Abstract
Somatostatin is known to mediate its actions through five G-protein-coupled receptors (sst1–sst5). We have studied the expression of the sst1 receptor in the rat hypothalamus by using a subtype-specific antiserum. In Western blotting, the antiserum reacted specifically with a band with an apparent molecular weight of 80,000 in membranes prepared from hypothalamic tissue.
The localization of the sst1 receptor was investigated by immunohistochemistry in hypothalamus sections. Additionally, an immunofluorescent double-labeling was performed for the sst1 receptor and somatostatin. Light microscopy revealed that the sst1 receptor is located in perikarya and nerve fibers in the rostral periventricular area surrounding the third ventricle as well as in nerve fibers projecting from the perikarya to the external layer of the median eminence. In these neuronal structures, sst1 immunoreactivity was found to be colocalized with somatostatin. Furthermore, the location of sst1 receptors was studied by immunoelectron microscopy in the median eminence. In the external layer, receptor immunoreactivity was confined to nerve terminals. Immunoreactive nerve terminals were seen to make synapse-like junctions with other both stained and unstained nerve terminals. Thus, the sst1 receptor is present in the classic somatostatinergic hypothalamic parvocellular system inhibiting hormone secretion from the anterior pituitary gland. These findings indicate that the sst1 receptor may act as an autoreceptor and inhibit the release of somatostatin from periventricular neurons projecting to the median eminence.
Keywords: somatostatin receptor, sst1, immunohistochemistry, ultrastructure, autoreceptor, hypothalamus, median eminence, synapse
From hypothalamic extracts, Brazeau et al. (1973) purified a tetradecapeptide, somatostatin, which was shown to inhibit the release of growth hormone (GH) from the anterior pituitary. Immunohistochemical studies demonstrated that this peptide is located in perikarya in the rostral periventricular area of the third ventricle and is part of the classic parvocellular hypothalamic system (Elde and Parsons, 1975; Hökfelt et al., 1975). The periventricular somatostatinergic neurons project to the median eminence where somatostatin is released into the hypophyseal portal circulation and carried to the anterior pituitary to inhibit the release of GH, thyroid-stimulating hormone, and prolactin (for review, see Lamberts, 1988). Somatostatin and GH-releasing hormone (GHRH) act in concert to regulate the pulsatile secretion of GH. In addition to the direct action on the pituitary gland, somatostatin and GHRH have been reported to inhibit their own neurosecretion and also to regulate the secretion of other peptides, thereby indirectly modulating the level of GH release (Lumpkin et al., 1981, 1985; for review, seeEpelbaum, 1992).
In addition to playing a neuroendocrine role, somatostatin acts as a neurotransmitter or neuromodulator or both in the CNS, with diverse neurophysiological effects (for review, see Schindler et al., 1996).
The physiological effects of somatostatin are mediated by high-affinity membrane receptors. Effector mechanisms include the inhibition of adenylyl cyclase and modulation of ion channels and tyrosine phosphatase activity (for review, see Reisine and Bell, 1995). Five specific membrane receptors for somatostatin (sst1–sst5) have been identified by molecular cloning in human and rat (Bruno et al., 1992; Kluxen et al., 1992; Li et al., 1992; Meyerhof et al., 1992; O’Carroll et al., 1992;Yamada et al., 1992a,b, 1993; Demchyshyn et al., 1993).
The distribution of mRNA encoding sst1–sst5receptors has been investigated in rat and mouse brain by in situ hybridization. The different somatostatin receptor mRNAs are expressed at varying levels in different brain areas (for review, seeSchindler et al., 1996). In two studies, the location of one receptor subtype (sst2A) has been described by immunohistochemistry in rat brain (Dournaud et al., 1996; Schindler et al., 1997).
We have raised specific antibodies against the C-terminal part of the sst1 receptor. In this study, we show by use of double-immunofluorescent labeling the presence of the sst1receptor in the parvocellular somatostatin-containing neurons projecting from the hypothalamic periventricular nucleus to the median eminence. By immunocytochemistry at the electron microscopical level, immunoreactive terminals in the median eminence are shown to make presynaptic contacts with other nerve terminals.
MATERIALS AND METHODS
Immunoblot. Male Wistar rats weighing 180 gm were anesthetized by intraperitoneal injection of tribromethanol (400 mg/kg) and killed by decapitation. The hypothalamic areas were dissected out and homogenized in buffer 1 (50 mm Tris base, 1 mm EGTA, 5 mm MgCl2, pH 7.4; supplemented with proteinase inhibitors bacitracin 200 μg/ml, leupeptin 2 μg/ml, phenylmethylsulfonyl fluoride 100 μg/ml). The homogenate was pelleted and rehomogenized in buffer 1. Protein concentrations were determined using a Bio-Rad (Hercules, CA) protein assay kit.
Twenty-five micrograms of membrane protein were reduced with 2-mercaptoethanol and fractionated in SDS-PAGE (12%). Electrophoresed proteins were semi-dry-blotted onto nitrocellulose membranes. The blots were saturated with 5% w/v defatted dry milk in TBS (50 mmTris-HCl, 150 mm NaCl, pH 7.5) containing 0.1% Tween 20 (TBS-T) for 1 hr at room temperature and reacted with anti-sst1 antiserum diluted 1:1000 in 5% dry milk in TBS-T for 1 hr at room temperature. Immunoreactive bands were visualized by incubation with horseradish peroxidase-conjugated swine anti-rabbit IgG (Dako, Glostrup, Denmark) at 1:2000 for 1 hr at room temperature and detected by enhanced chemiluminescence (Amersham, Little Chalfont, UK). As a control, the antiserum was preabsorbed overnight at 4°C with 50 μg fusion protein/ml diluted serum.
