotensin converting enzyme inhibitor, CCBs: Calcium channel blockers, T2DM: Type 2 diabetes mellitus, SD: Standard deviation
Patients with T2DM were significantly differed regarding anthropometric, blood pressure profile, and lipid profile compared with control (P = 0.001). Regarding inflammatory biomarker, CRP was high in patients with T2DM (2.9 ± 1.51 mg/L) compared with (1.21 ± 0.72 mg/L) in healthy controls (P = 0.001). As well, PRL serum level was high in patients with T2DM (36.84 ± 6.29 ng/mL) compared with (10.66 ± 2.45 ng/mL) in healthy controls (P = 0.001), [Table 2].
Table 2.
Prolactin serum levels, anthropometric and biochemical variables in patients with type 2 diabetes mellitus compared with control
| Parameters | Control | T2DM | 95% CI | P |
|---|---|---|---|---|
| n (%) | 25 (23.80) | 80 (76.19) | 30.3640-67.1773 | 0.001* |
| BMI (kg/m2) | 25.30±1.55 | 31.05±1.66 | 5.0070-6.4930 | 0.001* |
| SBP (mmHg) | 123.65±6.78 | 148.82±11.92 | 20.1985-30.1415 | 0.001* |
| DBP (mmHg) | 79.44±6.84 | 90.57±8.97 | 10.1665-20.1415 | 0.001* |
| FBG (mg/dl) | 81.77±8.83 | 141.65±22.72 | 50.6290-69.1310 | 0.001* |
| HbA1c | 5.77±1.71 | 8.54±1.51 | 2.0616-3.47784 | 0.001* |
| Fasting insulin (mIU/l) | 8.64±2.55 | 17.49±4.19 | 7.0912-10.6088 | 0.001* |
| HOMA-IR | 1.09±0.11 | 6.11±2.39 | 4.0685-5.9715 | 0.001* |
| HOMA-β (%) | 125.90±6.89 | 80.05±6.62 | −48.8873-42.8127 | 0.001* |
| TG (mg/dl) | 112.89±9.62 | 185.44±11.45 | 67.5283-77.5717 | 0.001* |
| TC (mg/dl) | 129.11±11.80 | 196.52±12.62 | 61.7598-73.0602 | 0.001* |
| LDL (mg/dl) | 49.80±5.61 | 118.4±12.92 | 63.3130-73.8870 | 0.001* |
| HDL (mg/dl) | 56.73±3.63 | 41.58±8.61 | −18.6679-11.6321 | 0.001* |
| VLDL (mg/dl) | 22.60±4.89 | 37.08±4.31 | 12.4570-16.5030 | 0.001* |
| AI | 0.06±0.001 | 0.289±0.03 | 0.2171-0.2409 | 0.001* |
| CRP (mg/L) | 1.21±0.72 | 2.9±1.51 | 1.0687-2.3113 | 0.001* |
| Prolactin (ng/ml) | 10.66±2.45 | 36.84±6.29 | 23.6197-28.7403 | 0.001* |
*P<0.01. BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, FBG: Fasting blood glucose, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostatic model assessment-insulin resistance, β%: β-cell function, S%: Insulin sensitivity, TG: Triglyceride, TC: Total cholesterol, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, VLDL: Very low-density lipoprotein, AI: Atherogenic index, CRP: C-reactive protein, T2DM: Type 2 diabetes mellitus, SD: Standard deviation
Regarding the effect of diabetic pharmacotherapy on the metabolic, inflammatory and hormonal profile, metformin-treated patients illustrated better blood pressure and lipid profile compared with glyburide-treated patients in most of the parameters (P < 0.05) except for BMI, FSI, HbA1c, HOMA-IR, and VLDL which were insignificant (P > 0.05). In metformin group, CRP was low (2.61 ± 1.82 mg/L) compared with glyburide group (P = 0.006), and not differed compared with combination group (P > 0.05).
PRL serum level was low in metformin-treated group (32.88 ± 5.85 ng/ml) compared with high in glyburide group (38.71 ± 6.79 ng/mL) (P = 0.002), but this level was not significantly differed compared to the combination group (P > 0.05) [Table 3].
Table 3.
