Abstract
Sensitivity of GABAA receptors (GABARs) to inhibition by zinc and other divalent cations is influenced by the α subunit subtype composition of the receptor. For example, α6β3γ2L receptors are more sensitive to inhibition by zinc than α1β3γ2L receptors. We examined the role of a His residue located in the M2-M3 extracellular domain (rat α6 H273) in the enhanced zinc sensitivity conferred by the α6 subtype. The α1 subtype contains an Asn (N274) residue in the equivalent location. GABA-activated whole-cell currents were obtained from L929 fibroblasts after transient transfection with expression vectors containing GABAA receptor cDNAs. Mutation of α1 (α1(N274H)) or α6 (α6(H273N)) subtypes did not alter the GABA EC50 of αβ3γ2L receptors. α1(N274H)β3γ2L receptor currents were as sensitive to zinc as α6β3γ2L receptor currents, although α6(H273N)β3γ2L receptor currents had the reduced zinc sensitivity of α1β3γ2L receptor currents. We also examined the activity of other inhibitory divalent cations with varying α subtype dependence: nickel, cadmium, and copper. α6β3γ2L receptor currents were more sensitive to nickel, equally sensitive to cadmium, and less sensitive to copper than α1β3γ2L receptor currents. Studies with α1 and α6 chimeric subunits indicated that the structural dependencies of the activity of some of these cations were different from zinc. Compared with α6β3γ2L receptor currents, α6(H273N)β3γ2L receptor currents had reduced sensitivity to cadmium and nickel, but the sensitivity to copper was unchanged. Compared with α1β3γ2L receptor currents, α1(N274H)β3γ2L receptor currents had increased sensitivity to nickel, but the sensitivity to cadmium and copper was unchanged. These findings indicate that H273 of the α6 subtype plays an important role in determining the sensitivity of recombinant GABARs to the divalent cations zinc, cadmium, and nickel, but not to copper. Our results also suggest that the extracellular N-terminal domain of the α1 subunit contributes to a regulatory site(s) for divalent cations, conferring high sensitivity to inhibition by copper and cadmium.
Keywords: GABA, divalent cations, GABA receptor, zinc, cadmium, copper, nickel, recombinant, site-directed mutagenesis,
Divalent cations modulate the activity of many ligand-gated ion channels, including the GABAA receptor (GABAR). Zinc and copper appear to be released during synaptic activity and could be important in the regulation of synaptic transmission (Assaf and Chung, 1984; Howell et al., 1984; Hartter and Barnea, 1988; Kardos et al., 1989; Xie and Smart, 1991). Other divalent cations may also be involved in physiological or pathological conditions (Carpenter, 1994). Sensitivity of native GABARs to inhibition by divalent cations, including zinc, has regional and developmental dependence (Westbrook and Mayer, 1987; Smart and Constanti, 1990; Celentano et al., 1991; Legendre and Westbrook, 1991; Smart, 1992; Ma and Narahashi, 1993; Kume et al., 1994; Kumamoto and Murata, 1995; Trombley and Shepherd, 1996). Sensitivity of GABARs to zinc also changes with the onset of epilepsy, with decreased sensitivity after rapid onset of status epilepticus (Kapur and Macdonald, 1997) and increased sensitivity after chronic kindling-induced seizure activity (Buhl et al., 1996; Gibbs et al., 1997). Variations in zinc sensitivity of GABARs may be related to differences or changes in the subunit subtype composition of these receptors.
Native GABARs are believed to be composed of a pentameric combination of at least three different subunit families. Many of these subunit families have multiple subtypes, including α(1–6), β(1–3), γ(1–3), δ, and ε in mammals (Sieghart, 1995; Davies et al., 1997). Expression of different GABAR subtypes is regulated in the brain both regionally and developmentally (Laurie et al., 1992a,b;Wisden et al., 1992). In particular, expression of mRNAs for α1 and α6 subtypes is very different. Whereas α1 subtype mRNA is widely and highly expressed throughout the brain, α6 mRNA is restricted to the cerebellum. Recombinant receptors containing α4, α5, or α6 subtypes, along with a β and γ subunit, are more sensitive to zinc inhibition than those containing an α1 subtype (Burgard et al., 1996;Knoflach et al., 1996; Saxena and Macdonald, 1996). Both γ and ε subunits reduce zinc sensitivity compared with αβ or αβδ receptors, which are highly sensitive to zinc (Draguhn et al., 1990;Saxena and Macdonald, 1994; Whiting et al., 1997). Sensitivity to other divalent cations also varies with brain region and developmental stage of neurons. Cadmium, nickel, copper, lead, and cobalt have been shown to inhibit GABAR currents with varying affinities and rank orders of potency depending on the type and developmental stage of the neuron examined (Draguhn et al., 1990; Ma and Narahashi, 1993;Narahashi et al., 1994; Kumamoto and Murata, 1995). It has been suggested that the zinc and cadmium (Celentano et al., 1991;Kumamoto and Murata, 1995) or zinc and copper sites (Ma and Narahashi, 1993) may interact or overlap. However, these findings vary depending on the type of neuron preparation. Except for zinc, there is little information regarding the GABAR subunit subtype dependence of the actions of divalent cations.
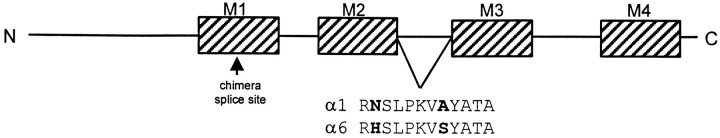
Previous work with rat α1 and α6 subtype chimeras suggested that the extracellular bridge between the M2 and M3transmembrane domains might contribute to the difference in zinc sensitivity between the α1 and α6 subtypes (Fisher et al., 1997). We focused on a His residue found only in the α4 and α6 subtypes (Fig. 1). This residue is near the M2 putative transmembrane domain that may form the lining of the channel pore. However, consistent with the voltage-independence of zinc inhibition (Westbrook and Mayer, 1987; Smart and Constanti, 1990), it is probably not within the pore itself (Xu and Akabas, 1996). Single-point mutations were made in the α1 subtype, converting the wild-type Asn (N) to either the α6 His (H) or Asp (D), and in the α6 subtype, converting the wild-type His to the α1 Asn or to Asp. Asp would be expected also to interact with divalent cations, thus controlling for alterations in the secondary, tertiary, or quaternary structure of the mutant receptors. We transiently transfected L929 fibroblasts with cDNAs encoding wild-type, mutant, or chimeric α subunits, along with β3 and γ2L, and determined the role of the α6 H273 in regulating the sensitivity of the receptors to inhibition by zinc, nickel, cadmium, and copper.
