Abstract
Background
To investigate the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and systemic immune‐inflammation index (SII) as prognostic biomarkers in intrahepatic cholangiocarcinoma (ICC) with a focus on viral hepatitis and liver status.
Methods
In this retrospective cohort study, patients from the institutional cancer registry with ICC from 2005 to 2016 were stratified by treatment group. Baseline inflammatory markers were dichotomized at the median. Overall survival (OS) was assessed via Kaplan‐Meier curves and Cox proportional hazard models. Multiple patient, liver, and tumor factors were included in the multivariable analysis (MVA).
Results
About 131 patients (median age 65 years, 52% male, 76% Caucasian) had a median OS of 13.0 months. Resection/interventional oncology with/without systemic therapy had improved survival vs systemic therapy alone in Child‐Pugh A patients (P < 0.01). In Child‐Pugh B/C patients, this survival difference became nonsignificant (P = 0.22). Increased NLR and SII were associated with decreased survival (P < 0.01), while dichotomized PLR was not (P = 0.3). On MVA, increased NLR remained an independent prognostic factor (HR 1.6, P < 0.05). In Child‐Pugh class A (n = 94), low‐NLR had higher OS vs high‐NLR (25.4 vs 12.2 months, P < 0.01). In Child‐Pugh class B/C (n = 28), NLR did not have a significant effect on median OS (low‐ vs high‐NLR: 6.7 vs 2.9 months, P = 0.2). Child‐Pugh class acted as an effect modifier on MVA for NLR (P = 0.0124).
Conclusions
The NLR has a stronger impact as a prognostic marker in ICC over the PLR and SII. This survival effect is decreased in advanced liver disease.
Keywords: bile ducts, cholangiocarcinoma, intrahepatic, liver cirrhosis, lymphocytes, neutrophils, platelets
The neutrophil‐to‐lymphocyte ratio (NLR) has the strongest impact as a prognostic marker in intrahepatic cholangiocarcinoma (ICC) versus the platelet‐to‐lymphocyte ratio and systemic immune‐inflammation index, and increased NLR is associated with decreased survival in ICC. The NLR has decreased prognostic utility in patients with advanced liver disease.
1. INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common form of primary liver cancer,1 comprising 5%‐10% of cases,2 with an annual age‐standardized incidence rate in western countries of <1.5 cases per 100 000 persons.3 Major risk factors for ICC include primary sclerosing cholangitis, intrahepatic lithiasis, biliary tree anomalies, liver fluke infection, chronic liver disease, and cirrhosis.4 More recently, it has been confirmed that exposure to viral hepatitis,5 nonalcoholic fatty liver disease, and diabetes6 may also increase the risk of ICC development.
ICC carries a dismal prognosis, with 3‐ and 5‐year survival rates of 30% and 18%.7 Surgical resection is recommended whenever possible8; however, as few as 15% of patients may present with inoperable disease.9 For those who are not surgical candidates, systemic chemotherapy is the standard of treatment.8 Recently, interventional oncology (IO) therapies have been used in ICC for palliation10, 11 and as an adjuvant therapy.12, 13 Additional work has examined the feasibility of IO therapies in unresectable disease.14, 15, 16
Chronic inflammation and the immune response are integral to the development of cancers, including ICC.17, 18 The risk factors associated with ICC create a neoplasia‐prone environment through first liver injury and then inflammation, leading to the generation of free radicals and the stimulation of cytokines, chemokines, and other growth factors.17 Activation of cellular proliferation and increased cellular DNA synthesis may accelerate the rate of cellular mutations and advancement along the cancer pathway.19
The neutrophil‐to‐lymphocyte ratio (NLR) has been studied as a biomarker in multiple solid tumors, such as hepatocellular carcinoma, renal, breast, and metastatic melanoma.20, 21, 22, 23 Several researchers have also investigated the NLR in ICC24, 25, 26, 27; however, little work has been done on the relative importance of the NLR, the platelet‐to‐lymphocyte ratio (PLR), and the systemic immune‐inflammation index (SII). Furthermore, this study seeks to examine the interplay between inflammatory markers and underlying liver disease in ICC, with the hypothesis that chronic inflammation may affect the prognostic value of these biomarkers.
2. METHODS
2.1. Study population
The protocols and methods were conducted in compliance with the Health Insurance Portability and Accountability Act and were approved by the institutional review board. Patients diagnosed with ICC from 2005 to 2016 were retrospectively identified from the institutional cancer registry of a single urban academic center. Treatment allocation had been determined by a multi‐disciplinary tumor board. Exclusion criteria included individuals under the age of 18, incomplete treatment or survival data, and those with a histopathologic diagnosis of hepato‐cholangiocarcinoma.
2.2. Data acquisition
The variables reported by the cancer registry included age, gender, race/ethnicity, insurance status, marital status, American Joint Committee on Cancer (AJCC) staging, and basic treatment status. Furthermore, electronic medical record review was conducted to identify multiple other tumor and liver factors at the time of diagnosis along with the temporal sequence of treatments. Along with liver factors, baseline laboratory values were used to calculate Model for End‐Stage Liver Disease (MELD) and Child‐Pugh scores were missing from the medical record. The Charlson Comorbidity Index 28 (CCI) was calculated to quantify the burden of non‐ICC‐related disease. Viral hepatitis was defined via International Classification of Diseases 9th Edition codes as well as HBsAg and HCV Ab laboratory data and included patients at various stages of treatment, including the untreated.
Treatment allocation was first stratified into systemic therapy (chemotherapy and/or radiation), IO (comprised of transarterial chemoembolization, radioembolization, and thermal ablation), resection, and supportive therapy (palliative care or no treatment). Patients who received IO or resection following or in conjunction with systemic therapy were classified as IO and resection, respectively. “Nonsurgical treatment” consisted of IO and systemic therapies. Patients who received treatment were further reclassified into three groups: those who received resection or IO first with/without subsequent systemic therapy (Group 1); patients who received systemic therapy followed by resection or IO (Group 2); and patients who received systemic therapy alone (Group 3). Overall survival (OS) was defined as time from diagnosis to time of death, regardless of etiology.
