Abstract
Animals can locate their present position in relation to a starting point and return to that starting point using cues generated by self-movement, a navigation strategy called dead-reckoning. Because contemporary research on spatial navigation suggests that some aspects of spatial navigation depend on the integrity of the hippocampal formation, whereas others do not, the present study examined whether dead-reckoning is hippocampally dependent. The task capitalized on the proclivity of foraging rats to carry large food pellets to a shelter for eating. Control rats and rats with fimbria–fornix (FF) lesions left a hidden burrow to search for one piece of food located somewhere on a circular table. The accuracy with which they returned to the burrow with the food was measured. In three experiments, rats received probe trials in which they (1) started from novel locations, (2) wore blindfolds to obscure visual cues, and (3) foraged under a condition in which surface cues, e.g., odors left by their outward searches, were displaced. Both sighted control and FF rats preferentially used visual cues for guidance when foraging from a familiar location. Control rats were accurate and FF rats were impaired in returning to novel starting locations (1) when sighted, (2) when blindfolded, and (3) when blindfolded in tests in which surface cues were displaced. These results, as well as detailed observations on the behavior of the animals, are consistent with the hypothesis that rats can use dead-reckoning to solve spatial problems, and this ability depends on the integrity of the hippocampal formation.
Keywords: fimbria–fornix, hippocampus, hippocampal lesions, path integration, spatial learning, spatial navigation
Charles Darwin’s (1873) suggestion that animals might use “dead-reckoning” to navigate has been confirmed by experiments with humans and other animals (Barlow, 1964;Etienne, 1987; Gallistel, 1990). In dead-reckoning, an animal computes its position relative to a starting location by integrating cues generated by its movements between a starting position and present location. If self-movement cues are integrated twice, an animal can compute the straight-line path from its current position back to the starting point, which is useful in allowing it to return home. An animal can record self-motion by cues from the vestibular system, muscle and joint receptors, and efference copies of commands that generate movement. It may also compute its speed by monitoring flows in visual, auditory, and olfactory stimuli caused by the movements. The internal and external cues that provide information about self-movement are referred to as idiothetic cues (Mittelstaedt and Mittelstaedt, 1973). The computations used to derive a location and a return path are referred to as path integration. The ability to monitor one’s location and to return home is colloquially called “sense of direction.”
An animal can also navigate using stable external stimuli, a behavior called piloting (O’Keefe and Nadel, 1978; Gallistel, 1990). Such stimuli, collectively called allothetic cues, can also be used to remember a starting point, know present location, and return home. Because an animal can navigate either by integrating idiothetic cues or by learning allothetic cue relationships, it is ordinarily difficult to determine which strategy is used in any situation. If either idiothetic or allothetic cues are manipulated experimentally, e.g., by making them uninformative, it is possible to determine which strategy is being used. For example, a subject deprived of allothetic cues by loss of vision is likely to use idiothetic cues and path integration (Landeau et al., 1984; Etienne et al., 1996).
Presently, it is not known what brain structures allow an animal to navigate by dead-reckoning, but behavioral (Whishaw, 1988; Whishaw et al., 1997), modeling (Worden, 1992; Samsonovich and McNaughton, 1997), and electrophysiological studies (O’Mara et al., 1994; Sharp et al., 1995; Taube and Burton, 1995; Blare and Sharp, 1996i; McNaughton et al., 1996; Wiener, 1996; Golob and Taube, 1997) suggest that the hippocampal formation [entorhinal cortex, dentate gyrus, hippocampus proper or Ammon’s horn, and subicular complex and fimbria–fornix (FF)] is involved. We examine this idea by contrasting the foraging performance of rats with sham lesions to rats with FF lesions, which disrupt information flow in the hippocampal formation (Bland, 1986), impair memory (Gaffan and Gaffan, 1991), and produce spatial deficits (Whishaw, 1993; Whishaw and Jarrard, 1995). The rats leave a hidden home base to find a large food pellet on an open field (Whishaw and Tomie, 1996). The accuracy with which they return to the home base is the dependent variable. Allothetic cues are restricted by starting the animals from novel locations, blindfolding them, and rotating the apparatus to displace olfactory cues left on the surface of the apparatus.
MATERIALS AND METHODS
Animals
Adult female Long–Evans rats (University of Lethbridge vivarium), weighing 250–300 gm, were housed in groups in wire mesh cages in a laboratory with room temperature maintained at 20–21°C and lighted on a 12 hr light/dark cycle (8 A.M.–8 P.M.)
Surgery
For surgery, the rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and atropine methyl nitrate (5 mg/kg). To make FF lesions, 1.5 mA cathodal current was passed for 40 sec through 00 stainless steel insect pins, insulated with Epoxylite except at the surface of their tips. Lesions were made at two sites in each hemisphere using coordinates in reference to bregma and the surface of the dura: 1.3 mm posterior, 1.5 mm lateral, and 3.6 mm ventral; and 1.5 mm posterior, 0.5 mm lateral, and 3.3 mm ventral (Whishaw and Jarrard, 1996). The control rats received anesthesia only.
Feeding
Feeding was restricted to maintain the rats at 90% of their expected body weights. Large (750 mg) rodent pellets (Bio-Serv Inc., Frenchtown, NJ), were used for reward during behavioral testing. Rats reliably carry these pellets to a refuge for eating (Whishaw et al., 1995a). After testing each day, the rats were supplementally fed with LabDiet laboratory rodent pellets in their home cage.
