Abstract
Background
Osteoarthritis (OA) is a joint disease characterized by articular cartilage degeneration and inflammation. We have previously clarified that a xanthan gum (XG) preparation exerts ameliorating effect on a rabbit OA model by regulating matrix metalloproteinase (MMP)-1 and MMP-3, which are critical proteins in the Wnt3a/β-catenin pathway. Thus, it is reasonable to predict that the Wnt3a/β-catenin pathway is involved in the treatment of OA with XG.
Material/Methods
The effect of XG in OA model animals were observed by hematoxylin and eosin staining (HE), Safranin O staining, and Fast Green staining. Articular cartilage degradation on the medial plateau sides was quantified using the modified Pritzker OARSI score. The levels of IL-6, TNF-α, and IL-1β in synovial fluid were determined with ELISA. The protective effect of XG in rat chondrocytes was assessed by CCK8 assay. Moreover, activation of the Wnt3a/β-catenin pathway and the expression of MMP13, ADAMTS5, aggrecan, and collagen II under the influence of XG was measured by Western blot and qRT-PCR.
Results
Our results showed that XG reduced the OARSI score and the concentration of inflammatory cytokines in OA after intra-articular injection. XG acted on Wnt3a/β-catenin in ATDC5 cells in a dose-dependent manner and exhibited a protective effect. XG also decreased the expression of MMP13 and ADAMTS5 and rescued the inhibition of aggrecan and collagen II expression in SNP-stimulated chondrocytes.
Conclusions
These results indicate that the effects of XG are related to the Wnt3a/β-catenin pathway and XG suppresses matrix degradation by inhibiting the expression of MMPs and ADAMTS and promotes aggrecan and collagen II content in the ECM, indicating its favorable potential for use in OA therapy.
MeSH Keywords: Aggrecans; Cell- and Tissue-Based Therapy; Collagen Type II; Matrix Metalloproteinase 1; Osteoarthritis, Knee; Wnt3A Protein
Background
Osteoarthritis (OA) is a disease characterized pathologically by articular cartilage loss in focal areas of the synovial joints, accompanied by various levels of synovitis, subchondral bone change, and osteophyte formation [1]. Extracellular matrix (ECM) degradation and death of articular cartilage are the primary characteristics of OA. The ECM is responsible for the structure of chondrocytes, which is maintained by remodeling [2]. An important cause of OA pathogenesis is cartilage homeostasis disorder [3]. Chondrocyte apoptosis and ECM degradation occur due to increased expression of MMPs and ADAMTS (A Disintegrin And Metalloproteinase With Thrombospondin Motifs 5), which are the predominant matrix-degrading enzymes that degrade ECM components and hydrolyze aggrecan core proteins [4]. Human articular cartilage is composed of chondrocytes and lacks blood vessels. Articular chondrocyte repair is generally considered a core component of OA treatment [5–7]. Although many risk factors have been identified and researchers have elucidated the chemical pathways associated with inflammation and wear, the pathological mechanism of OA is still controversial and not fully understood [8]. The main symptoms of OA are joint pain and swelling and stiffness in the limbs. In the clinic, nonsteroidal anti-inflammatory drugs (NSAIDs) are the preferred alternative for OA therapy, even though these drugs only relieve pain and cannot protect cartilage from further injury. Effective treatments need to be explored [9].
Wnt3a/β-catenin signaling has been shown to participate in OA pathological progression and may play a vital role in joint tissues through the production of MMPs in chondrocytes [10], osteoblasts [11], and synovial cells [12]. Previous studies have shown the upregulation of Wnt3a ligands, confirming the important role of Wnt3a in regulating OA pathogenesis [13]. Recent research has shown that triggering of the canonical Wnt3a signaling pathway by Wnt3a and β-catenin plays a central role in OA [14]. Wnt3a is a key gene and protein in chondrocytes in mechanically induced animal models and humans [15]. Wnt3a-stimulated chondrosarcoma cells can cause OA in vitro. The release of proteoglycans from the matrix by chondrocytes is increased by stimulation of canonical Wnt3a [10]. Dickkopf-1 (Dkk-1) and Dkk-3, which are Wnt3a antagonists, are structurally and functionally significant members of the Dkk family and show abnormal production in OA [16]. Dkk-1 inhibits the interaction between Wnt3a and its receptors Frizzled (Fz) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) [17]. The increased expression of Dkk-3 in human OA cartilage inhibits the loss of proteoglycans and collagen in bovine cartilage explants mediated by IL-1β and oncostatin-M [18]. Inhibiting Wnt3a signaling is considered a potential therapeutic strategy for treating OA. However, determining the best approach for targeting the canonical Wnt3a pathway remains challenging.
XG (PubChem CID: 7107), which contains 5 repeating monosaccharide patterns, is a natural microbial extracellular heteropolysaccharide. The molecular weights of injected polymer preparations range in size from 1500 to 2500 kDa [19]. XG is similar to sodium hyaluronate (SH) in viscosity and rheology. Previous studies have demonstrated the safety and ameliorative effects of XG on chondrocytes based on assessments of cell proliferation and MMP-1, MMP-3, MMP-13, and TIMP-1 (Tissue Inhibitor Of Metalloproteinases 1) protein production in IL-1β-induced rabbit chondrocytes [20]. Despite previous trials, the mechanism of XG-related agents in OA remains debated and uncertain.
The purpose of the present study was to explore the mechanism by which XG regulates the metabolism of articular cartilage and alters the OA process in OA models via the canonical Wnt3a/β-catenin pathway.
