Abstract
Combined hepatocellular‐cholangiocarcinoma (cHCC‐CC) and intrahepatic cholangiocarcinoma (ICC) are classified into one category, but comparison of prognosis of the two carcinomas remains controversial. The aim of the current study was to investigate surgical outcomes for patients with ICC or cHCC‐CC who underwent resection in order to elucidate whether the classification of ICC and cHCC‐CC is justified. Subjects were 61 patients with ICC and 29 patients with cHCC‐CC who underwent liver resection from 2001 to 2017. Clinic‐pathological data from the two groups were compared. Tumor number and vascular invasion were independent risk factors for recurrence‐free survival (RFS) in both groups (P < .001 for both). Of note, for patients with ICC, tumor cut‐off size of 5 cm showed statistical significance in median RFS (>5 cm vs ≤5 cm, 0.5 years vs 4.0 years, P = .003). For patients with cHCC‐CC, tumor cut‐off size of 2 cm showed statistical significance in median RFS (>2 cm vs ≤2 cm, 0.6 years vs 2.6 years, P = .038). The median RFS of patients with cHCC‐CC was 0.9 years (95% confidence interval: 0.3‐1.6), which was poorer than that of patients with ICC (1.3 years, 0.5‐2.1) (P = .028); the rate of RFS at 5 years was 0% and 37.7% respectively. Our study supports the concept of classifying ICC and cHCC‐CC into different categories because of a significant difference in RFS between the two.
Keywords: cHCC‐CC, ICC, prognosis, tumor number, tumor size, vascular invasion
Our study supports the concept of classifying ICC and cHCC‐CC into different categories because of a significant difference in RFS between the two.

1. INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumor following hepatocellular carcinoma (HCC) and accounts for 5‐15% of cases of primary liver cancer.1 Combined hepatocellular‐cholangiocarcinoma (cHCC‐CC) originates from cells that have the histological features of HCC and CC, with an incidence rate from 0.4% to 14.2% in different regions.2, 3, 4, 5, 6
Patients with cHCC‐CC are most often diagnosed based on pathological findings, and after surgery in particular. The 7th and 8th editions of the American Joint Committee on Cancer (AJCC) staging7, 8 and International Union for Cancer Control (UICC) tumor‐nodes‐metastasis staging9, 10 classify cHCC‐CC and ICC into one category, but there is debate over the clinical features of cHCC‐CC in comparison to those of ICC and assessment of their prognosis. Some studies have suggested that patients with cHCC‐CC have a poorer prognosis than those with ICC,3, 11 while others have either reported the opposite12 or no significant difference in survival.2, 13, 14 Liver transplantation is also one of choice for ICC and cHCC‐CC.15, 16, 17, 18 However, surgical outcomes of ICC patients after liver transplantation is quite poor due to higher recurrence and shorter survival compared to HCC patients.17 Liver transplantation also cannot contribute to better prognosis of patients with cHCC‐CC compared to those with HCC, but may improve surgical outcomes than ICC patients.15
Furthermore, the previous edition of the AJCC/UICC staging system excluded tumor size as a prognostic factor from the tumor (T) classification,7, 9 but the current (8th) edition clearly indicates that a tumor size of 5 cm is a factor for determining the T classification of ICC.8, 10 Besides, based on the HCC staging system,19 the Liver Cancer Study Group of Japan (LCSGJ) staging system determines the T classification based on a tumor size of 2 cm, the tumor number, and the presence of vascular/serosal invasion. A nationwide study by the LCSGJ revealed that a solitary ICC ≤2 cm in size without vascular or major biliary invasion can have an excellent prognosis.20
In order to elucidate whether classifying ICC and cHCC‐CC into one category is justified, the current study investigated the clinical features of and prognosis for the two types of liver cancer.
2. PATIENTS AND METHODS
2.1. Study population
Data from patients with primary liver cancer undergoing liver resection at the Department of Digestive Surgery of Nihon University Itabashi Hospital in Tokyo from 2001 to 2017 were retrospectively analyzed. Of them, the patients with ICC or cHCC‐CC who underwent initial and curative resection were included in this study (Figure 1).
Figure 1.
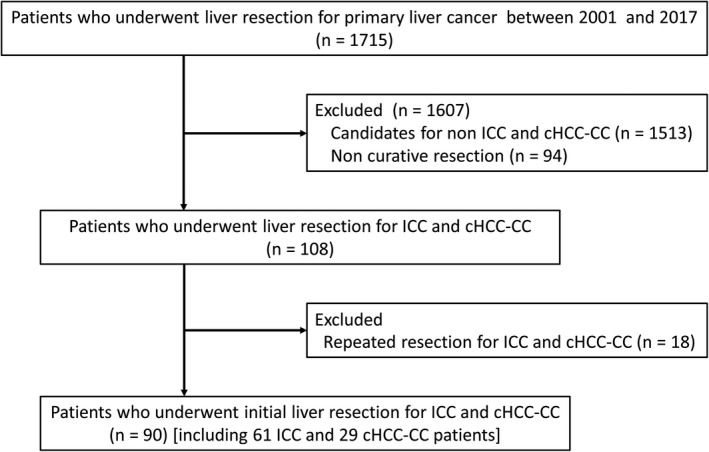
Flow‐chart for patient selection. cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma
For consecutive patients with ICC or cHCC‐CC, the diagnosis was based on imaging studies and clinical data, and confirmed by pathological findings. Combined hepatocellular‐cholangiocarcinoma was classified as the mixed type in accordance with the Allen and Lisa classification.21 Clinical and pathological data on patients were retrospectively collected. This study was approved by this facility's research ethics committee.
