Abstract
The neuropeptide galanin is overexpressed in the basal forebrain in Alzheimer’s disease (AD). In rats, galanin inhibits evoked hippocampal acetylcholine release and impairs performance on several memory tasks, including delayed nonmatching to position (DNMTP). Galanin(1–13)-Pro2-(Ala-Leu)2-Ala-NH2(M40), a peptidergic galanin receptor ligand, has been shown to block galanin-induced impairment on DNMTP in rats. M40 injected alone, however, does not improve DNMTP choice accuracy deficits in rats with selective cholinergic immunotoxic lesions of the basal forebrain. The present experiments used a strategy of combining M40 with an M1 cholinergic agonist in rats lesioned with the cholinergic immunotoxin 192IgG-saporin. Coadministration of intraventricular M40 with intraperitoneal 3-(3-S-n-pentyl-1,2,5-thiadiazol-4-yl)-1,2,5,6-tetrahydro-1-methylpyridine (TZTP), an M1 agonist, improved choice accuracy significantly more than a threshold dose of TZTP alone. These results suggest that a galanin antagonist may enhance the efficacy of cholinergic treatments for the cognitive deficits of AD.
Keywords: Alzheimer’s disease, acetylcholine, muscarinic receptors, galanin, neuropeptide, lesion model, memory
Galanin is a well characterized peptide neurotransmitter and is widely distributed in mammalian brain (Tatemoto et al., 1983; Melander et al., 1986; Skofitsch and Jacobowitz 1985, 1986; Merchenthaler et al., 1993). Studies in rodents and monkeys demonstrate that galanin inhibits presynaptic evoked acetylcholine release in hippocampal slices and in hippocampal microdialysate (Fisone et al., 1987, 1991; Consolo et al., 1991; Bartfai et al., 1992;Robinson et al., 1996b). In addition, galanin inhibits postsynaptic carbachol-stimulated phosphatidyl inositol hydrolysis in hippocampal slices (Fisone et al., 1991; Palazzi et al., 1991). Central galanin injections impair performance of learning and memory tasks, including delayed nonmatching to position (DNMTP), delayed alternation, Morris water maze, starburst maze, and passive avoidance (Sundstrom et al., 1988; Givens et al., 1992; Malin et al., 1992; Robinson and Crawley, 1993, 1994; Ukai et al., 1995; McDonald and Crawley, 1996; McDonald et al., 1997). This growing literature supports the interpretation that galanin acts as an inhibitory modulator of cholinergic function in the septohippocampal pathway (Hökfelt et al., 1987; Crawley, 1996;McDonald and Crawley, 1997).
Alzheimer’s disease (AD) is a neurodegenerative disorder diagnosed clinically by the progressive loss of cognitive function and is confirmed at autopsy by the presence of neuritic plaques, neurofibrillary tangles, and cholinergic cell loss (Richter et al., 1980; Whitehouse et al., 1982). AD has recently been linked to several candidate genes (Citron et al., 1992; Levy-Lahad et al., 1995; Rogaev et al., 1995; Sherrington et al., 1995). Treatments for AD are designed to increase cholinergic transmission, but clinical improvements have been modest (Davis et al., 1992; Bodick et al., 1997;Robbins et al., 1997). The limited efficacy of cholinergic treatments could be attributable to insufficient bioavailability at critical synapses, deleterious side effects, or changes in other neurochemical systems during the degenerative process in AD.
One of these neurochemical changes is the dramatic overexpression of galanin in the basal forebrain in AD. Human galanin, a 30-amino acid peptide, is localized in interneurons of the basal forebrain and in fibers and terminals surrounding cholinergic cell bodies of the nucleus basalis of Meynert (NBM) (Chan-Palay, 1988b). In AD, galanin-immunoreactive fibers and terminals hyperinnervate the surviving neurons of the NBM (Chan-Palay, 1988a,b, 1990; Beal et al., 1990; Mufson et al., 1993; Bowser et al., 1997). The inhibitory actions of galanin on cholinergic function and memory in rats suggest that galanin overexpression in AD may contribute to memory loss and reduce the efficacy of cholinergic treatments (Hökfelt et al., 1987;Crawley and Wenk, 1989; Bartfai et al., 1992; Crawley, 1996; McDonald and Crawley, 1997).
Selective peptidergic galanin receptor ligands have been developed that antagonize the physiological and behavioral actions of galanin (Langel et al., 1992; Bartfai et al., 1993; Crawley et al., 1993; Xu et al., 1995). Galanin(1–13)-Pro2-(Ala-Leu)2-Ala-NH2(M40) effectively blocks galanin-induced impairment on a delayed nonmatching to position task in normal rats (McDonald and Crawley, 1996). The present experiments were designed to test the hypothesis that blocking the putative inhibitory action of endogenous galanin could improve the efficacy of an M1 agonist on a memory task in cholinergically lesioned rats.
MATERIALS AND METHODS
Subjects. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Subjects were male Sprague Dawley rats (Taconic Farms, Germantown, NY), 2 months old, and weighing 143–175 gm at the beginning of the experiment. Animals were housed in groups of three; after surgery they were housed in individual cages. The colony room was maintained at 22°C on a 12 hr light/dark cycle with lights on at 6:00 A.M. Animals were fed ad libitum and were given access to water for 30 min after their daily lever press sessions. Behavioral sessions were typically conducted between 8:00 A.M. and 6:00 P.M. 5 d/week. Animals were fed food and water ad libitum on the weekends.
Apparatus. Behavioral testing was conducted in eight identical operant chambers, 29.2 cm length × 24.1 cm width × 21.0 cm height (MED Associates, Lafayette, IN). Each chamber was equipped with two response levers on the front wall (front levers) and a single response lever on the rear wall (rear lever). All levers were 4.8 cm wide and were 6.7 cm above the grid floor of the operant chamber. A force of 25.0 g was required to operate the levers. A 2.5-cm-diameter 2.8 W stimulus lamp was centered 5.8 cm above each of the three levers. A 2.8 W house light on the rear wall provided a constant source of low-level illumination. A recessed well for the delivery of reinforcers was located in the center of the front wall at equal distance from each lever. A liquid dripper behind the front wall was used to dispense reinforcers of 0.05 ml of water into the recessed well. The operant chambers were controlled by a DOS-based microcomputer running MED-PC software (MED Associates). The software monitored choice accuracy, as well as secondary measures such as number of trials completed, session duration, and rear-lever response rate, as described below.