Material and Methods
Cells, animals, and reagents
The L-Wnt3a cells (CRL-2647) were obtained from the American Type Culture Collection (Manassas, USA) and ATDC5 cells (RCB-0565) were purchased from Riken Cell Bank (Tsukuba, Japan). New Zealand White rabbits (weight, 2.5±0.5 kg; age, 6 months) and Sprague-Dawley rats (weight, 180±20 g; age, 7 weeks) were purchased from the Model Animal Research Center of Jinzhou Medical University (Jinzhou, China). IL-1β, TNF-α, and IL-6 enzyme-linked immunosorbent assay kits were obtained from Boster (Wuhan, China). Dulbecco’s modified Eagle’s medium (#11965118, DMEM) was from Gibco BRL (Grand Island, NY, USA), fetal bovine serum (#10099, FBS) was from Gibco BRL (Grand Island, NY, USA), and Cell Counting Kit-8 (#HY-K0301, CCK-8) was from MCE (NY, USA). The XG batch fermentation was purified and made into injection preparation as previously described [21] and supplied by the Postdoctoral Scientific Research Workstation, Institute of Biopharmaceuticals of Shandong Province (Jinan, China). SNP original powder was purchased from Beyotime (#S0015, Sodium Nitroprusside, Dihydrate, Shanghai, China), and a quantitative polymerase chain reaction (qPCR) kit (SYBR® Premix Ex Taq™ II, Takara Bio, Inc., Otsu, Japan) was obtained from Takara. Antibodies against Wnt3a, β-catenin, MMP13, ADAMTS5, aggrecan, and collagen II, as well as the secondary antibody, were purchased from Abcam (#ab28472, Wnt3a; #ab32572, β-catenin; #ab39012, MMP13; #ab41037, ADAMTS5; #ab3778, aggrecan; #ab34172, collagen II, #ab150055, anti-rabbit IgG; #ab150117, anti-mouse IgG, Cambridge, UK). qPCR primers were obtained from GENEWIZ (NJ, USA). Hematoxylin, eosin, Safranin O, and Fast Green were obtained from Baso (Zhuhai, China). The Dual-Luciferase® Reporter Assay System and FuGENE® 6 were purchased from Promega (Wisconsin, USA).
Surgically induced OA models and treatment regimens with XG
Animal and cell experiments were approved by the relevant committee and adhered to the institutional regulations on animal research of Jinzhou Medical University, Jinzhou city, China. All rabbits were provided free access to food and water and were housed separately in single cages with natural light. The rabbits were divided into the control group, sham group, OA group, and XG-treated group by the random number table method, with 15 rabbits in each group.
The rabbits were deeply anaesthetized with ear margin intravenous injection of phenobarbital sodium (50 mg/kg) and auxiliary inhalation of ether if necessary until the flexor withdrawal reflex was abolished. Animals receiving medial meniscus destabilization (MMD) operation as described by Arunakul et al. [22] were randomly assigned to the OA and XG groups, with 15 animals in each group. The sham group underwent the same operation except for MMD, and the control group did not undergo surgery. Intra-articular injections began 28 days after surgery. The OA group animals were injected with saline solution (0.1 mL/kg) once every 7 days into the left knee for 35 days. The XG-treated group received injections with the XG preparation (1.0% w/v; Mw is 1500 kDa to 2500 kDa). Intra-articular injections started 28 days after surgery and occurred once every 7 days for 35 days. The control group (non-operated group) and the sham group (sham-operated) were separately and intra-articularly injected with saline solution in a volume of 0.1 mL/kg.
Histological analysis
Cartilage tissues were fixed in 10% neutral buffered formalin overnight and decalcified in 13% EDTA for 3 weeks. After complete decalcification, dehydration in graded ethanol, permeabilization in xylene, and embedding in paraffin were carried out, and the tissues were cut into 3-μm sections. The sections were hydrated in xylene and graded ethanol and then stained with hematoxylin and eosin. The specimens were also stained by Safranin O and Fast Green. All slides were analyzed by 2 pathologists via light microscopy. Articular cartilage degradation on the medial plateau sides was quantified using the modified Pritzker OARSI score as previously described [23].
Enzyme-linked immunosorbent assay
The synovial fluid in the articular cavity of each group of animals was collected when the animals were killed, and the concentrations of interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin 1β (IL-1β) were determined using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions.
Cell isolation and culture
Animals were raised in natural light with free access to water and food. Articular cartilage was collected from the knee joints and femoral heads of SpragueDawley rats that had been sacrificed using intraperitoneal injection of 10% phenobarbital sodium. Under sterile conditions, articular cartilage tissues of normal rats were chopped and completely digested with 0.15% collagenase. The digestion was terminated by the addition of DMEM containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 60 μg/mL neomycin solution. The tissue mass was homogenized, and chondrocytes were obtained by centrifugation at 1000 rpm. The collected chondrocytes were cultivated in 5% CO2 in DMEM containing 10% FBS at 37°C. After incubation for 24 h, the cells were digested, collected, and subcultured. These chondrocytes were considered passage 0 (P0); P3 cells were used for analysis.
CCK8 assay
Chondrocytes were cultured in 96-well cell culture plates with 5×103 cells in each well and then treated with various concentrations of XG (0, 0.1, 1, 2, and 4 mg/mL) and co-treatment with or without 1 mM SNP for 24 h as previously reported [24]. Then, CCK-8 solution (Cell Counting Kit-8, 10 mL/100 mL) was added to the culture medium and incubated at 37°C for 4 h. The optical density (OD) at 450 nm was detected a using an iMark microplate reader (Bio-Rad, USA), and cell viability was calculated via reference to a standard curve plotting cell number against the absorbance value. This analysis was repeated in triplicate.