Fig. 1.
Schematic representation of a GABAR subunit and comparison of the sequence of the M2-M3extracellular domain of the rat α1 and α6 subtypes. The structure of the GABAR is believed to consist of a large N-terminal extracellular domain, four transmembrane domains (hatched boxes), a large intracellular region between M3 and M4, and a short extracellular C-terminal domain. The sequence of the 12 amino acid extracellular link between the M2 and M3 domains is given (Tyndale et al., 1995). The sequences of the rat α4 and α6 subtypes are identical in this region. The splice site in the M1 domain for the chimeric constructs of the α1 and α6 subtypes is shown by thearrow (Fisher et al., 1997).
MATERIALS AND METHODS
Construction of mutant and chimeric α subtype cDNAs. Point mutations were generated using QuikChange mutagenesis procedure and products (Stratagene, La Jolla, CA). Rat subunit cDNAs subcloned into the pCMVneo expression vector (Huggenvik et al., 1991) were used for creation of the mutants. Chimeras were constructed as described by Fisher et al. (1997). Oligonucleotide primers were synthesized by the University of Michigan DNA synthesis core facility (Ann Arbor, MI). Single amino acid changes were created using two nucleotide primers, 35 or 36 nucleotides in length, complementary to one another and encoding the desired amino acid mutation. The α1 N274 mutations were created by replacing the sequence 5′-AAT-3′ with 5′-CAT-3′ (N274H) or 5′-GAT-3′ (N274D). The α6 H273 mutations were created by replacing the sequence 5′-CAC-3′ with 5′-AAC-3′ (H273N) or 5′-GAC-3′ (H273D). The sequence of the primer region surrounding the mutations was verified for all constructs with DNA sequencing (University of Michigan sequencing core).
Transfection of L929 cells. Full-length cDNAs for rat GABAR α1 (Dr. A. Tobin, University of California, Los Angeles), β3 (Dr. D. Pritchett, University of Pennsylvania, Philadelphia), α6, and γ2L (F. Tan, University of Michigan) subtypes were subcloned into the pCMVNeo expression vector and transfected into the mouse fibroblast cell line L929 (American Type Culture Collection, Rockville, MD). Chimeric constructs and mutant subtypes were prepared as described above. For selection of transfected cells, the plasmid pHook-1 (Invitrogen, San Diego, CA) containing cDNA that encodes the surface antibody sFv was also transfected into the cells. L929 cells were maintained in DMEM plus 10% heat-inactivated horse serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were passaged by a 5 min incubation with 0.5% trypsin/0.2% EDTA solution in PBS (10 mm Na2HPO4, 0.15 mm NaCl, pH 7.3).
The cells were transfected using a modified calcium phosphate method (Chen and Okayama, 1987; Angelotti et al., 1993). Plasmids encoding GABAR subtype cDNAs were added to the cells in 1:1 ratios of 4 μg each plus 4–8 μg of the plasmid-encoding sFv. After a 4–6 hr incubation at 3% CO2, the cells were treated with a 15% glycerol solution in BBS buffer [50 mm BES (N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid), 280 mm NaCl, 1.5 mmNa2HPO4] for 30 sec. The selection procedure for sFv antibody expression was performed 20–28 hr later as described by Greenfield et al. (1997). Briefly, the cells were passaged and mixed with 5 μl of magnetic beads coated with hapten (∼7.5 × 105 beads) (Invitrogen). After 30–60 min of incubation to allow the beads to bind to positively transfected cells, the beads and bead-coated cells were isolated using a magnetic stand. The selected cells were resuspended into DMEM, plated onto 35 mm culture dishes, and used for recording 18–28 hr later.
Electrophysiological recording solutions and techniques. For whole-cell recording the external solution consisted of (in mm): 142 NaCl, 8.1 KCl, 6 MgCl2, 1 CaCl2, 10 glucose, 10 HEPES, pH 7.4, and osmolarity adjusted to 295–305 mOsm. Recording electrodes were filled with an internal solution of (in mm): 153 KCl, 1 MgCl2, 5 K-EGTA, 10 HEPES, 2 MgATP, pH 7.4, and osmolarity adjusted to 295–305 mOsm. These solutions provided a chloride equilibrium potential near 0 mV. Patch pipettes were pulled from thick-walled borosilicate glass with an internal filament (World Precision Instruments, Pittsburgh, PA) on a P-87 Flaming Brown puller (Sutter Instrument Co., San Rafael, CA) and fire-polished to a resistance of 5–10 MΩ. Series resistance was compensated 75–85%. Drugs were applied to cells using a modified U-tube delivery system with a 10–90% rise time of 70–150 msec (Greenfield and Macdonald, 1996). Currents were recorded with a List EPC-7 (Darmstandt) patch-clamp amplifier and stored on Beta videotape (Sony, Tokyo, Japan). All experiments were performed at room temperature.
Analysis of whole-cell currents. Whole-cell currents were analyzed off-line using the programs Axoscope (Axon Instruments, Foster City CA) and Prism (Graphpad, San Diego, CA). Normalized concentration–response data for the different isoforms were fit with a four-parameter logistic equation (Current = Maximum Current/(1 + ([drug]/EC50 or IC50)n), where n represents the Hill number. All fits were made to normalized data with the current expressed as a percentage of the maximum current elicited by saturating GABA concentrations for each cell for GABA concentration–response curves or, in the case of modulators, as a percentage of the response to GABA alone. Data are given as averages of the individual results ± SEM unless noted otherwise. Statistical tests were performed using the Instat program (Graphpad). Comparisons of the receptor properties were performed with one-way ANOVA, Tukey-Kramer multiple comparisons test, and Student’st test (p = 0.05). For comparisons of sensitivity, the logs of individual EC50 or IC50 values were compared.