Tissue preparation. For light microscopical immunohistochemistry, adult male Wistar rats weighing 250 gm were anesthetized by intraperitoneal injection of tribromethanol (400 mg/kg) and fixed by vascular perfusion with 4% cold paraformaldehyde in 0.1m phosphate buffer, pH 7.4, for 15 min. The brains were removed, post-fixed overnight in the same fixative, and transferred to PBS. Brains were cryoprotected in 30% sucrose in PBS, sectioned into 40-μm-thick cryostat sections, and transferred to PBS.
Immunohistochemistry. All reactions were performed on free-floating coronal sections. Endogenous peroxidase activity was quenched by incubating the sections in 1% H2O2in PBS. This was followed by a 20 min preincubation with 5% normal swine serum and 1% BSA in PBS/0.3% Triton X-100. Sections were incubated overnight at 4°C with an anti-sst1 antiserum diluted 1:10,000 in PBS/1%BSA/0.3% Triton X-100. This antiserum was raised in rabbit against the C-terminal part of the human sst1 receptor expressed as fusion proteins with glutathioneS-transferase. The antiserum was shown not to cross-react with any of the other somatostatin receptor subtypes (Helboe et al., 1997).
The sections were incubated for 1 hr with biotinylated swine anti-rabbit immunoglobulins (Dako) at 1:500 followed by 45 min with horseradish peroxidase-conjugated streptavidin–biotin complex (strept-ABC) (Dako). Biotinylated tyramide (DuPont NEN, Boston, MA) was applied to the sections at 1:50 for 10 min, and sections were finally incubated an additional 45 min with strept-ABC. Immunoreactivity was visualized with 0.05% diaminobenzidine (DAB) and 0.01% H2O2. The sections were mounted on glass slides using 0.5% gelatin in distilled water and then air-dried and coverslipped with Depex.
For controls, the diluted antiserum was preabsorbed with 50 μg sst1 fusion protein/ml overnight at 4°C before incubation of the sections.
Double-immunofluorescent labeling. For double-visualization of sst1 and somatostatin immunoreactivity, sections were incubated overnight at 4°C with anti-sst1 antiserum at 1:10,000 and anti-somatostatin antibody raised in sheep (American Research Products, Belmont, MA) diluted to 20 μg/ml. This was followed by incubation with biotinylated anti-rabbit antibodies 1:500, strept-ABC, and biotinylated tyramide 1:50 (as described above). The sections were then incubated with streptavidin fluorescein (Amersham) at 1:50 and Texas Red-conjugated donkey anti-sheep IgG (Jackson ImmunoResearch, West Grove, PA) at 1:100. The sections were mounted on glass slides with fluorescent mounting medium (Dako) and examined in the light microscope equipped with an epifluorescence system. Identical fields of sections were photographed for the two fluorescent markers.
To test for possible cross-reactions between primary and secondary antibodies, control sections were first incubated with either anti-sst1 antiserum or anti-somatostatin antibodies. Incubation with each of the primary antibodies was followed by biotinylated antibodies, strept-ABC, and biotinylated tyramide. The unrelated fluorescent marker was then added to the sections, i.e., Texas Red-conjugated donkey anti-sheep IgG to sections with the anti-sst1 antiserum and streptavidin fluorescein to sections with the anti-somatostatin antibody.
Immunocytochemistry. For ultrastructural localization of sst1 immunoreactivity in the median eminence, male Wistar rats weighing 250 gm were anesthetized and perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 mphosphate buffer. The brains were removed, post-fixed overnight in the same fixative, and transferred to PBS. Coronal sections (100-μm-thick) of the hypothalamus, including the median eminence, were cut on a vibratome. Before the immunoreaction, the sections were treated with 1% sodium borohydride and 0.1% sodium periodate in PBS for 45 min, cryoprotected in 20% sucrose, and snap-frozen in liquid nitrogen. Immunolabeling was performed as described above (see Immunohistochemistry) except that the anti-sst1 antiserum was 1:1000. The signal was visualized with DAB. The sections were post-fixed for 1 hr in 2% osmium tetroxide, dehydrated, and embedded in Epon. Thin sections with a gray to silver interference color were cut on a Reichert ultratome and contrasted with lead citrate. The sections were examined and photographed in a Philips EM208 electron microscope operated at 60 kV.
Thin sections of material in which the immunocytochemical staining was omitted served as controls.
RESULTS
Immunoblot
To establish whether the anti-sst1 antiserum recognizes the sst1 receptor in hypothalamic tissue, we performed Western blot analysis on membrane preparations from rat hypothalamus. Immunoreaction with the anti-sst1 antiserum resulted in a major band of an apparent molecular weight of ∼80,000 (Fig. 1). This immunoreaction was abolished when the antiserum was preabsorbed with the sst1antigen before the reaction.
Fig. 1.