Baseline pretreatment absolute neutrophil, lymphocyte, and platelet numbers were determined from complete blood count with differential values drawn within 30 days prior to treatment or 30 days prior to diagnosis (for supportive treatment patients only) and were used to calculate the various inflammatory markers. In instances where the absolute neutrophil count (ANC) or absolute lymphocyte count (ALC) were not reported in the electronic medical record, neutrophils were calculated by multiplying the white blood cell count by the differential percentage of the neutrophils and similarly for the lymphocytes. The NLR ratio was defined as ANC/ALC. The PLR ratio was defined as platelet count/ALC, and the SII was defined as ANC*platelet count/ALC.
2.3. Statistical analysis
Continuous variables were compared using the Wilcoxon rank sum test and categorical variables using the chi‐squared test. Kaplan‐Meier methods and log‐rank tests were used to estimate OS. In instances where survival curves crossed, the Wilcoxon test was employed to account for early survival losses. Inflammatory markers were dichotomized at the median for visualization purposes but were log‐transformed and evaluated as linear factors for all additional survival analyses. Predictors of OS were identified using univariate and multivariable Cox proportional hazard models. Factors that were significant on univariate models were included in the multivariable analysis (MVA) to address confounding. Separate MVA was modeled including all inflammatory markers (not reported). Among the biomarkers, only NLR retained statistical significance and was therefore considered the most relevant variable for further MVA modeling in order to avoid overfitting. A multiplicative interaction term between Child‐Pugh class and log‐transformed NLR (Child_Pugh*log_NLR) was included in multivariable Cox models to assess for heterogenous effects of NLR according to Child‐Pugh class. Patients with missing variables were conservatively excluded from analyses to account for bias.
All P‐values reported are two‐sided, with an alpha level of 0.05 considered statistically significant. Statistical analyses were completed using JMP Pro v.13.0.0 (SAS Institute Inc, Cary, NC), and GraphPad Prism v.7.0a for Mac (GraphPad Software, La Jolla, CA). Additional figure preparation was completed using R v.3.4.3 (R Core Development Team, Vienna, Austria).
3. RESULTS
3.1. Demographics
One hundred and thirty‐one patients (median age 65 years, 51.9% male, 75.6% Caucasian) met the inclusion criteria, with median follow‐up time of 12.0 months (IQR 4.5‐25.2 months) and 109 deaths during the study period (See Figure S1 for study design). Seventy‐seven percent of patients (n = 101) had confirmed histopathologic evidence of ICC. The majority of these biopsies were poorly or moderately‐to‐poorly differentiated (n = 58, 57.4%). Median CCI was 7.0 (IQR 5.0‐9.0), and median MELD was 8.0 (IQR 6.0‐11.0). 17.6% of patients (n = 23) had viral hepatitis, and 26% (n = 34) had cirrhosis. Seventeen patients had hepatitis C (74%), five had hepatitis B (22%), and one had coinfection with hepatitis B and hepatitis C (4%). The annual number of new ICC cases increased over time, from four in 2005 to 18 in 2016. Twelve patients were presented with AJCC Stage I disease (9.2%); 27 were Stage II (20.6%); 11 were Stage III (8.4%); and 56 patients were Stage IV (42.7%). At diagnosis, 72 patients (55.0%) had multifocal ICC, 64 patients (48.9%) had bilobar cholangiocarcinoma, and 60 patients (45.8%) had metastatic disease. Median tumor size was 6.5 cm (IQR 4.1‐8.8). Refer to Table 1 for additional baseline data.
Table 1.
Patient, liver, and tumor factors by neutrophil‐to‐lymphocyte group
| Factor | Entire cohort (n = 131) | NLR ≤ 3.95 (n = 66) |
NLR > 3.95 (n = 65) |
P‐value | |
|---|---|---|---|---|---|
| Age | 65.0 (57.0‐71.0) | 62.0 (56.0‐70.3) | 66.0 (59.0‐72.0) | 0.0670 | |
| Male Gender | 68 (51.9%) | 37 (56.1%) | 31 (47.7%) | 0.3376 | |
| Race/Ethnicity | 0.2509 | ||||
| Caucasian, non‐Hispanic | 99 (75.6%) | 47 (71.2%) | 52 (80.0%) | ||
| Black, Non‐Hispanic | 16 (12.2%) | 8 (12.1%) | 8 (12.3%) | ||
| Hispanic | 10 (7.6%) | 8 (12.1%) | 2 (3.1%) | ||
| Other/Unknown | 6 (4.6%) | 3 (4.6%) | 3 (4.6%) | ||
| Primary Insurance | 0.2290 | ||||
| Private | 75 (60.0%) | 40 (66.7%) | 35 (53.9%) | ||
| Medicare | 36 (28.8%) | 13 (21.7%) | 23 (35.4%) | ||
| Medicaid | 14 (11.2%) | 7 (11.7%) | 7 (10.8%) | ||
| Charlson Comorbidity Index | 7.0 (5.0‐9.0) | 7.0 (5.0‐8.3) | 8.0 (5.5‐10.0) | 0.0250 | |
| Viral Hepatitis | 23 (17.6%) | 17 (25.8%) | 6 (9.2%) | 0.0126 | |
| Cirrhosis | 34 (26.0%) | 19 (28.8%) | 15 (23.1%) | 0.0838 | |
| Child‐Pugh Class | 0.1043 | ||||
