Abstract
In the past decade, considerable progress has been made in the understanding of the biology of rodent oligodendrocyte precursor cells and their role in the generation of oligodendrocytes in the developing and adult rodent CNS. Much less is known about human oligodendrocyte lineage cells and about the reasons for the failure of the regeneration of the oligodendrocyte population during chronic stages of multiple sclerosis (MS). In particular, the fate of the oligodendrocyte precursor population in MS has remained elusive. The present study examined the possibility that oligodendrocyte regeneration ultimately fails because of the local destruction of both oligodendrocytes and their precursor cells. Analysis of chronic stage MS tissue suggested that this is not the case, because all chronic MS lesions studied contained significant numbers of oligodendrocyte precursor cells, identified as process-bearing cells that bound the O4 antibody but not antibodies to GalC and GFAP. The oligodendrocyte precursor cells appeared, however, to be relatively quiescent, because none expressed the nuclear proliferation antigen recognized by the Ki-67 antibody, and because most lesions lacked myelinating oligodendrocytes in their centers. Thus, it appears that the regeneration of the oligodendrocyte population fails during chronic stages of MS because of the inability of oligodendrocyte precursor cells to proliferate and differentiate rather than because of the local destruction of all oligodendrocyte lineage cells. The identification of ways of stimulating the endogenous oligodendrocyte precursor population to expand and generate remyelinating cells may represent an alternative to transplantation of oligodendrocyte lineage cells to promote myelin repair in MS.
Keywords: demyelination, Ki-67, lesion, myelin, multiple sclerosis, O4 antibody, oligodendrocyte, precursor cell, regeneration, remyelination, tissue repair
The inability of the adult human CNS to compensate adequately for neuronal and/or glial cell death is the underlying cause of many neurological diseases. In the most common human demyelinating disease, multiple sclerosis (MS), for example, the population of myelin-forming cells, i.e., oligodendrocytes, fails to regenerate successfully after damage, resulting in the formation of lesions that remain chronically devoid of myelin and oligodendrocytes (Prineas and McDonald, 1997) and neurological dysfunction. Some myelin repair may occur, however, during early stages of MS, as suggested by the presence of immature oligodendrocytes and thinly myelinated axons at the edges of early lesions (Prineas and Connel, 1979; Raine et al., 1981; Prineas et al., 1989; Brück et al., 1994; Ozawa et al., 1994). Insights into the reasons for the ultimate failure of myelin repair in MS are of paramount importance for the development of strategies to promote CNS remyelination.
Recovery from demyelinating damage in experimental animals is generally successful (Ludwin, 1981) and is associated with proliferation and subsequent differentiation of immature oligodendrocyte lineage cells (Ludwin, 1979; Aranella and Herndon, 1984; Godfraind et al., 1989;Carrol et al., 1990; Rodriguez et al., 1991; Gensert and Goldman, 1997). It is likely that these immature cells are oligodendrocyte precursor cells, because such cells are known to be present in the adult CNS (ffrench-Constant and Raff, 1986; Wolswijk and Noble, 1989;Armstrong et al., 1990; Levine et al., 1993; Engel and Wolswijk, 1996), and because both populations resemble each other ultrastructurally (Wolswijk et al., 1991a).
Tissue culture studies have shown that adult CNS-derived oligodendrocyte precursor cells generally divide, migrate, and differentiate at very slow rates (Wolswijk and Noble, 1989; Wolswijk et al., 1991b; Wren et al., 1992; Engel and Wolswijk, 1996), a behavior that is consistent with indications that only small numbers of oligodendrocytes are generated in the healthy adult CNS (McCarthy and Leblond, 1988). However, exposure of adult oligodendrocyte precursor cells to both basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) causes a marked increase in their rates of division and migration (Wolswijk and Noble, 1992; Engel and Wolswijk, 1996). Because both factors are expressed at increased levels after injury to the adult CNS (Logan et al., 1992; Lotan and Schwartz, 1992; Gehrmann et al., 1996), this particular behavior thus may represent a mechanism whereby adult oligodendrocyte precursor cells generate large numbers of remyelinating oligodendrocytes after experimentally induced myelin damage (Wolswijk and Noble, 1995;Wolswijk, 1997).
Oligodendrocyte precursor cells are also present in the adult human CNS (Armstrong et al., 1992; Gogate et al., 1994; Scolding et al., 1995;Wolswijk, 1997), but little is known about their fate in MS, and this is mainly because of the lack of a suitable marker to identify these cells in situ. Using the O4 antibody and appropriately fixed MS material, the present study provides evidence that oligodendrocyte precursor cells survive the demyelinating damage in MS, but that they apparently fail to proliferate and to differentiate during chronic stages of the disease.
MATERIALS AND METHODS
Preparation of sections of postmortem control and MS tissue. The MS and control tissue were obtained from the Netherlands Brain Bank (NBB; coordinator, Dr. R. Ravid); the clinical history of the subjects who donated their brain and spinal cord to the NBB is available (see Table 1), and the material is evaluated by a neuropathologist (Dr. W. Kamphorst, Pathology Department, Academic Hospital of the Free University, Amsterdam, The Netherlands). Blocks of CNS tissue (0.5–2 cm3) from 14 MS subjects and one control subject that were used in the present study were collected during the course of the study and were obtained at autopsy within 3 hr 45 min–9 hr 35 min (mean ± SD, 6 hr 10 min ± 1 hr 45 min) after death; in most cases, tissue was taken from areas around the ventricles. The tissue was immersion-fixed for 2–12 d in 4% paraformaldehyde in PBS, pH 7.4, at 4°C, incubated in 30% sucrose (until the tissue had sunk to the bottom of the vial), and then cut into smaller pieces. Each piece was transferred separately to an aluminum boat containing Tissue Tek OCT compound (Sakura Finetek Europe BV), frozen on solid CO2, and stored at −80°C. Ten-micrometer sections were cut from each tissue block and mounted onto either SuperFrost*/Plus microscope slides (Menzel-Gläser) or onto SuperFrost microscope slides (Menzel-Gläser) coated with a solution of 5 g/l gelatin and 1 mm chromium potassium sulfate. Immunolabelings were performed on cryostat sections of 4% paraformaldehyde-fixed tissue, because the staining pattern observed with the O4 antibody in this material is oligodendrocyte lineage-specific (Wolswijk, 1995), in contrast to that observed in formalin-fixed, paraffin-embedded material, as noted previously by others (Wu and Raine, 1992). Brain tumor tissue (glioblastoma and astrocytoma grade III tissue) was obtained from Dr. U. Engel (Klinikum Buch, Pathologisches Institut, Berlin, Germany) and was fixed and processed in the same manner as the MS tissue.
Table 1.
