Abstract
NMDA receptors play important roles in synaptic plasticity and neuronal development. The functions of NMDA receptors are modulated by many endogenous substances, such as external pH (pHe), as well as second messenger systems. In the present study, the nerve–muscle cocultures of Xenopusembryos were used to investigate the effects of both external and intracellular pH (pHi) changes on the functional responses of presynaptic NMDA receptors. Spontaneous synaptic currents (SSCs) were recorded from innervated myocyte using whole-cell recordings. Local perfusion of NMDA at synaptic regions increased the SSC frequency via the activation of presynaptic NMDA receptors. A decrease in pHe from 7.6 to 6.6 reduced NMDA responses to 23% of the control, and an increase in pHe from 7.6 to 8.6 potentiated the NMDA responses in increasing SSC frequency. The effect of NMDA on intracellular Ca2+ concentration ([Ca2+]i) was also affected by pHe changes: external acidification inhibited and alkalinization potentiated [Ca2+]iincreases induced by NMDA. Intracellular pH changes of single soma were measured by ratio fluorometric method using 2,7-bis (carboxyethyl)-5,6-carboxyfluorescein (BCECF). Cytosolic acidification was used in which NaCl in Ringer’s solution was replaced with weak organic acids. Acetate and propionate but not methylsulfate substitution caused intracellular acidification and potentiated NMDA responses in increasing SSC frequency, intracellular free Ca2+ concentration, and NMDA-induced currents. On the other hand, cytosolic alkalinization with NH4Cl did not significantly affect these NMDA responses. These results suggest that the functions of NMDA receptors are modulated by both pHeand pHi changes, which may occur in some physiological or pathological conditions.
Keywords: NMDA receptor, NMDA-induced current, intracellular alkalinization, cytosolic acidification, extracellular pH change, developing motoneuron
l-Glutamate plays an important role as an excitatory synaptic neurotransmitter in the mammalian CNS (Watkins and Evans, 1981; Foster and Fagg, 1984). Evidence has accumulated that at least three types of ionotropic receptors for glutamate exist on neuronal postsynaptic membranes: AMPA, kainate, and NMDA receptors (Monaghan et al., 1989). NMDA receptors possess high Ca2+ permeability and serve many functions in neuronal development, synaptic plasticity, and acquisition of memory (Hollmann and Heinemann, 1994; McBain and Mayer, 1994). However, activation of these receptors also can contribute to the pathology of epilepsy (Dingledine et al., 1990; Meldrum, 1992), stroke (Choi, 1990, 1992), and neurodegenerative disorders such as Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease (Beal, 1992). Therefore, the regulation of NMDA receptor activity is important in the CNS.
NMDA receptors are controlled by many endogenous substances as well as second messenger systems (Hollmann and Heinemann, 1994; McBain and Mayer, 1994). Among the ionotropic glutamate receptors, the NMDA receptor displays a unique sensitivity to extracellular pH (pHe). In mammalian central neurons, NMDA-evoked currents were significantly enhanced by extracellular alkaline shifts in pH of a few tenths. On the contrary, a decrease in pHewas shown to reduce NMDA receptor-mediated cation conductance dramatically in cultured central neurons (Tang et al., 1990; Vyklicky et al., 1990; Traynelis and Cull-Candy, 1991; Takahashi and Copenhagen, 1996). Both extracellular and intracellular pH are modulated in many physiological and pathological conditions. Excitatory synaptic transmission in the CNS has been reported to be associated with a rapid alkalinization of the extracellular space (Chesler, 1990; Chesler and Kaila, 1992). On the other hand, under normal conditions the pHi is decreased after prolonged neuronal activity (Chesler and Kaila, 1992). In addition, NMDA is reported to reduce neuronal pHi in central neurons and in spinal motoneurons (Edres et al., 1986; Dixon et al., 1993; Hartley and Dubinsky, 1993; Irwin et al., 1994). Whether the NMDA receptor is modulated by intracellular pH shifts is still unknown. Recently, we found that glutamate, which is also reported to be co-released from some cholinergic nerve terminals (Vyas and Bradford, 1987; Israel et al., 1993; Meister et al., 1993), markedly increased the frequency of spontaneous synaptic currents (SSCs) at embryonic neuromuscular synapses via the activation of NMDA and non-NMDA receptors (Fu et al., 1995). SSC frequency increased markedly in response to the local perfusion of glutamate at the synaptic regions, whereas only a slight increase was observed when perfusion was performed at the soma. Furthermore, local perfusion of NMDA to the growth cone induced an inward current (our unpublished observation). These results indicate the existence of NMDA receptors at the developing motor nerve terminals. In this study, we further investigated the functional regulation of presynaptic NMDA receptors by both pHe and pHi changes. It was found that intracellular acidification potentiated the responses to NMDA in increasing SSC frequency, intracellular free Ca2+concentration, and NMDA-induced currents, whereas intracellular alkalinization did not significantly affect these responses.
MATERIALS AND METHODS
Cell culture. Xenopus nerve–muscle cultures were prepared as reported previously (Tabti and Poo, 1991). Briefly, the neural tube and the associated myotomal tissues of 1-d-oldXenopus embryos (stages 20–22) were dissociated in Ca2+- and Mg2+-free Ringer’s solution supplemented with EDTA. The cells were plated onto clean glass coverslips and were used for experiments after 24 hr at room temperature (20–22°C). The culture medium consisted of 50% (v/v) Ringer’s solution (115 mm NaCl, 2 mmCaCl2, 1.5 mm KCl, 10 mmHEPES, pH 7.6), 49% L-15 Leibovitz medium (Sigma, St. Louis, MO), 1% fetal bovine serum (Life Technologies, Grand Island, NY), and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin).
