Abstract
Studies on animal models of stress, anxiety, aggression, and sensorimotor gating have linked specific monoamine neurotransmitter abnormalities to the cognitive and behavioral disturbances associated with many affective neuropsychiatric disorders. Although α2-adrenoceptors (α2-ARs) have been suggested to have a modulatory role in these disorders, the specific roles of each α2-AR subtype (α2A, α2B, and α2C) are largely unknown. The restricted availability of relevant animal models and the lack of subtype-selective α2-AR drugs have precluded detailed studies in this area. Therefore, transgenic mice were used to study the possible role of the α2C-AR subtype in two well established behavioral paradigms: prepulse inhibition (PPI) of the startle reflex and isolation-induced aggression. The α2C-AR-altered mice appear grossly normal, but subtle changes have been observed in their brain dopamine (DA) and serotonin (5-HT) metabolism. In this study, the mice with targeted inactivation of the gene encoding α2C-ARs (α2C-KO) had enhanced startle responses, diminished PPI, and shortened attack latency in the isolation–aggression test, whereas tissue-specific overexpression of α2C-ARs (α2C-OE) was associated with opposite effects. Correlation analyses suggested that both the magnitude of the startle response and its relative PPI (PPI%) were modulated by the mutations. In addition, the differences in PPI, observed between drug-naive α2C-OE mice and their wild-type controls, were abolished by treatment with a subtype nonselective α2-agonist and antagonist. Thus, drugs acting via α2C-ARs might have therapeutic value in disorders associated with enhanced startle responses and sensorimotor gating deficits, such as schizophrenia, attention deficit disorder, post-traumatic stress disorder, and drug withdrawal.
Keywords: α2C-adrenoceptor, gene-targeting, isolation-induced aggression, schizophrenia, sensorimotor gating, startle, transgenic mice
The α2-adrenoceptors (α2-ARs), which include three subtypes (α2A, α2B, and α2C) encoded by three genes, mediate many of the CNS effects of norepinephrine (NE) and regulate the release of NE, but also dopamine (DA), serotonin (5-HT), and other brain neurotransmitters (Ruffolo et al., 1993; MacDonald et al., 1997). The expression of α2C-AR is distinct; it is restricted mainly to the CNS, being prominent in the striatum and hippocampus (Nicholas et al., 1996;MacDonald et al., 1997). The α2-ARs are known to have modulatory roles in various neuropsychiatric disorders, such as schizophrenia, post-traumatic stress disorder, depression, and various cognitive abnormalities (Hornykiewicz, 1982; Coull, 1994; Nutt, 1994;Ahmed and Takeshita, 1996; Arnsten et al., 1996), but the significance of the α2-ARs, especially the role of each α2-AR subtype in these disorders, is poorly known. However, the most evident pharmacological effects attributed to CNS α2-ARs, i.e., sedation, hypotension, hypothermia, and analgesia, appear to be mediated via α2A-ARs, whereas the role of α2C-ARs has remained obscure (MacMillan et al., 1996; Hunter et al., 1997; Lakhlani et al., 1997; MacDonald et al., 1997; Stone et al., 1997).
The startle reflex is a short-latency response of the skeletal musculature elicited by a sudden auditory stimulus (Davis et al., 1982;Yeomans and Frankland, 1995). The startle reflex can be modulated by fear, stress, and other negative affective states (Davis, 1989; Howard and Ford, 1992), and by different types of immediately preceding stimuli. Prepulse inhibition (PPI), i.e., the attenuation of the startle reflex response produced by a prepulse, is an important measure of sensorimotor gating (Geyer et al., 1990). Like the startle reflex itself, PPI is also a cross-species phenomenon. Deficits in PPI are observed in schizophrenic patients (Braff et al., 1978), and deficits in PPI can be induced in the rat with administrations of various schizophrenomimetics, such as d-amphetamine (d-Amph), 2,5-dimethoxy-4-iodoamphetamine (DOI), and phencyclidine (PCP), and after social isolation (Geyer et al., 1993;Bakshi et al., 1994; Swerdlow et al., 1994; Varty and Higgins, 1995). Disrupted PPI can be normalized in rats by antipsychotics (Swerdlow et al., 1994), and the PPI model is used in the development of new CNS drugs.
Because subtype-selective α2-AR agonists or antagonists are not available, we have investigated the role of the α2C-AR in the startle reflex and its PPI in two genetically engineered mouse strains, one with targeted inactivation of the α2C-AR gene (α2C-KO) and the other with tissue-specific overexpression of α2C-ARs (α2C-OE) (Link et al., 1995; Sallinen et al., 1997). Because these mice have subtle alterations in their brain DA and 5-HT metabolism that are specifically associated with the mutations, the startle and PPI responses were first determined from drug-naive mice with or without preceding isolation, and after d-Amph and PCP. In addition, the effects of subtype-nonselective α2-AR drugs on startle and PPI were studied in α2C-OE mice and their wild-type controls. Finally, because social isolation and changes in brain 5-HT metabolism are also known to affect aggressive behavior, the mice were tested in the isolation-induced aggression paradigm.
MATERIALS AND METHODS
Animals. A total of 449 8- to 17-week-old male mice were used; 64 mice from the BALB/c strain (BOM, Bomholtgård, Denmark) were used as targets in isolation–aggression tests. All other mice were from the breeding colony maintained in the Central Laboratory Animal Facility of the University of Turku, Finland. They represented two strains of genetically engineered mice and their wild-type controls, either littermates or those closely related to the mutants. The mutations were generated at Stanford University (Stanford, CA). One mutant strain had a targeted disruption of the α2C-AR gene (α2C-KO), and the other had tissue-specific overexpression of α2C-ARs (α2C-OE). The wild-type controls are designated as α2C-KO-wt and α2C-OE-wt, respectively.
The generation of both mutant strains has been described previously (Link et al., 1995; Sallinen et al., 1997). Briefly, the α2C-AR gene was inactivated in 129/Sv embryonic stem cells (Nagy et al., 1993), which were injected into C57BL/6J blastocysts, and the resulting chimeric mice were bred to F1 (C57BL/6J × DBA/2J) animals. These animals were back-crossed for several generations to C57BL/6J mice and then intercrossed, and the tested α2C-KO mice were offspring of closely related F11–12 pairs, which were combinations of mice with wild-type, heterozygous, or homozygous genotypes for the α2C-AR mutation. All tested α2C-KO mice were homozygous for the mutation. The germline transmission of the mutation was monitored from mouse tail biopsies by Southern (DNA) analysis.
The α2C-OE mice were generated by pronuclear microinjection to one-cell fertilized eggs from the FVB/N strain and are thus congenic. A tyrosinase minigene construct was coinjected for visual identification of the transgenic progeny on the basis of coat color (Overbeek et al., 1991). The tested α2C-OE mice were heterozygous. According to brain in situ mRNA hybridization results and receptor autoradiograms, the overexpression of α2C-ARs is approximately threefold in α2C-OE mice in areas that normally express the receptor (Sallinen et al., 1997). Correspondingly, α2C-AR binding is absent in the brains of α2C-KO mice (Link et al., 1995).