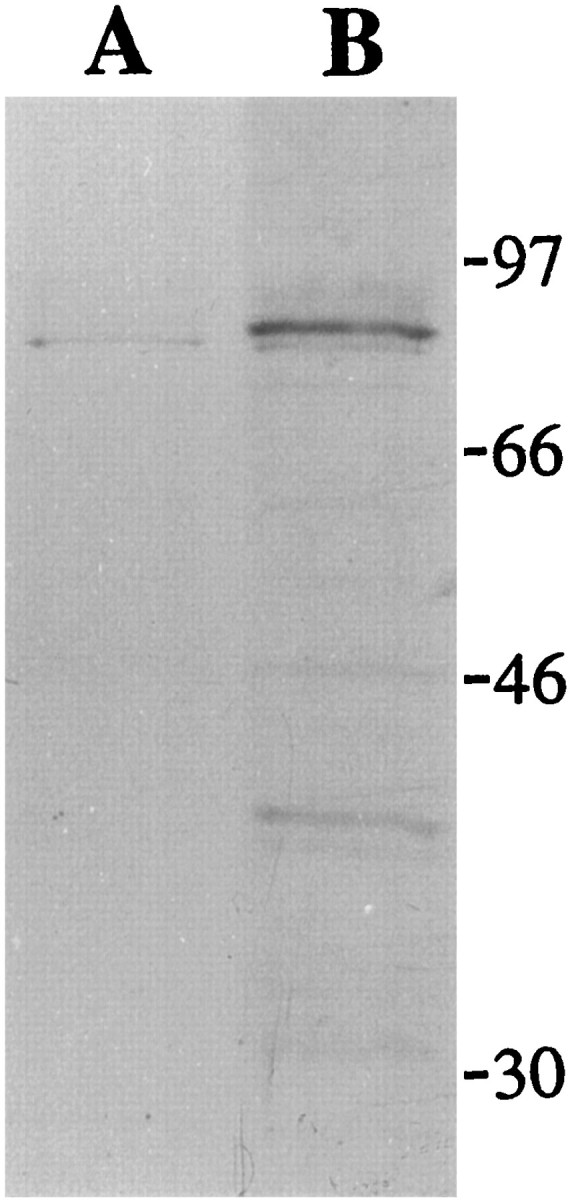
Western blot detection of the sst1receptor in rat hypothalamus. Membranes prepared from hypothalamic tissue (25 μg/lane) were separated by SDS-PAGE, transferred to nitrocellulose, and reacted with anti-sst1 antiserum diluted 1:1000. A band of an apparent molecular weight of ∼80,000 reacted specifically with the anti-sst1 antiserum (lane B). This band was not detected using antiserum preabsorbed with the sst1 antigen (lane A). A weak band placed immediately below the 80,000 molecular weight band was detected also with the preabsorbed antiserum and thus probably does not represent the sst1 receptor. Molecular weights are indicated in kilodaltons.
Immunohistochemistry
Strongly stained perikarya and nerve fibers positive for sst1 were detected with DAB visualization in the periventricular area of the hypothalamus and the median eminence. In control sections where the antiserum had been preabsorbed with the sst1 antigen before the immunoreaction, this staining was abolished completely (data not shown).
In the area surrounding the third ventricle, many sst1-immunoreactive perikarya and fibers with boutons en passage were observed. This immunostaining was limited to the rostral part of the periventricular nucleus from the organum vasculosum laminae terminalis extending caudally to the rostral part of the median eminence (Fig. 2). Some perikarya and nerve fibers were located within the ependymal and subependymal layers adjacent to the third ventricle (Fig. 2E).
Fig. 2.
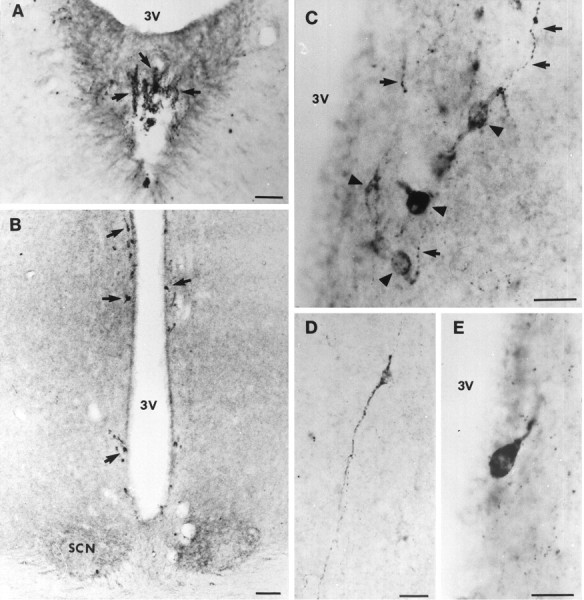
Immunohistochemical visualization of sst1 receptor in coronal sections of the rat hypothalamus.A, Organum vasculosum laminae terminalis showing sst1 labeling of nerve fibers in the ependyma (arrows). B, A strong staining for sst1 in perikarya (arrows) in the periventricular area surrounding the anterior part of the third ventricle (3V). In the suprachiasmatic nucleus (SCN), a moderate labeling of nerve fibers is observed. C, Immunostained nerve fibers (arrows) and neuronal perikarya (arrow heads) showing varying labeling intensity in the periventricular nucleus. D, In the hypothalamus, single-labeled perikarya and nerve fibers were observed.E, Micrograph showing a strongly labeled cell body positioned within the ependymal layer of the third ventricle. Scale bars: A, B, 200 μm; C–E, 50 μm.
