Abstract
In cultured nerve cord explants from the crayfish (Procambarus clarkii), the normal impulse activity levels of growing motor axons determine their response to Ca2+ influx. During depolarization or Ca2+ ionophore application, normally active tonic motor axons continue to grow, whereas inactive phasic motor axons retract and often degenerate. To determine the role of Ca2+ regulation in this difference, we measured the intracellular free Ca2+ concentration ([Ca2+]i) with fura-2. Growth cones from tonic axons normally had a higher [Ca2+]i than those from phasic axons. When depolarized with 60 mm K+, growth cones and neurites from phasic axons had a [Ca2+]i three to four times higher than did those from tonic axons. This difference in Ca2+ regulation includes greater Ca2+-handling capacity for growing tonic axons; the increase in [Ca2+]i produced by the Ca2+ ionophore 4-bromo-A23187 (0.25 μm) is four to five times greater in phasic than in tonic axons, and the decline in [Ca2+]i at the end of a depolarizing pulse is three to four times faster in tonic axons than phasic ones. Blocking impulses in growing tonic axons for 2–3 d with tetrodotoxin reduces their capacity to regulate [Ca2+]i. Thus, growing tonic and phasic axons have differences in Ca2+ regulation that develop as a result of their different activity levels. These activity-dependent differences in Ca2+ regulation influence axon growth and degeneration and probably influence other neuronal processes that are mediated by changes in [Ca2+]i.
Keywords: calcium regulation, activity-dependent, fura-2, cell culture, growth cones, crayfish
Intracellular Ca2+ is important in the control of many neuronal processes, such as transmitter release (Katz, 1969), membrane excitability (Turrigiano et al., 1994), gene transcription (Morgan and Curran, 1991), and neuron growth (Kater and Mills, 1991) and survival (Nishi and Berg, 1981). The regulation of intracellular free Ca2+ concentration ([Ca2+]i) involves a number of processes, including influx through membrane channels, release from intracellular stores, chelation by Ca2+-binding proteins, uptake into organelles, and extrusion across the plasma membrane. The development and modulation of the mechanisms involved in Ca2+ regulation are of considerable interest, because they are likely to influence the developmental fate of a neuron and its subsequent function.
Studies of crayfish motor axons growing in cell culture suggest that the development of differences in Ca2+ regulation are related to the level of impulse activity. Depolarization inhibits the growth of normally inactive phasic motor axons, eventually producing retraction and often degeneration, whereas growing tonic motor axons normally continue to advance during depolarization (Arcaro and Lnenicka, 1997). These different responses to depolarization are dependent on Ca2+ influx. It appears likely that depolarization results in higher [Ca2+]i in phasic axons than in tonic axons, because high levels of intracellular Ca2+ can trigger neurite retraction and degeneration (Cohan et al., 1987; Silver et al., 1989; Mills and Kater, 1990). Differences in [Ca2+]i could result from a lower Ca2+ current density in growing tonic axons, because increased impulse activity produces a reduction in voltage-dependent Ca2+ currents in crayfish motoneurons (Hong and Lnenicka, 1995, 1997). An additional possibility is that the tonic axons have greater Ca2+-handling capacity, because application of a Ca2+ ionophore also produces greater inhibition of growing tonic axons than phasic axons (Arcaro and Lnenicka, 1997).
In previous studies, differences in Ca2+ regulation have been shown to influence neuronal growth and survival. CulturedHelisoma neurons with weak Ca2+regulation show greater neurite retraction and degeneration during Ca2+ influx than those with stronger Ca2+ regulation (Mills and Kater, 1990). There is a positive correlation between the presence of Ca2+-binding proteins and resistance to excitotoxicity in cultures of rat hippocampal and cortical neurons (Mattson et al., 1991; Lukas and Jones, 1994). In cultured hippocampal neurons, increases in Ca2+ channel density are correlated with decreased neuronal survival (Porter et al., 1997).
To examine the relationship between impulse activity and the development of Ca2+ regulation, we have examined the increase in [Ca2+]i produced by high-K+ solutions and Ca2+ionophores in the growth cones and neurites from phasic and tonic axons. Our results show that during prolonged depolarization [Ca2+]i goes much higher in growing phasic axons than in tonic axons. This difference in Ca2+ regulation involves greater Ca2+-handling capacity for tonic axons than for phasic axons. The development of these differences in Ca2+ regulation are activity-dependent, because eliminating impulse activity in growing tonic axons reduces their Ca2+-handling capacity. The implications of these activity-dependent changes in Ca2+ regulation for the growing axon are discussed.
MATERIALS AND METHODS
Preparation of cultures. Nerve cord explant cultures were prepared from crayfish (Procambarus clarkii), which were obtained from Atchafalaya Biological Supply (Raceland, LA), and maintained at 20°C in shallow, aerated tanks. Abdominal nerve cords were removed from crayfish with carapace lengths of 1.5–2.5 cm and plated in defined culture medium, as described previously (Arcaro and Lnenicka, 1995). Briefly, the nerve cord was plated on a coverslip with the deep and superficial third roots arranged such that growth from the phasic and tonic motor axons was easily distinguished. The culture medium consisted of L-15 Medium Leibovitz (l-4386; Sigma, St. Louis, MO) that was diluted 1:1 and contained (in mm): 13.5 CaCl2, 2.6 MgCl2, 5.4 KCl, 206.0 NaCl, 5.6 d-glucose, and 10 Na-HEPES, pH 7.4. In experiments in which we blocked impulse activity during growth, 1 μm TTX was added to the medium when the nerve cords were plated (Arcaro and Lnenicka, 1995). All measurements of [Ca2+]i were performed on cultures that were 2–3 d old.