2.2. Preoperative evaluation
A routine preoperative evaluation for primary liver cancer was performed.20, 22, 23, 24 Computed tomography (CT) and gadolinium‐ethoxybenzyl‐diethylenetriamine pentaacetic acid‐enhanced magnetic resonance imaging (EOB‐MRI) were performed to evaluate the characteristics of each condition such as tumor size, tumor number, the presence of vascular invasion, and the presence of extrahepatic metastasis. Tumor markers including alpha‐fetoprotein (AFP), lens culinaris agglutinin‐reactive AFP (AFP‐L3), des‐gamma‐carboxy prothrombin (DCP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19‐9 (CA 19‐9) were evaluated.
2.3. Surgical procedures
All patients underwent open liver resection, and hilar lymphadenectomy was not routinely performed unless lymph node metastasis was suspected preoperatively. The indications for liver resection and the procedure were determined by assessing the liver functional reserve according to Makuuchi's criteria for liver resection.25 Briefly, liver resection is contraindicated in patients who have refractory ascites, hyperbilirubinemia (>2 mg/dL), or both. The extent of liver resection is determined on the basis of the serum total bilirubin level and indocyanine green retention rate at 15 minutes (ICG‐R15) value. Anatomical resection of Couinaud's segment was the first‐line operative procedure. Major liver resection was defined as removal of two or more segments, and minor resection was defined as resection of not more than one segment. R0 resection was defined as liver resection with “clear resection margin”, and R1 as liver resection with “resection margin touching inked tumor”.26 Patients undergoing R2 resection were excluded in this study.
2.4. Postoperative pathological factors
Each pathological factor was defined in accordance with the General Rules for the Clinical and Pathological Study of Primary Liver Cancer.19 Tumor size and number were determined based on pathological findings. Based on the LCSGJ classification system,19 the impact of portal vein invasion, hepatic vein invasion, arterial invasion, biliary invasion, and serosal invasion was evaluated according to the microscopic grade of each factor. Lymph node metastasis, distant metastasis, and intrahepatic metastasis were also analyzed.
2.5. Postoperative follow‐up
In general, patients were followed up every 1‐3 months during the first year after surgery and every 3 months thereafter. Levels of tumor markers such as AFP, DCP, CEA, and CA19‐9 were measured, and imaging studies, including CT and ultrasonography, were performed every 3 months on all patients. Recurrence was identified based on dynamic CT and/or EOB‐MRI. In patients with recurrent ICC or cHCC‐CC, the time between the date of surgery and recurrence was defined as the recurrence‐free period. Recurrent ICC or cHCC‐CC was treated with repeated liver resection, transcatheter arterial chemoembolization (TACE), radiofrequency ablation, or chemotherapy depending on the status of the ICC or cHCC‐CC and liver function at the time of recurrence. The final follow‐up was completed on September 12, 2018.
2.6. Statistical analysis
Continuous variables are expressed as the median and range and were compared using the Mann‐Whitney U test. Categorical variables are expressed as frequencies and percentages and were compared using the Chi‐square test. Cumulative survival curves were plotted using the Kaplan‐Meier method, and curves were compared using the log‐rank test. The predictive ability of tumor size was assessed by receiver operating characteristic (ROC) curve analysis and the corresponding area under the curve (AUC). The optimal cut‐off size was set as the value maximizing the sum of sensitivity and specificity.
The significance of clinical and pathological characteristics was assessed using univariate analysis. Variables that were significantly associated with survival and recurrence‐free survival (RFS) in univariate analysis were subjected to multivariate analysis using Cox's proportional hazards regression model (with a backward stepwise procedure), and the corresponding 95% confidence intervals (CI) were calculated. Two‐tailed P values <.05 were considered statistically significant.
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc).
3. RESULTS
3.1. Clinicopathological features
Based on pathological findings, 61 patients were diagnosed with ICC and 29 patients were diagnosed with cHCC‐CC (Figure 1). Levels of both AFP (P < .001) and AFP‐L3 (P = .006) were higher in patients with cHCC‐CC compared to levels in patients with ICC. The number of R1 was more frequent in cHCC‐CC group, but there was no significance between the two groups (cHCC‐CC vs ICC, 27.6% vs 24.6%, P = .799). The median tumor size was 4.0 cm (range: 1.0‐25.0 cm) for ICC and 3.0 cm (range: 1.0‐11.0 cm) for cHCC‐CC (Table 1).
Table 1.
