Abstract
The influence of overtraining on the magnitude of fear-conditioning deficits produced by neurotoxic lesions of the basolateral amygdala (BLA) was examined. Either 1 d before or 1 week after the administration of neurotoxic BLA lesions, rats received either 1 or 25 conditioning trials consisting of the delivery of unsignaled foot shock in a novel observation chamber; freezing served as the measure of conditional fear. In this conditioning paradigm, asymptotic performance is reached in five conditioning trials, and 25 conditioning trials constitutes an overtraining procedure. The results revealed that overtraining does not affect the magnitude of the contextual freezing deficits produced by post-training BLA lesions. Similarly, overtraining did not influence the level of reacquisition obtained by rats with post-training BLA lesions after 10 reacquisition trials. A similar pattern of results was observed in rats with pretraining BLA lesions. Neurotoxic BLA lesions did not alter either motor activity or shock reactivity. These results indicate that overtraining does not limit the important role of the BLA in the acquisition and expression of contextual fear conditioning.
Keywords: Pavlovian fear conditioning, amygdala, overtraining, NMDA, context, freezing, rats
It is now generally agreed that the basolateral amygdala (BLA), comprising the lateral, basolateral, and basomedial nuclei, plays an essential role in the acquisition and expression of Pavlovian fear conditioning (Davis et al., 1994; Maren and Fanselow, 1996; Rogan and LeDoux, 1996). Neurotoxic BLA lesions produce deficits in the acquisition and expression of conditional freezing to both context and tone conditional stimuli (CSs) (Maren et al., 1996a) and the acquisition and expression of fear-potentiated startle to auditory and visual CSs (Sananes and Davis, 1992; Campeau and Davis, 1995). The BLA has an enduring role in the expression of conditional fear insofar as neurotoxic lesions made in the BLA up to 1 month after conditioning produce severe deficits in conditional freezing (Maren et al., 1996a) and fear-potentiated startle (Lee et al., 1996). Inactivation of the BLA with muscimol produces deficits in the acquisition and expression of contextual fear conditioning (Helmstetter and Bellgowan, 1994; Muller et al., 1997), and intra-BLA infusions of NMDA receptor antagonists produce impairments in both first-order (Miserendino et al., 1990; Campeau et al., 1992; Fanselow and Kim, 1994; Maren et al., 1996b) and second-order (Gewirtz and Davis, 1997) fear conditioning. Moreover, BLA neurons exhibit electrophysiological correlates of conditional fear (Maren et al., 1991; Quirk et al., 1995) and synaptic plasticity in context (Maren and Fanselow, 1995; Maren, 1996) and tone (Clugnet and LeDoux, 1990;Rogan and LeDoux, 1995) CS pathways.
Although the involvement of the BLA in Pavlovian fear conditioning is established, it has recently been suggested that the amount of training (i.e., number of conditioning trials) may be an important determinant of the role of the amygdala in Pavlovian fear conditioning. For example, Kim and Davis (1993) have reported that rats given extensive training can reacquire fear-potentiated startle after post-training lesions of the central nucleus of the amygdala (CEA). Although not explicitly tested, they suggested that the ability of rats with CEA lesions to reacquire fear-potentiated startle was a function of the extensive training used during acquisition (Kim and Davis, 1993). Similarly, neurotoxic BLA lesions do not impair the acquisition of conditioned bar press suppression, a task that requires extensive training (Killcross et al., 1997). Thus, it is possible that superasymptotic training or overtraining mitigates the role of the BLA in the acquisition and expression of conditional fear. If true, this would force a revision of current thinking concerning the role of the BLA in fear conditioning and would suggest that the BLA is not essential for performing Pavlovian fear memories, at least after extensive training. To address this issue, the present experiments directly assessed the influence of overtraining on the acquisition, expression, and reacquisition of contextual fear conditioning in rats with pretraining or post-training lesions of the BLA. The results revealed that overtraining does not limit the important role of the BLA in the acquisition and expression of contextual fear conditioning.
EXPERIMENT 1: NUMBER OF CONDITIONING TRIALS REQUIRED FOR OVERTRAINING
Contextual fear conditioning to an aversive foot shock unconditional stimulus (US) is rapidly learned and rarely requires more than a few conditioning trials to establish. However, for any given US, it is not known how many trials are required for asymptotic performance and, therefore, how many trials constitute an overtraining procedure. To determine the number of conditioning trials required for asymptotic performance, the present experiment assessed contextual freezing after training with 1, 5, 25, or 50 conditioning trials. This information was used to develop an overtraining procedure for the remainder of the experiments.
Materials and methods
Subjects. The subjects were 16 adult male Long–Evans rats (200–224 gm) obtained from a commercial supplier (Harlan Sprague Dawley, Indianapolis, IN). After arrival, the rats were individually housed in standard stainless-steel hanging cages on a 14/10 hr light/dark cycle (lights on at 7:00 A.M.) and were provided free access to food and tap water. After housing, the rats were handled daily (10–20 sec/rat) for 5 d to acclimate them to the experimenter.
Behavioral apparatus. Eight identical observation chambers (30 × 24 × 21 cm; MED-Associates, Burlington, VT) were used for both conditioning and contextual fear testing. The chambers were constructed from aluminum (side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and were situated in chests located in a brightly lit and isolated room. The floor of each chamber consisted of 19 stainless steel rods (4 mm diameter) spaced 1.5 cm apart (center to center). The rods were wired to a shock source and solid-state grid scrambler (MED-Associates) for the delivery of foot shock USs. The chambers were cleaned with a 5% ammonium hydroxide solution, and stainless steel pans containing a thin film of the same solution were placed underneath the grid floors before the rats were placed inside. Background noise (65 dB, A scale) was supplied by ventilation fans in each chest.
Each conditioning chamber rested on a load cell platform that was used to record chamber displacement in response to each rat’s motor activity. To ensure interchamber reliability, each load cell amplifier was calibrated to a fixed chamber displacement. The output of the load cell of each chamber was set to a gain (vernier knob, 8) that was optimized for detecting freezing behavior. Load cell amplifier output from each chamber was digitized and acquired on-line using Threshold Activity software (MED-Associates).
Procedure. The rats were randomly assigned to groups that received 1, 5, 25, or 50 conditioning trials (n = 4 per group). On the conditioning day, the rats were transported to the laboratory in squads of eight and placed in the conditioning chambers; the chamber position was counterbalanced for each squad and group. The rats received 1, 5, 25, or 50 foot shocks (2 sec, 1.0 mA, 60 sec intershock interval) 3 min after being placed in the chambers. Sixty seconds after the final shock, the rats were returned to their home cages. Twenty-four hours after training, fear conditioning to the context was assessed by returning the rats to the conditioning chambers and measuring freezing behavior (somatomotor immobility except that necessitated by breathing) during a 4 min extinction test.