The mice of both mutant strains are viable and fertile and appear grossly normal, and their diurnal patterns of locomotor activity do not differ from their wild-type littermates, although the background strains show different behavior. Compared with α2C-KO-wt mice, α2C-OE-wt mice have been shown to be more active, and the potency of α2-AR agonists to induce locomotor inhibition is different between these strains of wild-type mice but not between respective mutant and wild-type mice. However, both mutant strains have subtle alterations in brain monoamine metabolism that are specifically associated with the mutations (Sallinen et al., 1997).
The mice were housed in groups of 7–10 in standard polypropylene cages (38 × 22 × 15 cm) at 22 ± 1°C and kept on a 12 hr light/dark cycle with light onset at 6 A.M. Some of the tested mice, however, were isolated for the experiments at the age of 6–10 weeks; these mice were subsequently housed alone in smaller cages (22 × 16 × 13 cm). Their bedding was changed once a week, but otherwise they were not disturbed. Experiments were conducted between 8:30 A.M. and 4 P.M.
The animal care was in accordance with the regulations of the International Council for Laboratory Animal Science, and the experiments had approval of the local committee for laboratory animal welfare.
Drugs. The following drugs were used: dexmedetomidine hydrochloride (3 or 10 μg/kg) (Orion Corporation, Orion Pharma, Turku, Finland), atipamezole hydrochloride (100 μg/kg) (Orion Pharma), d-amphetamine sulfate (0.25, 0.5, or 1.0 mg/kg) (Sigma, St. Louis, MO), and phencyclidine hydrochloride (0.3, 1.0, or 3.0 mg/kg) (RBI, Natick, MA). All drugs were dissolved in distilled water, and the injection volume was 5 ml/kg (s.c.).
Startle apparatus and experimental design. The startle responses were measured with four identical ventilated and illuminated startle chambers [39 × 38 × 58 cm (length × width × height)] (SR-LAB system, San Diego Instruments, San Diego, CA). Each chamber consisted of a Plexiglas cylinder (3.9 cm in diameter) mounted on a removable frame on a base unit. Movement of the mouse within the cylinder was detected by a piezoelectric accelerometer attached below the frame. A loudspeaker (Radio Shack Supertweeter, San Diego, CA) mounted 25 cm above the cylinder, provided the background white noise and the acoustic stimuli. Presentation of acoustic stimuli and the piezoelectric responses from the accelerometer were controlled and digitized by the SR-LAB software and interface system. Sensitivity of the chambers was adjusted at average readings of 250 using the standardization unit from San Diego Instruments. Sound levels within each chamber were measured repeatedly using the A weighting scale (Radio Shack Sound Level Meter, Fort Worth, TX) and were found to remain constant.
At the beginning of each startle session, the mice were placed in the startle chambers and exposed to 5 min of 72 dB background noise, which continued for the remainder of the session. The PULSE intensity was 118 dB, and the PREPULSE intensity was 3, 6, 9, or 15 dB above the 72 dB background level. The duration of both prepulses and pulses was 40 msec, and the prepulse–pulse interval was 100 msec. The allocation of different trial types and the intertrial intervals (7–30 sec) was pseudorandomized and kept unchanged within a study. The startle amplitudes from PULSE ALONE, PREPULSE ALONE, and PREPULSE + PULSE stimuli were determined by averaging 100 readings of 1 msec each taken from the beginning of the PULSE stimulus onset in PULSE and PREPULSE + PULSE trials, or from the beginning of the PREPULSE in PREPULSE ALONE trials.
Three startle experiments were conducted with separate groups of mice. In the first experiment, 73 male α2C-OE and 68 male α2C-OE-wt mice were divided randomly into four groups and administered either the subtype nonselective α2-agonist dexmedetomidine hydrochloride (Dex) (3 or 10 μg/kg), the subtype nonselective α2-antagonist atipamezole (Ati) (100 μg/kg), or vehicle (distilled water) subcutaneously 20 min before the test session (Haapalinna et al., 1997). In this first drug challenge, the startle session consisted of 30 trials with 10 PULSE ALONE, 10 PREPULSE + PULSE, and 10 PREPULSE ALONE stimuli during a period of 11 min, and the prepulse intensity was set at 15 dB above background. These mice were not used in subsequent experiments.
In the second experiment, groups of α2C-KO (n = 49), α2C-KO-wt (n = 49), α2C-OE (n = 42), and α2C-OE-wt (n = 40) mice underwent three separate startle challenges as follows. In the first step, drug-naive mice were exposed to a 5 min startle session with 10 PULSE and 6 PREPULSE + PULSE trials, with prepulses 9 dB above background to determine the average baseline startle and PPI levels of each genotype group. Results from this experiment were then used to establish four matched treatment groups within each genotype group according to their responses to PULSE ALONE stimuli. In the second step on the next day, the same mice were given 0.25, 0.5, 1.0 mg/kg of d-Amph or vehicle 30 min before a startle session, which consisted of 30 PULSE ALONE, 10 PREPULSE ALONE (15 dB above background), and 40 PREPULSE + PULSE trials (the prepulse level was 3, 6, 9, or 15 dB above background, 10 repetitions of each); these 80 stimuli were presented during a period of 18 min. In the third step, after at least 10 d, the same 18 min startle session was presented to the same mice starting 30 min after PCP injections (0.3, 1.0, or 3.0 mg/kg or vehicle). In this third challenge, the mice were rearranged to new matched treatment groups according to their initial startle responses: those mice that had received the same d-Amph doses in the previous experiment were put into different dose groups.
In the third startle experiment, the effect of isolation on startle responses and PPI was investigated in individually housed mice (see below) that had been studied once in the isolation–aggression test at least 1 week earlier. These mice were exposed to the same 5 min startle session that was used in the study with drug-naive group-housed mice in the first step of the second experiment.
Isolation–aggression tests. A total of 64 drug-naive male mice (17 α2C-KO, 18 α2C-KO-wt, 14 α2C-OE, and 15 α2C-OE-wt) were analyzed for aggression after 6 weeks of isolation. On the basis of our preliminary tests, the aggressiveness of α2C-OE and α2C-OE-wt mice remaining in their home cages after isolation was extremely high, and the rating of attacks was difficult because of almost continuous fighting. To decrease the aggression level and to increase the sensitivity of the experiment, the tests were subsequently performed in a fresh cage identical to the home cages. Each isolated mouse was put into the test cage 1 min before the target (BALB/c) mouse. The attack latency and number of attacks on the target mouse were measured during a 10 min session, starting from the introduction of the target mouse. An attack was given a score of one point when the isolated mouse bit the target mouse. If fighting continued for at least 10 sec, the same attack was scored as 2 points. The mice were analyzed for aggression only once. The observer was unaware of the expected results, but α2C-OE and α2C-OE-wt mice have different coat color and were tested first. α2C-KO and α2C-KO-wt mice could not be identified by visual inspection, and the observer was unaware of the randomized order of the mutant and wild-type mice.
