Abstract
Rat pinealocytes, melatonin-secreting endocrine cells, contain peripheral glutaminergic systems. l-Glutamate is a negative regulator of melatonin synthesis through a metabotropic receptor-mediated inhibitory cAMP cascade. Previously, we reported that depolarization of pinealocytes by externally added KCl and activation of L-type Ca2+ channels resulted in secretion ofl-glutamate by microvesicle exocytosis. What is unknown is how and what kinds of stimuli trigger glutamate exocytosis under physiological conditions. Here, we report that the nicotinic acetylcholine receptor can trigger glutamate exocytosis from cultured rat pinealocytes. Moreover, acetylcholine or nicotine inhibited norepinephrine-dependent serotonin N-acetyltransferase activity, which results in decreased melatonin synthesis. These activities were blocked by (2S,3S,4S)-2-methyl-2-(carboxycyclopropyl)glycine, an antagonist of the metabotropic glutamate receptor. These results suggest that cholinergic stimulation initiates the glutaminergic signaling cascade in pineal glands and that parasympathetic neurons innervating the gland exert negative control over melatonin synthesis by way of the glutaminergic systems.
Keywords: microvesicle (synaptic-like microvesicle), acetylcholine, nicotinic acetylcholine receptor (nAchR), exocytosis, glutamate, pinealocyte, pineal gland, L-type Ca2+ channel, serotonin N-acetyltransferase, parasympathetic neuron, melatonin synthesis
The mammalian pineal gland is a photoneuroendocrine transducer that rhythmically synthesizes and secretes melatonin at night in response to photoperiodic stimuli and signals from endogenous circadian oscillators (for review, see Axelrod, 1974; Klein, 1985; Reiter, 1991). In the rat, this process is controlled by sympathetic neurons projecting into the glands. These neurons secrete norepinephrine (NE), which binds to adrenergic α1 and β1 receptors. Stimulation of β1 receptors causes increased intracellular cAMP leading to transcriptional activation of the serotonin N-acetyltransferase (NAT) gene, which results in increased melatonin output (for review, see Foulkes et al., 1997). Furthermore, stimulation of α1 receptors potentiates the activation effect of β1 receptors by increasing intracellular Ca2+concentration ([Ca2+]i) by release from intracellular stores (Klein, 1985). On the basis of this information, the role of sympathetic innervation as a positive regulatory mechanism for melatonin synthesis has been firmly established.
In addition to adrenergic pathways, there is evidence for other types of neuronal control of synthesis and secretion of melatonin (Reiter, 1991; Korf et al., 1996). For example, morphological and immunohistochemical evidence suggests parasympathetic innervation of mammalian pineal glands (for review, see Moller, 1992; Laitinen et al., 1995). These neurons may originate from the pterygopalatine ganglion and use acetylcholine as a neurotransmitter (Moller, 1992; Laitinen et al., 1995) to activate nicotinic and muscarinic acetylcholine receptors that have been identified in the pineal gland (Wada et al., 1989;Pujito et al., 1991a; Reuss et al., 1992; Stankov et al., 1993). After binding of acetylcholine to receptors, NE-dependent melatonin synthesis is markedly inhibited, which suggests a negative regulatory role of parasympathetic innervation (Pujito et al., 1991b; Stankov et al., 1993; Drijfhout et al., 1996). The mechanism by which acetylcholine inhibits melatonin synthesis is not known, and the question of whether muscarinic or nicotinic receptors participate in this inhibitory processes is still controversial.
Peripheral glutaminergic systems recently identified in pineal glands are also involved in negative regulation of melatonin synthesis (for review, see Moriyama et al., 1996). Pinealocytes secretel-glutamate through microvesicle-mediated exocytosis (Yamada et al., 1996a) to affect inhibition of NE-dependent melatonin synthesis. In this case, activation of metabotropic type 3 glutamate receptors (mGluR3) initiates an inhibiting cAMP cascade and results in decreased NAT activity (Yamada et al., 1998). Under in vitroexperimental conditions, glutamate exocytosis can be triggered by the addition of external KCl, which depolarizes the cell membrane and activates L-type Ca2+ channels (Yamada et al., 1996a,b; Yatsushiro et al., 1997). The in vivo stimuli that initiate glutamate exocytosis remain to be understood.
Recently, Korf and colleagues (Letz et al., 1997) found that acetylcholine will depolarize pinealocyte membranes by activation of nicotinic acetylcholine receptor (nAchR). Combining this observation with our recent findings (Yamada et al., 1996b), we hypothesized that acetylcholine- and glutamate-evoked signaling cascades overlap. Here, we report that in rat pineal glands acetylcholine triggers glutamate exocytosis via nAchR and inhibits NE-dependent melatonin synthesis through an inhibitory cAMP cascade. Our results suggest that parasympathetic neurons negatively control melatonin synthesis by way of endogenous glutaminergic systems in pineal glands.
MATERIALS AND METHODS
Cell culture. Cells were isolated from the pineal glands of Wistar rats at 7 weeks postnatal and were cultured by published procedures (Yamada et al., 1996a,b). Briefly, pineal glands were dissected into small pieces, treated with a 0.1% collagenase solution (Life Technologies, Gaithersburg, MD) at 37°C for 30 min with gentle shaking, and then washed with PBS. After centrifugation at 180 × g for 2 min, the pieces were treated with a 0.025% trypsin solution (Life Technologies) at 37°C for 20 min and then centrifuged at 180 × g for 5 min. The dispersed cells were washed three times with DMEM supplemented with 6% fetal calf serum, 55 mg/ml sodium pyruvate, 6 mg/ml glucose, 0.1 mg/ml streptomycin, 100 U/ml penicillin G, and 0.25 mg/l fungizone and placed in a culture dish (35-mm-diameter) coated with type I collagen (Corning, Corning, NY) at 1.3 × 106cells/dish. Cells were cultured in the above medium at 37°C under 10% CO2. More than 99.5% cells that became attached to the dish were pinealocytes, as shown by the positive reactivity toward anti-melatonin and anti-synaptophysin antibodies (Yamada et al., 1996a). The viability of the cells did not change after treatment with the various reagents used in this study, as judged by staining with 0.3% Trypan blue and vital staining with acridine orange.