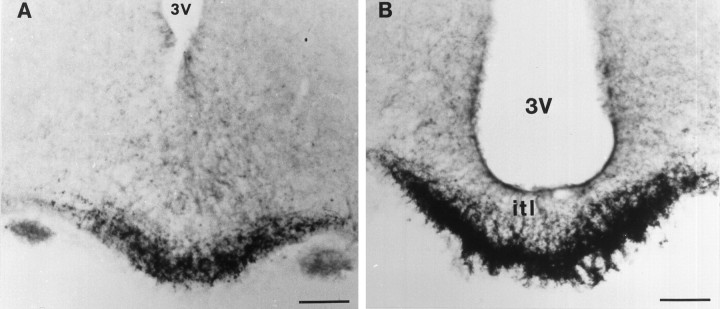
In the median eminence, a strong staining was observed in the external layer over the entire length of the structure (Fig.3). The immunoreactivity was confined to nerve fibers endowed with large swellings. The fibers were located mainly around the portal capillaries.
Fig. 3.
Detection of sst1 receptor immunoreactivity in coronal sections of the median eminence. An intense staining of nerve fibers endowed with large swellings is seen in the rostral part of the organ (A). In the more caudal part of the median eminence (B), the labeling is clearly confined to the external layer surrounding the portal capillaries. itl, Internal layer. Scale bars, 200 μm.
A medium-strong immunoreactivity was observed in fibers throughout the suprachiasmatic nucleus (Fig. 2B). No stained perikarya were detected in this structure. Single perikarya and nerve fibers were occasionally observed scattered in the cortex and hypothalamus (Fig. 2D).
Double-immunofluorescent labeling
With the fluorescent detection system using fluorescein and Texas Red, a widespread labeling of both sst1 and somatostatin-immunoreactive neuronal structures was observed. There was no cross-reactivity between the anti-sst1 antiserum and the Texas Red fluorescent marker, or between the anti-somatostatin antibody and the streptavidin fluorescein (not shown).
Compared with the DAB detection system, fewer perikarya were marked by using fluorescent markers. However, the number of positive nerve fibers was generally the same. This may reflect a less abundant signal for sst1 in perikarya compared with fibers; thus it was not detected in a less sensitive fluorescent visualization.
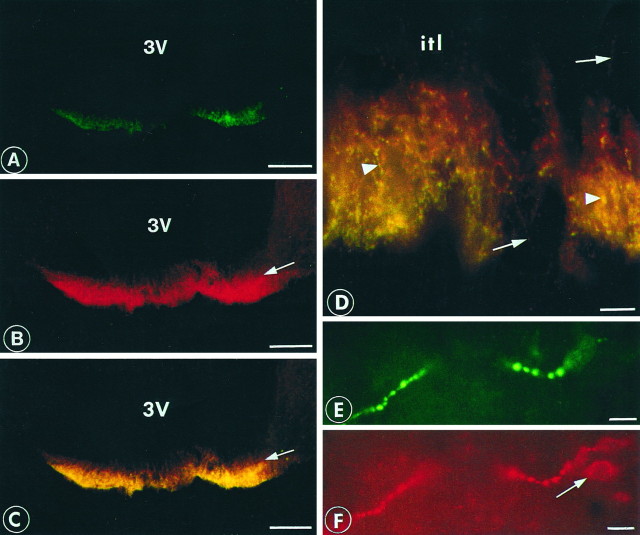
The largest concentration of labeled structures was located in the periventricular nucleus and the external part of the median eminence. There was an extensive colocalization of sst1 and somatostatin immunoreactivity (Fig. 4). Some somatostatin-positive perikarya and fibers did not contain sst1 immunoreactivity (Fig.4D–F), whereas no sst1 was detected outside somatostatinergic perikarya or fibers.
Fig. 4.
Double-immunofluorescent labeling of the rat median eminence. sst1 is visualized by fluorescein (green) and somatostatin with Texas Red (red). A strong labeling was observed in the external layer for sst1 (A) and somatostatin (B). A double-exposure micrograph is shown (C) displaying colocalization of somatostatin and sst1. Arrows mark the border between the internal and external layers of the median eminence. Double-exposure micrograph of high magnification of the median eminence (D) shows nerve fibers with boutons containing both sst1 and somatostatin (arrowheads) or somatostatin alone (arrows). itl marks the position of the internal layer. A periventricular nerve fiber endowed with large boutons shows colocalization of sst1(E) and somatostatin (F). Note the perikaryon exhibiting a positive immunoreaction for somatostatin (arrow) but not for sst1. Scale bars: A–C, 200 μm;D–F, 25 μm.
Ultrastructural immunocytochemistry
Thin sections of the median eminence showed the sst1immunoreactivity to be confined to the nerve terminals located predominantly in the external layer of the median eminence (Fig.5A). The number of immunoreactive terminals was roughly estimated to be below 5% of the total number of boutons. Perikarya in the median eminence were never stained. Nonreactive nerve fibers could be observed to terminate in positive-stained nerve terminals (Fig. 5B).
Fig. 5.