High-K+ depolarization and ionophore application. Motoneurons were depolarized with medium containing 60 mm K+. Correct osmolarity was maintained by compensating for the increased KCl with an equal reduction in NaCl. Recordings of resting membrane potentials from the motor giant, the largest of the phasic motor neurons, showed that the addition of 60 mm K+ reduced the resting membrane potential from approximately −80 to −30 mV (Arcaro and Lnenicka, 1997). The Ca2+ ionophore 4-bromo-A23187 (Br-A23187) was prepared in DMSO at a concentration of 0.1 mm. This solution was diluted (1:400) in normal medium to give a final Br-A23187 concentration of 0.25 μm and was sonicated before application. To eliminate the effect of impulse activity on [Ca2+]i, TTX was added initially during incubation in fura-2 AM (see below) and included in all perfusion solutions for both the high-K+ and Br-A23187 experiments. Solutions were exchanged by gravity flow perfusion at a rate of 3 ml/min for the chronic depolarization studies and 13 ml/min for the ionophore and brief depolarization studies (chamber volume, <0.5 ml).
Measurement of [Ca2+]i.Growing axons were loaded with fura-2 by incubating the cultures in medium containing 2 μm fura-2 AM (Molecular Probes, Eugene, OR) for 50–60 min. Fura-2 AM (1 mm) in DMSO was added to culture medium at a 1:500 dilution. After loading with fura-2, growing axons were imaged with a 40× objective (Nikon Fluor; numerical aperture, 1.3) on an inverted microscope (Nikon Diaphot 200) equipped with an intensified CCD camera (VS-2525 intensifier and 200E CCD camera; Video Scope International, Herndon, VA). Fura-2 was excited by passing light from a 75 W xenon arc lamp through bandpass filters of 340 ± 7 or 380 ± 8 nm (Chroma Technology, Brattleboro, VT). A Lambda-10 optical filter changer (Sutter Instrument Co., Novato, CA) was used to switch between excitation wavelengths. Typically, illumination intensity was attenuated with an ND4 filter. The excitation and emission wavelengths were separated with a 410 nm dichroic mirror, and emitted light was then passed through a 510 ± 20 nm barrier filter. Metafluor software (Universal Imaging, West Chester, PA) was used for controlling the shutter, filter wheel, and image acquisition, as well as subsequent analysis.
The fura-2 fluorescence ratio (340:380) was used to estimate [Ca2+]i using standard techniques (Grynkiewicz et al., 1985). Ratio pairs were acquired from 16 frame averages, and background values from a blank region of the slide were subsequently subtracted. We used 865 nm as the dissociation constant for fura-2 in crayfish axoplasm (Delaney et al., 1991; Mulkey and Zucker, 1992) and a viscosity correction factor of 0.7 (Poenie et al., 1986). As in previous studies of crayfish axons (Delaney et al., 1989), we measured ratios at zero Ca2+(Rmin) and saturating Ca2+(Rmax) in vitro using solutions similar to crayfish cytoplasm (Wallin, 1967). Typical calibration values were Rmin = 0.32, Rmax = 10.62, and F0/Fs = 16.00. Note that small errors in estimating the absolute Ca2+ concentrations should not affect the major findings of this study, which are based on relative Ca2+ concentrations.
In many types of cells (e.g., mammalian) fura-2 is not a reliable indicator of Ca2+ concentrations higher than a couple of micromolar because of its high affinity for Ca2+ (Tsien, 1988). Fura-2 has a lower affinity for Ca2+ in crayfish neurons compared with mammalian cells (Kd, 865 vs 220 nm), and thus higher levels of Ca2+ can be measured. However, in some cases the Ca2+ concentrations in crayfish phasic axons were still higher than what could be reliably measured with fura-2. To eliminate these unreliable measurements, we did not use data in which the ratio exceeded 4 (Ca2+concentration of ∼12 μm).
The average Ca2+ concentration in neurites or growth cones was determined for individual neurons. These average values were used to determine the overall mean and for statistical analysis using a two-tailed Student’s t test. All values were mean ± SE.
RESULTS
[Ca2+]i in growing phasic and tonic axons
Growing neurites extend from the cut ends of the cultured phasic and tonic axons. Growth cones of phasic and tonic neurites advance at similar rates despite greater impulse activity in the tonic axons (Egid and Lnenicka, 1993; Arcaro and Lnenicka, 1997). Either tonic axons grow with higher [Ca2+]i than phasic axons, or the impulse activity does not produce elevations in [Ca2+]i in the tonic neurites. This was examined by comparing [Ca2+]i in growing phasic and tonic axons.
Ca2+ levels were measured during days 2–3 of growth, which is a period when the neurites are generally elongating (Egid and Lnenicka, 1993). The [Ca2+]iin tonic growth cones (192 ± 36 nm; n= 17 neurons; n = 31 growth cones) and neurites (189 ± 32 nm; n = 15 neurons;n = 16 neurites; n = 8 animals) was significantly higher than in phasic growth cones (110 ± 11 nm; n = 16 neurons; n = 36 growth cones; p < 0.05) and neurites (111 ± 18 nm; n = 14 neurons; n = 16 neurites; n = 10 animals; p < 0.05). In some cases, we verified whether the growth cones were advancing at the time the Ca2+ measurements were performed. These results were similar to the previous ones; [Ca2+]i was significantly higher in tonic growth cones (163 ± 31 nm; n = 9 neurons; n = 12 growth cones) than in phasic growth cones (90 ± 14 nm; n = 9 neurons;n = 14 growth cones; p < 0.05). These results show that the active tonic axons normally grow with a higher [Ca2+]i than the silent phasic axons.
Depolarization produces a larger increase in [Ca2+]i in growing phasic axons than in tonic ones
When advancing phasic growth cones are depolarized with 60 mm K+ for 40 min, they are initially inhibited and often retract (Arcaro and Lnenicka, 1997). During this depolarization, most tonic growth cones continue to advance. To determine the role of intracellular Ca2+, the [Ca2+]i was compared in growing phasic and tonic axons during depolarization.
