Abstract
A paradigm based on measuring short-term memory for spatial location information as a function of spatial similarity between distal cues was developed to examine the role of pattern separation in the modulation of short-term memory for spatial information. A delayed-match-to-sample for spatial location task using a dryland version of the Morris water maze was used to assess spatial pattern separation in male Long–Evans rats. In the sample phase, animals were trained to displace an object that covered a baited food well in one of 15 spatial locations along a row of food wells perpendicular to a start box. In the ensuing choice phase, the animal was allowed to choose between two objects identical to the sample phase object. One covered the same baited food well as did the object in the study phase (correct choice), and another foil object (incorrect choice) covered a different unbaited food well along the row of wells. Five spatial separations were randomly used to separate the correct object from the foil object. After reaching a criterion before the operation, animals were given either hippocampal or cortical control lesions. In trials after the operation, control animals matched their performance before the operation across all spatial separations. In contrast, hippocampal-lesioned animals displayed impairments across all spatial separations with the exception of the longest (105 cm) spatial separation. The results suggest that the hippocampus may serve to separate incoming spatial information by temporarily storing one place separate from another. It is proposed that hippocampal lesions decrease efficiency in pattern separation, resulting in impairments in trials with increased spatial similarity among working-memory representations.
Keywords: hippocampus, pattern separation, spatial memory, spatial location, working memory, dentate gyrus
The hippocampus has been assumed to subserve a number of processes including (1) spatial and temporal separation of events associated with temporary memory representations of new spatial information (Shapiro and Olton, 1994), (2) short-term memory or working-memory representations of new information (Olton et al., 1979; Kesner, 1990), (3) consolidation or elaborative rehearsal of new information (Milner, 1966; Squire, 1992; Schacter et al., 1996), (4) retrieval of new information based on flexibility and action (Eichenbaum, 1994; Johnson and Chalfonte, 1994), and (5) the formation of cognitive maps (O’Keefe and Nadel, 1979). There is ample evidence indicating an important role for the hippocampus in mediating consolidation, working-memory, retrieval, and cognitive map processes, but its role in the separation of patterns of incoming spatial information is in need of a more-detailed behavioral analysis (Milner, 1966; Olton et al., 1979; Kesner, 1990; Squire, 1992; Eichenbaum, 1994;Johnson and Chalfonte, 1994; Schacter et al., 1996).
Several computational models of hippocampal function suggest that the hippocampus is involved in pattern separation or orthogonalization of sensory input information (McNaughton, 1989; McNaughton and Nadel, 1990; Rolls, 1990; O’Reilly and McClelland, 1994; Shapiro and Olton, 1994). Based on sparse connections and strong inhibitory interactions within the hippocampus, these models posit that relevant sensory information is processed by hippocampal neurons perhaps by providing spatial and temporal markers for the coding of sensory information. This would ensure that new highly processed sensory information is organized within the hippocampus in such a way that remembering and temporarily storing one place separate from another place in time and space is enhanced. Similarly, Nadel (1994) suggested that one process function of the hippocampus is to separate and organize spatial representations within memory.
Enhanced spatial similarity between distal cues and decreased efficiency in pattern separation could represent a key process deficiency in hippocampal-lesioned rats. One of the most popular means for testing hippocampal function is the water maze. In this task, enhanced spatial similarity between distal cues, because of different start locations, could account for impairments in the acquisition of the task. Support for this idea comes from the observations ofEichenbaum et al. (1990) who demonstrated that when fimbria–fornix-lesioned rats were trained on the water maze task from only a single starting position (a condition in which there is less spatial similarity among spatial cues), there were minimal learning deficits, whereas training from many different starting points resulted in learning difficulties. It is also interesting to note that the deficits observed in the fornix-lesioned group occurred only in trials requiring the flexible use of novel working-memory information as in the variable-start condition. In a somewhat similar study, it was shown that when only one spatial location was correct on an eight-arm maze, total hippocampal-lesioned rats learned the task rather readily. However, they were impaired when the correct arm varied from trial to trial (Hunt et al., 1994). Thus, pattern separation may play a role in working memory as well as in acquisition of new spatial information.
To instantiate the role of the hippocampus in separating spatial events based on the overlapping similarity of distal cues, we developed a paradigm in which rats are required to remember a spatial location dependent on spatial cues and to differentiate between the to-be-remembered location and a different location with different degrees of similarity or overlap among spatial cues.
MATERIALS AND METHODS
Subjects
Ten male, Long–Evans rats, each weighing ∼350 gm at the beginning of the study, were used as subjects. Each rat was initially food-deprived to 80% of its free-feeding weight and allowed access to water ad libitum. The animals were housed individually in standard rodent cages and were maintained on a 12 hr light/dark cycle. All testing was performed during the light phase of the cycle.