| A | 94 (71.8%) | 50 (75.8%) | 44 (67.7%) | ||
| B | 26 (19.8%) | 10 (15.2%) | 16 (24.6%) | ||
| C | 2 (1.5%) | 0 (0.0%) | 2 (3.1%) | ||
| Missing/Unknown | 9 (6.9%) | 6 (9.0%) | 3 (4.6%) | ||
| MELD Score | 8.0 (6.0‐11.0) | 7.0 (6.0‐9.0) | 8.0 (6.0‐13.0) | 0.0643 | |
| AJCC Stage | 0.3482 | ||||
| I | 12 (15.3%) | 9 (13.4%) | 3 (4.6%) | ||
| II | 27 (20.6%) | 15 (22.7%) | 12 (18.5%) | ||
| III | 11 (8.4%) | 5 (7.6%) | 6 (9.2%) | ||
| IV | 56 (42.7%) | 27 (40.9%) | 29 (44.6%) | ||
| Missing/Unknown | 25 (19.1%) | 10 (15.2%) | 15 (23.1%) | ||
| Tumor Size (cm) | 6.5 (4.1‐8.8) | 5.8 (4.0‐7.2) | 7.5 (5.4‐12.0) | 0.0007 | |
| Tumor Location | 0.0104 | ||||
| Unilobar | 65 (50.4%) | 40 (60.6%) | 25 (38.5%) | ||
| Bilobar | 64 (49.6%) | 25 (37.9%) | 39 (60.0%) | ||
| Multifocal Disease | 72 (55.0%) | 36 (54.6%) | 36 (55.4%) | 0.8173 | |
| Vascular Invasion | 33 (25.2%) | ||||
| Metastatic Disease | 60 (45.8%) | 37 (40.9%) | 33 (50.8%) | 0.3913 | |
| ANC (*103/µL) | 5.3 (3.5‐7.7) | 4.0 (3.0‐5.3) | 7.1 (5.3‐10.2) | <0.0001 | |
| Patient characteristics by NLR | |||||
| ALC (*103/µL) | 1.4 (1.0‐1.8) | 1.7 (1.3‐2.2) | 1.2 (0.8‐1.4) | <0.0001 | |
| Platelets (*103/µL) | 229 (161‐298) | 210.5 (149.8‐280.5) | 253.0 (162.0‐324.0) | 0.0885 | |
| NLR | 3.95 (2.36‐6.19) | 2.37 (1.81‐3.09) | 6.19 (4.77‐12.68) | <0.0001 | |
| PLR | 156 (111‐226) | 118 (86‐152) | 214 (162‐290) | <0.0001 | |
| SII | 867 (455‐1422) | 462 (326‐746) | 1406 (970‐2243) | <0.0001 | |
| Treatment | 0.0381 | ||||
| Resection | 28 (21.4%) | 20 (30.3%) | 8 (12.3%) | ||
| IO | 16 (12.2%) | 8 (12.1%) | 8 (12.3%) | ||
| Systemic Therapy | 65 (49.6%) | 31 (47.0%) | 34 (52.3%) | ||
| Supportive Therapy | 22 (16.8%) | 7 (10.6%) | 15 (23.1%) | ||
Data presented as median (IQR) or N (%).
Abbreviations: AJCC, American Joint Committee on Cancer; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; IO, interventional oncology; MELD, Model for End‐stage Liver Disease; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
Bold indicates P‐values falling below the level of significance (P < 0.05).
3.2. Treatment allocation
Sixteen patients received IO (12.2%), 65 patients received systemic therapy (49.6%), 28 patients underwent resection (21.4%), and 22 patients received supportive therapy (16.8%). Thirty‐five patients received resection or IO with/without subsequent systemic therapy (Group 1, 26.7%); nine patients received systemic therapy followed by IO or resection (Group 2, 6.9%); and 70 patients received systemic therapy alone (Group 3, 49.6%).
Patients who received surgical treatment were more likely to be younger, Child‐Pugh class A, AJCC Stage I, have solitary tumors, unilobar disease, and to not have metastases (P < 0.01). Supportive therapy patients had higher MELD and CCI scores than nonsurgical or surgical treatment (P < 0.01). Gender, race, insurance, viral hepatitis status, and the presence or absence of cirrhosis did not affect the likelihood of receiving treatment (P > 0.05). Surgical treatment was associated with lower median tumor size (4.2 cm) vs nonsurgical treatment (7.0 cm) or supportive care (7.5 cm, P = 0.0006).
3.3. Overall survival and recurrence
Median OS for the entire cohort was 13.0 months (95% CI: 9.2‐16.8 months) and was highest in resection (median OS 43.8 months), followed by IO (33.1 months), systemic therapy (11.0 months), and supportive therapy (1.3 months, overall P < 0.0001). Improved survival was also seen on MVA for IO as compared to systemic therapy (HR 8.32; 95% CI: 2.70‐29.81, P = 0.0001).
OS was highest in Group 1 (39.3 months), followed by Group 2 (33.1 months) and Group 3 (11.0 months, P < 0.01) (Figure 1A). In patients with Child‐Pugh A disease, Group 2 demonstrated the highest survival (Group 1 vs 2 vs 3: median OS 36.4 vs 54.6 vs 13.3 months, P < 0.0001) (Figure 1B). In patients with Child‐Pugh class B disease, there was a nonsignificant trend toward improved survival for patients in Group 1 vs Group 3 (P = 0.22) (Figure 1C).
Figure 1.
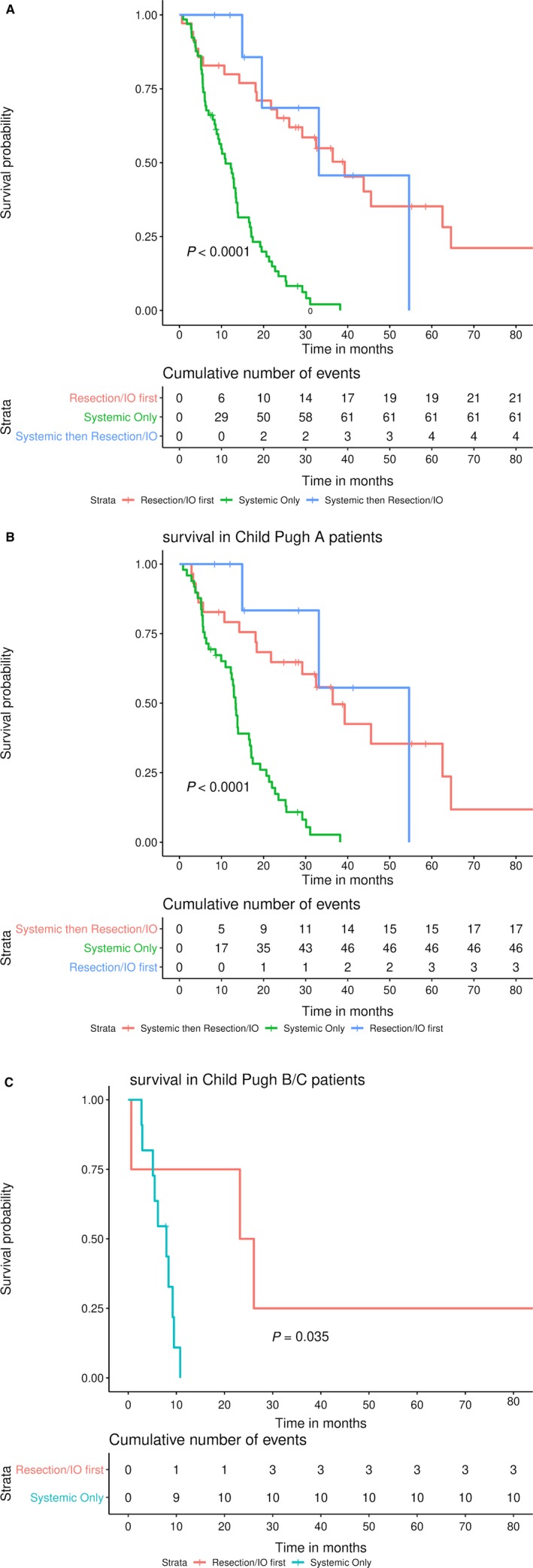
Overall survival by treatment group. A, Entire cohort; B, Child‐Pugh A patients; C, Child‐Pugh B patients
3.4. Prognostic factors
Factors significant on univariate analysis (P < 0.05) included age, insurance, CCI, Child‐Pugh stage, MELD score, tumor size, tumor location, multifocal disease, extrahepatic metastases, AJCC staging, treatment allocation, ANC, ALC, and log‐transformed NLR, SII, and PLR (Table 2). Nonsignificant factors included gender, race, viral hepatitis status, vascular invasion, the presence or absence of cirrhosis, and platelet counts. Significant factors (P < 0.05) on MVA included treatment allocation, insurance type, and log‐transformed NLR (Table 3).