RESULTS
GABA sensitivity of wild-type and mutant α1β3γ2L and α6β3γ2L receptors
Wild-type and all four mutant α subtypes produced functional GABARs when cotransfected with β3 and γ2L in L929 fibroblasts (Fig.2A). α1β3γ2L receptors were less sensitive to GABA (average GABA EC50 = 10.7 ± 1.8 μm; Hill slope = 1.6 ± 0.1;n = 5) than were α6β3γ2L receptors (average GABA EC50 = 1.8 ± 0.2 μm; Hill slope = 1.4 ± 0.2; n = 6) (Fig. 2B). The α subtype mutations did not affect the sensitivity of the GABARs to GABA (Fig. 2B). The GABA EC50 values for receptors containing the α1 mutants were not significantly different from the EC50 values for receptors containing the α1 wild-type, with average EC50 values of 11.9 ± 2.7 μm (α1(N274H)β3γ2L, average Hill slope = 1.3 ± 0.1; n = 4) and 9.1 ± 0.6 μm) (α1(N274D)β3γ2L, average Hill slope = 1.2 ± 0.2; n = 4) (data not shown). The α6 mutants also did not affect GABA sensitivity, with average GABA EC50 values of 1.2 ± 0.4 μm(α6(H273N)β3γ2L, average Hill slope = 1.1 ± 0.2; n = 5) and 1.1 ± 0.1 μm(α6(H273D)β3γ2L, average Hill slope = 1.5 ± 0.2; n = 4) (data not shown).
Fig. 2.
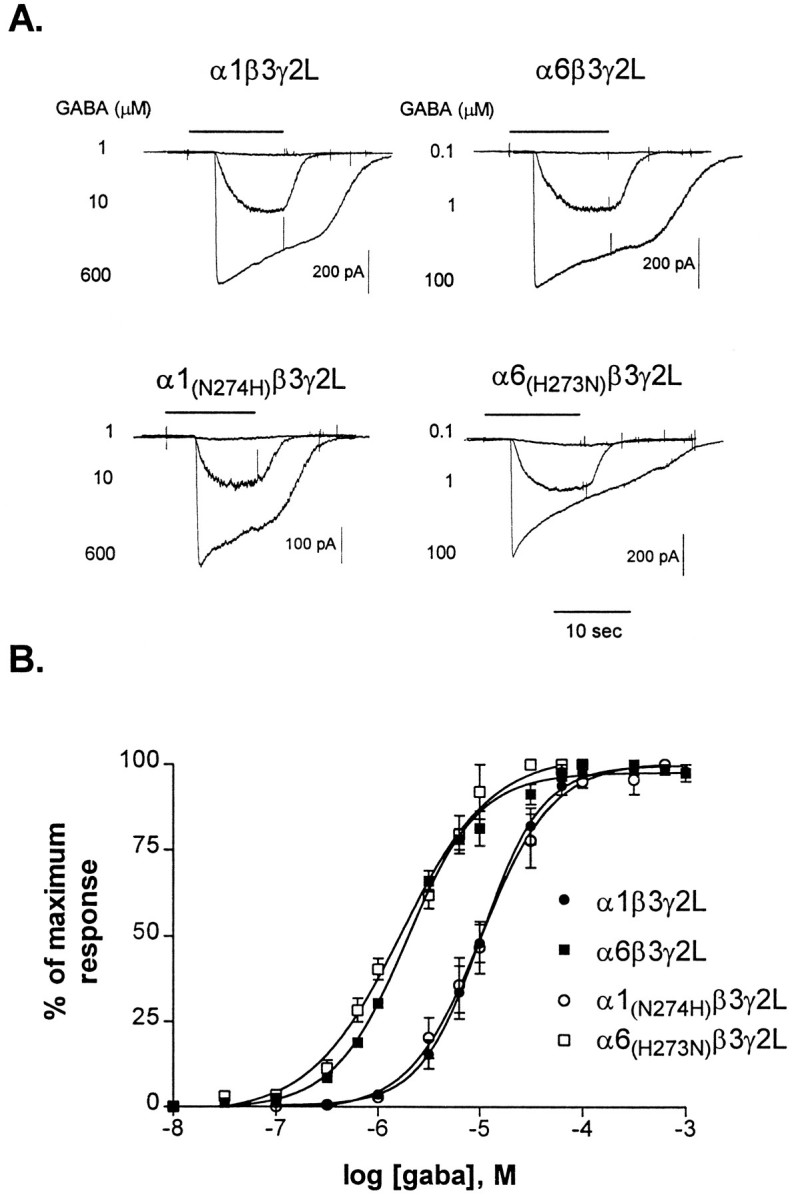
Sensitivity of GABARs to GABA. A, Representative whole-cell traces from transfected L929 fibroblasts. Cells transfected with wild-type or mutant subunits produced current responsive to GABA in a concentration-dependent manner. Varying concentrations of GABA were applied for 10–15 sec, as indicated, to cells voltage-clamped to −50 mV. The same time scale applies to all traces. B, Concentration–response relationships were constructed by normalizing the peak response to each concentration of GABA to the maximum current–response for each cell.Points shown are mean ± SEM. Data were fit with a four-parameter logistic equation. EC50 values and Hill slopes for the fits shown are as follows: α1β3γ2L (10.4 μm; Hill slope = 1.4), α6β3γ2L (1.9 μm; Hill slope = 1.2), α1(N274H)β3γ2L (10.4 μm; Hill slope = 1.2), and α6(H273N)β3γ2L (2.6 μm; Hill slope = 1.2).
