Abstract
Neurite outgrowth is a central aspect of the ontogenetic formation of neural networks and is regulated by distinct groups of cell surface molecules. One protein involved in neurite elongation and fasciculation is the neural Ig superfamily member F11/contactin. We have shown previously that F11 promotes neurite extension of chick tectal neurons by interaction with the tectal receptor NrCAM, a member of the L1 subgroup of the Ig superfamily. By contrast, it does not induce outgrowth of retinal neurons despite the fact that these cells also express NrCAM, suggesting that in retinal cells the F11–NrCAM interaction alone is not sufficient to induce neurite extension. In this report we present a novel image analysis procedure to quantify neurite outgrowth and use it to demonstrate that F11 enhances the fibronectin-induced outgrowth response of embryonic retinal neurons. We reveal that NrCAM is the neuronal receptor mediating the enhanced outgrowth of retinal neurons, whereas the related F11-binding molecule NgCAM is not involved. Furthermore, we provide evidence that a β1-integrin may represent the fibronectin-dependent receptor that cooperates indirectly with the F11–NrCAM pathway. Our results support the concept of a combinatorial labeling of cells in nervous system histogenesis by different classes of cell surface proteins, in particular by integrins and molecules of the Ig superfamily.
Keywords: retina, neurite growth, neural development, Ig superfamily, integrins, F11, NrCAM
Outgrowth of neurites in nervous system ontogenesis can be divided into at least two aspects, pathfinding of elongating axons on their way to their target regions and formation of the dendritic tree that is typical for a particular neuron. In the last decade several classes of ligands and receptors have been described to regulate neurite outgrowth and axon guidance (Dodd and Schuchardt, 1995; Keynes and Cook, 1995; Goodman, 1996). On the one hand, the ligands have been categorized, on the basis of their mode of action, in long- and short-range cues, which may have repulsive or attractive effects (Culotti and Kolodkin, 1996; Tessier-Lavigne and Goodman, 1996; Wadsworth and Hedgecock, 1996). On the other hand, receptors known so far have been grouped according to structural properties in integrins, cadherins, members of the Ig superfamily (IgSF), receptor protein tyrosine kinases, and phosphatases (Reichardt and Tomaselli, 1991; Bixby, 1992; Sonderegger and Rathjen, 1992;Doherty and Walsh, 1994; Schachner and Martini, 1995; Brümmendorf and Rathjen, 1996; Chien, 1996; Friedman and O’Leary, 1996; Hall et al., 1996; Müller et al., 1996; Stoker, 1996). Because the number of specific cellular interactions that have to be coordinated in the period of neurite outgrowth is likely to exceed the coding capacity of the genome, it is reasonable to assume that cell surfaces in the nervous system are labeled on the basis of combinatorial principles. To investigate a putative cooperativity of distinct receptor–ligand pairs in the regulation of neurite outgrowth, we have chosen chick retinal neurons as a model system and have focused on their integrin- and IgSF-mediated neurite outgrowth response in the present study.
Retinal neurons of embryonic day 6 (E6) are well suited to studying the interplay between integrin- and IgSF-mediated neurite outgrowth for two reasons. First, these neurons simultaneously express molecules of both protein classes on their surface during the period of neurite outgrowth. For instance, identified integrin subunits in embryonic chick retina include α1, α2, α4, α6, α8, αv, β1, β3, β5, and β8 (Bossy et al., 1991; de Curtis et al., 1991; Neugebauer et al., 1991; Duband et al., 1992;Reichardt et al., 1992; de Curtis and Reichardt, 1993; Cann et al., 1996; Gervin et al., 1996). Additionally, a large set of IgSF members is also expressed on these neurons, including NCAM, NrCAM, L1/NgCAM, neurofascin, F11/contactin, axonin-1, Thy-1, and DM-GRASP (for review, see Brümmendorf and Rathjen, 1995). Second, these neurons were shown to actually use these receptors to extend neurites in vitro. For example, the retinal integrin α6β1 is involved in laminin-stimulated neurite extension (de Curtis et al., 1991), and retinal IgSF members promote outgrowth by homophilic binding or by heterophilic interactions with other IgSF members (for review, see Brümmendorf and Rathjen, 1995, 1996).