During both the conditioning and extinction sessions, each rat’s activity was monitored continuously using the data acquisition system described above. For each chamber, load cell activity was digitized at 5 Hz, yielding one observation per rat every 200 msec (300 observations per rat per minute). In all experiments, freezing was quantified by computing the number of observations for each rat that had a value less than the freezing threshold. The freezing threshold was determined in a separate group of pilot animals by comparing load cell output with an observer’s ratings of freezing behavior. To achieve a sensitive freezing threshold, the load cell gain for all chambers was set to yield a freezing threshold that correlated with the observer’s ratings of freezing behavior. Thus, movements such as grooming, head turning, and sniffing that would not be scored by an observer as freezing produced load cell output that exceeded the freezing threshold. Importantly, the freezing threshold was absolute and was used for each rat and experiment in the present study. To avoid counting momentary inactivity as freezing, an observation was only scored as freezing if it fell within a contiguous group of at least five observations that were all less than the freezing threshold. Thus, freezing was only scored if the rat was immobile for at least 1 sec. For each session, the freezing observations were transformed to a percentage of total observations. In addition to freezing, locomotor activity was quantified in some experiments using the raw load cell output. In the present experiment, freezing was quantified before foot shock on the conditioning day and during the 4 min extinction test.
Data analysis. For each session, the freezing data were transformed to a percentage of total observations, a probability estimate that is amenable to analysis with parametric statistics. These probability estimates of freezing were analyzed using ANOVA. Planned comparisons in the form of Fisher’s LSD tests were performed after a significant omnibus F ratio. All data are represented as mean ± SEM.
Results
Behavior
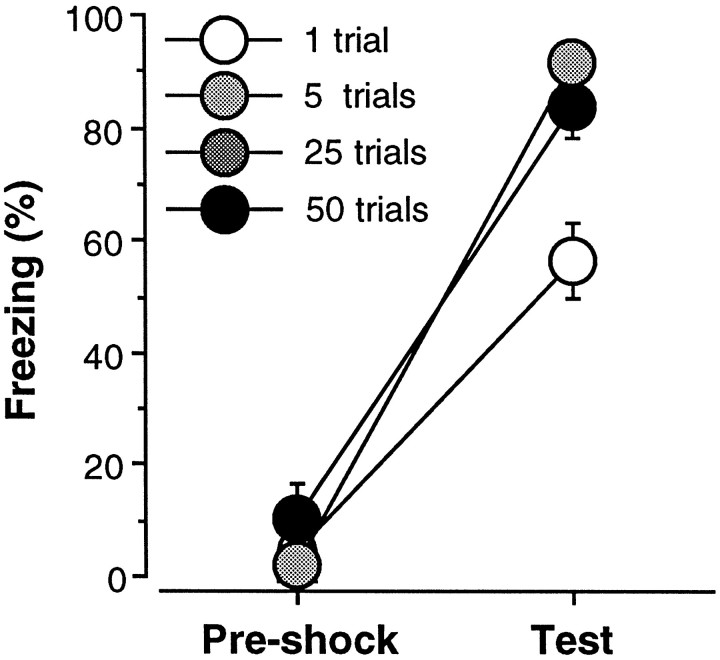
Freezing to the context of the conditioning chamber during the 3 min preshock period on the training day and during the 4 min extinction test the following day is shown in Figure1. The data were analyzed using a two-way ANOVA with factors of trial (1, 5, 25, and 50) and period (preshock and test). As shown, rats exhibited minimal levels of freezing (<10%) before foot shock, and the delivery of unsignaled shocks resulted in substantial freezing behavior during the extinction test. This observation was confirmed by a significant main effect of period [F(1,12) = 612.5; p < 0.0001] in the ANOVA. Not surprisingly, the level of freezing behavior varied as a function of the number of conditioning trials; this was indicated by a significant main effect of trial [F(3,12)= 6.6; p < 0.01] and a significant interaction of trial and period [F(3,12) = 8.2;p < 0.01]. Post hoc comparisons indicated that rats receiving one conditioning trial froze significantly less than rats receiving 5, 25, or 50 trials during the extinction test; rats receiving 5, 25, or 50 trials showed comparable levels of freezing during the extinction test. These results indicate that asymptotic freezing (∼90%) is produced by five conditioning trials. In light of these results, the following experiments used 25 conditioning trials, which is at least five times the number of trials required to produce asymptotic behavioral performance, as an overtraining procedure.
Fig. 1.
Number of conditioning trials required for overtraining. Mean ± SEM percentage of freezing during the 3 min period before foot shock on the conditioning day (Pre-shock) and during the 4 min context extinction test conducted 24 hr after conditioning (test). Rats received 1, 5, 25, or 50 conditioning trials consisting of the delivery of unsignaled foot shock. Asymptotic freezing was reached with five conditioning trials, and additional training did not increase the levels of freezing beyond the level obtained with five trials.
EXPERIMENT 2: OVERTRAINING AND POST-TRAINING NEUROTOXIC LESIONS
Post-training neurotoxic BLA lesions prevent the expression and severely attenuate the reacquisition of contextual fear conditioning in rats (Maren et al., 1996a). It is possible, however, that more extensive training would mitigate the effects of post-training BLA lesions on either the expression (Parent et al., 1992, 1994) or reacquisition (Kim and Davis, 1993) of contextual freezing. The following experiment directly addressed this issue by examining the influence of the degree of training on the expression and reacquisition of contextual fear after neurotoxic BLA lesions. Rats were trained with either 1 or 25 conditioning trials before receiving neurotoxic BLA lesions. As in previous studies, training was conducted in a single session (Parent et al., 1992, 1994). After recovery from surgery, the rats received an extinction test followed by reacquisition training.
Materials and methods
Subjects. The subjects were 64 adult male Long–Evans rats (200–224 gm) obtained and housed as described in experiment 1.
Apparatus and procedure. The behavioral apparatus was identical to that described in experiment 1. The rats were randomly assigned to a 2 × 2 (lesion × trial; n = 16 per group) design. For this design, rats received either NMDA lesions in the BLA or sham surgery within 2 d after contextual fear conditioning, which consisted of either 1 or 25 unsignaled shocks (2 sec, 1-mA, 60 sec intershock interval) delivered 3 min after the rats were placed in the conditioning chambers. All other conditioning procedures were identical to those described in experiment 1, and freezing data were collected during the 3 min preshock period. Contextual fear testing for all groups of rats was conducted during an 8 min context extinction test (test 1) 1 week after recovery from surgery. Twenty-four hours after the context extinction test, rats received two additional days of reacquisition training. On each reacquisition day, the rats received five unsignaled shocks of the same intensity and on the same schedule as described for acquisition. Twenty-four hours after the second reacquisition session, the rats received another 8 min context extinction test (test 2) to assess the level of conditional freezing generated by reacquisition training. Freezing behavior during both context extinction tests was quantified as described in experiment 1.