Behavioral testing. Rats were trained to press a lever in the operant chambers using a computer-controlled autoshaping program. Each lever press under the autoshaping program resulted in the delivery of a 0.05 ml water reinforcer. Rats were then gradually trained to perform the DNMTP contingencies. Each DNMTP trial consisted of a sample phase, a delay, and a choice phase. At the beginning of each DNMTP trial (the sample phase), a cue lamp above one of the two levers (designated the sample lever) was illuminated on the front wall. The lever designated as the sample for a given trial was randomly selected across trials with the constraint that each block of four trials contained two left-lever samples and two right-lever samples. After pressing the sample lever, rats were required to turn around and press the lever on the rear wall. During training, a 1 sec delay was interposed between the sample and choice phases. The first rear-lever press after expiration of the delay resulted in the immediate illumination of both cue lamps on the front wall and initiation of the choice phase. Rats were then required to press one of the two front levers to end the trial. Pressing the lever that was not the sample lever in the beginning of the trial (i.e., the nonmatching lever) was considered a correct response. Each correct response resulted in the delivery of a 0.05 ml water reinforcer. The house light remained illuminated for 3 sec after a correct response to allow time for water consumption. Making an incorrect choice response (i.e., choosing the sample lever during the choice phase) resulted in immediate darkness and no reinforcer. Each daily session was 60 trials or 60 min, whichever came first, with an intertrial interval of 20 sec. Rats were trained to a criterion of 80% correct for three consecutive sessions using trials with a 1 sec delay, at which time 10 and 20 sec delays were added. The sequence of delay intervals was randomly selected by the computer program such that each 60-trial session included 20 trials at each delay value with the constraint that each 6-trial block contained two trials at each of the three delay values. Rats were trained to a criterion of 80% at the 1 sec delay and 70% at the 10 sec delay for three consecutive sessions, at which time surgery was performed.
In addition to choice accuracy, eight secondary measures were examined, as described previously (Robinson and Crawley, 1993, 1994; McDonald and Crawley, 1996; McDonald et al., 1997). Five were measures of general slowing: session duration (60 min maximum), number of trials completed (60 maximum), percentage of long-latency trials (the percentage of trials in which the elapsed time from the end of the delay to the moment of choice exceeded 10 sec), mean sample latency per trial (elapsed time from trial onset to sample lever response), and mean choice latency per trial (the elapsed time from the rear-lever response to the choice-lever response). Other measures included percentage of correct discriminations (the percentage of trials in which the first lever pressed in the sample phase was the correct lighted lever), percentage of errors that followed errors (a measure of response perseveration), and rear-lever response rate (the rate of responding on the rear lever during the delays, expressed as responses per minute). Long-latency trials were excluded from all analyses.
Behavioral testing resumed 2 weeks after surgery. Drug treatments were typically administered twice weekly. Behavioral testing was conducted with no treatments on intervening days. This injection schedule allowed assessment of baseline performance before and after each treatment day to control for any residual drug effects or performance problems of the subjects.
Surgery. Surgery was performed under chloral hydrate anesthesia (350 mg/kg, i.p.). An incision was made at the midline, and the scalp was exposed. Stereotaxic coordinates for cannulas in the left lateral ventricle were 0.5 mm posterior and 1.0 mm lateral to bregma and 3.0 mm ventral to the top of the skull (Paxinos and Watson, 1986). Cannulas, fabricated from 24 gauge hypodermic stainless steel, were lowered through holes drilled in the skull and secured with dental acrylic and stainless steel screws. Injectors, fabricated from 31 gauge hypodermic stainless steel, extended 2.0 mm below the ventral tip of the indwelling cannulas. After the intracerebroventricular (icv) cannulas were secured in place, rats in the lesion group were given a single icv injection of 4.0 (experiment 1) or 5.0 (experiment 2) μg of 192IgG-saporin (Chemicon, Temecula, CA) in 5.0 μl of PBS. 192IgG-saporin is an immunotoxin that selectively lesions cholinergic cell bodies in the basal forebrain without damage to support cells, fibers of passage, or noncholinergic cells (Heckers et al., 1994; Lee et al., 1994; Torres et al., 1994; Waite et al., 1994; Wenk et al., 1994; Leanza et al., 1995; Steckler et al., 1995).192IgG is a monoclonal antibody against the p75 neurotrophin receptor. Saporin is a ribosome-inactivating protein that, when conjugated to 192IgG and injected into the lateral ventricle, selectively destroys basal forebrain cholinergic cells (Wiley et al., 1991). Central injection of 192IgG-saporin induces deficits in some learning and memory tasks, including DNMTP (McDonald et al., 1997). The higher dose of 192IgG-saporin was used in experiment 2, because some animals are unimpaired after injection with the 4.0 μg dose, despite significant reductions in choline acetyltransferase (McDonald et al., 1997). Rats in the sham group were injected with 5.0 μl of PBS. Injections of192IgG-saporin were administered over 2 min, and injectors were left in place an additional 2 min to allow for dispersion of the injectate. Subjects were allowed a 2 week recovery period before resumption of behavioral testing. Although p75 receptors are primarily located on basal forebrain cholinergic neurons, some are also found in the cerebellum. Because of this, any rat exhibiting movement abnormalities was excluded from the study.
Drug preparation and administration. M40 (kindly provided byÜlo Langel and Tamas Bartfai, University of Stockholm, Stockholm, Sweden) was dissolved in 0.9% physiological saline. The entire amount of M40 needed for each experiment was made at one time, separated into aliquots, and frozen at −80°C for later use. A thawed aliquot was used during a single test day; any amount left over was discarded at the end of the day. M40 was administered at doses shown previously to completely block galanin-induced choice accuracy deficits on DNMTP (McDonald and Crawley, 1996). M40 or saline vehicle was administered icv in a volume of 5.0 μl immediately before the behavioral testing session. The injector was left in place an additional 60 sec before withdrawal. The selective muscarinic M1 agonist 3-(3-S-n-pentyl-1,2,5-thiadiazol-4-yl)-1,2,5,6-tetrahydro-1-methylpyridine (TZTP; kindly provided by Per Sauerberg, Novo Nordisk, Måløv, Denmark) (Sauerberg et al., 1992) or saline vehicle was administered intraperitoneally in a volume of 1.0 mg/ml 45 min before the behavioral testing session.
Before the start of combination drug treatments, dose–response curves were generated for TZTP alone and for M40 alone to determine threshold doses of these compounds on DNMTP choice accuracy in lesioned rats when administered alone. For the dose–response curves, TZTP was administered at doses of 0.0, 0.1, 0.2, 0.3, and 0.5 mg/kg; M40 was administered at doses of 0.0, 1.0, and 3.0 nmol. Subsequent drug combinations were given at doses of 0.1 mg/kg TZTP plus 1.0 or 3.0 nmol of M40 (experiment 1) or 0.3 mg/kg TZTP plus 1.0 or 3.0 nmol of M40 (experiment 2). All drug administrations during dose–response curves and drug combination experiments were randomized across subjects.