Baseline characteristics of patients with ICC or cHCC‐CC
| Variables, n (%) | Total patients (n = 90) | ICC (n = 61) | cHCC‐CC (n = 29) | P values |
|---|---|---|---|---|
| Age (y)a | 68 (23‐84) | 69 (23‐84) | 64 (45‐80) | .080 |
| Gender, male | 64 (71.1) | 41 (67.2) | 23 (79.3) | .321 |
| Underlying liver disease | <.001 | |||
| Normal liver | 36 (40.0) | 31 (50.8) | 5 (17.2) | |
| Chronic hepatitis | 41 (45.6) | 27 (44.3) | 14 (48.3) | |
| Liver cirrhosis | 13 (14.4) | 3 (4.9) | 10 (34.5) | |
| Tumor markersa | ||||
| AFP (ng/mL) | 4.2 (0.8‐3689.4) | 3.7 (0.8‐808.1) | 10.9 (1.3‐3689.4) | <.001 |
| AFP‐L3 (%) | 0.5 (0.0‐90.9) | 0.5 (0‐90.6) | 5.7 (0.0‐90.9) | .006 |
| DCP (mAU/mL) | 21.0 (1.0‐15933.0) | 20.0 (1.0‐13380.0) | 26.0 (9.0‐15933.0) | .089 |
| CEA (ng/mL) | 3.0 (0.2‐175.1) | 3.6 (1.1‐175.1) | 2.6 (0.2‐32.7) | .067 |
| CA 19‐9 (U/mL) | 31.3 (0.1‐117800.0) | 31.3 (0.1‐117800.0) | 35.2 (0.1‐915.0) | .626 |
| ICR‐R15 (%)a | 10.0 (1.9‐34.0) | 9.9 (1.9‐33.4) | 11.5 (5.16‐34.0) | .113 |
| Child‐Pugh Score, A | 83 (92.2) | 55 (90.2) | 28 (96.6) | .421 |
| Operating time (min)a | 384.5 (145.0‐869.0) | 419.0 (191.0‐869.0) | 315.0 (145.0‐567.0) | .024 |
| Amount of bleeding (mL)a | 366.0 (29.0‐11002.0) | 364.0 (29.0‐11002.0) | 385.0 (35.0‐2740.0) | 1.000 |
| Blood transfusion, performed | 9 (10.0) | 7 (11.5) | 2 (6.9) | .712 |
| Extent of resection, major | 46 (51.1) | 39 (63.9) | 7 (24.1) | .001 |
| Surgical margin, R1 | 23 (25.6) | 15 (24.6) | 8 (27.6) | .799 |
| Tumor number, solitary | 72 (80.0) | 49 (80.3) | 23 (79.3) | 1.000 |
| Tumor sizea | 3.5 (1.0‐25.0) | 4.0 (1.0‐25.0) | 3.0 (1.0‐11.0) | .070 |
| Vascular invasion, present | 41 (45.6) | 28 (45.9) | 13 (44.8) | 1.000 |
| Serosal invasion, present | 22 (24.4) | 19 (31.1) | 3 (10.7) | .037 |
| Lymph node metastases, present | 10 (11.1) | 6 (9.8) | 4 (13.8) | .721 |
| Distant metastasis, present | 1 (1.1) | 0 (0.0) | 1 (3.4) | .322 |
| Intrahepatic metastasis, present | 13 (14.4) | 9 (14.8) | 4 (13.8) | 1.000 |
| Histologic differentiation, poor | 31 (34.4) | 12 (19.7) | 19 (65.5) | <.001 |
| Recurrence, present | 50 (55.6) | 29 (47.5) | 21 (72.4) | .040 |
Abbreviations: AFP, alpha‐fetoprotein; AFP‐L3, lens culinaris agglutinin‐reactive alpha‐fetoprotein; CA 19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; DCP, des‐gamma‐carboxy prothrombin; ICC, intrahepatic cholangiocarcinoma; ICR‐R15, indocyanine green retention rate at 15 min.
Median (range).
3.2. Patient survival
After a median follow‐up of 1.2 years (range: 0.1‐10.5 years) for 90 patients, recurrence was noted in 50 patients (55.6%), including 29 (47.5%) with ICC and 21 (72.4%) with cHCC‐CC (P = .040).
The median overall survival (OS) of 61 patients with ICC was 3.4 years (95% CI: 2.4‐4.4) and that of 29 patients with cHCC‐CC was 4.2 years (95% CI: 0.9‐7.6) (P = .200). The median RFS was 1.3 years (95% CI: 0.5‐2.1) for patients with ICC and 0.9 years (95% CI: 0.3‐1.6) for patients with cHCC‐CC (P = .028). The rates of OS and RFS at 5 years were 42.6% and 37.7%, respectively, in patients with ICC; and 25.8% and 0%, respectively, in patients with cHCC‐CC (Figure 2).
Figure 2.
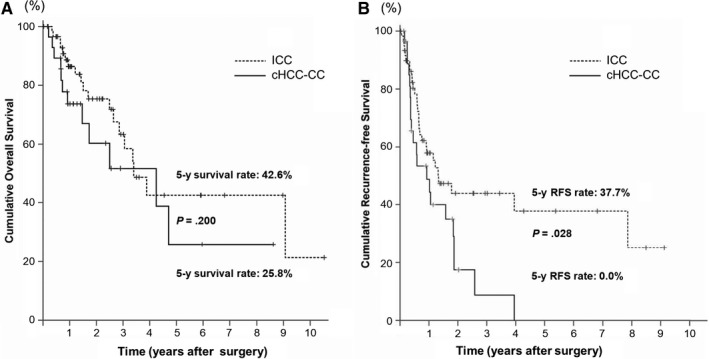
The cumulative OS (A) and RFS (B) curves for patients with ICC and patients with cHCC‐CC who underwent resection. cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; OS, overall survival; RFS, recurrence‐free survival
3.3. Prognostic factors
Cox's proportional model indicated that tumor number was the strongest independent risk factor for OS and RFS in both groups. Vascular invasion was also an independent risk factor for RFS in both groups (Tables 2 and 3).
Table 2.