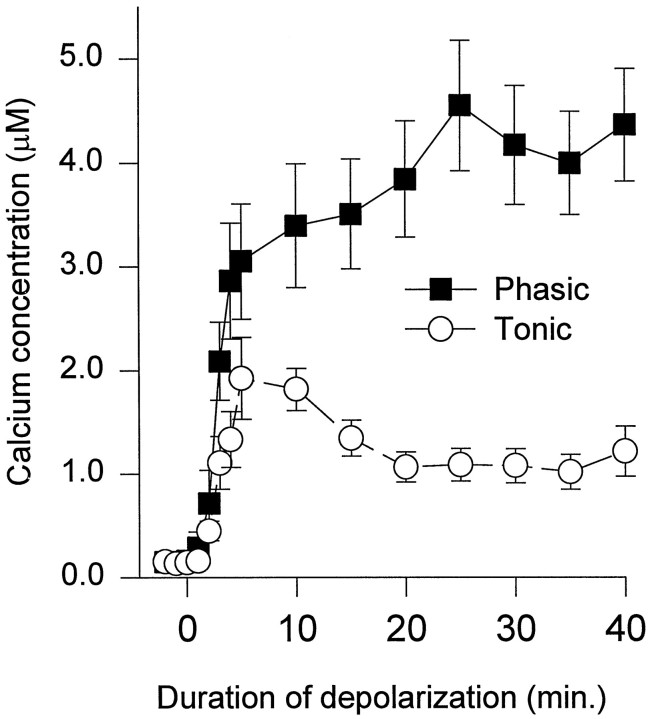
Intracellular Ca2+ was measured in phasic and tonic growth cones during depolarization produced by 60 mmK+ (Fig. 1,top). During the first 10 min of depolarization, [Ca2+]i reached higher levels in phasic than in tonic growth cones (Fig.2). Subsequently, [Ca2+]i in phasic growth cones gradually increased, whereas [Ca2+]iin the tonic growth cones decreased and plateaued at a lower level. The [Ca2+]i in the neurites was similar to that in the growth cones during this period of depolarization. A number of growth cones and neurites were examined 40–60 min after the beginning of the depolarization. The [Ca2+]i in phasic growth cones (4.61 ± 0.37 μm; n = 37 neurons;n = 70 growth cones) and neurites (5.54 ± 0.33 μm; n = 33 neurons; n = 43 neurites; n = 16 animals) was significantly higher than in tonic growth cones (1.38 ± 0.21 μm;n = 24 neurons; n = 71 growth cones;p < 0.0001) and neurites (1.56 ± 0.33 μm; n = 21 neurons; n = 32 neurites; n = 14 animals; p < 0.0001).
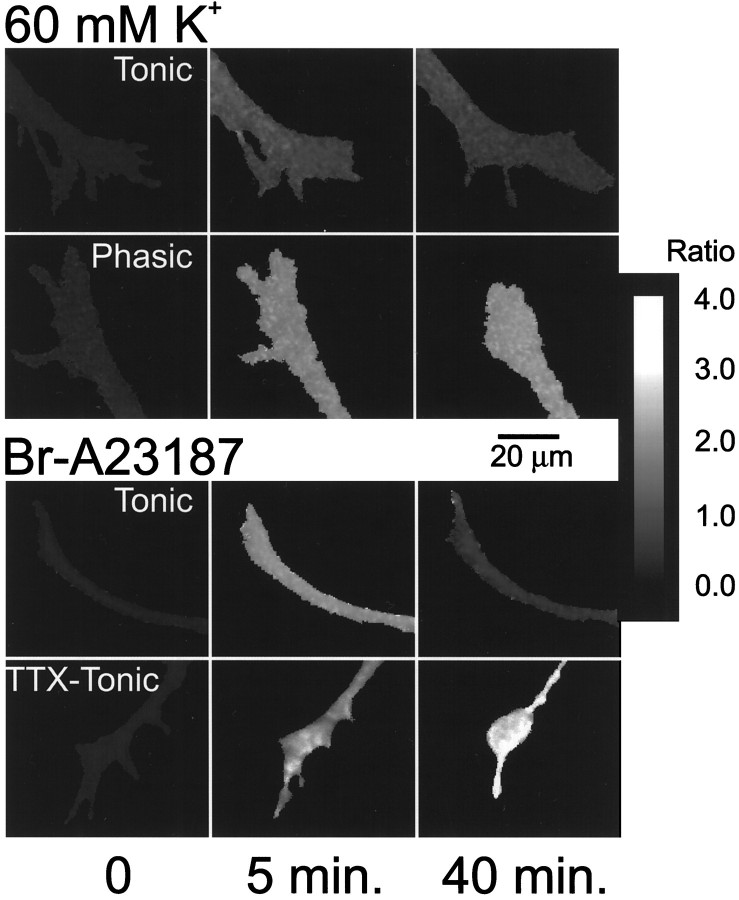
Fig. 1.
Ratio map of fura-2 fluorescence showing changes in [Ca2+]i in neurites and growth cones during application of medium containing 60 mmK+ or Br-A23187. Top, Medium containing 60 mm K+ was applied to growing phasic and tonic axons. After 5 min, [Ca2+]i increased in both phasic and tonic growth cones, although the increase was greater in the phasic growth cone than the tonic one. Between 5 and 40 min, [Ca2+]i continued to rise in the phasic growth cone, whereas [Ca2+]idecreased in the tonic growth cone. During this period of depolarization, the phasic growth cone clearly retracts, and the tonic growth cone continues to advance, although some of the finer processes are withdrawn. Bottom, Br-A23187 (0.25 μm) was added to tonic axons grown with normal impulse activity (Tonic) and to tonic axons grown with no impulse activity (TTX-Tonic). After 5 min of Br-A23187 application, [Ca2+]i increased in both tonic and TTX-tonic growth cones. During 5–40 min, [Ca2+]i continued to rise in the TTX-tonic growth cones; however, [Ca2+]i decreased in the tonic growth cone. The TTX-tonic growth cone retracts and the tonic growth cone advances slightly. Thus, the previous history of impulse activity determines the ability of the growing axon to regulate intracellular Ca2+. The scale on the right shows the relationship between the ratio and the gray level.