Table 2.
Significant and nonsignificant prognostic markers on univariate analysis
| Factor | HR | Lower 95% | Upper 95% | P‐value |
|---|---|---|---|---|
| Age | 1.02 | 1.001 | 1.038 | 0.0391 |
| Male gender | 1.20 | 0.820 | 1.764 | 0.3498 |
| Race | ||||
| Caucasian (reference) | ||||
| African American | 0.88 | 0.441 | 1.600 | 0.6972 |
| Hispanic | 1.08 | 0.525 | 1.991 | 0.8166 |
| Other/Unknown | 2.19 | 0.845 | 4.646 | 0.0995 |
| Insurance | ||||
| Private (reference) | ||||
| Medicare | 1.90 | 1.207 | 2.930 | 0.0062 |
| Medicaid | 1.54 | 0.809 | 2.726 | 0.1773 |
| Charlson Comorbidity Index | 1.26 | 1.172 | 1.355 | <0.0001 |
| Cirrhosis | 1.54 | 0.978 | 2.361 | 0.0618 |
| Viral hepatitis | 0.93 | 0.517 | 1.601 | 0.7935 |
| Child Pugh class | ||||
| A (reference) | ||||
| B/C | 2.84 | 1.758 | 4.437 | <0.0001 |
| MELD | 1.08 | 1.046 | 1.102 | <0.0001 |
| AJCC stage | ||||
| I (reference) | ||||
| II | 2.14 | 0.934 | 5.543 | 0.0727 |
| III | 2.58 | 0.900 | 7.598 | 0.0770 |
| IV | 6.39 | 2.88 | 16.49 | <0.0001 |
| Tumor size | 1.11 | 1.045 | 1.177 | 0.0005 |
| Tumor location | ||||
| Unilobar (reference) | ||||
| Bilobar | 2.30 | 1.542 | 3.437 | <0.0001 |
| Multifocal Tumor | 2.31 | 1.547 | 3.486 | <0.0001 |
| Vascular invasion | 1.74 | 0.906 | 3.485 | 0.0968 |
| Extrahepatic metastases | 2.97 | 1.970 | 4.514 | <0.0001 |
| Treatment | ||||
| Resection (reference) | ||||
| IO | 1.76 | 0.703 | 4.113 | 0.2156 |
| Systemic | 5.62 | 3.070 | 11.063 | <0.0001 |
| Supportive | 21.71 | 10.674 | 45.836 | <0.0001 |
| ANC (continuous) | 1.14 | 1.067 | 1.2016 | 0.0001 |
| ALC (continuous) | 0.58 | 0.403 | 0.811 | 0.0013 |
| Platelets (continuous) | 1.00 | 0.998 | 1.002 | 0.8154 |
| log(NLR) | 2.11 | 1.555 | 2.847 | <0.0001 |
| log(PLR) | 1.43 | 1.007 | 2.036 | 0.0454 |
| log(SII) | 1.38 | 1.106 | 1.732 | 0.0043 |
Abbreviations: AJCC, American Joint Committee on Cancer; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; IO, interventional oncology; MELD, Model for End‐stage Liver Disease; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
Table 3.
Significant and nonsignificant prognostic markers on multivariate analysis
| Factor | HR | Lower 95% | Upper 95% | P‐value |
|---|---|---|---|---|
| Age | 0.98 | 0.942 | 1.026 | 0.4281 |
| Insurance | ||||
| Private (reference) | ||||
| Medicare | 2.46 | 1.251 | 4.851 | 0.0093 |
| Medicaid | 1.31 | 0.535 | 3.001 | 0.5366 |
| Charlson Comorbidity Index | 1.13 | 0.855 | 1.466 | 0.3694 |
| Child‐Pugh class | ||||
| A (reference) | ||||
| B/C | 0.62 | 0.190 | 2.031 | 0.4246 |
| MELD | 1.03 | 0.971 | 1.092 | 0.2851 |
| AJCC stage | ||||
| I (reference) | ||||
| II | 1.40 | 0.442 | 5.102 | 0.5749 |
| III | 1.46 | 0.322 | 6.786 | 0.6234 |
| IV | 2.16 | 0.535 | 9.457 | 0.2819 |
| Tumor size | 1.06 | 0.956 | 1.170 | 0.2562 |
| Tumor location | ||||
| Unilobar (reference) | ||||
| Bilobar | 1.54 | 0.776 | 3.036 | 0.2154 |
| Multifocal Tumor | 1.34 | 0.734 | 2.525 | 0.3430 |
| Extrahepatic metastases | 0.68 | 0.166 | 2.914 | 0.5946 |
| Treatment Allocation | ||||
| Resection (reference) | ||||
| IO | 0.95 | 0.263 | 3.273 | 0.9406 |
| Systemic | 4.40 | 1.550 | 13.360 | 0.0051 |
| Supportive | 54.5 | 10.952 | 286.738 | <0.0001 |
| log(NLR) | 1.58 | 1.010 | 2.512 | 0.0469 |
| Interaction term: Log(NLR)*Child‐Pugh score | 0.0124 | |||
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; IO, interventional oncology; MELD, Model for End‐stage Liver Disease; NLR, neutrophil‐to‐lymphocyte ratio; SII, systemic immune‐inflammatory index.