Univariate and multivariate analysis of prognostic factors in patients with ICC
| Variables | Cases | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| OS n (%) | P | HR (95% CI) | P | RFS n (%) | P | HR (95% CI) | P | ||
| Age >65 y | 39 | 28 (71.8) | .783 | 24 (61.5) | .056 | ||||
| Gender, male | 41 | 31 (75.6) | .127 | 21 (51.2) | .619 | ||||
| Underlying liver disease, presenta | 30 | 18 (60.0) | .201 | 14 (46.7) | .352 | ||||
| AFP >20 ng/mL | 6 | 3 (50.0) | .185 | 2 (33.3) | .234 | ||||
| AFP‐L3 >10% | 8 | 4 (50.0) | .082 | 5 (62.5) | .713 | ||||
| DCP >40 mAU/mL | 5 | 1 (20.0) | .074 | 2 (40.0) | .963 | ||||
| CEA >5 ng/mL | 20 | 14 (70.0) | .770 | 10 (50.0) | .778 | ||||
| CA 19‐9 >40 U/mL | 27 | 17 (63.0) | .094 | 13 (48.1) | .183 | ||||
| ICR‐R15 >10% | 30 | 22 (73.3) | .132 | 18 (60.0) | .047 | .261 | |||
| Child‐Pugh Score, A | 55 | 37 (67.3) | .533 | 27 (49.1) | .109 | ||||
| Operating time >419 minb | 30 | 20 (66.7) | .439 | 13 (43.3) | .695 | ||||
| Amount of bleeding >364 mLb | 30 | 22 (73.3) | .556 | 15 (50.0) | .949 | ||||
| Blood transfusion, Performed | 7 | 2 (28.6) | .026 | .522 | 3 (42.9) | .796 | |||
| Extent of resection, major | 39 | 28 (71.8) | .803 | 22 (56.4) | .483 | ||||
| Surgical margin, R1 | 15 | 11 (73.3) | .605 | 7 (46.7) | .942 | ||||
| Tumor number, solitary | 49 | 39 (79.6) | <.001 | 22.929 (6.018‐87.365) | <.001 | 31 (63.3) | <.001 | 15.013 (5.321‐42.359) | <.001 |
| Tumor size ≤ 2 cm | 8 | 7 (87.5) | .364 | 6 (75.0) | .274 | ||||
| Tumor size ≤ 5 cm | 39 | 32 (82.1) | .003 | .481 | 25 (64.1) | .003 | .673 | ||
| Vascular invasion, present | 28 | 16 (57.1) | .001 | .122 | 11 (39.3) | .002 | 2.574 (1.136‐5.832) | .023 | |
| Serosal invasion, present | 19 | 10 (52.6) | .159 | 7 (36.8) | .285 | ||||
| Lymph node metastases, present | 6 |
3 (50.0) |
.126 | 2 (33.3) | .169 | ||||
| Intrahepatic metastasis, present | 9 | 2 (22.2) | <.001 | .606 | 1 (11.1) | <.001 | .413 | ||
| Histologic differentiation, poor | 12 | 10 (83.3) | .922 | 7 (58.3) | .657 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma; ICG‐R15, indocyanine green retention rate at 15 min; OS, overall survival; RFS, recurrence‐free survival.
Chronic hepatitis or liver cirrhosis.
Median value.
Table 3.
Univariate and multivariate analysis of prognostic factors in patients with cHCC‐CC
| Variables | Cases | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
|
OS n (%) |
P | HR (95% CI) | P |
RFS n (%) |
P | HR (95% CI) | P | ||
| Age >65 y | 14 | 6 (42.9) | .295 | 3 (21.4) | .440 | ||||
| Gender, male | 23 | 13 (56.5) | .525 | 7 (30.4) | .796 | ||||
| Underlying liver disease, presenta | 24 | 14 (58.3) | .843 | 7 (29.2) | .184 | ||||
| AFP >20 ng/mL | 11 | 6 (54.5) | .047 | .335 | 3 (27.3) | .005 | .696 | ||
| AFP‐L3 >10% | 12 | 8 (66.7) | .260 | 4 (33.3) | .019 | .785 | |||
| DCP >40 mAU/mL | 11 | 7 (63.6) | .768 | 4 (36.4) | .855 | ||||
| CEA >5 ng/mL | 5 | 0 (0.0) | .001 | .078 | 0 (0.0) | .006 | .065 | ||
| CA 19‐9 >40 U/mL | 13 | 6 (46.2) | .182 | 3 (23.1) | .325 | ||||
| ICR‐R15 >10% | 15 | 7 (46.7) | .325 | 2 (13.3) | .728 | ||||
| Child‐Pugh Score, A | 28 | 17 (60.7) | .629 | 8 (28.6) | .865 | ||||
| Operating time >315 minb | 14 | 8 (57.1) | .965 | 4 (28.6) | .590 | ||||
| Amount of bleeding >385 mLb | 14 | 8 (57.1) | .964 | 4 (28.6) | .506 | ||||
| Blood transfusion, performed | 2 | 1 (50.0) | .646 | 1 (50.0) | .932 | ||||
| Extent of resection, minor | 22 | 13 (59.1) | .231 | 7 (31.8) | .015 | 4.063 (1.223‐13.497) | .022 | ||
| Surgical margin, R1 | 8 | 5 (62.5) | .516 | 2 (25.0) | .656 | ||||
| Tumor number, solitary | 23 | 15 (65.2) | .002 | 7.382 (1.628‐33.468) | .010 | 7 (30.4) | <.001 | 10.631 (2.553‐44.273) | .001 |
| Tumor size >2 cmc | 24 | 13 (54.2) | .259 | 4 (16.7) | .038 | .188 | |||
| Vascular invasion, present | 13 | 6 (46.2) | .228 | 3 (23.1) | .036 | 3.247 (1.023‐10.299) | .046 | ||
| Serosal invasion, present | 3 | 3 (100.0) | .310 | 0 (0.0) | .325 | ||||
| Lymph node metastases, present | 4 | 3 (75.0) | .694 | 1 (25.0) | .819 | ||||
| Intrahepatic metastasis, present | 4 | 1 (25.0) | .083 | 0 (0.0) | .200 | ||||
| Histologic differentiation, poor | 19 | 9 (47.4) | .378 | 6 (31.6) | .411 | ||||
Abbreviations: AFP, alpha‐fetoprotein; AFP‐L3, lens culinaris agglutinin‐reactive alpha‐fetoprotein; cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; CI, confidence interval; HR, hazard ratio; OS, overall survival; RFS, recurrence‐free survival.