Choline acetyltransferase assays. Rats were killed by decapitation under chloral hydrate anesthesia. Brains were removed and dissected on wet ice, as described previously (Harrington et al., 1994;Robinson et al., 1996a), to obtain five brain regions: anterior cortex, posterior cortex, and hippocampus in experiment 1, and these brain areas plus olfactory bulbs and caudate in experiment 2. Choline acetyltransferase (ChAT) activity was assayed as the marker for cholinergic activity in these regions. ChAT activity was measured by the formation of [14C]acetyl-coenzyme A and choline (Fonnum, 1969), as described previously (Harrington et al., 1994; McDonald et al., 1997). Protein levels were determined according to standard methods (Lowry et al., 1951).
Statistical analysis. Two-group comparisons were made using Student’s t tests. Analyses of TZTP and M40 individual dose–response curves were performed using repeated measures ANOVA, with delay as the repeated measure and drug condition as a between-subjects factor. Follow-up analyses of dose–response data were made using Fisher’s least significant difference (LSD). Analyses of TZTP plus M40 coadministration were performed using two-factor repeated-measures ANOVA, with drug condition and delay as repeated measures. Follow-up analyses of drug coadministration data were performed between pairs of drugs using single-factor repeated-measures ANOVA, with delay as the repeated measure. Subjects that did not receive all four M40 plus TZTP drug combinations or that did not complete at least 20 trials on all four drug co-injections were excluded from the data analysis. Drug treatment effects on secondary measures during the DNMTP task were analyzed using repeated measures ANOVA, with drug condition as the repeated measure. Group differences on secondary measures were analyzed using multivariate ANOVA (MANOVA). Degrees of freedom on all repeated measures ANOVA were corrected for sphericity using the Greenhouse–Geisser ε (Greenhouse and Geisser, 1959). ChAT data were analyzed using MANOVA, with brain region as the multivariate factor. Two separate experiments were conducted. In experiment 1, rats received TZTP plus M40 co-injections 3–4 weeks after the cholinergic immunotoxic lesion. In experiment 2, a separate group of rats received the TZTP plus M40 co-injections 11–12 weeks after lesion. Statistical tests were conducted separately for the two experiments.
RESULTS
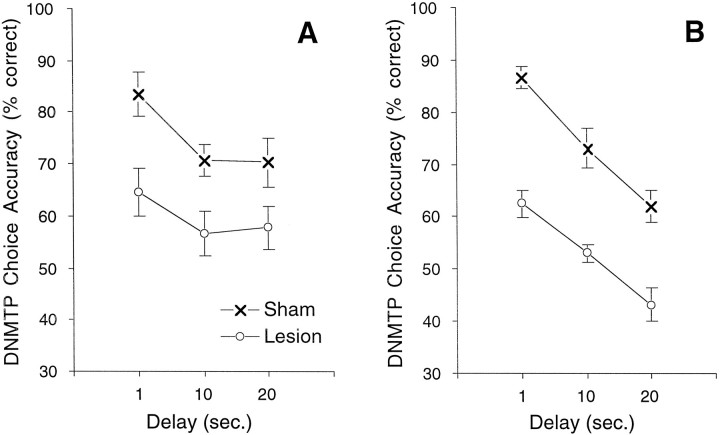
Rats that had received 192IgG-saporin were significantly impaired on the DNMTP task relative to saline-injected sham controls: experiment 1, F(1,17) = 10.9;p = .0042; experiment 2, F(1,20)= 52.3; p < 0.0001. Figure1 illustrates the delay-independent performance deficit induced by the cholinergic lesions. The group × delay interactions were not significant: experiment 1, ε = 0;F(1,25) = 0.66; p = 0.139; experiment 2, ε = 0.82; F(1,32) = 0.44;p = 0.648.
Fig. 1.
Choice accuracy on the DNMTP task for rats lesioned with the selective cholinergic immunotoxin192IgG-saporin and saline-injected sham controls.192IgG-saporin or saline was administered through indwelling cannulas after rats met criterion-level performance on the DNMTP task. Data represent mean ± SEM DNMTP choice accuracy for the first 2 weeks of behavioral testing after the 2 week lesion recovery period. A choice accuracy of 50% correct responses represents chance performance in this two-lever choice task. A, In experiment 1, lesioned rats (○; n = 9) were significantly impaired compared with sham controls (×;n = 10). B, Similarly, in an independent replication with another set of animals in experiment 2, lesioned rats (○; n = 11) treated with a higher dose of 192IgG-saporin were significantly impaired compared with sham controls (×; n = 11).
As shown in Table 1, ChAT activity was significantly reduced in lesioned rats in both experiment 1 (Wilks’ λ = 0.289; F(3,15) = 12.3; p = 0.0003) and experiment 2 (Wilks’ λ = 0.339;F(5,15) = 5.8; p = 0.0034). Follow-up analyses showed that significant differences were observed in the hippocampus (experiment 1, t(17) = 6.24; p < 0.0001; experiment 2,t(19) = 5.43; p < 0.0001), anterior cortex (experiment 1, t(17) = 6.02;p < 0.0001; experiment 2, t(19)= 4.59; p = 0.0002), posterior cortex (experiment 1,t(17) = 3.37; p = 0.0036; experiment 2, t(19) = 3.16; p = 0.0052), and olfactory bulbs (experiment 2,t(19) = 3.25; p = 0.0042). No significant differences in ChAT activity were observed in the caudate (t(19) = 0.65; p = 0.523) in experiment 2. These data are consistent with previous reports (Robinson et al., 1996a; McDonald et al., 1997) and with the anatomical distribution of basal forebrain cholinergic projections to the cortex, hippocampus, and olfactory bulbs but not the striatum (Butcher and Woolf, 1986).
Table 1.
ChAT levels in sham versus192IgG-saporin-lesioned rats
| Brain region | ChAT Level (mean ± SEM) | |||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||
| Sham (n = 10) | Lesion (n = 9) | Sham (n = 11) | Lesion (n = 10) | |
| Hippocampus | 37.8 ± 2.0 | 12.8 ± 3.6*** | 48.3 ± 4.1 | 15.2 ± 4.5*** |
| Anterior cortex | 35.2 ± 1.4 | 15.3 ± 3.1*** | 39.9 ± 2.7 | 19.1 ± 3.8** |
| Posterior cortex | 35.3 ± 1.3 | 20.1 ± 4.6* | 39.6 ± 4.0 | 23.6 ± 3.0* |
| Olfactory bulbs | 56.0 ± 9.8 | 20.9 ± 3.5* | ||
| Caudate | 76.2 ± 7.6 | 68.0 ± 10.1 | ||
ChAT activity is presented as nanomoles of ACh formed per hour per milligram of protein. Significant reductions were detected in hippocampus, anterior cortex, posterior cortex, and olfactory bulbs.