Fig. 2.
[Ca2+]i in phasic and tonic growth cones as a function of time during depolarization with medium containing 60 mm K+. The [Ca2+]i goes higher in phasic growth cones than in tonic growth cones. Mean values were obtained from 19 phasic growth cones from eight animals and 22 tonic growth cones from eight animals.
Thus, the greater depolarization-induced inhibition and degeneration of growing phasic neurites compared with tonic neurites is correlated with higher [Ca2+]i. The fact that [Ca2+]i reaches higher levels in phasic axon growth suggests that the growing phasic axons have greater Ca2+ influx and/or a lower rate of Ca2+ removal than the growing tonic axons.
Ca2+ levels go higher in growing phasic axons than tonic axons after addition of the Ca2+ ionophore Br-A23187
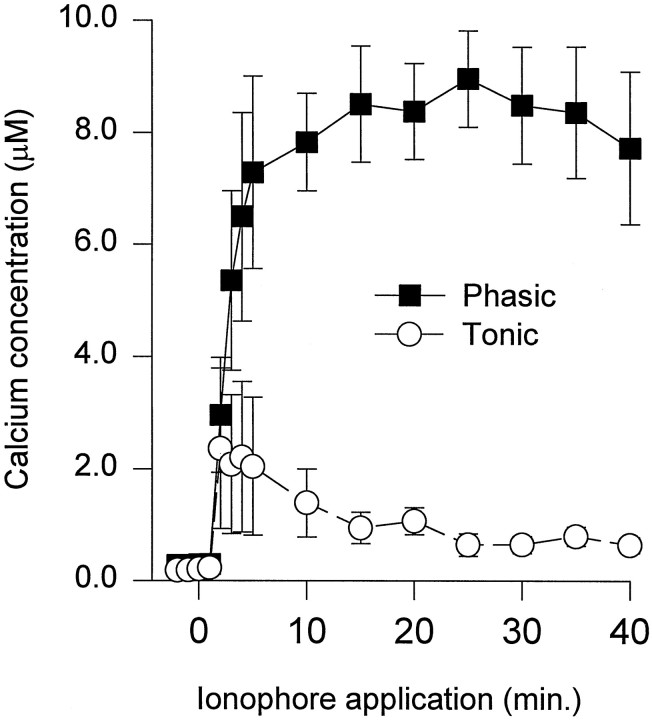
To determine whether the differences in Ca2+regulation involved differences in Ca2+ removal, we measured [Ca2+]i during perfusion of the Ca2+ ionophore Br-A23187. Although Br-A23187 produced a rapid increase in [Ca2+]iin both phasic and tonic growth cones, the [Ca2+]i reached higher levels in phasic growth cones. During the first 5 min of Br-A23187 application, [Ca2+]i was three to four times greater in phasic growth cones than in tonic ones (Fig.3). Subsequently, [Ca2+]i gradually increased and then plateaued in phasic growth cones, whereas [Ca2+]i in tonic growth cones decreased before leveling off. After 40 min of ionophore application, [Ca2+]i was approximately eight times greater in phasic growth cones than in tonic growth cones.
Fig. 3.
[Ca2+]i in phasic and tonic growth cones plotted as a function of time during application of the Ca2+ ionophore Br-A23187 (0.25 μm). After addition of Br-A23187, [Ca2+]i immediately goes higher in phasic growth cones than in tonic ones. The [Ca2+]i remains extremely high in the phasic growth cones for the duration of the ionophore application. In tonic growth cones, [Ca2+]i decreases after its initial increase. Mean values represent seven phasic growth cones from three animals and nine tonic growth cones from three animals.
The remaining neurites and growth cones in the cultures were examined 40–60 min after the beginning of ionophore application. The [Ca2+]i in phasic growth cones (4.78 ± 0.83 μm; n = 18 neurons;n = 35 growth cones) and neurites (3.47 ± 1.02 μm; n = 12 neurons; n = 18 neurites; n = 6 animals) was significantly higher than in tonic growth cones (1.28 ± 0.31 μm;n = 17 neurons; n = 35 growth cones;p < 0.001) and neurites (0.91 ± 0.16 μm; n = 12 neurons; n = 15 neurites; n = 6 animals; p < 0.05). These results indicate that the growing phasic axons have a reduced capacity to regulate intracellular Ca2+ compared with tonic axons.
The differences in [Ca2+]i for the growing phasic and tonic axons are likely to be underestimations during both depolarization and Br-A23187 application. In both experiments, some phasic axon data were omitted, because the [Ca2+]i went too high to be reliably measured (see Materials and Methods), whereas none of the tonic axon data had to be omitted. In addition, some phasic growth had completely degenerated after 40 min of Br-A23187 treatment, and Ca2+ measurements could not be made during the 40–60 min period. Because the growth cones that degenerated likely had the highest [Ca2+]i, this explains why the measurements of [Ca2+]i in phasic growth cones were higher during the first 40 min than during 40–60 min.
