Abstract
Neurotrophins are known to promote the survival, differentiation, and neurite outgrowth of developing neurons. Here we report that acutely applied brain-derived neurotrophic factor (BDNF) induces rapid growth cone collapse and neurite retraction of embryonicXenopus spinal neurons in culture. The collapsing effect of BDNF depends on the activation of Trk receptor tyrosine kinase, requires an influx of extracellular Ca2+, and is regulated by cAMP-dependent activity. Elevation of intracellular cAMP levels ([cAMP]i) by forskolin or (Sp)-cAMP completely blocked the collapsing effect, whereas inhibition of protein kinase A (PKA) by (Rp)-cAMP potentiated the collapsing action. BDNF-induced growth cone collapse was only observed in 6 hr cultures but not in 24 hr cultures. However, inhibition of PKA by (Rp)-cAMP restored the collapsing response of these “old” neurons in 24 hr cultures, suggesting that embryonic Xenopus spinal neurons may upregulate their endogenous cAMP-dependent activity during development in culture, leading to the blockade of their collapsing response to BDNF. Taken together, our results suggest the presence of cross-talk between Ca2+- and cAMP-signaling pathways involved in the collapsing action of neurotrophins, in which the cAMP-pathway regulates the Ca2+-mediated signal transduction required for BDNF-induced collapse. By modulating the cAMP-dependent activity through the intrinsic programming or interaction with other factors present in the environment, a neuron thus could respond to the same extracellular factors with different morphological and cellular changes at different stages during development.
Keywords: growth cone, collapse, neurotrophin, calcium, cAMP, guidance, repulsion
Formation of neuronal circuitry during development requires the growth and guidance of developing axons to their correct target cells. This process depends on the response of the growing tip of an axon, the growth cone, to a variety of extracellular factors present in developing embryos, including surface-bound as well as diffusible molecules (Bray and Hollenbeck, 1988; Keynes and Cook, 1995a; Tessier-Lavigne and Goodman, 1996). Guidance by positive cues that promote axonal extension and attract growth cones has been considered the main mechanism in vivo. However, recent studies have established an equally important role for inhibitory or repulsive cues in axonal guidance (Luo and Raper, 1994;Pini, 1994; Dodd and Schuchardt, 1995; Keynes and Cook, 1995b;Tessier-Lavigne and Goodman, 1996). By inhibiting axonal growth in “wrong” directions and by collapsing the growth cones entering inappropriate regions, these molecules can repel elongating axons and thus help them to reach the correct destination (Pini, 1993; Fan and Raper, 1995; Messersmith et al., 1995; Püschel et al., 1995).
Neurotrophins are a family of neurotrophic factors with profound influence on neuronal proliferation, survival, and differentiation (for review, see Barde, 1990; Thoenen, 1991; Davies, 1994b; Klein, 1994;Snider, 1994; Lindsay, 1996). Recent studies have revealed a number of novel biological actions of neurotrophins on various aspects of developing nervous systems, including axonal (Diamond et al., 1992;Cohen-Cory and Fraser, 1995) and dendritic (Cohen-Cory et al., 1991;McAllister et al., 1995, 1996) morphology, synaptic activity (Lohof et al., 1993; Kim et al., 1994; Kang and Schuman, 1995; Levine et al., 1995; Figurov et al., 1996) and maturation (Wang et al., 1995), and synaptic patterning (Cabelli et al., 1995). A role for neurotrophins in the regulation of axonal growth is supported by the remarkable ability of neurotrophins to promote neurite outgrowth of sensitive populations of neurons (Snider and Johnson, 1989; Kuffler, 1994; Lundborg et al., 1994). Furthermore, the chemoattractive effect of nerve growth factor (NGF) was demonstrated previously in vitro (Letourneau, 1978; Gundersen and Barrett, 1980) as well as in vivo(Menesini-Chen et al., 1978), suggesting a role for neurotrophins in axonal guidance. Despite subsequent evidence that NGF is not responsible for long-range growth cone guidance (Lumsden and Davies, 1983; Davies et al., 1987), a role for NGF in local regulation of axonal growth has been proposed (Gallo et al., 1997). Recent findings that brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are expressed relatively early during development (Maisonpierre et al., 1990; Hallböök et al., 1993) further raise the possibility for neurotrophins to play a role in the early development of the nervous system. The demonstration of chemoattractive effects of BDNF and NT-3 in culture (Ming et al., 1997; Song et al., 1997) suggests that they could be candidates involved in axonal growth and guidance.
We now report a collapsing effect of neurotrophins on growth cones of embryonic Xenopus spinal neurons in early stages in culture. Acute application of BDNF induces rapid growth cone collapse followed by neurite retraction of Xenopus neurons in 6 hr cultures. The collapsing effect of BDNF depends on the activation of Trk receptor tyrosine kinase and requires an influx of extracellular Ca2+. Furthermore, the Ca2+-mediated BDNF-induced collapse is regulated by cytosolic cAMP-dependent activity; elevation or inhibition of cAMP-dependent activity blocked or enhanced the collapsing effect, respectively. Such cAMP-dependent regulation is found to be responsible for the disappearance of the collapsing effect of BDNF on neurons in 24 hr cultures. Our results suggest that neurotrophins may play important roles in the regulation of growth cone motility and extension; such regulation may well depend on the developmental stages of the neurons and could be influenced by other extracellular factors that modulate the cytosolic cAMP activity, leading to differential responses of the neuron to the same factor.
MATERIALS AND METHODS
Cell culture. Cultures of embryonicXenopus spinal neurons were prepared according to procedures reported previously (Spitzer and Lamborghini, 1976; Tabti and Poo, 1990). The neural tube tissue from developing embryos at stage 20–22 (Nieuwkoop and Faber, 1967) was dissociated in a Ca2+- and Mg2+-free Ringer’s solution supplemented with EDTA (in mm: 115 NaCl, 2.5 KCl, 10 HEPES, and 0.5 EDTA, pH 7.6), plated on clean glass coverslips, and incubated at room temperature (20–22°C). The culture medium consisted of 50% (vol/vol) Leibovitz medium (Life Technologies, Gaithersburg, MD), 1% (v/v) fetal bovine serum (Life Technologies), and 49% (v/v) Ringer’s solution (in mm: 115 NaCl, 2 CaCl2, 2.5 KCl, and 10 HEPES, pH 7.6).
Neurotrophins and chemicals. Human recombinant BDNF and NGF were generously provided by Regeneron Pharmaceuticals, (Tarrytown, NY). All neurotrophins were aliquoted at 10 mg/ml and stored at −85°C. Working stock solutions of neurotrophins at 100 μg/ml were prepared and used within 1 week. Neurotrophins at working concentrations were prepared in culture medium before each experiment. A water-soluble derivative of forskolin (7-deacetyl-7-(O-N-methylpiperazino)-γ-butyryl-dihydrochloride), K252a, (Rp)-cAMP, (Sp)-cAMP, and staurosporine were all purchased from CalBiochem (La Jolla, CA). Cytochalasin D was purchased from Sigma (St. Louis, MO).
Collapsing experiments. Most of the experiments were performed on a Nikon TMS inverted microscope equipped with phase-contrast optics and a 20× objective (Nikon, Tokyo, Japan). The images of individual neurons were acquired through a inch CCD video camera (Coordinated Systems, East Hartford, CT) and digitized by a SNAPPY video digitizer (Play, Rancho Cordova, CA). For high-resolution imaging, cells grown on a glass coverslip were mounted on a microscopy chamber using silicon vacuum grease (Dow Corning, Midland, MI) and examined on a Nikon Diaphot 300 inverted microscope equipped with differential interference contrast (DIC) optics and a 40×, numerical aperture 1.3 oil-immersion objective. A ½ inch CCD video camera (C2400–75i; Hamamatsu Photonics System, Bridgewater, NJ) was used for video imaging in conjunction with an Argus-20 image processor (Hamamatsu) for image enhancement. The video images were background-subtracted, averaged over four video frames, and contrast-enhanced in real time using Argus-20. The enhanced video images were digitized and acquired by a personal computer at a standard rate of one frame every 2 min, although faster rates were sometimes used to examine the dynamic changes of growth cone morphology. For all the experiments, a control period of 5 min observation was performed on each neuron to assess the normal neurite extension before the neurotrophin addition. Neurotrophins at their final working concentrations were applied to the culture by rapid perfusion of the culture medium. Immediately after the perfusion, 10 min of time-lapse recording at the standard rate was performed. For each pharmacological treatment, the cells were pretreated for 20 min with various chemicals before the addition of neurotrophins, and the effect of these chemicals alone on growth cone extension was monitored during the 20 min pretreatment. Only those chemicals that did not significantly affect growth cone extension were used for further experiments.