Surgery. Within 2 d after conditioning, the rats were treated with atropine methyl nitrate (0.4 mg/kg body weight), anesthetized with Nembutal (sodium pentobarbital, 65 mg/kg body weight, i.p.), and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The scalp was incised and retracted, and head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes (2 mm diameter) were drilled bilaterally in the skull for the placement of 28-gauge cannula in the basolateral amygdala (3.3 mm posterior to bregma, 5.0 mm lateral to the midline). A 10 μl Hamilton syringe was mounted in an infusion pump (Harvard Apparatus, South Natick, MA) and connected to the injection cannula with polyethylene tubing. NMDA (20 μg/μl; Sigma, St. Louis, MO) in 100 mm PBS, pH 7.4, was infused (0.1 μl/min) 8.0 mm ventral to brain surface (0.2 μl) and 7.5 mm ventral to brain surface (0.1 μl) for each penetration. Five minutes were allowed after each infusion for diffusion of the drug. After surgery, the incision was closed with stainless steel wound clips, and the rats were allowed to recover on a heating pad before returning to their home cage.
Histology. Histological verification of lesion location was performed after behavioral testing. Rats were perfused across the heart with 0.9% saline followed by 10% formalin. After extraction from the skull, the brains were post-fixed in 10% formalin for 2 d and 10% formalin and 30% sucrose until sectioning. Coronal sections (50 μm thick, taken every 200 μm) were cut on a cryostat (−16°C) and wet-mounted on glass microscope slides with 70% ethanol. After drying, the sections were stained with 0.25% thionin to visualize neuronal cell bodies. Lesions were verified by visual inspection of the stained brain sections.
Data analysis. The data analysis was performed as described in experiment 1.
Results
Histology
Photomicrographs of coronal brain sections from representative rats in the sham and lesion groups are shown in Figure2. The pattern of damage produced by intra-amygdala NMDA was similar to that reported previously (Maren et al., 1996a). In general, the lesion was associated with extensive cellular damage in the lateral, basolateral, and basomedial amygdaloid nuclei; the central nucleus was spared. There was minimal damage to extra-amygdaloid structures, although there was some damage to rostral entorhinal cortex and the amygdalo–hippocampal transition zone; perirhinal cortex was not damaged in any of the subjects. There was variation in the degree of damage to the BLA, particularly with respect to the rostral–caudal extent of the lesions. Many rats had partial BLA lesions in which the rostral aspect of the BLA was largely spared. Rats with total BLA lesions had damage that extended throughout the rostral–caudal extent of the BLA. Of the 32 rats in the BLA groups, 8 had largely unilateral lesions (4 each from the 1 and 25 trial groups), 12 had partial bilateral BLA damage (6 each from the 1 and 25 trial groups), and 12 had total BLA lesions (6 each from the 1 and 25 trial groups). The rats with unilateral lesions were excluded from the statistical analysis, and rats with partial or total BLA lesions were analyzed as separate groups. Figure 3shows the extent of the lesions in rats with either partial or total BLA lesions.
Fig. 2.
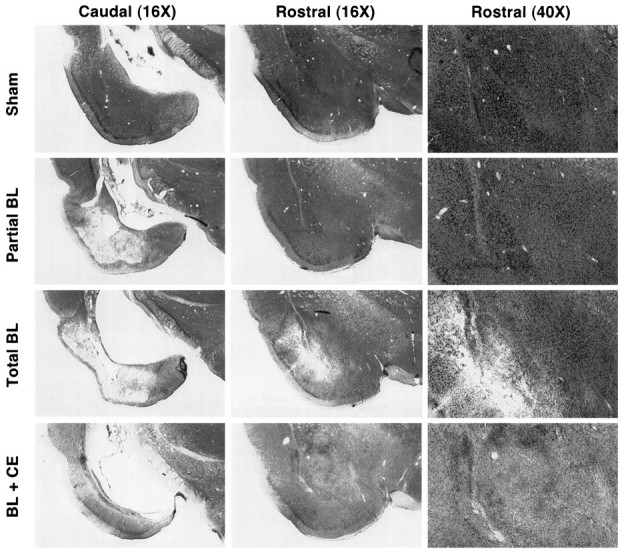
Photomicrographs showing representative thionin-stained coronal brain sections at two rostral–caudal levels from a rat receiving sham surgery (sham), a rat with a partial lesion of the basolateral amygdala (Partial BL), a rat with a total BLA lesion (Total BL), and a rat with a combined lesion of the BLA and CEA (BL + CE). Rostral sections are shown at two magnifications. Note the spared BLA neurons in the partial BL rat and the complete loss of these neurons in the total BL rat. Refer to Figure 3 for the position and labeling of the amygdaloid nuclei.
Fig. 3.
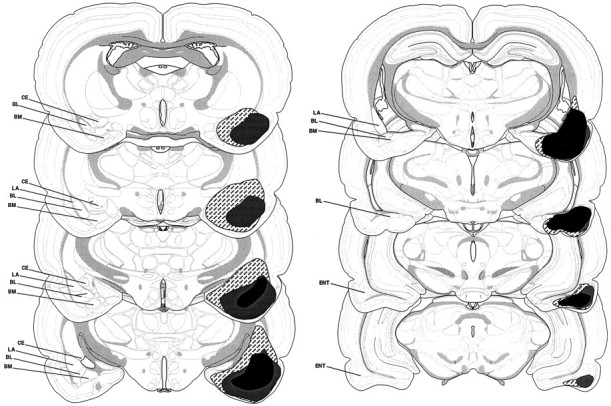
Schematic representation of the extent of partial lesions of the BLA (black), total BLA lesions (gray), and combined lesions of the BLA and CEA (hatched). Unilateral representations of bilateral lesions are shown so that the damaged areas can be visualized with respect to the labeled areas. BL, Basolateral nucleus;BM, basomedial nucleus; CE, central nucleus; ENT, entorhinal cortex; LA, lateral nucleus. Coronal brain section images adapted from Swanson (1992).