The F11 molecule, also referred to as F3 (Buttiglione et al., 1996) or contactin (Peles et al., 1997), is a member of a growing subgroup of neural glycosyl-phosphatidylinositol (GPI)-linked molecules that most likely arose from a common ancestor by gene duplication events (Plagge and Brümmendorf, 1997). The molecule has been shown to be involved in the induction of chick tectal neurite outgrowth by interaction with NrCAM, a member of the L1 subgroup of the IgSF (Morales et al., 1993; Volkmer et al., 1996; Sakurai et al., 1997). However, in contrast to tectal neurons, embryonic retinal neurons do not respond to F11 despite the fact that they express the NrCAM receptor (see below). This suggested to us that there may be additional outgrowth-mediating receptors on the retinal neurons, for instance, integrins, which are not stimulated if F11 alone is offered as a substrate. To investigate a potential co-stimulation of F11-binding partners and integrins on retinal neurons, we cultured retinal cells on mixtures of F11 with fibronectin (FN). This extracellular matrix (ECM) molecule promotes outgrowth of retinal neurons in vitro(Akers et al., 1981; Thompson and Pelto, 1982; Leifer et al., 1984), most likely by triggering retinal integrins. Therefore, we used FN as a model integrin ligand in the present study, because it binds to a large set of different integrins, among them α2β1, α3β1, α4β1, α4β7, α5β1, α7β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, and αIIbβ3 (for review, see Haas and Plow, 1994; Müller et al., 1995, and references therein), which raises the chances of detecting integrin-dependent co-stimulation of retinal neurite outgrowth. In the present study, we describe a functional cooperativity between the NrCAM pathway and a β1-integrin with respect to neurite outgrowth induction.
MATERIALS AND METHODS
Antibodies. The preparation and specificity of antibodies directed to F11, NCAM, NgCAM, and NrCAM have been described (Rathjen et al., 1987; Wolff et al., 1987; de la Rosa et al., 1990;Pollerberg and Beck Sickinger, 1993; Volkmer et al., 1996). Fab fragments of antibodies were prepared using agarose-coupled papain (Pierce, Rockford, IL) followed by retention of Fc fragments and residual whole antibody on a protein A-Sepharose column (Pharmacia, Freiburg, Germany). Hybridomas producing JG22 were obtained from the Developmental Studies Hybridoma Bank (Johns Hopkins University School of Medicine, Baltimore, MD). To control whether the NgCAM antibodies (which had no effect in our assay) are functional under the conditions used, tectal cells were seeded on an NgCAM substrate (isolated as outlined previously; Rathjen et al., 1987), which promotes neurite outgrowth of tectal cells (Brümmendorf et al., 1993). Inhibition of neurite extension on this substrate confirmed that the NgCAM antibodies are functional (data not shown).
Recombinant soluble F11. Because we intended to evaluate hexahistidine tagging as a means of isolating native, soluble recombinant protein from tissue culture supernatants, we inserted a DNA sequence encoding this tag after the leader sequence of F11. To this end, the DNA sequence corresponding to mature F11 (Thr22–Ala988) was amplified by PCR on the plasmid pSG5/F11 (Brümmendorf et al., 1993) using the primers 5′-gtg gcg agc tct acc cat ttt tca gag gaa gga-3′ and 5′-agt cga agc tta agc agt ggc acc tga aat-3′. PCR was performed as described (Spaltmann and Brümmendorf, 1996); however, the annealing temperature and extension time were adjusted to the requirements of this experiment, 65° and 3 min, respectively. In this PCR, restriction enzyme cleavage sites for SacI andHindIII were added and were used to subclone the product into the plasmid pQE31 (Qiagen, Hilden, Germany), which provides the hexahistidine tag. In a second PCR, the DNA sequence corresponding to histidine-tagged F11 was amplified on this plasmid using the primers 5′-gag aaa tta cgc gtt cta gaa tct cac cat cac cat-3′ and 5′-ctg gat tga tca aca gga gtc caa gct cag cta-3′ and was subcloned, making use of the restriction enzyme cleavage sites MluI andBclI, into the MluI- andBamHI-digested plasmid pSG5/FN (Brümmendorf et al., 1993). The hexahistidine-tagged F11 molecule is referred to as cHisF11 in this study. Sequencing of the F11 coding region of the plasmid with an automated laser fluorescent DNA sequencer (Pharmacia, Uppsala, Sweden) did not reveal PCR-caused sequence deviations.