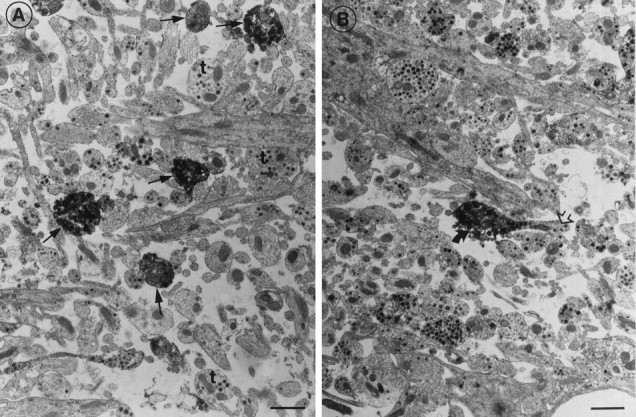
Electron microscopical detection of sst1 receptor immunoreactivity in the median eminence.A, Several immunoreactive nerve terminals (arrows) are seen between unstained nerve terminals (t). B, An unstained nerve fiber (open arrow) terminates in an immunoreactive nerve terminal (bent arrow). Scale bars, 1 μm.
The immunoreactive terminals contained both 20–40 nm clear transmitter vesicles and larger 100–300 nm dense-core vesicles (Fig.6).
Fig. 6.
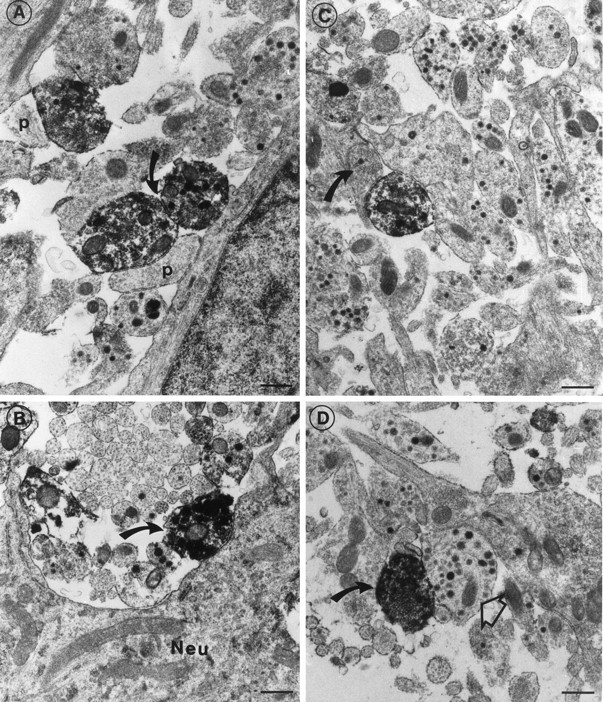
Electron micrographs of the median eminence immunoreacted for the sst1 receptor. A, Immunoreactive nerve terminals making synaptic contacts with unstained postsynaptic structures (p). Both presynaptic and postsynaptic structures contain clear neurotransmitter vesicles. Note the synaptic junction (bent arrow) between two immunoreactive terminals. B, Positive nerve terminal (bent arrow) making synapse-like contact with a neuronal perikaryon (Neu). C, Electron micrograph of an immunoreactive terminal making a synaptic contact with an unstained bouton termineaux (bent arrow) with small clear and a single large dense-core vesicle.D, Immunoreactive nerve terminal (bent arrow) with small clear and large dense-core vesicles. A classic peptidergic nerve terminal (open arrow) with many dense-core vesicles is seen close to the immunoreactive terminal. Scale bars, 0.5 μm.
The immunoreactive terminals often made presynaptic contacts with unstained nerve terminals containing small clear and single large dense-core vesicles (Fig. 6C,D). Sometimes two positive nerve terminals were observed to be connected by synapse-like junctions (Fig. 6A). Positive terminals also made synaptic contacts with neuronal perikarya in the median eminence (Fig.6B).
DISCUSSION
In Western blotting, the anti-sst1 antiserum specifically recognized a band in preparations of membranes isolated from rat hypothalamic tissue. The 80,000 apparent molecular weight band detected in the hypothalamus is larger than a broad band centered around 63,000 that was observed previously in BHK cells transfected with the human sst1 receptor (Helboe et al., 1997). This may reflect differences in the pattern of post-translational modifications between BHK cell lines and hypothalamic cells, including varying degrees of phosphorylation and glycosylation.
Retrograde neuronal tracings combined with immunohistochemical detection of somatostatin have shown that somatostatin-containing nerve fibers projecting to the median eminence originate in somatostatinergic perikarya in the hypothalamic periventricular region (Ishikawa et al., 1987; Kawano and Daikoku, 1988; Romero and Phelps, 1997). In the present study, we show that sst1 receptors are abundantly present in this neuronal projection system, on the perikarya as well as on the nerve fibers projecting from these perikarya to the external layer of the median eminence. This is in accordance with previous findings by in situ hybridization in the rat and mouse where mRNA encoding the sst1 receptor was found in periventricular-located perikarya (Breder et al., 1992; Pérez et al., 1994; Beaudet et al., 1995; Guo et al., 1996).
The double-labelings performed in this study revealed that sst1-immunoreactive neurons also contained somatostatin. This colocalization indicates that the sst1 receptor acts as an autoreceptor. Several studies suggest that somatostatin is able to inhibit its own secretion. Thus, intraventricular injection of somatostatin induced a paradoxical increase in the plasma concentration of GH (Abe et al., 1978; Lumpkin et al., 1981), and somatostatin or its analogs have been shown to suppress somatostatin release in cultured hypothalamic cells (Peterfreund and Vale, 1984; Richardson and Twente, 1986) and in anterior periventricular tissues (Epelbaum et al., 1986).