Effects of metformin and/or glyburide on prolactin serum level and other metabolic profile in patients with type 2 diabetes mellitus
| Variables | Metformin | Glyburide | Combination | A | B | C | ANOVA |
|---|---|---|---|---|---|---|---|
| n | 29 | 30 | 21 | ||||
| BMI (kg/m2) | 30.10±3.81 | 31.45±3.66 | 30.40±2.98 | NS | NS | NS | 0.32 |
| SBP (mmHg) | 145.79±4.64 | 150.75±5.90 | 146.79±4.48 | 0.001* | NS | 0.02× | 0.001 |
| DBP (mmHg) | 88.95±7.67 | 95.11±5.22 | 90.39±5.29 | 0.0009* | NS | 0.02× | 0.0009 |
| MAP (mmHg) | 107.89±6.68 | 113.65±5.83 | 109.19±4.81 | 0.001* | NS | 0.02× | 0.001 |
| FBG (mg/dl) | 144.52±7.65 | 141.22±6.11 | 133.11±5.22 | NS | 0.0001* | 0.0001* | 0.0001 |
| HbA1c | 8.6±2.32 | 9.99±2.56 | 7.11±2.12 | NS | NS | 0.0002* | 0.0003 |
| FSI (mIU/l) | 16.81±3.22 | 19.33±3.66 | 17.84±3.11 | 0.01× | NS | NS | 0.01 |
| HOMA-IR | 2.39±1.01 | 2.72±1.05 | 2.49±1.01 | NS | NS | NS | NS |
| HOMA-β% | 66.4±4.95 | 76.4±7.95 | 80.3±5.29 | 0.0001* | 0.0001* | NS | 0.0001 |
| TG (mg/dL) | 188.24±12.5 | 201.63±13.85 | 182.71±12.63 | 0.0005* | NS | 0.0001* | 0.00001 |
| TC (mg/dL) | 191.61±11.8 | 207.12±13.92 | 199.73±13.43 | 0.0001* | NS | NS | 0.0001 |
| LDL (mg/dL) | 110.14±9.71 | 126.83±10.49 | 114.44±9.47 | 0.0001* | NS | 0.0001* | 0.0001 |
| HDL (mg/dL) | 43.82±7.83 | 39.96±5.53 | 48.74±4.12 | 0.04× | 0.01× | 0.0001* | 0.00001 |
| VLDL (mg/dL) | 37.64±5.56 | 40.32±6.39 | 36.54±3.47 | NS | NS | 0.04× | 0.03 |
| AI | 0.27±0.01 | 0.34±0.02 | 0.21±0.01 | 0.0001* | 0.0001* | NS | 0.0001 |
| CRP (mg/L) | 2.61±1.82 | 3.96±1.94 | 2.33±0.41 | 0.006* | NS | 0.002* | 0.0009 |
| Prolactin (ng/ml) | 32.88±5.85 | 38.71±6.79 | 35.89±6.51 | 0.002* | NS | NS | 0.003 |
*P<0.01, ×P<0.05. BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, MAP: Mean arterial pressure, FSI: Fasting serum insulin, FBG: Fasting blood glucose, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostatic model assessment-insulin resistance, β%: β-cell function, S%: Insulin sensitivity, TG: Triglyceride, TC: Total cholesterol, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, VLDL: Very low-density lipoprotein, AI: Atherogenic index, CRP: C-reactive protein, NS: Not significant, A: Metformin versus glyburide, B: Metformin versus combination, C: Glyburide versus combination, ANOVA: Analysis of variance
PRL serum level was positively correlated with FBG, HbA1c, insulin levels, HOMA-IR, and CRP levels. Moreover, PRL levels were significantly correlated with BMI (r = 0.56, P = 0.001), TG levels (r = 0.82, P = 0.001), and TC levels (r = 0.65, P = 0.001), but it negatively correlated with HDL levels (r = 0.54, P = 0.001), [Table 4].
Table 4.