Electrophysiology. Whole-cell patch-clamp recording methods followed those described by Hamill et al. (1981). Patch pipettes were pulled with a two-stage electrode puller (pp-83, Narishige, Tokyo, Japan), and the tips were polished immediately before the experiment using a microforge (MF-83, Narishige). SSCs were recorded from innervated myocytes by whole-cell recording in the voltage-clamp mode. Recordings were made at room temperature in the Ringer’s solution. For whole-cell recordings of myocytes, the solution inside the recording pipette contained 150 mm KCl, 1 mm NaCl, 1 mm MgCl2, and 10 mm HEPES, pH 7.2. The extent of the potentiation was measured by the frequency ratio of SSCs, which is defined as the ratio of SSC frequency at peak level observed during application of drugs compared with the mean frequency observed before drug treatment. NMDA-induced currents were recorded from soma. The solution inside the recording pipette contained 145 mm CsCl, 0.1 mm CaCl2, 3 mm MgCl2, 5 mm EGTA, 5 mm HEPES, pH 7.2, and 1 mm amphotericin B was added to internal solution to get perforated patch. The membrane currents passing through the patch pipette were recorded with a patch-clamp amplifier (Dagan 8900). The data were digitized using Neuro-corder (Neuro Data DR 390) and stored on a videotape for later playback onto a storage oscilloscope (Tektronix 5113, Beaverton, OR) or an oscillographic recorder (Gould RS3200, Valley View, OH). The Data 6100 waveform analyzer (Data Precision, Danvers, MA) was used to analyze the frequency of SSCs. For analysis of spontaneous events at high frequency when overlaps of current events occurred, the events were counted visually from chart records of an oscillographic recorder driven at high chart speeds (25–100 mm/sec). Data are expressed as mean ± SEM. Statistical significance was evaluated by Student’st test.
Measurement of pHi. Measurement of pHi has been described in detail elsewhere (Wu et al., 1994). In brief, cultures were loaded with 5 μm 2,7-bis (carboxyethyl)-5,6-carboxyfluorescein (BCECF-AM, Molecular Probes, Eugene, OR) for 5–10 min at room temperature in Ringer’s solution and then washed with the same solution three times. A single soma was isolated by adjusting the slit width of the photomultiplier tube and excited by alternate flashes of 490 and 440 nm wavelength light, using a filter wheel (Cairn Research, Kent, England) rotating at 32 Hz. The excitation light was transmitted to the cell under study using a 510 nm dichroic mirror under the microscope nosepiece, and the resulting fluorescence was collected via a 40× magnification oil-immersion lens. The overall sampling rate was 0.5 Hz. The 490/440 emission ratio from the intracellular BCECF was calculated and converted to a linear pH scale using in situ calibration data obtained at the end of the experiment by the nigericin technique. A calibration curve similar to that used by Wu et al. (1994) was established by measuring the fluorescence ratio values between pHi 4.5 and 9.5. Between pHi 5.5 and 9.0, the response is linear and fits the equation pHi-pK + log [(Rmax−R)/(R− Rmin] + log(F440min/F440max), where R is the ratio of 530 nm fluorescence at 490 nm excitation to 530 nm fluorescence at 440 nm excitation.Rmax and Rmin are the maximum and minimum ratio values from the data curve, and Kis the dissociation constant for the dye, taken as 55 nm.
Measurement of intracellular Ca2+ levels.The intracellular concentration of free Ca2+([Ca2+]i) in the soma was measured using the Ca2+-sensitive fluorescent dye fura-2. Cultures plated onto a 25 mm round coverslip (No. 1001, Assistent) were loaded with 2 μm fura-2 AM (Molecular Probes) in Ringer’s solution for 1 hr at room temperature. The cells were then washed with the same solution. The fluorescence of a single soma was measured as indicated above except that the cell was excited by alternate flashes of 340 and 380 nm wavelength. The 340/380 emission ratio from the intracellular fura-2 was calculated and converted to a linear Ca2+ scale by in situ calibration at the end of the experiment using the Ca2+ionophore ionomycin (5 μm, Sigma). The following equation (Grynkiewicz et al., 1985) was used to convert the fluorescence ratio into the intracellular Ca2+ concentration [Ca2+]i = Kd[(R −Rmin)/Rmax −R](Sf2/Sb2), where R is the ratio of 510 nm fluorescence at 340 nm excitation to 510 nm fluorescence at 380 nm excitation,Rmax (10 mm Ca2+) and Rmin (10 mm EGTA in Ca2+-free Ringer’s solution) are the maximum and minimum ratio values from the data curve, Kd is the dissociation constant for the dye, taken as 135 nm at room temperature, andSf2/Sb2 is the ratio of the 380 nm signals determined at Rminand Rmax.
Because Ca2+ and H+ are presumably competitive, we have tested whether the 340/380 signal was affected by the change of pHi because of the possible pH sensitivity of fura-2. Three levels of pH (6.8, 7.2, and 7.8) were tested in Ca2+-free Ringer’s solution supplemented with 2 mm EGTA using the free acid form of fura-2, and we found that there was little change in the 340/380 ratio when the pH was changed (6.8, 7.8, and 7.2); the first two points were the two extreme levels of pHi we have observed in our experiments. We used Ca2+-free Ringer’s solution supplemented with 2 mm EGTA in the above test to mimic a similar level of resting [Ca2+]i (∼70 nm) in Xenopus cultured spinal neurons.
Chemicals. The following chemicals were used: NH4Cl and sodium acetate (Sigma), sodium methylsulfate (Aldrich, Milwaukee, WI), sodium propionate (Hayashi, Osaka, Japan), and NMDA (RBI, Natick, MA).