Chronic hepatitis or liver cirrhosis.
Median value.
A tumor cut‐off size of 5 cm (P = .001) was also significantly associated with RFS according to univariate analysis; when a tumor cut‐off size of 5 cm was used in multivariate analysis, multiple tumors (HR, 11.463; 95% CI, 2.536‐51.819; P = .002) and a tumor size > 5 cm (HR, 6.240; 95% CI, 1.619‐24.054; P = .008) were identified as significant predictors of RFS.
In ICC patients with multiple tumors, the rates of OS and RFS at 5 years were 0% respectively, which were lower than those of patients with a solitary tumor (54.1% and 47.2%, respectively; P < .001 for both) (Figure 3A,B). In ICC patients with vascular invasion, the rate of RFS at 5 years was 20.4%, which was lower than those of patients without vascular invasion (52.7%) (P = .002).
Figure 3.
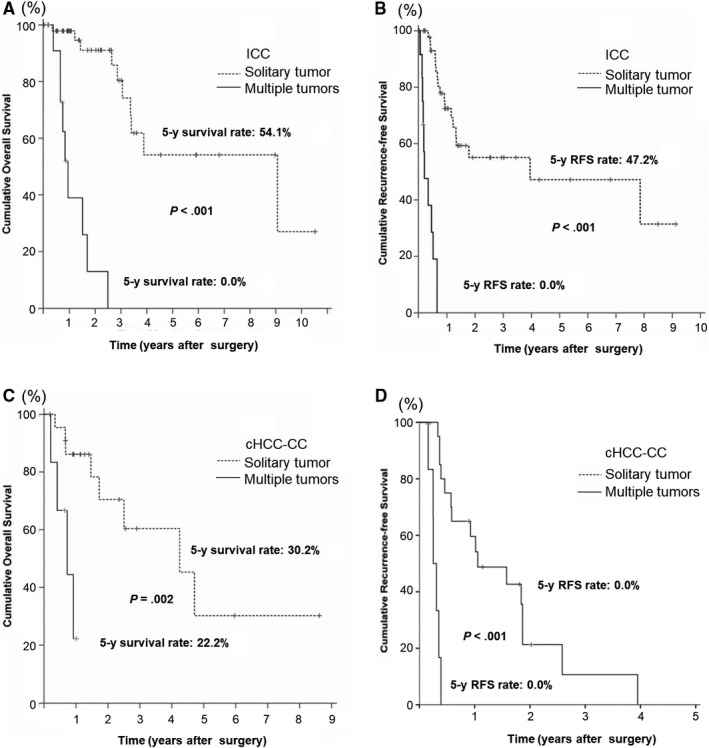
The cumulative OS and RFS curves for patients with ICC (A, B) and patients with cHCC‐CC (C, D) stratified by tumor number. cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; OS, overall survival; RFS, recurrence‐free survival
In cHCC‐CC patients with multiple tumors, the rate of OS at 5 years was 22.2%, which was lower than those of patients with a solitary tumor (30.2%, P = .002) (Figure 3C). The RFS at 5 years were poor in both groups. The median RFS was 0.3 years (95% CI: 0.2‐0.4) in patients with multiple tumors, which was shorter than 1.1 years (95% CI: 0.2‐1.9) in patients with a solitary tumor (P < .001) (Figure 3D). The median RFS was 0.4 years (95% CI: 0.1‐0.7) in patients with vascular invasion, which was shorter than 1.8 years (95% CI: 1.1‐2.6) in patients without vascular invasion (P = .019).
The resection margin status was not identified as an independent risk factor for OS and RFS in ICC and cHCC‐CC (P > .05, respectively) (Tables 2 and 3). The extent of resection was identified as an independent risk factor for RFS in patients with cHCC‐CC (P = .022) (Table 3). In cHCC‐CC patients undergoing major resection, the rate of RFS at 5 years was 0%, which was lower than those of patients undergoing a minor resection (10.8%, P = .022).