Bold indicates P‐values falling below the level of significance (P < 0.05).
3.5. Inflammatory marker selection
Median ANC for the cohort was 5.3 × 103/L (IQR 3.5‐7.7), with median ALC of 1.4 × 103/L (IQR 1.0‐1.8), and median platelets of 229 × 103/L (IQR 161‐298). When dichotomized at the median, increased neutrophils were associated with poorer survival (median OS 9.2 months, 95% CI: 5.3‐13.4 months) vs decreased neutrophils (median OS 16.8 months, 95% CI: 12.4‐22.7 months, P = 0.0126). Conversely, patients with an ALC above the median had a higher median OS of 16.5 months (95% CI: 9.9‐23.5 months) vs patients with lymphocytes below or equal to the median (median OS 10.1 months, 95% CI: 5.2‐13.8 months, P = 0.0222). Increased vs decreased platelets did not appear to affect survival (median OS 14.2 months, 95% CI: 9.5‐19.5 months; vs 12.2 months, 95% CI: 6.4‐13.9 months, P = 0.5922). Similarly, on univariate proportional hazards analyses, increased neutrophils and decreased lymphocytes were associated with decreased survival (P < 0.05), while there was no association between platelet count and survival (P > 0.05).
Median NLR for the cohort was 3.95 (IQR 2.36‐6.19), with median PLR of 156.43 (IQR 110.6‐225.6) and median SII of 867.4 (455.0‐1421.5). Correlation between inflammatory markers ranged from good (Spearman's ρ = 0.65) to very strong (ρ = 0.84). Decreased NLR and SII were associated with increased survival (P < 0.01) (Figure 2A,B), while dichotomized PLR was not (P = 0.3) (Figure 2C). ANC, ALC, log‐transformed PLR, and log‐transformed SII were not independent prognostic factors on MVA (P > 0.05). However, an increase in log‐transformed NLR continued to be associated with decreased survival (HR 1.96, P = 0.0001) on MVA. Given that the NLR was the only inflammatory marker to remain significant on MVA, the NLR was used for further subgroup analyses.
Figure 2.
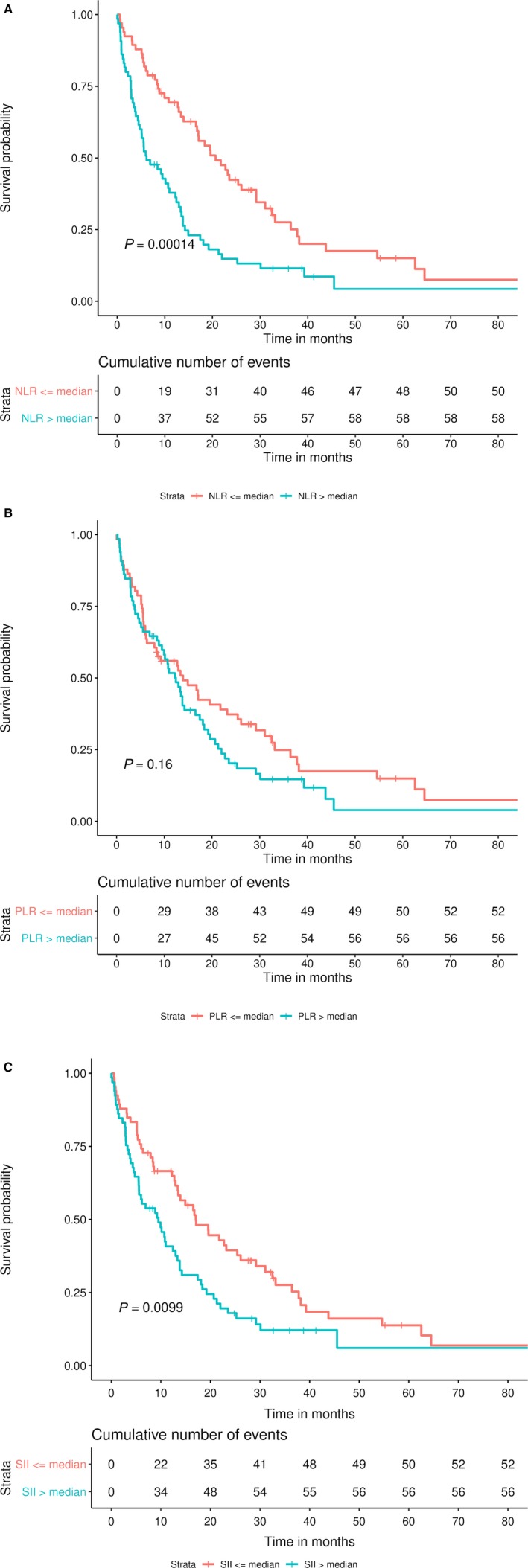
Overall survival by inflammatory marker groups. A, Neutrophil‐to‐lymphocyte ratio. B, Platelet‐to‐lymphocyte ratio. C, Systemic immune‐inflammation index
3.6. Neutrophil‐to‐lymphocyte ratio
Patients with NLR greater than the median had larger median tumor size (7.5 vs 5.8 cm, P = 0.0007) and were more likely to have viral hepatitis, bilobar disease, or to receive systemic or supportive therapy (P < 0.05). Patients across NLR groups were similar in terms of age, gender, race/ethnicity, insurance status, cirrhosis status, Child‐Pugh score, MELD score, and AJCC stage. The low‐NLR group had improved median OS (NLR ≤ 3.96, 20.7 months) vs the high‐NLR group (NLR > 3.96, OS 6.2 months). When stratified by treatment allocation, the NLR remained a significant factor in patients treated with systemic therapy (NLR ≤ 3.96 vs NLR > 3.96; median OS 13.4 vs 9.2 months; log‐rank test P = 0.0130). Higher survival was also seen in resection (n = 28; low‐ vs high‐NLR; median OS 36.4 vs 42.4 months; P = 0.83) and IO (n = 16; low‐ vs high‐NLR; median OS 33.1 vs 10.7 months; P = 0.0674), although this did not reach statistical significance.