Apparatus
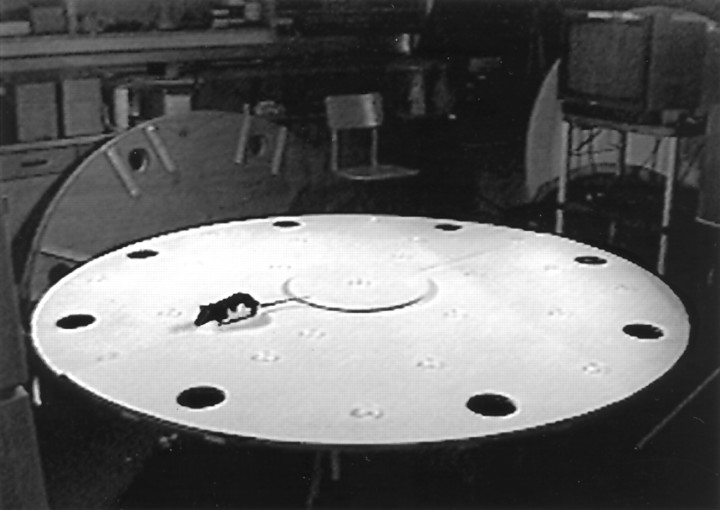
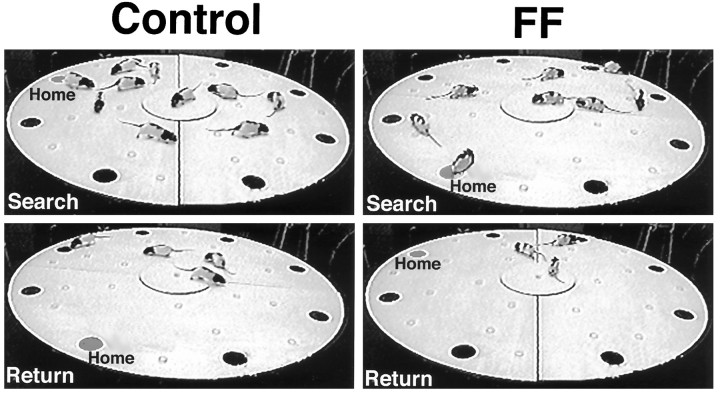
The open field consisted of a 204-cm-diameter circular wooden table, similar to a Barnes spatial testing apparatus (Barnes, 1979) that was painted white and was elevated 64 cm above the floor (Fig.1). Eight 11.5-cm-diameter holes were cut in the table, spaced equidistant around its perimeter, and centered 13.5 cm from the edge of the table. The apparatus was mounted on a central bearing that allowed it to be rotated between or during trials. A fixed central platform 45.5 cm in diameter, mounted 1 cm above its surface, could be located in the center of the table. This allowed the main table to be rotated while a rat was relatively stationary on the central table. A food pellet was hidden in one of the 23 translucent white food cups (4.5-cm-diameter and 1-cm-high plastic weigh boats) attached to the table. The apparatus was located in a test room in which many cues, including windows covered by blinds, counters, a refrigerator, cupboards, and a desk with computers, were present. A camera was located above the center of the table so that the behavior of the animals could be video-recorded (Whishaw and Tomie, 1997).
Fig. 1.
The apparatus consists of a large circular table. It is mounted to the table legs by a central bearing so that it can be turned. A cage is placed beneath one of the holes into which a rat can escape. Nothing is located beneath the other holes so that a rat cannot escape the surface through them. Small plastic food cups, into which food pellets can be placed, are located on the table. An elevated circular platform can be fixed in the center of the table, allowing the table to be rotated while the central zone is fixed.
Masks and blindfolds
Masks (Fig. 2A) and blindfolds (Fig. 2B), used to control the rat’s use of visual cues, were constructed of felt and attached by a velcro collar fixed around a rat’s neck. They were fastened across a rat’s face by an elastic chin strap that was attached to the neck collar. The elastic strap was flexible so that a rat could grasp food pellets with its mouth and chew and swallow them. A mask allowed the rats to see, whereas a blindfold occluded vision. The effectiveness of the blindfolds was tested on rats trained to swim to a visible platform located in a swimming pool. Well-trained rats wearing a mask swam directly to the platform from any starting point on the periphery of the pool, whereas rats wearing the blindfold swam around the edge of the pool or swam in a haphazard manner. The rats were adapted to wearing masks and blindfolds by having them wear the apparel for at least 30 min/d for 5 d before testing. Before the formal tests, a mask or blindfold was placed on the animals for 30 min before the test.
Fig. 2.
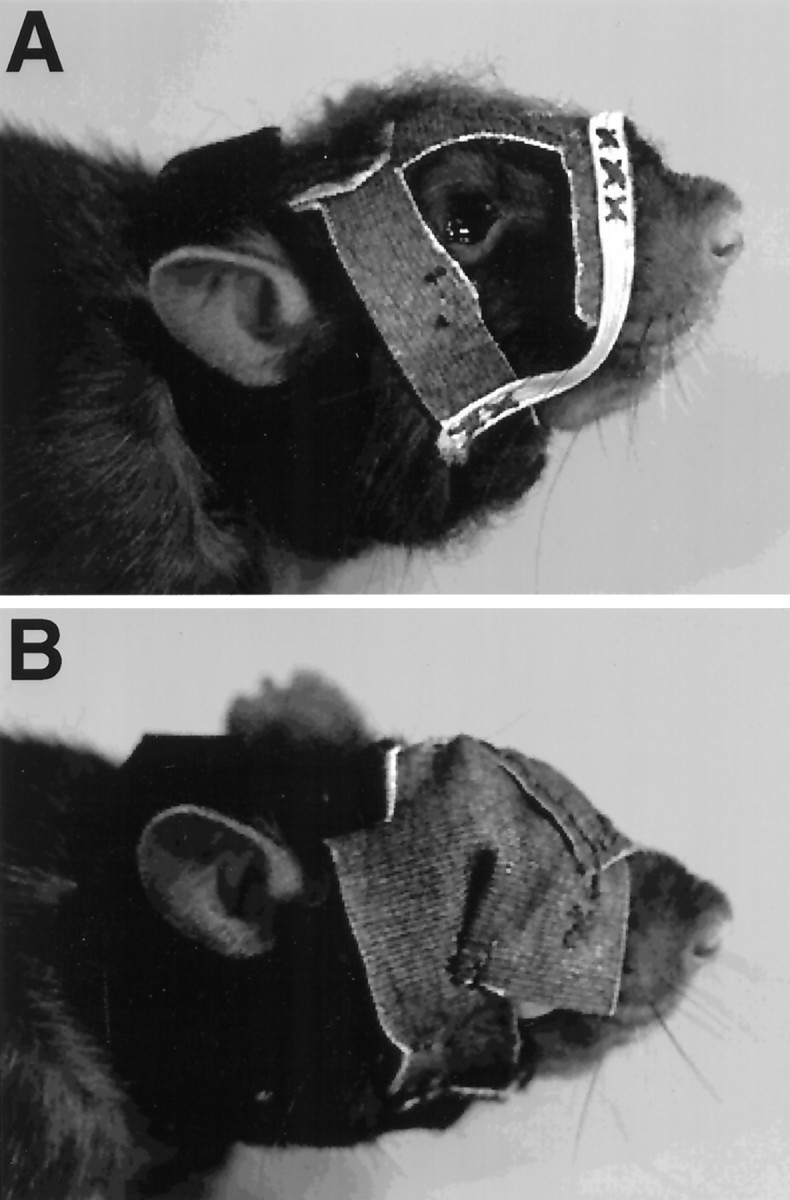
Masks (A) and blindfolds (B). Both kinds of headwear are fixed to a rats head by Velcro collars. An elastic chin strap holds the headwear against a rat’s face but still allows the rat to open its mouth and chew.