Assay of glutamate exocytosis. Pinealocytes (1.3 × 106 cells/dish) were preincubated at 28°C for 30 min and then washed with Ringer’s solution composed of 128 mm NaCl, 1.9 mm KCl, 1.2 mmKH2PO4, 2.4 mmCaCl2, 1.3 mm MgSO4, 26 mm NaHCO3, 10 mm glucose, and 10 mm HEPES, pH 7.4, or −Ca2+-Ringer’s solution composed of 128 mm NaCl, 1.9 mm KCl, 1.2 mmKH2PO4, 0.2 mmCaCl2, 1 mm EGTA, 3.8 mmMgSO4, 26 mm NaHCO3, 10 mm glucose, and 10 mm HEPES, pH 7.4. After cells were incubated in 2 ml of the above medium at 28°C, exocytosis was stimulated by the addition of either 50 mm KCl (Yamada et al., 1996a) or 0.2 mm acetylcholine. When necessary, various Ca2+ channel agonists or antagonists were included in the incubation medium. Finally, 10 μl aliquots were taken at intervals, and the amount of extracellular glutamate was determined by HPLC with precolumn o-phthalaldehyde (OPA) derivatization, separation by a reverse-phase RESOLVE C18 column (3.9 × 150 mm; Waters Associates, Milford, MA), and fluorescence detection (Godel et al., 1984).
Treatment of pinealocytes with botulinum neurotoxin type E (BoNT/E). Pinealocytes were treated with BoNT/E by a procedure similar to that described previously (Yamada et al., 1996a;Yatsushiro et al., 1997). In brief, pinealocytes (1.3 × 106 cells/dish) were incubated at 37°C for 24 hr in a low ionic strength buffer consisting of 5 mm NaCl, 4.8 mm KCl, 2.2 mm CaCl2, 1.2 mm MgSO4, 20 mm HEPES-NaOH, pH 7.4, 5.6 mm glucose, 220 mm sucrose, and 0.5% bovine serum albumin in the presence or absence of either 15 nm or 50 nm BoNT/E. After treatment, cells were washed with fresh culture medium and incubated an additional 12 hr at 37°C. Finally, acetylcholine-evoked glutamate release was measured as described above.
Measurement of intracellular [Ca2+].Pinealocytes were cultured for 3 d on a thin glass coverslip precoated with type I collagen (thickness 0.12 mm and diameter 40 mm; 8.0 × 105 cells/coverslip) in the medium as described (Ogura et al., 1990). After changing the culture medium to DMEM, cells were treated with 5 μm fura-2 AM (Dojin Company) for 50 min at 37°C in DMEM and then washed twice with the same medium. The cells were perfused with warmed Ringer’s solution or −Ca2+-Ringer’s solution, and images were continuously taken at 28°C with a silicon-intensified target camera (Hamamatsu Photonics C-2400-08) at a sampling rate of 30 sec-1, and recorded on videotape. The output of the camera or of the videotape recorder (Sony V0–5850) was fed into a Hamamatsu DVS-3000 image analyzer. The software enabled subtraction of background fluorescence, pixel-to-pixel division of F340 × F360 images, fitting of the F340/F360 ratios to a [Ca2+] calibration curve prepared separately, and digital averaging of [Ca2+] in multiple cells.
Other procedures. Melatonin was measured by HPLC with an IRICA RP-18T column and an amperometric detector (E-558) as described by Sagara et al. (1988). NAT activity was measured as described byThomas et al. (1990). Immunoblotting and PAGE were performed as described previously (Moriyama and Yamamoto, 1995a,b). cAMP concentrations were measured with an Amersham cAMP enzyme-immunoassay kit (Amersham, Arlington Heights, IL), and protein was measured by the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA).
Materials. Antagonists and agonists of acetylcholine receptor were from Sigma (St. Louis, MO). 1,4-Dihydro-2,6-dimethyl-5-nitro-4[trifluoromethyl-phenyl]−3-pyridinecarboxylic acid methylester (BAY K8644) was obtained from Research Biochemicals International (Natick, MA). (2S,3S,4S)-2-methyl-2-(carboxycyclopropyl)glycine (MCCG), a specific antagonist for class II mGluRs (Sekiyama et al., 1996), was obtained from Tocris Neuramin. BoNT/E was kindly provided by Professor S. Kozaki (Osaka Prefecture University). Monoclonal antibodies against synaptic vesicle-associated protein 25 (SNAP25) (BR05) and polyclonal antibodies against synaptobrevin 2 (VAMP2) were obtained from Wako Chemicals (Osaka, Japan). Other chemicals were of the highest grade commercially available.