Somatostatin is present in the CSF (Patel et al., 1977), from which the peptide can diffuse into the periventricular neuropil where the somatostatin-containing perikarya are located. This is possible because the ependymal cells of the third ventricle are connected by gap and intermediate junctions (Brightman and Reese, 1969; Weindl and Joynt, 1972) that do not obstruct the diffusion of somatostatin from the ventricle into the brain. However, in the region of the median eminence, tight junctions connect the ependymal cells (Weindl and Joynt, 1972; Krisch and Leonhardt, 1978), preventing a diffusion directly into the brain parenchyma of this region. The presence of this barrier will create a considerable time delay for somatostatin action on nerve terminals in the median eminence. Therefore, one might speculate that somatostatin in the CSF may not bind to the autoreceptors on the somatostatinergic nerve terminals in this region to cause an increase in GH level but more likely may mediate this effect via binding to periventricular somatostatinergic neurons. In contrast, an ultrashort loop of inhibitory feedback may be present in the median eminence. Somatostatin released into the extracellular space may bind to sst1 receptors on the same terminal from which somatostatin is released and thus inhibit further release of the hormone.
The ultrastructural analysis of the median eminence in this study showed the sst1 receptor to be confined to nerve terminals. Because of diffusion of the DAB reaction product, the receptor protein could not be localized to any cellular compartment or structure. The ultrastructural staining of nerve terminals and lack of staining of the nerve fibers indicate a higher concentration of the sst1receptor on the terminals compared with nerve fibers.
Our study also shows that sst1-immunoreactive terminals make presynaptic contacts on other nerve terminals, indicating a regulatory function of somatostatin on the release of neurotransmitters or neurohormones from these terminals. Such presynaptic contacts were not observed in a previous cytochemical study (Daikoku et al., 1988). However, in our study of the Wistar rat median eminence, presynaptic contacts formed by sst1-immunoreactive neurons were not uncommon. This difference might be caused by the high sensitivity of the biotinylated tyramide immunohistochemical technique used in the present study.
Inhibitory autoreceptors located on the presynaptic membrane are known in other neurotransmitter receptor systems. Thus, the α2-adrenergic receptor is an inhibitory receptor located on the presynaptic membrane (Hertting et al., 1990; Aoki et al., 1994). In the hippocampus, the metabotropic glutamate receptor (mGluR7) is located presynaptically (Bradley et al., 1996). Serotonergic inhibitory autoreceptors are found both in the raphe system (el Mansari and Blier, 1996) and in the hippocampus (Schlicker et al., 1996). Also, presynaptic autoregulatory H3-receptors are present within the histaminergic system (Fujimoto et al., 1991).
Somatostatin is believed to be secreted in an oscillary manner out of phase with GHRH from the hypothalamus into the hypophyseal portal blood, thereby contributing to the pulsatile secretion of GH in rat (Tannenbaum and Ling, 1984; Plotsky and Vale, 1985). An autocrine regulation of somatostatin secretion through the sst1receptor may add to this pulsativity by a short loop feedback in somatostatinergic neurons in both the hypothalamus and the median eminence.
The concept of the sst1 receptor being a somatostatinergic autoreceptor is not new. Earlier observations of sst1 mRNA located in the hypothalamic periventricular area suggested colocalizations with somatostatin, thereby indicating an autoregulatory function (Beaudet et al., 1995). This finding was further substantiated by Viollet et al. (1997). By use of RT-PCR and selective somatostatin analogs, they found hypothalamic neurons to express mainly the sst1 and sst2 receptor subtypes, sst1 being the predominant receptor. In our present study, we provide further evidence for sst1 as an autoreceptor being located on somatostatinergic neurons. With regard to the sst2 receptor, immunohistochemical localization suggests that the sst2A receptor is absent or very poorly expressed in the rat periventricular nucleus and median eminence (Dournaud et al., 1996; Schindler et al., 1997). Therefore, the sst2Areceptor is unlikely to be involved in autoregulation in the somatostatinergic hypothalamus/median eminence system. Interestingly, however, expression of the sst2 receptor has been reported to increase the production of somatostatin in other systems. Thus, expression of sst2 in a mouse fibroblast cell line (Rauly et al., 1996) or restoration of lost sst2 receptors in human pancreatic tumor cells (Delesque et al., 1997) induced an endogenous somatostatin production in these cells. This led to a constitutive activation of the sst2 receptor and thus supposedly negative regulation of cell proliferation. To our knowledge, there are no reports on somatostatin autocrine functions via sst3, sst4, or sst5receptor subtypes.
In conclusion, we found that the sst1 receptor is colocalized with somatostatin in periventricular neurons projecting to the median eminence. Therefore, we suggest that sst1 plays an autocrine role in inhibiting the release of somatostatin from the hypothalamus. This autocrine mechanism of neuroendocrine secretion may provide an alternative approach for a clinical upregulation of GH release. Therefore, the use of selective sst1 receptor agonists will be valuable for further investigation of this issue.
Footnotes
Correspondence should be addressed to Lone Helboe, Institute of Medical Anatomy Section B, The Panum Institute, Blegdamsvej 3, DK-2200 Copenhagen, Denmark.