TOPFlash reporter assay
Wnt3a-CM (mouse Wnt3a in conditioned medium) was collected from L-Wnt3a cells, which steadily produce Wnt3a, as previously described [25]. The intensity of β-catenin-mediated transcriptional activation was detected in ATDC5 cells exposed to Wnt3a-CM. Transfection was performed with the plasmid vector phRL-TK according to the manufacturer’s protocol. At 24 h after transfection, 2×104 cells/well was added to the wells of a 96-well culture plate, and each well received the indicated final concentrations (0, 0.1, 1, 2, and 4 mg/mL) and 25% Wnt3a-CM. After incubation for 24 h, luciferase activity was detected with the Dual-Luciferase® Reporter Assay System. Relative luciferase units (RLUs) were normalized to control Renilla activity. The mean value of 6 wells in an experiment was calculated at first, and mean ±SD in 6 wells of 3 experiments were calculated.
Protein extraction and Western blot analysis
Proteins were extracted from chondrocytes with a whole-cell lysis assay kit. The protein concentration was estimated with a protein assay kit. Equal 50-μg protein samples were separated by 10% SDS-PAGE and electroblotted onto a PVDF membrane, which was then blocked with PBS buffer containing 5% non-fat dry milk and 0.1% Tween-20. The following primary goat-anti-rabbit antibodies were used to probe the membranes at 4°C overnight: Wnt3a (1: 200), β-catenin (1: 800), MMP13 (1: 500), ADAMTS5 (1: 100), aggrecan (1: 100), and collagen II (1: 800). Then, the secondary antibody (1: 1000) was added and the mixture was incubated at 26°C for 1 h. Protein bands were detected and β-actin was used as a loading control.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from Sprague-Dawley rat articular chondrocytes treated with or without 1 mg/mL XG and with or without SNP (1 mM) for 24 h and reverse-transcribed to obtain cDNA. cDNA was amplified by qRT-PCR using primers for Wnt3a, β-catenin, MMP13, ADAMTS5, collagen II, and aggrecan, which are shown in Table 1. qRT-PCR was performed with an iCycler (Bio-Rad Laboratories, Hercules, CA, USA).
Table 1.
Primer sequences used in qRT-PCR.
| Gene | Forward primer | Reverse primer | Origin |
|---|---|---|---|
| wnt3a | 5′-TGCCAGGAGTGTATTCGCATC-3′ | 5′-TTCCCATGAGACTTCGCTGA-3′ | Human |
| β-catenin | 5′-GACACCAAGAAGCAGAGATGG-3′ | 5′-CGAATCAATCCAACAGTAGCC-3′ | Human |
| MMP-13 | 5′-CCAGAACTTCCCAACCAT-3′ | 5′-ACCCTCCATAATGTCATACC-3′ | Human |
| ADAMTS-5 | 5′-GCAGAACATCGACCAACTCTACTC-3′ | 5′-CCAGCAATGCCCACCGAAC-3′ | Human |
| Collagen II | 5′-CTCAAGTCGCTGAACAACCA-3′ | 5′-GTCTCCGCTCTTCCACTCTG-3′ | Human |
| Aggrecan | 5′-AAGTGCTATGCTGGCTGGTT-3′ | 5′-GGTCTGGTTGGGGTAGAGGT-3′ | Human |
| GAPDH | 5′-TCTCCTCTGACTTCAACAGCGAC-3′ | 5′-CCCTGTTGCTGTAGCCAAATTC-3′ | Human |
Statistical analysis
Experimental data are reported as the mean ± standard deviation (SD). Differences among groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Graphpad Prism (version 5.01) was used for statistical analysis. A p value less than 0.05 was considered significant.
Results
XG reduces articular cartilage damage and proinflammatory cytokines in vivo
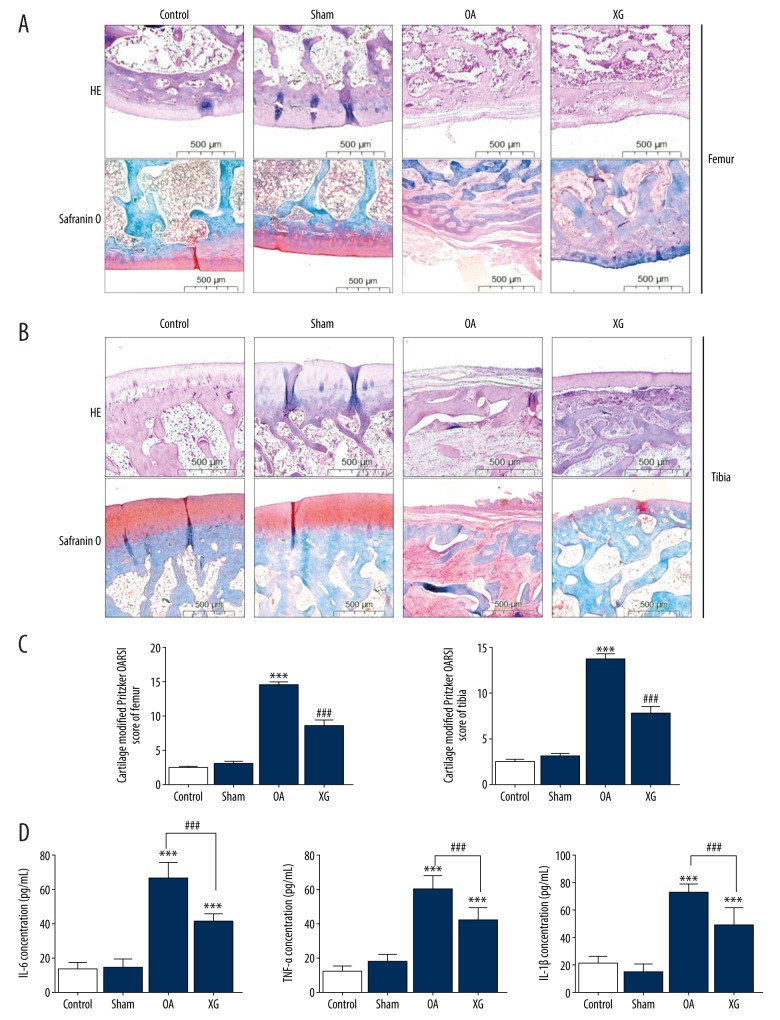
The results from HE, Safranin O, and Fast Green staining are shown in femurs (Figure 1A) and tibias (Figure 1B) for each group. The control group and the sham group showed normal cartilage structures. In the OA group, the surface layer consisted of damaged cartilage, with reduced and irregular chondrocyte aggregation, and these degenerative changes extended to the deeper region. By contrast, these results were not observed in the XG-treated group; cartilage destruction was significantly attenuated, and the structure of the cartilage surface layer was restored. This result means that XG could protect articular cartilage from OA damage in the MMD surgery-induced OA model. Furthermore, we examined the modified Pritzker OARSI score of each group, which revealed significant differences among the groups. In the OA group, the cartilage OARSI score of femurs and tibias was greater than 14.47±1.51 and 13.67±2.02, and after XG treatment for 5 weeks, the score decreased to 8.53±2.83 and 7.73±3.06 (Figure 1C). These findings also indicate that XG could protect against articular cartilage damage. To investigate the anti-inflammatory effects of XG in mechanically induced OA animals, we detected the IL-6, TNF-α, and IL-1β concentrations in joint synovial fluid when the animals were killed. The concentrations of IL-1β, IL-6, and TNF-α were significantly increased in the OA group. However, after XG treatment, proinflammatory factors IL-1β, IL-6, and TNF-α were significantly reduced (Figure 1D).