Apparatus
The test apparatus was a dryland version of the Morris water maze. The apparatus was painted white, stood 65 cm above the floor, and was kept in a well-lighted room with no windows, one door, a chair, a long shelf, and four pictures of various sizes placed on the walls of the room.
The surface of the apparatus was 119 cm in diameter and 3.5 cm in thickness. One-hundred seventy-seven food wells (2.5 cm in diameter and 1.5 cm in depth) were drilled into the surface of the maze in evenly spaced parallel rows and columns 2 cm apart. A black start box (24 cm long, 15 cm wide, and 17 cm high) was placed on top of the maze surface centered perpendicular to the rows of food wells with the posterior edge of the box placed along the edge of the apparatus. The box was equipped with a hinged top for transferring animals into and out of the box, and the front of the box was equipped with a guillotine door that could be raised and lowered manually by the experimenter.
Procedure
Shaping. During the first week of training, each animal was handled for ∼0.25 hr daily and was then allowed to explore individually the test apparatus for 0.25 hr. During this exploration period, ∼10 pieces of Froot Loops cereal (Kellogg, Battle Creek, MI) were spread out on the surface of the maze, and the guillotine door to the start box remained open. Beginning the second week of training, a single object was introduced into the testing environment. The object consisted of a hollow cylindrical metal pipe 3 cm in diameter and 4 cm tall welded onto a round, thin metal plate 5 cm in diameter. The object was placed over the food well directly in the center of the maze. In each trial, a piece of cereal was placed in front of the object on the maze surface. The animal was placed in the closed start box, and then the door was opened. The animal was allowed to exit the box, retrieve the reward, and then return to the box to consume the food reward with the door closed. This procedure was followed 16 times daily. Once the animal was eating the reward consistently, the food reward was placed in the food well with the object adjacent to the well. In each ensuing trial, the object was positioned to cover a larger portion of the food well until the base of the object covered the baited well completely. Once the animal consistently displaced the object in any position along the center row of food wells, the animal began the spatial task.
Spatial task training before the operation. A delayed-match-to-sample for spatial location task was used to assess spatial pattern separation in the animals. Each animal received 16 trials per day. Each trial consisted of a sample phase followed by a choice phase. During the sample phase, a randomly positioned object covered a baited food well in one of fifteen spatial locations along the center-most row of food wells perpendicular to the start box. The rat was placed in the start box with the guillotine door in the closed position. The door was then opened; the animal exited the box, displaced the object to receive a food reward, and returned to the box. The same food well was then quickly rebaited, the object was replaced, and a second identical object was placed in a different location along the row of food wells covering a different unbaited food well. The object used in the sample phase was randomly assigned to cover either the correct food well or the incorrect food well on the choice phase, thus eliminating the possibility of using object odor cues to choose the correct object. The interval separating the sample phase from the choice phase was ∼5–7 sec, or the time required to rebait the correct food well and to position the two objects. In the ensuing choice phase, the animal was allowed to choose between the two objects. Both objects were identical to the object described in the previous “shaping” section. The object that covered the same food well as did the object in the sample phase was the correct choice, and the second foil object was the incorrect choice. Five spatial distances (15, 37.5, 60, 82.5, and 105 cm) were randomly used to separate the correct object from the foil object. For each spatial separation, the distance between the two objects was held constant; however, the two objects were in different positions along the row of wells in different trials. The position of the correct object relative to the foil object was counterbalanced with regard to left versus right and closer versus farther with respect the animal across all separations. If an animal made an incorrect choice, it was immediately returned to the start box for a 20 sec intertrial interval. Once an animal established a criterion of 75% correct choices based on 80 trials across all spatial separations, the training period before the operation was ended.
Surgery. Each animal was randomly assigned to receive either a hippocampal lesion (n = 5) or a cortical control lesion (n = 5). The animal was given atropine sulfate (0.2 mg/kg, i.p.) and anesthetized with sodium pentobarbital (Nembutal; 50 mg/kg, i.p.). The animal was then placed in a stereotaxic instrument, and an incision was made along the midline in the skin covering the skull. The periosteal fascia covering the skull was scraped away to expose the skull, and the instrument was adjusted to level the head. Both the hippocampal lesion group (HIP) and the cortical control lesion group (CON) had the bone overlying the dorsal hippocampus removed with a dental burr; the HIP animals also had the bone removed above the ventral hippocampus. The HIP group then received bilateral electrolytic lesions, produced by passing a 1.2 mA anodal current for 10 sec through a stainless steel electrode (0.35 mm in diameter) insulated with Epoxylite except for ∼0.50–0.75 mm at the tip of the electrode. The lesion coordinates for the HIP group were 3.5 mm posterior to bregma; 1.0, 2.2, and 3.4 mm lateral to midline; and 2.8 mm below dura for the dorsal hippocampus. For the ventral hippocampus, the coordinates were 4.6 mm posterior to bregma, 5.2 mm lateral to midline, and 5.6 and 8.1 mm ventral to dura. The CON group was lesioned at the same coordinates used for the dorsal hippocampus lesion, except the electrode tip was only lowered to 1 mm below dura.