Details of MS subjects and lesions
| MS subject NBB1-a no. | Age (yr) | Sex | Disease duration (yr) | Postmortem delay (hr:min) | Lesion | Lesion diameter (mm) |
|---|---|---|---|---|---|---|
| 94-042 | 32 | M | 8 | 9:35 | A | 13 |
| 95-095 | 56 | M | 12 | 5:24 | B | 10 |
| 96-025 | 34 | F | 10 | 6:50 | C | >19 |
| D | >3 | |||||
| 96-026 | 69 | F | 19 | 9:15 | E | 3 |
| 96-039 | 57 | F | 19 | 5:45 | F | >12 |
| 96-040 | 35 | F | 11 | 5:45 | G | 4 |
| H | 5 | |||||
| I | >3 | |||||
| J | >3 | |||||
| K | >3 | |||||
| L | >4 | |||||
| M | >3 | |||||
| 96-074 | 40 | F | 14 | 7:00 | N | >11 |
| 96-076 | 81 | F | 49 | 4:15 | O | 9 |
| 96-104 | 73 | M | 22 | 4:45 | P | >10 |
| 96-121 | 53 | F | 18 | 7:16 | Q | >11 |
| 97-006 | 62 | F | 25 | 6:45 | R | >14 |
| 97-070 | 82 | F | 25 | 4:30 | S | 3 |
| T | 3 | |||||
| 97-077 | 50 | M | 17 | 5:40 | U | >4 |
| 97-123 | 46 | M | 23 | 3:45 | V | 14 |
NBB, Netherlands Brain Bank.
Antibodies. Sections were immunolabeled with the following antibodies: the mouse monoclonal antibody O4 (Sommer and Schachner, 1981) and antibodies to galactocerebroside (GalC; the Ranscht mouse monoclonal antibody; Ranscht et al., 1982), glial fibrillary acidic protein (GFAP; rabbit antiserum; Dako, Glostrup, Denmark), vimentin (mouse monoclonal antibody; Boehringer Mannheim, Mannheim, Germany), myelin basic protein (MBP; rabbit antiserum; a gift from Dr. H. van Noort, TNO, Leiden, The Netherlands), the NG2 chondroitin sulfate proteoglycan (rabbit antiserum; a gift from Dr. J. Levine, State University of New York), human PDGF-α receptor (rabbit antiserum to a peptide corresponding to the cytoplasmic domain of the human PDGF-α receptor; a gift from Dr. C.-H. Heldin, Ludwig Institute for Cancer Research, Uppsala, Sweden) human leukocyte common antigen (the 2B11 and PD7/26 mouse monoclonal antibodies; Dako) and human Ki-67 (rabbit antiserum; Dako).
Immunohistochemistry. In most experiments, sections of MS tissue were immunolabeled using indirect immunofluorescence procedures, as described in detail previously (Wolswijk, 1995). Sections were labeled with either two or three different primary antibodies, and their binding was visualized using two or three different fluorochromes, i.e., fluorescein, rhodamine, or coumarin; these reagents were purchased from either Southern Biotechnology Associates, Inc. (Birmingham, AL) or Molecular Probes (Eugene, OR). For confocal laser-scanning microscopic analysis, the coumarin-labeled reagents were replaced by Cy5-conjugated reagents (Jackson ImmunoResearch, West Grove, PA). To reduce nonspecific binding of the primary antibodies, sections were first incubated 15–30 min in the presence of 50% heat-inactivated horse serum (HS). All reagents were diluted in Tris-buffered saline (TBS), pH 7.6, supplemented with 1% HS. To visualize internal antigens, sections were incubated in methanol for 10 min at −20°C. Nuclei were visualized by incubating sections for 30 min at room temperature in 1 mg/ml Hoechst dye 33258 (Sigma, St. Louis, MO) (for conventional fluorescence microscopic analysis) or 10 nm TO-PRO-3 iodide (Molecular Probes) (for confocal laser scanning microscopic analysis) in PBS. After each incubation, sections were rinsed several times in TBS, followed by an incubation in TBS for at least 30 min. At the end of the immunolabelings, a drop of antifade (glycerol containing 22 mm1,4-diazobicyclo-[2,2,2]octane; Sigma) was placed onto the sections, followed by a glass coverslip.
The binding of the 2B11 and PD7/26 mouse monoclonal antibody mix and the rabbit anti-NG2 chondroitin sulfate and anti-human PDGF-α receptor antisera was visualized using a more sensitive indirect immunoperoxidase technique. After blocking of the endogenous peroxidase activity and the application of the primary antibodies, sections were incubated sequentially in the presence of biotinylated anti-mouse or anti-rabbit IgG (H+L) (Vector Laboratories, Burlingame, CA), a mixture of Vectastain ABC kit reagents A and B (Vector), and substrate [0.15 mg/ml 3,3′-diaminobenzidine (DAB; Sigma) and 0.006% H2O2 (Merck, Darmstadt, Germany) in TBS]; nuclei were visualized with hematoxylin.
The heat treatment necessary to obtain staining with the Ki-67 antibody completely destroyed labeling with the O4 antibody. Sections were therefore first labeled with the O4 antibody using the indirect immunoperoxidase technique described above, incubated in the presence of 3.0% H202 (to destroy any remaining peroxidase activity), and then heat-treated (the DAB precipitate withstands this treatment). Sections were then incubated with the Ki-67 antibody, goat anti-rabbit Ig, peroxidase–anti-peroxidase complex, and substrate (TBS containing 0.15 mg/ml DAB, 0.006% H2O2, and 0.2% ammonium nickel sulfate). After this procedure, the surface of the O4-positive cells was stained brown, whereas Ki-67-positive nuclei were stained purple/black (see Fig. 3). The Ki-67 labeling was optimized using human brain tumor tissue; up to 900 nuclei/mm2 section expressed Ki-67 in this tissue. The Ki-67 expression in some of the chronic MS lesions, in the control tissue, and in the tumor tissue was checked independently by the Pathology Department of the Academic Medical Center (Amsterdam, The Netherlands); identical levels of expression were found.
Fig. 3.
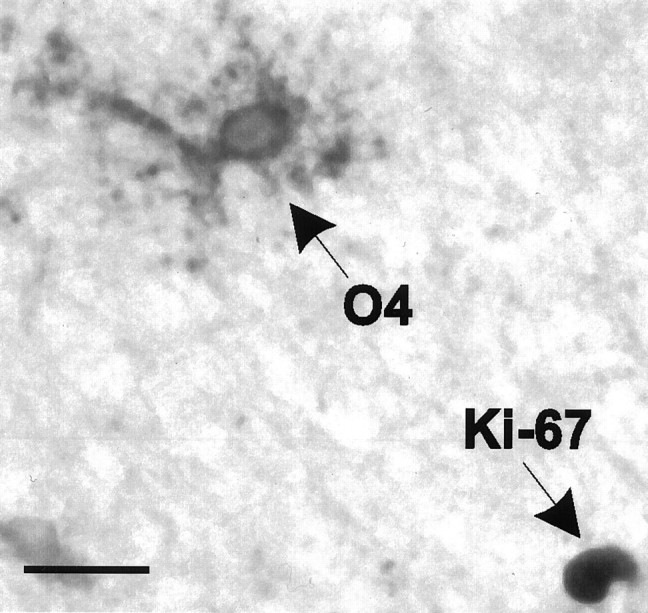
O4-positive oligodendrocyte precursor cells in the chronic MS lesions failed to bind the Ki-67 antibody. Some lesions did contain some Ki-67-immunoreactive nuclei, such as the one shown here, which was present in lesion N from subject 96-074. Scale bar, 25 μm.