Data analysis. The results are presented as mean ± SEM. Statistical analysis was performed using STATISTICA 4.5 computer software (StatSoft, Tulsa, OK). ANOVA for repeated measurements showed that statistically significant habituation effects were occasionally present only in the second and third step of the second startle experiment (d-Amph and PCP challenges). These effects, however, were minor: reductions of the startle amplitude in the course of the session were between 10 and 20% in the vehicle group. In addition, alterations in responses during a session could have arisen from differences in drug action, in habituation, or in their interaction. Therefore, and because the startle sessions were not designed to explore habituation, the habituation effects were not evaluated further. The startle responses of each animal were summed to give one integrated startle response value for each animal and trial type; these were then used in subsequent analyses. The PREPULSE ALONE responses were barely measurable and were omitted from further analyses.
Pearson product-moment correlation analysis was performed for each genotype group of drug-naive mice to assess the relationship between the startle response magnitude and PPI. The extent of PPI is usually determined as PPI%, according to the formula [100 − (mean startle amplitude on PREPULSE + PULSE − trials/mean startle amplitude on PULSE ALONE trials) × 100]. Because both the startle amplitudes and the PPI% appeared to be altered by the mutations, and because startle magnitude after PULSE ALONE is used in the denominator of PPI%, the relationship between the startle reflex and PPI phenomena was examined further. The correlation coefficients were calculated for relationships between absolute startle magnitudes after PULSE ALONE and PREPULSE + PULSE as well as the absolute PPI (= startle magnitude after PULSE ALONE − startle magnitude after PREPULSE + PULSE). It should also be stressed that the wild-type control mice representing two different background strains had different startle and PPI levels, as expected (Logue et al., 1997; Paylor and Crawley, 1997), and that statistical comparisons were made only between the appropriate mutant and wild-type groups. Before the calculation of linear correlation, the data were plotted on a scatter plot to detect possible nonlinear associations; such associations were not found.
Results from isolation–aggression tests and the startle experiments with drug-naive mice were analyzed with Mann–Whitney Utests between mutant and respective wild-type groups, because startle responses and attack latencies were not normally distributed. However, nonparametric statistical methods for multivariate analysis are not available, and results from drug challenges were thus analyzed with parametric statistics using two- or three-way ANOVA. Three-way ANOVA for repeated measurements revealed that in the second experiment, in which multiple prepulse intensities (3, 6, 9, and 15 dB above background) were used, the PPI was dependent on prepulse intensity, as expected (prepulse intensity × PPI% interaction:p < 0.0001). However, the different prepulse intensities had no effect on the differences between drug doses or genotypes. Therefore, for clarity, only the PPI results after the 9 dB above background prepulses are shown from these experiments.
RESULTS
Startle responses and PPI in drug-naive mice with altered α2C-adrenoceptor expression
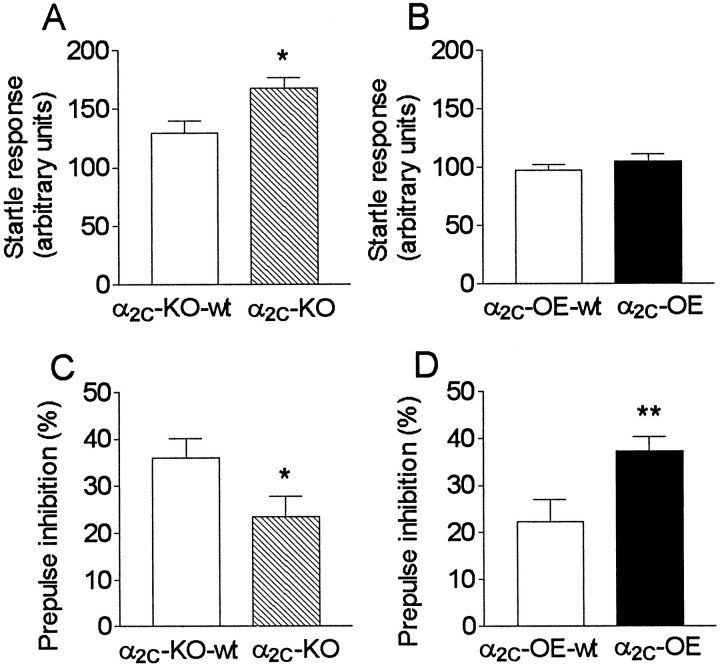
The average startle amplitudes to PULSE ALONE stimuli were greater in α2C-KO mice compared with α2C-KO-wt mice (Z = 2.1; p = 0.034), whereas α2C-OE and α2C-OE-wt mice had similar startle responses (Fig.1A,B). The prepulses inhibited startle responses less in α2C-KO mice (Z = 2.6; p = 0.011) and more in α2C-OE mice (Z = 3.0; p = 0.0028), when compared with α2C-KO-wt and α2C-OE-wt mice (Fig. 1C,D).
Fig. 1.
A–D, Startle responses without prepulses (A, B) and prepulse inhibition (PPI) of the startle reflex (C, D) in drug-naive mice with altered α2C-AR expression. Data are presented as mean ± SEM (n = 42–49). The mean startle response amplitude of mice with targeted disruption of the α2C-AR (α2C-KO) (hatched bars) were higher than in their respective wild-type control group (open bars). In addition, α2C-KO mice had diminished PPI, and mice overexpressing the α2C-ARs (α2C-OE mice) (closed bars) had increased PPI compared with respective wild-type controls (Mann–Whitney U test). *p < 0.05; **p < 0.01.
Effects of subtype nonselective α2-adrenoceptor drugs on startle responses and PPI
The specific, subtype nonselective α2-antagonist Ati increased the startle response in α2C-OE and α2C-OE-wt mice compared with respective vehicle-treated mice (mean change: +22%; two-way ANOVA, dose effect:F(1,67) = 5.0; p = 0.028) (Fig.2A). A low dose of the subtype nonselective α2-AR agonist Dex also slightly increased the startle response (+24%; F(1,65) = 6.0; p = 0.017), but a larger, slightly sedative dose of Dex clearly decreased the startle response (−55%;p < 0.0001) (Fig. 2A). Although the drug effects seemed to be slightly different between the genotypes (Fig. 2A), two-way ANOVA did not indicate any significant drug × genotype interactions between α2C-OE and α2C-OE-wt mice in the startle responses.
Fig. 2.