RESULTS
Regulation of NMDA responses in increasing SSC frequency by pHe changes
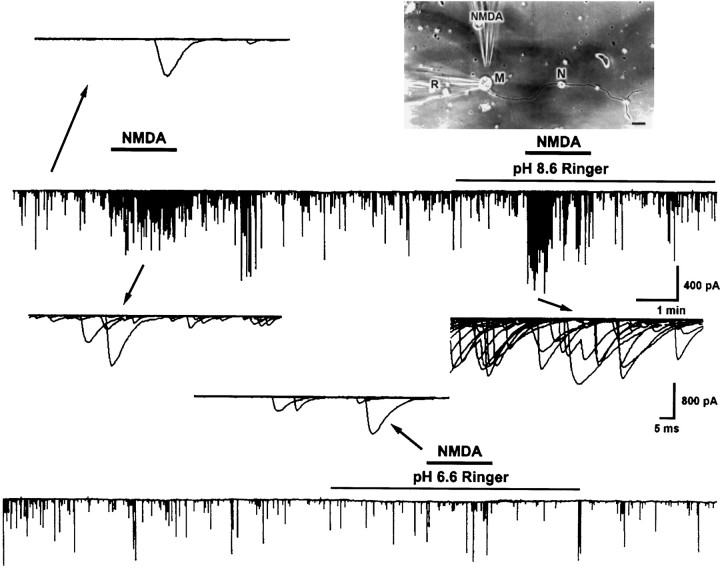
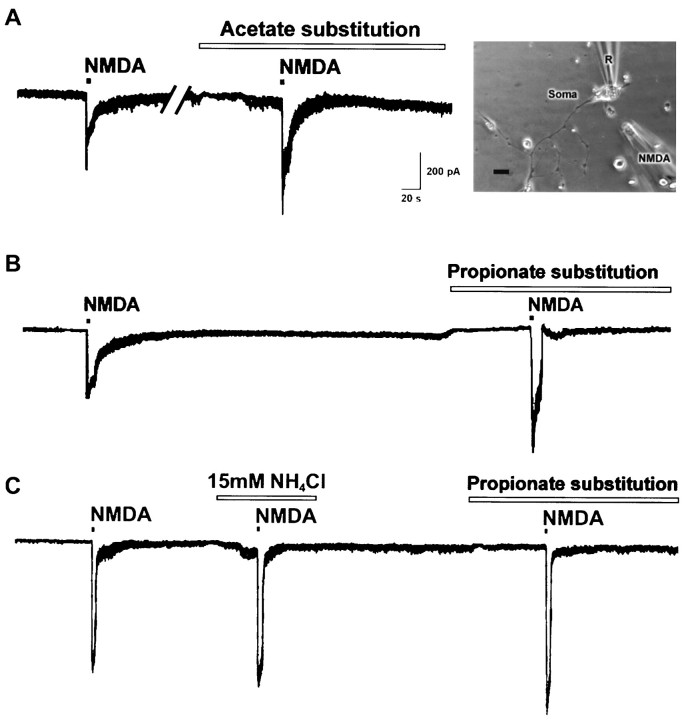
In nerve–muscle cultures prepared from 1-d-old Xenopusembryos, synaptic contacts were established between dissociated spinal neurons and myocytes within the first day of culture. SSCs were readily detectable from the innervated myocytes by whole-cell voltage-clamp recording. These currents have been shown to be caused by spontaneous ACh secretion from the neuron, because they are abolished by bath application of d-tubocurarine and unaffected by tetrodotoxin (Xie and Poo, 1986). It was found recently that there are NMDA receptors in the nerve terminals of developing motoneurons (Fu et al., 1995; Liou et al., 1996). We thus used these nerve–muscle cocultures to investigate the functional regulation of presynaptic NMDA receptors by pH changes. Figure 1 shows the recording of SSCs from an innervated myocyte. Local application of NMDA by pressure at synaptic regions with another glass microelectrode as indicated in the inset (pipette opening, 9 μm; pipette NMDA concentration, 150 μm) reversibly increased the SSC frequency (SSC frequency ratio was 13.7 ± 3.4; n= 21). The effects of changes in pHe on the NMDA responses were investigated by replacing normal Ringer’s solution with pHe 6.6 or 8.6 Ringer’s solution. The pH was adjusted before the experiment with 1N HCl or NaOH, respectively, and the pH value remained unchanged throughout the experiment (within 1 hr). The change in extracellular pH from 7.6 to 6.6 or 8.6 did not by itself significantly affect the frequency of spontaneous ACh secretion (SSC frequency ratio was 1.2 ± 0.1, n = 5, and 0.9 ± 0.1, n = 6, respectively). The mean SSC amplitude in pH 6.6 and 8.6 Ringer’s solution also showed no significant change before NMDA application (146.5 ± 35.0 pA,n = 5, and 154.7 ± 19.3 pA, n = 4, respectively; control SSC amplitude was 167.3 ± 14.3 pA,n = 21). However, changes in pHe range between 6.6 and 8.6 had different effects on the SSC potentiating action of NMDA. Alkalinization of external pH from 7.6 to 8.6 potentiated the NMDA effect by 195 ± 34% of the control (SSC frequency ratio was 26.5 ± 4.7, n = 4) (Figs. 1,2A). On the other hand, acidification of external medium from pH 7.6 to 6.6 antagonized the NMDA action to 23 ± 12% of the control (SSC frequency ratio was 3.2 ± 1.7, n = 4) (Figs. 1,2A). The time course-response curves of the NMDA action in different external pH are shown in Figure2B.
Fig. 1.
Effect of external pH changes on the SSC-increasing action of NMDA. Shown is continuous tracing of SSCs recorded from an innervated muscle cell in 1-d-oldXenopus culture. The myocyte was voltage-clamped at a potential of −60 mV. Downward deflections are SSCs (filtered at 150 Hz). The top right panel shows the relative position of patch pipette (R) and NMDA pipette (NMDA). Local perfusion of NMDA at the synaptic region with another micropipette increased SSC frequency. NMDA responses were potentiated in pH 8.6 Ringer’s solution and antagonized in pH 6.6 Ringer’s solution. Samples of superimposed SSCs in 1 sec before and after NMDA treatment are shown at higher time resolution. Scale bar, 40 μm. M, Myocyte; N, neuron.
Fig. 2.
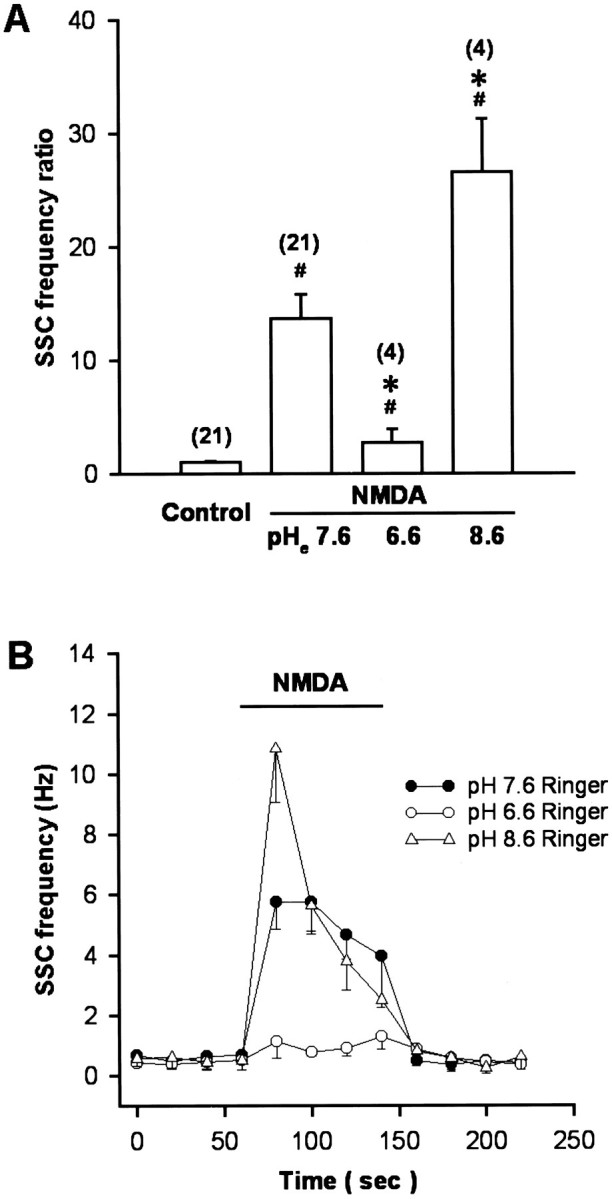
Summary of the effect of external pH changes on the SSC-increasing action of NMDA. Note that NMDA increased SSC frequency in normal pH 7.6 Ringer’s solution and the change of external pH to 8.6 potentiated NMDA action, whereas the reduction of external pH to 6.6 antagonized the SSC-increasing action of NMDA (A). B, Time course-response curves in response to NMDA application. Data are presented as mean ± SEM (n). *p < 0.05 compared with control.