Pretraining
For pretraining, the rats received four trials each day for 10 d. A food pellet was located in each of the 23 food cups for the first few days, but the number of pellets was quickly reduced until only one food cup was baited. The refuge cage was located beneath the south hole throughout pretraining. During pretraining, the rats learned to climb up onto the table, find a food pellet, and carry it back to the cage. After the rat obtained a food pellet, a new dish was baited while the rat was eating. Pretraining was complete when the rats quickly executed the four search and retrieval trials in succession.
Analysis
The behavior of the rats was filmed on all of the tests. From the ongoing trials and the video recordings the following behavioral measures were made:
Retrieval. A retrieval was defined as an exit from the home cage and a return with a food pellet.
Correct trial. A correct trial was a trial in which a rat found a food pellet and returned directly to the starting hole without stopping at any other potential exit hole.
Error. An incorrect trial was one in which a rat found a food pellet but stopped at one of the other potential exits before returning to the exit from which its excursion began. A rat was deemed to have stopped at an exit if its snout was brought to within ∼2 cm of a hole (errors were usually unambiguous as the rats stopped and inserted their heads into the holes).
First choice. The first choice was defined as the first hole a rat visited after finding a food pellet.
Second choice. The second choice was defined as the second hole that a rat visited, given that the first choice was incorrect, and a second choice could include a perseverative return to the first choice.
Exit and return routes. The route taken as a rat left the home base and the route taken as a rat returned to the home base were drawn on maps of the field. The exit and return routes were drawn with different color pens.
Response times. Using a stopwatch, an observer recorded separately the time taken to find a food pellet and the time taken to return to the home cage with the food.
Statistical analysis
The directional selectivity of choices was assessed by circular statistics (Batschelet, 1981). Group comparisons were made by converting directional headings to deviational degrees from a direct heading and subjecting the results to ANOVAs (Winer, 1962).
Histology
At the completion of the experiments, the rats were deeply anesthetized and perfused with saline and saline–formalin, and the brains were removed and stored in a 30% sucrose–formalin solution. The brains were cut in 40 μm sections on a cryostat, and alternate sections were stained with cresyl violet and stained for acetylcholinesterase (AChE).
Procedure
Three experiments were performed. The first experiment examined the ability of control and FF rats to return accurately to a starting location in a visually rich environment. The second experiment controlled the rat’s use of visual cues by placing either a mask or blindfold on the rat. The third experiment controlled the rat’s use of olfactory cues by rotating the table, to displace the odor of the home cage and a rat’s odor trail relative to the starting point.
Experiment 1: novel starting locations. Twelve rats were pretrained to forage for a food pellet with the refuge burrow located at the south position. Once pretrained, six rats received sham and six received FF lesions followed by 10 d of recovery. The rats then received additional pretraining for ∼5 d to ensure that they still performed the task. On the following day the test began. The test took place over the next 7 d during which the rats received one trial each day. Each trial began from one of the unused holes, i.e., a new starting location, and starting locations were selected semirandomly. The test procedure was completed after each rat had started once from each of the seven starting locations. For the tests, the starting cage was placed under the “hole of the day” and no cages were located under other holes.
Experiment 2: masks and blindfolds. Six control and six FF rats were used. They received sham or FF lesions before training and testing. The home cage was located at the south location during pretraining. The rats were pretrained by giving them four trials a day for 15 d, by which time they all returned accurately to the south exit hole. They then received two probe trials, interspersed with 5 d of baseline training from the south hole. (1) Mask probe: for the masked probe, the masks were fitted to the rats for 30 min. The rats then received a single trial in which the home cage containing the animal was placed beneath the northeast hole. The food pellet was located at a position that was diagonally opposite the starting position. (2) Blindfold probe: for the blindfold probe, the blindfolds were fitted to the rats for 30 min. The rats then received a single trial in which the home cage containing the animal was placed beneath the northeast hole. The food pellet was located at a position that was diagonally opposite the starting position.
Experiment 3: table rotation. Ten control and 10 FF rats were trained from the south position and were adapted to wearing blindfolds. The rats received four sets of four trials, with each set of trials beginning at the same starting point. On three trials, the food was in one of the food locations chosen in a semirandom manner, and the table remained at a fixed position. On the fourth trial, a probe trial, the food pellet was located in the center of the fixed platform. When the rat reached the center, and as it was grasping the food, the table was quickly rotated so that the starting hole was moved 90° to the left or right of its initial location. On the training trials, the measure of the rat’s performance was the hole that it first contacted after finding the food. On the probe trials, the measure of the rat’s performance was the deviation in degrees from the most direct route back to the starting position. The angle measures were made at a point 83 cm from the center of the table, the point that marked the inner circle of the refuge holes. Thus, if a rat’s deviation angle was 0°, it would have returned to the point in space from which it had begun and would have found a new hole at that location.
RESULTS
Histological results
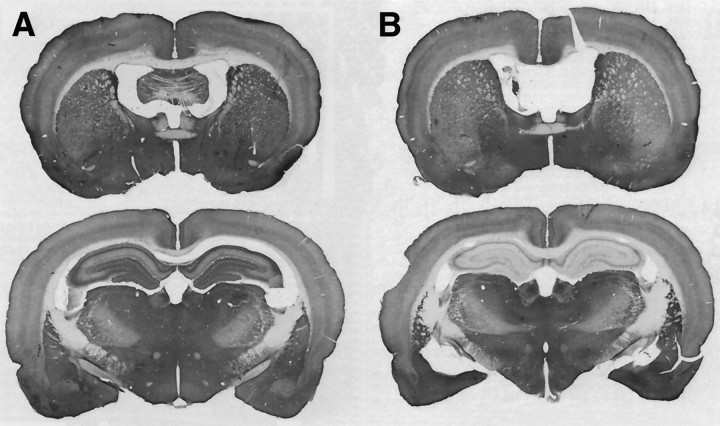
The dorsal fimbria–fornix was completely sectioned in all of the rats that were given lesions (Fig.3A, top vsbottom). The lesions were selective and did not damage the septum, the septal portions of the hippocampus, or the hippocampal commissure. The tract made by the electrodes and the lesion did little damage to the supracallosal septohippocampal pathways or cortex other than the path made by the penetration. Previous work has shown that supracallosal damage does not produce additional impairments on spatial tasks (Sutherland and Rodriguez, 1989; Jeltsch et al., 1994). Stains for AChE revealed extensive depletion of AChE in the hippocampus (Fig.3A,B, bottom). From previous work it is known that the lesion used in the present experiment reduces cholinergic markers by ∼70% in the dorsal hippocampus (Cassel et al., 1991; Jeltsch et al., 1994).