RESULTS
Identification of oligodendrocyte precursor cells
Oligodendrocyte precursor cells were identified using a combination of the O4 antibody (Sommer and Schachner, 1981), which recognizes sulfatide and an unidentified antigen (Bansal et al., 1992), and antibodies to GalC (Ranscht et al., 1982). The reasons for using this combination of antibodies were twofold. First, oligodendrocyte precursor cells derived from the adult rodent and human CNS are O4-positive, GalC-negative in tissue culture (Wolswijk and Noble, 1989;Armstrong et al., 1992; Gogate et al., 1994; Scolding et al., 1995;Engel and Wolswijk, 1996; Wolswijk, 1997) (oligodendrocytes are O4-positive, GalC-positive) (Sommer and Schachner, 1981; Wolswijk and Noble, 1989). Second, the O4–anti-GalC antibody combination has been used previously to identify successfully oligodendrocyte precursor cells in sections of developing rodent and human CNS tissue (Sommer and Schachner, 1981; Warrington and Pfeiffer, 1992; Wolswijk, 1995;Hajihosseini et al., 1996). The only other cells in the CNS that are known to bind the O4 antibody are the olfactory nerve-ensheathing cells of the olfactory bulb (Barnett et al., 1993). Surface labeling with both antibodies is obtained in cryostat sections of 4% paraformaldehyde-fixed tissue (Wolswijk, 1995). Because most of the available MS tissue is either embedded in paraffin or snap-frozen, it was necessary to built up a collection of appropriately fixed MS material.
Details of chronic MS lesions
The presence of oligodendrocyte precursor cells in MS lesions was examined in a series of 22 distinct demyelinated lesions (≥3 mm in diameter; Tables 1, 2, lesions A–R) obtained at autopsy from 14 MS subjects; they died during chronic stages of MS (9–49 years of disease duration) (Table 1). The chronic MS lesions, i.e., lesions derived from subjects with chronic MS, were collected during the course of the study and were characterized in detail using antibody markers for oligodendrocytes–myelin (anti-GalC–myelin basic protein), axons (antineurofilament), and leukocytes (anti-human leukocyte common antigen), i.e., (activated) microglia, macrophages, and lymphocytes. Most of the MS lesions in the collection were collected from white matter (WM) regions around the ventricles, an area where MS lesions are encountered frequently (Prineas and McDonald, 1997).
Table 2.
Cell densities in the 15 chronic MS lesions that mostly lacked myelin and oligodendrocytes in their center
| Lesion (MS subject) | Tissue block | Size demyelinated area in section (mm2) | Number of cells/mm2 | |||
|---|---|---|---|---|---|---|
| Oligodendrocyte precursor cells (% total) | Oligodendrocytes (% total) | Phase-bright macrophages (% total) | Total | |||
| A (94-042) | I | 9 | 19.0 ± 0.3 | 0.1 ± 0.1 | 1.1 ± 0.4 | 466.4 ± 23.2 |
| (4.1 ± 0.2) | (<0.1) | (0.2 ± 0.1) | ||||
| II | 20 | 19.0 ± 2.4 | 1.3 ± 1.1 | 3.7 ± 1.1 | 528.7 ± 56.2 | |
| (3.6 ± 0.3) | (0.2 ± 0.2) | (0.7 ± 0.2) | ||||
| B (95-095) | I | 3 | 12.2 ± 2.3 | <0.1 | 0.6 ± 0.3 | 373.5 ± 56.4 |
| (3.4 ± 0.9) | (<0.1) | (0.2 ± 0.1) | ||||
| II | 5 | 5.7 ± 0.5 | <0.1 | 0.2 ± 0.2 | 307.2 ± 20.6 | |
| (1.9 ± 0.2) | (<0.1) | (<0.1) | ||||
| III | 7 | 12.1 ± 3.9 | <0.1 | 0.7 ± 0.3 | 437.0 ± 28.2 | |
| (2.8 ± 1.1) | (<0.1) | (0.2 ± 0.1) | ||||
| IV | 18 | 9.8 ± 1.1 | 0.2 ± 0.2 | 0.2 ± 0.1 | 385.8 ± 21.2 | |
| (2.5 ± 0.2) | (<0.1) | (<0.1) | ||||
| V | 8 | 12.5 ± 3.8 | <0.1 | 0.4 ± 0.2 | 388.7 ± 16.7 | |
| (3.2 ± 0.9) | (<0.1) | (≤0.1) | ||||
| C (96-025) | I | 16 | 17.4 ± 0.8 | 0.6 ± 0.7 | 31.7 ± 9.1 | 588.4 ± 26.1 |
| (3.0 ± 0.1) | (0.1 ± 0.1) | (5.4 ± 1.8) | ||||
| II | 24 | 17.1 ± 7.0 | 1.5 ± 1.2 | 118.2 ± 13.6 | 634.9 ± 26.8 | |
| (2.8 ± 1.2) | (0.2 ± 0.2) | (18.7 ± 2.8) | ||||
| E (96-026) | 5 | 4.6 ± 0.8 | 0.1 ± 0.1 | 1.2 ± 0.3 | 704.8 ± 51.2 | |
| (0.7 ± 0.1) | (<0.1) | (0.2 ± 0.1) | ||||
| F (96-039) | I | 21 | 15.3 ± 1.1 | 0.7 ± 0.5 | 34.7 ± 18.0 | 455.7 ± 32.3 |
| (3.4 ± 0.3) | (0.2 ± 0.1) | (7.5 ± 3.4) | ||||
| II | 16 | 21.0 ± 1.2 | 0.6 ± 0.7 | 62.2 ± 21.6 | 529.4 ± 34.0 | |
| (4.0 ± 0.4) | (0.2 ± 0.1) | (11.6 ± 3.4) | ||||
| III | 17 | 34.0 ± 3.8 | <0.1 | 323.6 ± 52.1 | 795.4 ± 34.0 | |
| (4.3 ± 0.3) | (<0.1) | (40.6 ± 4.8) | ||||
| J (96-040) | 6 | 18.2 ± 2.3 | 0.2 ± 0.3 | 7.0 ± 6.3 | 524.8 ± 30.2 | |
| (3.5 ± 0.5) | (<0.1) | (1.3 ± 1.2) | ||||
| K (96-040) | 2 | 20.5 ± 2.3 | 0.6 ± 0.3 | 54.4 ± 11.7 | 587.5 ± 57.7 | |
| (3.5 ± 0.6) | (≤0.1) | (9.4 ± 2.9) | ||||
| L (96-040) | 6 | 10.5 ± 2.0 | 0.3 ± 0.2 | 21.4 ± 6.9 | 484.4 ± 23.5 | |
| (2.2 ± 0.3) | (≤0.1) | (4.4 ± 1.6) | ||||