Behavior
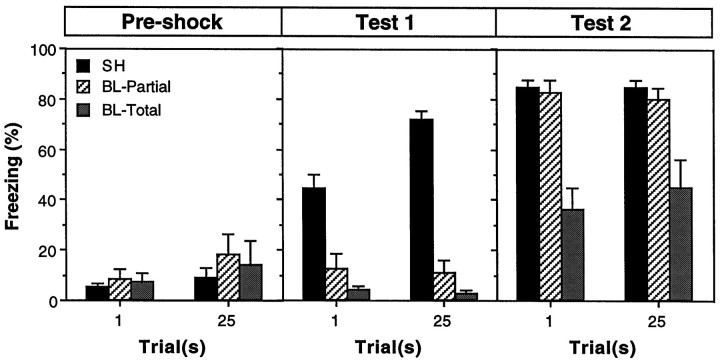
Freezing during three stages of training for rats in the sham, partial BLA, and total BLA groups is shown in Figure4. Before receiving foot shock on the conditioning day (preshock), all of the rats showed low levels of freezing behavior. After conditioning and recovery from surgery, rats in the sham groups exhibited robust freezing during the first extinction test (test 1). As expected, conditional freezing during test 1 was abolished by either partial or total BLA lesions made shortly after training. The impairments in freezing produced by partial or total BLA lesions were equivalent in test 1. Importantly, the amount of freezing in rats with BLA lesions was also equivalent in groups receiving either 1 or 25 conditioning trials. In fact, the magnitude of the freezing deficits in rats with BLA lesions was actually greater in the 25 trial groups (because of the greater level of freezing in the sham rats). Thus, overtraining did not mitigate the effects of post-training BLA lesions on the expression of contextual freezing; rats with BLA lesions were severely impaired with either 1 or 25 trials of training. After extinction testing, all rats received two reacquisition sessions. Reacquisition training increased the level of freezing in all of the groups. Rats with partial BLA lesions exhibited complete reacquisition and reached a level of freezing that was equivalent to that in sham rats. In contrast, rats with total BLA lesions increased their level of freezing after reacquisition training, but still exhibited a pronounced freezing deficit. Again, the level of freezing in rats with either partial or total BLA lesions was not influenced by the number of original training trials. Moreover, BLA rats receiving 1 or 25 trials approached their level of reacquired fear at the same rate (data not shown). Thus, overtraining did not mitigate the deficits in contextual freezing observed after reacquisition training.
Fig. 4.
Post-training neurotoxic lesions of the BLA and freezing. Mean ± SEM percentage of freezing during the 3 min period before foot shock on the conditioning day (Pre-shock) and during the 8 min context extinction tests conducted 1 week after conditioning and recovery from surgery (test 1) and 24 hr after reacquisition (test 2). Rats received either 1 or 25 conditioning trials consisting of the delivery of unsignaled foot shock. Data are shown from rats that received sham surgery (SH; black bars), or intra-amygdaloid infusion of NMDA. Intra-amygdaloid NMDA resulted in either partial lesions of the basolateral amygdala (BL-Partial; hatched bars) or total lesions of the basolateral amygdala (BL-Total;gray bars).
All of these observations were confirmed by a three-way ANOVA with factors of lesion (sham, partial BL, or total BL), trial (1 or 25), and training stage (preshock, test 1, or test 2). For clarity and brevity, only a subset of the ANOVA will be reported. The ANOVA revealed a significant main effect of lesion [F(1,24) = 45.1; p < 0.0001] and a significant lesion × training stage interaction [F(2,48) = 30.2;p < 0.0001], which indicates that the impairments in freezing produced by neurotoxic BLA lesions varied across training stages. Planned comparisons (p < 0.05) indicated that the freezing deficits in rats with BLA lesions were greater in test 1 compared with test 2. Importantly, there was not a significant group × trial interaction [F(2,50) = 0.9; p = 0.43], indicating that the number of training trials did not influence the magnitude of the freezing deficits in rats with BLA lesions. There was a significant interaction of group, trial, and training stage [F(4,100) = 3.0; p < 0.05], but this interaction was generated by the greater effects of BLA lesions in the 25 trial condition compared with the 1 trial condition in test 1, and the greater effects of BLA lesions in the 25 trial condition in test 1 compared with either the 1 or 25 trial conditions in test 2. Planned comparisons (p < 0.05) indicated that freezing in rats with total BLA lesions was not significantly different in the 1 and 25 trial groups in both tests 1 and 2 and was significantly impaired relative to shams in the 1 and 25 trial groups in both tests 1 and 2. Freezing in rats with partial lesions did not differ from that in rats with total BLA lesions in test 1. However, rats with partial BLA lesions showed complete reacquisition of contextual freezing, whereas rats with total BLA lesions showed impaired reacquisition. These results indicate that total post-training BLA lesions produce severe deficits in both the expression and reacquisition of contextual freezing, and that these deficits are not ameliorated by prior overtraining. Furthermore, the data indicate that although spared tissue in the rostral BLA is not sufficient for expression of contextual fear after post-training lesions, it is sufficient to support complete reacquisition of contextual fear.
EXPERIMENT 3: OVERTRAINING AND PRETRAINING NEUROTOXIC BLA LESIONS
Experiment 2 indicates that overtraining does not influence the impact of post-training BLA lesions on the expression or reacquisition of contextual freezing. Interestingly, however, rats with BLA lesions did acquire some conditional freezing after 10 reacquisition trials, a result that was also noted in our previous report (Maren et al., 1996a). Because BLA lesions prevent the acquisition of contextual fear conditioning after three conditioning trials (Maren et al., 1996a), these results suggest that the degree of training may be an important factor in determining the magnitude of fear conditioning deficits in rats with pretraining BLA lesions. To assess this possibility, experiment 3 examined the influence of overtraining on the acquisition and reacquisition of contextual freezing in rats with BLA lesions made before training. Rats received neurotoxic BLA lesions and, after recovery from surgery, were trained with either 1 or 25 conditioning trials. This was followed by an extinction test and reacquisition training.
Materials Materials and methods
Subjects, surgery, and behavioral apparatus. The subjects were 32 adult male Long–Evans rats (200–224 gm) obtained and housed as described in experiment 1. All surgical procedures were the same as those described for experiment 1, except that surgery preceded conditioning by 1 week. The behavioral apparatus was identical to that described in experiment 1.
Procedure. The rats were randomly assigned to a 2 × 2 (lesion × trial; n = 8 per group) design. All of the procedures for fear conditioning, reacquisition training, and extinction testing were identical to those described in experiment 2. However, unlike in experiment 2, rats received either BLA lesions or sham surgery 1 week before conditioning.
Histology and data analysis. Histological verification of lesion location and data analysis were performed as described in experiment 2.
Results
Histology
The pattern of damage induced by intra-amygdala NMDA was similar to that reported for experiment 2. Of the 16 rats in the BLA groups, 4 had partial bilateral BLA damage (2 rats from each of the 1 and 25 trial groups), and 12 had total BLA lesions (6 each from the 1 and 25 trial groups). The rats with partial lesions were excluded from the statistical analysis, because there were not enough of these subjects to form a “partial lesion” group.