Fig. 3.
Photomicrographs of the control and lesion brain.A, Control. The top photomicrograph shows the intact fimbria–fornix at the level of the anterior commissure, and the bottom figure shows the hippocampus at the level of the thalamus. B, Corresponding photomicrographs in a rat with a fimbria–fornix lesion. Top, Most of the tissue in the cavity left by the removal of the fimbria–fornix is choroid plexus. Bottom, Note the absence of cholinesterase staining in the hippocampus. The cut in the cortex on thetop is a path made by an electrode penetration.
General behavioral observations
Whether placed at a familiar or novel location, a rat typically poked its head out of the hole a number of times before it exited. It exited by pulling itself up with its forepaws and pushing with its hindpaws. Once on the table, it typically paused briefly before setting off in search of food. Its outward journeys typically consisted of short darts followed by pauses. The direction taken from trial to trial was inconsistent across both rats and days. As a rat traversed the surface of the table, it scanned the food dishes with lateral head scans, or it actually stopped and sniffed the dish. By observing a rat’s behavior, it was clear that the rat had to be within ∼10 cm of the dish to detect the food. Thus, as well as approaching the dishes, a rat would occasionally stop and sniff, although some distance away from a dish. Once it found a piece of food, a rat grasped it in its mouth and set off quickly, often at a gallop, for the home base. If the home base had been moved, or if the rat had difficulty locating it, it stopped and inspected other holes. Inspections consisted of quite long pauses during which the rat thrust its head into the hole, often repeatedly, and sniffed. Once it arrived at the home base, the rat inserted its head into the hole and adjusted the position of its feet so that it could drop down into the cage beneath the hole. Individual rats required ∼25–40 sec to eat the food before making another foraging trip.
Experiment 1: return accuracy
When started from novel locations, the control rats all initially returned to the south location, which was the base used for pretraining. When they did not find the home cage at that location, they then correctly returned to the starting location of that day. With each successive daily trials, they were increasingly likely to return to the location from which they emerged, i.e., the new location, or the location of the previous day. FF rats also initially returned to the south, the training location, but their behavior was perseverative as they returned again as their second choice (and many subsequent choices). As training progressed, their performance became haphazard.
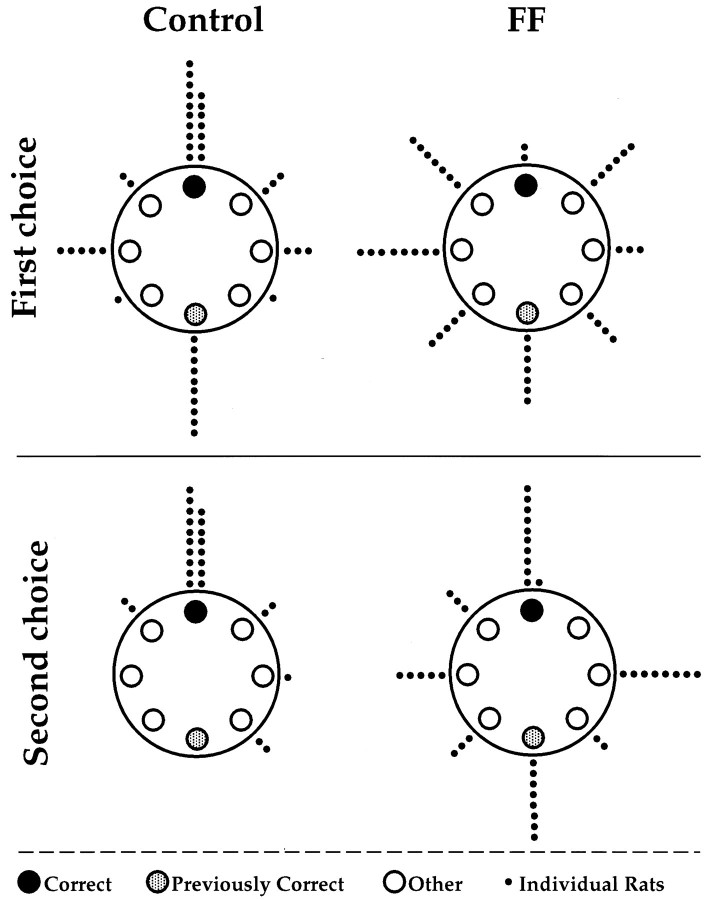
By the end of pretraining, both control and FF rats returned to the south location on every trial. On the test, using new starting locations, the control rats returned to the starting hole on 40.5% of trials versus 4.67% of trials for FF rats, giving a group difference that was significant, (F(1,10) = 12.87;p < 0.001). The accuracy in returning to the starting hole improved across trials as indicated by a significant effect of trials (F(6,60) = 6.75; p < 0.001), but improvement was limited to the control rats, as indicated by a significant group by trial interaction (F(6,60) = 2.60; p < 05). When control rats did make an error, their second choice was the correct hole on 72% of trials versus 27.5% for FF rats. On those trials in which the control rats failed to return to the departure location, they most frequently went first to the training location. This was also initially true for the FF rats, but as training progressed their choices became almost random. By representing the correct choice as the north hole, the training location as the south hole, and other choices as a function of distance from the correct hole, choices were plotted as vectors on a diagram of the apparatus (Fig.4). Circular statistics indicated that the control rats’ choices were concentrated on the correct location as a first choice (p < 0.001) and a second choice (p < 0.001). FF rats showed no overall preference (p > 0.10).
Fig. 4.
Vector diagrams of location choices by control and fimbria–fornix (FF) rats in seven tests in which rats received one trial a day from a new location. First choice(top), The control rats mainly returned to the starting hole (top, correct) or to the hole that was correct on the previous day (bottom, previously correct), whereas choices by the FF rats are randomly distributed.Second choice (bottom), After an incorrect choice, the control rats mainly chose the starting location as a second choice, whereas fimbria–fornix rats still make choices at random. Although each trial began from a different location, the correct hole is represented by the top location, and the training hole is represented by the bottom location. Other locations represent distances and direction from the correct location.