The plasmid was used to transfect COS cells transiently by the DEAE-dextran method as outlined previously (Brümmendorf et al., 1996). Under conditions not disrupting the tertiary structure of the molecule, however, we did not succeed in isolating pure preparations of cHisF11 by chelate affinity chromatography using its histidine tag. Recombinant F11 was therefore isolated by immunoaffinity chromatography using a monoclonal antibody essentially as outlined (Rathjen et al., 1987) but with an additional high-salt washing step with PBS containing 1.2 m NaCl. A typical transfection experiment in 16 dishes of 140 cm2 yielded ∼50 μg of F11, as estimated in SDS-PAGE followed by silver staining (performed as described byRathjen et al., 1987). The protein proved to be functional in the attachment and outgrowth assay with tectal cells reported previously (Morales et al., 1993).
Neurite outgrowth assay. To prepare fibronectin substrates, a solution containing 20 μg/ml fibronectin (Dianova, Hamburg, Germany) in PBS was spotted as 3 μl spots on Petriperm dishes (20 cm2; Heraeus, Osterode, Germany) and incubated for 3 hr in a humidified atmosphere at 37°C. To generate a fibronectin–F11 substrate, fibronectin (20 μg/ml) and F11 (4 μg/ml, recombinant or isolated from adult chick brain as described; Rathjen et al., 1987) were mixed before coating. With respect to neurite outgrowth essentially the same effect was observed up to 50 μg/ml of both proteins in the mixture, suggesting that the concentrations chosen are saturating. If coated alone, F11 did not promote outgrowth of retinal cells in this range of concentrations but supported cell adhesion at >4 μg/ml. As a negative control protein, the Fc fragment of chick IgY (4 μg/ml, Dianova) was used, because it is very similar to F11 in biochemical terms (Warr et al., 1995). Coating efficiencies of FN in the presence of F11 or IgY were monitored by indirect immunofluorescence analysis with polyclonal antibodies specific for human fibronectin (Sigma, Deisenhofen, Germany), followed by quantification with a digital image analysis system. The amount of FN bound to the substrate was not found to be significantly influenced by the presence of F11 or IgY in the protein mixtures. After coating, each spot was washed separately with 5 μl of PBS, followed by a final wash of the whole dish with 5 ml of PBS. Before plating of neurons, the dishes were blocked for at least 1 hr at 37°C with serum-free N2 neuronal cell culture medium (Bottenstein and Sato, 1979) containing 1 mg/ml essentially fatty acid-free BSA (Sigma).
To isolate retinal cells, retinas were dissected from E6 chick embryos in HBSS without Ca2+ and Mg2+ and incubated for 15 min at 37°C with 0.05% trypsin in PBS and 0.02% EDTA (Boehringer Mannheim, Mannheim, Germany). After aspiration of the trypsin solution, cells were dissociated by trituration in PBS containing 1 mg/ml soybean trypsin inhibitor (Sigma), 1 mg/ml DNase (Worthington, Freehold, NJ) and 3 mg/ml BSA (Sigma). The cells were pelleted and carefully resuspended in serum-free N2 culture medium with 0.5–1.5 × 105 cells/ml, followed by seeding 0.5–4.5 × 105 cells in a protein-coated Petriperm dish. The dishes were incubated at 37°C in an atmosphere of 5% CO2 for 20 hr. In contrast to tectal cells, which extend neurites if grown on a pure F11 substrate in serum-free N2 medium or DMEM with 10% FCS, retinal cells do not in either medium (our unpublished observations).
For antibody perturbation or peptide inhibition experiments, spots were prepared, and cells were seeded as described. After attachment of the cells (2 hr at 37°C), medium was aspirated, and the region between spots was dried with wicks. The medium was replaced with 100 μl of fresh medium containing antibodies or the GRGDSP peptide (Nova-Biochem, Bad Soden, Germany). Polyclonal rabbit antibodies directed to F11, NgCAM, or NrCAM or their Fab fragments were used at 200 μg/ml. JG22 hybridoma culture supernatant was dialyzed against PBS and diluted 1:10 in N2 culture medium. This resulted in a functionally saturating (with respect to retinal ganglion cell binding to FN) concentration of the monoclonal antibody (mAb), as determined by serial dilution experiments (data not shown). The peptide GRGDSP was used with 200 μg/ml, a functionally saturating concentration if retinal cell binding to FN is examined.