Image analysis was performed using the ImageTool program (developed at the University of Texas Health Science Center, San Antonio, TX, and available from the Internet by anonymous file transfer protocol fromftp://maxrad6.uthscsa.edu). The lengths of neurite processes were measured by tracing the entire trajectory of neurite extension, including all major branches. Because of the dynamic nature of filopodia and lamellipodia, they were not included in the length measurement. Therefore, the length measurement for each neurite process ends at the center of the phase-dark “palm” of the growth cone.
RESULTS
Neurotrophin collapsing effects
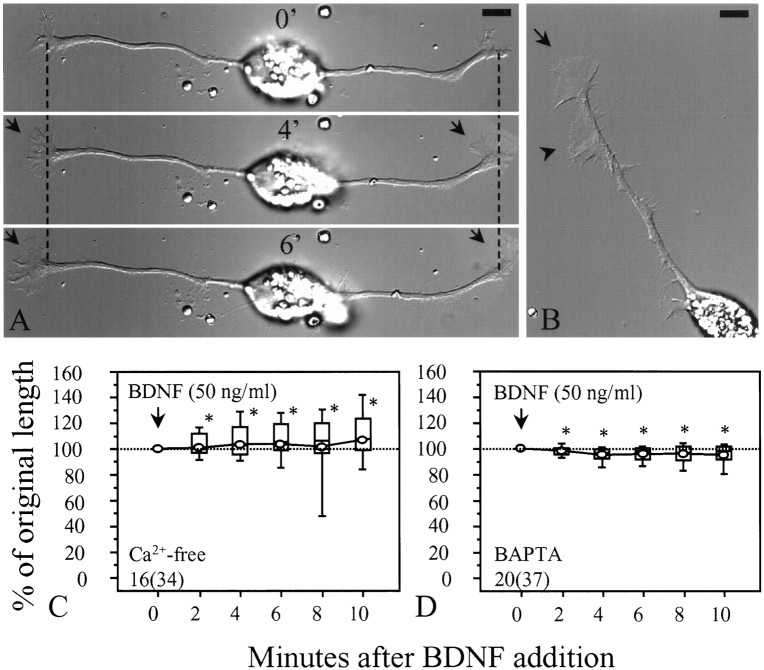
Isolated spinal neurons in 6 hr Xenopus cultures were used for these experiments. Active neurite extension at a rate of ∼15 μm/hr is observed in these cultures (Zheng et al., 1996b). Time-lapse video imaging was performed to monitor the changes in growth cone motility and neurite lengths before and after the addition of BDNF. In a typical experiment, a cell was monitored for 5 min before and 10 min after BDNF application. BDNF was applied to the culture by rapid perfusion with the culture medium containing the final concentration of BDNF. Exposure of cultured Xenopus neurons to 50 ng/ml BDNF caused rapid collapse of growth cones and subsequent withdrawal of neurite processes (Fig. 1). The collapsing response was observed as early as 1 min after BDNF addition, and the maximal neurite retraction was observed within the next 4 min. Whereas some neurons responded to BDNF by drastically withdrawing their processes (Fig. 1A), others displayed moderate neurite retraction and were able to resume extension after the washout of BDNF (Fig. 1B). Growth cones undergoing drastic collapse appeared to maintain some adhesion to the substratum, as evidenced by the thin membrane traces left behind (Fig.1A, double arrowheads), suggesting that the collapse did not result from a complete loss of cell adhesion. The growth cone collapse induced by BDNF is accompanied by a number of morphological changes at the growth cone, most notably including the transient loss of filopodia (Fig. 1, arrows) and the later appearance of lamellipodial protrusion (arrowheads), an effect of BDNF that was also observed in 24 hr cultured Xenopus spinal neurons (Ming et al., 1997).
Fig. 1.
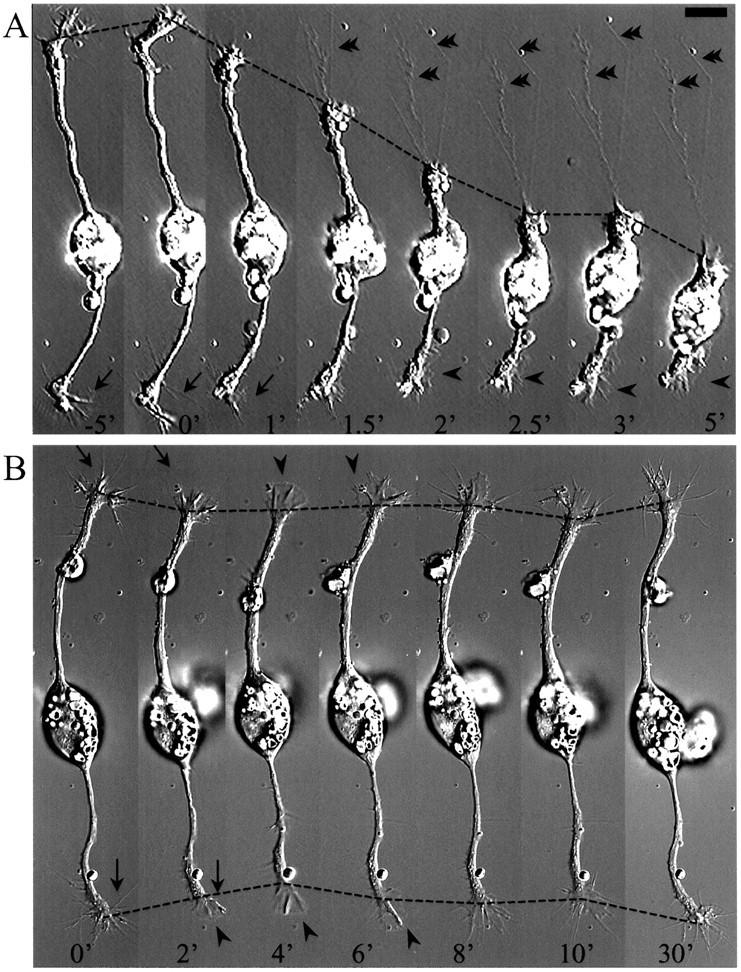
BDNF-induced collapse of growth cones of cultured embryonic Xenopus spinal neurons. Time-lapse DIC sequences showing growth cone collapse followed by dramatic (A) or moderate (B) neurite retraction induced by BDNF. Numbers represent minutes at various times before (negative numbers) and after (positive numbers) the application of 50 ng/ml BDNF (at time 0). In B, BDNF was washed out at 10 min after the application, and neurite extension was observed thereafter. Note the transient loss of filopodia (arrows) after BDNF application and the appearance of lamellipodial protrusion at later times (arrowheads). In A, also note that some adhesion remained during growth cone collapse (double arrowheads). Dashed lines depict the positions of the growth cones at various times. Scale bar, 15 μm.