The accuracy measures of the rats’ performance were paralleled by measures of search and return latencies. The control rats took longer to find the food than the FF rats (control, 39.5 sec; vs FF, 14.4 sec;F(1,10) = 8.3; p < 0.01), but they also returned more quickly to the correct hole than the FF rats (control, 10.8 sec; vs FF, 23.9 sec; F(1,10) = 10.3; p < 0.01). The latency results, along with inspection of the video records, indicated that the control rats spent more time pausing and making scanning movements as well as inspecting the apparatus than did the FF rats on the outward trips. For example, control rats would also stop and inspect the starting location of the previous day before searching for food, a behavior that was seldom displayed by the FF rats. The high return latencies of the FF rats reflect the time that elapsed as they made errors and their tendency to stop and eat the food on the table when they were unable to find the correct hole.
Experiment 2: masks and blindfolds
At the end of preliminary training, all of the rats in the control group and in the FF group left the refuge hole, searched the table for food, and after they found the food pellet, carried it directly back to the refuge hole. They were also able to find and carry food when wearing masks and blindfolds. Their behavior while wearing masks was very similar to their behavior on training trials, but when wearing blindfolds, their movements were deliberate, they made few head scans in the air, and they walked with a hunched posture with their nose to the surface of the table.
Mask probe
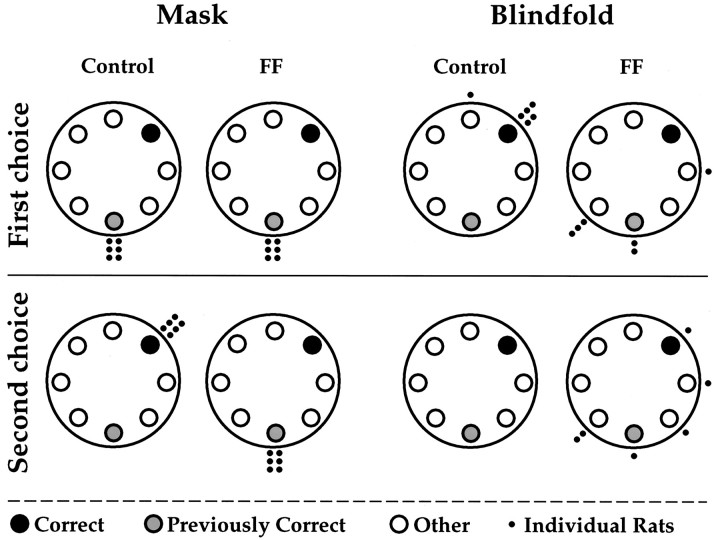
When the masks were placed on the rats and the rats were placed at the northeast hole, all of the control rats and all of the FF rats carried the food back to the south hole (the training hole). On not finding the refuge cage at the south hole, all of the control rats then went to the northeast location, the point from which they departed. All of the rats with FF lesions perseverated in returning to the south hole and only made it back to the northeast hole after committing numerous repetitive errors. First and second choices by control and FF rats are shown in (Fig. 5). There was one other interesting difference between the control and FF rats. When the control rats first emerged from the novel location, all six rats first went to the south (training) location and investigated that hole, before searching for the food pellet. Only two of the six FF rats returned to the south location before searching for food. Measures of latency showed that the control rats were slower in finding the food pellet than were the FF rats (t(10) = 7.21;p < 0.001) but faster in returning to the starting location with the food (t(10) = 12.2;p < 0.001).
Fig. 5.
Performance of control and fimbria–fornix rats on mask and blindfold probes. The rats were pretrained to go to the previously correct location and given one probe trial while wearing a mask in which they started from a new location (correct) and one probe trial while wearing a blindfold in which they started from the new location. Note that in the mask condition, control and fimbria–fornix rats first chose the previously correct hole, but as a second choice the control rats chose the new hole, whereas the fimbria–fornix rats perseverated in returning to the previously correct hole. In the blindfold condition, five of the six control rats returned to the new starting location, whereas the responses of the fimbria–fornix rats seemed random.
Blindfold probe
On the blindfold probe trial, five of the control rats returned correctly to the northeast starting location with the food, whereas none of rats in the FF group did so (t(10) = 5.91; p < 0.001). As a second choice the one control rat that missed the correct hole found it on its second choice, but only one FF rat found the starting location as a second choice. Interestingly, when blindfolded, five of the six FF rats navigated toward the south portion of the table. They may have been guided by auditory cues or surface olfactory cues, because no attempt was made to mask auditory cues, and the table was not washed to mask olfactory cues. Measures of latency showed that there were no differences in finding the food (t(10) = 0.78;p < 0.05), but the control rats were faster in returning with the food (t(10) = 5.32;p < 0.001).
Experiment 3: table rotation
All of the rats wore blindfolds during final training and on testing for this experiment. Their selection of the hole was the dependent measure on the training trials, and their selection of a direction was the dependent measure on the probe trials on which the starting location was rotated. All rats were able to find food pellets, and there were no significant group differences in their latency to do so. The control rats were more accurate and quicker in returning to their starting point on both training and probe trials, whereas the FF rats were inaccurate on both kinds of trials.
Training trials
The control rats were much more accurate in returning to the starting position on the training trials than were the FF rats. The starting hole was the first hole contacted by the control rats on 88.3% of trials versus 33.4% for the FF rats (F(1,18) = 68.2; p < 0.001).
Probe trials
Measures of heading accuracy on the probe trials, on which the board was rotated by 90°, while the rat was on the fixed central portion of the table retrieving the food, also showed that the control rats were significantly more accurate in their returns to the starting location than FF rats (control, 17.2 ± 3°; vs FF, 86 ± 11°; F(1,18) = 9.22; p < 0.001). There was no effect of probe number, nor was there an interaction effect.
Figure 6 illustrates a typical search route and return by a control and FF rat, each of which wore a blindfold. Both the control and FF rat (Fig. 6, top) took a circuitous route on the search for food before locating the food pellet in the center of the table. At this time the periphery of the table was rotated. The control rat then returned to the starting location, and the FF rat did not (Fig. 6, bottom). Actual paths taken by the control rats and the FF rats on the first probe trial are illustrated in Figure 7.
Fig. 6.