Figure 1.
XG reduced articular cartilage damage and proinflammatory cytokines in vivo. (A, B) Hematoxylin and eosin, Safranin O, and Fast Green staining were performed on femurs (A) and tibias (B) of rabbit articular cartilage samples. (C) The degree of cartilage destruction was evaluated by the modified Pritzker OARSI score in the femurs and tibias of rabbit articular cartilage samples. (D) The concentrations of IL-6, TNF-α, and IL-1β in joint synovial fluid were determined by ELISA. The values are presented as the mean±SD, n=15, *** p<0.001 vs. Control group, ### p<0.001 vs. OA group.
XG has protective effects on SNP-stimulated rat chondrocyte viability
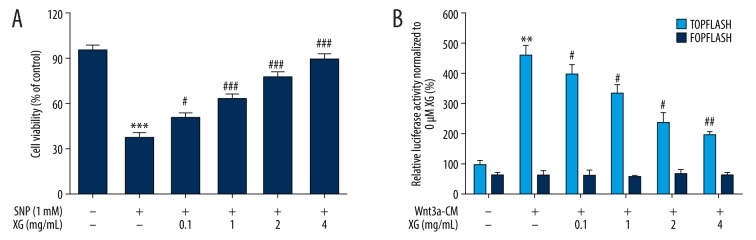
The protective effect of XG was assessed in an in vitro model with 1 mM SNP stimulated for 24 h in rat chondrocytes (Figure 2A). The results of the CCK8 assays for rat chondrocyte viability revealed that chondrocytes incubated with 1 mM SNP for 24 h had clearly reduced cell viability compared with the control group. Concurrently, XG significantly increased cell viability at various concentrations (0.1, 1, 2, and 4 mg/mL) compared with the SNP-induced only group, and XG at different concentrations raised cell viability in a dose-dependent manner.
Figure 2.
Assessment of the protective effect of XG on SNP-stimulated chondrocytes and the suppressive effect of XG on Wnt/β-catenin signaling activity. (A) SD rat chondrocytes were treated with various concentrations of XG (0, 0.1, 1, 2, and 4 mg/mL) with or without SNP (1 mM, 24 h) followed by CCK8 assay analysis. n=6, *** p<0.001 compared with control group; # p<0.05, ### p<0.001 compared with the SNP-induced only group. (B) XG suppressed activation of the Wnt3a/β-catenin signaling pathway by 25% Wnt3a-CM in a dose-dependent manner. To examine the ability of XG to inhibit Wnt3a/β-catenin signaling, ATDC5 cells were treated with Wnt3a-CM (25%) and different concentrations of XG (0, 0.1, 1, 2 and 4 mg/mL) 24 h after transfection with the TOPFlash plasmid. FOPFlash was used as a negative control. Data represent the mean ±SD, n=3, ** p<0.01, compared with untreated group, # p<0.05, ## p<0.01, compared with Wnt3a-CM group.
XG suppresses Wnt3a/β-catenin signaling activity in ATDC5 cells according to the TOPFlash reporter assay
The L-Wnt3a cells were derived from mice showing high Wnt3a expression [26], and mouse-derived ATDC5 cells epitomize chondrogenic differentiation [27]; therefore, we investigated the suppression effect caused by XG preparation via the downstream β-catenin pathway. Using the TOPFlash reporter assay, we found that XG suppressed activation of the Wnt3a/β-catenin signaling pathway by 25% Wnt3a-CM (Figure 2B). Moreover, XG inhibited TOPFlash reporter gene activity in a dose-dependent manner. Firefly luciferase activity was normalized to Renilla luciferase activity with 0 mg/mL XG driven by the TK promoter.
XG regulates cartilage degradation by downregulating overexpression of the Wnt3a/β catenin signaling pathway in vitro
To determine if Wnt3a/β-catenin signaling pathway activation stimulates the occurrence of OA and to quantify the effects of XG, chondrocytes were incubated for 24 h with SNP to simulate an inflammatory environment. SNP, a donor of exogenous NO, has become a common experimental reagent for investigating cartilage degeneration via the Wnt/β-catenin signaling pathway [28]. Previous research determined the dose of XG that had a protective effect on articular cartilage and reduced OA pain; the same treatment regimen was applied in this study [29].