| M (96-040) | 8 | 20.4 ± 9.2 | 0.3 ± 0.2 | 10.3 ± 3.0 | 605.9 ± 91.8 | |
| (3.3 ± 1.0) | (<0.1) | (1.6 ± 0.3) | ||||
| N (96-074) | I | 29 | 12.1 ± 2.1 | 1.7 ± 0.9 | 42.6 ± 14.2 | 467.2 ± 34.1 |
| (2.6 ± 0.3) | (0.4 ± 0.2) | (9.1 ± 2.7) | ||||
| II | 21 | 14.2 ± 0.1 | 0.5 ± 0.1 | 100.4 ± 21.4 | 495.4 ± 1.4 | |
| (2.9 ± 0.1) | (≤0.1) | (20.3 ± 4.4) | ||||
| III | 25 | 13.5 ± 0.7 | 0.3 ± 0.3 | 108.9 ± 18.2 | 565.3 ± 28.7 | |
| (2.4 ± 0.2) | (0.1 ± 0.1) | (19.2 ± 2.5) | ||||
| O (96-076) | I | 3 | 6.8 ± 1.5 | 0.1 ± 0.2 | 1.4 ± 0.1 | 380.8 ± 66.3 |
| (1.8 ± 0.6) | (<0.1) | (0.4 ± 0.1) | ||||
| II | 15 | 4.5 ± 1.1 | <0.1 | 0.4 ± 0.3 | 275.1 ± 5.0 | |
| (1.6 ± 0.5) | (<0.1) | (0.1 ± 0.1) | ||||
| III | 9 | 7.8 ± 1.1 | 0.3 ± 0.1 | 0.1 ± 0.2 | 322.4 ± 30.6 | |
| (2.4 ± 0.6) | (≤0.1) | (<0.1) | ||||
| IV | 9 | 7.8 ± 0.8 | 0.3 ± 0.2 | 0.2 ± 0.3 | 435.6 ± 53.2 | |
| (1.8 ± 0.2) | (<0.1) | (<0.1) | ||||
| Q (96-121) | Ia | 14 | 21.7 ± 4.8 | 0.8 ± 0.9 | 2.4 ± 1.6 | 656.4 ± 45.8 |
| (3.3 ± 0.6) | (0.1 ± 0.1) | (0.4 ± 0.3) | ||||
| Ib | 5 | 14.3 ± 1.5 | 1.8 ± 1.3 | 155.8 ± 40.6 | 643.5 ± 64.9 | |
| (2.2 ± 0.2) | (0.3 ± 0.2) | (24.0 ± 4.7) | ||||
| IIa | 19 | 13.3 ± 1.8 | 0.4 ± 0.7 | 1.3 ± 0.8 | 657.6 ± 37.9 | |
| (2.0 ± 0.3) | (0.1 ± 0.1) | (0.2 ± 0.1) | ||||
| IIb | 22 | 10.1 ± 2.5 | <0.1 | 130.1 ± 21.4 | 614.9 ± 50.9 | |
| (1.7 ± 0.5) | (<0.1) | (21.2 ± 4.1) | ||||
| IIIa | 23 | 15.8 ± 5.0 | <0.1 | 3.2 ± 2.3 | 722.4 ± 80.2 | |
| (2.2 ± 0.6) | (<0.1) | (0.5 ± 0.4) | ||||
| IIIb | 17 | 11.4 ± 0.9 | 0.2 ± 0.3 | 180.5 ± 16.5 | 667.6 ± 39.2 | |
| (1.7 ± 0.2) | (<0.1) | (27.2 ± 3.8) | ||||
| T (97-070) | 6 | 2.3 ± 0.7 | 0.1 ± 0.1 | 0.5 ± 0.1 | 409.7 ± 75.6 | |
| (0.6 ± 0.1) | (<0.1) | (0.1 ± 0.1) | ||||
| U (97-077) | 8 | 18.8 ± 5.2 | 0.2 ± 0.3 | 14.5 ± 1.0 | 385.1 ± 70.2 | |
| (5.1 ± 1.9) | (<0.1) | (3.9 ± 0.5) | ||||
| V (97-123) | I | 19 | 18.0 ± 1.8 | 0.1 ± 0.2 | 10.1 ± 5.9 | 408.1 ± 51.1 |
| (4.4 ± 0.2) | (<0.1) | (2.5 ± 1.5) | ||||
| II | 16 | 25.0 ± 3.3 | 0.7 ± 0.3 | 43.2 ± 5.9 | 496.0 ± 40.1 | |
| (4.8 ± 0.3) | (0.1 ± 0.1) | (9.8 ± 1.7) | ||||
Cell densities were determined in the 15 MS lesions that contained very few oligodendrocytes in their centers; border regions were excluded from the cell counts. The numbers of oligodendrocyte precursor cells, oligodendrocytes, and debris-laden macrophages (identified using phase-contrast optics; see Fig. 1) per square millimeter section were determined by analyzing the complete lesion center or by counting 6–10 semi-randomly selected microscope fields of 0.20 or 0.30 mm2; nuclei were visualized using Hoechst dye 33258, which aided the counting. Similarly, the total number of cells per square millimeter section was determined by counting the total number of Hoechst dye 33258-positive nuclei present in two to eight fields; fields containing blood vessels with perivascular cuffs (which consisted of mainly macrophages and lymphocytes) were excluded from the cell counts. Of the larger MS lesions, several tissue blocks were analyzed to determine cell densities. When lesions contained debris-laden macrophages, they were found throughout the lesions, with the exception of lesion Q (subject 96-121), in which they were only present in a sharp band (regions Ib, IIb, and IIIb) surrounding the center of the lesion (regions Ia, IIa, and IIIa). Results are expressed as mean ± SD of three to five sections.
The immunocytochemical studies revealed that the collection of chronic MS lesions consisted of different types of lesions (Brück et al., 1994; Ozawa et al., 1994; Lucchinetti et al., 1996; Prineas and McDonald, 1997) (Tables 1,2). Four lesions lacked myelin but still contained many GalC-positive oligodendrocyte cell bodies (lesions D, G, H, and I); they also contained numerous debris-laden macrophages. Fifteen of the chronic MS lesions comprised confluent areas lacking both myelin and oligodendrocytes. Ten of these had a relatively wide border region and contained significant numbers of macrophages, both in their centers and borders (lesions C, F, J–N, Q, U, and V); rounded oligodendrocytes were also observed in the borders of these lesions. The remaining five such lesions had a sharp lesion border and almost completely lacked debris-laden macrophages (lesions A, B, E, O, and T). Finally, three lesions in the collection were only partially demyelinated (lesions P, R, and S); few, if any, debris-laden macrophages were observed in these lesions. Some features of the 22 chronic MS lesions in the collection are shown in Figure1.
Fig. 1.