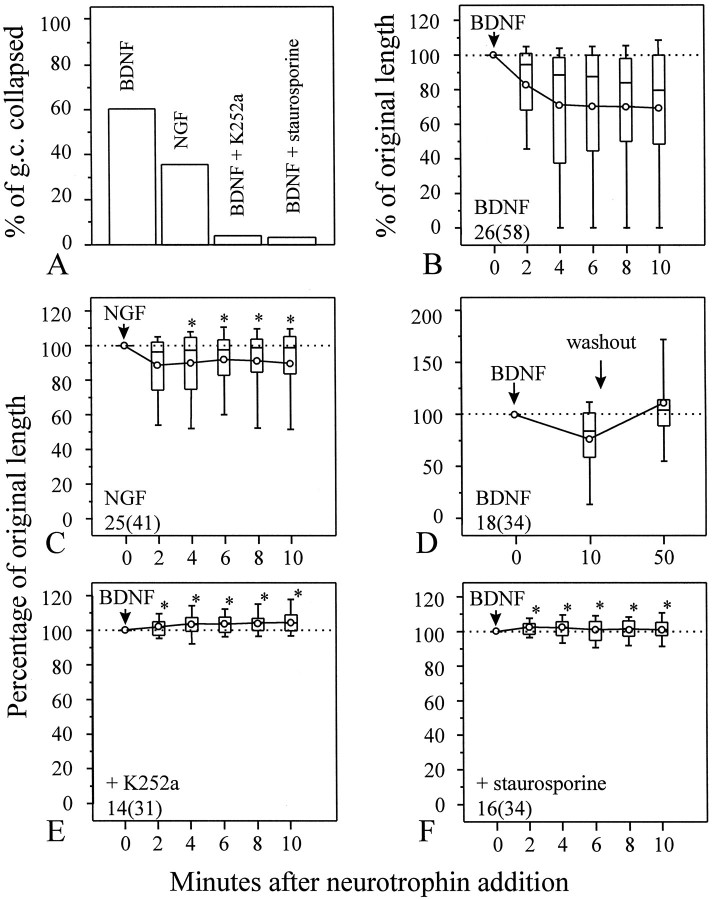
To quantify the collapsing effect, we directly measured the length of each neurite process at the beginning and at various times after the addition of BDNF. A growth cone is considered exhibiting collapsing response when it displays the morphological changes described above and withdraws at least 10 μm (approximately the average size ofXenopus growth cones) at any time point during the 10 min observation after exposure to BDNF. The percentage of growth cones exhibiting collapse was determined and used to assess the overall responsiveness of Xenopus growth cones to neurotrophins (Fig. 2A). To further quantitatively analyze the collapsing response of growth cones from a population of neurons, the length of each neurite process at various times after BDNF addition was measured and normalized against the length before BDNF application and presented as the percentage of original length (POL). POL did not exhibit a normal distribution; therefore, results from populations of neurons are presented as box whisker plots to illustrate the overall response from a population of cells (Fig. 2B). The boxes enclose the 25th and 75th percentiles, the horizontal lines mark the median, and the error bars denote the 10th and 90th percentiles of the distributions. The mean POL at various times after BDNF application is also presented on the same graph as symbol line plots. The majority of neurons responded to acutely applied BDNF by collapsing their growth cones followed by neurite withdrawal within 4 min after BDNF addition; the neurite retraction induced by BDNF was attenuated thereafter. Although some neurons largely withdrew their neurite processes within the 10 min period (indicated by the small POL at the 25th percentile: 41% at 4 min and 49% at 10 min after BDNF addition), most neurons only showed growth cone collapse with a small amount of neurite retraction (median POL, 88% at 4 min and 80% at 10 min), indicating selective responsiveness by different neurons. The collapsing effect seems to be specific for BDNF. The related factor, NGF, at the same concentration was ineffective in inducing collapse of the majority of growth cones (35% of growth cones showing collapse, compared with 60% for BDNF; see Fig. 2A). This is further evidenced by the near 100% median POL after NGF addition (Fig. 2C; median POL, 97% at 4 min and 99% at 10 min), which is significantly higher than that of BDNF alone (*p < 0.05 when compared at corresponding time points, Mann–Whitney test). The collapsing effect of BDNF appears to be reversible. Although regrowth of these retracted processes was not observed during the 10 min period, retracted neurite processes did reextend after BDNF washout (Fig.2D).
Fig. 2.
Quantitative analysis of growth cone collapse induced by neurotrophins. A, The percentage of growth cones collapsed in response to various neurotrophins is determined by measuring the lengths of each neurite at various times after the addition of neurotrophins. A growth cone is considered to exhibit collapse when it withdraws at least 10 μm, in addition to the morphological changes described in Results. B–F, The growth cone collapse was quantified by measuring the lengths of each neurite at various times after the addition of neurotrophins, normalized against the lengths before the neurotrophin addition, and presented as the percentage of original length (POL). POL did not follow a normal distribution; therefore, the data are presented as the box whisker plots. The boxes enclose the 25th and 75th percentiles; the horizontal lines mark the median; and the error bars denote the 10th and 90th percentiles of the distributions. Circles represent the mean POL.B, Acute application of 50 ng/ml BDNF induced rapid growth cone collapse and neurite retraction, but the related factor NGF had little effect on the neurite length (C). When compared with the POL of BDNF (B) at corresponding times after the neurotrophin addition, NGF caused significantly less collapse than BDNF (*p < 0.05, Mann–Whitney test). D, BDNF-induced growth cone collapse is reversible. When BDNF was washed out at the end of 10 min application, growth cones recovered and resumed extension. E, F, BDNF collapsing effect seems to be mediated by Trk receptor tyrosine kinases. Incubation of cells with 200 nm K252a (E) or 100 nm staurosporine (F) completely blocked BDNF-induced collapse [*p < 0.0001, Mann–Whitney test, compared with the POL of BDNF alone (B) at corresponding times after BDNF addition].Numbers indicate the numbers of cells (growth cones) examined.
Involvement of Trk receptor tyrosine kinase
Most of the biological effects of neurotrophins are believed to be mediated by the high-affinity Trk receptor tyrosine kinases: NGF activates TrkA, BDNF and NT-4 activate TrkB, and NT-3 activates TrkC (for review, see Chao, 1992; Barbacid, 1994; Dechant et al., 1994). Binding of neurotrophins to Trk receptors induces dimerization of the receptors followed by autophosphorylation, leading to cascades of signaling events (Kaplan and Stephens, 1994; Barbacid, 1995). The growth cone collapsing effect of BDNF appeared to be mediated by Trk receptors, because it was completely blocked by 200 nmK252a (Fig. 2A,E) or 100 nm staurosporine (Fig. 2A,F). These two potent, structurally related inhibitors for a broad range of serine and threonine protein kinases have been shown to effectively block the activity of Trk receptor tyrosine kinases (Knusel and Hefti, 1992; Nye et al., 1992; Tapley et al., 1992). All neurotrophins also bind to a low-affinity receptor p75, which could modulate Trk activity (Chao and Hempstead, 1995). The potential contribution of p75 receptors in the BDNF-induced growth cone collapse remains to be elucidated.
Ca2+ mediates the collapsing effect
Activation of Trk receptor tyrosine kinases by neurotrophins is known to elicit a range of second messenger responses, including increases in intracellular Ca2+ (Nikodijevic and Guroff, 1991; Berninger et al., 1993; De Bernardi et al., 1996; Stoop and Poo, 1996), cAMP (Knipper et al., 1993a,b), cGMP (Laasberg et al., 1988), and phosphoinositide turnover (Contreras and Guroff, 1987), as well as to activate the protein kinases Src, Raf, and the GTP-binding protein Ras (D’Arcangelo and Halegoua, 1993; Heumann, 1994; for review, see Kaplan and Stephens, 1994; Greene and Kaplan, 1995; Segal and Greenberg, 1996). Ca2+ and cAMP are two important second messengers that are known to affect growth cone motility and behavior (Kater and Mills, 1991; Kim and Wu, 1996). To test whether Ca2+ is involved in BDNF-induced growth cone collapse, we removed extracellular Ca2+ by using a Ca2+-free medium (in mm: 115 NaCl, 2.5 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES, pH 7.4). BDNF-induced growth cone collapse was completely blocked in the Ca2+-free medium (Fig.3A–C). Results from a population of neurons show that growth cones actually extended in the Ca2+-free medium during the 10 min exposure to BDNF (Fig. 3C). The possibility that the removal of extracellular Ca2+ may have interfered with the activation of Trk receptors by BDNF is argued against by the finding that BDNF-induced lamellipodial protrusion was still observed in the Ca2+-free medium (Fig. 3A,B; also seeMing et al., 1997), suggesting that the action of Ca2+-free medium on collapse is likely downstream of the receptor activation. To further test whether an influx of Ca2+ is involved in BDNF-induced growth cone collapse, we loaded the cells with BAPTA (10 μm BAPTA AM for 20 min), a rapid Ca2+ chelator (Tsien, 1980), before the application of BDNF. Loading neurons with BAPTA appeared to slow down the growth cone extension but did not cause growth cone collapse or retraction. However, the BDNF collapsing effect was diminished by BAPTA (Fig. 3D). Taken together, our results suggest that an influx of extracellular Ca2+ is required for the collapse, and that the Ca2+signaling pathway mediates the collapsing effect of BDNF.
Fig. 3.