3.4. Tumor size and prognosis
When patients were stratified using a tumor cut‐off size of 2 cm, the prognosis for patients with ICC did not differ significantly. However, a tumor cut‐off size of 5 cm was significantly associated with OS and RFS (P = .003 for both) according to a univariate analysis of patients with ICC (Table 2). In patients with an ICC tumor >5 cm in size, the median OS and RFS were 2.5 years (95% CI: 0.7‐4.2) and 0.5 years (95% CI: 0.2‐0.7), respectively; the median OS and RFS were shorter than those in patients with a tumor ≤5 cm in size (6.5 years [95% CI: 4.8‐8.3] and 4.0 years [95% CI: 0.1‐8.1], respectively) (P = .003 for both). The OS and RFS rates at 5 years were 21.7% and 28.1% in patients with an ICC tumor >5 cm in size and 59.5% (P = .001) and 44.3% (P = .002) in patients with a tumor ≤5 cm in size.
In contrast, a tumor cut‐off size of 2 cm was significantly associated with RFS (P = .038) according to a univariate analysis of patients with cHCC‐CC (Table 3). In patients with a cHCC‐CC tumor >2 cm in size, the median RFS was 0.6 years; the median RFS was shorter than that of patients with a tumor ≤2 cm in size (2.6 years, P = .038).
In order to determine the optimal cut‐off value of tumor size for survivals, we performed ROC curve analysis (Figure S1). The area under the ROC curve was 0.706 (95% CI: 0.568‐0.844; P = .010) for OS of ICC patients. The optimal cut‐off value of tumor size was 4.55 cm, with sensitivity of 63.2% and specificity of 81.0%. On the other hand, the optimal cut‐off size was 2.35 cm for OS of cHCC‐CC patients, with sensitivity of 91.7% and specificity of 41.2% (Table 4).
Table 4.
The clinical utility of tumor size with different cut‐off values in predicting survival and recurrence for patients with ICC and cHCC‐CC
| Tumor cut‐off values | Sensitivity (%) | Specificity (%) | Youden index (%) | Area under ROC curve (95% CI) | P |
|---|---|---|---|---|---|
| ICC survival | |||||
| Optimal cut‐off size: 4.55 cm | 63.2 | 81.0 | 44.1 | 0.706 (0.568‐0.844) | .010 |
| Cut‐off size of 2 cm | 94.7 | 16.7 | 11.4 | ||
| Cut‐off size of 5 cm | 63.2 | 76.2 | 39.3 | ||
| ICC recurrence | |||||
| Optimal cut‐off size: 3.75 cm | 72.4 | 62.5 | 34.9 | 0.683 (0.548‐0.818) | .014 |
| Cut‐off size of 2 cm | 93.1 | 18.7 | 11.9 | ||
| Cut‐off size of 5 cm | 51.7 | 78.1 | 29.8 | ||
| cHCC‐CC survival | |||||
| Optimal cut‐off size:2.35 cm | 91.7 | 41.2 | 32.8 | 0.588 (0.376‐0.801) | .425 |
| Cut‐off size of 2 cm | 91.7 | 29.4 | 21.1 | ||
| Cut‐off size of 5 cm | 25.0 | 82.4 | 7.4 | ||
| cHCC‐CC recurrence | |||||
| Optimal cut‐off size: 2.15 cm | 95.2 | 62.5 | 57.7 | 0.708 (0.442‐0.974) | .088 |
| Cut‐off size of 2 cm | 95.2 | 62.5 | 57.7 | ||
| Cut‐off size of 5 cm | 23.8 | 87.5 | 11.3 | ||
Abbreviations: cHCC‐CC, combined hepatocellular‐cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma.
4. DISCUSSION
Even though the 7th and 8th editions of the AJCC/UICC staging system classify cHCC‐CC and ICC into one category,7, 8, 9, 10 patients with cHCC‐CC had a poorer prognosis than those with ICC and the prognoses for the two types of liver cancer differ when stratified by the tumor size in the current study.
Differences in prognosis for ICC and cHCC‐CC may be due to their distinct mechanisms of carcinogenesis and biological behavior. The disadvantageous behavior of cHCC‐CC may be related to tumor cells that originate from pluripotent hepatic precursor cells.27, 28 According to one hypothesis, the ICC components in cHCC‐CC do not originate from standard HCC and hepatic progenitor cells that undergo malignant transformation; instead, those cells may exhibit dual differentiation.29, 30, 31, 32 In the current study, chronic hepatitis or liver cirrhosis and poorly differentiated cancer were more frequently noted in patients with cHCC‐CC. Consequently, the rate of recurrence was higher in the cHCC‐CC group. Differences in prognosis for ICC and cHCC‐CC may be due to differences in the aforementioned background liver factors and the grade of cancer differentiation. Therefore, it might be justified to propose that ICC and cHCC‐CC be clinically regarded as different categories.
Tumor number is regarded as one of the important factors for determining the T classification according to the current staging systems of the AJCC/UICC8, 10 and LCSGJ.19 A greater number of tumors may result in satellite nodules or intrahepatic metastasis, both of which are reported to be associated with a worse outcome.33, 34, 35, 36 Consistent to the previous study, our data showed that tumor number was a significant predictor of OS and RFS in both groups, and patients with multiple ICCs had a poorer prognosis than those with a solitary tumor. It was also true for patients with cHCC‐CC. Similarly, numerous studies have reported on the prognostic importance of vascular invasion in ICC and cHCC‐CC.6, 14, 37, 38 In the current study, vascular invasion was also identified as a significant predictor of RFS in both groups. Patients with ICC with vascular invasion had a poorer prognosis than those without vascular invasion, and it was also true for patients with cHCC‐CC.