Inhibition of GABAR currents by zinc
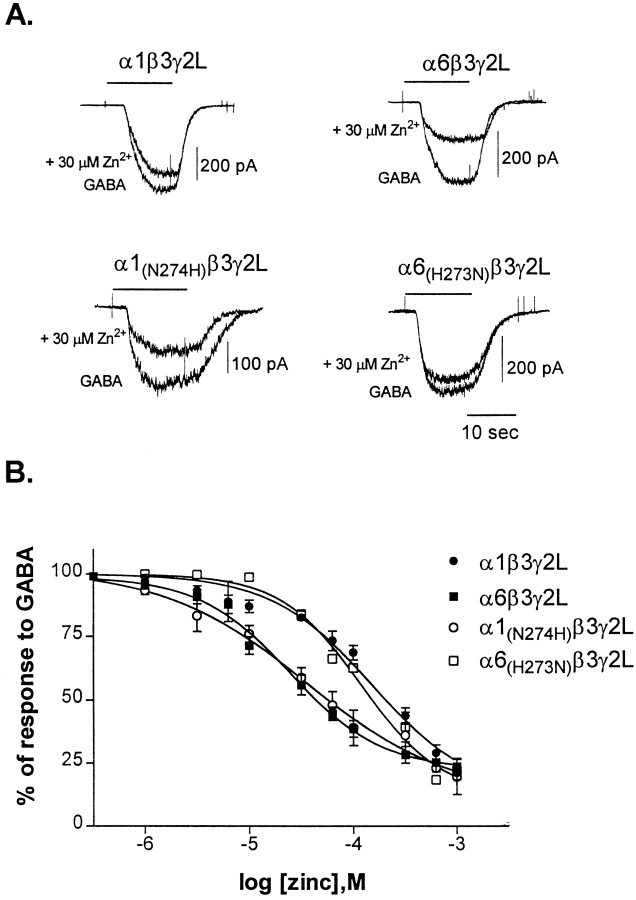
Both α1β3γ2L and α6β3γ2L receptor currents were reduced by zinc (Fig.3A), but α1β3γ2L receptor currents were less sensitive to zinc (average IC50= 151 ± 34 μm; n = 5) than α6β3γ2L receptor currents (average IC50 = 26 ± 4 μm; n = 7) (Fig. 3B). However, both receptors were inhibited to the same extent (∼80% of the current) by maximally effective zinc concentrations. The difference in zinc sensitivity between these isoforms, therefore, was in their affinity for zinc and not in its efficacy. Replacement of H273 in the α6 subtype with the Asn found in the equivalent location in the α1 subtype (N274) reduced the sensitivity of the receptor for zinc (IC50 of 114 ± 12 μm; n= 4) to near that of the wild-type α1β3γ2L receptor (Fig.3A,B). Replacement of N274 in the α1 subtype with the His found in the equivalent location in the α6 subtype (H273) increased the sensitivity of the receptor for zinc (IC50 = 38 ± 6 μm; n = 4) to that of the wild-type α6β3γ2L receptor (Fig.3A,B). Exchanging Asp for either of these amino acids also produced high sensitivity to zinc, with IC50 values for zinc of 28 ± 9 μm(α1(N274D)β3γ2L, n = 4) and 17 ± 4 μm (α6(H273D)β3γ2L,n = 4) (data not shown). This was consistent with the ability of Asp to contribute to binding sites for divalent cations. These results indicated that replacing the α6 subtype H273 with Asn prevented the higher zinc sensitivity conferred by the α6 subtype and that a His residue in this location was sufficient to convert the α1 subunit from low to high zinc sensitivity.
Fig. 3.
Sensitivity of GABARs to zinc.A, Representative whole-cell traces from transfected L929 fibroblasts. The response to GABA and GABA plus 30 μm zinc is shown for each receptor isoform. GABA concentrations were near the EC50 value for each receptor: 1 μm for α6 and α6 mutants, or 10 μmfor α1 and α1 mutants. Cells were voltage-clamped to −50 mV.B, Concentration–response relationships were constructed by expressing the inhibition by zinc as a percentage of the response to GABA alone (1 μm or 10 μm) for each cell. Points shown are mean ± SEM. Data were fit with a four-parameter logistic equation. IC50 values and Hill slopes for the fits shown are as follows: α1β3γ2L (190 μm; Hill slope = −1.0), α6β3γ2L (25 μm; Hill slope = −1.0), α1(N274H)β3γ2L (36 μm; Hill slope = −0.8), and α6(H273N)β3γ2L (120 μm; Hill slope = −1.1).
Structural dependence of modulation of GABAR currents by other divalent cations
By using chimeras of α1 and α6 subtypes with a splice site within the first transmembrane domain (M1), we demonstrated previously that the increased zinc sensitivity conferred by the α6 subtype was associated with C-terminal regions, including the M2-M3 extracellular domain (Fisher et al., 1997). This finding led us to focus on H273 in the α6 subtype M2-M3 domain as a potentially important site for influencing the zinc sensitivity of GABARs. Other divalent cations, however, also inhibit the activity of GABARs, and it is not known whether all of these divalent cations act at the same site or whether multiple allosteric regulatory sites exist. It is also not known whether all divalent cations show the same α subunit subtype dependence shown by zinc. Therefore, to determine whether there was a common structural dependence of GABARs for inhibition of currents by these divalent cations, we measured the responsiveness of the α1/α6 chimeras and the His and Asn mutations on the sensitivity of recombinant receptors to inhibition by nickel, copper, and cadmium. The α1/α6 chimera contains α1 sequence in the large extracellular N-terminal domain through the first half of the M1transmembrane domain to the splice site (Fig. 1) and α6 sequence for the remainder of the subunit. The α6/α1 chimera is the opposite, containing α6 sequence in the N terminus and α1 sequence C terminal to the splice site. A single-point mutation was introduced into the M1 domain of the α1/α6 chimera to create the chimeric receptors. L258 was converted to the Thr present in the α6 subtype. This mutation alone did not affect the properties of the α1 subtype (Fisher et al., 1997).
Inhibition of GABAR currents by nickel
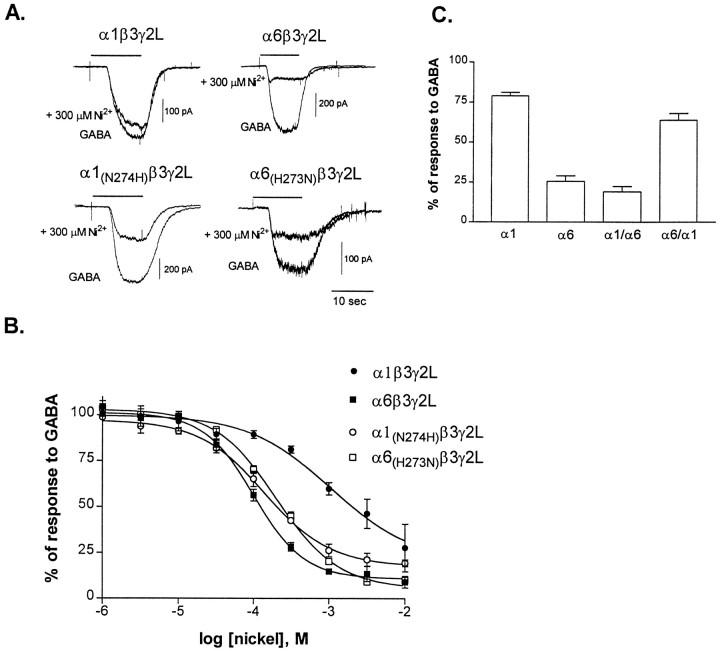
Both α1β3γ2L and α6β3γ2L receptor currents were reduced by nickel (Fig.4A), and as with zinc, α6β3γ2L receptor currents (average IC50 = 108 ± 9 μm; n = 5) were more sensitive to inhibition by nickel than α1β3γ2L receptor currents (average IC50 = 1.3 ± 0.3 mm; n = 6) (Fig. 4B).