Ca2+ removal after brief depolarizing pulses
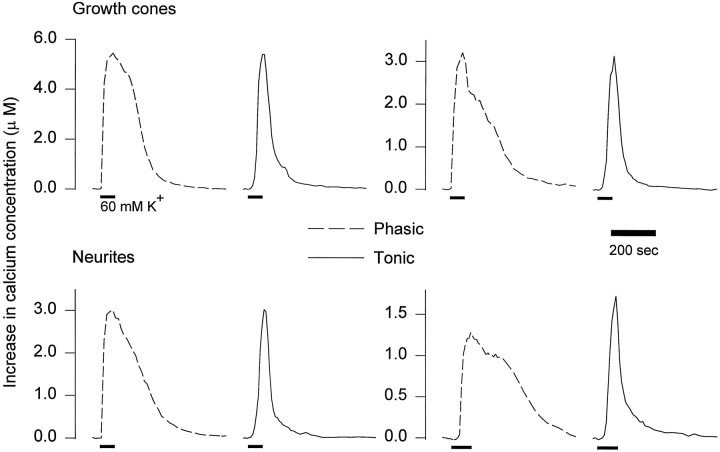
To compare Ca2+ regulation in phasic and tonic growth cones further, we examined Ca2+ removal after brief depolarizing pulses. The [Ca2+]iwas measured in neurites and growth cones during 60–90 sec depolarizing pulses (60 mm K+). At the end of the pulse, [Ca2+]i declined more rapidly in tonic growth cones than in phasic ones (Fig.4). Because the decline in [Ca2+]i did not follow a single exponential, we measured the time required for [Ca2+]i to decay to one-half of peak values. The time to one-half decay was significantly greater for phasic growth cones (167 ± 47 sec; n = 7 neurons;n = 17 growth cones) than for tonic growth cones (59 ± 10 sec; n = 5 neurons; n = 14 growth cones; p < 0.05). The differences in Ca2+ removal were not attributable to differences in the peak [Ca2+]i, because the peaks were similar in phasic (3.90 ± 0.83 μm) and tonic growth cones (3.65 ± 1.07 μm). In addition, even when the peak [Ca2+]i in phasic growth cones was lower, Ca2+ removal was still slow (Fig. 4, bottom right).
Fig. 4.
Changes in [Ca2+]i in phasic and tonic growth cones and neurites produced by brief depolarizations. Pulses of 60 mm K+ (bars) were applied to growing phasic and tonic axons in six animals, and changes in [Ca2+]i were measured.Top, Representative results for phasic and tonic growth cones. Phasic and tonic growth cones with similar peak [Ca2+]i are compared.Bottom, Representative results from phasic and tonic neurites. Phasic and tonic neurites with similar widths and peak [Ca2+]i are compared. On theleft, the phasic and tonic neurites are 7 and 6 μm wide, respectively. On the right, phasic and tonic neurites are 5 and 6 μm wide, respectively. Note that at the end of the pulse, [Ca2+]i consistently declined more rapidly in tonic growth cones and neurites than in phasic ones.
These differences in Ca2+ removal do not appear to result from differences in growth cone size or shape. That is, if Ca2+ removal results from extrusion of Ca2+ across the plasma membrane, the surface-to-volume ratio would be expected to influence the rate at which [Ca2+]i declines. When Ca2+ removal was compared in phasic and tonic neurites, which generally have a uniform shape, we obtained results similar to those for growth cones (Fig. 4). Ca2+levels took significantly longer to decay to one-half of peak values in phasic neurites (216.5 ± 58.9 sec; n = 5 neurons;n = 11 neurites), which had a mean width of 7 μm compared with tonic neurites (60.5 ± 19.4 sec; n= 5 neurons; n = 14 neurites; p < 0.05), which had a mean width of 5 μm. Similar differences were also seen when neurites of similar width were compared. Eliminating all phasic neurites >7 μm resulted in a mean width of 5 μm and a time to one-half decay of 187.3 ± 18.3 sec (n = 3 neurons; n = 7 neurites), which was also significantly greater than the tonic neurite values (p < 0.01). These results support faster Ca2+ removal by growing tonic axons compared with growing phasic axons.
Differences in Ca2+ regulation are activity-dependent
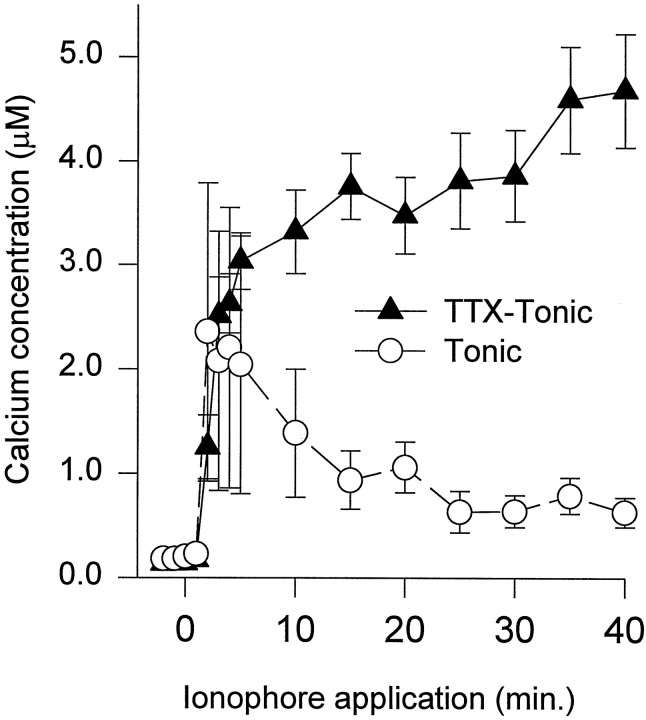
To determine whether the differences in Ca2+regulation were activity-dependent, the impulse activity in the tonic axons was eliminated by adding 1 μm TTX during their growth. After 2–3 d of growth in TTX, the Ca2+-handling capacity of these inactive tonic axons was examined by measuring changes in [Ca2+]i after the addition of Br-A23187 (Fig. 1, bottom). These results were compared with the previous measurements obtained from tonic axons grown in normal medium. During 40 min of ionophore application, the inactive tonic growth cones showed weak Ca2+ regulation; [Ca2+]i continued to increase during ionophore application (Fig. 5). These results were different from the control tonic axons and more similar to the normally inactive phasic axons. Similar differences between inactive and control tonic axons were also seen when [Ca2+]i was compared 40–60 min after the beginning of ionophore application. The [Ca2+]i in the inactive tonic growth cones (3.15 ± 0.60 μm; n = 15 neurons; n = 37 growth cones) and neurites (2.96 ± 0.56 μm; n = 14 neurons;n = 21 neurites) was significantly greater than in the control tonic growth cones (1.28 ± 0.31 μm;n = 17 neurons; n = 35 growth cones;p < 0.01) and neurites (0.91 ± 0.16 μm; n = 12 neurons; n = 15 neurites; p < 0.01). These results clearly show that axons growing with greater impulse activity develop greater Ca2+-handling capacity.