A point worth noting is that the prognostic importance of tumor size in patients with ICC or cHCC‐CC is still a subject of debate. Based on the analysis of population‐based data of patients with ICC in the United States, a study indicated that tumor size was not a prognostic factor, and it was unable to confirm the prognostic impact of a tumor cut‐off size of 2 or 5 cm on survival.39 Using Japanese data from the LCSGJ, a report indicated that an ICC cut‐off size of 2 cm in the largest dimension readily predicted patient survival and that a solitary ICC ≤2 cm in size without vascular or major biliary invasion can have an excellent prognosis.20 Several studies have similarly revealed the prognostic importance of tumor size in patients with cHCC‐CC,6, 40, 41 while others have found that tumor size was not associated with survival.42, 43 The reason why the prognostic impact of a tumor cut‐off size of 2 or 5 cm differs in the literature is unclear, but detecting a tumor ≤2 cm in size is very difficult due to the lack of symptoms. Surveillance is crucial for patients with hepatic disease. Moreover, the prognostic role of tumor size needs to be evaluated in additional studies.
In the current study, tumor size was significantly associated with prognosis in both groups according to univariate analysis. However, the prognoses of ICC and cHCC‐CC differed when the two forms of liver cancer were stratified according to tumor size. When stratified by a tumor cut‐off size of 2 cm, the prognosis for patients with ICC did not differ significantly. However, patients with cHCC‐CC >2 cm in size had a poorer prognosis than those with cHCC‐CC ≤2 cm in size. When stratified by a tumor cut‐off size of 5 cm, patients with ICC >5 cm in size had a poorer prognosis than those with ICC ≤5 cm in size. Given that the optimal cut‐off values of tumors size were approximately 5 cm for patients with ICC and 2 cm for those with cHCC‐CC in this study, tumor factor defined by AJCC/UICC and LCSGJ staging systems maybe appropriate for ICC and cHCC‐CC, respectively. However, patient number was too small in this study, and therefore it should be determined using a large cohort such as nationwide study.
The current study had several limitations. First, the current study was a retrospective, single‐center study. Consequently there were some differences of the background between the ICC and cHCC‐CC patients. Although propensity‐score matching is undoubtedly one of the best solution methods for observational data, the sample size was not large enough due to the low prevalence of ICC and cHCC‐CC, and therefore the statistical matching was impossible. Second, all patients in this study underwent surgical resection. Given that substantial proportion of patients with ICC and cHCC‐CC are not to be candidates for resection, the clinical features and prognosis for patients receiving nonsurgical treatment should be evaluated and compared.
5. CONCLUSION
The current study revealed that patients with cHCC‐CC had a poorer prognosis than those with ICC and that the prognosis differed significantly when cHCC‐CC was stratified by a tumor cut‐off size of 2 cm and when ICC was stratified by a tumor cut‐off size of 5 cm. This difference is likely to be due to the difference in the biological behavior of the two types of carcinoma. Taken together, the current findings suggest that ICC and cHCC‐CC should be classified into different categories. Moreover, the differences in tumor size, tumor characteristics, and tumor biology need to be further evaluated in order to accurately predict the prognosis for patients with ICC or cHCC‐CC.
CONFLICT OF INTEREST
There is no conflict of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
This research was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP19hk0102049s0302 and a grants‐in‐aid of The 106th Annual Congress of JSS Memorial Surgical Research Fund, Tokyo Japan.
Song P, Midorikawa Y, Nakayama H, et al. Patients' prognosis of intrahepatic cholangiocarcinoma and combined hepatocellular‐cholangiocarcinoma after resection. Cancer Med. 2019;8:5862–5871. 10.1002/cam4.2495
REFERENCES
- 1. Nakanuma Y, Curado MP, Fanceschi S, et al. Intrahepatic cholangiocarcinoma In: Bosman FT, Carnerio F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, Vol. 3 4th ed. International Agency for Research on Cancer; 2010:217‐214. [Google Scholar]
- 2. Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040‐2046. [DOI] [PubMed] [Google Scholar]
- 3. Yano Y, Yamamoto J, Kosuge T, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283‐287. [DOI] [PubMed] [Google Scholar]
- 4. Liu CL, Fan ST, Lo CM, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003;138:86‐90. [PubMed] [Google Scholar]
- 5. Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular‐cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34‐40. [DOI] [PubMed] [Google Scholar]
- 6. Kim SH, Park YN, Lim JH, et al. Characteristics of combined hepatocellular‐cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2014;40:976‐981. [DOI] [PubMed] [Google Scholar]
- 7. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual, 7th ed. New York, NY: Springer; 2010:201‐205. [Google Scholar]
- 8. Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual, 8th ed. New York, NY: Springer; 2017:288‐292. [Google Scholar]
- 9. Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours, 7th ed. New York, NY: Wiley Blackwell; 2009:108‐110. [Google Scholar]
- 10. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours, 8th ed. New York, NY: Wiley Blackwell; 2017:83‐84. [Google Scholar]