Correlation of prolactin serum level with metabolic variables in patients with type 2 diabetes mellitus
| Biochemical and anthropometric variables | Patients with T2DM | |
|---|---|---|
| r | P | |
| BMI (kg/m2) | 0.56 | 0.0001* |
| TC (mg/dL) | 0.65 | 0.0001* |
| TG (mg/dL) | 0.82 | 0.00001* |
| HDL (mg/dL) | -0.54 | 0.0001* |
| LDL (mg/dL) | 0.66 | 0.0001* |
| VLDL (mg/dL) | 0.86 | 0.0001* |
| AI | 0.77 | 0.0001* |
| FBG (mg/dL) | 0.84 | 0.0001* |
| HbA1c | 0.81 | 0.0001* |
| Insulin (mIU/L) | 0.99 | 0.000001* |
| HOMA-IR | 0.98 | 0.000001* |
| CRP (mg/L) | 0.99 | 0.000001* |
*P<0.01. BMI: Body mass index, TC: Total cholesterol, TG: Triglyceride, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, VLDL: Very low-density lipoprotein, AI: Atherogenic index FBG: Fasting blood glucose, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostatic model assessment-insulin, CRP: C - reactive protein, r: Pearson correlation, P: Significant value
DISCUSSION
The present study illustrated that patients with T2DM have different cardiometabolic disorders such as hyperglycemias, dyslipidemia, and inflammatory changes compared with controls since; T2DM is a metabolic disorder associated with cardiovascular morbidity and mortality as well as different complications.[20]
The chief finding of the present study was high PRL serum levels in patients with T2DM as reported by Wang et al., study that confirms a positive association between high PRL levels and IR. This high PRL serum level is to overcome IR and development of T2DM since; high circulating PRL levels are associated with a lower prevalence of diabetes and impaired glucose regulation in middle-aged and elderly Chinese men and postmenopausal women.[21]
Physiologically, elevated PRL could protect against the impairment of glucose homeostasis, whereas excessively high levels of PRL exacerbate IR and impair insulin-secretory capacity since; pituitary prolactinoma often accompanied by hyperglycemia, obesity, and IR, and a dopamine-2 agonist, such as bromocriptine, can be used to reverse these symptoms.[22]
It has been reported that PRL is a protective factor against the development of T2DM. LaPensee et al.'s study showed that PRL knockout mice is accompanied by β-cell hypoplasia, a reduced pancreatic insulin mRNA level, a blunted insulin secretory response to glucose, and mild glucose intolerance.[23] Physiologically, elevated PRL levels induce normal adaptive increases in glucose-stimulated insulin secretion through expanding β-cell mass and improving hepatic insulin sensitivity and have an indirect action by increasing hypothalamic dopamine synthesis to contribute to the improved energy and glucose homeostasis.[24] It is worth mentioning that the effect of a physiologically high PRL level and pathological hyperprolactinemia on glucose metabolism could be different. Excessive high levels of PRL exacerbate whole-body and hepatic IR and impair the insulin secretory capacity in patients with hyperprolactinemia caused by prolactinoma.[22]
Moreover, the treatment of neonatal rat islets with PRL enhances islet insulin content and early insulin secretion also increasing islet sensitivity to glucose. This effect may be partly explained by the increased β-cell and liver glucose transporter GLUT2 in the membrane of cultured neonatal rat islets.[25]
Indeed, Bordin et al. confirmed that PRL induces expression of several genes in the pancreatic islets such as macrophage migration inhibitory factor and Ca2+/calmodulin-dependent protein kinase IV. These genes have been shown to play a crucial role in insulin secretion. Treatment with PRL also modifies the expression of AKT2 and bone morphogenetic protein receptor 1A that control glucose homeostasis.[26]
Moreover, PRL is also produced from adipose tissue and acts as a cytokine in the regulation of body metabolism. Adipose tissue-derived PRL level appears to be correlated with the body fat,[27] this incident coincides with our results since; most of the enrolled patients were with high BMI. Furthermore, adipose tissue macrophages also produce PRL in reaction to hyperglycemia and diabetic-induced inflammation[11] seeing as CRP levels, FBG, and HbA1c were elevated in our patients. CRP level reflects the inflammatory changes in the patients with T2DM since; CRP level is correlated with diabetic complications. Thus, hyperglycemia, obesity, and inflammation seem to be responsible for elevated PRL levels in patients with T2DM.[28] What is more, elevated PRL levels in T2DM may be a compensatory mechanism against hyperglycemia and metabolic impairments to overcome IR. Physiological high PRL in diabetic rat leads to an augmentation of insulin sensitivity, reduced inflammatory cytokine expression in visceral fat, prevent adipocyte hypertrophy, and increase expression of GLUT4.[29]
It has been reported that PRL activates adiposity PPAR-γ similar to thiazolidinediones leading to a reduction in blood glucose and lipids through activation of lipogenesis. As well, PRL improves endogenous antioxidant capacity through the activation of paraoxonase-1 gene which prevents lipid peroxidation and endothelial dysfunction in T2DM.[30] Alternatively, pathological or very high levels of PRL leading to hyperglycemia, IR, dyslipidemia, and metabolic complications. Therefore, the administration of bromocriptine or other D2-agonist ameliorates IR and metabolic complications through central inhibition of PRL secretion.[31] Hence, the effect of PRL on blood glucose is concentration-dependent.