Fig. 4.
Sensitivity of GABARs to nickel. A, Representative whole-cell traces from transfected L929 fibroblasts. The responses to GABA and GABA plus 300 μm nickel are shown for each receptor isoform. GABA concentrations were near the EC50 for each receptor: 1 μm for α6 and α6 mutants, or 10 μm for α1 and α1 mutants. Cells were voltage-clamped to −50 mV. B, Concentration–response relationships were constructed by expressing the inhibition by nickel as a percentage of the response to GABA alone (1 μm or 10 μm) for each cell.Points shown are mean ± SEM. Data were fit with a four-parameter logistic equation. IC50 values for the fits shown are as follows: α1β3γ2L (1.1 mm), α6β3γ2L (102 μm), α1(N274H)β3γ2L (142 μm), and α6(H273N)β3γ2L (208 μm). C, Sensitivity of the chimeric constructs of the α1 and α6 subtypes to 600 μmnickel. GABA concentration was near the EC50 for each receptor (60 μm for α1/α6β3γ2L and 0.3 μm for α6/α1β3γ2L). The inhibition by nickel was normalized to the response to GABA for each cell. Error bars represent mean + SEM for four cells.
To localize the α subtype functional domain that determined the sensitivity of the receptors to inhibition by nickel, we compared the extent of inhibition by 600 μm nickel of currents from αβ3γ2L receptors containing wild-type or chimeric α subtypes (Fig. 4C). For each GABAR isoform, currents were evoked by EC50 GABA concentrations. Wild-type α1β3γ2L receptor currents were less inhibited by nickel than wild-type α6β3γ2L receptor currents. The extent of inhibition by 600 μmnickel of α1/α6 chimeric subunit receptor currents was not significantly different from that of wild-type α6β3γ2L receptor currents (Fig. 4C). The extent of inhibition by 600 μm nickel of α6/α1 chimeric receptor currents was not significantly different from that of wild-type α1β3γ2L receptor currents. This pattern was comparable to that of zinc, suggesting that high nickel sensitivity was associated with domains of the α6 subtype C terminal to the M1 domain.
To determine whether the α6 H273 was responsible for the higher sensitivity to nickel of α6β3γ2L receptors, we examined the nickel sensitivity of the mutant α6(H273N)β3γ2L and α1(N274H)β3γ2L receptor currents (Fig.4A,B). In contrast to the result obtained for zinc, the sensitivity of α6(H273N)β3γ2L receptor currents to nickel (average IC50 = 212 ± 16 μm; n = 5) was only slightly but significantly reduced compared with wild-type α6β3γ2L currents. This indicated that this His residue was not required for high sensitivity to nickel but that it might contribute to or influence the sensitivity. The α1(N274H) mutant subtype increased the sensitivity to nickel (average IC50 of 142 ± 21 μm; n = 5) compared with the wild-type α1β3γ2L receptor (Fig. 4B). The degree of inhibition by 300 μm nickel of the α1(N274H)β3γ2L receptor was significantly different from either of the wild-type receptors, again suggesting that a His in this location contributed to but was not solely responsible for the higher sensitivity to nickel associated with the α6 subtype.
Inhibition of GABAR currents by cadmium
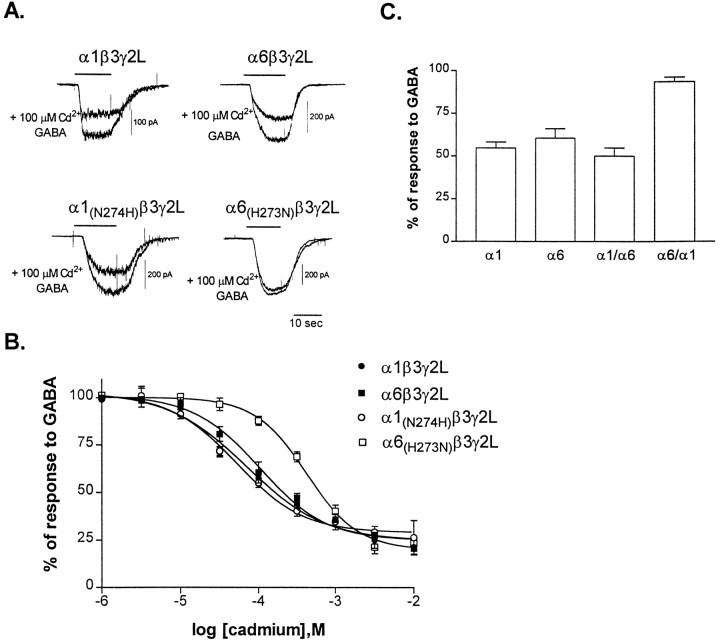
Both α1β3γ2L and α6β3γ2L receptor currents were reduced by cadmium (Fig. 5A). However, unlike the difference in sensitivity seen with zinc, α1β3γ2L and α6β3γ2L receptor currents had similar sensitivity to inhibition by cadmium, with average IC50values of 102.6 ± 34.4 μm (n = 5) and 134.1 ± 19.3 μm (n = 5), respectively (Fig. 5B). Although previous work suggested that the zinc and cadmium binding sites might overlap or interact (Celentano et al., 1991; Kumamoto and Murata, 1995), these data suggested that the structural dependence of cadmium sensitivity might be different from that of zinc.
Fig. 5.
Sensitivity of GABARs to cadmium.A, Representative whole-cell traces from transfected L929 fibroblasts. Responses to GABA and GABA plus 100 μmcadmium are shown for each receptor isoform. GABA concentrations were near the EC50 for each receptor: 1 μm for α6 and α6 mutants, or 10 μm for α1 and α1 mutants. Cells were voltage-clamped to −50 mV. B, Concentration–response relationships were constructed by expressing the inhibition by cadmium as a percentage of the response to GABA alone (1 μm or 10 μm) for each cell.Points shown are mean ± SEM. Data were fit with a four-parameter logistic equation. IC50 values for the fits shown are as follows: α1β3γ2L (64 μm), α6β3γ2L (103 μm), α1(N274H)β3γ2L (52 μm), and α6(H273N)β3γ2L (425 μm). C, Sensitivity of the chimeric constructs of the α1 and α6 subtypes to 100 μmcadmium. GABA concentration was near the EC50 for each receptor (60 μm for α1/α6β3γ2L and 0.3 μm for α6/α1β3γ2L). The inhibition by cadmium was normalized to the response to GABA for each cell. Error bars represent mean + SEM for n ≥ four cells.