Intracellular acidification enhances the responses to NMDA in increasing SSC frequency
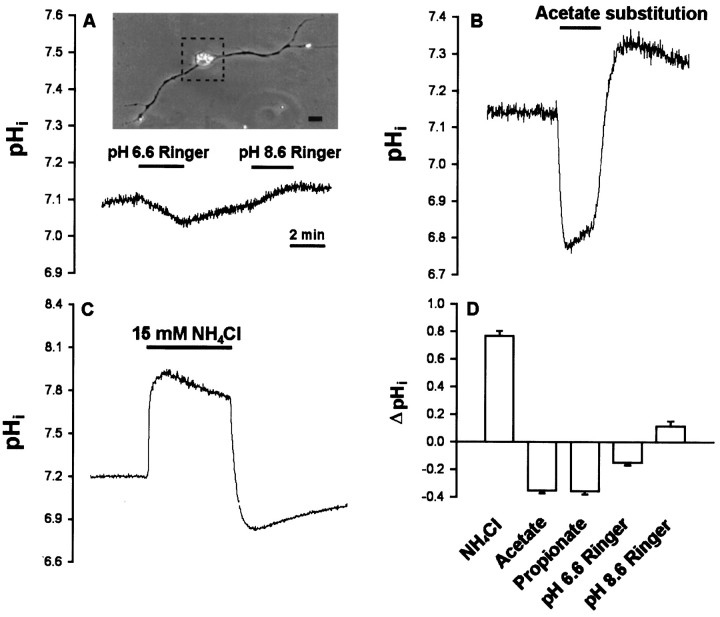
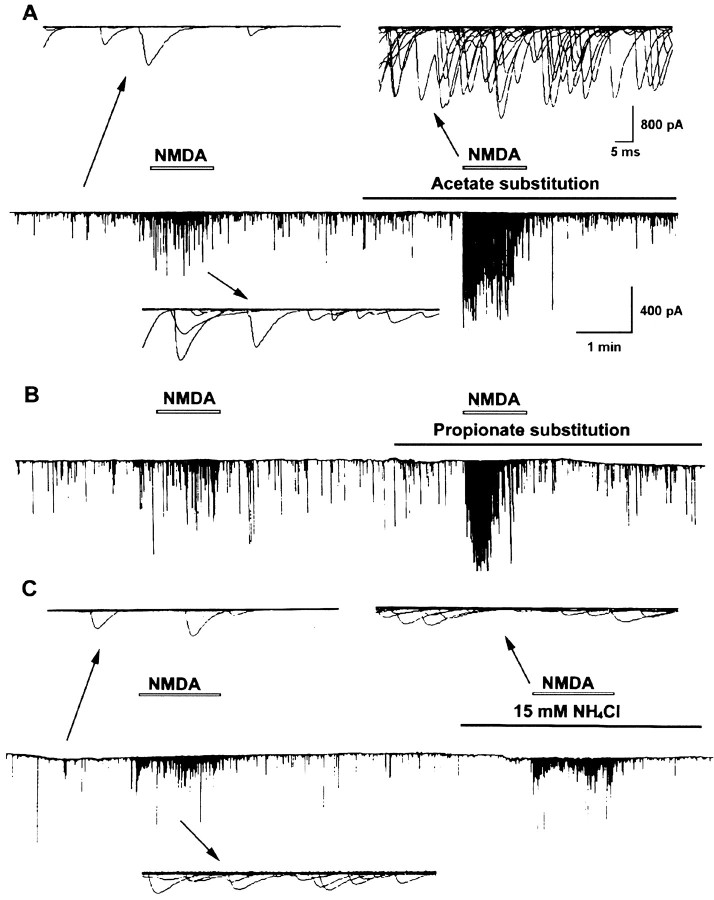
Cytosolic acidification was induced by weak organic acid substitution. Weak organic acid with pKa values in the range of 4–6 might allow selective acidification of cells. The protonated forms of such weak acids are more membrane permeable than their charged anionic forms and then dissociate intracellularly to liberate H+. Exposure of cells to weak acids is thus expected to result in an acute acid load to the cytoplasm. For these experiments, all the extracellular NaCl was replaced by the sodium salts of organic acids in normal Ringer’s solution. Cytosolic pH in neuronal soma was monitored by the BCECF fluorescence method. The baseline pHi value was 7.17 ± 0.03 (n= 46). The change of external solution from pHe 7.6 to 6.6 or 8.6 caused only a slight cytosolic acidification of 0.14 ± 0.02 units (n = 6) or intracellular alkalinization of 0.11 ± 0.04 units (n = 4), respectively (Fig.3A,D). However, a rapid intracellular acidification occurred after exposure with either acetate or propionate at a fixed pH of external medium (pHe 7.6) (Fig. 3B,D). The pHi reached peak values of 6.86 ± 0.12 (n = 8) with acetate and 6.78 ± 0.15 (n = 7) with propionate. The intracellular fluid revealed an acidic shift of 0.2–0.4 pH units. Removal of acetate or propionate results in a rebound cytosolic alkalinization. On the other hand, the peak value of pHi after exposure to methylsulfate did not show significant change (pHi was 7.09 ± 0.03;n = 6). The frequency and amplitude of SSC did not show significant change after exposure to organic acids alone for 1–2 min (SSC frequency ratio was 1.3 ± 0.2, n = 6, with acetate; 1.4 ± 0.3, n = 6, with propionate; and 1.1 ± 0.2, n = 4, with methylsulfate, respectively; mean SSC amplitude was 150.9 ± 21.6 pA with acetate; 186.3 ± 46.9 pA with propionate; and 142.2 ± 13.5 pA with methylsulfate, respectively). However, substitution with either acetate or propionate markedly potentiated SSC frequency, increasing the action of NMDA (SSC frequency ratio was 35.5 ± 7.2,n = 6, and 34.8 ± 10.9, n = 5, respectively) (Figs.4A,B,5A), while substitution of NaCl with strong organic acids such as methylsulfate resulted in no significant effect on the NMDA response (SSC frequency ratio was 8.5 ± 2.6, n = 4) (Fig. 5A). We further investigated the effect of intracellular alkalinization on the NMDA responses. Exposure of the soma to 15 mmNH4Cl resulted in a rapid increase of pHi(ΔpH = 0.77 ± 0.04; n = 8), and the pHi in peak value was 7.67 ± 0.14 (Fig.3C,D). The alkalinization seen during the first moments of exposure to NH4+ is presumably caused by the rapid, passive entry of NH3 and its subsequent hydration to form NH4+ and OH− (Roos and Boron, 1981). Cytosolic alkalinization with NH4Cl alone for 2 min also did not significantly affect SSC frequency and amplitude (SSC frequency ratio was 0.9 ± 0.2; mean SSC amplitude was 145.4 ± 28.5 pA;n = 6). As shown in Figures 4C and5A, the NMDA responses were not affected after intracellular alkalinization (SSC frequency ratio was 12.6 ± 3.6,n = 6). The time course-response curves of the NMDA action on the cytosolic acidification and alkalinization are shown in Figure 5B.