Spatial task testing after the operation. After a 7–10 d recovery period from surgery, each animal was again tested on the task following the same procedure used in the training trials before the operation. Each animal was tested on two blocks of 80 trials each over a 2 week period. The performance after the operation of a subset of animals was video recorded using a video camera that was mounted to the ceiling above the apparatus.
Transfer task. A transfer task was implemented to assess the ability of each animal to solve the task, while removing the possibility of using an alternate response strategy based on making the same turn in the choice phase that was made in the sample phase. During the third week of testing after the operation, HIP and CON animals were tested on a transfer task for one block of 80 trials. The procedure on the transfer task was identical to the procedure used on the spatial task; however, after the sample phase, the start box was picked up by the experimenter, carried around the maze 180°, and placed facing the row of food wells in a position opposite that of the box in the sample phase. The choice phase of the task was then performed as described for the initial task. Each animal was tested using the transfer procedure for 5 consecutive days consisting of 16 trials each day.
Strategy probe tasks. Three probe tasks were designed to characterize the strategies that control animals used to solve the task. The tests were conducted on control animals (n = 4) that were added to the original group of subjects. These animals were pretrained on the spatial task to the criterion used before the operation and were then tested on the probe trials. The first two probe tasks were designed to assess the ability of the animals to solve the task using ideothetic cues while removing external allothetic cues. On the first probe task, the procedure was the same as the original task procedure; however, all testing was conducted in the dark. On the second probe task, the procedure was again the same; however, the sample phase was conducted in the light, and the choice phase was conducted in the dark. A third probe task was designed to characterize the use of environmental cues by the animals. On this task, after the sample phase, the entire maze was shifted either to the right or to the left the distance of the separation. Thus, on the choice phase, the foil object was in the same position relative to the environmental cues as was the object in the sample phase, whereas the correct object was in the same position as was the sample phase object relative to the maze but in a different position relative to the environmental cues. The distances used to separate the correct object from the foil object were 15, 30.5, and 38.5 cm. The correct object was randomly positioned with respect to the incorrect object and to the approach position of the animals as described in the original task to eliminate position-biased strategies.
Histology. At the conclusion of all testing, each animal was deeply anesthetized with 1.5 ml of sodium pentobarbital (50 mg/kg, i.p.) and perfused intracardially with normal saline followed by a 10% formalin solution. Each rat was then decapitated; the brain was removed from the skull and stored in a 10% formalin and 30% sucrose solution. Each brain was then frozen and cut at 24 μm sections starting at bregma and extending through the region of the ventral hippocampus. Every third section was mounted on a glass slide, stained with cresyl violet, and examined for histological verification of the lesion placement.
RESULTS
Histology
A representative cortical control lesion is shown in Figure1A. Cortical control lesions did not invade the hippocampus in any of the animals but caused fairly extensive damage to the cortex above the dorsal hippocampus. A representative dorsal hippocampal lesion is shown in Figure1B. In four of the HIP animals, lesions of the dorsal hippocampus were complete, and there was fairly extensive damage to the cortex dorsal to the dorsal hippocampus. However, in one of the HIP animals, there were minimal savings in the dorsal hippocampus region. With the exception of one animal, all lesions of the ventral hippocampus (Fig. 1C) were complete with minimal savings and limited damage to surrounding tissue.
Fig. 1.