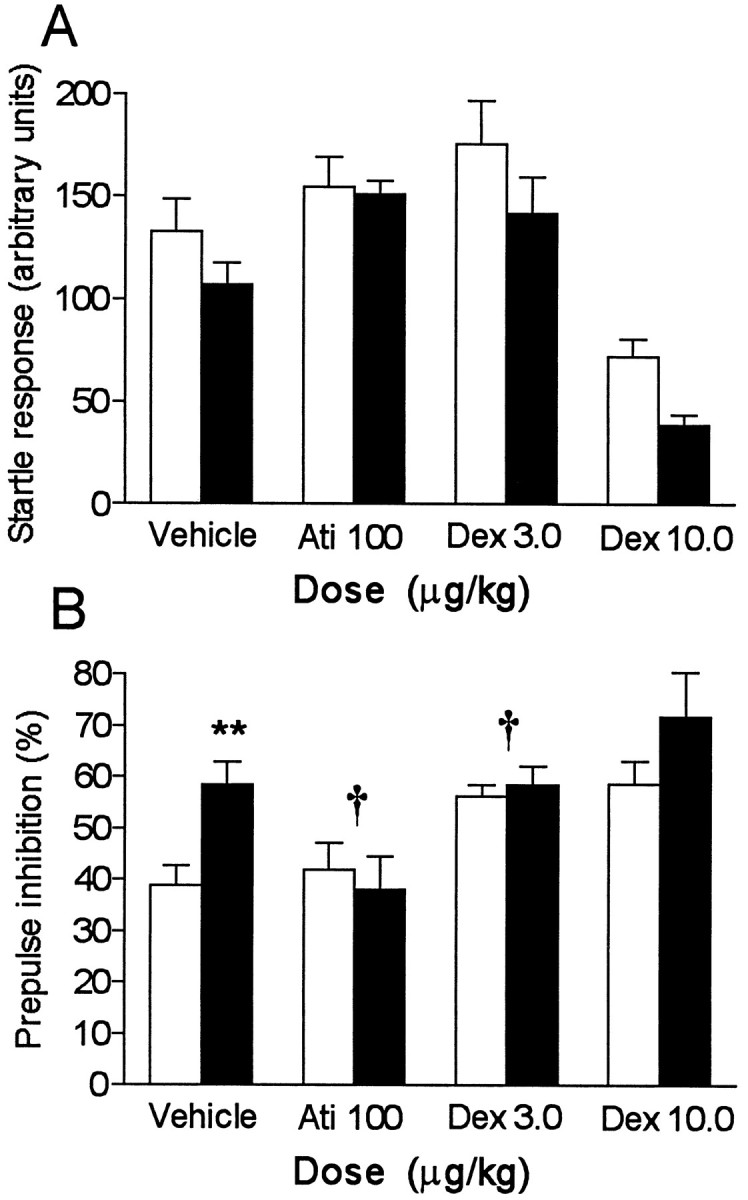
A, B, The effects (mean ± SEM; n = 14–20) of vehicle, the α2-antagonist atipamezole (Ati), and the α2-subtype-nonselective agonist dexmedetomidine (Dex) on startle responses without prepulses (A) and prepulse inhibition (PPI) (B) in mice overexpressing the α2C-ARs (filled bars) and their wild-type controls (open bars). Two-way ANOVA revealed significant genotype × dose interactions between doses of Ati 100 and vehicle and Dex 3.0 and vehicle; these are indicated as † (p < 0.05). The difference in PPI between vehicle-treated α2C-OE and α2C-OE-wt mice was also significant, which is indicated as ** (Z = 2.9; p = 0.0035; Mann–Whitney Utest).
Ati abolished the PPI differences between α2C-OE and α2C-OE-wt mice that were observed after vehicle injections and in the drug-naive mice (two-way ANOVA, drug × genotype interaction: F(1,67) = 5.5;p = 0.022) (Fig. 2B). Also the small dose of Dex (3 μg/kg) abolished the PPI difference between the genotypes, enhancing only the PPI of α2C-OE-wt mice (dose × genotype: F(1,65) = 4.8;p = 0.033). The larger dose of Dex (10 μg/kg) increased PPI in both α2C-OE and α2C-OE-wt mice (dose effect: F(1,67) = 16;p = 0.0001; dose × genotype:F(1,67) = 0.57; p = 0.44).
Effects of d-amphetamine and phencyclidine on startle responses and PPI
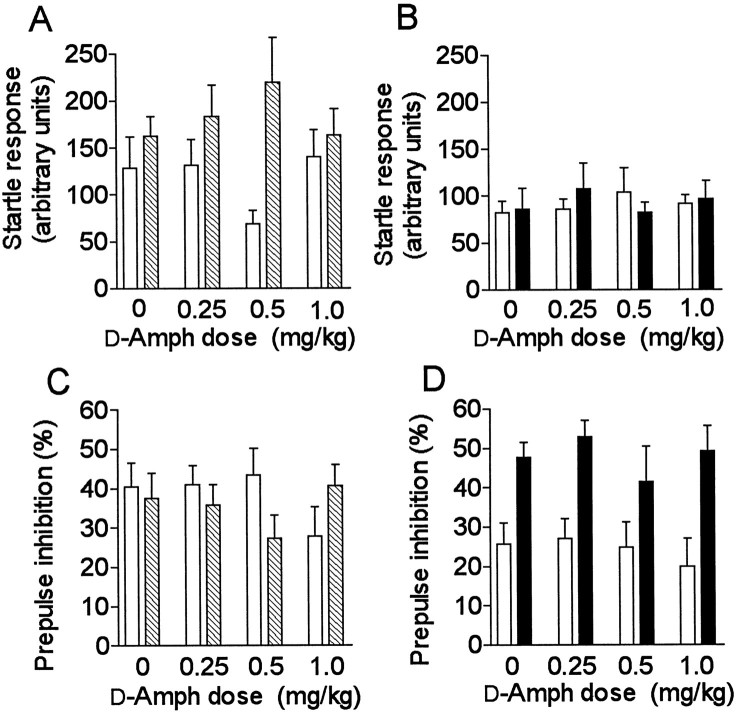
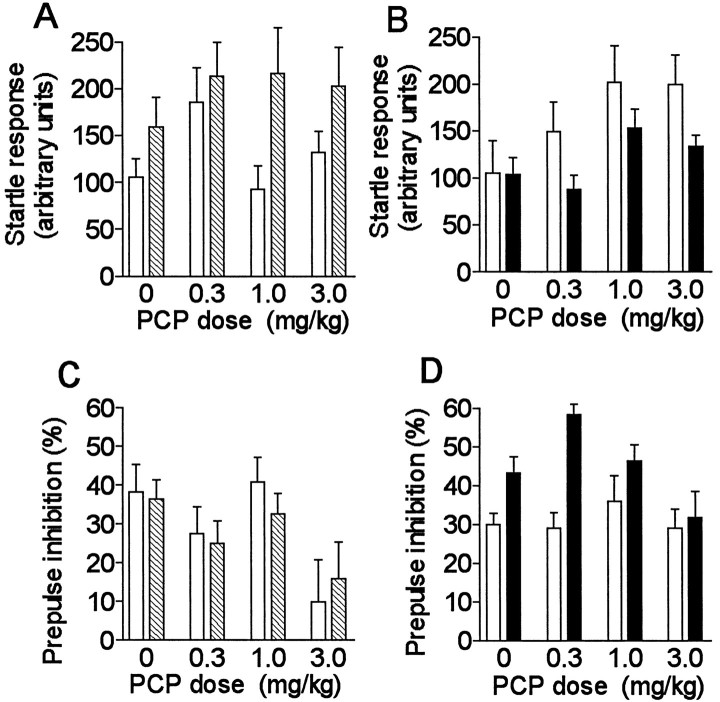
The results from d-Amph and PCP challenges are presented in Figures 3A–D and4A–D, respectively. A significant genotype difference was observed between α2C-KO and α2C-KO-wt mice in their average startle responses to pulse alone stimuli after d-Amph (F(1,90) = 9.0; p = 0.0034); the responses differed between genotypes, especially at the 0.5 mg/kg dose (Fig. 3A) (Tukey’s post hoc test:p = 0.018). Startle amplitudes were similar in α2C-OE and α2C-OE-wt mice and were not influenced by d-Amph. There was also a significant difference in startle amplitudes between α2C-KO and α2C-KO-wt mice after PCP (F(1,90)= 8.3; p = 0.0049) (Fig.4A). PCP clearly increased the startle amplitudes of α2C-OE-wt mice, but had a smaller effect in α2C-OE mice (Fig.4B) (dose effect: F(3,77) = 3.2; p = 0.027; genotype difference:F(1,77) = 5.1; p = 0.027). Interestingly, this difference in startle responses between α2C-OE mice and their wild-type controls was opposite to the difference observed between α2C-KO mice and their controls.