*–***Significantly different compared with sham controls. *p < 0.01; **p < 0.001; ***p < 0.0001.
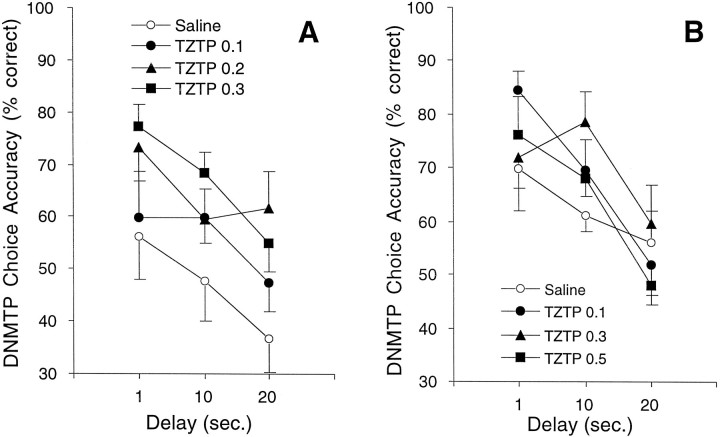
As shown in Figure 2, TZTP injected alone at 2 weeks after lesion produced significant delay-independent improvement in DNMTP choice accuracy in lesioned rats in experiment 1 (F(3,21) = 3.42; p= 0.0361). Follow-up analyses showed that TZTP significantly improved choice accuracy at doses of 0.2 (LSD = 14.75; p = 0.0193) and 0.3 (LSD = 14.75; p = 0.0103) mg/kg compared with saline. In experiment 2, there was no significant effect of TZTP alone on choice accuracy in lesioned rats injected 10 weeks after lesion (F(3,25) = 0.47; p= 0.708). On the basis of these results, subthreshold doses of 0.1 (experiment 1) and 0.3 (experiment 2) mg/kg were chosen for coadministration with M40. Lesion-induced choice accuracy deficits were greater at the earlier time points than at the later time points after lesion. Because it is common for animals to partially recover function with continued training after lesions of this type (McDonald et al., 1997), a higher dose of 192IgG-saporin was used in experiment 2. The difference in TZTP responses in experiments 1 and 2 may be attributable to the different doses of192IgG-saporin used or to the difference in time after lesion that the dose–effect curves were established.
Fig. 2.
Choice accuracy on DNMTP for lesioned rats receiving injections of the muscarinic M1 receptor agonist TZTP. A dose–response curve for TZTP was generated to determine a threshold dose of TZTP that minimally improved DNMTP choice accuracy and that could be used for subsequent coadministration with M40. TZTP or saline was injected intraperitoneally 45 min before behavioral testing. A, In experiment 1, TZTP administered 2 weeks after lesion at doses of 0.2 (▴; n = 6) and 0.3 (▪; n = 6) mg/kg significantly improved DNMTP choice accuracy compared with saline (○;n = 8). Performance under 0.1 mg/kg TZTP (•;n = 5) was not significantly different from saline vehicle. B, In experiment 2, when administered 10 weeks after lesion, none of the doses of TZTP [0.1 (•;n = 6), 0.3 (▴; n = 5), or 0.5 (▪; n = 5) mg/kg] significantly affected DNMTP choice accuracy compared with saline vehicle (○;n = 13).
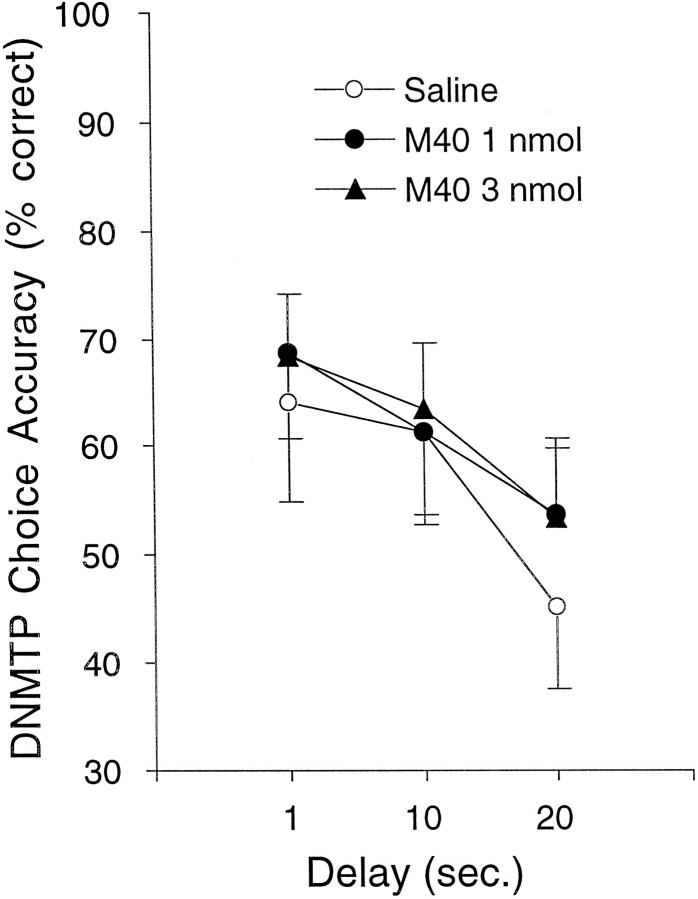
As shown in Figure 3, M40 administered alone did not affect choice accuracy at any of the doses tested. The lack of effect of M40 alone in the present study is consistent with previous findings from our laboratory (McDonald et al., 1997).
Fig. 3.
Choice accuracy on DNMTP for lesioned rats receiving injections of the galanin receptor antagonist M40 in experiment 1. A dose–response curve for M40 was generated to determine a threshold dose of M40 that minimally improved DNMTP choice accuracy and that could be used for subsequent coadministration with TZTP. M40 or saline was administered icv immediately before behavioral testing. Neither of the doses of M40 [1.0 (•; n = 7) or 3.0 (▴; n = 7) nmol] significantly affected DNMTP choice accuracy compared with saline vehicle (○;n = 7).