Immunofluorescence analysis of retinal ganglion cell cultures. Cultures were fixed in PBS containing Ca2+ and Mg2+ (PBSCM) and 4% formaldehyde for 1 hr at room temperature. After three washes with PBSCM containing 0.02% BSA, cultures were processed for immunofluorescence analysis. Cell bodies and neurites were detected by incubating for at least 1 hr with NCAM-specific antibodies, followed by Cy3-conjugated secondary antibodies from goat (Dianova). To reveal cellular nuclei, the DNA-staining reagent bisbenzimide (H33258, Boehringer Mannheim) was added to the secondary antibodies at a final concentration of 50 ng/ml. Alternatively, to ensure that effects measured on neurite growth were attributable to increased growth of axons originating from retinal ganglion cells, polyclonal antibodies specific for NgCAM, a marker predominantly expressed on retinal ganglion cell axons at stage E6 (de la Rosa et al., 1990; Rager et al., 1996), were used instead of the NCAM antibodies.
Quantification of neurite growth by digital image analysis.To determine parameters reflecting neurite outgrowth, images of neurons and their nuclei were captured at the appropriate wavelength by an intensified CCD camera (Proxitronic, Bensheim, Germany) attached to a fluorescence microscope (Axiophot; Zeiss, Oberkochen, Germany). Spectral overlap of the images was not observed. Images with a resolution of 512 × 512 pixels were processed with an image analysis card [Machine Vision System IV120(C); AIT Göhner, Stuttgart, Germany] on a standard personal computer. With the help of the JL Genias image analysis software (AIT Göhner) an algorithm was developed that was suited to evaluating automatically the number of attached cells, the number of putative neurites, and their individual length (see Results and Fig. 1). Raw data generated by JL Genias were processed with a spreadsheet program (Excel; Microsoft, Unterschleißheim, Germany) and evaluated statistically using the Mann–Whitney U test implemented in the SPSS (Chicago, IL) package. The final layout of figures was created with graphics programs (Freehand; Altsys Inc., Richardson, TX; and Photoshop; Adobe Systems, Mountain View, CA).
Fig. 1.
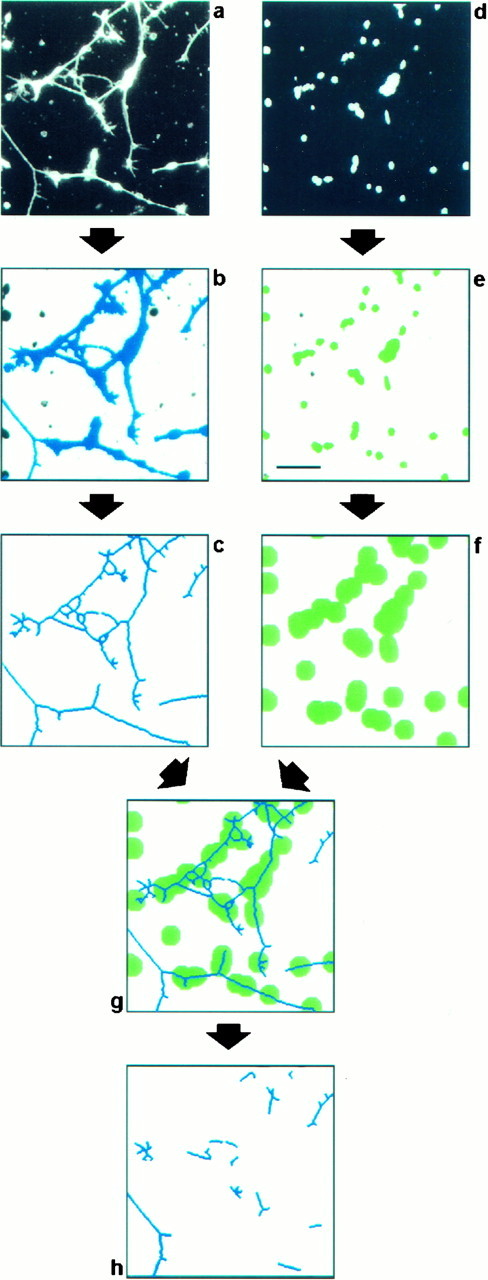
Digital image analysis procedure to evaluate neurite outgrowth in vitro. Retinal cells were seeded on a substratum composed of a mixture of FN and F11. After cultivation for 20 hr, cell bodies and processes were detected by immunofluorescence analysis using antibodies directed to NCAM (a), and cellular nuclei were identified by bisbenzimide staining of DNA (d). To discriminate neurites from cell bodies, both fields were processed as follows. The field showing cells and neurites (a) was processed by applying a staining intensity threshold and size exclusion criteria to distinguish the significant structures representing neurons from background and from small artifactual signals of subcellular size (b, blue objects). These structures were then eroded to a skeleton of a single-pixel width (c). The field comprising cellular nuclei (d) was also processed by applying a staining intensity threshold and size exclusion criteria to label cellular nuclei (e, green objects). These were used to estimate the number of attached cells by dividing the total area representing cell nuclei by the average area covered by a single nucleus. Because we are interested in quantifying primarily the long neurites with a length of more than two cell diameters, all structures within this distance of every cell nucleus had to be excluded from the analysis. To this end, every object that represents a cell nucleus was enlarged correspondingly (f). A comparison of the resulting image with the image representing putative neurites (c) allows the identification of long neurites and fragments thereof, because they do not