Fig. 3.
A–D, Startle responses (A, B) and prepulse inhibition (PPI) (C, D) in mice with altered α2C-AR expression after different doses ofd-amphetamine (d-Amph). Error bars represent mean ± SEM (n = 10–14) results from mice with targeted disruption of α2C-ARs (α2C-KO) (hatched bars), overexpression of the α2C-AR (α2C-OE) (closed bars), and corresponding wild-type mice (α2C-KO-wt or α2C-OE-wt) (open bars).
Fig. 4.
A–D, The effects of different doses of phencyclidine (PCP) on startle responses and PPI in mice with altered α2C-AR expression.Symbols are as described in Figure3A–D.
PPI was not statistically significantly different between α2C-KO and α2C-KO-wt mice afterd-Amph in overall ANOVA. PCP potently disrupted PPI in both α2C-KO and α2C-KO-wt mice (dose effect:F(3,90) = 4.9; p = 0.0035) without significant differences in responses between the genotypes (Fig. 4C).
In α2C-OE and α2C-OE-wt mice,d-Amph did not have any detectable effect on the PPI. The difference in PPI between genotypes was perhaps even more prominent than earlier without drug (Figs. 1D, 3D) (overall genotype difference: F(1,83) = 29;p < 0.0001). PCP diminished the PPI also in α2C-OE-mice, but not in α2C-OE-wt mice (dose effect: F(3,77) = 3.1; p = 0.034; dose × genotype interaction:F(3,77) = 2.8; p = 0.048) (Fig.4D).
The effect of isolation on startle and prepulse inhibition
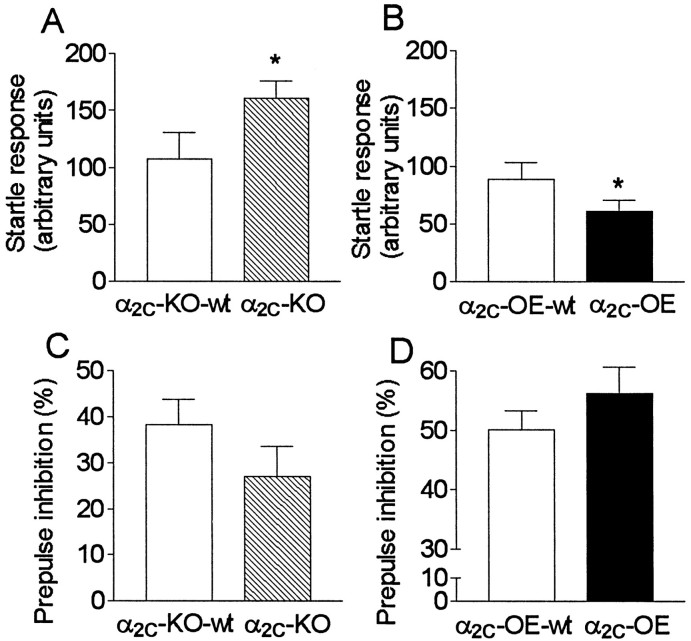
After isolation, the average startle responses to PULSE ALONE stimuli were larger in α2C-KO mice than in α2C-KO-wt mice, as observed earlier in group-housed mice (Figs. 1A,5A). The startle responses of isolated α2C-OE mice were slightly attenuated compared with isolated α2C-OE-wt mice (Fig. 5B) (Z = 2.0; p = 0.049) in contrast to group-housed α2C-OE and α2C-OE-wt mice, in which no differences between genotype groups were seen in their PULSE ALONE responses (Fig. 1B).
Fig. 5.
A–D, Prepulse inhibition (PPI) of the startle reflex in drug-naive mice with altered α2C-AR expression after 7–8 weeks of isolation (n = 14–18). Compared with group-housed mice (Fig.1A–D), isolation had no clear effect in mice with targeted disruption of the α2C-AR gene (α2C-KO) (hatched bars) or in their controls (α2C-KO-wt) (open bars), but it reduced startle responsiveness in mice overexpressing the α2C-AR (α2C-OE) (closed bars) and increased the PPI in both α2C-OE mice and in their wild-type controls (α2C-OE-wt) (open bars).
The differences in PPI, previously observed between genotypes, were now evident only as trends without significance. This probably resulted from lack of statistical power, because the differences in the average responses were similar to those observed earlier in group-housed mice; the group sizes of the studied isolated mice were considerably smaller (n = 14–18) than those of the group-housed mice (n = 40–49). Somewhat surprisingly, compared with group-housed mice, PPI was increased in both α2C-OE and α2C-OE-wt mice after isolation (51–56% vs 23–38%, respectively; two-way ANOVA, effect of isolation:F(1,117) = 32; p < 0.0001) (Figs. 1D, 5D). In addition, startle responses to PULSE ALONE stimuli were decreased by isolation (effect of isolation: F(1,117) = 6.0; p = 0.016).
Association of startle amplitude and prepulse inhibition
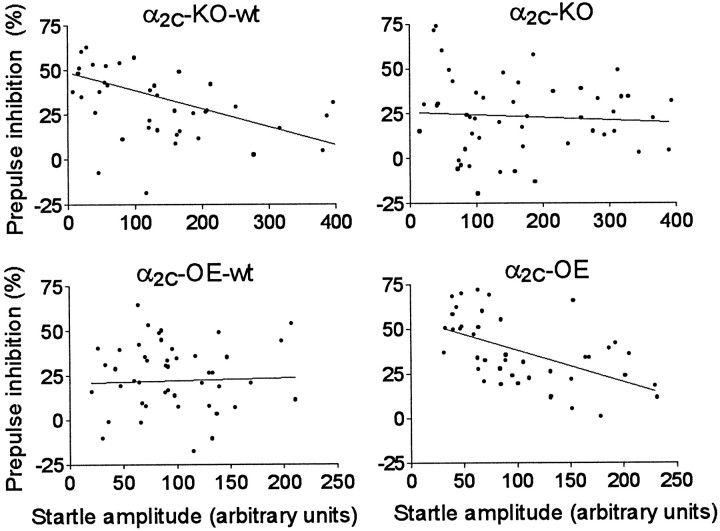
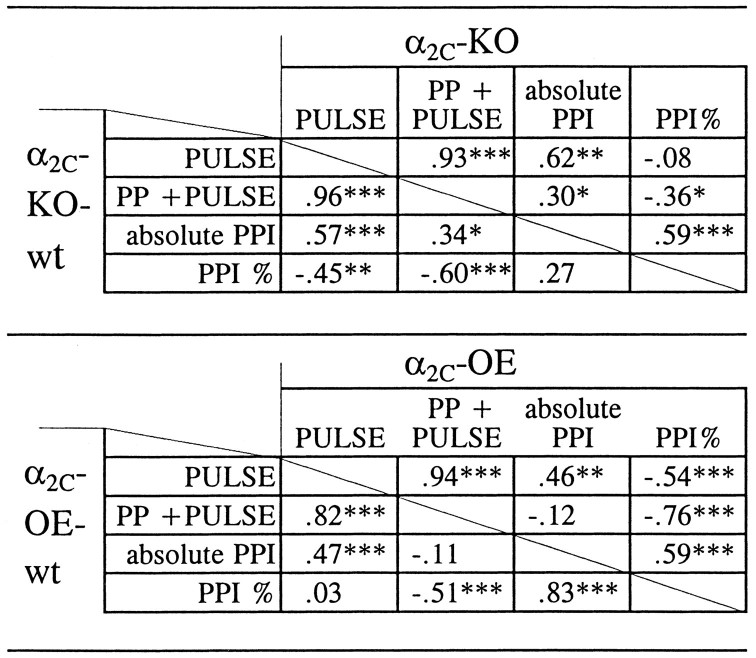
Linear regression analysis of the results from group-housed drug-naive mice revealed substantial negative correlation between individual mean startle responses to PULSE ALONE stimuli and PPI% values in the genotype groups of α2C-KO-wt (r = −0.45; p = 0.0014) and α2C-OE mice (r = −0.54;p = 0.0002), but these variables were independent of each other in the groups of α2C-KO (r = −0.078; p = 0.82) and α2C-OE-wt mice (r = + 0.028; p = 0.61) (Fig.6, Table1). The relationship between absolute startle magnitudes after PULSE ALONE and PREPULSE and PULSE trials was highly dependent (r = 0.82 in α2C-OE-wt mice and r > 0.93 in other genotype groups;p < 0.001), and a weaker but substantial positive correlation was observed also between the PULSE ALONE and absolute PPI responses (r = 0.46–0.62; p < 0.01) (Table 1).