Fig. 3.
Cytosolic pH changes induced by organic acids and NH4Cl in neuronal soma. One-day-old Xenopuscultures were loaded with BCECF-AM, and ratio fluorometric measurements of intracellular pH were made on single soma. A,Top panel: the area of single soma for the measurement of pHi was outlined by the dotted box. Scale bar, 20 μm. Bottom panel: change of external pH slightly affected the intracellular pH. B, Replacement of all NaCl in Ringer’s solution with sodium acetate resulted in a marked cytosolic acidification. C, Addition of NH4Cl to the bath caused cytosolic alkalinization.D, Summarized effect on cytosolic pH by the changes of external pH or by the addition of organic acids or NH4Cl. Data are presented as mean ± SEM (n = 4–8).
Fig. 4.
Effect of intracellular pH changes on the SSC-increasing action of NMDA. Shown is a continuous tracing of SSCs recorded from an innervated muscle cell in 1-d-old Xenopus laevis culture. The myocyte was voltage-clamped at a potential of −60 mV. Downward deflections are SSCs (filtered at 150 Hz). Perfusion of NMDA at synaptic region with another micropipette increased SSC frequency. NMDA responses were potentiated by cytosolic acidification with organic acid substitution and not significantly affected by cytosolic alkalinization with NH4Cl. Samples of superimposed SSCs in 1 sec before and after NMDA treatment are shown at higher time resolution.
Fig. 5.
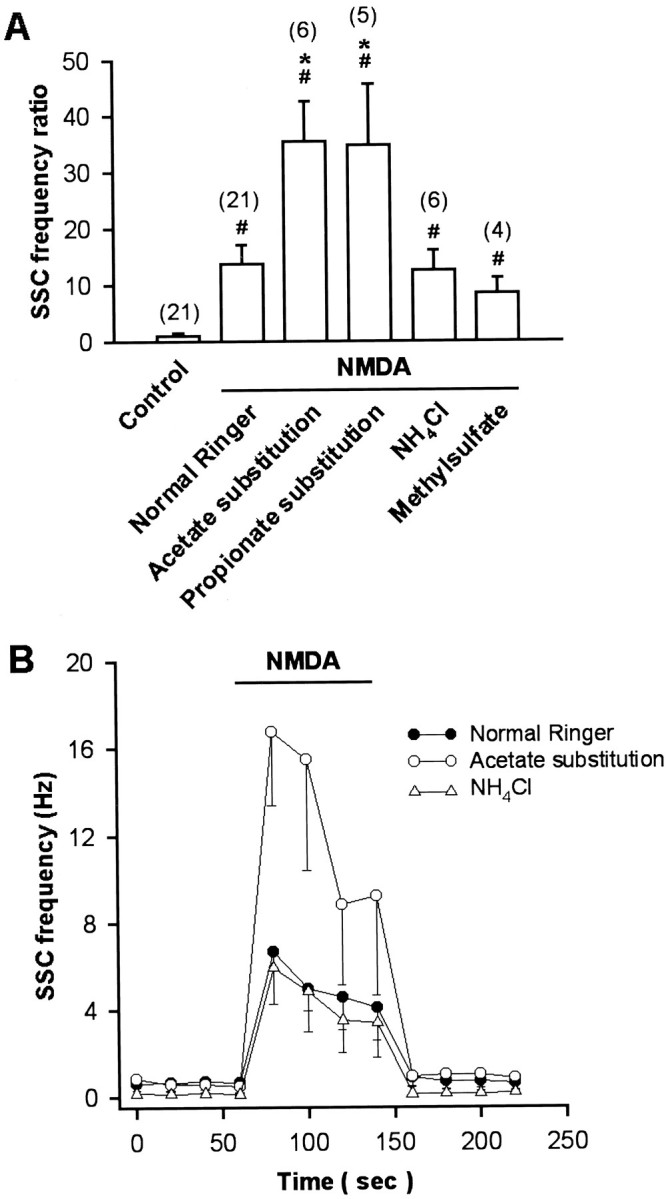
Summary of the effects of intracellular pH changes on the SSC-increasing action of NMDA. Note that NMDA increased SSC frequency in normal Ringer’s solution, and intracellular acidification with either acetate or propionate potentiated NMDA responses (A). B, Time course-response curves of the NMDA action on the cytosolic pH changes. Data are presented as mean ± SEM (n). # p < 0.05 compared with control. *p < 0.05 compared with NMDA in normal Ringer’s solution.