Ca2+ mediates the collapsing effect of BDNF. A, Representative images showing the changes induced by BDNF (50 ng/ml) in Ca2+-free medium. No growth cone collapse and neurite retraction were observed after BDNF addition. However, BDNF-induced lamellipodial protrusion was still observed (arrows). Numbersrepresent minutes after BDNF addition. Dashed linesindicate corresponding positions along the neurite. B, BDNF-induced lamellipodial protrusion was observed not only at the growth cone (arrow) but also along the neurite shaft (arrowhead). The image was taken 10 min after BDNF application. C, Data from populations of cells in the Ca2+-free medium are presented as a box whisker plot. Apparently no collapse was observed after BDNF application. D, Loading cells with 10 μmBAPTA AM for 20 min also blocked the collapsing effect of BDNF. No substantial changes in neurite length were observed. *Significantly different from the POL of BDNF in culture medium (Fig.2B) at corresponding times after BDNF addition (p < 0.001, Mann–Whitney test).Numbers in C and Dindicate the numbers of cells (growth cones) examined. Scale bars, 10 μm.
cAMP regulates the collapsing effect
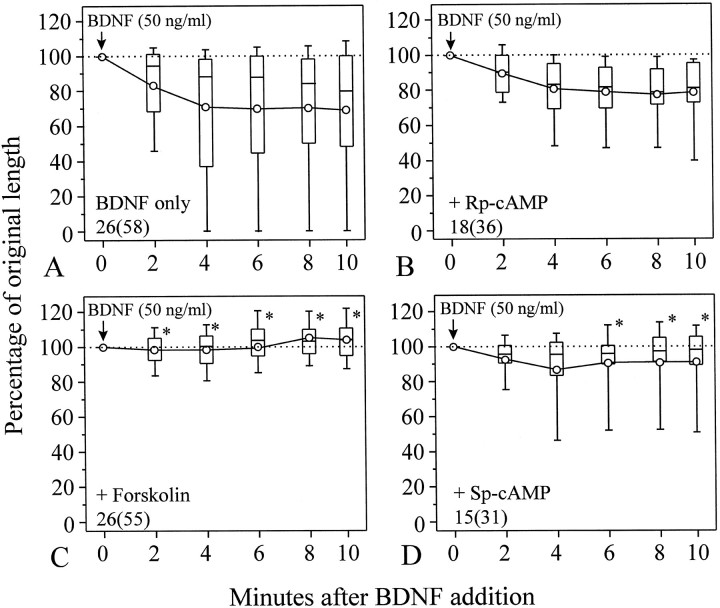
We further tested the role of cAMP in BDNF-induced growth cone collapse. Application of 100 μm(Rp)-cAMP, a competitive inhibitor of protein kinase A (Rothermel and Parker Botelho, 1988; Dostmann et al., 1990), did not affect normal neurite extension, nor did it inhibit growth cone collapse induced by 50 ng/ml BDNF (Fig.4B). Approximately 66% of (Rp)-cAMP-treated growth cones were collapsed by the addition of BDNF, a percentage not significantly different from that observed for growth cones exposed to BDNF alone (60%; see Fig. 2A). However, the collapsing effect of BDNF was completely blocked by 10 μm water-soluble derivative of forskolin (Fig. 4C, *p < 0.05, Mann–Whitney test), an agent known to stimulate membrane-bound adenylate cyclase, resulting in an elevation of [cAMP]i(Laurenza et al., 1987). To further confirm that forskolin blockade of the collapse is directly associated with an elevation of [cAMP]i, we applied (Sp)-cAMP, a membrane-permeant cAMP analog that is resistant to hydrolysis (Rothermel and Parker Botelho, 1988; Dostmann et al., 1990). Although not as effective as forskolin, application of 500 μm(Sp)-cAMP blocked BDNF-induced growth cone collapse (Fig. 4D, *p < 0.05, Mann–Whitney test). The blockade of the BDNF collapsing effect by the elevation of [cAMP]i is further supported by the small percentage of growth cones collapsed after exposure to 50 ng/ml BDNF (14.5 and 19.4% for forskolin- and (Sp)-cAMP-treated growth cones, respectively, compared with 60% for growth cones exposed to BDNF only).
Fig. 4.
cAMP regulation of BDNF-induced growth cone collapse. A, Growth cone collapse induced by BDNF alone (from Fig. 2B). B, The culture was preincubated with 100 μm(Rp)-cAMP for 20 min before BDNF application, and (Rp)-cAMP was present throughout the experiment. No significant difference fromA was detected (*p > 0.5, Mann–Whitney test). C, D, Application of 10 μm forskolin (C) or 500 μm (Sp)-cAMP (D) blocked the collapsing effect. Both chemicals were preincubated with the culture for 20 min before the application of BDNF and present throughout the experiment. Numbersrepresent numbers of cells (growth cones) examined. *Significantly different from BDNF alone (A) at corresponding times (p < 0.05, Mann–Whitney tests).
Although inhibition of protein kinase A did not significantly affect the collapse induced by 50 ng/ml BDNF, (Rp)-cAMP did exert influence on the collapse induced by BDNF at lower concentrations. For example, BDNF at 5 ng/ml was ineffective to induce growth cone collapse. The percentage of growth cones collapsed (25%) and the extent of neurite retraction induced by 5 ng/ml BDNF were small (Fig.5A). When 100 μm(Rp)-cAMP was applied to the culture, the collapsing effect was potentiated (Fig. 5B). The percentage of growth cones collapsed in response to 5 ng/ml BDNF was also increased to 46% when growth cones were treated with (Rp)-cAMP. To better illustrate the potentiation of the BDNF collapsing effect by (Rp)-cAMP, the distribution of POL from populations of growth cones at 10 min after BDNF addition was plotted as a histogram. The presence of (Rp)-cAMP caused more growth cones to respond to BDNF by collapse, as indicated by an increased number of growth cones with smaller POL values when compared with that of 5 ng/ml BDNF alone (Fig. 5C, arrow). Statistical analysis further confirms the significance of the potentiation effect on BDNF-induced collapse by the inhibition of PKA (Fig. 5B, *p < 0.05, Mann–Whitney test).
Fig. 5.
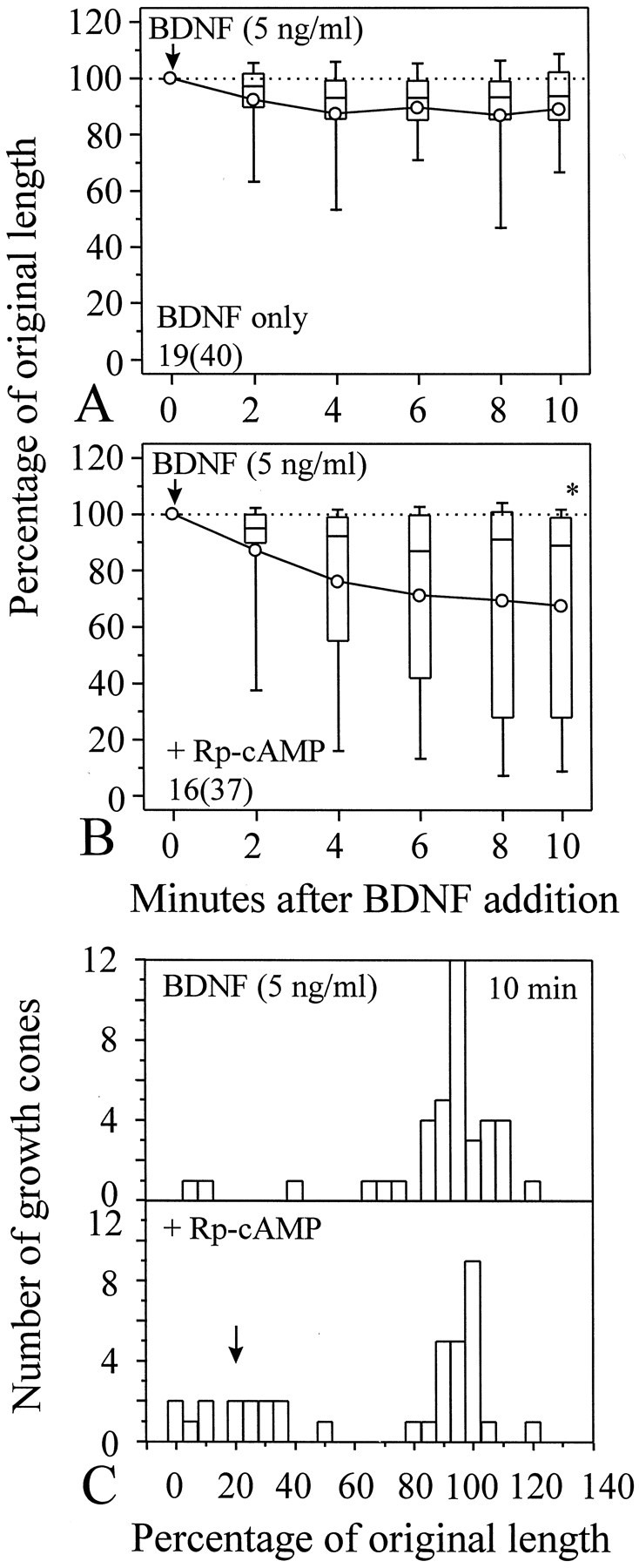
Potentiation of BDNF-induced growth cone collapse by (Rp)-cAMP. A, Growth cone collapse induced by 5 ng/ml BDNF alone. B, One hundred micromolar (Rp)-cAMP was added to the culture 20 min before the application of 5 ng/ml BDNF. Increased collapse was observed [*p < 0.05, Mann–Whitney test, compared with the POL of BDNF alone (A) at the corresponding time].Numbers in A and Brepresent numbers of cells (growth cones) examined. C, Histogram showing the difference in the distribution of POL between BDNF alone (top panel) and BDNF with (Rp)-cAMP treatment (bottom panel) at 10 min after BDNF application (5 ng/ml). Presence of (Rp)-cAMP in the medium caused more growth cones to respond to BDNF with collapse, as evidenced by the appearance of a peak of the POL distribution at smaller POL (arrow).