overlap with the enlarged nuclei (g). To enhance the stringency of the procedure further, objects below a defined length threshold were discarded. The remaining objects (h) were counted, and their individual lengths were measured automatically. The values obtained were normalized with respect to the cell number to correct for intra-assay variations in the number of attached cells. In this procedure we did not attempt to measure the real number of neurites in the observed field or their real length, but we were merely interested in parameters that reflect the outgrowth and can be used to quantify the response of a large number of neurons. Scale bar, 50 μm.
RESULTS
Quantification of neurite outgrowth in vitro by an automatic image analysis procedure
The study of potential synergistic or cooperative effects between different adhesion receptor—ligand pairs with respect to neurite outgrowth requires the quantification of process outgrowth of a large number of neurons. To this end we developed an automatic digital image analysis algorithm, which is suited to evaluation of several hundreds of neurons for each experimental condition by a standardized procedure. This enabled us to measure parameters reflecting neurite outgrowth without any manual processing of the micrographs to be compared with each other. Two different parameters have been chosen to quantify neurite outgrowth in this study, namely, the number of neurites observed and their average length.
Retinal cells were grown in a tissue culture system in vitroon different substrates composed of the proteins to be analyzed. After incubation for 20 hr, the cultures were fixed, and the neurons were detected by immunofluorescence analysis with NCAM-specific antibodies (Fig. 1a). The nuclei of the cells were simultaneously stained with bisbenzimide, a reagent intercalating in DNA (Fig. 1d). Two digitized images representing the neurons and their nuclei, respectively, were then captured separately by a CCD camera. The images were processed automatically to estimate the number of attached cells, the number of neurites, and their average length (Fig. 1).
To examine the performance of this automatic procedure, we compared it with a manual method that we had used earlier to measure neurite lengths of tectal neurons. In our previous studies, we had shown that immobilized F11 or NgCAM promotes neurite outgrowth of tectal neurons and that NgCAM is a more potent substratum than F11 (Morales et al., 1993). Evaluation of such cultures with the manual method and with the automatic procedure shows that both reveal the outgrowth-promoting effect of the molecules and that both lead to the same result, namely, that NgCAM is a stronger substratum than F11 (Fig.2). We therefore conclude that our method is suited to evaluating neurite outgrowth at least as well as the manual method.
Fig. 2.
Comparison of methods to evaluate neurite outgrowth. Neurite extension of tectal neurons on immobilized F11 was compared with that on immobilized NgCAM. A manual evaluation, which was performed as described (Brümmendorf et al., 1993; Morales et al., 1993), determines the percentage of neurons with neurites longer than a given length (a). The automatic procedure, which is illustrated in Figure 1, estimates the number of adhered cells (b), the number of neurites per cell (c), and their average length (d). Each histogram bar represents the average of 10 values and their SDs (error bars) derived from 10 distinct images captured in a stereotyped layout: three in the upper third, four in the middle, and three in the lower third of each protein spot. For each parameter, arbitrary units are given at theordinates, rather than real values. **Statistical significance of the difference (Mann–Whitney U test,p < 0.001). Both methods show that NgCAM is a better substrate than F11.