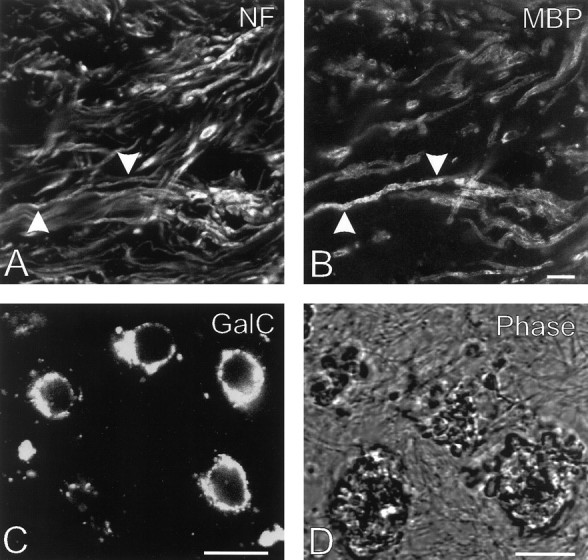
Some characteristics of the chronic MS lesions in the collection. A, B, Variable numbers of neurofilament (NF)-positive axons (A) that were surrounded by MBP-positive myelin segments (arrowheads) (B) were observed in the center of three of the chronic lesions. Detail of lesion R, subject 97-006. C, Numerous GalC-positive oligodendrocytes lacking processes were present in four of the chronic MS lesions analyzed. Such rounded oligodendrocytes were also present in the borders of the lesions with an oligodendrocyte-free center. Detail of lesion H, subject 96-040. D, Many of the chronic MS lesions contained debris-laden macrophages, which were easily identifiable when sections were examined under phase-contrast optics; this semi-phase contrast image was generated using a confocal scanning laser microscope. Detail of lesion Q, subject 96-121. Scale bars:B, C, D, 10 μm.
Chronic MS lesions contain oligodendrocyte precursor cells
O4-positive, GalC-negative cells were found in all 22 chronic MS lesions investigated, and they fully resembled in their properties oligodendrocyte precursor cells (Fig. 2, Table 2). For example, they had an oval or round cell body from which variable numbers of fine, and sometimes long, processes emanated; these processes were arranged predominantly in an asymmetrical manner, which suggested that they were immature and not at a stage just before oligodendrocytic differentiation (Wolswijk and Noble, 1989). Their nuclei were often irregular in shape, had a maximum diameter of 9.8 ± 1.5 μm (n = 34), and were surrounded by only a small rim of cytoplasm. Triple immunofluorescence combined with confocal laser-scanning microscopy indicated that the O4-positive, GalC-negative cells lacked both vimentin and the astrocyte-specific intermediate filament GFAP, like adult rodent and human oligodendrocyte precursor cells in tissue culture (Wolswijk and Noble, 1989; Armstrong et al., 1992; Gogate et al., 1994; Scolding et al., 1995; Engel and Wolswijk, 1996; Wolswijk, 1997). Thus, all of the available evidence indicated that the O4-positive, GalC-negative cells in the chronic MS lesions studied were oligodendrocyte precursor cells. They were identified most easily in areas with only small numbers of myelin segments and oligodendrocytes, such as in the 15 lesions that almost completely lacked myelin and oligodendrocytes in their centers. The O4–anti-GalC antibody combination was thus not useful in detecting O4-positive, GalC-negative oligodendrocyte precursor cells in unaffected WM, as noted previously (Armstrong et al., 1992; Warrington and Pfeiffer, 1992; Wolswijk, 1995). Triple immunofluorescence studies showed that the oligodendrocyte precursor cells in the lesions were in close proximity to large numbers of demyelinated, neurofilament-positive axons. In some of the MS lesions, the oligodendrocyte precursor cells were also surrounded by numerous debris-laden macrophages.
Fig. 2.
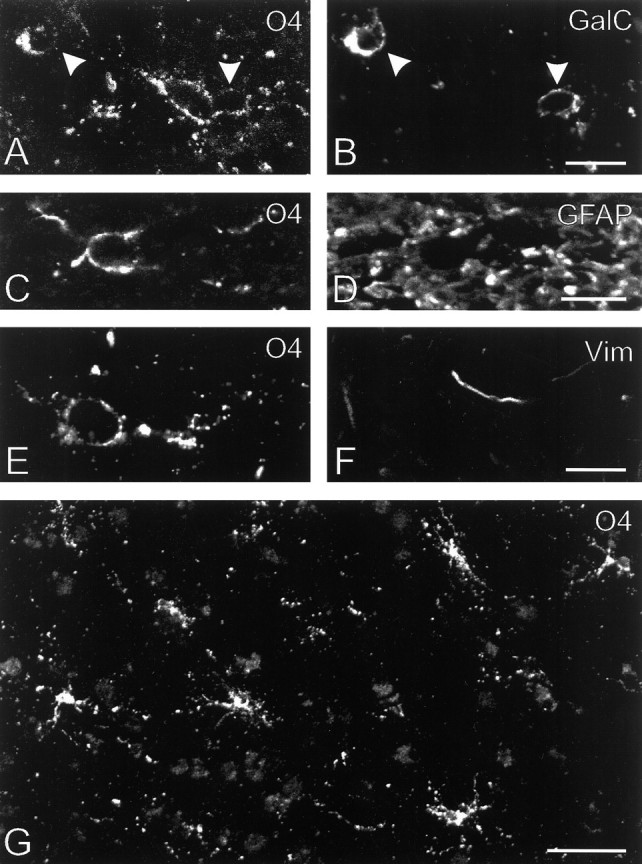
Antigenic and morphological characteristics of oligodendrocyte precursor cells in chronic MS lesions. A, B, An O4-positive, GalC-negative oligodendrocyte precursor cell and two GalC-positive, weakly O4-positive, rounded oligodendrocytes (arrowheads) that were present in the center of lesion H (subject 96-040). C, D, Although the O4-positive (GalC-negative) cells were surrounded by large numbers of GFAP-positive filaments, confocal laser-scanning microscopic analysis suggested that they themselves lacked GFAP and thus were oligodendrocyte precursor cells. Detail of lesion Q, subject 96-121. E, F, The O4-positive (GalC-negative) oligodendrocyte precursor cells also lacked the intermediate filament vimentin (Vim).G, Low-power view of an area of lesion F (subject 96-039) that contained large numbers of O4-positive (GalC-negative) oligodendrocyte precursor cells (Table 2). This lesion also contained numerous debris-laden macrophages (Table 2), and because the debris within these cells was slightly autofluorescent, they are vaguely visible in the background. Scale bars: B, D, F, 10 μm;G, 50 μm.
In initial experiments, some sections of MS tissue were immunolabeled with antibodies to both the human PDGF-α receptor and the NG2 chondroitin sulfate proteoglycan, two other putativein situ markers for human oligodendrocyte precursor cells (Pringle et al., 1992; Levine et al., 1993; Nishiayama et al., 1996;Oumesmar et al., 1997); some neuronal populations also express PDGF-α receptors (Oumesmar et al., 1997). The anti-human PDGF-α receptor antibodies only labeled an occasional oligodendrocyte precursor-like cell in some of the chronic MS lesions (plus some axonal sprout-like structures and some structures associated with blood vessels), whereas no staining was observed with the anti-NG2 antibodies. Because of these results, the expression of the PDGF-α receptor and NG2 chondroitin sulfate proteoglycan in chronic MS lesions was not pursued further.