Loss of collapsing response of old neurons to BDNF in 24 hr cultures
The collapsing effect of BDNF was only observed on neurons in 6–8 hr cultures in which motile growth cones and active neurite extension are observed. In 24 hr cultures, although active neurite extension was still observed but at a slightly reduced rate (Ming et al., 1997), no apparent collapsing effect of BDNF was observed (Fig.6A). Conversely, BDNF was found to elicit extensive lamellipodial protrusion along the neurite shaft and to induce chemoattractive turning of the growth cone (Ming et al., 1997; Song et al., 1997). These results suggest that neurons may respond to the same tropic factors differently at different developmental stages. Because a clear cAMP regulation of BDNF-induced collapse was observed in 6 hr cultures, it is possible that neurons in 24 hr cultures may have higher resting levels of endogenous cAMP-dependent activity, which could block the collapsing effect of BDNF. To test this hypothesis, we applied (Rp)-cAMP (100 μm) to 24 hr cultures to inhibit the activity of PKA. When 50 ng/ml BDNF was applied to these (Rp)-cAMP-treated neurons, significant collapse of growth cones was observed in these 24 hr cultures (Fig. 6B, *p < 0.001, Mann–Whitney test). It appears that (Rp)-cAMP restored the collapsing response of 24 hr cultures to a level similar to that of 6 hr cultures. The percentage of growth cones collapsed by 50 ng/ml BDNF was also increased from 25 to 81% after growth cones were treated with (Rp)-cAMP in 24 hr cultures. These results suggest that the loss of the collapsing response to BDNF by neurons in 24 hr cultures is tightly associated with the endogenous cAMP-dependent activity, which may be elevated during the development.
Fig. 6.
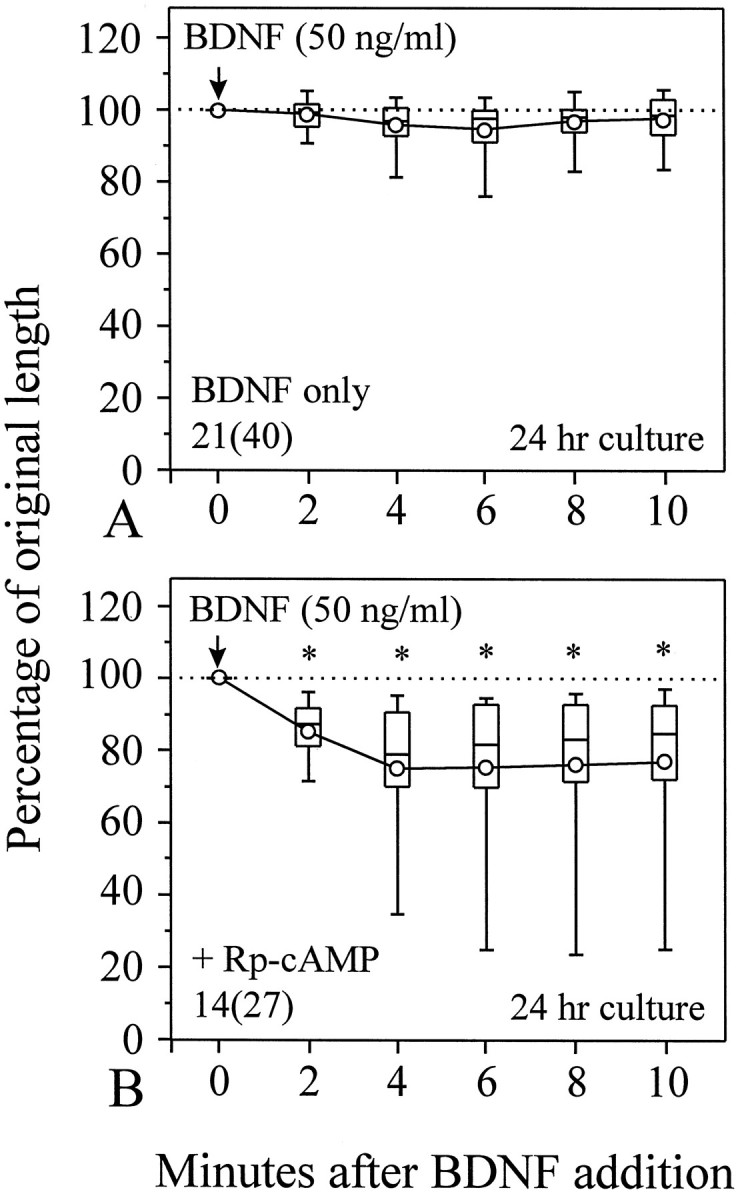
Endogenous cAMP-dependent activity plays a key role in the disappearance of the BDNF collapsing effect on neurons in 24 hr cultures. A, BDNF was apparently ineffective in inducing growth cone collapse in 24 hr cultures. However, when endogenous cAMP-dependent activity was inhibited by 100 μm (Rp)-cAMP, the collapsing effect of BDNF was clearly observed (B). *Significantly different from the data inA at corresponding times (p< 0.001, Mann–Whitney test).
Involvement of the actin cytoskeleton in BDNF-induced growth cone collapse
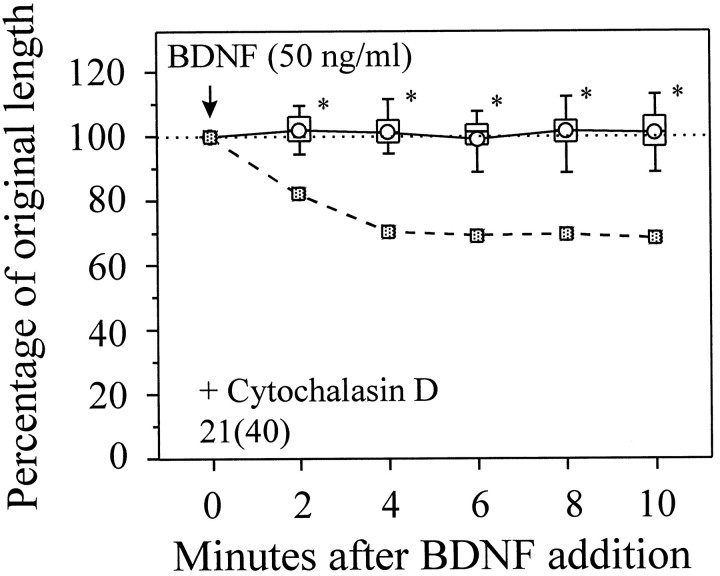
Although the signaling events downstream of second messengers are unknown, the actin cytoskeleton seems to be one of the final targets in BDNF-induced growth cone collapse. Cytochalasins are fungal metabolites that bind to the barbed ends of actin filaments to prevent further polymerization, leading to the disassembly of actin filaments over time. Application of cytochalasin D at 1 μm did not cause neurite retraction but effectively abolished BDNF-induced growth cone collapse (Fig. 7) as well as lamellipodial protrusion (Ming et al., 1997). Dependence on actin polymerization has been observed previously in NGF-induced rapid neurite retraction of cultured sensory neurons (Griffin and Letourneau, 1980). It was suggested that extensive membrane ruffling induced at such thin neurite processes by growth factors may directly cause neurite retraction (Griffin and Letourneau, 1980), mainly as the result of cytoskeletal reorganization. However, the presence of lamellipodial protrusion and the blockade of collapsing effect in the Ca2+-free medium (Fig. 3) argue strongly against this possibility. Other actin-based mechanism(s) must be involved in the collapse.