Modulation of NMDA effects on neuronal cytoplasmic Ca2+ by the changes of pHe and pHi
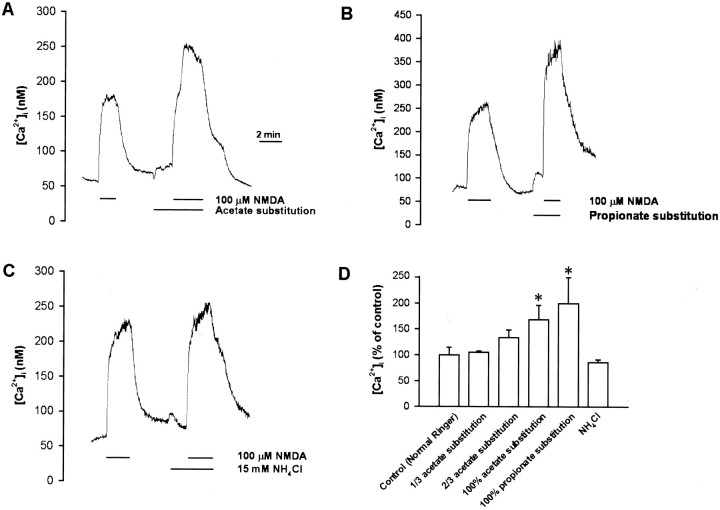
The intracellular concentration of free Ca2+([Ca2+]i) in the soma was measured using the Ca2+-sensitive fluorescent dye fura-2. The basal neuronal [Ca2+]ivalue was 70.2 ± 4.6 nm (n = 32). Superfusion with 100 μm NMDA in pH 7.6 Ringer’s solution increased [Ca2+]i by 131.3 ± 26.9 nm (n = 27). Responses to NMDA were affected by changing the pH of external medium. Lowering the pHe from 7.6 to 7.0 or 6.6 decreased the [Ca2+]i responses of NMDA to 64 ± 10% and 23 ± 7% of the control (n = 4 for each), respectively (Fig.6A,B), whereas increasing the pHe to 8.6 potentiated the [Ca2+]i response of NMDA to 156 ± 18% of the control (n = 4) (Fig.6A,B). By contrast, intracellular acidification by either acetate or propionate substitution enhanced the [Ca2+]i response to NMDA. NMDA response was enhanced concentration-dependently by acetate substitution. Two-thirds of acetate substitution (two-thirds of the NaCl in Ringer’s solution was replaced with equimolar sodium acetate) nonsignificantly increased NMDA response to 134 ± 15% of control (n = 7), whereas 100% acetate substitution significantly increased the NMDA response to 168 ± 28% (n = 8) (Fig.7A,D). Substitution with another weak acid propionate (100% substitution) also increased NMDA response to 200 ± 50% (n = 5) (Fig.7B,D). However, intracellular alkalinization by 15 mm NH4Cl had no significant effect (Fig.7C,D).
Fig. 6.
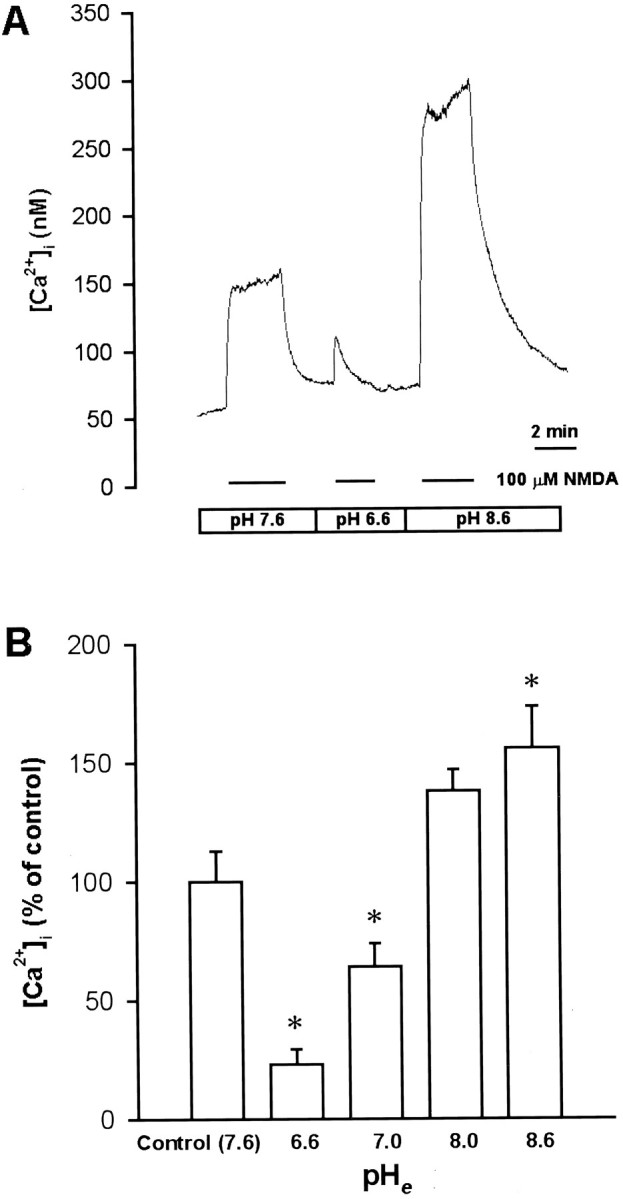
Effect of external pH changes on the [Ca2+]i-increasing action of NMDA.A, Xenopus nerve–muscle cultures were loaded with fura-2 AM, and ratio fluorometric measurement of intracellular Ca2+ was made on a single soma. Superfusion with NMDA increased [Ca2+]i, and this response was potentiated in pH 8.6 Ringer’s solution and antagonized in pH 6.6 Ringer’s solution. B, Summary of the effect of external pH changes on the [Ca2+]i-increasing action of NMDA. Data are presented as mean ± SEM (n = 4). *p < 0.05 compared with control.
Fig. 7.
Effect of intracellular pH changes on the [Ca2+]i-increasing action of NMDA.Xenopus nerve–muscle cultures were loaded with fura 2-AM, and ratio fluorometric measurement of intracellular Ca2+ was made on a single soma. Superfusion with NMDA increased [Ca2+]i, and this response was enhanced by cytosolic acidification with either acetate (A) or propionate (B) but not affected by cytosolic alkalinization with NH4Cl (C). D, Summary of the effects of intracellular pH changes on the [Ca2+]i-increasing action of NMDA. Data are presented as mean ± SEM (n = 3–22). *p < 0.05 compared with control.
Potentiation of NMDA-induced currents by intracellular acidification
To determine directly the effect of intracellular acidification on the NMDA receptor, the soma of isolated spinal neurons was whole-cell voltage-clamped at −60 mV by using perforated patch. Local perfusion of NMDA with another micropipette (intrapipette concentration, 150 μm), as indicated in the right panel of Figure8A, induced an inward current in the soma. Intracellular acidification by the substitution of all NaCl of Ringer’s solution with either sodium acetate (Fig.8A) or sodium propionate (Fig. 8B) enhanced the NMDA-induced currents (134.6 ± 6.4% and 162.6 ± 14.0% of the control, respectively; n = 7 for each). On the other hand, cytosolic alkalinization with NH4Cl did not significantly affect the NMDA-induced currents (96.1 ± 4.8% of the control, n = 6) (Fig. 8C). However, changes of external pH from 7.6 to 8.6 or 6.6 enhanced and inhibited the NMDA-induced currents, respectively [149.4 ± 8.5% (n = 6) and 37.1 ± 6.6% (n = 4) of the control, respectively].