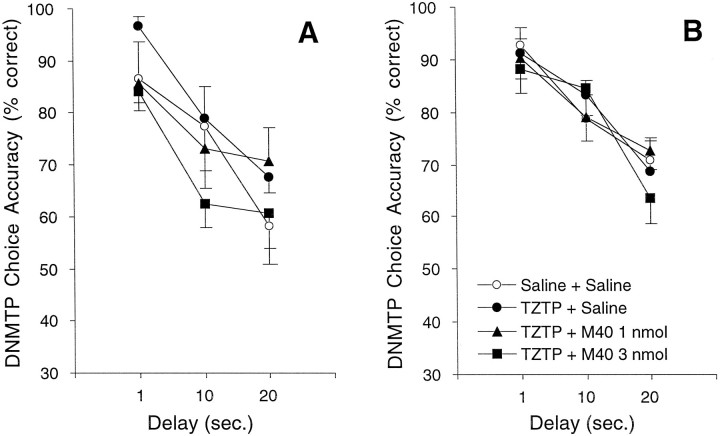
As shown in Figure 4, there was no significant effect of TZTP or any combination of TZTP and M40 in experiments 1 and 2 in the sham animals.
Fig. 4.
Choice accuracy on DNMTP for sham control rats receiving combinations of the muscarinic agonist TZTP and the galanin antagonist M40. TZTP was injected intraperitoneally 45 min before behavioral testing, and M40 was administered icv immediately before behavioral testing. A, In experiment 1, coadministration of TZTP (0.1 mg/kg) and M40 (1.0 or 3.0 nmol) did not affect DNMTP choice accuracy in sham control animals at 3–4 weeks after lesion (n = 8). B, Similarly, in experiment 2, coadministration of TZTP (0.3 mg/kg) and M40 (1.0 or 3.0 nmol) did not affect DNMTP choice accuracy in sham control animals at 11–12 weeks after lesion (n = 7).
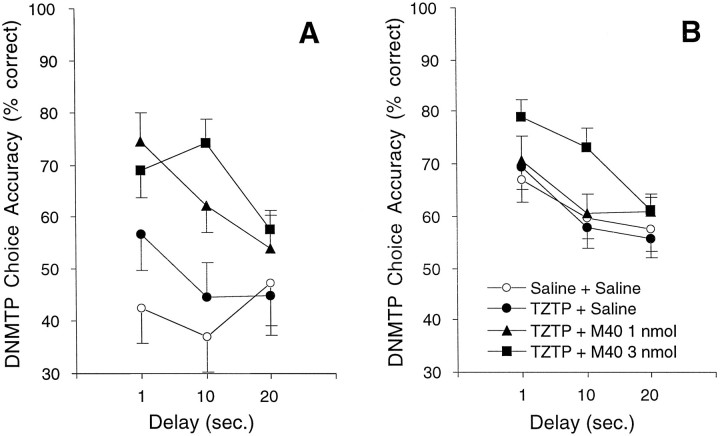
In lesioned rats, however, as shown in Figure 5, coadministration of TZTP and M40 produced significant delay-independent improvement in DNMTP choice accuracy at 3–4 weeks after lesion in experiment 1 (ε = 0.61; F(1,14) = 6.4; p = 0.0114) and at 11–12 weeks after lesion in experiment 2 (ε = 0.78;F(2,23) = 8.06; p = 0.0015). Follow-up analyses showed that in experiment 1 subjects performed significantly better after treatment with TZTP plus 1.0 nmol of M40 (ε = 1.0;F(1,8) = 11.9; p = 0.0087) and TZTP plus 3.0 nmol of M40 (ε = 1.0; F(1,8) = 21.1; p = 0.0018) compared with TZTP plus saline. In experiment 2, subjects under TZTP plus 3.0 nmol of M40 performed significantly better than under TZTP plus saline (ε = 1.0;F(1,10) = 22.1; p = 0.0008).
Fig. 5.
Choice accuracy data on the DNMTP task for lesioned rats receiving combinations of the muscarinic agonist TZTP and the galanin antagonist M40. TZTP was injected intraperitoneally 45 min before behavioral testing, and M40 was administered icv immediately before behavioral testing. A, In experiment 1, 0.1 mg/kg TZTP combined with either 1.0 (▴) or 3.0 (▪) nmol of M40 significantly improved DNMTP choice accuracy compared with TZTP plus saline (•) at 3–4 weeks after lesion (n = 9).B, In experiment 2, TZTP (0.3 mg/kg) combined with 3.0 nmol of M40 (▪) significantly improved DNMTP choice accuracy compared with TZTP plus saline (•) at 11–12 weeks after lesion (n = 11).
None of the secondary measures was significantly affected by the drug treatments in lesioned rats in either experiment (Table2). In addition, drug treatment did not affect secondary measures in the sham rats in either experiment (data not shown). In experiment 1, there was no effect of the lesion on secondary measures (Wilks’ λ = 0.384; F(8,8)= 1.60; p = 0.2599). In experiment 2, using a higher dose of the neurotoxin, there was a significant effect of the neurotoxin on the secondary measures (Wilks’ λ = 0.205;F(8,9) = 4.36; p = 0.0207). Follow-up analyses showed that the only significant difference between lesion and sham subjects was percentage of errors that followed errors (t(16) = 2.63; p < 0.0183). Lesioned rats had a significantly higher baseline rate of errors after errors (56.8 ± 4.6, mean ± SEM) compared with sham rats (29.3 ± 7.4).
Table 2.