Fig. 5.
Differences in Ca2+ regulation for tonic axons grown with or without impulse activity. The Ca2+-handling capacity was compared in tonic axons grown in the presence of TTX with tonic axons grown in normal medium. The [Ca2+]i was measured in inactive tonic growth cones during 40 min of Br-A23187 application. These values are compared with those obtained from tonic growth cones grown in normal medium (Fig. 3). Note that the [Ca2+]i continued to increase in the inactive tonic growth cones in contrast to control tonic growth cones in which [Ca2+]i declined after the initial increase.
DISCUSSION
Depolarization produced higher Ca2+ levels in the phasic axons and growth cones than in tonic ones
During maintained depolarization with 60 mmK+, [Ca2+]i reaches higher levels in phasic neurites and growth cones than in tonic ones; [Ca2+]i in phasic axon growth is three to four times greater than in tonic axon growth at the end of 40 min of depolarization. The [Ca2+]i in phasic growth cones and axons during depolarization or ionophore application was very high and presumably was responsible for their retraction and eventual degeneration (Arcaro and Lnenicka, 1997). It is unclear what [Ca2+]i is necessary to produce degeneration or the extent to which increases in [Ca2+]i actually reflect the early stages of degeneration. Very high levels of intracellular Ca2+ (>5 μm) appear to be required to trigger excitotoxic death of mouse cortical neurons (Hyrc et al., 1997). During depolarization of growing tonic axons, [Ca2+]i stabilizes at values that are not high enough to trigger retraction or degeneration. In fact, although there is a loss of filopodia, growth cones usually continue to advance. Thus, the different responses of the growing phasic and tonic axons to depolarization involve differences in Ca2+regulation.
There may also be differences in the sensitivity of phasic and tonic growth to Ca2+ because normally the [Ca2+]i in the tonic growth cones is ∼70% greater than in phasic growth cones, although they advance at a similar rate (Arcaro and Lnenicka, 1997). The greater [Ca2+]i in growing tonic axons apparently results from differences in impulse activity (Egid and Lnenicka, 1993), because the differences in [Ca2+]i are not seen when TTX is added to the cultures before perfusing high-K+ or Br-A23187 solutions. Tonic growth cones may have a low sensitivity to intracellular Ca2+ because when depolarized they continue to advance, although the increase in [Ca2+]i appears sufficient to inhibit growth cone advance in many other neurons (Cohan et al., 1987; Silver et al., 1989; Lankford and Letourneau, 1991; Fields et al., 1993). It appears that not all growth cones are equally sensitive to intracellular Ca2+; neurites from rat superior cervical ganglion neurons continue to elongate with elevated [Ca2+]i (Garyantes and Regehr, 1992), and identified neurons in Helisoma show differences in their sensitivity to intracellular Ca2+ during growth (Torreano and Cohan, 1997). In fact, a prolonged increase in impulse activity can produce a reduction in the sensitivity of the growth cone to intracellular Ca2+ (Fields et al., 1993). Further studies are required to determine whether Ca2+sensitivity is different in growing phasic and tonic axons.
Tonic neurites and growth cones have a greater Ca2+-handling capacity than phasic ones
Ca2+ regulation was compared in phasic and tonic growth by adding the Ca2+ ionophore Br-A23187 and monitoring changes in [Ca2+]i. After Br-A23187 application, [Ca2+]iin phasic growth cones plateaued at levels four to five times greater than that observed in tonic growth cones. Thus, under conditions in which the density of Ca2+ influx was presumably similar, the resultant [Ca2+]i is dramatically different. Of course this assumes that the ionophore is incorporated into growing phasic and tonic axons at equal densities, which seems likely. Further evidence for stronger Ca2+ regulation by tonic axon growth was provided by examining Ca2+ removal at the end of a brief depolarizing pulse. After a 60–90 sec depolarizing pulse, the [Ca2+]i decreases more rapidly in tonic growth cones and neurites than in phasic ones. Thus, the differences in Ca2+ regulation include differences in Ca2+ removal; however, we do not know whether there are also differences in Ca2+ entry during depolarization.
The removal of intracellular free Ca2+ involves chelation by Ca2+-binding proteins, sequestration by intracellular organelles, and extrusion across the plasma membrane by the Na+–Ca2+ exchange and Ca2+ATPase (for review, see Miller, 1991). Generally, Ca2+-binding proteins have a low capacity for buffering Ca2+; therefore, they probably contribute little to buffering the large Ca2+ loads imposed in these experiments. In addition, greater cytoplasmic buffering should slow the removal of Ca2+, whereas Ca2+ is removed more rapidly from tonic than phasic growth cones. Mitochondria are likely to be more effective than the endoplasmic reticulum at sequestering these large Ca2+ loads because of their greater capacity. Mitochondrial Ca2+ uptake can reduce the peak [Ca2+]i during a depolarizing pulse and accelerate the initial decay of [Ca2+]i at the end of the pulse; however, mitochondria then release Ca2+, which causes the [Ca2+]i to plateau before returning to resting levels (Friel and Tsien, 1994; Werth and Thayer, 1994; Herrington et al., 1996). Again, because Ca2+transients decay more rapidly in tonic growth cones than phasic ones, the greater Ca2+-handling capacity of tonic growth cones is unlikely to be attributable simply to greater mitochondrial Ca2+ uptake.