RESULTS
Acetylcholine-evoked l-glutamate exocytosis
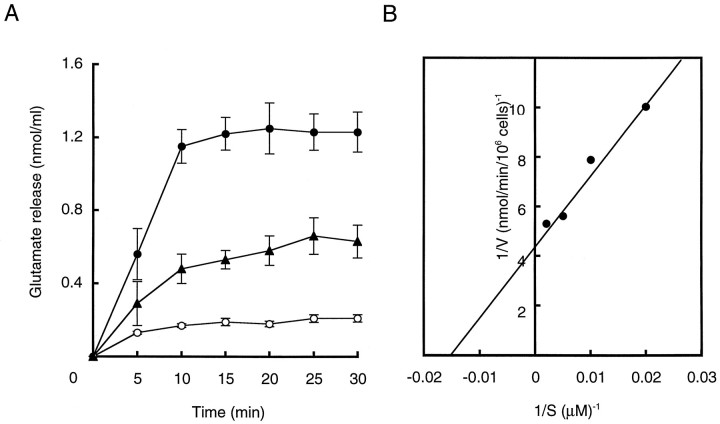
Our previous studies indicated that l-glutamate in microvesicles was exocytized from pinealocytes after depolarization of the cell membrane evoked by the addition of 50 mm or more KCl (Yamada et al., 1996a). A rise of intracellular [Ca2+] via voltage-gated L-type Ca2+ channels is responsible for the secretion process (Yamada et al., 1996b). We report here that acetylcholine can also stimulate appreciable amounts of l-glutamate exocytosis (Fig. 1A). The rate of secretion increases in a saturable manner with increasing concentrations of acetylcholine with a calculated Km value of 63 μm (Fig. 1B). Approximately 1.8 nmol of glutamate was released from 106 cells after a 10 min incubation with acetylcholine. Acetylcholine-induced glutamate secretion was dependent on temperature: release was observed at 28°C, decreased gradually with decreasing temperature, and disappeared below 18°C (Fig. 1A). Above 32°C, pinealocytes became sensitive to mechanical stimulation such as medium replacement and readily released glutamate even in the absence of acetylcholine. At 28°C, essentially no glutamate was released by mechanical stimulation such as medium replacement. Because of this behavior, subsequent experiments were performed at 28°C to ensure quantitative secretion measurements. Involvement of either eserine or neostigmine at 50 μm, inhibitors of acetylcholine esterase, did not affect the acetylcholine-induced glutamate secretion. This excludes a possibility that the enzyme perturbed the acetylcholine effect under the assay condition. After a round of glutamate release, successive additions of acetylcholine did not induce further secretion. The ability to secrete glutamate gradually returned during a 12 hr period (data not shown). These properties were consistent with those of KCl-evoked glutamate secretion (Yamada et al., 1996a), suggesting that acetylcholine-evoked glutamate secretion is also mediated through microvesicle exocytosis.
Fig. 1.
Characterization of acetylcholine-evokedl-glutamate secretion from cultured pinealocytes.A, Glutamate concentration in the medium at the indicated times after the addition of 200 μmacetylcholine was monitored as described in Materials and Methods. Cells were incubated at 28°C (•), 22°C (▴), or 18°C (○). Results were expressed as mean ± SEM (4 independent experiments). B, Lineweaver-Burk plot of acetylcholine dose-dependence after 10 min. The derived Kmand Vmax values were 63 μm and 0.22 nmol/min per 106 cells, respectively.
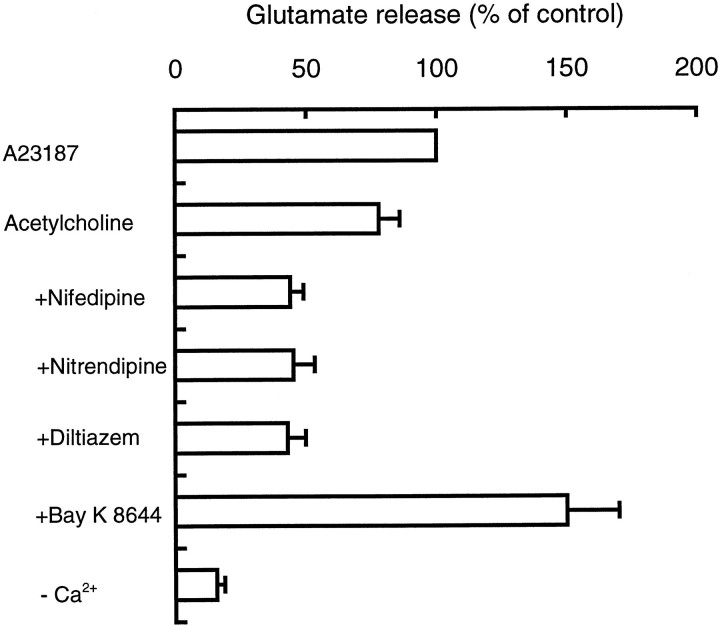
We conducted two lines of experiments to show that acetylcholine-evoked exocytosis involved microvesicles. First, we assessed the role of Ca2+ (Fig. 2). Removal of Ca2+ from the medium reduced acetylcholine-evoked glutamate secretion by 80%. Diltiazem, nifedipine, and nitrendipine, L-type Ca2+ channel blockers (Tsien et al., 1988), inhibited ∼50% of acetylcholine-evoked glutamate release, whereas BAY K8644, an L-type Ca2+ channel agonist (Bellemann and Frankowiak, 1985; Nowycky et al., 1985), stimulated secretion twofold over the acetylcholine response. These results strongly suggest the involvement of L-type Ca2+ channels in the secretion mechanism.
Fig. 2.
Involvement of L-type Ca2+channels in acetylcholine-evoked glutamate secretion. Cells were treated with the indicated L-type Ca2+ channel antagonists (20 μm each) or BAY K8644 (1 μm) for 10 min and washed once with medium. Cells were then stimulated with 200 μm acetylcholine for 10 min, and aliquots of medium were taken for determination of glutamate. The results were expressed as relative values ± SEM (three independent experiments). The amount of glutamate released on addition of 5 μm Ca2+ ionophore A23187 (2.2 nmol glutamate/106 cells) was taken as 100%, and glutamate secretion in the absence of Ca2+ in the medium (−Ca2+-Ringer’s solution) is shown to indicate background levels (control levels).