Our findings also illustrated that PRL was positively correlated with TG and TC levels but it negatively correlated with HDL as documented by the previous study.[32]
Concerning the effect of diabetic pharmacotherapy on PRL serum levels, the glyburide-treated group demonstrated high IR and high PRL serum levels compared with metformin-treated group or their combination because; glyburide increases appetite and bodyweight that worse IR which positively correlated with high PRL levels. What's more glyburide increased PRL secretion from anterior pituitary through inhibition of somatostatin (endogenous inhibitor of prolactin).[33]
Moreover, findings of the present study illustrated that the metformin-treated group had low PRL and low IR compared with glyburide group. Low PRL levels in this group might be due to relatively low IR or due to metformin effect. It has been reported that metformin improves endogenous hypothalamic dopaminergic tone, which inhibits endogenous PRL secretion and ameliorate IR in obese women with polycystic ovary syndrome.[34]
Furthermore, metformin plus glyburide in the present study led to better glycemic control than either metformin or glyburide monotherapy; but, PRL serum levels were not decreased more than metformin-treated group since; glyburide antagonize metformin effect on PRL secretion.[35]
Alongside, high PRL levels in patients with T2DM might be due to low dopaminergic activity seeing as; dopamine receptor was decreased in obese and hyperinsulinemic status.[36,37]
Consequently, high PRL level in patients with T2DM is regarded as beneficial incident to overcome IR and diabetic complications.
Limitations of the present study were relatively small sample size which may limit the significance of findings, all of enrolled patients were from single-center, newly diagnosed patients with T2DM were not included in this study to observe PRL levels, dopaminergic activity was not evaluated separately in the present study, T2DM patient with chronic kidney disease or diabetic patients on verapamil were not involved in the present study since; these two conditions affect PRL serum levels and finally gender differences were not evaluated in this study.
In spite of these limitations, this study is considered as the initial step for a large-scale study to observe the association between PRL levels and T2DM-induced complications. Alongside, this study established that high PRL levels in patients with T2DM is the results of diabetic complication and not a causative factor in the pathogenesis of T2DM as known previously.
CONCLUSION
This study concludes that elevated PRL serum level in patients with T2DM is associated with diabetic complications. Diabetic pharmacotherapy mainly metformin reduced PRL serum level in patients with T2DM through amelioration of IR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical conduct of research
This study was approved by the Institutional Review Board / Ethics Committee. The authors followed applicable EQUATOR Network (http://www.equator-network.org/) guidelines during the conduct of this research project.
Acknowledgment
The authors would like to express deep thanks for all enrolled patients and volunteers.