F11 enhances FN-induced neurite outgrowth of retinal cells
Because retinal cells do not extend neurites on F11 (data not shown) and only poorly on FN, these neurons are well suited to study synergistic promotion of outgrowth elicited by a mixture of both proteins. Retinal cells of embryonic day 6 were isolated and plated on FN alone or on an FN–F11 mixture. On the FN substrate, a significant neurite outgrowth response is elicited (Fig.3a). If retinal cells are plated on a mixed substrate of FN and F11, the number of attached cells is almost indistinguishable from that on FN alone (Fig.3c,g). However, the neurite outgrowth response on the FN–F11 mixture is enhanced if compared with FN alone (Fig.3a,e). To quantify this enhancement, the image analysis procedure that was outlined in detail above (Fig. 1) was applied. Cell bodies and neurites that had been detected by immunofluorescence analysis (Fig. 3a,e) were identified by application of a staining intensity threshold and size exclusion criteria (Fig.3b,f). In parallel, cell bodies were identified analogously by staining of their nuclei (Fig. 3c,g). Both images were then processed to identify the neurites (Fig.3d,h) and to estimate their number and length by applying the stringent criteria outlined in Figure 1.
Fig. 3.

Enhancement of FN-based retinal neurite outgrowth by F11. Retinal cells of embryonic day 6 were cultured for 20 hr on FN alone (left column) or on a mixture of FN and F11 (right column). Cells and neurites were identified by immunofluorescence analysis using NCAM-specific antibodies, and cellular nuclei were identified by bisbenzimide staining. Thefirst row shows immunofluorescence micrographs, and thesecond through fourth rows show objects that have been processed by the image analysis procedure outlined in detail in Figure 1. In the second row cells and neurites are depicted; in the third row, the cellular nuclei; and in the fourth row, the identified neurites. Scale bar, 100 μm.
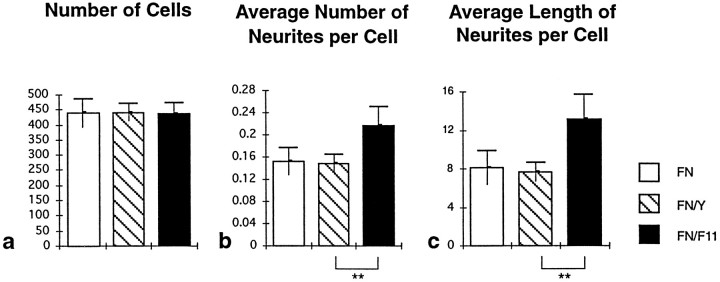
To compare the neurite outgrowth-promoting effect of FN and the FN–F11 mixture, 10 different images were evaluated by the image analysis procedure for each experimental condition. For each image the number of attached cells, the number of observed neurites, and the sum of the lengths of these neurites were determined to estimate the number of neurites per cell and the average length of neurites per cell. In a typical experiment, the average number of cells attached to the FN substrate is not significantly different from that on an FN–F11 mixture (Fig. 4a, open bar, filled bar). However, the neurite outgrowth response is enhanced in the presence of F11 (Fig. 4b,c). Mixing of FN with an equal amount of the biochemically similar control protein IgY did not modulate the outgrowth-promoting effect of FN (Fig. 4, open bars, hatched bars).
Fig. 4.
F11 enhances FN-dependent neurite outgrowth of retinal ganglion cells. Retinal cells were cultured on FN alone, on an FN–IgY mixture, or on an FN–F11 mixture. Cultures were evaluated, and data are presented as outlined in the legend of Figure 2. The average number of attached cells is indistinguishable on the three substrates (a). If F11 is added to FN before coating, the average number of neurites per cell (b) and the average length of neurites per cell (c) are larger (filled bars) on this mixture than on FN alone (open bars). By contrast, substitution of F11 by an equal amount of IgY (hatched bars) did not enhance the outgrowth response of retinal neurons. Data from one representative experiment of at least four independent experiments are depicted. **Statistical significance of the difference (Mann–WhitneyU test, p < 0.001).