The density of oligodendrocyte precursor cells in the chronic MS lesions varies considerably
The relative size of the precursor population in lesion sites was determined by counting the number of O4-positive, GalC-negative cells in sections labeled with the O4–anti-GalC antibody combination and the nuclear Hoechst dye 33258, which aided the counting. These experiments, which were performed on the 15 lesions that mostly lacked myelin and oligodendrocytes in their centers, showed that there was a large variation between lesions in the density of the oligodendrocyte precursor population (Table 2). A complete cross-section of lesion E (from subject 96-026), for example, only contained 4.6 ± 0.8 oligodendrocyte precursor cells/mm2 (sections were 10 μm thick), whereas as many as 34.0 ± 3.8 precursor cells/mm2 were present in an area of lesion F (from subject 96-039) (Table 2, Fig. 2). This variation in precursor densities was also reflected in the proportion of all cells in the lesions that were oligodendrocyte precursor cells (range, 0.6–5.1%; Table 2). Of the larger lesions, several tissue blocks were analyzed, and these studies indicated that the oligodendrocyte precursor cells were found throughout the lesions, although their density varied sometimes from region to region (Table 2). As shown in Table 2, these 15 chronic MS lesions contained very few GalC-positive oligodendrocytes in their centers. The few oligodendrocytes that were present either resembled the oligodendrocyte precursor cells in their morphology or were large cells with large nuclei and often long processes (data not shown). The cell counts showed further that the total cell density in the lesions also varied considerably. This variability was partially attributable to the presence of large numbers of debris-laden macrophages in some of the lesions (Table 2).
The lesions derived from the three oldest MS subjects (lesions E, O, and T) contained the smallest number of oligodendrocyte precursor cells/mm2 section (only 2.3–7.8 cells/mm2; Table 2). However, there was no clear correlation between the density of the oligodendrocyte precursor population in a lesion and the age of the subject from which it was derived. In addition, the presence and number of debris-laden macrophages [which is indicative of the relative age of MS lesions (Ozawa et al., 1994; Brück et al., 1995; Prineas and McDonald, 1997; Lucchinetti et al., 1996)] also did not appear to influence the density of the precursor population in the chronic lesions. For example, lesions A and C contained similar relative numbers of oligodendrocyte precursor cells, but lesion A contained only an occasional phase-bright macrophage, whereas the center of lesion C contained up to 130 debris-laden macrophages/mm2(Table 2). The oligodendrocyte precursor density was also not correlated with the length of the disease process, the size of the lesion, or the length of the postmortem delay (Tables 1, 2).
Oligodendrocyte precursor cells in the chronic MS lesions do not express the nuclear proliferation antigen recognized by the Ki-67 antibody
To assess whether the oligodendrocyte lineage cells in the chronic MS lesions studied were proliferatively active, sections were double-labeled with the O4 antibody and the Ki-67 antibody, which recognizes a nuclear protein expressed in proliferating cells in G1, S, G2, and M phases but not the G0 phase of the cell cycle (Gerdes et al., 1984; Brown and Gatter, 1990). Although >7000 O4-positive cells were examined in the 15 lesions that were used to determine cell densities (482 ± 334 cells per lesion), none were found to have a Ki-67-positive nucleus (Fig. 3); as can be seen in Table 2, the vast majority of the O4-positive cells in the center of these lesions were GalC-negative. Of the 15 MS lesions, only three contained some O4-negative, Ki-67-positive cells in their centers [lesion B, 0.4 ± 0.1 cells/mm2; lesion N, 2.1 ± 0.6 cells/mm2 (Fig. 3); lesion Q, 0.4 ± 0.1 cells/mm2]. Also, very few Ki-67-positive nuclei were present in the partially demyelinated lesions and in the lesions that contained large numbers of oligodendrocytes lacking processes. The periplaque WM of most lesions lacked Ki-67-positive nuclei, with the exception of lesion B (from subject 95-095), which contained 22.9 ± 3.5 immunoreactive nuclei/mm2 in a band ∼2 mm away from the lesion border. Only a very occasional Ki-67-positive nucleus was encountered in the WM derived from a 78-yr-old female control subject (three immunoreactive nuclei in an area of 240 mm2, a density of 0.013 Ki-67-positive nuclei/mm2), whereas large numbers of Ki-67-positive nuclei were present in sections of a glioblastoma and an astrocytoma grade III tumor sample (both contained up to 900 Ki-67-positive nuclei/mm2 section; this relatively high number is partially attributable to the high cellular density of tumors).
DISCUSSION
This study shows for the first time that MS lesions derived from subjects who died during chronic stages of MS, a stage at which myelin repair is scant or absent, contain significant numbers of O4-positive, GalC-negative, GFAP-negative oligodendrocyte precursor cells (between 2.3 and 34.0 precursor cells/mm2 section). These observations were made in 22 demyelinated lesions derived from 14 subjects with chronic MS. The collection of MS lesions included both lesions with recent demyelinating activity (as evidence by the presence of numerous debris-laden macrophages) and old, inactive lesions. The MS material was collected in a random manner and became available during the course of the study. No tissue became available from subjects suffering from acute MS or from subjects who died during early stages of MS (such material becomes only rarely available), and it was thus not possible to examine acute and early MS lesions for the presence of oligodendrocyte precursor cells.
Two observations suggested that the oligodendrocyte precursor population in the chronic MS lesions analyzed was relatively quiescent. First, none of the precursor cells in the lesions expressed a proliferation antigen recognized by the Ki-67 antibody; this is in contrast to the fetal human spinal cord, in which up to 60% of oligodendrocyte precursor cells were found to express the proliferating cell nuclear antigen (PCNA) (Hajihosseini et al., 1996), which is a different nuclear proliferation marker (Bravo et al., 1987). Second, the majority of the chronic MS lesions in the collection lacked myelinating oligodendrocytes in their center. Thus, either oligodendrocyte precursor cells are intrinsically not capable of contributing to the repair process during chronic stages of MS, or the environment of the chronic MS lesion is nonpermissive for CNS remyelination; i.e., it either lacks factors that are necessary to stimulate regenerative events in the oligodendrocyte lineage or contains factors that hamper this.
Although the O4–anti-GalC antibody combination proved extremely useful for the identification of oligodendrocyte precursor cells in MS lesions lacking myelin and oligodendrocytes, it was not useful in visualizing O4-positive, GalC-negative precursor cells in areas packed with O4-positive, GalC-positive oligodendrocytes, such as unaffected WM. Very little staining was observed with two other putative in situ makers for oligodendrocyte precursor cells, i.e., antibodies to the NG2 chondroitin sulfate proteoglycan and the human PDGF-α receptor. Because a previous study failed to detect PDGF-α receptor mRNA-positive cells in human fetal tissue (Hajihosseini et al., 1996), no attempts were made to examine the expression of PDGF-α receptor mRNA in postmortem CNS tissue; using the same antibody as was used in the present study, many PDGF-α receptor antibody-positive cells were observed in this study (Hajihosseini et al., 1996).
Although no data are available about the normal density of the oligodendrocyte precursor population in myelinated areas of the adult human CNS, studies in rodents have suggested that ∼4% of all cells in WM regions are oligodendrocyte precursor cells (Fulton et al., 1992;Pringle et al., 1992). Normal human WM contains between 450 and 600 cells/mm2 section (10-μm-thick sections) (data from a 78-yr-old female control subject). If four percent of the WM cells in the human CNS are also oligodendrocyte precursor cells, then their density would range from 18 to 24 cells/mm2; this is within the range found in the chronic MS lesions (2–34 precursor cells/mm2).