Second, we assessed participation of SNAP25, a synaptic vesicle protein that is a component of the SNARE (SNAP receptor) complex (Damer and Creutz, 1994; Rothman, 1994; Scheller, 1995; Südhof, 1995). Botulinum toxin cleaves SNAP25 and interferes with microvesicle docking and fusion during the exocytotic process (Yamada et al., 1996b;Yatsushiro et al., 1997). Indeed, immunoblot analysis using SNAP25 antibodies shows that BoNT/E treatment of pinealocytes resulted in cleaved SNAP25 without affecting VAMP2 (Fig.3, top panel). Significantly, BoNT/E treatment also resulted in inhibited acetylcholine-evoked glutamate secretion in a toxin concentration-dependent manner (Fig. 3). Together with the results indicating the role of Ca2+, these results are fully consistent with the properties of microvesicle exocytosis (Yamada et al., 1996a,b) and strongly suggest that acetylcholine triggers glutamate secretion through this process.
Fig. 3.
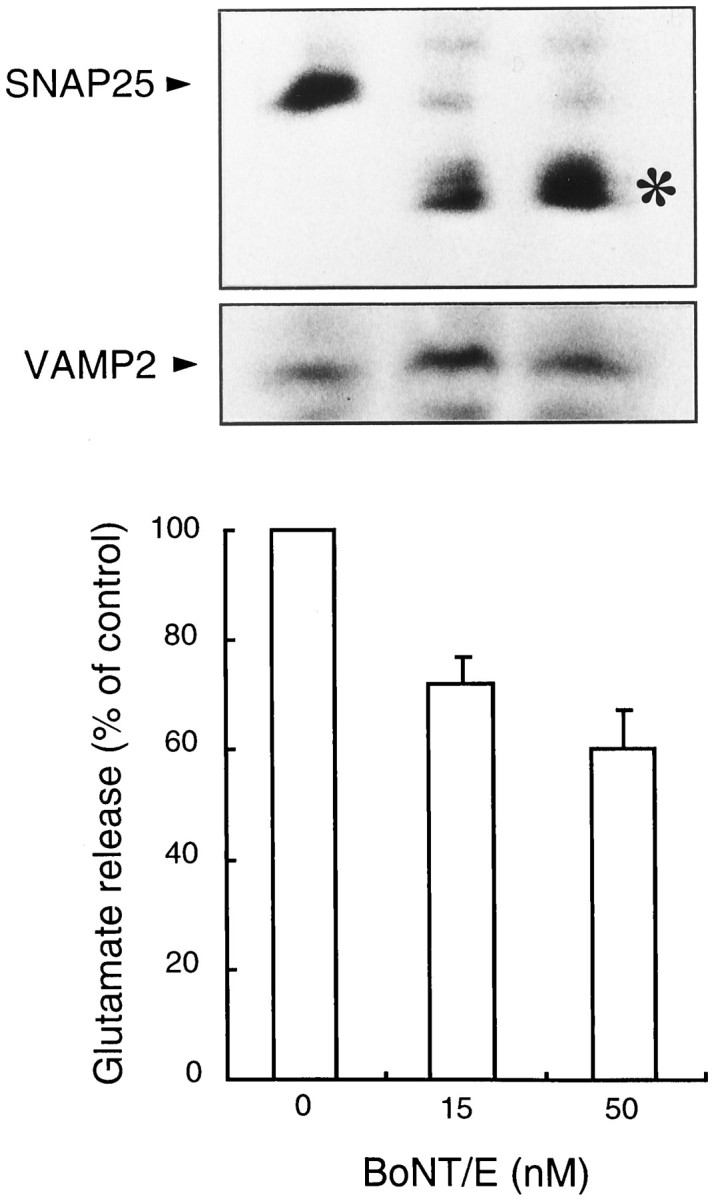
Effects of BoNT/E on acetylcholine-evoked glutamate secretion. Cells were treated with BoNT/E at the indicated concentrations as described in Materials and Methods. After treatment, acetylcholine-evoked glutamate release for 10 min was determined. Results in the bottom panel are expressed as relative values ± SEM (4 independent experiments); 100% value is 1.8 nmol glutamate/106 cells. In the top panel, the same cells were washed with PBS containing DNase and dissolved in sample buffer containing 10% SDS and β-mercaptoethanol. After dissociation of the proteins, samples were separated over a 13% polyacrylamide gel in the presence of SDS and analyzed by immunoblotting using antibodies against SNAP-25 and VAMP2 as indicated. The position of cleaved SNAP-25 is indicated by *.
Involvement of nAchR in glutamate secretion
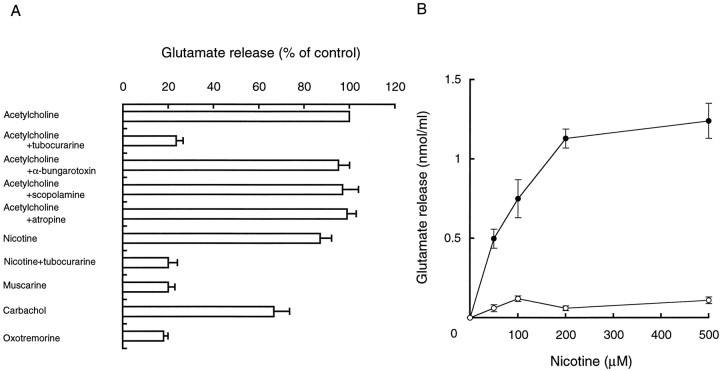
Acetylcholine binds to both nicotinic and muscarinic acetylcholine receptors. To determine which type of receptor is involved in pinealocyte glutamate secretion, pharmacological analysis was conducted. As shown in Figure4A, glutamate secretion was triggered by the addition of nicotine but not muscarine: theKm and Vmax values for nicotine were 77 μm and 0.21 nmol/min per 106 cells, respectively (Fig. 4B). Both nicotine- and acetylcholine-evoked glutamate secretion were inhibited 80% by the addition of tubocurarine, a competitive inhibitor of the nAchR, whereas scopolamine and atropine, antagonists of the muscarinic receptor (Pujito et al., 1991b), or α-bungarotoxin, a selective inhibitor for some subtypes of nAchRs, were ineffective (Fig.4A). Carbachol at 0.2 mm, an agonist for both muscarinic and nicotinic receptors (Pujito et al., 1991b), stimulated secretion. Neither scopolamine nor atropine at 400 μm affected the carbachol-induced glutamate secretion (data not shown). These results specify that an α-bungarotoxin-insensitive nAchR is responsible for glutamate secretion.