REFERENCES
- 1.Al-Kuraishy HM, Al-Gareeb AI, Awad MS, Alrifai SB. Assessment of serum prolactin levels in acute myocardial infarction: The role of pharmacotherapy. Indian J Endocrinol Metab. 2016;20:72–9. doi: 10.4103/2230-8210.172240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip SH, Romanò N, Gustafson P, Hodson DJ, Williams EJ, Kokay IC, et al. Elevated prolactin during pregnancy drives a phenotypic switch in mouse hypothalamic dopaminergic neurons. Cell Rep. 2019;26:1787–99.e5. doi: 10.1016/j.celrep.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 3.Al-Maiahy TJ, Al-Gareeb AI, Al-kuraishy HM. Prolactin and risk of preeclampsia: A single institution, cross-sectional study. Asian Pacific Journal of Reproduction. 2019;8:112. [Google Scholar]
- 4.Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. Med Hypotheses. 2011;76:553–9. doi: 10.1016/j.mehy.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Xu Y, Xu M, Ning G, Lu J, Dai M, et al. Circulating prolactin and risk of type 2 diabetes: A prospective study. Am J Epidemiol. 2016;184:295–301. doi: 10.1093/aje/kwv326. [DOI] [PubMed] [Google Scholar]
- 6.Shao S, Yao Z, Lu J, Song Y, He Z, Yu C, et al. Ablation of prolactin receptor increases hepatic triglyceride accumulation. Biochem Biophys Res Commun. 2018;498:693–9. doi: 10.1016/j.bbrc.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Marshania Z. P-03-053 A rare case of combined treatment of erectile dysfunction in conjunction with hyperprolactinemia and testosterone deficiency in men with diabetes mellitus and'syndrome of desactualization'. J Sex Med. 2016;13:S198. [Google Scholar]
- 8.Luo C, Wang X, Huang H, Mao X, Zhou H, Liu Z, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: The potential role of gut-brain axis. Front Pharmacol. 2019;10:371. doi: 10.3389/fphar.2019.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Braak EW, Appelman AM, van der Tweel I, Erkelens DW, van Haeften TW. The sulfonylurea glyburide induces impairment of glucagon and growth hormone responses during mild insulin-induced hypoglycemia. Diabetes Care. 2002;25:107–12. doi: 10.2337/diacare.25.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Mingrone G, Manco M, Iaconelli A, Gniuli D, Bracaglia R, Leccesi L, et al. Prolactin and insulin ultradian secretion and adipose tissue lipoprotein lipase expression in severely obese women after bariatric surgery. Obesity (Silver Spring) 2008;16:1831–7. doi: 10.1038/oby.2008.297. [DOI] [PubMed] [Google Scholar]
- 11.Brandebourg T, Hugo E, Ben-Jonathan N. Adipocyte prolactin: Regulation of release and putative functions. Diabetes Obes Metab. 2007;9:464–76. doi: 10.1111/j.1463-1326.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, et al. Targeted deletion of the PRL receptor: Effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–85. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association. 64th WMA General Assembly. Fortaleza, Brazil: World Medical Association; 2013. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. [Google Scholar]
- 14.Al-Kuraishy HM, Al-Gareeb AI. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J Intercult Ethnopharmacol. 2016;5:408–14. doi: 10.5455/jice.20160815080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Kuraishy HM, Al-Gareeb AI. Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J Lab Physicians. 2017;9:163–9. doi: 10.4103/0974-2727.208263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nery EW, Kubota LT. Evaluation of enzyme immobilization methods for paper-based devices – A glucose oxidase study. J Pharm Biomed Anal. 2016;117:551–9. doi: 10.1016/j.jpba.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Larsson A, Hagström E, Nilsson L, Svensson MK. Treatment target re-classification of subjects comparing estimation of low-density lipoprotein cholesterol by the friedewald equation and direct measurement of LDL-cholesterol. Ups J Med Sci. 2018;123:94–9. doi: 10.1080/03009734.2018.1465496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengupta S, Jaseem T, Ambalavanan J, Hegde A. Homeostatic model assessment-insulin resistance (HOMA-IR 2) in mild subclinical hypothyroid subjects. Indian J Clin Biochem. 2018;33:214–7. doi: 10.1007/s12291-017-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J Clin Diagn Res. 2016;10:FC21–FC26. doi: 10.7860/JCDR/2016/19971.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Guo T, Tao Y, Wang Q, Song Y, Huang W. Association between serum adipocyte factor level and insulin resistance in polycystic ovarian syndrome. Gynecol Endocrinol. 2011;27:931–4. doi: 10.3109/09513590.2011.569597. [DOI] [PubMed] [Google Scholar]