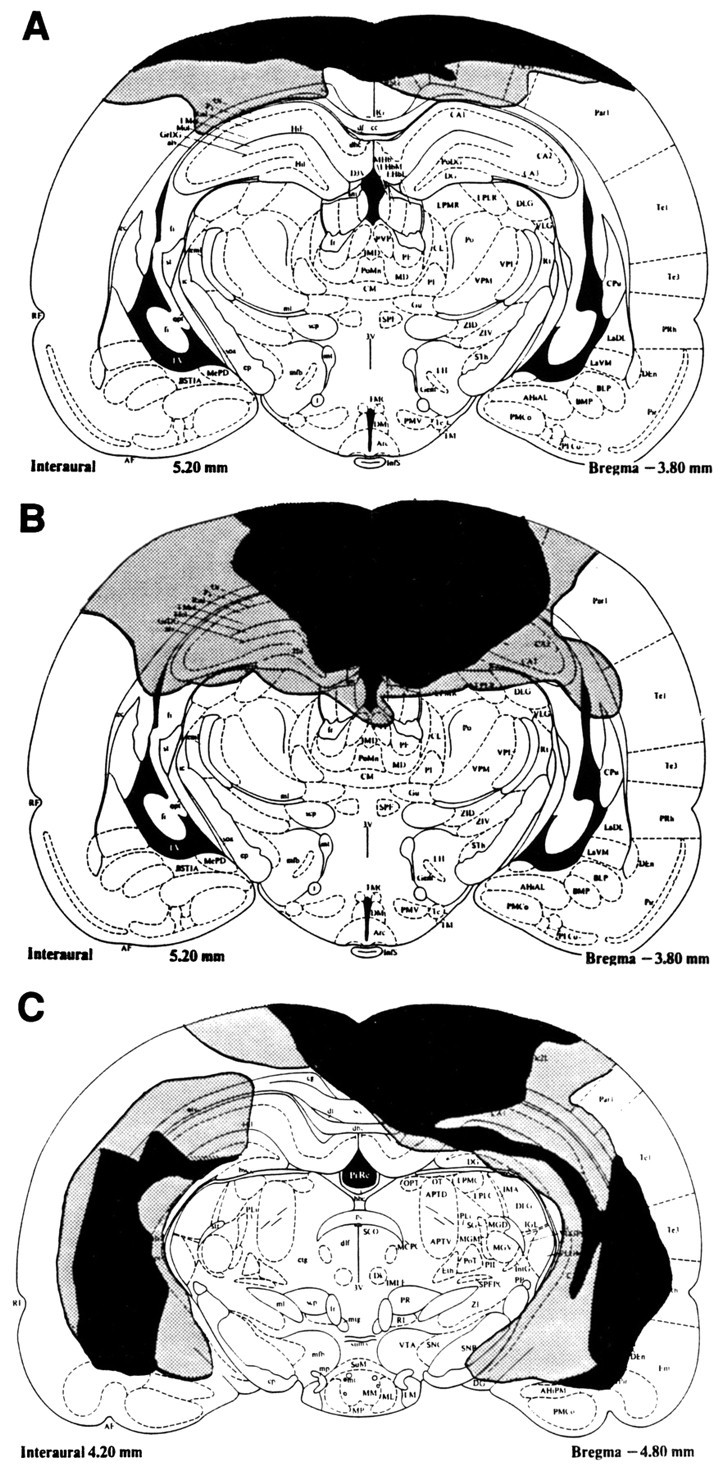
A schematic drawing of the largest (stippled) and the smallest (black) cortical control lesion (A), dorsal hippocampal lesion (B), and ventral hippocampal lesion (C).
Strategy probe tasks
The results of the first probe task, in which animals were tested in the dark, revealed that the mean performance of the animals across separations was 54% or approximately chance based on 32 trials. Similarly, on the second probe task, in which the sample phase was conducted in the light and the choice phase in the dark, the performance of the animals was at chance based on 16 trials. These findings indicate that the animals were not able to solve the task using only ideothetic cues, thus indicating that the animals relied on environmental cues to accurately solve the task.
If the animals were not using environmental cues to solve the third probe task, in which the maze was shifted the distance of the separation, then it would be predicted that shifting the maze relative to the cues would have had no effect on the performance of the animals. However, a binomial test (one-tailed) indicated that the performance of the animals on this task (29% correct) was significantly below chance (p < 0.05) based on 24 trials. These data indicate that the animals tended to choose the object that was in the same position, relative to the environmental cues, as the sample phase object (foil) but not the object in the same position, relative to the maze, as the sample phase object (correct). The results from this task indicate not only that the animals used environmental cues to solve this task but that the animals were likely to use relationships among cues to identify the correct location. Because hippocampal-lesioned animals perform poorly on close separations, we were unable to test these animals on this probe test.
Spatial task
All animals reached a 75% correct criterion before the operation across all spatial distances and maintained this performance for 80 trials. The mean number of trials needed to learn this task to a 75% correct criterion was 300 for the CON group and 394 for the HIP group. A one-way ANOVA revealed no significant difference between the groups with regard to the number of trials to reach criterion.
The data were grouped into blocks of 80 trials for analysis. These included the criterion trials before the operation (PRE) and two sets of trials after the operation (POST 1 and POST 2). The data are shown in Figure 2A and indicate that the performance of the CON group after the operation (POST 1 and POST 2) matched their performance before the operation (PRE) across all spatial separations. In contrast, the data in Figure2B indicate that the HIP group was significantly impaired in trials after the operation (POST 1 and POST 2) across all spatial separations with the exception of the largest (105 cm) separation. The errors committed by the HIP group tended to be quite random with no particular pattern such as always choosing the object on the left, the nearest object, or a particular object, thus indicating that the deficit did not seem to be a result of perseverative-type behavior. Behaviorally, control and lesioned animals tended to quickly exit the start box, scan the row of food wells and objects, and then make a direct trajectory toward one of the objects. Based on the video recordings made after the operation, the CON animals tended to make a direct trajectory toward the chosen object on >90% of the trials. Hippocampal-lesioned animals also tended to make a direct trajectory on ∼90% of the trials. We believe that the reason for the straight trajectory is because the objects provide intramaze landmarks in conjunction with the extramaze cues to facilitate the cue-guided component of a navigational strategy, whereas in a task such as the water maze, these intramaze landmarks are not likely to be present.