Fig. 4.
Pharmacological (A) and nicotine (•) and muscarine (○) dose-dependent (B) effects on glutamate secretion in the presence or absence of acetylcholine receptor antagonists at 10 min were determined as described in the legend of Figure 1. Results are expressed as relative values (A) or means (B) ± SEM (4 independent experiments); 100% activity is 1.7 nmol glutamate/106 cells. Reagents at the following concentrations were used: acetylcholine and receptor agonists, 200 μm; tubocurarine, scopolamine and atropine, 400 μm; and α-bungarotoxin, 100 nm.
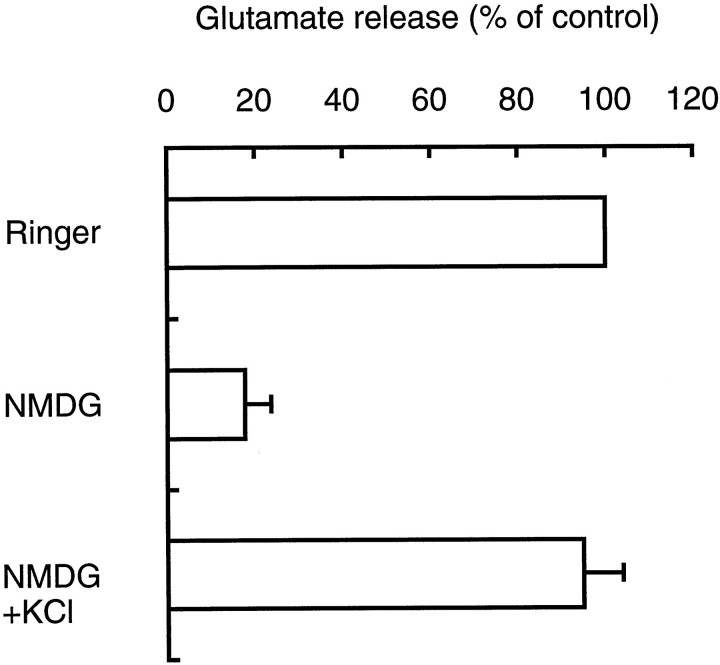
The requirement for extracellular Na+ will also distinguish nicotinic and muscarinic receptors. Replacement of Na+ with the nonpermeating cationN-methyl-d-glucamine (NMDG) (Letz et al., 1997) significantly decreased acetylcholine-evoked glutamate secretion (Fig.5). The same pinealocytes exhibited normal levels of KCl-evoked glutamate secretion, indicating that the cells retain microvesicle exocytosis capability. Because Na+ is required for nAchR function (see below), these results further supported the involvement of nAchR but not muscarinic receptor in acetylcholine-evoked glutamate secretion.
Fig. 5.
Na+ requirement for acetylcholine-evoked glutamate secretion. Cells were incubated in Ringer’s solution (Ringer) or Ringer’s solution containing NMDG (NMDG). The acetylcholine-evoked glutamate released at 10 min was determined. In NMDG + KCl, 50 mm KCl was added in Ringer’s solution containing NMDG to initiate glutamate secretion. Results are expressed as relative values ± SEM (4 independent experiments); 100% activity is 1.8 nmol glutamate/106 cells.
Functional coupling between nAchR and L-type Ca2+ channel
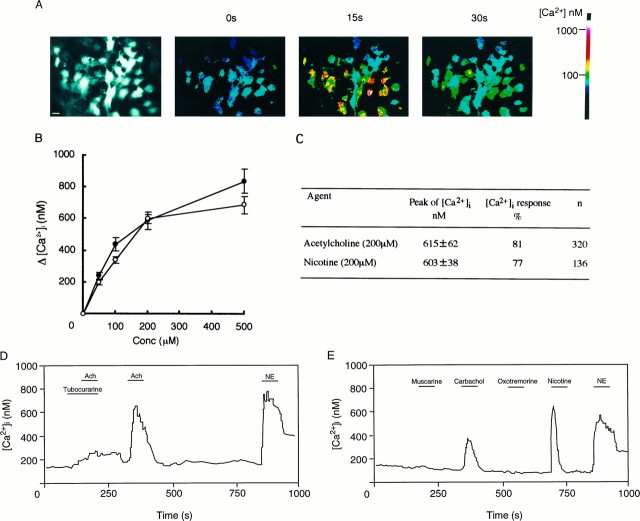
After activation, the nAChR channel increases Na+ conductance and depolarizes the pinealocyte membrane (Letz et al., 1997). Depolarization activates L-type Ca2+ channels, and the resultant increase of intracellular [Ca2+] should trigger microvesicle exocytosis. To demonstrate that the above cascade is operating in our experimental conditions, we observed acetylcholine- or nicotine-evoked intracellular [Ca2+] transients in fura-2 AM-loaded pinealocytes in the presence or absence of various channel blockers. Either acetylcholine or nicotine evoked increased intracellular [Ca2+] in pinealocytes in a dose-dependent manner (Fig.6A–C). ApparentKm values for acetylcholine and nicotine are 86 and 90 μm, respectively (Fig. 6B). A rise of intracellular [Ca2+] required extracellular Ca2+ and was inhibited by L-type Ca2+ channel blockers such as nifedipine (data not shown). Removing Na+ from the medium also blocked the acetylcholine effect (data not shown), as did tubocurarine (Fig.6D). Although carbachol stimulated increased intracellular [Ca2+], no muscarinic acetylcholine receptor agonists caused increased intracellular [Ca2+] (Fig. 6E). Essentially the same effects on the requirement of extracellular Na+ and Ca2+ and on inhibitor sensitivities were observed in the case of nicotine-evoked [Ca2+] transients (data not shown). These results were consistent with the previous observations by Letz et al. (1997). We conclude that nAchR and L-type Ca2+ channel are functionally coupled in pinealocytes.