Fig. 7.
Cytochalasin D blocks growth cone collapse induced by BDNF. The mean POL from growth cones exposed to BDNF alone (Fig.2B) is presented as the reference (squares, dashed line). Cytochalasin D at 1 μm did not reduce the neurite length but blocked the collapsing effect of BDNF. *Significantly different from that of BDNF alone (Fig. 2B) at corresponding times (p < 0.05, Mann–Whitney test).
DISCUSSION
Acute, inhibitory effects of neurotrophins on growth cone motility
An increasing number of studies have established a “positive” role for neurotrophins in axonal and dendritic growth. In addition to the key activity of neurotrophins in promoting neurite outgrowth of responsive neuronal populations, neurotrophins have been shown to increase axonal sprouting (Diamond et al., 1992; Cohen-Cory and Fraser, 1995) and to regulate morphological development of dendrites (Cohen-Cory et al., 1991; McAllister et al., 1995, 1996). In ourXenopus cultures, addition of neurotrophins enhances neuronal survival and promotes neurite outgrowth of embryonic spinal neurons; when applied as concentration gradients, BDNF and NT-3 also exert chemotropic effects on growth cones in 18–24 hr cultures (Ming et al., 1997; Song et al., 1997). In this study, we provide evidence on a “negative” effect of BDNF on growth cone extension of embryonicXenopus neurons in 6 hr cultures: acute application of BDNF induces rapid growth cone collapse and neurite retraction. The effective neurotrophin concentrations for growth cone collapse are similar to those known to promote neuronal survival as well as to elicit a number of acute effects on these neurons (Ming et al., 1997). The collapsing effect appears to be relatively specific for BDNF, because NGF was ineffective in inducing growth cone collapse. Only a small portion of growth cones collapsed in response to NGF (35% of total growth cones examined, compared with 60% for BDNF). The lack of the collapsing effect of NGF is further demonstrated by the significantly smaller extent of neurite retraction than that of BDNF (Fig. 2C). Given the heterogeneity of Xenopuscultures, it is not surprising for the existence of subpopulations of neurons that may respond to different neurotrophins differently (Lohof et al., 1993; Ming et al., 1997). Nevertheless, the significant difference between BDNF and NGF in the ability of inducing growth cone collapse further supports the conclusion that the high-affinity Trk receptor (TrkB for BDNF), rather than the low-affinity p75 receptor, mediates the collapsing effect; otherwise, similar extents of growth cone collapse would have been observed for BDNF and NGF, because both can activate p75 effectively. However, our results do not exclude the possibility that p75 may be involved, but not required, in the collapsing action of neurotrophins. The possible contribution from the p75 receptor activation in BDNF-induced growth cone collapse remains to be determined.
Ca2+ is an important second messenger involved in regulation of a wide range of cellular activities, including growth cone motility (Kater and Mills, 1991; Zheng et al., 1996a). In BDNF-induced growth cone collapse, an influx of Ca2+is required for the collapse, suggesting that the Ca2+-dependent signaling pathway mediates the collapse. Furthermore, the collapsing response is regulated by cAMP-dependent activity, because elevation or inhibition of cytosolic cAMP-dependent activity attenuated or potentiated the collapse, respectively. This cAMP-mediated regulation appears to play a key role in the loss of the collapsing response to BDNF by old neurons in 24 hr cultures. The endogenous cAMP-dependent activity in these neurons may be elevated to a higher level than that in neurons from 6 hr cultures, therefore leading to the blockade of the collapsing effect of BDNF. Our finding that inhibition of PKA by (Rp)-cAMP restored the collapsing response of these old neurons confirms such a notion. The cellular mechanism underlying the elevated levels of cAMP-dependent activity in these old neurons remains to be studied. Nevertheless, these results thus resolve the apparent contradiction between the negative collapsing effect and the positive effects of neurotrophins on neurite growth observed on same Xenopus cultures. By modulating its cytosolic levels of cAMP-dependent activity at different stages during development, a neuron could thus exhibit different responses to the same extracellular signals.
Although the cellular mechanism underlying BDNF-induced growth cone collapse is largely unknown at this moment, the actin cytoskeleton seems to play an important role. The result that cytochalasin D completely blocked the collapse induced by BDNF suggests that actin polymerization may be required for the collapse. However, in growth cone collapse induced by collapsin-1, actin depolymerization was observed (Fan et al., 1993), indicating that the collapse induced by BDNF and collapsin-1 may involve different cellular processes. On the other hand, cytochalasins are known not only to prevent actin polymerization but also to disrupt the existing actin cytoskeletal network. Therefore, the blockade of BDNF-induced collapse by cytochalasins could also suggest that an intact actin network is required for BDNF collapsing action, either for signal transduction or for force generating. In neurite retraction induced by lysophosphatidade (LPA) and thrombin, a similar dependence on the actin cytoskeleton was observed, and an actomyosin contractile mechanism was suggested to mediate the neurite retraction (Jalink and Moolenaar, 1992; Jalink et al., 1993). However, whether the same mechanism operates in BDNF-induced growth cone collapse and neurite retraction remains to be determined.
Mechanism(s) underlying the cAMP regulation of BDNF-induced collapse: a tentative model
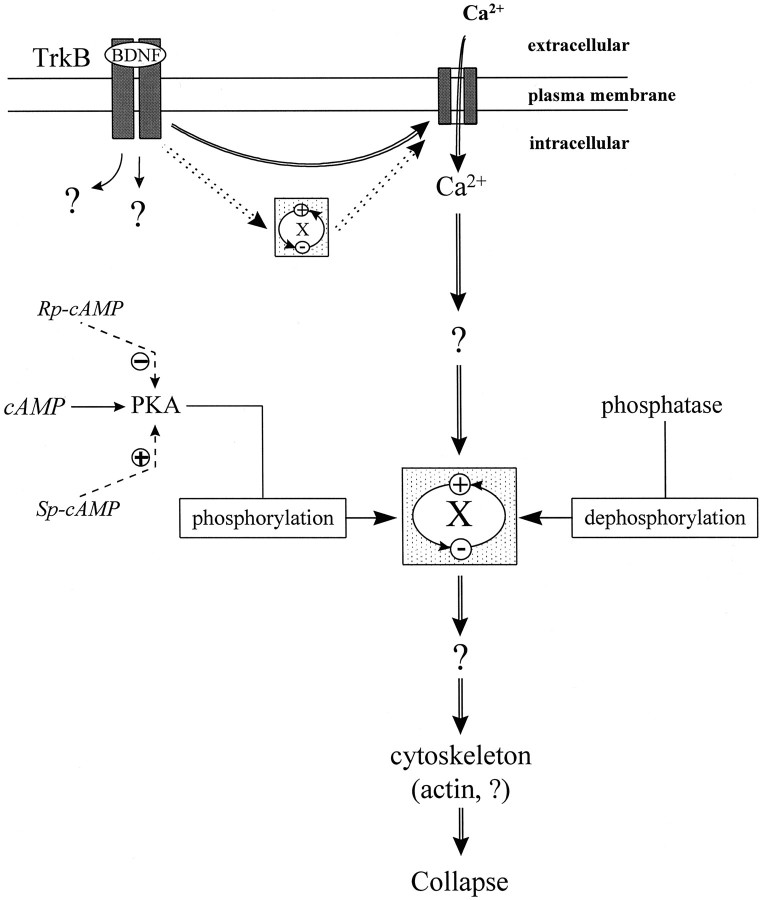
The cAMP pathway has been suggested to regulate the activity of other signal transduction pathways (for review, see Iyengar, 1996). In smooth muscle, contractility is regulated by myosin light-chain kinase (MLCK), which can be phosphorylated by PKA, cGMP-dependent protein kinases, and Ca2+-/calmodulin-dependent protein kinase II (for review, see Burridge and Chrzanowska-Wodnicka, 1996). Phosphorylation of MLCK decreases its affinity for Ca2+ and calmodulin, inhibiting MLCK activity and contractility (Nishikawa et al., 1984; Tansey et al., 1994). It was suggested that LPA-induced neurite retraction is mediated by the contraction of cortical actin cytoskeleton, which may also be subjected to this kind of regulation (Tigyi et al., 1996). We hypothesize a similar cAMP-dependent regulation in BDNF-induced growth cone collapse (see Fig. 8). Elevation of [cAMP]i in these cultured Xenopus neurons activates protein kinase A, which in turn phosphorylates the unknown protein “X” that might be involved in the main signaling pathway (Ca2+-mediated) in BDNF-induced collapse. The phosphorylation inactivates X and blocks the collapse; dephosphorylation of X by phosphatases could reactivate the protein. The resting levels of PKA and phosphatase activity in the cell are likely to reach a balance, leading to an equilibrium between active and inactive forms of X. Inhibition of PKA thus can change the equilibrium and increase the level of active X, leading to the potentiation of the collapsing effect induced by low concentrations of BDNF, which was observed in this study. At high concentrations of BDNF, the collapsing effect may already be saturated, and further enhancement by PKA inhibitors cannot be produced. Although our hypothesized model places cAMP-mediated regulation downstream of Ca2+, it is possible that such cAMP regulation is upstream of Ca2+; that is, X mediates BDNF-induced Ca2+ influx to induce collapse that can be regulated by cAMP-dependent activity in a similar matter.