Behavior
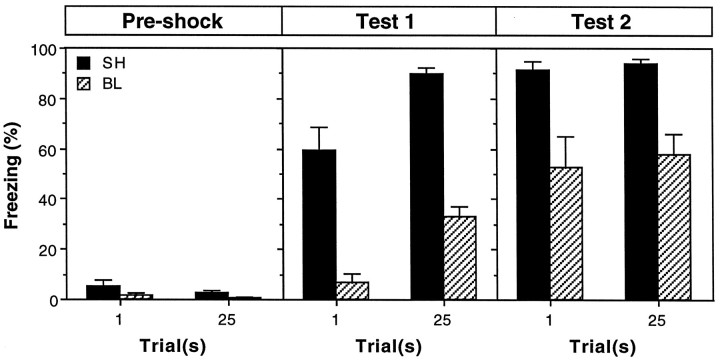
Freezing during three stages of training for rats in the sham and BLA groups is shown in Figure 5. As before, all of the rats showed low levels of freezing behavior before receiving foot shock. After conditioning, rats in the sham groups exhibited robust freezing during the first extinction test (test 1), and, as expected, conditional freezing was severely impaired by pretraining BLA lesions. Reacquisition training increased freezing in all of the rats, although rats with BLA lesions continued to exhibit significant impairments. The magnitude of the freezing deficits produced by pretraining BLA lesions during both tests 1 and 2 was similar in rats receiving either 1 or 25 trials. These observations were confirmed in three-way ANOVA with factors of lesion (sham or BL), trial (1 or 25) and training stage (preshock, test 1, or test 2); only a subset of the analysis will be reported. The ANOVA revealed a significant main effect of lesion [F(1,24) = 79.2; p < 0.0001] and a significant lesion × training stage interaction [F(2,48) = 27.8;p < 0.0001], which indicates that the impairments in freezing produced by neurotoxic BLA lesions varied across training stages. Planned comparisons (p < 0.05) indicated that the freezing deficits in rats with BLA lesions were greater in test 1 compared with test 2. Importantly, there was not a significant interaction of trial and lesion [F(1,24) = 0.0; p = 0.98], nor was there a significant interaction of lesion, trial, and training stage [F(2,48) = 0.1; p = 0.88]. This indicates that the magnitude of freezing deficits in rats with BLA lesions was not influenced by the number of training trials the rats received. Thus, rats with pretraining BLA lesions exhibited impairments in contextual freezing that were not overcome by overtraining.
Fig. 5.
Pretraining neurotoxic lesions of the BLA and freezing. Mean ± SEM percentage of freezing during the 3 min period before foot shock on the conditioning day (Pre-shock) and during the 8 min context extinction tests conducted 24 hr after conditioning (test 1) and reacquisition (test 2). Surgery was performed 1 week before conditioning. On the conditioning day, rats received either 1 or 25 conditioning trials consisting of the delivery of unsignaled foot shock. Data are shown from rats that received sham surgery (SH; black bars) or BLA lesions (BL; hatched bars).
EXPERIMENT 4: LOCOMOTOR ACTIVITY AND SHOCK REACTIVITY AFTER NEUROTOXIC BLA LESIONS
To interpret the results of experiments 2 and 3, it is important to determine whether the deficits in the acquisition, expression, and reacquisition of conditional freezing after BLA lesions are caused by impaired learning and/or memory processes or are precipitated by perceptual or motor deficits. For instance, deficits in the expression of conditional freezing in rats with neurotoxic BLA lesions might be caused by increased locomotor activity, a response that may interfere with conditional freezing. Similarly, deficits in the acquisition of contextual fear in rats with BLA lesions might be caused by impaired shock sensitivity. To address these issues, locomotor activity before foot shock and shock-induced activity across a range of shock intensities were measured in rats with neurotoxic BLA lesions.
Materials and methods
Subjects, surgery, and, behavioral apparatus. The subjects were 24 adult male Long–Evans rats (200–224 gm) obtained and housed as described in experiment 1. All surgical procedures were the same as those described for experiment 2. The chambers for shock reactivity testing were identical to those described in experiment 1, except that the load cell gain was reduced (vernier knob, 0.2) to prevent the amplifiers from saturating in response to the relatively large chamber displacements produced by shock-elicited activity bursts.
Procedure. For shock reactivity testing, rats were transported to the conditioning chambers in squads of eight. Two minutes after placement in the chambers the rats received a series of six foot shocks (2 sec duration, 10 sec intershock interval) that progressively increased in intensity (0, 0.2, 0.4, 0.6, 0.8, and 1.0 mA). This series of foot shocks was repeated twice after the initial series (10 sec interseries interval), yielding a total of three foot shocks at each intensity. The 2 min preshock period was used to quantify locomotor activity in rats with BLA lesions.
Data analysis. Locomotor activity during the 2 min preshock period was quantified by averaging the raw output of the load cell amplifiers from each conditioning chamber. These data were analyzed using ANOVA. Shock-elicited activity induced by each foot shock was quantified by averaging the load cell output during each shock (10 observations) and averaging these values across the three shocks of the same intensity. These shock-elicited activity data were analyzed using ANOVA. Planned comparisons in the form of Fisher’s LSD tests were performed after a significant omnibus F ratio. All data are represented as mean ± SEM.
Results
Histology
The pattern of damage induced by intra-amygdala NMDA was similar to that reported for experiment 2. Of the 12 rats in the BLA groups, 2 had partial bilateral BLA damage, and 10 had total BLA lesions. The rats with partial lesions were excluded from the statistical analysis.
Behavior
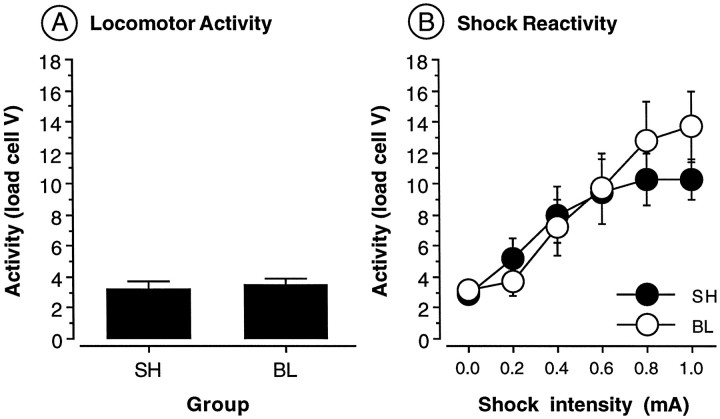
Preshock locomotor activity is shown in Figure6A. Neurotoxic BLA lesions did not affect preshock motor activity [F(1,22) = 0.1; p = 0.73]. This indicates that freezing deficits in rats with BLA lesions are not the result of a general increase in locomotor activity. Shock reactivity data are shown in Figure 6B. As shown, shock-induced activity increased monotonically across the range of shock intensities tested. This observation was confirmed by a significant main effect of shock intensity [F(5,110) = 27.1; p < 0.0001] in the ANOVA. Consistent with earlier reports (Sananes and Davis, 1992;Campeau and Davis, 1995), neurotoxic BLA lesions did not alter shock reactivity. This was confirmed by a nonsignificant main effect of lesion [F(1,22) = 0.1; p = 0.74] and a nonsignificant interaction of lesion and shock intensity [F(5,110) = 1.8; p = 0.12]. Thus, freezing deficits in rats with neurotoxic BLA lesions are not attributable to a change in perception of the foot shock US. This suggests that the deficits in conditional freezing exhibited by rats in experiments 2 and 3 result from learning and memory impairments, rather than impaired motor or perceptual processes.