Fig. 2.
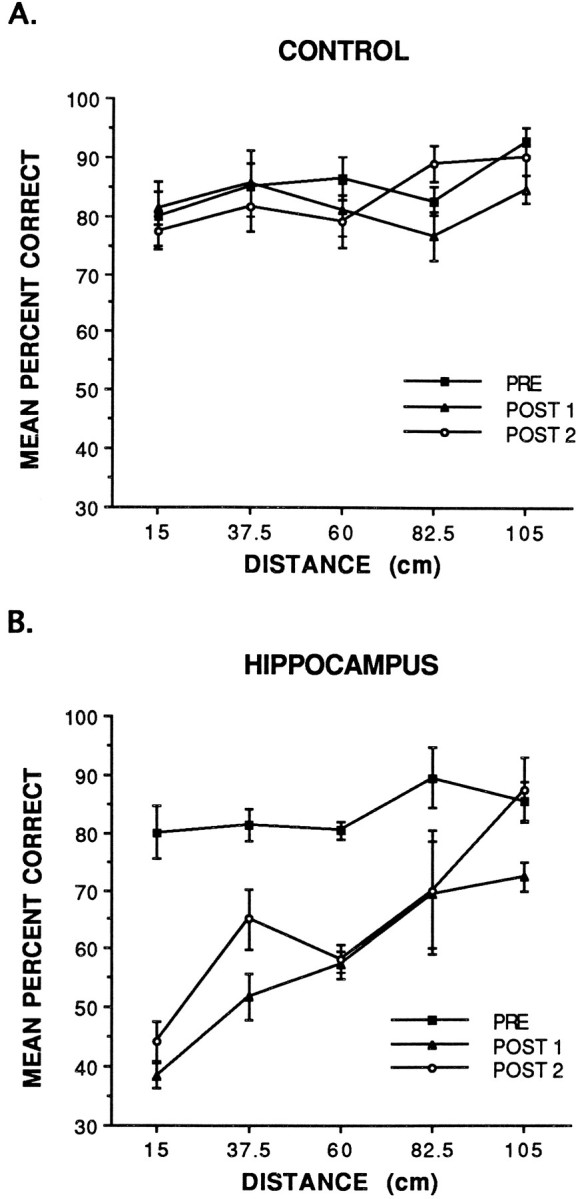
Mean percent correct performance of the cortical control lesion group (A) and the hippocampal lesion group (B) on trials before the operation (PRE) and two blocks of trials after the operation (POST 1 and POST 2).
A repeated-measures three-way ANOVA with lesion group (cortical control or hippocampus) as the between factor and block (PRE, POST 1, or POST 2) and distance (15, 37.5, 60, 82.5, or 105 cm) as the within factors revealed that there was a significant lesion effect [F(1,8) = 40.38; p < 0.001], a significant block effect [F(2,16) = 22.74;p < 0.001], and a significant distance effect [F(4,32) = 9.25; p < 0.001]. Furthermore, the analysis revealed a significant block × lesion interaction effect [F(2,16) = 13.40;p < 0.001], a significant distance × lesion interaction effect [F(4,32) = 3.11;p < 0.05], and a significant block × distance interaction effect [F(8,64) = 2.70;p < 0.05]. In addition, the analysis revealed a three-way block × distance × lesion interaction effect [F(8,64) = 4.36; p < 0.001].
A Newman–Keuls comparison test of the three-way block × distance × lesion interaction effect revealed that the PRE performance of the CON group did not differ significantly from the PRE performance of the HIP group. Furthermore, no significant differences were found between the PRE, POST 1, or POST 2 performances of the CON group with regard to block or distance. For the HIP group, the POST 1 and POST 2 performance across all distances, with the exception of 105 cm, was significantly different (p < 0.05) from the PRE performance of the HIP group on corresponding spatial distances. In contrast, the POST 1 and POST 2 performance of the HIP group on the 105 cm separation was not significantly different from the 105-cm-separation PRE performance of the HIP group or from the POST 1 and POST 2 performance of the CON group on the 105 cm separation. A Newman–Keuls comparison of the performance of the HIP group on POST 1 revealed that the performance on the 15 cm separation was significantly different (p < 0.05) from the performance on all other spatial separations for this group. The analysis also revealed that the performance of this group on the 105 cm separation was significantly different (p < 0.05) from all separations with the exception of the 82.5 cm separation. A Newman–Keuls analysis of POST 2 of the HIP group revealed that the performance on the 15 and the 105 cm separations was significantly different (p < 0.05) from the performance of this group on all other separations.