- 11. Lee JH, Chung GE, Yu SJ, et al. Long‐term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69‐75. [DOI] [PubMed] [Google Scholar]
- 12. Koh KC, Lee H, Choi MS, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120‐125. [DOI] [PubMed] [Google Scholar]
- 13. Chok K‐H, Ng K, Cheung TT, et al. An update on long‐term outcome of curative hepatic resection for hepatocholangiocarcinoma. World J Surg. 2009;33:1916‐1921. [DOI] [PubMed] [Google Scholar]
- 14. Lee SD, Park S‐J, Han S‐S, et al. Clinicopathological features and prognosis of combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Hepatobiliary Pancreat Dis Int. 2014;13:594‐601. [DOI] [PubMed] [Google Scholar]
- 15. Machairas N, Stamopoulos P, Kostakis ID, et al. Mixed hepatocellular cholangiocarcinoma: a review of long‐term outcomes following liver transplantation. Transplant Proc. 2019;51:437‐439. [DOI] [PubMed] [Google Scholar]
- 16. Sapisochin G, Facciuto M, Rubbia‐Brandt L, et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178‐1188. [DOI] [PubMed] [Google Scholar]
- 17. Lee DD, Croome KP, Musto KR, et al. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl. 2018;24:634‐644. [DOI] [PubMed] [Google Scholar]
- 18. Goldaracena N, Gorgen A, Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transpl. 2018;24:294‐303. [DOI] [PubMed] [Google Scholar]
- 19. Liver Cancer Study Group of Japan . General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Second English edtion. Tokyo, Japan: Kanehara & Co Ltd; 2003:23‐25. [Google Scholar]
- 20. Sakamoto Y, Kokudo N, Matsuyama Y, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647‐655. [PMC free article] [PubMed] [Google Scholar]
- 22. Kurokawa T, Yamazaki S, Mitsuka Y, Moriguchi M, Sugitani M, Takayama T. Prediction of vascular invasion in hepatocellular carcinoma by next‐generation des‐r‐carboxy prothrombin. Br J Cancer. 2016;114:53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamazaki S, Takayama T, Moriguchi M, et al. Validation of biological and clinical outcome between with and without thoracotomy in liver resection: a matched cohort study. World J Surg. 2012;36:144‐150. [DOI] [PubMed] [Google Scholar]
- 24. Yamazaki S, Takayama T, Kimura Y, et al. Transfusion criteria for fresh frozen plasma in liver resection: a 3 + 3 cohort expansion study. Arch Surg. 2011;146:1293‐1299. [DOI] [PubMed] [Google Scholar]
- 25. Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancer. Semin Surg Oncol. 1993;9:298‐304. [DOI] [PubMed] [Google Scholar]
- 26. Ma KW, Cheung TT, She WH, et al. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: a single‐center experience. Medicine (Baltimore). 2016;95:e4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang F, Chen X‐P, Zhang W, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double‐fluorescence immunostaining evidence. Histopathology. 2008;52:224‐232. [DOI] [PubMed] [Google Scholar]
- 28. Tang D, Nagano H, Nakamura M, et al. Clinical and pathological features of Allen's type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg. 2006;10:987‐998. [DOI] [PubMed] [Google Scholar]
- 29. Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253‐260. [DOI] [PubMed] [Google Scholar]
- 30. Kojiro M. Pathomorphology of advanced hepatocellular carcinoma In: Tobe T, Kameda H, Okudaira M, Ohto M, Endo Y, Mito M, Okamoto E, Tanikawa K, Kojiro M, eds. Primary Liver Cancer in Japan. Tokyo, Japan: Springer‐Verlag; 1992:31‐37. [Google Scholar]
- 31. Robrechts C, De Vos R, Van den Heuvel M, et al. Primary liver tumor of intermediate (hepatocyte‐bile duct cell) phenotype: a progenitor cell tumour? Liver. 1998;18:288‐293. [DOI] [PubMed] [Google Scholar]
- 32. Shiraishi M, Takushi Y, Simoji H, et al. Combined hepatocellular and cholangiocellular carcinoma in a non‐cirrhotic liver. J Gastroenterol. 1998;33:593‐596. [DOI] [PubMed] [Google Scholar]
- 33. Isaji S, Kawarada Y, Taoka H, Tabata M, Suzuki H, Yokoi H. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6:108‐116. [DOI] [PubMed] [Google Scholar]
- 34. Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jan YY, Jeng LB, Hwang TL, et al. Factors influencing survival after hepatectomy for peripheral cholangiocarcinoma. Hepatogastroenterology. 1996;43:614‐619. [PubMed] [Google Scholar]
- 36. Roayaie S, Guarrera JV, Ye MQ, et al. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187:365‐372. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188‐1195. [DOI] [PubMed] [Google Scholar]
- 38. Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB (Oxford). 2015;17:244‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nathan H, Aloia TA, Vauthey J‐N, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14‐22. [DOI] [PubMed] [Google Scholar]
- 40. He C, Mao Y, Wang J, et al. The predictive value of staging systems and inflammation scores for patients with combined hepatocellular cholangiocarcinoma after surgical resection: a retrospective study. J Gastrointest Surg. 2018;22:1239‐1250. [DOI] [PubMed] [Google Scholar]
- 41. Chu K‐J, Lu C‐D, Dong H, Fu X‐H, Zhang H‐W, Yao X‐P. Hepatitis B virus‐related combined hepatocellular‐cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192‐199. [DOI] [PubMed] [Google Scholar]
- 42. Park H, Choi KH, Choi S‐B, et al. Clinicopathological characteristics in combined hepatocellular‐cholangiocarcinoma: a single center study in Korea. Yonsei Med J. 2011;52:753‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin G, Toh C‐H, Wu R‐C, et al. Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract. 2008;62:1199‐1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