Fig. 6.
Locomotor activity and shock sensitivity in rats with BLA lesions. A, Mean ± SEM activity (load cell V) in rats receiving either sham surgery (SH) or BLA lesions (BL).B, Mean ± SEM activity (load cell V) across six foot shock intensities in rats receiving either sham surgery (SH) or BLA lesions (BL).
EXPERIMENT 5: COMBINED NEUROTOXIC LESIONS OF THE BLA AND CEA
Experiments 2 and 3 indicate that rats with BLA lesions exhibit contextual freezing after reacquisition training. This is somewhat surprising, because the BLA is thought to be essential for the acquisition of contextual fear (Davis et al., 1994; Maren and Fanselow, 1996; Rogan and LeDoux, 1996). The ability of rats with BLA lesions to acquire contextual fear suggests that there may be other neural systems that can mediate fear conditioning (albeit deficient fear conditioning) in the absence of the BLA. It is unlikely that the reacquisition of contextual fear is mediated by spared tissue in the BLA, because the “total” BLA lesions in experiments 2 and 3 were associated with nearly complete loss of BLA neurons. Another possibility is that the CEA mediates fear conditioning in rats with BLA lesions. To test this hypothesis, experiment 5 examined the effect of combined lesions of BLA and CEA made either before or after training on the acquisition, expression, and reacquisition of contextual fear conditioning. To promote acquisition of freezing in rats with amygdala lesions, all rats underwent an overtraining procedure that consisted of 25 conditioning trials.
Materials and methods
Subjects, surgery, and, behavioral apparatus. The subjects were 32 adult male Long–Evans rats (200–224 gm) obtained and housed as described in experiment 1. Neurotoxic lesions of the BLA were induced as described in experiment 1, except that the lesions were made with a solution of NMDA (10 μg/μl) and ibotenic acid (IBO, 10 μg/μl; Research Biochemicals International, Natick, MA) in 100 mm PBS. Lesions in the CEA were made by infusing the NMDA and IBO solution into the central nucleus (0.3 μl, 0.1 μl/min; 2.3 mm posterior to bregma, 4.5 mm lateral to bregma, and 6 mm ventral to dura). All of the subjects that received intra-amygdala NMDA and IBO exhibited a suppression in food and water intake after the surgery. Four rats died after the lesion procedure, and four other subjects were killed because of illness. The remaining subjects received daily injections (10 ml, i.p.) of lactated Ringer’s solution and were offered a high-fat liquid diet. The subjects required ∼3 d on the liquid diet before they began normal feeding and drinking. Because of the adipsia and aphagia in these rats, they were allowed 2 weeks to recover after surgery to assure that all rats were healthy at the time of conditioning. The behavioral apparatus was identical to that described in experiment 1.
Procedure. The rats were randomly assigned to a 2 × 2 design (lesion × time; n = 6 per cell) design. Rats received BLA and CEA lesions either 2 weeks before (pretraining) or 1 d after (post-training) fear conditioning. The conditioning procedures were the same as described in experiment 2, except that all of the rats received 25 conditioning trials and extinction testing followed conditioning by 2 weeks.
Histology and data analysis. Histological verification of lesion location and data analysis were performed as described in experiment 2.
Results
Histology
Photomicrographs of coronal brain sections from a representative rat in the lesion groups are shown in Figure 2. In general, the combined lesions were associated with extensive cellular damage in the central, lateral, basolateral, and basomedial amygdaloid nuclei. There was some damage to extra-amygdaloid structures, particularly structures rostral and dorsal to the central nucleus (e.g., the ventral caudate–putamen and globus pallidus). All 12 rats receiving intra-amygdala lesions exhibited complete loss of neurons in the BLA and CEA. Figure 3 illustrates the extent of these lesions.
Behavior
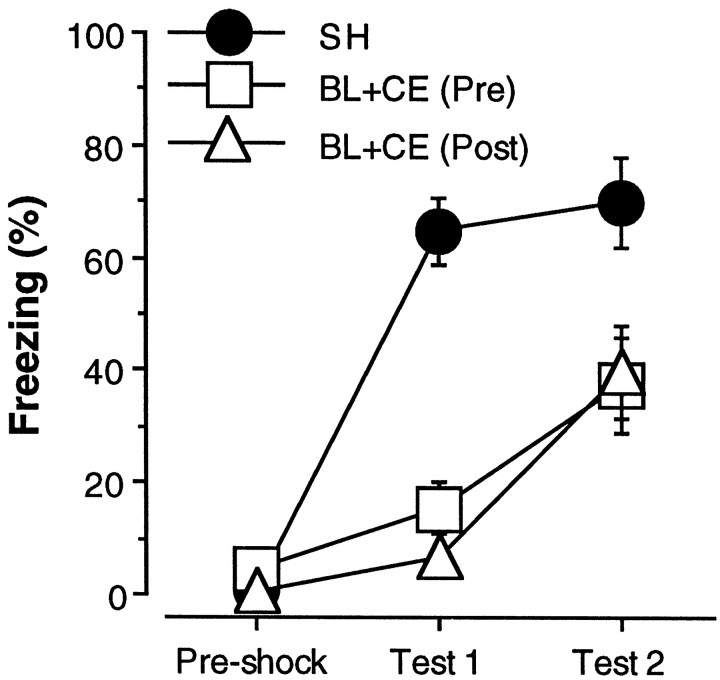
Freezing during the three stages of training for rats in the sham and BL groups is shown in Figure 7. Freezing in the sham rats in the pretraining and post-training groups did not differ and was collapsed. Similar to the results reported in experiments 2 and 3, rats with combined BLA and CEA lesions exhibited severe deficits in the expression of contextual freezing during test 1 but were able to acquire a small degree of conditional freezing that was exhibited during test 2. These observations were confirmed in a two-way ANOVA with factors of lesion (SH, BLA, and CEA), time (pretraining and post-training), and training stage (preshock, test 1, and test 2). The ANOVA revealed significant main effect of lesion [F(2,21) = 14.8; p < 0.0001] and a significant lesion × training stage interaction [F(4,42) = 11.4; p < 0.0001]. Planned comparisons (p < 0.05) indicated that freezing in rats with either pretraining or post-training BLA and CEA lesions was significantly lower than that in shams during both test 1 and 2; pretraining or post-training BLA and CEA lesions were associated with comparable deficits in conditional freezing.
Fig. 7.