Fig. 8.
Schematic diagram showing the proposed model involved in the cAMP regulation of BDNF-induced collapse. Activation of TrkB receptors by BDNF induces multiple intracellular signaling cascades including a Ca2+ influx through Ca2+ channels, and the Ca2+pathway mediates the collapsing effect (double lineswith arrows). Although the unknown protein X is involved in the main Ca2+-signaling pathway for collapse, its activity can be modulated by phosphorylation and dephosphorylation by cAMP-dependent activity (single lines witharrows). PKA phosphorylates and inactivates (−) X, whereas phosphotase(s) dephosphorylate(s) and activate(s) (+) X. An equilibrium between the active and inactive X is likely reached in the cell. Experimental manipulation of PKA activity by (Rp)-cAMP or (Sp)-cAMP (dashed lines with arrows) can change the equilibrium and thus regulate the collapsing effect of BDNF. Activation of PKA by (Sp)-cAMP causes more X to become inactive, leading to the blockade of the collapsing effect; inhibition of the resting level of PKA activity by (Rp)-cAMP results in more active X, leading to the potentiation of the collapsing effect induced by the low concentrations of BDNF. Alternatively, the cAMP-mediated regulation can be upstream of Ca2+. In this case, the protein X mediates BDNF-induced Ca2+ influx required for growth cone collapse (dotted double lines witharrows), and its activity can be regulated by cAMP-dependent activity in a similar way.
Collapsing effect and growth cone guidance: the in vivo relevance
Recent studies have identified an increasing number of receptor tyrosine kinases involved in axonal guidance (Tessier-Lavigne and Goodman, 1996). Although no in vivo evidence is available for a role of neurotrophic factors in axonal guidance, the chemoattractive effects of neurotrophins in culture suggest such a potential (Letourneau, 1978; Menesini-Chen et al., 1978; Gundersen and Barrett, 1980; Ming et al., 1997; Song et al., 1997). The collapsing effect observed here, together with previous observations of similar inhibitory effects of neurotrophins in vivo and in cell culture (Griffin and Letourneau, 1980; Zhang et al., 1994; Paves and Saarma, 1997), suggests that neurotrophins may exert acute, negative regulation on neuronal development. The observation that BDNF administered through a micropipette could also produce a similar inhibitory effect on Xenopus growth cones in 6 hr cultures (Q. Wang and J. Q. Zheng, unpublished observation) suggests that the negative regulation of axonal growth could be achieved by neurotrophins derived from point sources, such as those neurotrophin-expressing cells along developing axonal pathways, as well as target tissues (Elkabes et al., 1994; Hallböök et al., 1995). Such negative regulation of axonal growth, if occurring in the early stages during neuronal development in vivo, could be used to temporarily halt axonal extension to prevent developing axons from entering incorrect intermediate regions and targeting prematurely; by switching the dependence on specific neurotrophins (Davies, 1994a) or by modulating its endogenous cAMP activity in the later stages of development, a growth cone could then change its responsiveness to accommodate the positive influence of neurotrophins (O’Connor et al., 1990; Kuhn et al., 1995; Gallo et al., 1997).
The cAMP-dependent activity has recently been shown to regulate the turning direction of Xenopus growth cones exposed to BDNF gradients in 18–24 hr cultures (Song et al., 1997). A gradient of BDNF normally induces a chemoattractive response in these neurons (Ming et al., 1997; Song et al., 1997). It was proposed that a cytoplasmic Ca2+ gradient induced by the BDNF gradient produces the repulsive turning of the growth cone, which is normally overridden by a Ca2+-induced cAMP gradient that attracts growth cones. Inhibition of cAMP-dependent activity thus results in an opposite turning response. Because the repulsive turning of growth cones is thought to result from a local, asymmetric collapse of the growth cone (Fan and Raper, 1995), our finding of the collapsing effect of BDNF provides the direct evidence to support the repulsive action of BDNF on embryonic Xenopus neurons. However, our data show that such a collapsing and repulsive effect of BDNF is mainly blocked by the change of endogenous cAMP-dependent activity in these 18–24 hr neurons; the Ca2+-induced cAMP gradient, on the other hand, might be responsible for inducing attractive turning of the growth cone in BDNF gradients after the repulsive effect is blocked. When endogenous cAMP-dependent activity is inhibited in these 24 hr cultures, the collapsing (this study) and chemorepulsive (Song et al., 1997) effect of BDNF is observed. The cAMP-mediated regulation observed in this study might also play a role in repulsive guidance of growth cones by other molecules. Our preliminary study has shown that elevation of [cAMP]i by forskolin completely blocked the repulsive turning of Xenopus growth cones induced by extracellular gradients of semaphorin III in culture (Q. Wang and J. Q. Zheng, unpublished observations), suggesting that the cAMP-dependent activity could regulate the repulsive action of semaphorins. Developing neurons in vivo do encounter many extracellular factors that act through second messenger-dependent mechanisms, including the cAMP-dependent pathway. Concurrent presence or absence of these factors may thus regulate the different responses of growth cones to the guidance cues. Furthermore, a neuron may switch on or off the pathways responsible for different cellular responses to the same guidance cues by its intrinsic programming, allowing different responses at different stages during development.
Footnotes
This work was supported by a University of Medicine and Dentistry of New Jersey Foundation research seed grant and a startup fund from the Department of Neuroscience and Cell Biology at University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School. We thank Dr. Ann M. Lohof (Laboratoire de Neurobiologie, Ecole Normale Supérieure, Paris, France) for valuable input during manuscript preparation, Dr. Ira B. Black for review and comments on this paper, and Ms. Jean Gibney for technical assistance.
Correspondence should be addressed to Dr. James Q. Zheng, Department of Neuroscience and Cell Biology, University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, 675 Hoes Lane, Piscataway, NJ 08854.