3.7. Liver status and NLR
Patients with Child‐Pugh A disease had higher median ALC (P < 0.05), whereas patients with Child‐Pugh B/C disease had higher comorbidity indices (median 10.0 vs 6.5, P < 0.0001), MELD scores (13.0 vs 7.0, P < 0.0001), and higher rates of cirrhosis (P < 0.05). Patients with Child‐Pugh A disease also had higher rates of resection (22.4% vs 10.7%), IO (16.0% vs 3.6%), and systemic therapy (52.1% vs 39.3%) and lower rates of palliative therapy (8.5% vs 46.4%, overall P = 0.0001) as compared to Child‐Pugh B/C patients. Across both Child‐Pugh groupings, patients had similar distribution of age, gender, race/ethnicity, insurance, viral hepatitis, AJCC stage, tumor size, bilobar tumor burden, vascular invasion, metastatic disease, ANC, platelets, NLR, PLR, and SII and (P > 0.05). In Child‐Pugh class A (n = 94), low‐NLR had higher OS vs high‐NLR (25.4 vs 12.2 months, P < 0.01) (Figure 3A). In Child‐Pugh class B/C (n = 28), NLR did not have a significant effect on survival (low‐ vs high‐NLR: 6.7 vs 2.9 months, P = 0.2) (Figure 3B).The interaction term Child‐Pugh class*log(NLR) was also significant on MVA (P = 0.0124), demonstrating Child‐Pugh class to act as an effect modifier for NLR.
Figure 3.
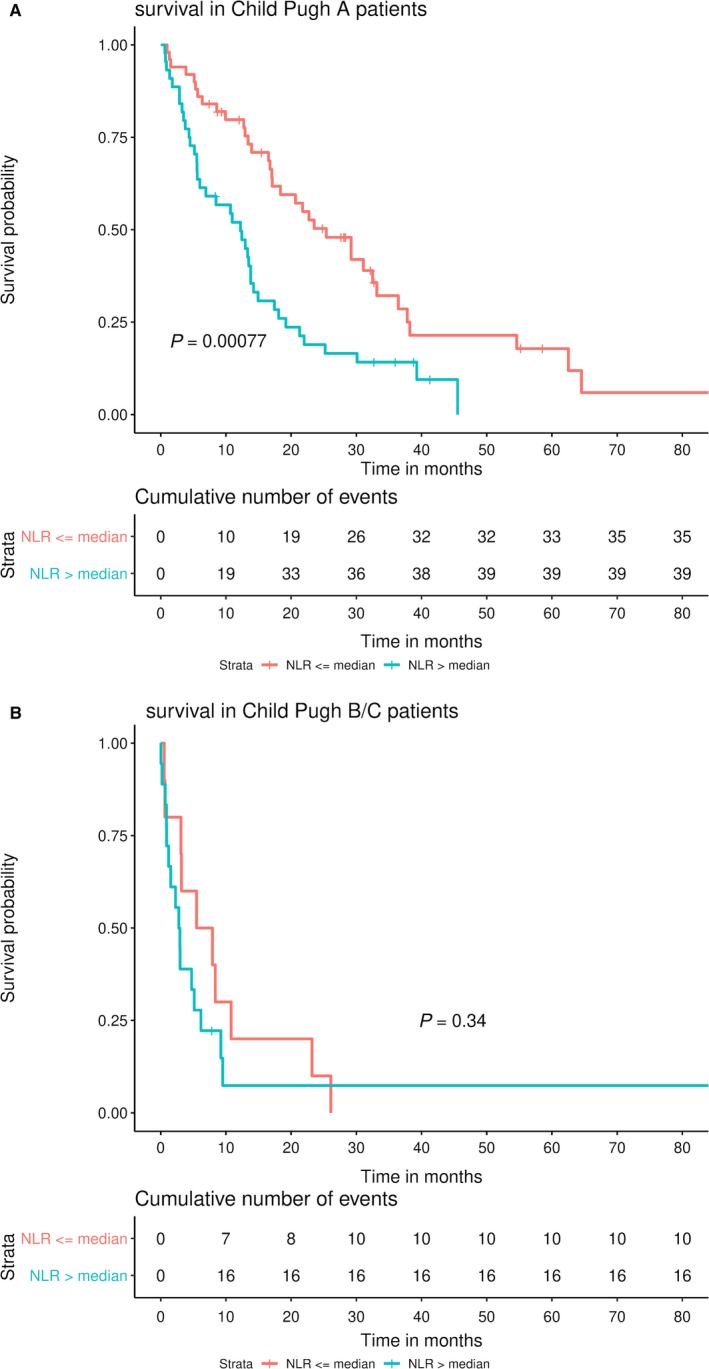
Overall survival by neutrophil‐to‐lymphocyte ratio and Child‐Pugh status. A, Child‐Pugh A patients. B, Child‐Pugh B patients
4. DISCUSSION
Intrahepatic cholangiocarcinoma is the second most common form of primary liver cancer1 and carries a very poor prognosis. ICC risk factors, including primary sclerosing cholangitis and intrahepatic lithiasis, predispose toward cancer development through injury, immune system activation, and cellular proliferation. This study sought to clarify the significance of three circulatory inflammatory markers and to examine the effect of chronic liver disease on their prognostic value.
During the study period, annual cases of newly diagnosed ICC increased from four in 2005 to 18 in 2016, a trend which is reflected on both a national29, 30 and a global scale.4 It is unclear if this is due to a true increase in incidence or to a possible change in referral patterns or increased rates of early detection. MVA demonstrated that patients treated with IO had improved survival vs those treated with systemic therapy alone. Furthermore, patients treated with resection or IO therapies with and without systemic therapy had greater survival than patients treated with systemic therapy alone. While this survival difference was also seen on subgroup analyses in patients with Child‐Pugh A disease, it became a nonsignificant trend among patients with Child‐Pugh B disease. This may in part be due to the smaller numbers of Child‐Pugh B patients included in this study (n = 26).