A higher rate of Ca2+ extrusion from growing tonic axons than from phasic ones could be responsible for their lower [Ca2+]i during ionophore application and more rapid decay of [Ca2+]i at the end of depolarizing pulses. According to a model describing the dynamics of residual Ca2+ in crayfish motor terminals, an increase in the rate of Ca2+ extrusion will decrease the [Ca2+]i plateau during a train of impulses and increase the rate of decay of [Ca2+]i at the end of the train (Tank et al., 1995). In crayfish motor terminals, the rate of Ca2+ extrusion appears to be a linear function of [Ca2+]i and can be modeled using a first-order Michaelis–Menten reaction (Tank et al., 1995). This formulation does not distinguish between the plasma membrane Ca2+ATPase or Na+–Ca2+ exchange (Sala and Hernandez-Cruz, 1990; Lagnado et al., 1992; Tank et al., 1995). Based primarily on studies of squid axons, the Na+–Ca2+ exchange has been classified as having a low affinity but high capacity for Ca2+ transport, whereas the Ca2+ATPase has a high affinity but low capacity (Baker and DiPolo, 1984).
It appears reasonable that the plasma membrane Na+–Ca2+ exchanger could play a major role in Ca2+ extrusion during the large Ca2+ loads imposed in our experiments. The Na+–Ca2+ exchanger was important in removing intracellular Ca2+ fromHelisoma neurons during a large Ca2+ load produced by Br-A23187 (Mills and Kater, 1990). There is evidence that Na+–Ca2+ exchange extrudes Ca2+ from rat brain synaptosomes (Sanchez-Armass and Blaustein, 1987), and based on immunocytochemistry the Na+–Ca2+ exchanger is concentrated at presynaptic terminals, as well as in growing neurites and growth cones (Luther et al., 1992). Na+–Ca2+ exchange, as well as mitochondrial Ca2+ uptake, play a role in removing cytoplasmic free Ca2+ from crayfish motor terminals (Mulkey and Zucker, 1992; Tang and Zucker, 1997). Differences in Na+–Ca2+ exchange in growing phasic and tonic axons could result from differences in the exchanger and/or their ability to extrude Na+ (Blaustein, 1988).
Differences in Ca2+ regulation are activity-dependent
The strong Ca2+ regulation seen in tonic axons appears to result from its high-impulse activity levels during growth. Tonic axons growing in TTX showed weaker Ca2+regulation than control ones during the application of Br-A23187. The strong Ca2+ regulation produced by high-impulse activity persists for at least 1 hr, and probably much longer, because impulse activity in the tonic axons was blocked for >1 hr before examining their Ca2+ handling (see Materials and Methods).
The activity-dependent strengthening of Ca2+regulation could involve changes in protein synthesis. For example, the synthesis of Ca2+-binding proteins can be upregulated by increased electrical activity (Lowenstein et al., 1991). In addition, there are activity-dependent differences in the density and activity of mitochondria in crayfish motor axons and terminals (Lnenicka et al., 1986, 1997; Nguyen and Atwood, 1994). Mitochondrial changes could play a role in modulating Ca2+regulation, if not through direct Ca2+ uptake, then indirectly through the production of ATP.
Relevance to neuronal physiology
Because [Ca2+]i is so important to neuronal function, the activity-dependent development of Ca2+ regulation could affect a number of neuronal properties. For the developing axon, Ca2+ regulation can affect how impulse activity and environmental cues shape the pattern of growth. In the adult, greater Ca2+-handling capacity could make neurons more resistant to Ca2+ neurotoxicity produced by insults such as excitotoxicity (Choi, 1988). Activity-dependent differences in Ca2+ regulation could influence cell excitability, e.g., intracellular Ca2+ levels influence the firing properties of lobster neurons (Turrigiano et al., 1994).
Activity-dependent differences in Ca2+ regulation at motor terminals are likely to influence transmitter release. For example, Ca2+ removal is likely to influence the production of post-tetanic potentiation (PTP), because PTP requires the buildup of residual Ca2+ (Delaney et al., 1989;Delaney and Tank, 1994). Because chronic in vivo stimulation of crayfish phasic motor terminals reduces their capacity to produce PTP (Pahapill et al., 1986), it may be that increased impulse activity strengthens Ca2+ regulation in mature motor terminals, as well as in growing axons.
Footnotes
This work was supported by National Science Foundation Grant IBN-9511558 (G.A.L.). We thank Drs. John Schmidt and Su Tieman for reviewing this manuscript.
Correspondence should be addressed to Gregory A. Lnenicka, Neurobiology Research Center, Department of Biological Sciences, University at Albany, State University of New York, Albany, NY 12222.