A linear trend analysis of the average performance of the HIP group after the operation across separations revealed a significant linear increase in performance as a function of spatial separation [F(1,4) = 10.03; p < 0.05]. Furthermore, of the five animals in the HIP group, four displayed a significant linear increase (p < 0.05) in performance as a function of increased spatial separation. The performance of the fifth animal was not statistically significant; however, it approached significance. It is critical to note that the performance of this particular animal was at chance across all separations with the exception of the 105 cm separation and, therefore, did not yield a significant linear trend. These data do not support the assumption that the deficits in the HIP group may be caused by an inability to form internal representations of the environment nor do they suggest that the animals may be solving the task based on a cue-approach strategy. Thus, the most parsimonious interpretation of the data is that hippocampal lesions are likely to decrease the efficiency of hippocampal pattern separation.
Transfer task
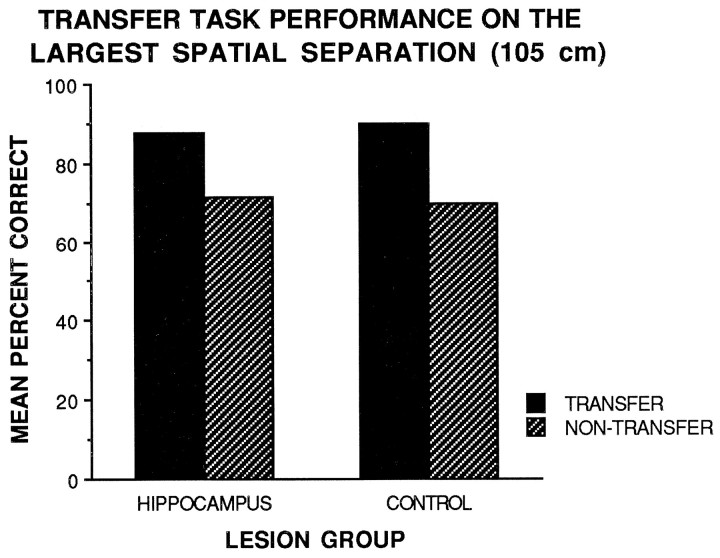
If an animal was indeed using a response strategy correctly, it is predicted that their performance would be lower than 50%, because making a turn during the test phase identical to the turn made during the sample phase would result in errors. However, the performance of the HIP and CON groups on the transfer task was above chance across all spatial separations. Figure3 shows that the HIP group matched the performance of the CON group on transfer and nontransfer 105-cm-separation trials. Furthermore, the performance of the HIP group on transfer task 105-cm-separation trials matched the performance of the group on nontransfer 105-cm-separation trials. A repeated-measures two-way ANOVA with lesion group (cortical control or hippocampus) as the between factor and task type (transfer or nontransfer) as the within factor revealed no significant main effects or interaction effects for the largest spatial separation (105 cm). The analysis revealed no significant difference between transfer and nontransfer 105-cm-separation trials for either group. Furthermore, the performance of the HIP group was not significantly different from the performance of the CON group on the 105 cm separation on either transfer or nontransfer trials. The results showed that the HIP group did not seem to be using a response strategy to solve the task at large spatial separations.
Fig. 3.
Mean percent correct performance of the cortical control and hippocampal lesion groups on transfer (solid bars) and nontransfer (hatched bars) task 105-cm-separation trials.
DISCUSSION
The results of the present study indicate that a function of the hippocampus may be to separate patterns of incoming spatial information and thus preserve the uniqueness of a memory representation. The results demonstrate that on short and medium separation trials (15–82.5 cm), with an increased overlap of distal cues and presumably increased spatial similarity among distal cues, hippocampal-lesioned animals were impaired. However, on trials with increased spatial separation (105 cm), less overlap of distal cues resulted in less similarity, and as a consequence the hippocampal-lesioned animals performed the task as well as the controls. The fact that the HIP group was able to perform the task well at large separations indicates that the deficits observed on 15–82.5 cm separations were not the result of an inability to remember the rule of the task and eliminates nonspecific sensory and motor deficits. Furthermore, this finding also indicates that the deficit was not simply caused by a deficit in working memory. The results suggest that the hippocampus may serve to separate patterns of incoming spatial information by temporarily storing one place separate from another place. It is proposed that hippocampal lesions result in a decrease in efficiency in pattern separation that may result in an impairment on trials with increased spatial similarity or interference among spatial working-memory representations. Similar spatial pattern separation deficits have been observed for new geographical information in patients with hippocampal damage caused by a hypoxic episode (Hopkins and Kesner, 1993).
Because it was not a certainty that the HIP group would perform the task poorly regardless of separation, the ability of this group to solve the task at the 105 cm separation raised the question of whether the group may have used an alternate strategy, such as an egocentric response strategy based on a right or left turn, to solve the task at large spatial separations. The results of the transfer task demonstrated that the HIP group did not seem to be using a response strategy to solve the task at large separations. The HIP group performed well on transfer task 105-cm-separation trials initially and maintained this performance throughout testing. There was no indication that the group simply altered their initial response strategy on transfer trials by making a turn in the choice phase opposite of the turn made in the sample phase to choose the correct object.