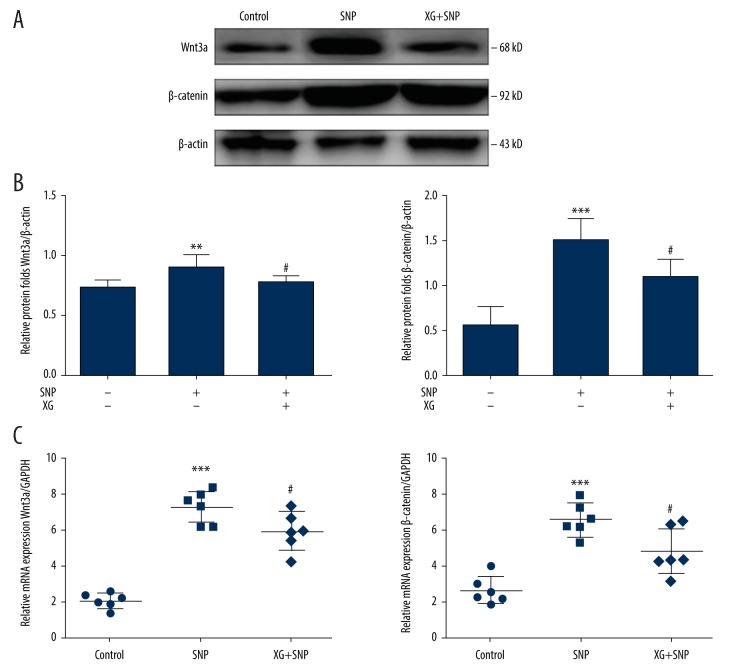
Compared with the control group, the protein expression levels of Wnt3a and β-catenin were higher in the SNP group, whereas the protein levels of Wnt3a and β-catenin were decreased in the XG group. Wnt3a and β-catenin mRNA expression levels were increased following stimulation with SNP but decreased following treatment with XG in chondrocytes (Figure 3).
Figure 3.
XG regulates cartilage degradation by downregulating the Wnt/β catenin signaling pathway in vitro. (A) Following treatment with or without SNP (1 mM, 24 h) in the absence or presence of XG (1 mg/mL, 24 h). Representative blots of Wnt3a and β-catenin. (B) Quantification of Wnt3a and β-catenin protein expression levels. n=6, **p<0.01, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group. (C) Quantification results obtained by qRT-PCR for Wnt3a and β-catenin mRNA expression levels in each group. n=6, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group.
XG decreased the levels of the downstream proteins MMP13 and ADAMTS5 in the Wnt3a/β-catenin pathway
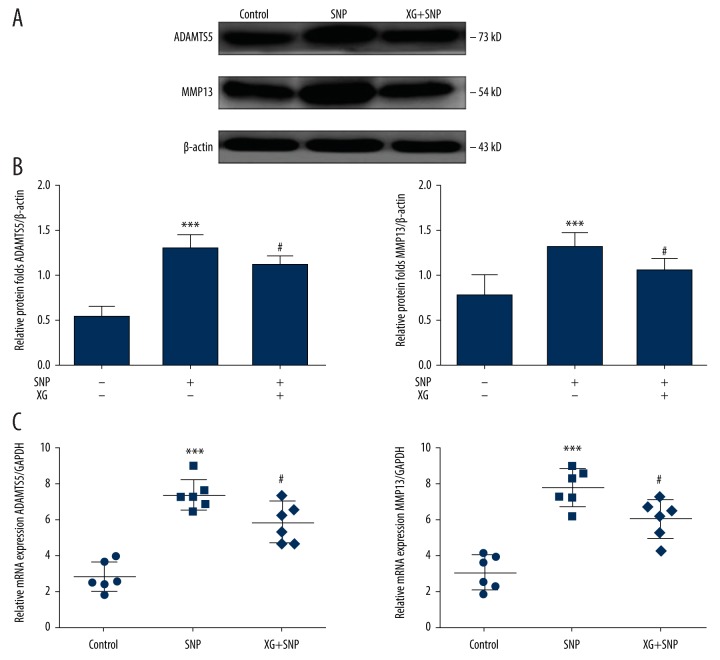
The Wnt3a/β-catenin pathway is activated in inflammatory environments, with subsequent increases in the levels of the downstream proteins MMP13 and ADAMTS5 and decreases in anabolic markers [30]. To further clarify the relevance of XG and the Wnt3a/β-catenin pathway downstream proteins MMP13 and ADAMTS5 in the inhibition of SNP-induced Wnt3a/β-catenin signaling activation, chondrocytes were further observed after SNP stimulation.
Chondrocytes exhibited obvious upregulation of mRNA levels of MMP13 and ADAMTS5 upon SNP stimulation relative to that in the control group. By contrast, XG treatment obviously suppressed MMP13 and ADAMTS5 mRNA expression. In accordance with the changes in mRNA expression, XG treatment clearly blocked the mRNA expression of MMP13 and ADAMTS5 (Figure 4). The protein expression levels of MMP13 and ADAMTS5, which are important cartilage catabolic factors, were significantly increased in the SNP group relative to the control group; furthermore, the XG-treated SNP group showed lower levels of MMP13 and ADAMTS5.
Figure 4.