REFERENCES
- 1.Abe H, Kato Y, Iwasaki Y, Chihara K, Imura H. Central effect of somatostatin on the secretion of growth hormone in the anaesthetized rat. Proc Soc Exp Biol Med. 1978;159:346–349. doi: 10.3181/00379727-159-40345. [DOI] [PubMed] [Google Scholar]
- 2.Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- 3.Beaudet A, Greenspun D, Raelson J, Tannenbaum GS. Patterns of expression of SSTR1 and SSTR2 somatostatin receptor in the hypothalamus of the adult rat: relationship to neuroendocrine functions. Neuroscience. 1995;65:551–561. doi: 10.1016/0306-4522(94)00486-o. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 6.Breder CD, Yamada Y, Yasuda K, Seino S, Saper CB, Bell GI. Differential expression of somatostatin receptor subtypes in brain. J Neurosci. 1992;12:3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci USA. 1992;89:11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daikoku S, Hisano S, Kawano H, Chikamori-Aoyama M, Kagotani Y, Zhang R, Chicara K. Ultrastructural evidence for neuronal regulation of growth hormone secretion. Neuroendocrinology. 1988;47:405–415. doi: 10.1159/000124955. [DOI] [PubMed] [Google Scholar]
- 10.Delesque N, Buscail L, Estève JP, Saint-Laurent N, Müller C, Weckbecker G, Bruns C, Vaysse N, Susini C. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997;57:956–962. [PubMed] [Google Scholar]
- 11.Demchyshyn LL, Srikant CB, Sunahara RK, Kent G, Seeman P, van Tol HHM, Panetta R, Patel YC, Niznik HB. Cloning and expression of a human somatostatin-14-selective receptor variant (somatostatin receptor 4) located on chromosome 20. Mol Pharmacol. 1993;43:894–901. [PubMed] [Google Scholar]
- 12.Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A. Localization of the somatostatin receptor sst2A in rat brain using a specific anti-peptide antibody. J Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elde RP, Parsons JA. Immunocytochemical localization of somatostatin in cell bodies of the rat hypothalamus. Am J Anat. 1975;144:541–548. doi: 10.1002/aja.1001440416. [DOI] [PubMed] [Google Scholar]
- 14.el Mansari M, Blier P. Functional characterization of 5-HT1D autoreceptors on the modulation of 5-HT release in guinea-pig mesencephalic raphe, hippocampus and frontal cortex. Br J Pharmacol. 1996;118:681–689. doi: 10.1111/j.1476-5381.1996.tb15454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelbaum J. Intrahypothalamic neurohormonal interactions in the control of growth hormone secretion. In: Chadwick DJ, Marsh J, editors. Functional anatomy of the neuroendocrine hypothalamus (Ciba Foundation Symposium 168) Wiley; Chichester, UK: 1992. pp. 54–68. [DOI] [PubMed] [Google Scholar]
- 16.Epelbaum J, Tapia-Arancibia L, Alonso G, Astier H, Kordon C. The anterior periventricular hypothalamus is the site of somatostatin inhibition on its own release: an in vitro and immunohistochemical study. Neuroendocrinology. 1986;44:255–259. doi: 10.1159/000124653. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto K, Mizuguchi H, Fukui H, Wada H. Presynaptic localization of histamine H3-receptors in rat brain. Biochem Biophys Res Commun. 1991;177:907–912. doi: 10.1016/0006-291x(91)90624-g. [DOI] [PubMed] [Google Scholar]
- 18.Guo F, Beaudet A, Tannenbaum GS. The effect of hypophysectomy and growth hormone replacement on sst1 and sst2 somatostatin receptor subtype messenger ribonucleic acids in the arcuate nucleus. Endocrinology. 1996;137:3928–3935. doi: 10.1210/endo.137.9.8756568. [DOI] [PubMed] [Google Scholar]
- 19.Helboe L, Møller M, Nørregaard L, Schiødt M, Stidsen CE. Development of selective antibodies against the human somatostatin receptor subtypes sst1-sst5. Mol Brain Res. 1997;49:82–88. doi: 10.1016/s0169-328x(97)00127-7. [DOI] [PubMed] [Google Scholar]
- 20.Hertting G, Wurster S, Allgaier C. Regulatory proteins in presynaptic function. Ann NY Acad Sci. 1990;604:289–304. doi: 10.1111/j.1749-6632.1990.tb32001.x. [DOI] [PubMed] [Google Scholar]
- 21.Hökfelt T, Efendic S, Hellerström C, Johansson O, Luft R, Arimura A. Cellular localization of somatostatin in endocrine-like cells and neurons of the rat with special references to the A1-cells of the pancreatic islets and to the hypothalamus. Acta Endocrinol. 1975;200:5–41. [PubMed] [Google Scholar]
- 22.Ishikawa K, Taniguchi Y, Kurosumi K, Suzuki M, Shinoda M. Immunohistochemical identification of somatostatin-containing neurons projecting to the median eminence of the rat. Endocrinology. 1987;121:94–97. doi: 10.1210/endo-121-1-94. [DOI] [PubMed] [Google Scholar]
- 23.Kawano H, Daikoku S. Somatostatin-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies. J Comp Neurol. 1988;271:293–299. doi: 10.1002/cne.902710209. [DOI] [PubMed] [Google Scholar]
- 24.Kluxen FW, Bruns C, Lübbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci USA. 1992;89:4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krisch B, Leonhardt H. The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res. 1978;192:327–339. doi: 10.1007/BF00220750. [DOI] [PubMed] [Google Scholar]