REFERENCES
- 1.Arcaro KF, Lnenicka GA. Intrinsic differences in axonal growth from crayfish fast and slow motoneurons. Dev Biol. 1995;168:272–283. doi: 10.1006/dbio.1995.1079. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro KF, Lnenicka GA. Differential effects of depolarization on the growth of crayfish tonic and phasic motor axons in culture. J Neurobiol. 1997;33:85–97. [PubMed] [Google Scholar]
- 3.Baker PF, DiPolo R. Axonal calcium and magnesium homeostasis. Curr Top Membr Transp. 1984;22:95–147. [Google Scholar]
- 4.Blaustein MP. Calcium transport and buffering in neurons. Trends Neurosci. 1988;11:438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 6.Cohan CS, Connor JA, Kater SB. Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci. 1987;7:3588–3599. doi: 10.1523/JNEUROSCI.07-11-03588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney KR, Tank DW. A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. J Neurosci. 1994;14:5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney KR, Zucker RS, Tank DW. Calcium in motor nerve terminals associated with post-tetanic potentiation. J Neurosci. 1989;9:3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney K, Tank DW, Zucker RS. Presynaptic calcium and serotonin-mediated enhancement of transmitter release at crayfish neuromuscular junction. J Neurosci. 1991;11:2631–2643. doi: 10.1523/JNEUROSCI.11-09-02631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egid K, Lnenicka GA. Regeneration from crayfish phasic and tonic motor axons in vitro. J Neurobiol. 1993;24:1111–1120. doi: 10.1002/neu.480240809. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD, Guthrie PB, Russell JT, Kater SB, Malhotra BS, Nelson PG. Accommodation of mouse DRG growth cones to electrically induced collapse: kinetic analysis of calcium transients and set-point theory. J Neurobiol. 1993;24:1080–1098. doi: 10.1002/neu.480240807. [DOI] [PubMed] [Google Scholar]
- 12.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-envoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garyantes TK, Regehr WG. Electrical activity increases growth cone calcium but fails to inhibit neurite outgrowth from rat sympathetic neurons. J Neurosci. 1992;12:96–103. doi: 10.1523/JNEUROSCI.12-01-00096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 15.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Lnenicka GA. Activity-dependent reduction in voltage-dependent calcium current in a crayfish motoneuron. J Neurosci. 1995;15:3539–3547. doi: 10.1523/JNEUROSCI.15-05-03539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong SJ, Lnenicka GA. Characterization of a P-type calcium current in a crayfish motoneuron and its selective modulation by impulse activity. J Neurophysiol. 1997;77:76–85. doi: 10.1152/jn.1997.77.1.76. [DOI] [PubMed] [Google Scholar]
- 18.Hyrc K, Handran SD, Rothman SM, Goldberg MP. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. J Neurosci. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz B. The release of neural transmitter substances. Thomas; Springfield, IL: 1969. [Google Scholar]
- 21.Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segment of retinal rods from the tiger salamander. J Physiol (Lond) 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lankford KL, Letourneau PC. Roles of actin filaments and three second-messenger systems in short-term regulation of chick dorsal root ganglion neurite outgrowth. Cell Motil Cytoskeleton. 1991;20:7–29. doi: 10.1002/cm.970200103. [DOI] [PubMed] [Google Scholar]
- 23.Lnenicka GA, Atwood HL, Marin L. Morphological transformation of synaptic terminals of a phasic motoneuron by long-term tonic stimulation. J Neurosci. 1986;6:2252–2258. doi: 10.1523/JNEUROSCI.06-08-02252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lnenicka GA, Case CJ, Travis JL. Activity-dependent differences in the mitochondrial density of crayfish phasic and tonic motor axons. J Exp Zool. 1997;280:18–27. [Google Scholar]
- 25.Lowenstein DH, Miles MF, Hatam F, McCabe T. Upregulation of calbindin-D28K mRNA in the rat hippocampus following focal stimulation of the perforant path. Neuron. 1991;6:627–633. doi: 10.1016/0896-6273(91)90065-8. [DOI] [PubMed] [Google Scholar]
- 26.Lukas W, Jones KA. Cortical neurons containing calretinin are selectively resistant to calcium overload and excitotoxicity in vitro. Neuroscience. 1994;61:307–316. doi: 10.1016/0306-4522(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 27.Luther PW, Yip RK, Bloch RJ, Ambesi A, Lindenmayer GE, Blaustein MP. Presynaptic localization of sodium/calcium exchangers in neuromuscular preparations. J Neurosci. 1992;12:4898–4904. doi: 10.1523/JNEUROSCI.12-12-04898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excitoprotective roles for the calcium-binding protein calbindin-D28K in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 29.Miller RJ. The control of neuronal calcium homeostasis. Prog Neurobiol. 1991;37:255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- 30.Mills LR, Kater SB. Neuron-specific and state-specific differences in calcium homeostasis regulate the generation and degeneration of neuronal architecture. Neuron. 1990;2:149–163. doi: 10.1016/0896-6273(90)90451-k. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–452. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 32.Mulkey RM, Zucker RS. Post-tetanic potentiation at the crayfish neuromuscular junction is dependent on both intracellular calcium and sodium ion accumulation. J Neurosci. 1992;12:4327–4336. doi: 10.1523/JNEUROSCI.12-11-04327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen PV, Atwood HL. Altered impulse activity modifies synaptic physiology and mitochondria in crayfish phasic motor neurons. J Neurophysiol. 1994;72:2944–2955. doi: 10.1152/jn.1994.72.6.2944. [DOI] [PubMed] [Google Scholar]
- 34.Nishi R, Berg DK. Effects of high K+ concentrations on the growth and development of ciliary ganglion neurons in cell culture. Dev Biol. 1981;87:301–307. doi: 10.1016/0012-1606(81)90153-6. [DOI] [PubMed] [Google Scholar]
- 35.Pahapill PA, Lnenicka GA, Atwood HL. Neuronal experience modifies synaptic long-term facilitation. Can J Physiol Pharmacol. 1986;64:1052–1054. doi: 10.1139/y86-180. [DOI] [PubMed] [Google Scholar]
- 36.Poenie M, Alderton J, Steinhardt R, Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986;33:886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- 37.Porter NM, Thibault O, Thibault V, Chen K-C, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. Neuroscience. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sala F, Hernandez-Cruz A. Calcium diffusion modeling in a spherical neuron, relevance of buffering properties. Biophys J. 1990;57:313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Armass S, Blaustein MP. Role of sodium-calcium exchange in regulation of intracellular calcium in nerve terminals. Am J Physiol. 1987;252:C595–C603. doi: 10.1152/ajpcell.1987.252.6.C595. [DOI] [PubMed] [Google Scholar]
- 40.Silver RA, Lamb AG, Bolsover SR. Elevated cytosolic calcium in the growth cone inhibits neurite elongation in neuroblastoma cells: correlation of behavioral states with cytosolic calcium concentration. J Neurosci. 1989;9:4007–4020. doi: 10.1523/JNEUROSCI.09-11-04007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 42.Tank DW, Regehr WG, Delaney KR. A quantitative analysis of presynaptic calcium dynamics that contribute to short-term enhancement. J Neurosci. 1995;15:7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torreano PJ, Cohan CS. Electrically induced changes in Ca2+ in Helisoma neurons: regional and neuron-specific differences and implications for neurite outgrowth. J Neurobiol. 1997;32:150–162. [PubMed] [Google Scholar]
- 44.Tsien RY. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988;11:419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- 45.Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- 46.Wallin BG. Intracellular ion concentrations in single crayfish axons. Acta Physiol Scand. 1967;70:419–430. doi: 10.1111/j.1748-1716.1967.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 47.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]