The ability of the HIP group to solve the task at large separations may be because of the reliance of the animals on one environmental cue, based on a single cue or an array of distal cues, to locate the correct place on the apparatus. On a 105-cm-separation trial, minimal overlap would exist among the distal cues, and it would be possible for an animal to identify the correct location based on a single distal cue. Thus, at large spatial separations, a hippocampal-lesioned animal could simply select a single environmental cue for the sample phase location and base its choice only on this cue. In contrast, on close separation trials, this strategy may not be available to hippocampal-lesioned animals because a single environmental cue may be common to both the correct and incorrect locations on the choice phase. The significant linear increase in performance as a function of increased spatial separation clearly demonstrates that the deficits observed in the HIP group on close separation trials were the result of decreased efficiency in pattern separation and not a cue-approach strategy deficit. If the animals were using a cue-approach strategy, a linear function would not be expected.
The results of the strategy probe tasks illustrate more clearly how animals use environmental cues to solve this task. Based on the probe tasks either completely conducted in the dark or with the choice phase conducted in the dark, it is clear that the animals rely on environmental cues to solve the task. Furthermore, even though spatial cues and ideothetic cues may be used when solving this task in the light, it is clear that ideothetic cues alone are not sufficient to accurately solve this task. The results of the third probe task, in which the maze was shifted to the left or to the right the distance of the separation, also demonstrate that the animals are relying on environmental cues to solve the task. In addition, the results also suggest that the animals may be using relationships among cues to solve the task.
It is also essential to demonstrate that the deficits observed in the HIP group were the result of a working-memory deficit and not simply a perceptual deficit. The finding of Long and Kesner (1996) that animals with hippocampal lesions could discriminate between simultaneously presented objects, separated by short spatial distances (2 or 7 cm), as well as controls demonstrated that hippocampal lesions do not impair the ability to perceive short distances. Therefore, it does not seem that the deficits observed in the HIP group were the result of a perceptual deficit. Because hippocampal-lesioned rats can clearly discriminate spatial distances, the inefficiency in the pattern separation problem manifests itself primarily in working memory.
Deficits in memory for proximal spatial locations, similar to those of the present study, were also found by McDonald and White (1995) who used a place preference procedure in an eight-arm maze. In this procedure, food is place at the end of one arm, and no food is placed at the end of another arm. In a subsequent preference task, normal rats prefer the arm that contains the food. In this study, fimbria–fornix-lesioned rats acquired the place preference task as quickly as did controls if the arm locations were opposite each other, but the fornix-lesioned rats were markedly impaired if the locations were adjacent to each other. It is likely that there would be greater overlap among distal cues when the locations were adjacent to each other rather than when separate; thus, inefficiency in spatial pattern separation may result in impairments on adjacent trials of this task. As mentioned previously, the results of Eichenbaum et al. (1990) may also indicate that decreased efficiency in pattern separation may be a key process deficiency in hippocampal-lesioned rats on the acquisition of spatial tasks.
The results of the present experiment offer evidence that complete lesions of the hippocampus may decrease efficiency in pattern separation, resulting in working-memory deficits on a spatial task. It has been suggested that specific subregions of the hippocampal system may actually serve as the mechanism for pattern separation (Marr, 1971;McNaughton and Nadel, 1990; Rolls, 1989, 1996; O’Reilly and McClelland, 1994; Shapiro and Olton, 1994). Rolls (1989) and Marr (1971) have suggested that pattern separation and vector orthogonalization may be a function of the dentate gyrus. Rolls’s (1989) model suggests that pattern separation takes place in the mossy fiber system that connects the granular cells of the dentate gyrus to CA3 pyramidal neurons. The separation of patterns is attributable to the low probability that any two CA3 neurons will receive mossy fiber synapses from a similar subset of dentate granular cells. The low probability of contact between dentate gyrus granular cells and pyramidal cells of CA3 facilitates pattern separation.
Based on the computational models, it is assumed that similar input patterns may activate similar subsets of neurons and dissimilar input patterns may activate dissimilar subsets of neurons. Furthermore, the input patterns, in the spatial task described in this paper, are assumed to be based on arrays of distal cues. If these two assumptions are true, then proximal spatial locations, based on similar cues, should result in similar input patterns, whereas distal locations should result in dissimilar input patterns. Rolls (1989) suggests that a function of the hippocampus may be to ensure that even though these similar, overlapping input patterns may activate similar populations of neurons within the dentate gyrus, they activate very dissimilar populations within CA3, hence preserving the uniqueness of each representation. A functional hippocampus may enable an animal to distinguish, in memory, between two proximal locations even though they share many common cues. However, when the hippocampus is damaged, the animal may no longer be able to distinguish, in memory, between the correct location and the foil when the two are proximal. Therefore, based on the assumptions of the models, there may be a firm parallel between spatial separation based on distal cues and pattern separation within layers of the hippocampus.