Secondary measures (mean ± SEM) during DNMTP performance for 192IgG-saporin-lesioned rats after M40 plus TZTP 0.1 mg/kg (experiment 1) or M40 plus TZTP 0.3 mg/kg (experiment 2) combination drug treatments
| Secondary measure | Drug condition | |||
|---|---|---|---|---|
| Saline + Saline | TZTP + Saline | TZTP + M40 1.0 | TZTP + M40 3.0 | |
| Experiment 1 (n = 9) | ||||
| Trials completed | 46.9 ± 3.6 | 50.6 ± 3.8 | 42.1 ± 5.6 | 48.8 ± 4.3 |
| Session duration | 58.3 ± 1.1 | 57.4 ± 1.4 | 57.4 ± 1.5 | 56.1 ± 2.3 |
| Percentage of longlatency trials | 23.4 ± 4.6 | 14.5 ± 3.8 | 24.5 ± 5.5 | 16.9 ± 3.0 |
| Sample latency per trial | 26.8 ± 2.4 | 25.4 ± 2.6 | 22.0 ± 2.8 | 22.6 ± 3.2 |
| Choice latency per trial | 3.3 ± 0.8 | 2.4 ± 0.2 | 2.6 ± 0.5 | 2.4 ± 0.3 |
| Correct discriminations | 78.6 ± 6.9 | 70.9 ± 6.7 | 87.1 ± 3.9 | 85.2 ± 4.1 |
| Rear-lever response rate | 17.7 ± 2.0 | 15.8 ± 1.5 | 15.4 ± 1.5 | 15.8 ± 1.3 |
| Errors following errors | 56.8 ± 4.6 | 49.7 ± 5.2 | 53.6 ± 5.3 | 45.0 ± 6.7 |
| Experiment 2 (n = 11) | ||||
| Trials completed | 51.3 ± 3.5 | 43.9 ± 4.7 | 50.9 ± 3.6 | 42.5 ± 4.4 |
| Session duration | 53.7 ± 2.5 | 55.2 ± 2.1 | 54.8 ± 1.9 | 58.2 ± 1.0 |
| Percentage of longlatency trials | 7.2 ± 1.8 | 8.8 ± 1.9 | 8.5 ± 2.4 | 14.1 ± 3.4 |
| Sample latency per trial | 23.6 ± 3.8 | 23.5 ± 4.0 | 23.8 ± 3.5 | 29.8 ± 2.9 |
| Choice latency per trial | 2.1 ± 0.1 | 1.9 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 |
| Correct discriminations | 84.3 ± 3.2 | 81.7 ± 5.2 | 79.3 ± 4.4 | 78.4 ± 4.7 |
| Rear-lever response rate | 22.3 ± 1.6 | 18.6 ± 1.1 | 20.2 ± 1.4 | 17.8 ± 1.3 |
| Errors following errors | 40.5 ± 4.0 | 37.0 ± 4.6 | 35.8 ± 3.3 | 30.6 ± 4.2 |
None of the drug treatments had a significant effect on any secondary measure.
DISCUSSION
The combination of M40 and TZTP significantly improved DNMTP choice accuracy in cholinergically lesioned rats compared with TZTP alone. These findings reveal the ability of a galanin receptor antagonist to potentiate the actions of a muscarinic M1agonist in restoring cognitive function in rats with selective cholinergic immunotoxic lesions of the basal forebrain. M40 potentiated the actions of TZTP at both the early and late time points after cholinergic lesion, although higher doses of M40 and TZTP appear to be required to significantly improve performance at a later time point. The improvement in choice accuracy in the lesioned rats was most striking when TZTP and M40 were given at 3–4 weeks after lesion when baseline performance was low. Lesioned rats typically exhibit a modest improvement in baseline DNMTP performance with repeated testing over time (McDonald et al., 1997). In the present experiments, this improvement is evident from the increase in choice accuracy on saline plus saline at the two time points. No effects of TZTP, M40, or their combinations were observed in sham controls. In addition, these compounds had no significant effect on any of the secondary measures in either sham or lesioned subjects, indicating no changes in visual discrimination, general speed of responding, perseveration, nor any general adverse effects of this drug combination.
The present data support the hypothesis that endogenous galanin exerts an inhibitory tone on cholinergic transmission under special conditions (Hökfelt et al., 1987; Bartfai et al., 1992; Crawley, 1996;Bowser et al., 1997; McDonald and Crawley, 1997). In normal rats, the putative inhibitory tone exerted by galanin may be relatively minor. A modulatory peptide, such as galanin, may exert its inhibitory actions only under extreme conditions of high neuronal firing rates. Such conditions could be triggered by the sharp reduction in cholinergic transmission after 192IgG-saporin lesions when surviving neurons may upregulate ACh release as a compensatory response (Hökfelt et al., 1987). Under these circumstances, the putative inhibitory action of endogenously released galanin could induce further reductions in cholinergic transmission and contribute to impaired memory processes.
A galanin antagonist could act to remove the hypothesized inhibitory actions of galanin induced by neuronal damage. However, a previous study showed that intraventricular M40 alone had no effect on DNMTP choice accuracy in lesioned rats, indicating that eliminating the putative inhibitory effects of endogenous galanin is not sufficient alone to reverse DNMTP choice accuracy deficits after cholinergic lesions (McDonald et al., 1997). Rather, the effect of a galanin antagonist may be detectable only in potentiating the improvement gained by replacing the missing acetylcholine.
The site of action for the observed M40 effect in the present experiment is likely to be at a postsynaptic M1 receptor through a common signal transduction mechanism. This interpretation is based on previous observations that galanin inhibits carbachol-stimulated phosphatidyl inositol hydrolysis in rats and monkeys (Fisone et al., 1991; Palazzi et al., 1991) and that TZTP selectively activates M1 receptors, which stimulate phosphatidyl inositol hydrolysis (Sauerberg et al., 1992). Our present working hypothesis is that excess galanin, released in the lesioned condition, attenuates the postsynaptic actions of an M1agonist on its effector system. Administration of a galanin antagonist, therefore, allows the M1 agonist to exert its full postsynaptic efficacy. Further experiments in our laboratory will be designed to test this hypothesis and to investigate other potential sites of galanin–acetylcholine interaction, using combinations of M40 with cholinesterase inhibitors, presynaptic M2 autoreceptor antagonists, and nicotinic agonists.
One implication of the present findings is that a galanin receptor antagonist may improve the efficacy of a cholinergic agonist for the treatment of the cognitive impairment of AD. Because of the excessive galaninergic hyperinnervation of the remaining NBM cholinergic cell bodies in AD (Chan-Palay, 1988a; Beal et al., 1990; Mufson et al., 1993; Bowser et al., 1997), overexpressed galanin may diffuse to postsynaptic sites to inhibit phosphatidylinositol hydrolysis. In addition, excess galanin may act on cell bodies of the basal forebrain to inhibit ACh release in terminal fields, as reported in rats in a recent microdialysis study (Robinson et al., 1996b). Prevention of the putative inhibitory actions of excess endogenous galanin in AD, when cholinergic transmission is already sharply reduced, may allow cholinergic drugs to more fully exert their therapeutic effects on the remaining cholinergic neurons and receptors.
This first demonstration of a synergistic cognitive improvement in cholinergically lesioned rats treated with a galanin antagonist and a cholinergic agonist supports previous proposals for a drug combination approach to address the changes in many neurotransmitter systems in AD (Sarter et al., 1990; Vitiello et al., 1997). The need to investigate the multiple neurotransmitter systems affected in AD is further indicated in the present experiments, in which the combination of an M1 agonist and a galanin antagonist was not sufficient to fully restore DNMTP choice accuracy in lesioned rats up to sham control performance levels. Alternatively, improved galanin receptor antagonists, with greater receptor subtype selectivity, better bioavailability, and longer half-life in vivo, may be necessary and sufficient to further increase the efficacy of this drug combination. Development of nonpeptide galanin receptor antagonists will provide the tools necessary to test the hypothesis that a galanin antagonist will potentiate the action of cholinergic agonists and improve cognitive function in AD.