- 22.Berinder K, Nyström T, Höybye C, Hall K, Hulting AL. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary. 2011;14:199–207. doi: 10.1007/s11102-010-0277-9. [DOI] [PubMed] [Google Scholar]
- 23.LaPensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben-Jonathan N. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology. 2006;147:4638–45. doi: 10.1210/en.2006-0487. [DOI] [PubMed] [Google Scholar]
- 24.Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: Novel feedback control mechanisms in the lactotrophic axis. J Neurosci. 2012;32:8074–83. doi: 10.1523/JNEUROSCI.0129-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Siqueira KC, de Lima FM, Lima FS, Taki MS, da Cunha CF, de Lima Reis SR, et al. MiR-124a expression contributes to the monophasic pattern of insulin secretion in islets from pregnant rats submitted to a low-protein diet. Eur J Nutr. 2018;57:1471–83. doi: 10.1007/s00394-017-1425-z. [DOI] [PubMed] [Google Scholar]
- 26.Bordin S, Amaral ME, Anhê GF, Delghingaro-Augusto V, Cunha DA, Nicoletti-Carvalho JE, et al. Prolactin-modulated gene expression profiles in pancreatic islets from adult female rats. Mol Cell Endocrinol. 2004;220:41–50. doi: 10.1016/j.mce.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: A population-based study. Diabetes Care. 2013;36:1974–80. doi: 10.2337/dc12-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouckenooghe T, Sisino G, Aurientis S, Chinetti-Gbaguidi G, Kerr-Conte J, Staels B, et al. Adipose tissue macrophages (ATM) of obese patients are releasing increased levels of prolactin during an inflammatory challenge: A role for prolactin in diabesity? Biochim Biophys Acta. 2014;1842:584–93. doi: 10.1016/j.bbadis.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Hwang YC, Morrow DA, Cannon CP, Liu Y, Bergenstal R, Heller S, et al. High-sensitivity C-reactive protein, low-density lipoprotein cholesterol and cardiovascular outcomes in patients with type 2 diabetes in the EXAMINE (Examination of cardiovascular outcomes with alogliptin versus standard of care) trial. Diabetes Obes Metab. 2018;20:654–9. doi: 10.1111/dom.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Herrera X, de Los Ríos EA, Díaz JM, Lerma-Alvarado RM, Martínez de la Escalera L, López-Barrera F, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. 2017;158:56–68. doi: 10.1210/en.2016-1444. [DOI] [PubMed] [Google Scholar]
- 31.Kotula-Balak M, Gorowska-Wojtowicz E, Milon A, Pawlicki P, Kaminska A, Pardyak L, et al. Towards understanding biology of leydiogioma. G protein-coupled receptor and peroxisome proliferator-activated receptor crosstalk regulates lipid metabolism and steroidogenesis in Leydig cell tumors. bioRxiv. 2018;1:477901. [Google Scholar]
- 32.Al-Gareeb AI, Abd Al-Amieer WS, Alkuraishy HM, Al-Mayahi TJ. Effect of body weight on serum homocysteine level in patients with polycystic ovarian syndrome: A case control study. Int J Reprod Biomed (Yazd) 2016;14:81–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Perić B, Kruljac I, Šundalić S, Pećina HI, Jović A, Štefanović M, et al. Obesity and hypercholesterolemia in patients with prolactinomas: Could DHEA-S and growth hormone be the missing link? Endocr Res. 2016;41:200–6. doi: 10.3109/07435800.2015.1135444. [DOI] [PubMed] [Google Scholar]
- 34.Hussien NR, Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Sulfonylurea and neuroprotection: The bright side of the moon. J Adv Pharm Technol Res. 2018;9:120–3. doi: 10.4103/japtr.JAPTR_317_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega-González C, Cardoza L, Coutiño B, Hidalgo R, Arteaga-Troncoso G, Parra A, et al. Insulin sensitizing drugs increase the endogenous dopaminergic tone in obese insulin-resistant women with polycystic ovary syndrome. J Endocrinol. 2005;184:233–9. doi: 10.1677/joe.1.05844. [DOI] [PubMed] [Google Scholar]
- 36.Lopez Vicchi F, Luque GM, Brie B, Nogueira JP, Garcia Tornadu I, Becu-Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016;109:74–80. doi: 10.1016/j.phrs.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Al-kuraishy HM, Al-Gareeb AI. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Crosssectional study. Emergency medicine international. 2015 doi: 10.1155/2015/283021. [DOI] [PMC free article] [PubMed] [Google Scholar]