Tissue culture studies have shown that adult human oligodendrocyte precursor cells do have the capacity to divide. When grown on monolayers of rat cortical astrocytes, small numbers of adult human oligodendrocyte precursor cells incorporate the thymidine analog bromodeoxyuridine (Scolding et al., 1995). However, no division was seen when they were exposed to PDGF and bFGF (Armstrong et al., 1992;Scolding et al., 1995), two potent mitogens for adult rat CNS-derived oligodendrocyte precursor cells (Wolswijk et al., 1991b; Wolswijk and Noble, 1992; Engel and Wolswijk, 1996). In this respect, it is important to note that, in contrast to our studies, other groups failed to find a mitogenic response of adult rodent oligodendrocyte precursor cells to these two defined growth factors (Armstrong et al., 1990; Chan et al., 1990). This thus suggests that the failure to find a significant response of adult human oligodendrocyte precursor cells to PDGF and bFGF may not be attributable to species differences but to, for example, differences in isolation and culture procedures.
Very few studies have examined thus far the expression of proliferation markers in MS lesions. Morris and colleagues (1994), using antibodies to the PCNA (a nuclear protein that is associated with DNA polymerase-δ and that is most abundant in S phase of the cell cycle;Bravo et al., 1987) found that only one of the six MS cases analyzed contained significant numbers of PCNA-positive nuclei (12–20 nuclei/mm2; Morris et al., 1994); control WM lacked PCNA-positive cells. In contrast to low levels of expression of nuclear proliferation markers in chronic MS lesions observed in the study of Morris and colleagues (1994) (six lesions) and the present study (22 lesions), Dowling and co-workers (1997) found that the five chronic MS lesions they analyzed contained relatively large numbers of perivascular inflammatory cells and parenchymal glial cells expressing Ki-67. The reasons for the varying results in the expression of proliferation markers in chronic MS lesions are unclear but may be a reflection of differences in disease activity. For example, the five chronic lesions examined in the study of Dowling and colleagues (1997) also contained large numbers of apoptotic nuclei, suggesting that they were relatively active.
A number of factors may contribute to the failure of myelin repair in MS, including the formation of an astrocytic scar and the presence of inhibitory factors. Two factors that are expressed in MS lesions, i.e., transforming growth factor-β (TGF-β) and interferon-γ (IFN-γ) (Woodroofe and Cuzner, 1993), potentially may be involved in keeping oligodendrocyte precursor cells in chronic MS lesions in a quiescent state. This suggestion has come from in vitro studies that have shown that both TGF-β and IFN-γ are capable of reducing the proliferative response of oligodendrocyte precursor cells derived from the developing rodent CNS to defined mitogens (McKinnon et al., 1993;Agresti et al., 1996); these effects are reversible. IFN-γ at the same time also inhibits their oligodendrocytic differentiation. Moreover, the effects of IFN-γ are potentiated by tumor necrosis factor-α, another factor that is expressed in MS lesions (Hofman et al., 1989; Selmaj et al., 1991; Woodroofe and Cuzner, 1993). It remains to be determined, however, whether these factors are capable of blocking the proliferation and/or differentiation of adult oligodendrocyte precursor cells both in vitro and in vivo.
In addition to treatments to prevent or to limit damage to myelin and oligodendrocytes in MS (and other demyelinating diseases), there is a need for the development of strategies aimed at repairing existing damage. Studies in experimental animals have demonstrated convincingly that myelin repair may be achieved by transplantation of purified populations of oligodendrocytes, oligodendrocyte precursor cells, or Schwann cells, the myelin-forming cells of the peripheral nervous system (Blakemore and Franklin, 1991; Groves et al., 1993; Archer et al., 1997). However, before transplantation can be performed in humans a number of obstacles have to be overcome, such as the limited availability of human oligodendrocyte lineage cells. Moreover, if chronic MS lesions indeed contain remyelination inhibitory factors, then the success of transplantation may be limited. Because chronic MS lesions contain a resident population of oligodendrocyte precursor cells, a potential strategy to promote CNS remyelination would be to identify ways of stimulating these cells to proliferate and to generate new myelin-forming cells.
Footnotes
This study was supported by grants from the Multiple Sclerosis Society of Great Britain and Northern Ireland and the Netherlands Foundation “Friends MS Research.” The team of the Netherlands Brain Bank (coordinator Dr. R. Ravid) is thanked for collecting the control and multiple sclerosis material and for advice. Dr. Ute Engel is thanked for providing human brain tumor tissue; Dr. H. van Noort, Dr. J. Levine, and Dr. C.-H. Heldin are thanked for their gift of antibodies; Dr. D. Troost, Dr. A. Walter, and M. Ramkema (Pathology Department, Academic Medical Center, Amsterdam, The Netherlands) are thanked for their advice on immunolabelings involving the Ki-67 antibody, and Gerben van der Meulen is thanked for assistance with the preparation of the figures. Thanks also to Mark Noble, Dick Swaab, Joost Verhaagen, and Stephan Guldenaar for critical reading of this manuscript.
Correspondence should be addressed to Dr. G. Wolswijk, Netherlands Institute for Brain Research, Meibergdreef 33, 1105 AZ Amsterdam ZO, The Netherlands.