Fig. 8.
Potentiation of NMDA-induced current by intracellular acidification. A, The soma of an isolated neuron was whole-cell voltage-clamped at −60 mV. Local perfusion with NMDA from a glass micropipette (NMDA) as indicated in the right panel induced an inward current. NMDA-induced current was enhanced by intracellular acidification with either acetate (A) or propionate (B) but not affected by cytosolic alkalinization (C). Scale bar, 10 μm. R, Patch pipette.
DISCUSSION
Many studies have revealed that second messengers such as Ca2+, cyclic nucleotides, and G-proteins modulate voltage-gated ion channels and ligand-gated receptor channels. Here we show that NMDA receptor-mediated synaptic potentiation, increase of intracellular Ca2+ concentration, and NMDA-induced currents are significantly influenced by the changes of either external or intracellular pH. We have observed that external acidification inhibited but intracellular acidification enhanced NMDA responses. On the other hand, external alkalinization potentiated the NMDA action, whereas intracellular alkalinization had no significant effect. The modulation of NMDA responses by both external and internal pH changes might mediate some of the physiological and pathological actions of glutamate.
Our results are different from those reported by Tang et al. (1990), who showed that NMDA-activated current was rather insensitive to the change in internal pH. The intracellular pH was altered in their study by filling the pipette with an internal solution of different values of pH. The pH of intracellular fluid may be different from that of patch pipette as a result of the buffering capacity of cytoplasm and improper diffusion of patch pipette solution. We here acidified intracellular fluid by using a weak organic acid substitution. It has long been known that weak acids can readily cross cell membranes in their uncharged forms and then dissociate, thereby acidifying the cell interior (Karuri et al., 1993). Once inside the cell, the pHi is well above pKa, leading to the dissociation of the protonated form and intracellular acidification. Therefore, the anions of weak acids such as acetate (pKa 4.75) or propionate (pKa 4.87) caused larger internal acidification (pHi ≒ 6.8), and the anions of strong acids such as methylsulfate (pKa < 1.0) induced little pHi change of the cells (Chesler, 1990).
The potentiation of NMDA action was in parallel with the levels of cytosolic acidification by these organic acids. We found no effect on NMDA response by methylsulfate substitution, ruling out the likelihood that the Cl− substitution itself was affecting NMDA receptor. Modulation of the NMDA-activated current by external pH changes resulted primarily from changes in the number of channel openings. The single-channel conductance of NMDA receptor was not significantly affected (Tang et al., 1990; Vyklicky et al., 1990;Traynelis and Cull-Candy, 1991). We here confirmed previous reports that external alkalinization enhances and external acidosis inhibits NMDA receptor activation (Tang et al., 1990; Vyklicky et al., 1990;Traynelis and Cull-Candy, 1991; Takahashi and Copenhagen, 1996). A lowering of pHe from 7.6 to 6.6 inhibits the functional activation of NMDA receptors, whereas a rise of pHe from 7.6 to 8.6 significantly enhanced NMDA responses. However, we provide additional evidence that the function of the NMDA receptor can also be regulated by the changes of intracellular pH. The potentiation of SSC and an increase in intracellular free Ca2+ by NMDA are regulated in parallel. We found previously that a larger decrease of pHi (∼1.4–1.6 pH units) is required for SSC frequency-increasing effect by weak organic acid substitution in pHe 6.6 Ringer’s solution (Chen et al., 1998). In the current study, the intracellular fluid revealed an acidific shift in pH of only 0.2–0.4 units by weak organic acid substitution in normal Ringer’s solution. Therefore, the potentiation effect on SSC frequency can be fully accounted for by changes in the NMDA-induced [Ca2+]i increase. Furthermore, direct evidence is shown in Figure 8 that NMDA-induced inward currents were increased in the presence of acetate or propionate. On the other hand, intracellular alkalinization had no significant effect on these NMDA responses. Because the NMDA receptor is activated in the absence of glycine, the modulation of intracellular H+ appears not to be related to the glycine recognition site of the NMDA receptor. How the internal H+ modulates the gating of the NMDA channel needs further investigation. Although pHi was reduced by 0.1 units after the pHe was lowered from 7.6 to 6.6, no potentiation of NMDA responses was observed, probably because of the larger inhibitory effect on NMDA receptors by external acidification and the insufficient intracellular acidification.
Changes in the concentration of H+ have been clearly shown to serve important biological roles by regulating the properties of protein macromolecules. Accumulating evidence suggests that extracellular and intracellular pH can change significantly under not only pathological but also physiological circumstances. The local pH shifts could be large in synaptic cleft and subsynaptic regions under these conditions. Repetitive neuronal activity may lower intracellular pH through several mechanisms. These include the metabolic production of CO2 and lactic acid, the release of protons from internal sites in response to elevation of free intracellular Ca2+ concentration, and the net entry of acid through ligand- or voltage-gated channels (Chesler and Kaila, 1992). Brief exposure of hippocampal neurons to NMDA results in a concentration-dependent pHi reduction (0.2–0.4 pH units) (Irwin et al., 1994). Acute focal electrical stimulation evoked an intracellular acidosis of approximately pH 6.8 in cortical tissue (Yaksh and Anderson, 1987). Changes in pHi and pHe have been known to modify several types of voltage-gated ion channels (Takahashi and Copenhagen, 1996). Modulation of excitatory transmission by pH shifts may be especially pertinent to forms of long-term potentiation (LTP) that have been linked to NMDA receptor activation (Blanton et al., 1989). Experimentally, LTP is often induced by trains of high frequency stimulation. Both the intracellular and external pH shifts may thus influence the relationship between LTP induction and stimulus frequency. Pathologically, hypoxia and glucose depletion in hippocampal slices have also been shown to result in a decrease in pHi within 5 min of exposure (Chesler, 1990). Furthermore, brain ischemia has been reported to lower brain pHe and pHi (Nedergaard et al., 1991; Fujiwara et al., 1992). Recent evidence suggests that mild extracellular acidosis protects central neurons from injury induced by oxygen and glucose deprivation (Giffard et al., 1990;Tombaugh and Sapolsky, 1990). Extracellular acidosis prevents the activation of NMDA receptors, which play a major role in mediating ischemic neuronal injury. By contrast, extracellular alkalosis can induce epileptiform activity, which is blocked by NMDA antagonists in mammalian brain slices (Aram and Lodge, 1987). Our results showed that intracellular acidic shift in pH of only 0.2–0.4 units enhanced NMDA responses. Therefore, intracellular acidosis may enhance NMDA responses in some physiological conditions and NMDA neurotoxicity in certain pathological diseases. The proton sensitivity of NMDA receptors could influence synaptic plasticity and seizure generation and may be relevant for glutamate-induced neurotoxicity during ischemia. These results add another level of complexity to the regulation of NMDA synaptic events by endogenous factors. Intracellular H+, acting as a second messenger, may influence the neuronal excitability via modulation of NMDA channel activity.