We observed strong correlation among the NLR, PLR, and SII; however, only the NLR remained an independent predictor of survival on MVA, where increased NLR was associated with decreased survival. This survival difference was also seen on subgroup analyses in patients who received systemic therapy, and there was a trend toward improved survival with decreased NLR in the surgery and IO treatment groups. Although this trend was nonsignificant, this may have been due to the smaller numbers in the surgery and IO subgroups.
Child‐Pugh status was demonstrated to be an effect modifier of the NLR. For patients with none‐to‐mild liver disease (Child‐Pugh A), the association between increased NLR and poorer survival remained significant. For those with moderate liver disease (Child‐Pugh B), however, there was no significant survival difference across NLR groups. It should be noted that the Child‐Pugh A and Child‐Pugh B groups in our cohort had similar distribution of inflammatory markers. Perhaps, in cases of advanced chronic inflammation, the immune system becomes fatigued, and the rates of increased neutrophils may be fewer. Furthermore, in light of the low numbers of Child‐Pugh class B patients present in this cohort, there may be smaller effects and nuances that are being missed due to low power.
4.1. Interventional oncology
It is thought that both transarterial chemoembolization (TACE)10 and Y‐9011 are safe and effective as palliative therapies for unresectable ICC. TACE has been used successfully as adjuvant therapy alongside chemotherapy12 and surgical resection.13 In a study comparing resection with TACE, Scheuermann et al31 found that there was no survival benefit for resection vs TACE in patients with positive lymph nodes or positive resection margins after surgery. Radiofrequency ablation has also been shown to prolong survival in inoperable ICC, particularly in tumors <5 cm.15, 32 In our study, we found that IO was associated with a survival benefit compared to systemic therapy. Collectively, this evidence suggests that IO therapies may be an area of future study for treatment of ICC, particularly in patients with comorbidities that prevent them from being surgical candidates or who are unable to tolerate the side effects of systemic chemotherapy.
4.2. Inflammation and ICC
Intrahepatic cholangiocarcinoma is a very heterogenous tumor, with multiple morphologies on gross,33 cellular,34 and molecular35 levels. Risk factors for ICC activate the inflammatory response, leading to increased cholangiocyte proliferation and the production of free radicals and multiple other cytokines and chemicals which continue the cycle of injury and growth,17, 19 creating an ideal environment for carcinogenesis.18, 36
As available knowledge about circulating inflammatory biomarkers grows, it is important to determine which markers carry the most prognostic value. Of the markers studied here, the NLR was most useful. Similarly, Ha and colleagues37 studied the NLR, PLR, and SII as well as soluble programmed cell death ligand‐1 in 158 patients with advanced biliary tract cancers, and concluded that only NLR and soluble PD‐L1 were independent prognostic factors.
Increased NLR ratios have been associated with poor prognosis following surgery24, 27, 38 and chemotherapy.25, 27 The NLR has been a component of potential prognostic systems39 and nomograms for the prediction of resection futility.40 A recent meta‐analysis comprising 26 studies and 4461 patients concluded that while increased NLR indicated a poor prognosis in primary liver cancers, subgroup analyses suggested that the predictive role of NLR in cholangiocarcinomas might be limited.41 Of note, only 29 of these 4461 patients had confirmed ICC.
The exact mechanism of how the NLR ratio affects survival is unclear. Lin et al26 examined 102 patients with ICC and saw that higher PD‐1+CD4+ and PD‐1+CD8+ T cells were found in the high‐NLR group while higher amounts of IFN+CD4+ and IFN+CD8+ T cells were seen in the low‐NLR group. Furthermore, the high‐NLR group experienced an increased density of tumor‐infiltrating CD3+T cells. However, in spite of these findings, the strengths of the NLR over PLR and SII37 suggest that it may be the neutrophil component and not the lymphocytic component which drives the effect of the NLR.
Given the vast heterogeneity of ICCs, further work is required to better understand the differences between patients with an increased NLR and those with a lower NLR on a cellular and molecular level. The decreased effectiveness of the NLR in advanced liver disease seen in this cohort suggests that a state of chronic inflammation not directly subject to the cancer may also affect how the body responds to the tumor. Perhaps in cases of significant or advanced chronic inflammation, the immune system becomes fatigued, and the rates of increased neutrophils may be fewer.
4.3. Limitations
Although our sample size is a limiting factor in subgroup analyses, our cohort was diverse and well‐characterized among gender, race, and treatment modalities. We limited the NLR, PLR, and SII values to within 30 days prior to ICC treatment or diagnosis (for supportive therapy patients) in order to improve standardization, as our data relied on archived records. Furthermore, this study lacks information whether patients with cirrhosis and viral hepatitis also suffered from portal hypertension or hypersplenism. However, only platelet counts should be directly affected by these sequelae of liver disease. As this study has been conducted among a United States (Western) population, results may not be globally generalizable.
5. CONCLUSION
The NLR ratio has the strongest impact as a prognostic marker in ICC versus the PLR and SII, and increased NLR is associated with decreased survival in ICC. This increased survival is modulated by liver status, suggesting that the interplay between acute tumor‐associated inflammation and chronic liver parenchymal dysfunction may further affect survival.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report. The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript. Hyun S. Kim served on Advisory boards for Boston Scientific and SIRTex.
AUTHOR CONTRIBUTIONS
Cortlandt M. Sellers: Conceptualization, data curation, formal analysis, writing—original draft; writing—reviewing and editing, final approval. Johannes Uhlig: Formal analysis and writing—reviewing and editing, final approval. Johannes M. Ludwig: Formal analysis and writing—reviewing and editing, final approval. Stacey M. Stein: Formal analysis and writing—reviewing and editing, final approval. Hyun S. Kim: Conceptualization, formal analysis, and writing—reviewing and editing, Resources, Funding, Supervision, final approval.