Fig. 6.
Scatter plot of associations between individual mean startle responses to PULSE ALONE stimuli and the percentage of prepulse inhibition (PPI%) of all studied group-housed drug-naive mice from each of the four α2C-AR genotype groups. A statistically significant negative correlation was observed in groups of α2C-KO-wt and α2C-OE mice (p < 0.0014), but not in α2C-KO and α2C-OE-wt mice (p > 0.61) (Pearson product-moment analysis).
Table 1.
Correlations between individual means of startle magnitudes after PULSE alone and PREPULSE (PP) + PULSE stimuli, relative prepulse inhibition (PPI%), and the absolute prepulse inhibition (absolute PPI = startle amplitude after PULSE alone minus startle amplitude after PP + PULSE stimuli)
Correlation coefficients are shown separately for each genotype group of drug-naive mice [= mice lacking the α2C-AR (α2C-KO), mice overexpressing the α2C-AR (α2C-OE), and respective wild-type (wt) controls;n = 40–49/group]. Pearson product-moment analysis: *p <0.05; **p <0.01; ***p<0.001.
Isolation-induced aggression tests
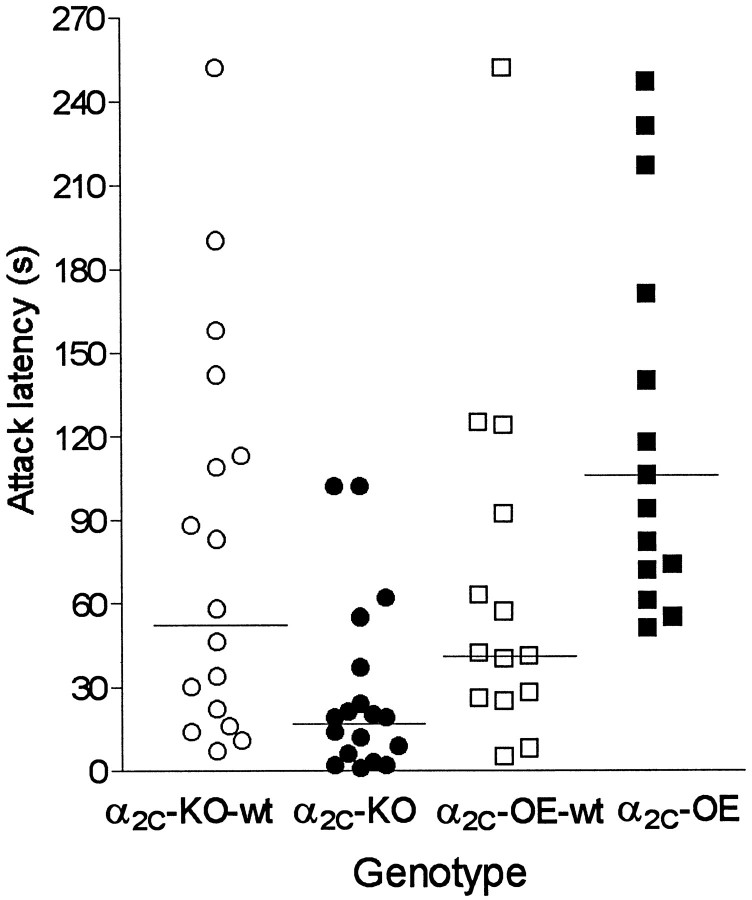
The onset of fighting was clearly dependent on the expression of α2C-ARs. α2C-AR overexpression was associated with increased latency to attack (Z = 2.7;p = 0.0078), and the lack of α2C-AR expression was associated with reduced latency to attack (Z = 2.7; p = 0.0070), when compared with the respective wild-type control mice (Fig.7). All mice attacked the BALB/c target mice within the first 4.5 min, except one α2C-OE mouse that did not attack at all (the latency was scored as 600 sec). In two cases, the BALB/c mice also attacked, but otherwise they performed only defensive behavior. The number of attacks during the 10 min observation period, however, showed no statistically significant differences between the genotype pairs (Table 2).
Fig. 7.
Isolation-induced aggression test: scatter plot of attack latencies. Horizontal lines indicate medians of each genotype group. All studied mice attacked during the first 270 sec, except one α2C-OE mouse that did not attack at all. The attack latency was shortened in α2C-KO mice and prolonged in α2C-OE mice (p < 0.01) (Mann–Whitney U test).
Table 2.
Isolation-induced aggression test
| Genotype | Latency to first attack (sec) | Number of attacks/10 min | Number of observations |
|---|---|---|---|
| α2C-KO-wt | 81 ± 17 | 16 ± 3 | 18 |
| α2C-KO | 28 ± 8* | 22 ± 3 | 17 |
| α2C-OE-wt | 66 ± 22 | 22 ± 6 | 14 |
| α2C-OE | 155 ± 21* | 21 ± 6 | 15 |
Mean ± SEM latency to first attack and number of attacks of individually housed mice with targeted disruption of the α2C-AR (α2C-KO), mice overexpressing the α2C-AR (α2C-OE), and respective wild-type mice (α2C-KO-wt; α2C-OE-wt) toward group-housed BALB/c mice. Asterisk denotes significant differences (*p <0.01; Mann–Whitney U test) between corresponding mutant and wild-type groups.