To localize the α subtype functional domain that determined the sensitivity of the receptors to inhibition by cadmium, we compared the extent of inhibition by 100 μm cadmium of currents from αβ3γ2L receptors containing wild-type or chimeric α subtypes (Fig. 5C). For each GABAR isoform, currents were evoked by EC50 GABA concentrations. Wild-type α1β3γ2L receptor currents were inhibited by cadmium to the same extent as wild-type α6β3γ2L receptor currents. Inhibition by cadmium of currents from receptors containing the α1/α6 chimeric subunit (n= 5) was not significantly different from inhibition of currents from the wild-type receptors. However, α6/α1β3γ2L receptor currents (average IC50 for cadmium of 696 ± 203 μm; n = 7) were significantly less sensitive to cadmium inhibition than wild-type receptor currents. These data suggested that regions of the α6 subtype C terminal to the first transmembrane domain and residue(s) in the N-terminal extracellular domain of the α1 subtype were required for cadmium sensitivity.
To determine whether the α6 H273 was responsible for the sensitivity to cadmium of α6β3γ2L receptors, we examined the cadmium sensitivity of the mutant α6(H273N)β3γ2L and α1(N274H)β3γ2L receptors (Fig.5A,B). The α6(H273N)mutation decreased the sensitivity of the receptor for cadmium, with an IC50 of 432.4 ± 55.8 μm(n = 4), suggesting that this His was important for cadmium sensitivity as well as for zinc sensitivity. Because the H273N mutation accounted for only part of the loss of sensitivity compared with the chimeric subunit, other residues might also contribute to cadmium sensitivity. The α1(N274H) mutation did not alter the inhibition by cadmium compared with the wild-type α1β3γ2L receptor, with an IC50 of 54.7 ± 15.3 μm (n = 4). Consistent with the findings from the chimeric receptors, these results suggested that although α1- and α6-containing receptors were equally sensitive to cadmium, the structural determinants of the properties of inhibition of these subtypes were different. It is interesting that the α1/α6 chimera did not confer significantly greater sensitivity to cadmium than the wild-type subtypes, although it presumably contained the domains responsible for cadmium sensitivity for both the α1 and the α6 subtypes. This suggests that these sites are not additive or that one of the sites was not functional.
Inhibition of GABAR currents by copper
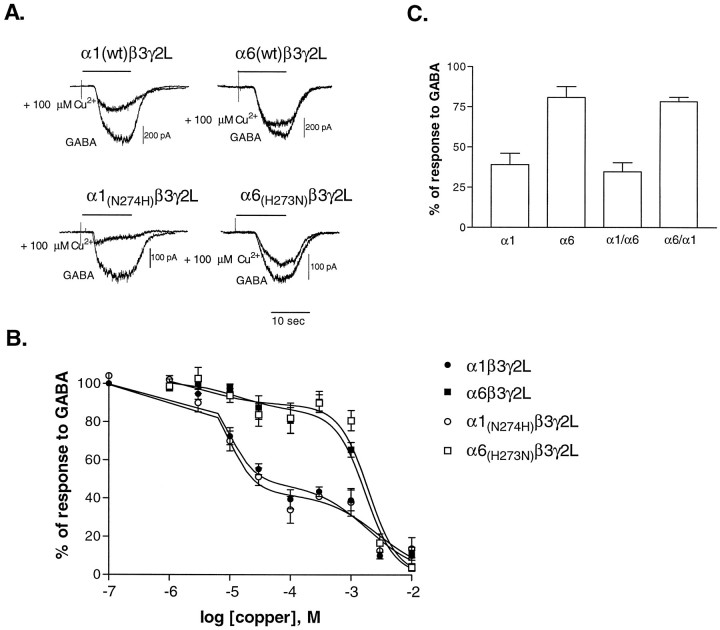
Both α1β3γ2L and α6β3γ2L receptor currents were reduced by copper (Fig.6A), but unlike the other divalent cations that we examined, α1β13γ2L receptor currents were more sensitive than α6β3γ2L receptor currents to inhibition by copper (Fig. 6B). The concentration–response curves were fitted best with a two-population logistic equation. The IC50 values (and relative contributions) ± SE of the fitting parameters for the α1β3γ2L receptor were 9.0 ± 2.6 μm (51.9 ± 5.7%) and 1.89 ± 0.81 mm (48.1 ± 4.8%) (n = 3). For the α6β3γ2L receptor the data were fit with IC50 values (and relative contributions) of 13.3 ± 2.1 μm (16.3 ± 5.7%) and 1.73 ± 0.25 mm (83.7 ± 5.2%) (n = 5). The difference in sensitivity of the isoforms appeared to be attributable primarily to the greater contribution of the higher affinity site for the α1-containing receptors.
Fig. 6.
Sensitivity of GABARs to copper. A, Representative whole-cell traces from transfected L929 fibroblasts. Responses to GABA and GABA plus 100 μm copper are shown for each receptor isoform. GABA concentrations were near the EC50 for each receptor: 1 μm for α6 and α6 mutants, or 10 μm for α1 and α1 mutants. Cells were voltage-clamped to −50 mV. B, Concentration–response relationships were constructed by expressing the inhibition by copper as a percentage of the response to GABA alone (1 μm or 10 μm) for each cell.Points shown are mean ± SEM. Data are fit with a two-population logistic equation. C, Sensitivity of the chimeric constructs of the α1 and α6 subtypes to 100 μm copper. GABA concentration was near the EC50 for each receptor (60 μm for α1/α6β3γ2L and 0.3 μm for α6/α1β3γ2L). The inhibition by copper was normalized to the response to GABA for each cell. Error bars represent mean + SEM for n ≥ three cells.