Supporting information
ACKNOWLEDGEMENTS
The Yale Cancer Center registry
Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: Effects of advanced liver disease. Cancer Med. 2019;8:5916–5929. 10.1002/cam4.2373
Funding information
Funding for this work was provided by the Office of Student Research, Yale School of Medicine, New Haven, CT. HSK is supported by the United States Department of Defense (CA160741). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 Suppl):171‐190. [DOI] [PubMed] [Google Scholar]
- 2. Shaib Y, El‐Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115‐125. [DOI] [PubMed] [Google Scholar]
- 3. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. Vol. VIII: IARC Scientific Publication No. 155I. Lyon: ARC Press; 2002. [Google Scholar]
- 4. Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379(2):198‐205. [DOI] [PubMed] [Google Scholar]
- 5. Zhou HB, Hu JY, Hu HP. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J Gastroentero. 2014;20(19):5721‐5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thinkhamrop K, Khuntikeo N, Phonjitt P, et al. Association between diabetes mellitus and fatty liver based on ultrasonography screening in the world's highest cholangiocarcinoma incidence region, Northeast Thailand. Asian Pac J Cancer Prev. 2015;16(9):3931‐3936. [DOI] [PubMed] [Google Scholar]
- 7. Bartella I, Dufour JF. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis. 2015;24(4):481‐489. [DOI] [PubMed] [Google Scholar]
- 8. Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic Cholangiocarcinoma: expert consensus statement. HPB. 2015;17(8):669‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buettner S, van Vugt J, Ijzermans J, Koerkamp BG . Intrahepatic cholangiocarcinoma: current perspectives. Oncotargets Ther. 2017;10:1131‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66(4):322‐328. [DOI] [PubMed] [Google Scholar]
- 11. Swinburne NC, Biederman DM, Besa C, et al. Radioembolization for unresectable intrahepatic cholangiocarcinoma: review of safety, response evaluation criteria in solid tumors 1.1 imaging response and. survival. Cancer Biother Radiopharm. 2017;32(5):161‐168. [DOI] [PubMed] [Google Scholar]
- 12. Poggi G, Amatu A, Montagna B, et al. OEM‐TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2009;32(6):1187‐1192. [DOI] [PubMed] [Google Scholar]
- 13. Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y, Yang GS. Postoperative adjuvant transcatheter arterial chemoembolisation improves survival of intrahepatic cholangiocarcinoma patients with poor prognostic factors: results of a large monocentric series. Ejso‐Eur J Surg Onc. 2012;38(7):602‐610. [DOI] [PubMed] [Google Scholar]
- 14. Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213‐220. [DOI] [PubMed] [Google Scholar]
- 15. Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196(2):W205‐209. [DOI] [PubMed] [Google Scholar]
- 16. Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead‐transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24(4):437‐443. [DOI] [PubMed] [Google Scholar]
- 17. Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF‐kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5(5):325‐344. [DOI] [PubMed] [Google Scholar]
- 18. Massarweh NN, El‐Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim HJ, Kim JS, Joo MK, et al. Hepatolithiasis and intrahepatic cholangiocarcinoma: a review. World J Gastroentero. 2015;21(48):13418‐13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baum YS, Patil D, Huang JH, et al. Elevated preoperative neutrophil‐to‐lymphocyte ratio may be associated with decreased overall survival in patients with metastatic clear cell renal cell carcinoma undergoing cytoreductive nephrectomy. Asian J Urol. 2016;3(1):20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du Z, Dong J, Bi J, et al. Predictive value of the preoperative neutrophil‐to‐lymphocyte ratio for the development of hepatocellular carcinoma in HBV‐associated cirrhotic patients after splenectomy. PLoS ONE. 2018;13(4):e0195336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyagawa Y, Araki K, Bun A, et al. Significant association between low baseline neutrophil‐to‐lymphocyte ratio and improved progression‐free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab‐paclitaxel. Clin Breast Cancer. 2018;18(5):400‐409. [DOI] [PubMed] [Google Scholar]
- 23. Pan M, Alavi M, Herrinton LJ. Association of inflammatory markers with disease progression in patients with metastatic melanoma treated with immune checkpoint inhibitors. Perm J. 2018;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez D, Morris‐Stiff G, Toogood GJ, Lodge J, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97(6):513‐518. [DOI] [PubMed] [Google Scholar]
- 25. Lee BS, Lee SH, Son JH, et al. Neutrophil‐lymphocyte ratio predicts survival in patients with advanced cholangiocarcinoma on chemotherapy. Cancer Immunol Immun. 2016;65(2):141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin G, Liu Y, Li S, et al. Elevated neutrophil‐to‐lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(32):50963‐50971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omichi K, Cloyd JM, Yamashita S, et al. Neutrophil‐to‐lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162(4):752‐765. [DOI] [PubMed] [Google Scholar]
- 28. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–283. [DOI] [PubMed] [Google Scholar]
- 29. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353‐1357. [DOI] [PubMed] [Google Scholar]
- 30. Shaib YH, Davila JA, McGlynn K, El‐Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472‐477. [DOI] [PubMed] [Google Scholar]
- 31. Scheuermann U, Kaths JM, Heise M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma ‐ A single‐center experience. Ejso‐Eur J Surg Onc. 2013;39(6):593‐600. [DOI] [PubMed] [Google Scholar]
- 32. Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta‐analysis. J Vasc Interv Radiol. 2015;26(7):943‐948. [DOI] [PubMed] [Google Scholar]
- 33. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288‐291. [DOI] [PubMed] [Google Scholar]
- 34. Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Modern Pathol. 2014;27(8):1163‐1173. [DOI] [PubMed] [Google Scholar]
- 35. Marcano‐Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5(5):61. [DOI] [PubMed] [Google Scholar]
- 36. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ha H, Nam A‐R, Bang J‐H, et al. Soluble programmed death‐ligand 1 (sPDL1) and neutrophil‐to‐lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604‐76612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Q, Yang L‐X, Li X‐D, et al. The elevated preoperative neutrophil‐to‐lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumor Biol. 2015;36(7):5283‐5289. [DOI] [PubMed] [Google Scholar]
- 39. Yoh T, Seo S, Hatano E, et al. A novel biomarker‐based preoperative prognostic grading system for predicting survival after surgery for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24(5):1351‐1357. [DOI] [PubMed] [Google Scholar]
- 40. Nam K, Hwang DW, Shim JH, et al. Preoperative nomogram for prediction of futile resection in patients undergoing exploration for potentially resectable intrahepatic cholangiocarcinoma. Sci Rep. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xue T‐C, Zhang L, Xie X‐Y, et al. Prognostic significance of the neutrophil‐to‐lymphocyte ratio in primary liver cancer: a meta‐analysis. PLoS ONE. 2014;9(5):e96072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