DISCUSSION
The lack of α2C-AR expression was associated with increased startle reactivity, reduced PPI, and reduced attack latency. The overexpression of α2C-AR resulted in opposite changes. These contrasting findings strongly support the significance of the observed differences and their causal relationship to altered α2C-AR expression, because the two pairs of mutant and wild-type mouse strains are independent of each other. In addition, the α2-AR agonist Dex and the antagonist Ati were capable of abolishing the PPI difference between α2C-OE and α2C-OE-wt mice. Taken together, these findings suggest that activation of α2C-ARs reduces the hyper-reactivity and impulsivity of mice, which are common features of the studied paradigms.
Current opinion generally assumes that the startle reflex amplitude and its prepulse inhibition, determined usually as PPI%, are independent variables and that startle amplitude and PPI are not necessarily correlated. In the present study, negative correlations were observed between the startle amplitude and the PPI%, i.e., the greater the reactivity to the PULSE ALONE stimuli, the smaller the relative inhibition in the startle magnitude by prepulse. These correlations were present only in those genotype groups with marked PPI efficacy (mean PPI% >35; α2C-KO-wt and α2C-OE), but not when PPI was weak (mean PPI% <24; α2C-KO and α2C-OE-wt). This suggests that the extent of PPI may be responsible for these now observed negative correlations, but it simultaneously supports the notion that α2C-ARs are involved in the modulation of both the startle reflex and its PPI. Also two recent studies with mice have explored differences in the startle and PPI of multiple inbred strains. In one study, which compared 12 different mouse strains, the average startle magnitude and PPI% were not correlated over the strains (Paylor and Crawley, 1997), whereas in another study in 20 strains a positive correlation (r = 0.57; p < 0.01) was observed between the average startle amplitude evoked by a weak auditory stimulus (90 dB) and PPI% (Logue et al., 1997). However, in these studies the correlations were analyzed using group means of the markedly different mouse strains, whereas in the present study the analyzed values were individual means of mice having similar strain backgrounds within a group, which obviously can explain, at least partly, the discrepancies. The results support the possibility that calculation of PPI% is the best simple way to control the relationships between startle magnitude and its PPI. Nevertheless, the association between startle and PPI is currently not clear, and the now observed negative correlations may raise the question of whether more sophisticated mathematical methods should be developed to explore the startle plasticity.
The interpretation of the results from d-Amph tests is limited by the failure of d-Amph to produce clear effects on startle responses or PPI% in any of the studied mouse strains. This was probably because of the mild dosage (Dulawa and Geyer, 1996). However, the startle amplitude difference between α2C-KO and α2C-KO-wt genotypes was pronounced afterd-Amph. After PCP stimulations, the startle responses of α2C-KO mice were again larger than those of their controls, whereas α2C-OE mice had smaller startle amplitudes than α2C-OE-wt mice. This suggests that the inhibitory contribution of α2C-ARs on the startle response may increase in stimulated conditions. In contrast to thed-Amph doses that were used, the PCP doses were sufficient to reduce PPI as expected. In conclusion, the stimulation experiments with d-Amph and PCP were in line with the results from drug-naive mice, but they provided only limited additional information on the mechanisms involved in the modulation of the startle response and PPI by α2C-ARs.
These results and current knowledge do not allow us to identify the probable multiple neural structures that are involved in the inhibitory role of α2C-ARs in CNS reactivity. The startle reflex itself may be partly spinally modulated by α2-ARs (Davis et al., 1989), but otherwise the mechanisms are obviously supraspinal, involving the cortico–striato–pallido–pontine circuitry (Geyer et al., 1990). According to in situ mRNA and immunohistochemical studies, α2A-ARs are expressed widely throughout the CNS and in peripheral tissues, α2B-ARs are present mainly in the periphery, and α2C-ARs have a distinct expression pattern in the brain (Nicholas et al., 1993;Scheinin et al., 1994; Nicholas et al., 1996; Rosin et al., 1996;Talley et al., 1996; Wang et al., 1996). Compared with α2A-ARs, the expression of α2C-ARs in the CNS is generally less abundant and their distribution is more concentrated into distinct structures, such as some hippocampal and cortical regions and the nucleus accumbens and other striatal nuclei, which are important structures in the pathophysiology of impaired PPI. Nevertheless, it is unclear in which types of neurons each of the α2-ARs are expressed. It is generally agreed that α2-ARs couple to adenylyl cyclases and ion channel regulation through inhibitory Gi/0-type proteins, which also supports the observed inhibitory characteristics of α2C-ARs in this study.
The results from previous aggression studies with α2-AR-subtype-nonselective drugs in rodents are inconsistent and difficult to interpret. One possible explanation for this is the complexity of adrenoceptor interactions when both pre- and postsynaptic α2-ARs are affected with drugs lacking subtype selectivity. In addition, it has been postulated that the drug injection per se may mask the possible aggression-heightening effects of small doses of α2-AR antagonists in mice (Haller et al., 1996); the genetic approach used in this study circumvents this problem. Targeted disruption of the mouse gene encoding neuronal nitric oxide synthase has recently been reported to enhance aggression without preceding isolation (Nelson et al., 1995). Of other targeted gene disruptions, those leading to lack of 5-HT1B receptors and the neuropeptide precursor pre-proenkephalin also enhance aggression, manifesting especially as shortened attack latency after isolation (Sadou et al., 1994; König et al., 1996). The mice with altered α2C-AR expression, when normally housed in groups, do not differ markedly from their wild-type cage mates in their aggressiveness. Therefore, NE and α2C-ARs may not have a specific role in the development of enhanced aggression. Rather, the isolation possibly induces a complex neural process in which α2C-ARs may subserve an inhibitory function when a strange stimulus is introduced (Bell and Hepper, 1987). On the other hand, 5-HT is known to be an important modulator of aggression; therefore, α2C-AR-dependent modulation of brain 5-HT systems may also explain these findings. Opposite to the now observed relationships between startle amplitude, PPI, and aggression is the finding that mice with targeted disruption of the 5-HT1Breceptor gene that were more aggressive than their wild-type controls (Sadou et al., 1994) had reduced startle amplitude and increased PPI (Dulawa et al., 1997). Another contradictory finding in this study was that the isolation increased or did not have an effect on PPI, whereas isolation has clearly disrupted PPI in rats (Geyer et al., 1993; Varty and Higgins, 1995). It is possible that mice and rats have significant differences in their startle and PPI responsiveness. This possibility is supported by a recent report in which the 5-HT1A agonist 8-OH-DPAT increased PPI in mice (Dulawa et al., 1997), whereas in previous studies with rats 8-OH-DPAT disrupted PPI (Rigdon and Weatherspoon, 1992; Sipes and Geyer, 1995).