Pretraining or post-training neurotoxic lesions of the BLA and CEA. Mean ± SEM percentage of freezing during the 3 min period before foot shock on the conditioning day (Pre-shock) and during the 8 min context extinction tests conducted 2 weeks after conditioning (test 1) and 24 hr after reacquisition (test 2). Surgery was performed 2 weeks before conditioning for rats in the pretraining condition [BL+CE (Pre); open squares] and 1 d after conditioning for rats in the post-training condition [BL+CE (Post); open triangles]. The sham group (SH; filled circles) is composed of rats that received sham surgery either 1 week before or 1 d after conditioning. On the conditioning day, all rats received 25 conditioning trials consisting of the delivery of unsignaled foot shock.
It is surprising that rats with pretraining lesions did not exhibit contextual freezing after 25 conditioning trials (measured during test 1) but did exhibit freezing after 10 conditioning trials (measured during test 2). The reason for this discrepancy probably relates to the shock test intervals; test 2 was conducted 1 d after the last reacquisition session, whereas test 1 was conducted 2 weeks after the conditioning session. Thus, the freezing that is apparent during test 2 may represent a short-term fear memory that decays during a 2 week retention interval. The ability of rats with amygdala lesions to exhibit some freezing during a 1 d retention test (test 2) suggests that the amygdala is not essential for short-term forms of fear memory produced by a large number of conditioning trials [note that the amygdala is required to express freezing on 1 d retention tests after either three (Maren et al., 1996a) or one (experiment 3) conditioning trial(s)]. However, the amygdala would appear to be essential for the establishment of long-term fear memories, even after overtraining.
DISCUSSION
The present experiments used an overtraining procedure to determine whether overtraining mitigates the devastating effects of neurotoxic BLA lesions on the acquisition and expression of Pavlovian fear conditioning. Collectively, these experiments revealed that overtraining does not mitigate the deficits in the expression or reacquisition of contextual freezing produced by BLA lesions. Consistent with earlier work, BLA lesions produced massive deficits in freezing to contextual cues paired with foot shock, and these deficits were not ameliorated by prelesion overtraining. The deficits in conditional freezing produced by BLA lesions were not attributed to either increased motor activity or blunted shock sensitivity, reaffirming the view that the BLA is critical for associative processes underlying the acquisition and expression of contextual fear conditioning.
The present results are surprising, because a recent report suggested that overtraining might permit unabated reacquisition of Pavlovian fear in rats with amygdala lesions (Kim and Davis, 1993). To briefly summarize, Kim and Davis (1993) reported that large, electrolytic lesions of the CEA produce severe impairments in the expression of fear-potentiated startle in rats that received an extended training protocol consisting of 64 acquisition trials. The surprising outcome of their study was that the rats with CEA lesions reacquired fear-potentiated startle after additional training. In fact, rats with CEA lesions came to exhibit a level of fear-potentiated startle that was no different from sham rats. Although not explicitly tested, the authors suggested that this pattern of results was caused by the use of an extended training protocol.
Consistent with Kim and Davis (1993), the present results reveal that post-training amygdala lesions produce profound deficits in the expression of Pavlovian fear independent of the degree of original training. However, the trial-independent, partial reacquisition of contextual freezing observed in rats with either BLA or combined BLA and CEA lesions in the present study does not accord with either the complete reacquisition of fear observed by Kim and Davis (1993) or their interpretation that reacquisition of fear in rats with CEA lesions requires overtraining. What accounts for this disparity? One possibility is that the locus of the lesion is critical. Complete reacquisition of Pavlovian fear in the report of Kim and Davis (1993)may have been mediated by spared tissue in the BLA. In support of this view, Falls and Davis (1995) found that CEA lesions that included rostral portions of the BLA did, in fact, prevent reacquisition of fear-potentiated startle. This accords with the results of the present study, in which it was found in experiment 2 that total BLA lesions (which damaged the rostral portion of the BLA) blocked the reacquisition of freezing. Partial BLA lesions (which spared the rostral portion of the BLA) were sufficient to produce severe impairments in the expression of contextual freezing but did not block the reacquisition of freezing. Thus, the anterior portion of the BLA may be essential for normal acquisition of Pavlovian fear.
Another possibility is that the distribution of training trials in the present study and the report by Kim and Davis (1993) were markedly different. In the present experiments, all of the training was conducted in a single session, whereas Kim and Davis (1993) distributed their training over either 4 or 30 d. The distributed training schedule used by Kim and Davis (1993) may have been a critical factor in permitting reacquisition in rats with amygdala lesions. Nonetheless, rats with complete BLA lesions trained on a distributed schedule (Falls and Davis, 1995) failed to reacquire fear-potentiated startle. This suggests that lesion extent is a more critical factor than training schedule in determining the conditions that favor reacquisition of fear in rats with amygdala lesions.
In general, training schedule does not appear to be a major determinant of overtraining effects in rats with amygdala damage. For instance, the design of the experiments of Parent et al. (1992, 1994) was quite similar to the design of the present experiments; both sets of experiments used a single-session training protocol and from 1 to 25 shocks. Nonetheless, only Parent et al. (1992, 1994) found that overtraining mitigated learning deficits in rats with amygdala lesions. Thus, it appears that the nature of what is learned is a more important factor than the schedule by which it is trained in determining the effects of post-training amygdala lesions on aversive memories.
In addition to preventing expression of fear-potentiated startle, Kim and Davis (1993) also found that pretraining CEA lesions completely prevented the acquisition of fear-potentiated startle, even with an overtraining procedure. In the present experiments, we also found that pretraining BLA lesions produced severe impairments in the acquisition of contextual freezing. However, we did find that rats with pretraining BLA lesions were able to acquire some contextual fear after extended training. Whereas Kim and Davis (1993) have not observed acquisition in rats with CEA lesions submitted to an overtraining procedure, they have found that overtraining supports fear-potentiated startle in rats with BLA lesions (M. Davis, personal communication). This pattern of results agrees with the present data, and suggests that the rats with BLA lesions acquire some Pavlovian fear after either overtraining or extensive reacquisition training. It is possible, though, that freezing in rats with BLA lesions represents a transient, short-term fear response that is enabled by a large number of strong USs. Consistent with this possibility, we found in experiment 5 that rats with combined BLA and CEA lesions did not exhibit contextual freezing during the extinction test conducted 2 weeks after the 25-trial conditioning session but did exhibit contextual freezing during the extinction test conducted 1 d after the 10-trial reacquisition session. This suggests that rats with amygdala damage have short-term retention of conditional fear after extensive training, but do not consolidate this learning into a long-term memory.