To localize the α subtype functional domain responsible for the sensitivity to inhibition by copper, we compared the inhibition by 100 μm copper of currents from αβ3γ2L receptors containing wild-type or chimeric α subtypes (Fig. 6C). For each GABAR isoform, currents were evoked by EC50 GABA concentrations. The extent of inhibition by 100 μm copper of α1/α6 chimeric receptor currents (n = 4) was not significantly different from that of wild-type α1β3γ2L receptor currents. The extent of inhibition by copper of α6/α1 chimeric subunit receptor currents (n = 4) was not significantly different from that of wild-type α6β3γ2L receptor currents. This suggested that regions in the N-terminal extracellular domain of the α1 subtype were responsible for the higher copper sensitivity.
As expected from the chimera data, the C-terminal mutations in the α1 and α6 subtypes had no effect on the sensitivity or the receptor currents to copper inhibition (Fig.6A,B). The α1(N274H)β3γ2L receptor data were fit with two populations with IC50 values (and relative contributions) of 9.7 ± 2.2 μm (57.8 ± 5.8%) and 2.78 ± 1.3 mm (43.2 ± 4.6%), whereas the fits of the α6(H273N)β3γ2L receptor data were 3.8 ± 1.12 μm (15.3 ± 13.6%) and 1.94 ± 0.30 mm (84.7 ± 0.44%). The degree of inhibition by 100 μm copper of the mutant receptors was not significantly different from the inhibition of wild-type receptors for either of the mutations, indicating that H273 of α6 and N274 of α1 did not influence copper inhibition of GABAR current. These data were consistent with data from the chimeric subunits, indicating that high sensitivity to inhibition of current by copper is associated with the extracellular N-terminal domain of the α1 subtype.
DISCUSSION
We examined the role of H273 of the rat α6 subtype in the sensitivity of recombinant GABARs to inhibition by divalent cations. Previous studies of α1/α6 chimeric subunits in our laboratory suggested that the extracellular domain between the second and third transmembrane domains in which this His is located may be important in the higher sensitivity to zinc of α6β3γ2L receptors compared with α1β3γ2L receptors (Fisher et al., 1997). GABARs containing the mutant receptors showed wild-type responsiveness to GABA, indicating that the GABA binding sites and transduction pathways were not substantially affected. Mutation of H273 to the Asn found in the homologous location in the α1 subtype reduced the zinc sensitivity, whereas exchanging a His for N274 in the α1 subtype produced α6-like sensitivity. This His could influence zinc sensitivity through several different mechanisms: it may contribute to the binding site for zinc, remotely influence the properties of the binding site by changing the structure of the receptor, or modify the transduction pathway through which zinc binding reduces the GABAR current. Substitution of Asp for the His in α6 or the Asn in α1 also produced high sensitivity to zinc. Because Asp is structurally unrelated to His but shares the ability to participate in zinc binding, this suggested that the residue in this location may contribute to the zinc binding site. However, because receptors containing α subunits that lacked the His residue were still sensitive to zinc but with higher IC50 values, this His was not required for zinc binding but instead apparently increased the attractiveness of the receptor for zinc. Our results do not rule out participation of other residues in the α subunits in zinc binding, and it is possible that the residues that contributed to the binding of other divalent cations could also contribute to one or more zinc sites. Additionally, although our findings may explain the higher sensitivity of α4- and α6-containing receptors to zinc, the α5 subtype also confers relatively high sensitivity to zinc (Burgard et al., 1996), but like α1 contains an Asn residue in this location. Because α4 and α6 subtypes share this His residue, the identical mutation of the α4 subtype would probably also reduce zinc sensitivity. However, it is possible that other residues in the α4 subtype influence zinc binding and that this His residue plays a role only in the zinc sensitivity of the α6 subtype.
A His residue responsible for inhibition by divalent cations has also been identified in the structurally related ρ1 subunit. ρ subunits are highly expressed in the retina and are believed to form the GABAC class of receptors (Tyndale et al., 1995). Homomeric ρ1 receptors are highly sensitive to block by zinc, nickel, and cadmium, and a His has been shown to be responsible for this inhibition (Wang et al., 1995). However, the location of this residue in the large N-terminal extracellular domain does not correlate with the location of the α6 His we have identified. This suggests that although His residues in both GABAA and GABAC receptors influence the sensitivity to inhibition by divalent cations, the structural domains responsible for the inhibition are different.
Contributions to zinc inhibition from other subunits
The GABAR has a complex structure, and native GABARs are believed to consist of a pentameric combination of two α, two β, and one γ, δ, or ε subunits. The α subunit alone clearly does not determine all the properties of zinc inhibition. Contributions from the β, γ, δ, and ε subunits also influence these properties. Because the His residue in the M2-M3extracellular bridge appears to be important in the α subunit contribution, it is possible that this region plays a role in the other subunits as well. At the equivalent location in β subunit is a Glu that is conserved among all β subtypes. Glu would be capable of participating in zinc binding, consistent with the high sensitivity of αβ heterodimers to inhibition by zinc (Draguhn et al., 1990). In addition, in all β subtypes there is a His that is only three amino acids N terminal to the Glu (H267 in β3) that has recently been shown to regulate zinc sensitivity in β3 homomers and α1β3 heterodimers expressed in Xenopus oocytes (Wooltorton et al., 1997). Both Glu and His residues may contribute to β subtype regulation of zinc inhibition. The γ(1–3) and ε subunits all contain a lysine residue at the M2-M3 location. The positive charge of this residue would repel cation binding, consistent with the reduced sensitivity to zinc of γ- and ε-containing GABARs (Draguhn et al., 1990; Whiting et al., 1997). The δ subunit has a serine residue that would not be expected to influence zinc binding, and αβδ receptors have an intermediate sensitivity to zinc between the αβ heterodimers and the αβγ heterotrimers (Saxena and Macdonald, 1996). Although a zinc binding pocket(s) could be formed by residues from many different regions of the subunits rather than a single homologous domain, it is interesting that this location in the M2-M3 extracellular bridge appears to be a location of heterogeneity among subunits and that characteristics of the residues are consistent with their contributions to zinc sensitivity of the receptor.
Multiple sites for divalent cations
We also examined the role of H273 of the α6 subtype in the sensitivity of GABARs to three other divalent cations, nickel, cadmium, and copper, to determine whether they shared a common structural dependence for activity with zinc. Although only copper and zinc are believed to play physiological roles in regulating synaptic activity, the effects of other divalent cations can be important in understanding their neurotoxicity, as well as helpful in understanding the structural contributions of the different GABAR subunits to the actions of divalent cations. Replacement of the His residue in α6 with Asn reduced the sensitivity to both cadmium and nickel, although not to the same level seen with the chimeric receptor. This suggests that although this His contributes to these sites, other residues also in the C-terminal extracellular domains significantly influence the sensitivity to cadmium and nickel. The α6 subtype confers a relatively low sensitivity to copper, and replacement of the H273 with Asn did not affect inhibition by copper.