Previous results from startle experiments in humans are in good agreement with the current results, because the classical subtype nonselective α2-AR agonist clonidine has been shown to reduce and the α2-AR antagonist idazoxan to facilitate the acoustic startle response (Morgan et al., 1993; Kumari et al., 1996), but the role of α2-ARs in PPI is not clear. Interestingly, clonidine has been suggested to be beneficial in the treatment of various neuropsychiatric disorders, although its clinical usefulness has not been proven (Ahmed and Takeshita, 1996). In recent studies performed with gene-targeted mice with dysfunctional α2A-ARs, the sedative and hypotensive effects of nonselective α2-AR agonists were blocked by the α2A-AR disruption (MacMillan et al., 1996; Hunter et al., 1997; Lakhlani et al., 1997), whereas the hypotensive or sedative responses to α2-AR agonists are not significantly altered in α2C-KO mice (Link et al., 1995; Sallinen et al., 1997). Thus, it is possible that the therapeutic benefit and clinical acceptance of clonidine in neuropsychiatric disorders might have been restricted by the adverse effects of hypotension and sedation, which seem to be mediated solely by α2A-ARs. Furthermore, some of the atypical neuroleptics, such as clozapine and risperidone, have been reported to have moderate affinity to α2-ARsin vivo and especially to the human α2C-AR subtype in vitro (Schotte et al., 1996). In light of the present results, it would be interesting to compare the functional effects of various antipsychotics at different α2-AR subtypes, and to screen for polymorphisms of the α2C-AR gene among various psychiatric patients.
In summary, α2C-ARs modulate the startle reflex and its prepulse inhibition and aggressive behavior in mice. Because symptomatology related to the now studied paradigms is present in several patient categories, it is possible that yet to be developed subtype-selective α2C-AR drugs might have therapeutic value in the treatment of schizophrenia and also other neuropsychiatric disorders with pathological hyper-reactivity and affective disturbances, such as schizophrenia, attention deficit hyperactivity disorder, post-traumatic stress disorder, and drug withdrawal symptoms.
Footnotes
We thank Dr. Richard Link for generating the strains of mice used in these studies, and Ms. Päivi Saikkonen for skillful technical assistance.
Correspondence should be addressed to Dr. Jukka Sallinen, Department of Pharmacology and Clinical Pharmacology, University of Turku, Kiinamyllynkatu 10, FIN-20520 Turku, Finland.
REFERENCES
- 1.Ahmed I, Takeshita J. Clonidine: a critical review of its role in the treatment of psychiatric disorders. CNS Drugs. 1996;6:53–70. [Google Scholar]
- 2.Arnsten AFT, Steere JC, Hunt RD. The contribution of α2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 3.Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- 4.Bell R, Hepper PG. Catecholamines and aggression in animals. Behav Brain Res. 1987;23:1–21. doi: 10.1016/0166-4328(87)90238-5. [DOI] [PubMed] [Google Scholar]
- 5.Braff D, Grillon C, Gallaway E, Geyer M, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 6.Coull JT. Pharmacological manipulations of the α2-noradrenergic system: effects on cognition. Drugs Aging. 1994;5:116–126. doi: 10.2165/00002512-199405020-00005. [DOI] [PubMed] [Google Scholar]
- 7.Davis M. Neural systems involved in fear-potentiated startle. Ann NY Acad Sci. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis M, Commissaris RL, Yang S, Wagner KR, Kehne JH, Cassella JV, Boulis NM. Spinal vs. supraspinal sites of action of the α2-adrenergic agonists clonidine and ST-91 on the acoustic startle reflex. Pharmacol Biochem Behav. 1989;33:233–240. doi: 10.1016/0091-3057(89)90455-3. [DOI] [PubMed] [Google Scholar]
- 10.Dulawa SC, Geyer MA. Psychopharmacology of prepulse inhibition in mice. Clin J Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- 11.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology. 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 12.Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 13.Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- 14.Haapalinna A, Viitamaa T, MacDonald E, Savola J-M, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the effects of a specific α2-adrenoceptor antagonist, atipamezole, on α1-and α2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:570–582. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- 15.Haller J, Makara GB, Kovács JL. The effect of α2 adrenoceptor blockers on aggressive behavior in mice: implications for the actions of adrenoceptor agents. Psychopharmacology. 1996;126:345–350. doi: 10.1007/BF02247386. [DOI] [PubMed] [Google Scholar]
- 16.Hornykiewicz O. Brain catecholamines in schizophrenia: a good case for noradrenaline. Nature. 1982;299:484–486. doi: 10.1038/299484a0. [DOI] [PubMed] [Google Scholar]
- 17.Howard R, Ford R. From the jumping Frenchmen of Maine to post-traumatic stress disorder: the startle response in neuropsychiatry. Psychol Med. 1992;22:695–707. doi: 10.1017/s0033291700038137. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Kassotakis L, Lewis R, Eglen RM. The relative contribution of α2-adrenoceptor subtypes to the antinociceptive action of dexmedetomidine and clonidine in rodent models of acute and chronic pain. Br J Pharmacol. 1997;120:229P. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.König M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–537. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 20.Kumari V, Cotter P, Corr PJ, Gray JA, Checkley SA. Effect of clonidine on the human acoustic startle reflex. Psychopharmacology. 1996;123:353–360. doi: 10.1007/BF02246646. [DOI] [PubMed] [Google Scholar]
- 21.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant α2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci USA. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link RE, Stevens MS, Kulatunga M, Scheinin M, Barsh GS, Kobilka BK. Targeted inactivation of the gene encoding the mouse α2C-adrenoceptor homolog. Mol Pharmacol. 1995;48:48–55. [PubMed] [Google Scholar]
- 23.Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald E, Kobilka BK, Scheinin M. Gene targeting: homing in on α2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 25.MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the α2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 26.Morgan CA, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine: facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110:342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- 27.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson RJ, Demas GE, Huang PL, Fishman LC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas AP, Pieribone V, Hökfelt T. Distributions of mRNA for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- 30.Nicholas AP, Hökfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- 31.Nutt DJ. Putting the “A” in atypical: does α2-adrenoceptor antagonism account for the therapeutic advantage of new antipsychotics? J Psychopharmacol. 1994;8:193–195. doi: 10.1177/026988119400800401. [DOI] [PubMed] [Google Scholar]
- 32.Overbeek PA, Aguilar-Cordova E, Hanten G, Schaffner DL, Patel P, Lebovitz RM, Lieberman MW. Coinjection strategy for visual identification of transgenic mice. Transgenic Res. 1991;1:31–37. doi: 10.1007/BF02512994. [DOI] [PubMed] [Google Scholar]
- 33.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 34.Rigdon GC, Weatherspoon J. 5HT1A receptor agonists block prepulse inhibition of the acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- 35.Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Ruffolo RRJ, Nichols AJ, Stadel JM, Hieble PJ. Pharmacologic and therapeutic applications of α2-adrenoceptor subtypes. Annu Rev Pharmacol Toxicol. 1993;32:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- 37.Sadou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 38.Sallinen J, Link RE, Haapalinna A, Viitamaa T, Kulatunga M, Sjöholm B, MacDonald E, Pelto-Huikko M, Leino T, Barsh GS, Kobilka BK, Scheinin M. Genetic alteration of α2C-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective α2-adrenoceptor agonist. Mol Pharmacol. 1997;51:36–46. doi: 10.1124/mol.51.1.36. [DOI] [PubMed] [Google Scholar]
- 39.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 40.Schotte A, Janssen PFM, Gommeren W, Luyten WHML, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 41.Sipes TA, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+) WAY 100,135 and localization of site of action. Psychopharmacology. 1995;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- 42.Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 44.Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, MacMillan LB, Fremeau RT, Jr, Magnuson MA, Lindner J, Limbird LE. Expression of α2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the α2AAR-receptor gene in transgenic animals. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- 47.Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]