- 26.Lamberts SWJ. The role of somatostatin in the regulation of anterior pituitary hormone secretion and the use of its analogs in the treatment of human pituitary tumors. Endocr Rev. 1988;9:417–436. doi: 10.1210/edrv-9-4-417. [DOI] [PubMed] [Google Scholar]
- 27.Li XJ, Forte M, North RA, Ross CA, Snyder SH. Cloning and expression of a rat somatostatin receptor enriched in brain. J Biol Chem. 1992;267:21307–21312. [PubMed] [Google Scholar]
- 28.Lumpkin MD, Negro-Vilar A, McCann SM. Paradoxical elevation of growth hormone by intraventricular somatostatin: possible ultrashort-loop feedback. Science. 1981;211:1072–1074. doi: 10.1126/science.6110244. [DOI] [PubMed] [Google Scholar]
- 29.Lumpkin MD, Samson WK, McCann SM. Effects of intraventricular growth hormone-releasing factor on growth hormone release: further evidence for ultrashort loop feedback. Endocrinology. 1985;116:2070–2074. doi: 10.1210/endo-116-5-2070. [DOI] [PubMed] [Google Scholar]
- 30.Meyerhof W, Wulfsen I, Schönrock C, Fehr S, Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Natl Acad Sci USA. 1992;89:10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Carroll AM, Lolait SJ, König M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992;42:939–946. [PubMed] [Google Scholar]
- 32.Patel YC, Rao K, Reichlin S. Somatostatin in human cerebrospinal fluid. N Engl J Med. 1977;296:529–533. doi: 10.1056/NEJM197703102961002. [DOI] [PubMed] [Google Scholar]
- 33.Pérez J, Rigo M, Kaupmann K, Bruns C, Yasuda K, Bell GI, Lübbert H, Hoyer D. Localization of somatostatin (SRIF) SSTR-1, SSTR-2 and SSTR-3 receptor mRNA in rat brain by in situ hybridization. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:145–160. doi: 10.1007/BF00169831. [DOI] [PubMed] [Google Scholar]
- 34.Peterfreund RA, Vale WW. Somatostatin analogs inhibit somatostatin secretion from cultured hypothalamus cells. Neuroendocrinology. 1984;39:397–402. doi: 10.1159/000124011. [DOI] [PubMed] [Google Scholar]
- 35.Plotsky PM, Vale W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science. 1985;230:461–463. doi: 10.1126/science.2864742. [DOI] [PubMed] [Google Scholar]
- 36.Rauly I, Saint-Laurent N, Delesque N, Buscail L, Estève JP, Vaysse N, Susini C. Induction of a negative autocrine loop by expression of sst2 somatostatin receptor in NIH 3T3 cells. J Clin Invest. 1996;97:1874–1883. doi: 10.1172/JCI118618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 38.Richardson SB, Twente S. Inhibition of rat hypothalamic somatostatin release by somatostatin: evidence for somatostatin ultrashort loop feedback. Endocrinology. 1986;118:2076–2082. doi: 10.1210/endo-118-5-2076. [DOI] [PubMed] [Google Scholar]
- 39.Romero MI, Phelps CJ. Identification of growth hormone-releasing hormone and somatostatin neurons projecting to the median eminence in normal and growth hormone-deficient ames dwarf mice. Neuroendocrinology. 1997;65:107–116. doi: 10.1159/000127170. [DOI] [PubMed] [Google Scholar]
- 40.Schindler M, Humphrey PPA, Emson PC. Somatostatin receptors in the central nervous system. Prog Neurobiol. 1996;50:9–47. doi: 10.1016/0301-0082(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 41.Schindler M, Sellers LA, Humphrey PPA, Emson PC. Immunohistochemical localization of the somatostatin sst2(A) receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- 42.Schlicker E, Fink K, Zentner J, Gothert M. Presynaptic inhibitory serotonin autoreceptors in the human hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:393–396. doi: 10.1007/BF00171075. [DOI] [PubMed] [Google Scholar]
- 43.Tannenbaum GS, Ling N. The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology. 1984;115:1952–1957. doi: 10.1210/endo-115-5-1952. [DOI] [PubMed] [Google Scholar]
- 44.Viollet C, Lanneau C, Faivre-Bauman A, Zhang J, Djordjijevic D, Loudes C, Gardette R, Kordon C, Epelbaum J. Distinct patterns of expression and physiological effects of sst1 and sst2 receptor subtypes in mouse hypothalamic neurons and astrocytes in culture. J Neurosci. 1997;68:2273–2280. doi: 10.1046/j.1471-4159.1997.68062273.x. [DOI] [PubMed] [Google Scholar]
- 45.Weindl A, Joynt RJ. The median eminence as a circumventricular organ. In: Knigge KM, Scott DE, Weindl A, editors. Brain-endocrine interaction. Median eminence: structure and function. Karger; Basel: 1972. pp. 280–297. [Google Scholar]
- 46.Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA. 1992a;89:251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, Kagimoto, Seino M, Seino Y, Bell GI, Seino S. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol. 1992b;6:2136–2142. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]
- 48.Yamada Y, Kagimoto S, Kubota A, Yasuda A, Masuda K, Sorneya Y, Ihara YU, Li Q, Imura H, Seino S, Seino Y. Cloning, functional expression and pharmacological characterization of a fourth (hSSTR4) and a fifth (hSSTR5) human somatostatin receptor subtype. Biochem Biophys Res Commun. 1993;195:844–852. doi: 10.1006/bbrc.1993.2122. [DOI] [PubMed] [Google Scholar]