Footnotes
This work was supported by research Grant NSC 87–2314-B002–303 from the National Science Council. We thank Mr. I. S. Peng for help in preparing this manuscript.
Correspondence should be addressed to Dr. Wen-Mei Fu, Department of Pharmacology, College of Medicine, National Taiwan University, Taipei, Taiwan 100, Republic of China.
REFERENCES
- 1.Aram JA, Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-d-aspartate. Neurosci Lett. 1987;83:345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- 2.Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- 3.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen YH, Wu ML, Fu WM. Regulation of acetylcholine release by intracellular acidification at developing motoneurons in Xenopus cell cultures. J Physiol (Lond) 1998;507.1:41–53. doi: 10.1111/j.1469-7793.1998.041bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 6.Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- 7.Choi DW. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990;10:2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 9.Dingledine R, McBain CJ, McNamara JO. Excitatory amino acid receptors in epilepsy. Trends Pharmacol Sci. 1990;11:334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- 10.Dixon DB, Takahashi K, Copenhagen DR. l-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- 11.Edres WK, Ballanyi K, Serve B, Grafe P. Excitatory amino acids and intracellular pH in motoneurons of the isolated spinal cord. Neurosci Lett. 1986;72:54–58. doi: 10.1016/0304-3940(86)90617-8. [DOI] [PubMed] [Google Scholar]
- 12.Foster AC, Fagg GE. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res Rev. 1984;7:103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- 13.Fu WM, Liou JC, Lee YH, Liou HC. Potentiation of neurotransmitter release by activation of presynaptic glutamate receptors at developing neuromuscular synapses of Xenopus. J Physiol (Lond) 1995;489.3:813–823. doi: 10.1113/jphysiol.1995.sp021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara N, Abe T, Endoh H, Warashina A, Shimoji K. Changes in intracellular pH of mouse hippocampal slices responding to hypoxia and/or glucose depletion. Brain Res. 1992;572:335–339. doi: 10.1016/0006-8993(92)90496-v. [DOI] [PubMed] [Google Scholar]
- 15.Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506:339–342. doi: 10.1016/0006-8993(90)91276-m. [DOI] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 17.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 18.Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate oritotoxicity. J Neurosci. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 20.Irwin RP, Lin SZ, Long RT, Paul SM. N-methyl-d-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci. 1994;14:1352–1362. doi: 10.1523/JNEUROSCI.14-03-01352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israel M, Lesbats B, Bruner J. Glutamate and acetylcholine release from cholinergic nerve terminals, a calcium control of the specificity of the release mechanism. Neurochem Int. 1993;22:53–58. doi: 10.1016/0197-0186(93)90068-g. [DOI] [PubMed] [Google Scholar]
- 22.Karuri AR, Dobrowsky E, Tannock IF. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br J Cancer. 1993;68:1080–1087. doi: 10.1038/bjc.1993.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liou HC, Yang RS, Fu WM. Potentiation of spontaneous acetylcholine release from motor nerve terminals by glutamate in Xenopus tadpoles. Neuroscience. 1996;75:325–331. doi: 10.1016/0306-4522(96)00280-1. [DOI] [PubMed] [Google Scholar]
- 24.McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 25.Meister B, Arvidsson U, Zhang X, Jacobsson G, Villar MJ, Hokfelt T. Glutamate transporter mRNA and glutamate-like immunoreactivity in spinal motoneurones. NeuroReport. 1993;5:337–340. doi: 10.1097/00001756-199312000-00040. [DOI] [PubMed] [Google Scholar]
- 26.Meldrum BS. Excitatory amino acids in epilepsy and potential novel therapies. Epilepsy Res. 1992;12:189–196. doi: 10.1016/0920-1211(92)90040-z. [DOI] [PubMed] [Google Scholar]
- 27.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–R588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 30.Tabti N, Poo MM. Culturing spinal neurons and muscle cells from Xenopus embryos. In: Banker G, Goslin K, editors. Cultures of nerve cells. MIT; Cambridge, MA: 1991. pp. 139–153. [Google Scholar]
- 31.Takahashi KI, Copenhagen DR. Modulation of neuronal function by intracellular pH. Neurosci Res. 1996;24:109–116. doi: 10.1016/0168-0102(95)00989-2. [DOI] [PubMed] [Google Scholar]
- 32.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-d-aspartate channel by extracellular H+. Proc Natl Acad Sci USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tombaugh GC, Sapolsky RM. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990;506:343–345. doi: 10.1016/0006-8993(90)91277-n. [DOI] [PubMed] [Google Scholar]
- 34.Traynelis SF, Cull-Candy SG. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol (Lond) 1991;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vyas S, Bradford HF. Co-release of acetylcholine, glutamate and taurine from synaptosomes of Torpedo electric organ. Neurosci Lett. 1987;82:58–64. doi: 10.1016/0304-3940(87)90171-6. [DOI] [PubMed] [Google Scholar]
- 36.Vyklicky L, Vlachova V, Krusek J. The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol (Lond) 1990;430:497–517. doi: 10.1113/jphysiol.1990.sp018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- 38.Wu ML, Tsai ML, Tseng YZ. DIDS-sensitive pHi-regulation in single cardiac myocytes in nominally HCO3−-free conditions. Circ Res. 1994;75:123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- 39.Xie ZP, Poo MM. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci USA. 1986;83:7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaksh TL, Anderson RE. In vivo studies on intracellular pH, focal flow, and vessel diameter in the cat cerebral cortex: effects of altered CO2 and electrical stimulation. J Cereb Blood Flow Metab. 1987;7:332–341. doi: 10.1038/jcbfm.1987.71. [DOI] [PubMed] [Google Scholar]