XG affects the levels of the downstream proteins MMP13 and ADAMTS5 of the Wnt/β-catenin pathway. (A) Representative blots of MMP13 and ADAMTS5. (B) Quantified proteins expression levels of Wnt3a and β-catenin in SD rat chondrocytes incubated with or without SNP (1 mM, 24 h) with or without XG treatment. n=6, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group. (C) Quantification results obtained by qRT-PCR for MMP13 and ADAMTS5 mRNA expression levels in each group. n=6, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group.
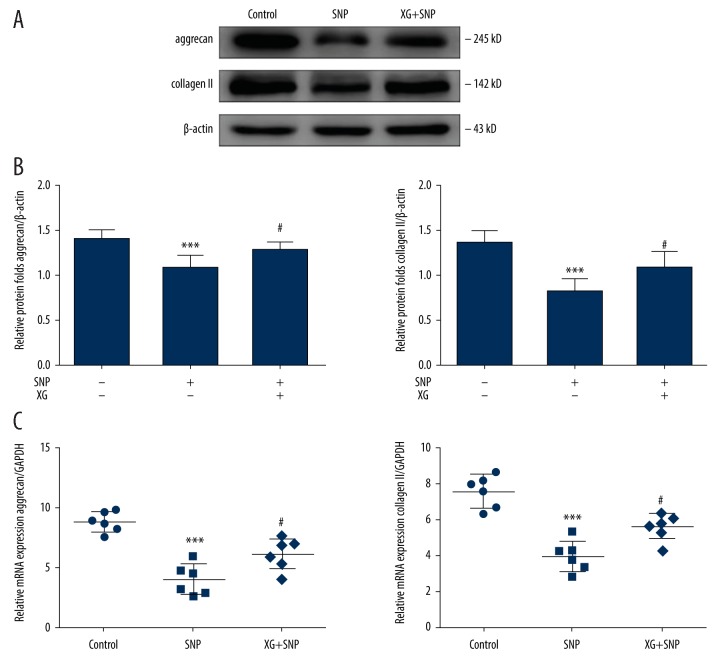
XG alters the aggrecan and collagen II contents in SNP-induced OA chondrocytes
Decreases in aggrecan and collagen II result in compression or calcification of the ECM, followed by accelerated arthrosis degeneration [31]. To assess the effects of XG on SNP-induced OA chondrocytes, aggrecan and collagen II mRNA and protein expression levels were evaluated. Following treatment with or without SNP (1 mM, 24 h) in the absence or presence of XG (1 mg/mL), qRT-PCR was performed on the control group, SNP group, and XG group chondrocytes to determine aggrecan and collagen II mRNA expression levels, and Western blotting was performed on chondrocytes to observe aggrecan and collagen II protein expression levels (Figure 5).
Figure 5.
XG affects the levels of aggrecan and collagen II in chondrocytes. (A) Representative blots of aggrecan and collagen II in SD rat chondrocytes incubated with or without SNP (1 mM, 24 h) with or without XG treatment. (B) The protein expression levels of aggrecan and collagen II were quantified. n=6, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group. (C) Quantification results obtained by qRT-PCR for aggrecan and collagen II mRNA expression levels. n=6, *** p<0.001 vs. control group, # p<0.05 vs. SNP-induced only group.
SNP-stimulated chondrocytes showed significantly lower aggrecan and collagen II mRNA expression. By contrast, XG treatment resulted in significant downregulation of aggrecan and collagen II mRNA expression. The anabolic markers aggrecan and collagen II were decreased following treatment with SNP. However, the expression of aggrecan and collagen II were altered by XG treatment. Consequently, XG treatment increased aggrecan and collagen II levels in SNP-induced cartilage destruction in vitro.
Discussion
The ECM is an important structure for maintaining the internal stability and structural integrity of cartilage. Protecting the ECM from degeneration is one way to maintain chondrocyte function. Polysaccharide pharmaceutical preparations, which were reported to play an active role in the treatment of OA, such as biotechnological chondroitin, are important in translational medicine [32]. XG is an extracellular polysaccharide that is produced by fermentation of Xanthomonas campestris in a stirred fermenter [33]. XG is a possible treatment for OA symptoms [34]; XG and low molecular weight XG (1000 kDa to 1500 kDa) can repair damaged cartilage though preventing apoptosis, as discussed above [35,36]. Moreover, XG is probably more stable and less easily degraded than SH in vivo [20]. In our present study, we found that XG reduced the MMD surgery-induced irregular chondrocyte aggregation and restored the cartilage surface layer. XG also has anti-inflammatory effects. The concentrations of IL-6, TNF-α, and IL-1β concentrations in joint synovial fluid were reduced. For better evaluation of XG treatment, the cartilage OARSI score of femurs and tibias were used, and they were assessed after 5 weeks of XG treatment.
Local XG administration can relieve the pain of arthritis and reduce the degeneration of articular cartilage, as illustrated in a rat model of monosodium iodoacetate-induced knee OA [19]. However, the pathway by which XG treatment affects OA is unclear. Various biochemical pathways contribute to insufficient modulation of cartilage matrix synthesis [37]. Increased Wnt3a signaling in synovial and subchondral bones is likely to contribute to development of the disease, reiterating the need to tightly regulate the activation of Wnt3a pathways in joints [38]. Wnt3a signaling promotes the OA process and negatively impacts the generation and molecular characteristics of articular cartilage cells and tissue loss-related enzymes [39]. Wnt8a and Wnt16, which act on the canonical Wnt3a signaling pathway, resulted in the initiation of erosive articular cartilage degeneration 7 days after overexpression [40]. Here, we show that XG maintains the chondrocyte phenotype by controlling the Wnt3a/β-catenin signaling pathway in vitro.