Fig. 6.
Acetylcholine or nicotine increases intracellular [Ca2+] in pinealocytes. A, Typical intracellular [Ca2+] transients as monitored by fura-2 AM fluorescence. A fluorescence micrograph was shown to indicate fura-2 AM-loaded pinealocytes (left). At 0 time, acetylcholine (200 μm) was added and the change in fura-2 AM fluorescence within individual pinealocytes was followed.B, Dose dependencies of acetylcholine (•) and nicotine (○) are shown. Results are expressed as means of peak values at ∼15 sec ± SEM obtained from 20 cells. C, The majority of pinealocytes respond after the addition of acetylcholine or nicotine (200 μm each). D, E, Traces of fura-2 AM fluorescence indicating intracellular [Ca2+] transients from a single pinealocyte. The following compounds were added as indicated by the bars: 200 μmacetylcholine, 200 μm nicotine, 1 μm NE, 400 μm tubocurarine, 200 μm muscarine, 200 μm carbachol, or 200 μm oxotremorine.
Acetylcholine-evoked inhibition of melatonin synthesis
We showed previously that l-glutamate secreted from pinealocytes may inhibit NE-dependent melatonin synthesis via the mGluR3-mediated inhibitory cAMP cascade (Yamada et al., 1998). As a final step of the study, we tested whether stimulation of nAchR would result in inhibition of NE-dependent melatonin synthesis as well.
As expected, either acetylcholine or nicotine strongly inhibited NE-dependent melatonin synthesis with 50% inhibition attained at 75 and 60 μm, respectively (Table1). Muscarine at 0.2 mm had only a slight effect. The nicotine-evoked inhibition was prevented by the treatment of 0.4 mm tubocurarine. Consistent with the glutaminergic signaling cascade, either acetylcholine or nicotine also inhibited NE-dependent NAT activity (Table 1). Inhibition of melatonin synthesis and NAT activity were recovered to control levels after treatment with 2 mm dibutylyl cAMP, a nonhydrolyzable cAMP analog (data not shown). Furthermore, the acetylcholine- or nicotine-evoked inhibition was blocked when pineal glands were treated with MCCG, a specific antagonist of class II mGluR. This compound also blocked glutamate action in the pineal gland (Yamada et al., 1998) (Table 1). These results clearly showed that the class II mGluR-mediated inhibitory cAMP cascade is involved in acetylcholine-evoked inhibition of melatonin synthesis.
Table 1.
Effects of AchR agonists on the NE-dependent increase of NAT activity and melatonin production
| Additions | Melatonin production (%) | NAT activity (%) |
|---|---|---|
| Experiment 1 | ||
| Control | 100 | 100 |
| Acetylcholine | ||
| 50 μm | 60.4 ± 2.9 | 65.3 ± 4.4 |
| 100 μm | 43.3 ± 3.8 | 51.3 ± 3.2 |
| 200 μm | 25.4 ± 1.8 | 32.6 ± 4.9 |
| 500 μm | 27.4 ± 0.9 | 32.7 ± 2.5 |
| Acetylcholine + tubocurarine | 86.3 ± 6.3 | 84.0 ± 7.9 |
| Acetylcholine + MCCG | 58.9 ± 8.3 | 79.0 ± 6.9 |
| Experiment 2 | ||
| Control | 100 | 100 |
| Nicotine | ||
| 50 μm | 53.2 ± 7.6 | 61.5 ± 5.8 |
| 100 μm | 40.1 ± 4.9 | 43.1 ± 6.2 |
| 200 μm | 26.3 ± 5.9 | 24.1 ± 2.9 |
| 500 μm | 28.4 ± 1.3 | 31.0 ± 2.7 |
| Muscarine | 76.3 ± 3.2 | 82.7 ± 2.4 |
| Carbachol | 53.4 ± 2.9 | 44.9 ± 4.2 |
| Oxotremorine | 73.0 ± 5.2 | 70.6 ± 3.8 |
| Nicotine + tubocurarine | 79.4 ± 8.3 | 65.8 ± 4.8 |
| Nicotine + MCCG | 72.8 ± 6.4 | 60.7 ± 6.4 |
Pineal glands (3 glands per experiment) were incubated in 1 ml of DMEM for 1 hr. After glands were washed with DMEM, 10 μmNE (experiments 1 and 2) plus the indicated compound (200 μm each, unless stated otherwise) were added. In some cases, organs were preincubated with 2 mm MCCG or 400 μm tubocurarine for 30 min, and then the indicated compounds were added. After further incubation for 6 hr, the medium was collected and melatonin content was determined. Simultaneously, glands were homogenized and NAT activity was assayed. The results are presented as relative activity ± SEM (4 independent experiments) with 100% activity of 0.60 and 0.65 nmol/ml of melatonin synthesis, and 2.4 and 2.1 nmol · min−1 · mg−1 protein of NAT activity in experiments 1 and 2, respectively. Neither acetylcholine nor AchR agonists affected the level of these activities in the absence of NE. In experiment 1, 50 μm eserine was also included in the assay medium.