Examples of a search and return by blindfolded control and fimbria–fornix rats on a probe trial on which the food pellet is located in the fixed center portion of the table. While the rat is grasping the food, the outer portion of the maze is rotated 90° to displace surface cues. Note that (1) the path taken by both rats on the outward trip indirectly leads them to the food pellet; (2) the return path taken by the control rat takes it directly to the starting location; and (3) the return path taken by the fimbria–fornix rat is incorrect.
Fig. 7.
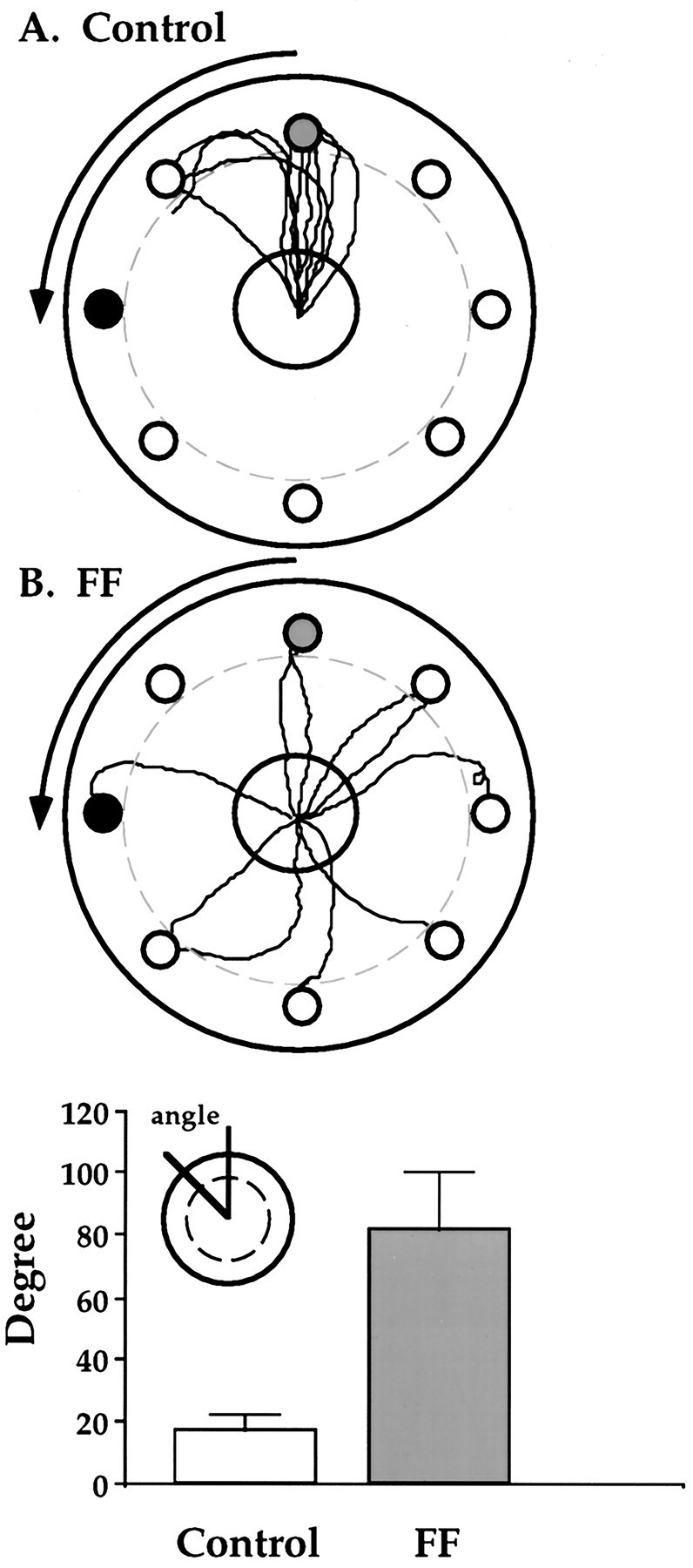
Return paths of blindfolded control (A) and fimbria–fornix (B) rats on a probe trial on which the outer portion of the table was rotated 90°, to displace surface cues on the table, while the rat was retrieving a food pellet from the fixed center portion of the table. Note that the control rats returned accurately to the starting point, whereas the fimbria–fornix rats did not. Bottom, average heading errors (mean + SE) of control and FF rats, with 0° representing the correct return and the angle between the correct return and the actual return (inset) representing performance.
DISCUSSION
We examined whether control and fimbria–fornix rats could return with food to a starting location after a foraging trip, and we manipulated the testing conditions to determine the strategy that they used. Both control and FF rats could forage from, and return to, a familiar starting location using vision. Only control rats (1) returned accurately to new starting locations under visually rich conditions, (2) returned accurately to starting points although blindfolded, and (3) returned accurately to starting points when olfactory cues were displaced. These results suggest that the control rats can use dead-reckoning to navigate and further suggest that the hippocampal formation or some portions of it is important for the use of this strategy.
Navigating to novel locations
There is accumulating evidence that in certain circumstances animals use dead-reckoning in conventional laboratory tests (Barlow, 1964; Alayn, 1996; Etienne et al., 1996; Dudenchenko et al., 1997;Martin et al., 1997). The present study provides evidence that control, not FF, rats may use dead-reckoning in a normal visually rich environment.
In the first experiment, both control and FF rats learned to return to a familiar starting location, but only the control rats returned to novel starting locations that they had experienced only on that trial. This finding suggests that the control rats could access both piloting and dead-reckoning strategies to guide their responses, whereas FF rats could only access a piloting strategy. That is, the initial preference of both groups of rats was to return to the old training location, suggesting that they ignored idiothetic cues generated on their outward trip from new locations in favor of using the allothetic cues that previously had guided their homeward trips to the training location. On finding that the refuge cage was not at that location, however, only the control rats could quickly abandon this strategy. With repeated exposure to new locations they were also able to abandon piloting completely. It is our suggestion that the control rats switched to using idiothetic cues and dead-reckoning, whereas the FF rats were not able to do so (Whishaw and Tomie, 1997). This suggestion is consistent with findings that control rats can flexibly select among a number of spatial strategies when solving spatial problems (Whishaw and Mittleman, 1986).
It is possible, of course, that the control rats noted the location of the new starting holes as they exited from the hole and then subsequently made the necessary transforms to generate a new route to the starting location. The ability to make such transforms is referred to as instantaneous transfer, but there is some doubt concerning the ability of rats to perform instantaneous transfer (Whishaw, 1991). In the present training conditions, the use of such transforms might be thought to be especially difficult. The rats would not have had an expectation that they would have to return to the novel starting location, they had not had previous experience in returning to new starting locations, and they had not previously viewed and approached the new location using the cues that marked its position. We emphasize, however, that these results are only suggestive of the use of a dead-reckoning strategy. Mapping theory does predict that an animal familiar with an environment could make transforms necessary to reach new locations in that environment (O’Keefe and Nadel, 1978; Muller et al., 1996).