Work in other aversive learning tasks indicates that overtraining mitigates conditioning deficits in animals with amygdala damage. It is well known, for example, that instrumental avoidance conditioning becomes resistant to amygdala disruption after overtraining, even though initial acquisition of the avoidance response requires an intact amygdala (Brady et al., 1954; Fonberg et al. 1962; Horvath, 1963;Thatcher and Kimble, 1966). Recent reports indicate that increased training in an inhibitory avoidance task mitigates the memory-impairing effects of post-training neurotoxic amygdala lesions on a avoidance response in rats (Parent et al., 1992, 1994). Furthermore, amygdala inactivation impairs the acquisition, but not maintenance, of a discriminative avoidance response in rabbits (Poremba and Gabriel, 1995), and learning-related, CS-evoked, multiple-unit activity in the BLA peaks early in training and subsides during overtraining of the avoidance response (Maren et al., 1991). In these cases, however, the mitigating influence of overtraining on the effects of amygdala lesions and learning-related neuronal firing in the amygdala can be explained by two-process models of avoidance learning (Mowrer, 1947). Specifically, two-process models of avoidance conditioning hold that the acquisition of avoidance responses involves sequential Pavlovian and instrumental processes. Animals first learn a Pavlovian CS–US association that endows the CS with aversive properties. They subsequently learn that their avoidance behavior terminates the aversive CS, a form of instrumental conditioning. By this model, the amygdala should only be involved in the early acquisition of the instrumental avoidance response, which requires Pavlovian fear (Mineka and Gino, 1980). The pattern of results in both instrumental and Pavlovian conditioning tasks appears consistent with this view.
What are the implications of the present results for understanding the role of the BLA in the neural mechanisms of Pavlovian fear conditioning? The abolition of conditional freezing by post-training BLA lesions in overtrained rats provides compelling support for the important role of the BLA in the expression of Pavlovian fear. Moreover, the severe deficits in the acquisition and reacquisition of conditional fear in rats with BLA lesions lend further support to the view that neurons in the BLA are critical for CS–US association in Pavlovian fear-conditioning tasks. Although these results provide further evidence for the emerging view that neurons in the BLA are required for mnemonic processes in Pavlovian fear conditioning tasks (Davis et al., 1994; Maren, 1996; Maren and Fanselow, 1996; Rogan and LeDoux, 1996), a recent report raises doubt about the role of the BLA in Pavlovian fear conditioning (Killcross et al., 1997). A number of questions have arisen about procedural aspects of this work (Nader and LeDoux, 1997), and the present data suggest that the ability of rats with BLA lesions to acquire some conditional fear after extensive training might explain preserved conditional suppression in rats with BLA lesions (Killcross et al., 1997). Nonetheless, it is clear from the present work that these studies must be reexamined with post-training lesions before a role for the BLA in the suppression paradigm can be ruled out.
Footnotes
This work was supported by National Institute of Mental Health Grants R03MH57360 and R29MH57865 and the University of Michigan. We thank Michael Kia, Lisa Randazzo, Lisa Diepenhorst, Rishi Gupta, Bill Holt, and Ki Goosens for technical assistance.
Correspondence should be addressed to Stephen Maren, Department of Psychology, University of Michigan, 525 East University Avenue, Ann Arbor, MI 48109-1109.
REFERENCES
- 1.Brady JV, Schreiner L, Geller I, Kling A. Subcortical mechanisms in emotional behavior: the effect of rhinencephalic injury upon the acquisition and retention of a conditioned avoidance response in cats. J Comp Physiol Psychol. 1954;47:179–186. doi: 10.1037/h0055426. [DOI] [PubMed] [Google Scholar]
- 2.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-d-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- 4.Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 6.Falls WA, Davis M. Lesions of the central nucleus of the amygdala block conditioned excitation, but not conditioned inhibition of fear as measured with the fear-potentiated startle effect. Behav Neurosci. 1995;109:379–387. doi: 10.1037//0735-7044.109.3.379. [DOI] [PubMed] [Google Scholar]
- 7.Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist d,l-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- 8.Fonberg E, Brutkowski S, Mempel E. Defensive conditioned reflexes and neurotic motor reactions following amygdalectomy in dogs. Acta Biol Exp (Wars) 1962;22:51–57. [PubMed] [Google Scholar]
- 9.Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- 10.Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 11.Horvath FE. Effects of basolateral amygdalectomy on three types of avoidance behavior in cats. J Comp Physiol Psychol. 1963;56:380–389. doi: 10.1037/h0041108. [DOI] [PubMed] [Google Scholar]
- 12.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Walker D, Davis M. Lack of a temporal gradient of retrograde amnesia following NMDA-induced lesions of the basolateral amygdala assessed with the fear-potentiated startle paradigm. Behav Neurosci. 1996;110:836–839. doi: 10.1037//0735-7044.110.4.836. [DOI] [PubMed] [Google Scholar]
- 15.Maren S. Synaptic transmission and plasticity in the amygdala: an emerging physiology of fear conditioning circuits. Mol Neurobiol. 1996;13:1–22. doi: 10.1007/BF02740749. [DOI] [PubMed] [Google Scholar]
- 16.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maren S, Fanselow MS. The amygdala and fear conditioning: Has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 18.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 19.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996a;110:718–717. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 20.Maren S, Aharonov G, Stote DL, Fanselow MS. N-Methyl-d-aspartate receptors in the basolateral amygdala are required for both the acquisition and expression of conditional fear in rats. Behav Neurosci. 1996b;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 21.Mineka S, Gino A. Dissociation between conditioned emotional response and extended avoidance performance. Learn Motiv. 1980;11:476–502. [Google Scholar]
- 22.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 23.Mowrer OH. On the dual nature of learning: a reinterpretation of “conditioning” and “problem solving.”. Harvard Educ Rev. 1947;17:102–150. [Google Scholar]
- 24.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 25.Nader K, LeDoux JE. Is it time to invoke multiple fear learning systems in the amygdala? Trends Cognit Sci. 1997;1:241–244. doi: 10.1016/S1364-6613(97)01078-4. [DOI] [PubMed] [Google Scholar]
- 26.Parent MB, Tomaz C, McGaugh JL. Increased training in an aversively motivated task attenuates the memory-impairing effects of post-training N-methyl-d-aspartate-induced amygdala lesions. Behav Neurosci. 1992;106:789–797. doi: 10.1037//0735-7044.106.5.789. [DOI] [PubMed] [Google Scholar]
- 27.Parent MB, West M, McGaugh JL. Memory of rats with amygdala lesions induced 30 days after footshock-motivated escape training reflects degree of original training. Behav Neurosci. 1994;108:1080–1087. doi: 10.1037//0735-7044.108.6.1080. [DOI] [PubMed] [Google Scholar]
- 28.Poremba A, Gabriel M. The amygdala is necessary for the initial acquisition but not for maintenance of discriminative avoidance behavior in rabbits. Soc Neurosci Abstr. 1995;21:1930. [Google Scholar]
- 29.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 30.Rogan M, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 31.Rogan M, LeDoux JE. Emotion: systems, cells, synaptic plasticity. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- 32.Sananes CB, Davis M. N-Methyl-d-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Swanson LW. Brain maps: structure of the rat brain. Elsevier; New York: 1992. [Google Scholar]
- 34.Thatcher RW, Kimble DP. Effect of amygdaloid lesions on retention of an avoidance response in overtrained and nonovertrained rats. Psychon Sci. 1966;6:9–10. [Google Scholar]