DISCUSSION
In this study, we report that acetylcholine triggers glutamate exocytosis from rat pinealocytes by way of nAchR and L-type Ca2+ channels. The data presented here and in previous reports are consistent with the following signaling cascade: activation of nAchR depolarizes the pinealocyte membrane by increasing Na+ conductance. Depolarization activates L-type Ca2+ channels resulting in increased intracellular [Ca2+], which in turn triggers microvesicle exocytosis. Secreted glutamate activates mGluR3 receptors leading to inhibited NE-dependent melatonin synthesis (Yamada et al., 1998). Although the physiological significance of nAchR has yet to be established, the results reported here clearly indicate functional coupling between nAchR and L-type Ca2+ channels and participation in the glutaminergic inhibitory mechanism of melatonin synthesis in pineal glands.
To our knowledge, this is the first example of nAchR regulation of glutamate secretion in endocrine cells. Enhancement of neurotransmitter release on stimulation of nAchR has been observed in various neurons. nAchRs are classified into two categories based on sensitivity to nicotine and α-bungarotoxin (for review, see Role and Berg, 1996). One class requires relatively high concentrations of nicotine (∼100 μm) for activation and is insensitive to α-bungarotoxin, whereas the other type activates at low nicotine concentrations (<1 μm) and is sensitive to α-bungarotoxin. Glutamate secretion in pinealocytes seems to be of the former type, because a high concentration of nicotine was required for activation and the receptor was insensitive to α-bungarotoxin (Fig. 4). This is consistent with in situ hybridization studies reporting the presence of α-bungarotoxin-insensitive α3β2 subunits in the pineal gland (Wada et al., 1989).
Recently, Pujito et al. (1991b) and Drijfhout et al. (1996) reported that muscarinic receptor agonists strongly inhibited melatonin synthesis in rat pineal gland; however, such inhibition was not observed in our hands (Table 1). Muscarinic receptor agonists appear to target presynaptic membranes and block NE release from sympathetic neurons. This effect causes decreased melatonin output (Drijfhout et al., 1996). Such muscarinic agonist action would not be observed in our assay conditions because exogenous NE was included to ensure full melatonin output. It is reasonable that acetylcholine has alternative pathways to inhibit NE-dependent melatonin synthesis. One pathway involves presynaptic modulation of NE release through muscarinic acetylcholine receptors (Drijfhout et al., 1996) and the other involves nAchR-mediated glutamate-evoked inhibitory cascade as shown here in pinealocytes.
We showed that cholinergic and glutaminergic signaling pathways overlap on the way to the end point of decreased melatonin output. Our conclusion immediately raises the fascinating hypothesis that the CNS is responsible for negative regulation of melatonin synthesis. Either parasympathetic neurons innervating the gland or cholinergic interneurons present in the gland (Pujito et al., 1991a; Wessler et al., 1997) may participate in regulation of the melatonin synthesis. Clearly, more extensive studies are necessary.
In summary, we presented evidence that acetylcholine initiates an intrinsic glutaminergic system via nAchR to inhibit melatonin synthesis in pineal glands. This finding may define the major physiological role of nicotinic acetylcholine receptors in the endocrine system.
Footnotes
H.Y. is supported by a research fellowship from the Japan Society for Promotion of Science. This study was supported in part by grants from the Japanese Ministry of Education, Science and Culture. We thank Professor H.-W. Korf for valuable discussion, Professor R. K. Nakamoto (University of Virginia) for critical reading of this manuscript, and Professor S. Kozaki for providing BoNT/E.
Correspondence should be addressed to Dr. Y. Moriyama, Department of Cell Membrane Biology, Institute of Scientific and Industrial Research (ISIR), Osaka University, Ibaraki, Osaka 567, Japan.
REFERENCES
- 1.Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974;184:1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- 2.Bellemann P, Frankowiak G. Different receptor affinities of enantiomers of Bay K 8644, a dihydropyridine calcium channel activator. Eur J Pharmacol. 1985;118:187–194. doi: 10.1016/0014-2999(85)90680-6. [DOI] [PubMed] [Google Scholar]
- 3.Damer CK, Creutz CE. Secretory and synaptic vesicle membrane proteins and their possible roles in regulated exocytosis. Prog Neurobiol. 1994;43:511–536. doi: 10.1016/0301-0082(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 4.Drijfhout WJ, Grol CJ, Westerink BHC. Parasympathetic inhibition of pineal indole metabolism by prejunctional modulation of noradrenaline release. Eur J Pharmacol. 1996;308:117–124. doi: 10.1016/0014-2999(96)00283-x. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes NS, Borjigin J, Snyder SH, Sassone-Corsi P. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997;20:487–492. doi: 10.1016/s0166-2236(97)01109-0. [DOI] [PubMed] [Google Scholar]
- 6.Godel H, Graser T, Foeldi P, Fuerst P. Measurements of free amino acids in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1984;297:49–61. doi: 10.1016/s0021-9673(01)89028-2. [DOI] [PubMed] [Google Scholar]
- 7.Klein DC. Photoneural regulation of the mammalian pineal gland (Ciba Foundation Symposium 117): Photoperiodism, melatonin, and the pineal gland (Evered D, and Clark S, eds), pp 38–56. Pitman; London: 1985. [DOI] [PubMed] [Google Scholar]