In support of Rolls’s (1989) model, preliminary results in our laboratory indicate that deficits were observed in animals with colchicine lesions to the dorsal dentate gyrus that completely mimicked the deficits observed in animals with large hippocampal lesions. Therefore, subregional specificity may exist within the hippocampus, and the mechanism for pattern separation may reside in the mossy fiber system connections between the dentate gyrus and CA3. The results of the present study are, however, essential to the examination of hippocampal pattern separation, because the results demonstrate that animals are able to solve the task when the separation is large, even though the entire hippocampus, including the dentate gyrus, is destroyed.
In conclusion, the present study represents the development of a paradigm to instantiate the role of the hippocampus in efficiently separating patterns of spatial information to decrease similarity between spatial cues, preserve the uniqueness of a memory representation, and thereby facilitate recall.
Footnotes
This work was supported by National Science Foundation Grant BNS 892-1532. We thank Robert Jones for his histological work and Jason Knight for assistance in conducting the experiment. We also thank Charles Shimp, Sheri Mizumori, Jeffrey Long, Thane Fremouw, and Michael Ragozzino for their helpful comments on this manuscript.
Correspondence should be addressed to Dr. Raymond P. Kesner, Department of Psychology, 502 SBS, University of Utah, Salt Lake City, UT 84112.
REFERENCES
- 1.Eichenbaum H. The hippocampal system and declarative memory in humans and animals: experimental analysis and historical origins. In: Schacter DL, Tulving E, editors. Memory systems. MIT; London: 1994. pp. 147–201. [Google Scholar]
- 2.Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins RO, Kesner RP. Memory for temporal and spatial distances for new and previously learned geographical information in hypoxic subjects. Soc Neurosci Abstr. 1993;19:1248. [Google Scholar]
- 4.Hunt ME, Kesner RP, Evans RB. Memory for spatial location: functional dissociation of entorhinal cortex and hippocampus. Psychobiology. 1994;22:186–194. [Google Scholar]
- 5.Johnson MK, Chalfonte BL. Binding complex memories: the role of reactivation and the hippocampus. In: Schacter DL, Tulving E, editors. Memory systems. MIT; London: 1994. pp. 311–350. [Google Scholar]
- 6.Kesner RP. Learning and memory in rats with an emphasis on the role of the hippocampal formation. In: Kesner RP, Olton DS, editors. Neurobiology of comparative cognition. Erlbaum; Hillsdale, NJ: 1990. pp. 179–204. [Google Scholar]
- 7.Long JM, Kesner RP. The effects of dorsal versus ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behav Neurosci. 1996;110:922–932. doi: 10.1037//0735-7044.110.5.922. [DOI] [PubMed] [Google Scholar]
- 8.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond [Biol] 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 9.McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- 10.McNaughton BL. Neural mechanisms for spatial computation and information storage. In: Nadel L, Cooper LA, Culicover P, Harnish RM, editors. Neural connection, mental computations. MIT; Cambridge, MA: 1989. pp. 285–350. [Google Scholar]
- 11.McNaughton BL, Nadel L. Hebb-Marr networks and the neurobiological representation of action in space. In: Gluck MA, Rumelhart DE, editors. Neuroscience and connectionist theory. Erlbaum; Hillsdale, NJ: 1990. pp. 1–63. [Google Scholar]
- 12.Milner B. Amnesia following operation on the frontal lobes. In: Whitty CWM, Zangwill OL, editors. Amnesia. Butterworths; London: 1966. pp. 109–133. [Google Scholar]
- 13.Nadel L. Multiple memory systems: what and why, an update. In: Schacter DL, Tulving E, editors. Memory systems. MIT; London: 1994. pp. 39–63. [Google Scholar]
- 14.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford; London: 1979. [Google Scholar]
- 15.Olton DS, Becker JT, Handelmann GH. Hippocampus, space and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- 16.O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 17.Rolls E. Functions of neuronal networks in the hippocampus and neocortex in memory. In: Byrne JH, Berry WO, editors. Neural models of plasticity: theoretical and empirical approaches. Academic; New York: 1989. pp. 240–265. [Google Scholar]
- 18.Rolls E. Spatial memory, episodic memory, and neural network functions in the hippocampus. In: Squire LR, Lindenlaub E, editors. The neurobiology of memory. Schattauer Verlag; New York: 1990. pp. 445–470. [Google Scholar]
- 19.Rolls E. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Schacter DL, Alpert NM, Savage CR, Rauch SL. Conscious recollection in the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory systems. MIT; London: 1994. pp. 141–146. [Google Scholar]
- 22.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]