Footnotes
This work was supported by the National Institute of Mental Health (NIMH) Intramural Research Program (M.P.M. and J.N.C.) and Alzheimer’s Association Grant IIRG-95-004 (G.L.W.). We thank NIMH student volunteers Gregory Goldstein, Simrun Kalra, Katherine Miller, and Kristlyn Araujo, who contributed excellent technical assistance with behavioral testing.
Correspondence should be addressed to Dr. Mike McDonald, Section on Behavioral Neuropharmacology, Experimental Therapeutics Branch, National Institute of Mental Health, Building 10, Room 4D11, Bethesda, MD 20892-1375. E-mail address: mikemc@codon.nih.gov
REFERENCES
- 1.Bartfai T, Fisone G, Langel Ü. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends Pharmacol Sci. 1992;13:312–317. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- 2.Bartfai T, Langel Ü, Bedecs K, Andell S, Land T, Gregersen S, Ahrén B, Girotti P, Consolo S, Corwin R, Crawley J, Xiaojun X, Wiesenfeld-Hallin Z, Hökfelt T. Galanin-receptor ligand M40 peptide distinguishes between putative galanin-receptor subtypes. Proc Natl Acad Sci USA. 1993;90:11287–11291. doi: 10.1073/pnas.90.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beal MF, MacGarvey U, Swartz KJ. Galanin immunoreactivity is increased in the nucleus basalis of Meynert in Alzheimer’s disease. Ann Neurol. 1990;28:157–161. doi: 10.1002/ana.410280207. [DOI] [PubMed] [Google Scholar]
- 4.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer’s disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 5.Bowser R, Kordower JH, Mufson EJ. A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer’s disease. Brain Pathol. 1997;2:723–730. doi: 10.1111/j.1750-3639.1997.tb01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher LL, Woolf HN. Central cholinergic systems: synopsis of anatomy and overview of physiology and pathology. In: Sheibel AB, Wechsler AF, editors. The biological substrates of Alzheimer’s disease. Academic; New York: 1986. pp. 73–86. [Google Scholar]
- 7.Chan-Palay V. Galanin hyperinnervates surviving neurons of the human basal nucleus of Meynert in dementias of Alzheimer’s and Parkinson’s disease: a hypothesis for the role of galanin in accentuating cholinergic dysfunction in dementia. J Comp Neurol. 1988a;273:543–557. doi: 10.1002/cne.902730409. [DOI] [PubMed] [Google Scholar]
- 8.Chan-Palay V. Neurons with galanin innervate cholinergic cells in the human basal forebrain and galanin and acetylcholine coexist. Brain Res Bull. 1988b;21:465–472. doi: 10.1016/0361-9230(88)90160-8. [DOI] [PubMed] [Google Scholar]
- 9.Chan-Palay V. Hyperinnervation of surviving neurons of the human basal nucleus of Meynert by galanin in dementias of Alzheimer’s and Parkinson’s disease. Adv Neurol. 1990;51:253–255. [PubMed] [Google Scholar]
- 10.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 11.Consolo S, Bertorelli R, Girotti P, La Porta C, Bartfai T, Parenti M, Zambelli M. Pertussis toxin-sensitive G-protein mediates galanin’s inhibition of scopolamine-evoked acetylcholine release in vivo and carbachol-stimulated phosphoinositide turnover in rat ventral hippocampus. Neurosci Lett. 1991;126:29–32. doi: 10.1016/0304-3940(91)90363-x. [DOI] [PubMed] [Google Scholar]
- 12.Crawley JN. Minireview. Galanin-acetylcholine interactions: relevance to memory and Alzheimer’s disease. Life Sci. 1996;58:2185–2199. doi: 10.1016/0024-3205(96)00093-8. [DOI] [PubMed] [Google Scholar]
- 13.Crawley JN, Wenk GL. Co-existence of galanin and acetylcholine: is galanin involved in memory processes and dementia? Trends Neurosci. 1989;21:278–282. doi: 10.1016/0166-2236(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN, Robinson JK, Langel Ü, Bartfai T. Galanin receptor antagonists M40 and C7 block galanin-induced feeding. Brain Res. 1993;600:268–272. doi: 10.1016/0006-8993(93)91382-3. [DOI] [PubMed] [Google Scholar]
- 15.Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, Drachman DA, Schneider LS, Whitehouse PJ, Hoover TM, Morris JC, Kawas CH, Knopman DS, Earl NL, Kumar V, Doody RS. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. The Tacrine Collaborative Study Group. N Engl J Med. 1992;327:1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- 16.Fisone G, Wu CF, Consolo S, Nordström Ö, Brynne N, Bartfai T, Melander T, Hökfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo and in vitro studies. Proc Natl Acad Sci USA. 1987;84:7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisone G, Bartfai T, Nilsson S, Hökfelt T. Galanin inhibits the potassium-evoked release of acetylcholine and the muscarinic receptor-mediated stimulation of phosphoinositide turnover in slices of monkey hippocampus. Brain Res. 1991;568:279–284. doi: 10.1016/0006-8993(91)91409-t. [DOI] [PubMed] [Google Scholar]
- 18.Fonnum F. Radiochemical microassays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969;115:465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Givens B, Olton DS, Crawley JN. Galanin in the medial septal area impairs working memory. Brain Res. 1992;582:71–77. doi: 10.1016/0006-8993(92)90318-4. [DOI] [PubMed] [Google Scholar]
- 20.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 21.Harrington CA, Mobley SL, Wenk GL. Nitric oxide formation does not underlie the memory deficits produced by ibotenate injections into the nucleus basalis of rats. Behav Neurosci. 1994;108:277–283. doi: 10.1037//0735-7044.108.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Heckers S, Ohtake T, Wiley RG, Lappi DA, Geula C, Mesulam MM. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci. 1994;14:1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hökfelt T, Millhorn D, Seroogy K, Tsuruo Y, Cecatelli S, Lindh B, Meister B, Melander T, Schalling M, Bartfai T, Terenius L. Coexistence of peptides with classical neurotransmitters. Experientia (Basel) 1987;43:768–780. doi: 10.1007/BF01945354. [DOI] [PubMed] [Google Scholar]
- 24.Langel Ü, Land T, Bartfai T. Design of chimeric peptide ligands to galanin receptors and substance P receptors. Int J Pept Protein Res. 1992;39:516–522. doi: 10.1111/j.1399-3011.1992.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 25.Leanza G, Nilsson OG, Wiley RG, Björklund A. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioural, biochemical and stereological studies in the rat. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 27.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu C-E, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Malin DH, Novy BJ, Lett-Brown A, Plotner RE, May BT, Radulescu SJ, Crothers MK, Osgood LD, Lake JR. Galanin attenuates retention of one-trial reward learning. Life Sci. 1992;50:939–944. doi: 10.1016/0024-3205(92)90171-k. [DOI] [PubMed] [Google Scholar]