Navigating wearing blindfolds
In the second experiment, the rats were trained to forage from one location and then given probe trials from a novel location. On the probe trials, they either wore masks, through which they could see, or blindfolds, which obscured vision. When wearing masks, both the control and FF rats returned with food accurately to the training location, not the probe location. This result shows that using vision, both groups used a piloting strategy to reach the training location. Then, only the control rats returned to the novel starting location on discovering their error. These results replicate the findings of the first experiment.
When wearing blindfolds, the control rats returned directly to the probe location, whereas the FF rats did not. Thus, the rats did not transfer any spatial information from the training trials to the probe trials. These results suggest that without vision, the control, but not FF, rats use dead-reckoning to make their accurate return trips. Our conclusion that the blindfolded control rats use dead-reckoning is supported by the results of a number of studies that suggest that when vision is restricted, dead-reckoning becomes a preferred navigational strategy (Barlow, 1964; Landeau et al., 1984; Etienne et al., 1996).
Although these results are suggestive that the control rats accessed idiothetic cues, we made no attempt to mask auditory or olfactory cues. Were the rats able to navigate using auditory cues, it would be expected that they should return to the training location and not to the probe location. This is because the mask test showed that when the rats used allothetic visual cues, they go to the training location. On this logic, the possibility that the rats used auditory cues can be minimized. There are also difficulties with any unambiguous suggestion that they used olfactory cues. Because we did not clean the test table between training and probe trials, were they to use enduring surface olfactory cues, it might be expected that these cues would lead the rats to the training location. Because the control animals returned to the probe location, the only available olfactory cues would be those that they left on their outward trip. Because self-tracking is not beyond the ability of animals, the purpose of experiment 3 was to rule out the possibility that the rats were following their own odor trails home.
Navigating with displaced olfactory cues
In the third experiment, animals were tested only with blindfolds, and they were tested on a table with a fixed central portion and a movable outside portion. On probe trials, the food pellet was located on the central platform, and as the rat retrieved it, the periphery of the table was rotated 90°. Thus, the rats were not able to use visual cues, and olfactory cues were displaced. Because the control rats were accurate in returning to the starting location, and because the FF rats were inaccurate, this appears to unambiguously demonstrate that control rats are able to use a dead-reckoning, whereas the rats with FF lesions are unable to do so.
Implications for hippocampal function
Contemporary research suggests that the hippocampus plays some central role in spatial navigation, but here are divergent views concerning its role. Cognitive mapping theorists posit that the hippocampus develops a spatial map that is assembled through associationistic process in which the relations between allothetic cues are learned as an animal interacts with its environment (O’Keefe and Nadel, 1978; Muller et al., 1996). The present results show that the FF rats had some mapping ability, because they could return to a familiar starting location, a result that suggests there is an extrahippocampal map (Whishaw et al., 1997). Path integration theorists posit that the hippocampus contains an innate spatial reference frame within which it can generate vectors between points (Whishaw et al., 1995a, 1997;McNaughton et al., 1996; Samsonovich and McNaughton, 1997). Our finding that in the absence of visual cues control rats could continue to navigate, whereas FF rats could not, supports this position. Although the present results support a role for the hippocampal formation in path integration, they are not definitive in determining whether this is an exclusive function. Hippocampal animals are also impaired in learning new spatial problems in which the demands of working memory are high (Angeli et al., 1993; Whishaw, 1995; Whishaw and Jarrard, 1995, 1996; Whishaw et al., 1995b). Although it is potentially possible that path integration is an important component of working spatial memory, any relation between these functions is still to be worked out.
In conclusion, the hippocampal formation is complex of nuclei and pathways (Amaral and Witter, 1995), and there is behavioral evidence for heterogeneity in its function (Grey and McNaughton, 1983; Jarrard, 1993; Whishaw and Jarrard, 1995). Because FF lesions disconnect a number of hippocampal structures, it is not possible to be definitive concerning the structures that are involved in path integration. It is interesting that cells that code head direction are found in the subicular complex (Taube et al., 1990, 1995), parietal and posterior cingulate cortex (Chen et al., 1994), thalamus (Mizumori and Williams, 1993; Taube 1995), and striatum (Wiener, 1996), suggesting a role for these structures in a sense of direction. Place cells are more likely to be found within the hippocampus proper (O’Keefe, 1976; Jung and McNaughton, 1993; Muller et al., 1996), suggesting a role for this structure in “spatial awareness.” Perhaps these systems interact to produce seamless spatial behavior (Sharp, 1997). Future studies will have to separately assess the roles of different hippocampal structures on dissociative tasks such as the ones used in the present study.
Footnotes
This work was supported by the Medical Research Council of Canada.
Correspondence should be addressed to Ian Q. Whishaw, Department of Psychology, University of Lethbridge, Lethbridge, Alberta, Canada T1K 3M4.