REFERENCES
- 1.Agresti C, D’Urso D, Levi G. Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendrocyte lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 2.Aranella LS, Herndon RM. Mature oligodendrocyte division following experimental demyelination in adult animals. Arch Neurol. 1984;41:1162–1165. doi: 10.1001/archneur.1984.04050220060015. [DOI] [PubMed] [Google Scholar]
- 3.Archer DR, Cuddon PA, Lipsitz D, Duncan ID. Myelination of the canine central nervous system by glial cell transplantation: a model for repair of human myelin disease. Nat Med. 1997;3:54–59. doi: 10.1038/nm0197-54. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong R, Friedrich VL, Holmes KV, Dubois-Dalcq M. In vitro analysis of the oligodendrocyte lineage in mice during demyelination and remyelination. J Cell Biol. 1990;111:1183–1195. doi: 10.1083/jcb.111.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong RG, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal R, Stefansson K, Pfeiffer SE. Prooligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem. 1992;58:2221–2229. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- 7.Barnett SC, Hutchins A-M, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155:337–350. doi: 10.1006/dbio.1993.1033. [DOI] [PubMed] [Google Scholar]
- 8.Blakemore WF, Franklin RJM. Transplantation of glial cells in the CNS. Trends Neurosci. 1991;14:323–327. doi: 10.1016/0166-2236(91)90155-n. [DOI] [PubMed] [Google Scholar]
- 9.Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 10.Brown D, Gatter KC. Monoclonal antibody Ki67: its use in histopathology. Histopathology. 1990;17:489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 11.Brück W, Schmied M, Suchanek G, Brück Y, Breitschopf H, Poser S, Piddlesden S, Lassmann H. Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- 12.Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar H, Lassmann H. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 13.Carrol WM, Jennings AR, Mastaglia FL. The origin of remyelinating oligodendrocytes in antiserum-mediated demyelinative optic neuropathy. Brain. 1990;113:953–973. doi: 10.1093/brain/113.4.953. [DOI] [PubMed] [Google Scholar]
- 14.Chan CLH, Wigley CB, Berry M. Oligodendrocyte-type-2 astrocyte progenitor cells from neonatal and adult rat optic nerve differ in their responsiveness to platelet-derived growth factor. Dev Brain Res. 1990;55:275–282. doi: 10.1016/0165-3806(90)90209-h. [DOI] [PubMed] [Google Scholar]
- 15.Dowling P, Husar W, Menonna J, Donnenfeld H, Cook S, Sidhu M. Cell death and birth in multiple sclerosis brain. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- 16.Engel U, Wolswijk G. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: in vitro characteristics and response to PDGF, bFGF and NT-3. Glia. 1996;16:16–26. doi: 10.1002/(SICI)1098-1136(199601)16:1<16::AID-GLIA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in the adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 18.Fulton B, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gehrmann J, Lannes-Vieira J, Wekerle H. Differential expression of fibroblast growth factor-2 and receptor by glial cells in experimental autoimmune encephalomyelitis (EAE). Glia. 1996;16:93–100. doi: 10.1002/(SICI)1098-1136(199602)16:2<93::AID-GLIA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 21.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 22.Godfraind C, Friedrich VL, Holmes KV, Dubois-Dalcq M. In vivo analysis of glial cell phenotypes during a viral demyelinating disease in mice. J Cell Biol. 1989;109:2405–2416. doi: 10.1083/jcb.109.5.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogate N, Verma L, Zhou JM, Milward E, Rusten R, O’Conner M, Kufta C, Kim J, Hudson L, Dubois-Dalcq M. Plasticity in the adult human oligodendrocyte lineage. J Neurosci. 1994;14:4571–4587. doi: 10.1523/JNEUROSCI.14-08-04571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groves AK, Barnett SC, Franklin RJM, Crang AJ, Mayer M, Blakemore WF, Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- 25.Hajihosseini M, Tham TN, Dubois-Dalcq M. Origin of oligodendrocytes within the human spinal cord. J Neurosci. 1996;16:7981–7994. doi: 10.1523/JNEUROSCI.16-24-07981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumour necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine JM, Stincone F, Lee Y-S. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- 28.Logan A, Frautschy SA, Gonzalez A-M, Baird A. A time course for the local elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following localized cortical brain injury. J Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotan M, Schwarz M. Postinjury changes in platelet derived growth factor-like activity in fish and rat optic nerves: possible implications in regeneration. J Neurochem. 1992;58:1637–1642. doi: 10.1111/j.1471-4159.1992.tb10035.x. [DOI] [PubMed] [Google Scholar]
- 30.Lucchinetti CF, Brück W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity in pathogenesis. Brain Pathol. 1996;6:243–258. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwin SK. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am J Pathol. 1979;95:683–696. [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwin SK. Pathology of demyelination and remyelination. In: Waxman SG, Ritchie JM, editors. Demyelinating disease: basic and clinical electrophysiology. Raven; New York: 1981. pp. 123–168. [PubMed] [Google Scholar]
- 33.McCarthy GF, Leblond CP. Radiographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271:589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- 34.McKinnon RD, Piras G, Ida JA, Jr, Dubois-Dalcq M. A role for TGF-β in oligodendrocyte differentiation. J Cell Biol. 1993;121:1397–1407. doi: 10.1083/jcb.121.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris CS, Esiri MM, Sprinkle TJ, Gregson N. Oligodendrocyte reactions and cell proliferation markers in demyelinating disease. Neuropathol Appl Neurobiol. 1994;20:272–281. doi: 10.1111/j.1365-2990.1994.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 36.Nishiayama A, Lin X-H, Giese N, Heldin C-H, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF-α receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Ozawa K, Suchanek G, Breitschopf H, Brück W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendrocyte pathology in multiple sclerosis. Brain. 1994;117:1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- 38.Oumesmar BN, Vignais L, Baron-van Evercooren A. Developmental expression of platelet-derived growth factor-α receptor in neurons and glial cells of the mouse CNS. J Neurosci. 1997;17:125–139. doi: 10.1523/JNEUROSCI.17-01-00125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prineas JW, Connel F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 40.Prineas JW, McDonald WI. Demyelinating diseases. In: Graham DI, Lantos PL, editors. Greenfield’s neuropathology, Ed 6. Arnold; London: 1997. pp. 813–889. [Google Scholar]
- 41.Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis: oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- 42.Pringle N, Mudha HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 43.Raine CS, Scheinberg L, Waltz JM. Multiple sclerosis: oligodendrocyte survival and proliferation in active established lesions. Lab Invest. 1981;45:534–546. [PubMed] [Google Scholar]
- 44.Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez M, Pierce ML, Thiemann R. Immunoglobulins stimulate central nervous system remyelination: electron microscopic and morphometric analysis of proliferating cells. Lab Invest. 1991;64:358–370. [PubMed] [Google Scholar]
- 46.Scolding NJ, Rayner PJ, Sussman J, Shaw C, Compston DAS. A proliferative adult human oligodendrocyte progenitor. NeuroReport. 1995;6:441–445. doi: 10.1097/00001756-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumour necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cells surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 49.Warrington AE, Pfeiffer SE. Proliferation and differentiation of O4+ oligodendrocytes in postnatal cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. J Neurosci Res. 1992;33:338–353. doi: 10.1002/jnr.490330218. [DOI] [PubMed] [Google Scholar]
- 50.Wolswijk G. Strongly GD3+ cells in the developing and adult rat cerebellum belong to the microglial lineage rather than to the oligodendrocyte lineage. Glia. 1995;13:13–26. doi: 10.1002/glia.440130103. [DOI] [PubMed] [Google Scholar]
- 51.Wolswijk G. Perinatal to adult transition in precursor populations of the oligodendrocyte-type-2 astrocyte (O-2A) lineage. In: Herzenberg LA, Herzenberg LA, Weir A, editors. Handbook of experimental immunology. Blackwell; Cambridge, MA: 1997. pp. 186.1–186.12. [Google Scholar]
- 52.Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- 53.Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolswijk G, Noble M. In vitro studies of the development, maintenance and regeneration of the oligodendrocyte-type-2 astrocyte (O-2A) lineage in the adult central nervous system. In: Kettenmann H, Ransom BR, editors. Neuroglial cells. Oxford UP; Oxford: 1995. pp. 149–161. [Google Scholar]
- 55.Wolswijk G, Munro PMG, Riddle PN, Noble M. Origin, growth factor responses and ultrastructural characteristics of an adult-specific glial progenitor cell. Ann NY Acad Sci. 1991a;633:502–504. doi: 10.1111/j.1749-6632.1991.tb15640.x. [DOI] [PubMed] [Google Scholar]
- 56.Wolswijk G, Riddle PN, Noble M. Platelet-derived growth factor is mitogenic for O-2Aadult progenitor cells. Glia. 1991b;4:495–503. doi: 10.1002/glia.440040509. [DOI] [PubMed] [Google Scholar]
- 57.Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 58.Wren DR, Wolswijk G, Noble M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu E, Raine CS. Multiple sclerosis: interactions between oligodendrocytes and hypertrophic astrocytes and their occurrence in other, nondemyelinating conditions. Lab Invest. 1992;67:88–99. [PubMed] [Google Scholar]