- 8.Korf H-W, Schomerus C, Maronde E, Stehle JH. Signal transduction molecules in the rat pineal organ: Ca2+, pCREB, and ICER. Naturwissenshaften. 1996;83:535–543. doi: 10.1007/BF01141978. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen JT, Laitinen KSM, Kokkola T. Cholinergic signaling in the rat pineal gland. Cell Mol Neurobiol. 1995;15:177–192. doi: 10.1007/BF02073327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letz B, Schomerus C, Maronde E, Korf H-W, Korbmacher C. Stimulation of a nicotinic ACh receptor causes depolarization and activation of L-type Ca2+ channels in rat pinealocytes. J Physiol (Lond) 1997;499:320–340. doi: 10.1113/jphysiol.1997.sp021930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller M. Fine structure of pinealopetal innervation of the pineal gland. Microsc Res Tech. 1992;21:188–204. doi: 10.1002/jemt.1070210303. [DOI] [PubMed] [Google Scholar]
- 12.Moriyama Y, Yamamoto A. Microvesicles isolated from bovine pineal gland specifically accumulate L-glutamate. FEBS Lett. 1995a;367:233–236. doi: 10.1016/0014-5793(95)00559-r. [DOI] [PubMed] [Google Scholar]
- 13.Moriyama Y, Yamamoto A. Vesicular L-glutamate transporter in microvesicles from bovine pineal glands. J Biol Chem. 1995b;270:22314–22320. doi: 10.1074/jbc.270.38.22314. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama Y, Yamamoto A, Yamada H, Tashiro Y, Futai M. Role of endocrine cell microvesicles in intercellular chemical transduction. Biol Chem. 1996;377:155–165. doi: 10.1515/bchm3.1996.377.3.155. [DOI] [PubMed] [Google Scholar]
- 15.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–449. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 16.Ogura A, Myojo Y, Higashida H. Bradykinin-evoked acetylcholine release via inositol triphosphate-dependent elevation in free calcium in neuroblastoma x glioma hybrid NG108–15 cells. J Biol Chem. 1990;265:3577–3584. [PubMed] [Google Scholar]
- 17.Pujito PP, Mikkelsen JD, Govitrapong P, Moller M. A cholinergic innervation of the bovine pineal gland visualized by immunohistochemical detection of choline acetyltransferase-immunoreactive nerve fibers. Brain Res. 1991a;545:49–58. doi: 10.1016/0006-8993(91)91268-6. [DOI] [PubMed] [Google Scholar]
- 18.Pujito PP, Govitrapong P, Ebadi M. Inhibitory actions of muscarinic cholinergic receptor agonists on serotonin N-acetyltransferase in bovine pineal explants in culture. Neurochem Res. 1991b;16:885–889. doi: 10.1007/BF00965537. [DOI] [PubMed] [Google Scholar]
- 19.Reiter J (1991) Pineal gland: interaction between the photoperiodic environment and the endocrine system. Trends Endocrinol Metab 13–19. [DOI] [PubMed]
- 20.Reuss S, Schroder B, Schroder H, Maelicke A. Nicotinic cholino-ceptors in the rat pineal gland as analyzed by western blot, light- and electron microscopy. Brain Res. 1992;573:114–118. doi: 10.1016/0006-8993(92)90119-t. [DOI] [PubMed] [Google Scholar]
- 21.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 22.Rothman JE. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 23.Sagara Y, Okatani Y, Yamanaka S, Kiriyama T. Determination of plasma 5-hydroxytryptophan, 5-hydroxytryptamine, 5-hydroxyindoleacetic acid, tryptophan, and melatonin by HPLC with electrochemical detection. J Chromatogr. 1988;431:170–176. doi: 10.1016/s0378-4347(00)83081-9. [DOI] [PubMed] [Google Scholar]
- 24.Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 25.Sekiyama N, Hayashi Y, Nakanishi S, Jane DE, Tse H-W, Birse EF, Watkins JC. Structure-activity relationship of new agonists and antagonists of different metabotropic glutamate receptor subtypes. Br J Pharmacol. 1996;117:1493–1503. doi: 10.1111/j.1476-5381.1996.tb15312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stankov B, Cimino M, Marini P, Lucini V, Fraschini F, Clementi F. Identification and functional significance of nicotinic cholinergic receptors in the rat pineal gland. Neurosci Lett. 1993;156:131–134. doi: 10.1016/0304-3940(93)90456-u. [DOI] [PubMed] [Google Scholar]
- 27.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 28.Thomas KB, Zawilska J, Iuvone PM. Arylalkylamine (serotonin) N-acetyltransferase assay using high-performance liquid chromatography with fluorescence or electrochemical detection of Nacetyltryptamine. Anal Biochem. 1990;184:228–234. doi: 10.1016/0003-2697(90)90673-w. [DOI] [PubMed] [Google Scholar]
- 29.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 30.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 31.Wessler I, Reinheimer T, Bittinger F, Kirkpatrick CJ, Schenda J, Vollath L. Day-night rhythm of acetylcholine in the rat pineal gland. Neurosci Lett. 1997;224:173–176. doi: 10.1016/S0304-3940(97)00165-1. [DOI] [PubMed] [Google Scholar]
- 32.Yamada H, Yamamoto A, Yodozawa S, Kozaki S, Takahashi M, Michibata H, Morita M, Furuichi T, Mikoshiba K, Moriyama Y. Microvesicle-mediated exocytosis of glutamate is a novel paracrine-like chemical transduction mechanism and inhibits melatonin secretion in rat pinealocytes. J Pineal Res. 1996a;21:175–191. doi: 10.1111/j.1600-079x.1996.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, Yamamoto A, Takahashi M, Michibata H, Kumon H, Moriyama Y. The L-type Ca2+ channel is involved in microvesicle-mediated glutamate exocytosis from rat pinealocytes. J Pineal Res. 1996b;21:165–174. doi: 10.1111/j.1600-079x.1996.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamada H, Yastushiro S, Ishio S, Hayashi M, Nishi T, Yamamoto A, Futai M, Yamaguchi A, Moriyama Y. Metabotropic glutamate receptors negatively regulate melatonin synthesis in rat pinealocytes. J Neurosci. 1998;18:2056–2062. doi: 10.1523/JNEUROSCI.18-06-02056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatsushiro S, Yamada H, Kozaki S, Kumon H, Michibata H, Yamamoto A, Moriyama Y. L-Aspartate but not the D form is secreted through microvesicle-mediated exocytosis and is sequestered through Na+-dependent transporter in rat pinealocytes. J Neurochem. 1997;69:340–347. doi: 10.1046/j.1471-4159.1997.69010340.x. [DOI] [PubMed] [Google Scholar]