REFERENCES
- 1.Alyan SH. Evidence for resetting the directional component of path integration in the house mouse (Mus musculus). Ethology. 1996;102:629–638. [Google Scholar]
- 2.Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 443–493. [Google Scholar]
- 3.Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31:1021–1030. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- 4.Barlow JS. Inertial navigation as a basis for animal navigation. J Theor Biol. 1964;6:76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 6.Batschelet E. Circular statistics in biology. Academic; New York: 1981. [Google Scholar]
- 7.Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci. 1996;110:643–660. doi: 10.1037//0735-7044.110.4.643. [DOI] [PubMed] [Google Scholar]
- 8.Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 9.Cassel JC, Kelche C, Peterson GM, Ballough GP, Goepp I, Will B. Graft induced behavioral recovery from subcallosal septo-hippocampal damage in rats depends on maturity stage of donor tissue. Neuroscience. 1991;45:571–586. doi: 10.1016/0306-4522(91)90272-p. [DOI] [PubMed] [Google Scholar]
- 10.Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 11.Darwin C. On the origin of certain instincts. Nature. 1873;7:417–418. [Google Scholar]
- 12.Dudenchenko PA, Goodridge JP, Seiterle DA, Taube JS. Effects of repeated disorientation on the acquisition of spatial tasks in rats: dissociation between the appetitive radial arm maze and aversive water maze. J Exp Psychol Gen. 1997;23:194–210. doi: 10.1037//0097-7403.23.2.194. [DOI] [PubMed] [Google Scholar]
- 13.Etienne A, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J Exp Biol. 1996;199:201–209. doi: 10.1242/jeb.199.1.201. [DOI] [PubMed] [Google Scholar]
- 14.Etienne AS. The control of short-distance homing in the golden hamster. In: Ellen P, Thinus-Blanc C, editors. Cognitive processes in spatial orientation in animal and man. Nijhoff; Dordrecht, Netherlands: 1987. pp. 223–251. [Google Scholar]
- 15.Gaffan D, Gaffan EA. Amnesia in man following transection of the fornix. Brain. 1991;14:2611–2618. doi: 10.1093/brain/114.6.2611. [DOI] [PubMed] [Google Scholar]
- 16.Gallistel CR. The organization of learning. MIT; Cambridge, MA: 1990. [Google Scholar]
- 17.Golob EJ, Taube JS. Head direction cells and episodic spatial information in rats without a hippocampus. Proc Natl Acad Sci USA. 1997;94:7645–7650. doi: 10.1073/pnas.94.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grey JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- 19.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 20.Jeltsch H, Cassel JC, Jackisch R, Neufang B, Green PL, Kelche C, Hertting G, Will B. Lesions of supracallosal or infracallosal hippocampal pathways in the rat: behavioural, neurochemical, and histochemical effects. Behav Neural Biol. 1994;62:121–133. doi: 10.1016/s0163-1047(05)80033-0. [DOI] [PubMed] [Google Scholar]
- 21.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 22.Landeau B, Spelke E, Gleitman H. Spatial knowledge in a young blind child. Cognition. 1984;16:225–260. doi: 10.1016/0010-0277(84)90029-5. [DOI] [PubMed] [Google Scholar]
- 23.Martin GM, Harley CW, Smith AR, Hoyles ES, Hynes CA. Opaque transportation with rotation blocks reliable goal location on a plus maze but does not prevent goal location in the Morris maze. J Exp Psychol Gen. 1997;23:183–193. doi: 10.1037//0097-7403.23.2.183. [DOI] [PubMed] [Google Scholar]
- 24.McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung JJ, Knierim JJ, Kudrimoti H, Quin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 25.Mittelstaedt H, Mittelstaedt M-L (1973) Mechaniom der orientierung ohne richtende aubenreize. Fortsch der Zool 21:46–58.
- 26.Mizumori SJY, Williams JD. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J Neurosci. 1993;13:4015–4028. doi: 10.1523/JNEUROSCI.13-09-04015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller RU, Stead M, Pach J. The hippocampus as a cognitive graph. J Gen Physiol. 1996;107:663–694. doi: 10.1085/jgp.107.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Keefe J. Place units in the hippocampus of freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 29.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon; London, UK: 1978. [Google Scholar]
- 30.O’Mara S, Rolls ET, Berthoz A, Desner RP. Neurons responding to whole-body motion in the primate hippocampus. J Neurosci. 1994;14:6511–6523. doi: 10.1523/JNEUROSCI.14-11-06511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp PE. Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav Brain Res. 1997;85:71–92. doi: 10.1016/s0166-4328(96)00165-9. [DOI] [PubMed] [Google Scholar]
- 33.Sharp PE, Blair HT, Etkin D, Tzanetos DBJ. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. Neuroscience. 1995;15:173–189. doi: 10.1523/JNEUROSCI.15-01-00173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland RJ, Rodriguez AJ. The role of the fimbria-fornix and some related subcortical structures in place learning and memory. Behav Brain Res. 1989;32:129–144. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- 35.Taube JS. Head direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;172:49–84. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–85. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurosci. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- 38.Whishaw IQ. Latent learning in a swimming pool place task by rats: evidence for the use of associative and not cognitive mapping processes. Q J Exp Psychol Sect B Comp Physiol Psychol. 1991;43:83–103. [PubMed] [Google Scholar]
- 39.Whishaw IQ. Activation, travel distance, and environmental change influence food carrying in rats with hippocampal, medial thalamic and septal lesions: implications for studies on hoarding and theories of hippocampal function. Hippocampus. 1993;3:373–385. doi: 10.1002/hipo.450030311. [DOI] [PubMed] [Google Scholar]
- 40.Whishaw IQ (1998) Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Rev, in press. [DOI] [PubMed]
- 41.Whishaw IQ, Jarrard LE. Similarities versus differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- 42.Whishaw IQ, Jarrard LE. Evidence for extrahippocampal involvement in place learning and hippocampal involvement in path integration. Hippocampus. 1996;6:513–524. doi: 10.1002/(SICI)1098-1063(1996)6:5<513::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Whishaw IQ, Mittleman G. Visits to starts, routes, places by rats (Rattus norvegicus) in swimming pool navigation tasks. J Comp Psychol. 1986;100:422–431. [PubMed] [Google Scholar]
- 44.Whishaw IQ, Tomie J. Impairments on a movement-integration task in rats with fimbria-fornix lesion. Soc Neurosci Aabstr. 1996;26:269.13. [Google Scholar]
- 45.Whishaw IQ, Tomie J. Piloting and dead reckoning-dissociated by fimbria–fornix lesions in a rat food carrying task. Behav Brain Res. 1997;89:87–98. doi: 10.1016/s0166-4328(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 46.Whishaw IQ, Coles BKL, Bellerive CHM. Food carrying: a new method for naturalistic studies of spontaneous and forced alternation. J Neurosci Methods. 1995a;61:139–143. doi: 10.1016/0165-0270(95)00035-s. [DOI] [PubMed] [Google Scholar]
- 47.Whishaw IQ, Cassel J-C, Jarrard LE. Rats with fimbria–fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J Neurosci. 1995b;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whishaw IQ, McKenna J, Maaswinkel H. Hippocampal lesions and path integration. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- 49.Wiener SI. Spatial, behavioral and sensory correlates of hippocampal CA1 complex spike cell activity: implications for information processing functions. Prog Neurobiol. 1996;49:335–361. doi: 10.1016/0301-0082(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 50.Winer BJ. Statistical principles in experimental design. McGraw-Hill; London: 1962. [Google Scholar]
- 51.Worden R. Navigation by fragment fitting: a theory of hippocampal function. Hippocampus. 1992;2:165–188. doi: 10.1002/hipo.450020208. [DOI] [PubMed] [Google Scholar]