REFERENCES
- 1.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 3.Barde YA. The nerve growth factor family. Prog Growth Factor Res. 1990;2:237–248. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- 4.Berninger B, Garcia DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. NeuroReport. 1993;4:1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bray D, Hollenbeck PJ. Growth cone motility and guidance. Annu Rev Cell Biol. 1988;4:43–61. doi: 10.1146/annurev.cb.04.110188.000355. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 8.Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- 9.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 10.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J Neurosci. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras ML, Guroff G. Calcium-dependent nerve growth factor-stimulated hydrolysis of phosphoinositides in PC12 cells. J Neurochem. 1987;48:1466–1472. doi: 10.1111/j.1471-4159.1987.tb05687.x. [DOI] [PubMed] [Google Scholar]
- 13.D’Arcangelo G, Halegoua S. A branched signaling pathway for nerve growth factor is revealed by Src-, Ras-, and Raf-mediated gene inductions. Mol Cell Biol. 1993;13:3146–3155. doi: 10.1128/mcb.13.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies AM. Neurotrophic factors. Switching neurotrophin dependence. Curr Biol. 1994a;4:273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 15.Davies AM. The role of neurotrophins in the developing nervous system. J Neurobiol. 1994b;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- 16.Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 17.De Bernardi MA, Rabin SJ, Colangelo AM, Brooker G. TrkA mediates the nerve growth factor-induced intracellular calcium accumulation. J Biol Chem. 1996;271:6092–6098. doi: 10.1074/jbc.271.11.6092. [DOI] [PubMed] [Google Scholar]
- 18.Dechant G, Rodriguez-Tebar A, Barde YA. Neurotrophin receptors. Prog Neurobiol. 1994;42:347–352. doi: 10.1016/0301-0082(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 19.Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:454–466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodd J, Schuchardt A. Axon guidance: a compelling case for repelling growth cones. Cell. 1995;81:471–474. doi: 10.1016/0092-8674(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 21.Dostmann WRG, Taylor SS, Genieser HG, Jastorff B, Doskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- 22.Elkabes S, Dreyfus CF, Schaar DG, Black IB. Embryonic sensory development: local expression of neurotrophin-3 and target expression of nerve growth factor. J Comp Neurol. 1994;341:204–213. doi: 10.1002/cne.903410206. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Raper JA. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Mansfield SG, Redmond T, Gordon-Weeks PR, Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 26.Gallo G, Lefcort FB, Letourneau PC. The Trka receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene LA, Kaplan DR. Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin CG, Letourneau PC. Rapid retraction of neurites by sensory neurons in response to increased concentrations of nerve growth factor. J Cell Biol. 1980;86:156–161. doi: 10.1083/jcb.86.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87:546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallböök F, Ibanez CF, Ebendal T, Persson H. Cellular localization of brain-derived neurotrophic factor and neurotrophin-3 mRNA expression in the early chicken embryo. Eur J Neurosci. 1993;5:1–14. doi: 10.1111/j.1460-9568.1993.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 31.Hallböök F, Backstrom A, Kullander K, Kylberg A, Williams R, Ebendal T. Neurotrophins and their receptors in chicken neuronal development. Int J Dev Biol. 1995;39:855–868. [PubMed] [Google Scholar]
- 32.Heumann R. Neurotrophin signalling. Curr Opin Neurobiol. 1994;4:668–679. doi: 10.1016/0959-4388(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 33.Iyengar R. Gating by cyclic AMP: expended role for an old signaling pathway. Science. 1996;271:461–462. doi: 10.1126/science.271.5248.461. [DOI] [PubMed] [Google Scholar]
- 34.Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 36.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1562. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan DR, Stephens RM. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 38.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keynes RJ, Cook GM. Axon guidance molecules. Cell. 1995a;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 40.Keynes RJ, Cook GM. Repulsive and inhibitory signals. Curr Opin Neurobiol. 1995b;5:75–82. doi: 10.1016/0959-4388(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim HG, Wang T, Olafsson P, Lu B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci USA. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y-t, Wu CF. Reduced growth cone motility in cultured neurons from Drosophila memory mutants with a defective cAMP cascade. J Neurosci. 1996;16:5593–5602. doi: 10.1523/JNEUROSCI.16-18-05593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein R. Role of neurotrophins in mouse neuronal development. FASEB J. 1994;8:738–744. doi: 10.1096/fasebj.8.10.8050673. [DOI] [PubMed] [Google Scholar]
- 44.Knipper M, Beck A, Rylett J, Breer H. Neurotrophin induced cAMP and IP3 responses in PC12 cells. Different pathways. FEBS Lett. 1993a;324:147–152. doi: 10.1016/0014-5793(93)81382-a. [DOI] [PubMed] [Google Scholar]
- 45.Knipper M, Beck A, Rylett J, Breer H. Neurotrophin induced second messenger responses in rat brain synaptosomes. NeuroReport. 1993b;4:483–486. doi: 10.1097/00001756-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuffler DP. Promoting and directing axon outgrowth. Mol Neurobiol. 1994;9:233–243. doi: 10.1007/BF02816122. [DOI] [PubMed] [Google Scholar]
- 48.Kuhn TB, Schmidt MF, Kater SB. Laminin and fibronectin guideposts signal sustained but opposite effects to passing growth cones. Neuron. 1995;14:275–285. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 49.Laasberg T, Pihlak A, Neuman T, Paves H, Saarma M. Nerve growth factor increases the cyclic GMP level and activates the cyclic GMP phosphodiesterase in PC12 cells. FEBS Lett. 1988;239:367–370. doi: 10.1016/0014-5793(88)80953-0. [DOI] [PubMed] [Google Scholar]
- 50.Laurenza A, Khandelwal Y, De Souza NJ, Rupp RH, Metzger H, Seamon KB. Stimulation of adenylate cyclase by water-soluble analogues of forskolin. Mol Pharmacol. 1987;32:133–139. [PubMed] [Google Scholar]
- 51.Letourneau PC. Chemotactic response of nerve fiber elongation to nerve growth factor. Dev Biol. 1978;66:183–196. doi: 10.1016/0012-1606(78)90283-x. [DOI] [PubMed] [Google Scholar]
- 52.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 54.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 55.Lumsden AG, Davies AM. Earliest sensory nerve fibers are guided to peripheral targets by attractants other than nerve growth factor. Nature. 1983;306:786–788. doi: 10.1038/306786a0. [DOI] [PubMed] [Google Scholar]
- 56.Lundborg G, Dahlin L, Danielsen N, Zhao Q. Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg. 1994;10:345–354. doi: 10.1055/s-2007-1006604. [DOI] [PubMed] [Google Scholar]
- 57.Luo Y, Raper JA. Inhibitory factors controlling growth cone motility and guidance. Curr Opin Neurobiol. 1994;4:648–654. doi: 10.1016/0959-4388(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 58.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 59.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 60.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 61.Menesini-Chen MG, Chen JS, Levi-Montalcini R. Sympathetic nerve fibers ingrowth in the CNS of neonatal rodent upon intracerebral NGF injections. Arch Ital Biol. 1978;116:53–84. [PubMed] [Google Scholar]
- 62.Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 63.Ming GL, Lohof AM, Zheng JQ. Acute morphogenic and chemotropic effects of neurotrophins on cultured embryonic Xenopus spinal neurons. J Neurosci. 1997;17:7860–7871. doi: 10.1523/JNEUROSCI.17-20-07860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. Elsevier; Amsterdam: 1967. [Google Scholar]
- 65.Nikodijevic B, Guroff G. Nerve growth factor-induced increase in calcium uptake by PC12 cells. J Neurosci Res. 1991;28:192–199. doi: 10.1002/jnr.490280206. [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa M, Sellers JR, Adelstein RS, Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J Biol Chem. 1984;259:8808–8814. [PubMed] [Google Scholar]
- 67.Nye SH, Squinto SP, Glass DJ, Stitt TN, Hantzopoulos P, Macchi MJ, Lindsay NS, Ip NY, Yancopoulos GD. K-252a and staurosporine selectively block autophosphorylation of neurotrophin receptors and neurotrophin-mediated responses. Mol Biol Cell. 1992;3:677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connor TP, Duerr JS, Bentley D. Pioneer growth cone steering decisions mediated by single filopodial contacts in situ. J Neurosci. 1990;10:3935–3946. doi: 10.1523/JNEUROSCI.10-12-03935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290:285–297. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- 70.Pini A. Chemorepulsion of axons in the developing mammalian CNS. Science. 1993;261:95–98. doi: 10.1126/science.8316861. [DOI] [PubMed] [Google Scholar]
- 71.Pini A. Axon guidance. Growth cones say no. Curr Biol. 1994;4:131–133. doi: 10.1016/s0960-9822(94)00029-1. [DOI] [PubMed] [Google Scholar]
- 72.Püschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 73.Rothermel JD, Parker Botelho LH. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–389. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 75.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 76.Snider WD, Johnson EM., Jr Neurotrophic molecules. Ann Neurol. 1989;26:489–506. doi: 10.1002/ana.410260402. [DOI] [PubMed] [Google Scholar]
- 77.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 78.Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabti N, Poo MM. Culturing spinal cord neurons and muscle cells from Xenopus embryos. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1990. pp. 137–154. [Google Scholar]
- 81.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem. 1994;269:9912–9920. [PubMed] [Google Scholar]
- 82.Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- 83.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 84.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 85.Tigyi G, Fischer DJ, Sebök A, Marshall F, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-protective effects of cyclic AMP signaling. J Neurochem. 1996;66:549–558. doi: 10.1046/j.1471-4159.1996.66020549.x. [DOI] [PubMed] [Google Scholar]
- 86.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 87.Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Schmidt RE, Yan Q, Snider WD. NGF and NT-3 have differing effects on the growth of dorsal root axons in developing mammalian spinal cord. J Neurosci. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng JQ, Poo MM, Connor JA. Calcium and chemotropic turning of nerve growth cones. Perspect Dev Neurobiol. 1996a;4:205–213. [PubMed] [Google Scholar]
- 90.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996b;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]