- 30.McDonald MP, Crawley JN. Galanin receptor antagonist M40 blocks galanin-induced choice accuracy deficits on a delayed nonmatching-to-position task. Behav Neurosci. 1996;110:1025–1032. doi: 10.1037//0735-7044.110.5.1025. [DOI] [PubMed] [Google Scholar]
- 31.McDonald MP, Crawley JN. Galanin-acetylcholine interactions in rodent memory tasks and Alzheimer’s disease. J Psychiatry Neurosci. 1997;22:303–317. [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald MP, Wenk GL, Crawley JN. Analysis of galanin and the galanin antagonist M40 on delayed nonmatching-to-position performance in rats lesioned with the cholinergic immunotoxin 192IgG-saporin. Behav Neurosci. 1997;111:552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- 33.Melander T, Hökfelt T, Rökaeus Å. Distribution of galanin-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 34.Merchenthaler I, Lopez FJ, Negro-Vilar A. Anatomy and physiology of central galanin-containing pathways. Prog Neurobiol. 1993;40:711–769. doi: 10.1016/0301-0082(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 35.Mufson EJ, Cochran E, Benzing WC, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and the bed nucleus of the stria terminalis in aging, Alzheimer’s disease and Down’s syndrome. Dementia. 1993;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- 36.Palazzi E, Felinska S, Zambelli M, Fisone G, Bartfai T, Consolo S. Galanin reduces carbachol stimulation of phosphoinositide turnover in rat ventral hippocampus by lowering Ca2+ influx through voltage-sensitive Ca2+ channels. J Neurochem. 1991;56:739–747. doi: 10.1111/j.1471-4159.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney, Australia: 1986. [Google Scholar]
- 38.Richter JA, Perry EK, Tomlinson BE. Acetylcholine and choline levels in post-mortem human brain tissue: preliminary observations in Alzheimer’s disease. Life Sci. 1980;26:1683–1689. doi: 10.1016/0024-3205(80)90176-9. [DOI] [PubMed] [Google Scholar]
- 39.Robbins TW, McAlonan G, Muir JL, Everitt BJ. Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer’s disease. Behav Brain Res. 1997;83:15–23. doi: 10.1016/s0166-4328(97)86040-8. [DOI] [PubMed] [Google Scholar]
- 40.Robinson JK, Crawley JN. Intraventricular galanin impairs delayed nonmatching-to-sample performance in rats. Behav Neurosci. 1993;107:458–467. doi: 10.1037//0735-7044.107.3.458. [DOI] [PubMed] [Google Scholar]
- 41.Robinson JK, Crawley JN. Analysis of anatomical sites at which galanin impairs delayed nonmatching to sample in rats. Behav Neurosci. 1994;108:941–950. [PubMed] [Google Scholar]
- 42.Robinson JK, Wenk GL, Wiley RG, Lappi DA, Crawley JN. 192IgG-saporin immunotoxin and ibotenic acid lesions of nucleus basalis and medial septum produce comparable deficits on delayed nonmatching to position in rats. Psychobiology. 1996a;24:179–186. [Google Scholar]
- 43.Robinson JK, Zocchi A, Pert A, Crawley JN. Galanin microinjected into the medial septum inhibits scopolamine-induced acetylcholine overflow in the rat ventral hippocampus. Brain Res. 1996b;709:81–87. doi: 10.1016/0006-8993(95)01307-5. [DOI] [PubMed] [Google Scholar]
- 44.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St. George-Hyslop PH. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 45.Sarter M, Bruno JP, Dudchenko P. Activating the damaged basal forebrain cholinergic system: tonic stimulation versus signal amplification. Psychopharmacology. 1990;101:1–17. doi: 10.1007/BF02253710. [DOI] [PubMed] [Google Scholar]
- 46.Sauerberg P, Olesen PH, Nielsen S, Treppendahl S, Sheardown M, Honoré T, Mitch CH, Ward JS, Pike AJ, Bymaster FP, Sawyer BD, Shannon HE. Novel functional M1 selective muscarinic agonists. Synthesis and structure-activity relationships of 3-(1):2,5-thiadiazolyl)-1,2,5,6-tetrahydro-1-methylpyridines. J Med Chem. 1992;35:2274–2283. doi: 10.1021/jm00090a019. [DOI] [PubMed] [Google Scholar]
- 47.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St. George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 48.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 49.Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 50.Steckler T, Keith AB, Wiley RG, Sahgal A. Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: role of the septohippocampal projection. Neuroscience. 1995;66:101–114. doi: 10.1016/0306-4522(94)00603-3. [DOI] [PubMed] [Google Scholar]
- 51.Sundstrom E, Archer T, Melander T, Hökfelt T. Galanin impairs acquisition but not retrieval of spatial memory in rats studied in the Morris swim maze. Neurosci Lett. 1988;88:331–335. doi: 10.1016/0304-3940(88)90233-9. [DOI] [PubMed] [Google Scholar]
- 52.Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. Galanin: a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 53.Torres EM, Perry TA, Blockland A, Wilkinson LS, Wiley RG, Lappi DA, Dunnett SB. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 54.Ukai M, Miura M, Kameyama T. Effects of galanin on passive avoidance response, elevated plus-maze learning, and spontaneous alternation performance in mice. Peptides. 1995;16:1283–1286. doi: 10.1016/0196-9781(95)02009-l. [DOI] [PubMed] [Google Scholar]
- 55.Vitiello B, Martin A, Hill J, Mack C, Molchan S, Martinez R, Murphy D, Sunderland T. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology. 1997;16:15–24. doi: 10.1016/S0893-133X(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 56.Waite JJ, Wardlow ML, Chen AC, Lappi DA, Wiley RG, Thal LJ. Time course of cholinergic and monoaminergic changes in rat brain after immunolesioning with 192 IgG-saporin. Neurosci Lett. 1994;169:154–158. doi: 10.1016/0304-3940(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 57.Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. J Neurosci. 1994;14:5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 59.Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–153. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- 60.Xu XJ, Wiesenfeld-Hallin Z, Langel Ü, Bedecs K, Bartfai T. New high affinity peptide antagonists to the spinal galanin receptor. Br J Pharmacol. 1995;116:2076–2080. doi: 10.1111/j.1476-5381.1995.tb16414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]