The increases in apoptotic cells and ECM degradation in OA are due to increases in MMP and ADAMTS production and decreased collagen and proteoglycan levels, which lead to matrix degradation [41]. Excessive secretion of members of the MMP and ADAMTS protein families plays an important role in OA pathogenesis by activating the NF-κB and Wnt3a/β-catenin pathways [42]. XG in chondrocytes exerts a protective effect against cartilage injury markers and simultaneously inhibits the activated Wnt3a/β-catenin pathway in vitro. Our results show that XG treatment significantly suppressed the Wnt3a/β-catenin pathway and decreased the levels of the downstream proteins MMP13 and ADAMTS5 in SNP-induced cartilage destruction in vitro. XG also reduced aggrecan and collagen II mRNA and protein expression levels in SNP-induced OA chondrocytes in vitro.
Aggrecan (ACAN) and collagen II are significant factors in the response to mechanical load [32]. The load-induced modulation of matrix synthesis is largely confined to proteoglycans and the collagen response [43]. Following treatment with XG, MMP and ADAMTS expression levels were inhibited, and aggrecan and collagen II expression levels increased. These findings show that XG is beneficial in promoting matrix content and inhibiting cartilage degradation, in accordance with previous findings in chondrocyte cells in vitro.
In this study, we found that XG is an effective regulatory factor for Wnt3a/β-catenin signaling-mediated OA cartilage breakdown. However, the specific mechanism underlying the protective effect of XG against OA remains to be further investigated. The Wnt3a/β-catenin signaling pathway is certainly not the only signaling pathway through which XG relieves OA symptoms. Whether the NF-κB pathway and AKT pathway are also involved in the OA-ameliorating effects of XG should be examined in a future study. Downstream signaling pathways related to the OA-ameliorating effects of XG remain to be studied. XG may also exert positive effects against bursitis and synovitis through other signaling pathways. However, further evidence is needed. The role of XG in other intracellular pathways remains to be studied. It is unclear whether the lubricity and viscosity of XG play a role in protecting against degradation of the cartilage matrix. In our future research, we will deeply examine the Wnt3a/β-catenin signaling pathway effect on OA by overexpression of Wnt3a and assess the relationship between Wnt3a/β-catenin signaling pathway and downstream signaling in OA.
Our research provides new clues to explain the role of signaling pathway regulation in causing cartilage degradation. XG function and its advantages and disadvantages for protecting against cartilage degradation were analyzed, and the results provide a good foundation for the preparation of high-efficiency cartilage repair materials and relevant experiments.
Conclusions
In this study we demonstrated that XG can protect articular cartilage and reduce articular cartilage degradation and death in a rabbit OA model. XG acted on the Wnt3a/β-catenin signaling activity in ATDC5 cells in a dose-dependent manner and exhibited no cytotoxicity in rat chondrocytes. In addition, XG decreased the expression of downstream catabolic factors to induce anti-catabolic effects in OA chondrocytes. XG also suppressed the expression of the Wnt3a downstream target proteins MMP13 and ADAMTS5 in SNP-stimulated chondrocytes and rescued the inhibition of aggrecan and collagen II expression. Importantly, XG reversed the SNP-induced increase in matrix homeostasis toward catabolism by elevating collagen II and aggrecan transcription.
Acknowledgments
The authors express their thanks to American Journal Experts for English language editing.
Footnotes
Source of support: This study was supported by the Principal Fund of Jinzhou Medical University (grant no. xzjj20140123)
References
- 1.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 2.Ma B, Landman EB, Miclea RL, et al. WNT signaling and cartilage: Of mice and men. Calcif Tissue Int. 2013;92(5):399–411. doi: 10.1007/s00223-012-9675-5. [DOI] [PubMed] [Google Scholar]
- 3.Sandell L, Aigner T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001;3(2):107–13. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19(5):785–96. doi: 10.1016/S0736-0266(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 6.Yasuhara R, Miyamoto Y, Akaike T, et al. Interleukin-1beta induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389(Pt 2):315–23. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SW, Lee HJ, Chung WT, et al. TRAIL induces apoptosis of chondrocytes and influences the pathogenesis of experimentally induced rat osteoarthritis. Arthritis Rheum. 2004;50(2):534–42. doi: 10.1002/art.20052. [DOI] [PubMed] [Google Scholar]
- 8.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol. 2018;30(2):160–67. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: An update with relevance for clinical practice. Lancet. 2011;377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 10.Yuasa T, Otani T, Koike T, et al. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: Its possible role in joint degeneration. Lab Invest. 2008;88(3):264–74. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 11.Huang JF, Du WX, Chen JJ. Elevated expression of matrix metalloproteinase-3 in human osteosarcoma and its association with tumor metastasis. J BUON. 2016;21(5):1279–86. [PubMed] [Google Scholar]