The α1 subtype also appears to have a distinct site(s) for divalent cation binding, conferring high sensitivity to inhibition by copper and cadmium. The sensitivity to copper, and probably to cadmium, was associated with the large N-terminal extracellular domain of the subunit. This is in contrast to the α6 subtype in which all high sensitivity to zinc, cadmium, and nickel was associated with regions C terminal to this domain. The N-terminal extracellular domain has a high degree of sequence variability among the α subtypes, and there are numerous divergent amino acids in the α1 subtype compared with the α6 subtype, including His, Glu, and Asp residues that could contribute to high sensitivity to divalent cations. Further work may indicate which of these amino acid differences are responsible for the higher copper and cadmium sensitivity of the α1β3γ2L receptors.
Footnotes
This work was supported by National Institutes of Health Grant RO1-NS33300 (R.L.M.) and National Institute on Drug Abuse Training Grant 5T32-DA07268 (J.L.F.). We acknowledge the assistance of Dr. Naomi Nagaya.
Correspondence should be addressed to Dr. Robert L. Macdonald, 1103 East Huron Street, Neuroscience Lab Building, Ann Arbor, MI 48104-1687.
Dr. Fisher’s present address is Baylor College of Medicine, Division of Neuroscience, One Baylor Plaza, Houston, TX 77030-3498.
REFERENCES
- 1.Angelotti TP, Uhler MD, Macdonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaf SY, Chung S-H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 3.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 4.Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant γ-aminobutyric acidA receptor isoforms containing the α5 subunit subtype. Mol Pharmacol. 1996;50:119–127. [PubMed] [Google Scholar]
- 5.Carpenter DO. The public health significance of metal neurotoxicity. Cell Mol Neurobiol. 1994;14:591–597. doi: 10.1007/BF02088670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celentano JJ, Gyenes M, Gibbs TT, Farb DH. Negative modulation of the γ-aminobutyric acid response by extracellular zinc. Mol Pharmacol. 1991;40:766–773. [PubMed] [Google Scholar]
- 7.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 9.Draguhn A, Verdoorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JL, Zhang J, Macdonald RL. The role of α1 and α6 subtype amino-terminal domains in allosteric regulation of γ-aminobutyric acida receptors. Mol Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs JW, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 12.Greenfield LJ, Jr, Macdonald RL. Whole cell and single channel α1, β1, γ2s GABAA receptor currents elicited by a “multipuffer” drug application device. Pflügers Arch. 1996;432:1080–1090. doi: 10.1007/s004240050238. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield LJ, Jr, Sun F, Neelands TR, Burgard EC, Donnelly JL, Macdonald RL. Expression of functional GABAA receptors in the transfected L929 cells isolated by immunomagnetic bead separation. Neuropharmacology. 1997;36:63–73. doi: 10.1016/s0028-3908(96)00150-5. [DOI] [PubMed] [Google Scholar]
- 14.Hartter DE, Barnea A. Evidence for release of copper in the brain: depolarization-induced release of newly taken-up 67copper. Synapse. 1988;2:412–415. doi: 10.1002/syn.890020408. [DOI] [PubMed] [Google Scholar]
- 15.Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- 16.Huggenvik JI, Collard MW, Stofko RE, Seasholtz AF, Uhler MD. Regulation of the human enkephalin promoter by two isoforms of the catalytic subunit of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase. Mol Endocrinol. 1991;5:921–930. doi: 10.1210/mend-5-7-921. [DOI] [PubMed] [Google Scholar]
- 17.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardos J, Kovács I, Hajós F, Kálmán M, Simony M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- 19.Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4β2 γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 20.Kumamoto E, Murata Y. Characterization of GABA current in rat septal cholinergic neurons in culture and its modulation by metal cations. J Neurophysiol. 1995;74:2012–2027. doi: 10.1152/jn.1995.74.5.2012. [DOI] [PubMed] [Google Scholar]
- 21.Kume A, Sakurai SY, Albin RL. Zinc inhibition of t[3H]butylbicycloorthobenzoate binding to the GABAA receptor complex. J Neurochem. 1994;62:602–607. doi: 10.1046/j.1471-4159.1994.62020602.x. [DOI] [PubMed] [Google Scholar]
- 22.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurie DJ, Wisden W, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legendre P, Westbrook GL. Noncompetitive inhibition of γ-aminobutyric acidA channels by Zn. Mol Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- 25.Ma JY, Narahashi T. Differential modulation of GABAA receptor-channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Res. 1993;607:222–232. doi: 10.1016/0006-8993(93)91510-y. [DOI] [PubMed] [Google Scholar]
- 26.Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. GABA receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cell Mol Neurobiol. 1994;14:599–622. doi: 10.1007/BF02088671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 29.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 30.Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol (Lond) 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart TG, Constanti A. Differential effect of zinc on the vertebrate GABAA-receptor complex. Br J Pharmacol. 1990;99:643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trombley PQ, Shepherd GM. Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J Neurophysiol. 1996;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- 33.Tyndale RF, Olsen RW, Tobin AJ. GABAA receptors. In: North RA, editor. Ligand- and voltage-gated ion channels. CRC; Boca Raton, FL: 1995. pp. 265–290. [Google Scholar]
- 34.Wang T-L, Hackam A, Guggino WB, Cutting GR. A single His residue is essential for zinc inhibition of GABA ρ1 receptors. J Neurosci. 1995;15:7684–7691. doi: 10.1523/JNEUROSCI.15-11-07684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 36.Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O’Donnell R, Rigby MR, Sirinathsinghji DJS, Marshall G, Thompson SA, Wafford KA. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. J Neurosci. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wooltorton JRA, McDonald BJ, Moss SJ, Smart TG. Identification of a Zn2+ binding site on the murine GABAA receptor complex: dependence on the second transmembrane domain of β subunits. J Physiol (Lond) 1997;505:633–640. doi: 10.1111/j.1469-7793.1997.633ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie X, Smart TG. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349:521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Akabas MH. Identification of channel-lining residues in the M2 membrane-spanning segment of the GABAA receptor α1 subunit. J Gen Physiol. 1996;107:195–205. doi: 10.1085/jgp.107.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]