- 12.van den Bosch MH, Blom AB, van de Loo FA, et al. Brief report: Induction of matrix metalloproteinase expression by synovial Wnt signaling and association with disease progression in early symptomatic osteoarthritis. Arthritis Rheumatol. 2017;69(10):1978–83. doi: 10.1002/art.40206. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh V, Hu H, Barroga C, et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2018;26(1):18–27. doi: 10.1016/j.joca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Huang X, Yuan Y. MicroRNA-410 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells through down-regulating Wnt3a. Am J Transl Res. 2017;9(1):136–45. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SS, Zhou P, Zhang Y. Abnormal expression of key genes and proteins in the canonical Wnt/beta-catenin pathway of articular cartilage in a rat model of exercise-induced osteoarthritis. Mol Med Rep. 2016;13(3):1999–2006. doi: 10.3892/mmr.2016.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Bosch MH, Blom AB, van Lent PL, et al. Canonical Wnt signaling skews TGF-beta signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell Signal. 2014;26(5):951–58. doi: 10.1016/j.cellsig.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Bourhis E, Tam C, Franke Y, et al. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285(12):9172–79. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snelling SJ, Davidson RK, Swingler TE, et al. Dickkopf-3 is upregulated in osteoarthritis and has a chondroprotective role. Osteoarthritis Cartilage. 2016;24(5):883–91. doi: 10.1016/j.joca.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao H, Han G, Ling P, et al. Intra-articular injection of xanthan gum reduces pain and cartilage damage in a rat osteoarthritis model. Carbohydr Polym. 2013;92(2):1850–57. doi: 10.1016/j.carbpol.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Han G, Shao H, Zhu X, et al. The protective effect of xanthan gum on interleukin-1beta induced rabbit chondrocytes. Carbohydr Polym. 2012;89(3):870–75. doi: 10.1016/j.carbpol.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Han G, Wang G, Zhu X, et al. Preparation of xanthan gum injection and its protective effect on articular cartilage in the development of osteoarthritis. Carbohydr Polym. 2012;87(2):1837–42. [Google Scholar]
- 22.Arunakul M, Tochigi Y, Goetz JE, et al. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J Orthop Res. 2013;31(10):1555–60. doi: 10.1002/jor.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosenza S, Ruiz M, Toupet K, et al. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Wu G, Chen J, et al. Electroacupuncture inhibits sodium nitroprussidemediated chondrocyte apoptosis through the mitochondrial pathway. Mol Med Rep. 2018;18(6):4922–30. doi: 10.3892/mmr.2018.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto K, Ohkawara B, Ito M, et al. Fluoxetine ameliorates cartilage degradation in osteoarthritis by inhibiting Wnt/beta-catenin signaling. PLoS One. 2017;12(9):e0184388. doi: 10.1371/journal.pone.0184388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadanaka S, Kinouchi H, Taniguchi-Morita K, et al. Down-regulation of chondroitin 4-O-sulfotransferase-1 by Wnt signaling triggers diffusion of Wnt-3a. J Biol Chem. 2011;286(6):4199–208. doi: 10.1074/jbc.M110.155093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Wang Y. ATDC5: An excellent in vitro model cell line for skeletal development. J Cell Biochem. 2013;114(6):1223–29. doi: 10.1002/jcb.24467. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Tao H, Li Y, et al. Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/beta-catenin pathway. Eur J Pharmacol. 2016;789:109–18. doi: 10.1016/j.ejphar.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Mei L, Shen B, Xue J, et al. Adipose tissue-derived stem cells in combination with xanthan gum attenuate osteoarthritis progression in an experimental rat model. Biochem Biophys Res Commun. 2017;494(1–2):285–91. doi: 10.1016/j.bbrc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Takamatsu A, Ohkawara B, Ito M, et al. Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling. PLoS One. 2014;9(3):e92699. doi: 10.1371/journal.pone.0092699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hee H, Chuah Y, Tan B, et al. Vascularization and morphological changes of the endplate after axial compression and distraction of the intervertebral disc. Spine. 2011;36(7):505–11. doi: 10.1097/BRS.0b013e3181d32410. [DOI] [PubMed] [Google Scholar]
- 32.Stellavato A, Tirino V, de Novellis F, et al. Biotechnological chondroitin a novel glycosamminoglycan with remarkable biological function on human primary chondrocytes. J Cell Biochem. 2016;117(9):2158–69. doi: 10.1002/jcb.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohsin A, Zhang K, Hu J, et al. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr Polym. 2018;181:793–800. doi: 10.1016/j.carbpol.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(11):1040–47. doi: 10.1056/NEJMct1215534. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Shao X, Ling P, et al. Low molecular weight xanthan gum suppresses oxidative stress-induced apoptosis in rabbit chondrocytes. Carbohydr Polym. 2017;169:255–63. doi: 10.1016/j.carbpol.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Mei X, Han G, et al. Xanthan gum protects rabbit articular chondrocytes against sodium nitroprusside-induced apoptosis in vitro. Carbohydr Polym. 2015;131:363–69. doi: 10.1016/j.carbpol.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto M, Nakasa T, Hikata T, Asahara H. Molecular network of cartilage homeostasis and osteoarthritis. Med Res Rev. 2008;28(3):464–81. doi: 10.1002/med.20113. [DOI] [PubMed] [Google Scholar]
- 38.Monteagudo S, Lories RJ. Cushioning the cartilage: A canonical Wnt restricting matter. Nat Rev Rheumatol. 2017;13(11):670–81. doi: 10.1038/nrrheum.2017.171. [DOI] [PubMed] [Google Scholar]
- 39.Burr DB, Utreja A. Wnt signaling related to subchondral bone density and cartilage degradation in OA. Arthritis Rheumatol. 2018;70(2):157–61. doi: 10.1002/art.40382. [DOI] [PubMed] [Google Scholar]
- 40.van den Bosch MH, Blom AB, Sloetjes AW, et al. Induction of canonical Wnt signaling by synovial overexpression of selected wnts leads to protease activity and early osteoarthritis-like cartilage damage. Am J Pathol. 2015;185(7):1970–80. doi: 10.1016/j.ajpath.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Hwang S, Ryu J, Kim I, et al. Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J Biol Chem. 2004;279(25):26597–604. doi: 10.1074/jbc.M401401200. [DOI] [PubMed] [Google Scholar]
- 42.Ding Q, Ye C, Chen E, et al. Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting NF-κB and Wnt/β-catenin signaling in-vitro and in-vivo. Int. Immunopharmacol. 2018;61:222–30. doi: 10.1016/j.intimp.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Gan Y, Wang H, et al. Biological responses of the immature annulus fibrosus to dynamic compression in a disc perfusion culture. Cells Tissues Organs. 2016;202(5–6):296–306. doi: 10.1159/000446363. [DOI] [PubMed] [Google Scholar]