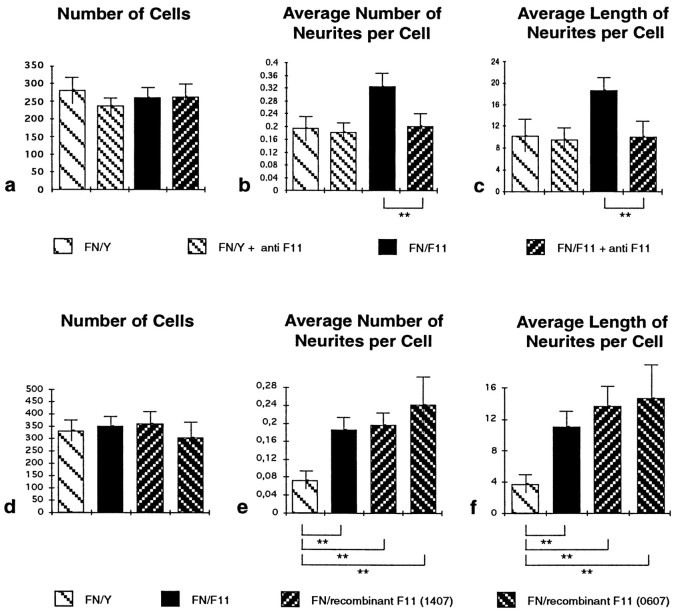
The F11 protein used in the previous experiment was isolated from embryonic chick brain by immunoaffinity chromatography as outlined previously (Rathjen et al., 1987). To demonstrate that the enhancement effect is specific for F11 and not caused by potential contaminants in the F11 preparation, the experiment was repeated in the presence of F11-specific antibodies. Incubation of substrate spots with polyclonal F11-specific antibodies before addition of neurons did not interfere significantly with cell adhesion to FN–IgY or to FN–F11 (Fig.5a). This shows that they have no unspecific toxic effect and confirms that F11 in the FN–F11 mixture does not contribute significantly to cell adhesion under these conditions. However, the antibodies were found to neutralize the F11-mediated enhancement of neurite outgrowth and to reduce it to the level observed with FN–IgY alone (Fig. 5b,c).
Fig. 5.
F11-induced enhancement of neurite outgrowth can be inhibited by F11-specific antibodies and can also be observed if recombinant F11 is analyzed. Retinal cells were cultured on an FN–IgY mixture and on an FN–F11 mixture in the presence or absence of polyclonal F11-specific antibodies. Cultures were evaluated, and data are presented as outlined in the legend of Figure 2. The number of substrate-bound cells was indistinguishable on both substrates and was not influenced by the application of the antibodies (a). On the FN–IgY substratum the antibodies did not interfere with the average number of neurites per cell (b) and the average length of neurites per cell (c). However, the antibody neutralized the outgrowth-promoting effect mediated by F11 contained in the FN–F11 substratum (b, c). In another set of experiments, the outgrowth-promoting effect of soluble recombinant F11, expressed in eukaryotic cells, was evaluated. Two different preparations of recombinant F11 were found to enhance neurite outgrowth to a similar extent as F11 isolated from embryonic chick brain (d–f). Data from one representative experiment of at least four independent experiments are depicted. **Statistical significance of the difference (Mann–Whitney U test,p < 0.001).
As a further specificity control, recombinant F11, which was heterologously expressed in eukaryotic cells, was also tested. To express soluble recombinant F11 in COS cells, the C-terminal hydrophobic stretch involved in the posttranslational attachment of the GPI anchor was deleted on the cDNA level in a eukaryotic F11 expression plasmid. Recombinant F11 was isolated from tissue culture supernatants by immunoaffinity chromatography using a monoclonal antibody (Rathjen et al., 1987). It has an apparent molecular mass of 135 kDa (Fig.6b,e), ∼5 kDa larger than F11 isolated from brain (Fig. 6a,d) which may be caused by aberrant glycosylation in the COS cells. A comparison of recombinant soluble F11 with embryonic chick brain F11 showed that identical amounts of both preparations enhance retinal neuron outgrowth to a similar extent (Fig. 5e,f) but do not affect cell adhesion (Fig. 5d). In conclusion, the enhancement of retinal neuron outgrowth can be inhibited by F11-specific antibodies and can also be observed if recombinant F11 is analyzed in the assay.
Fig. 6.
Characterization of heterologously expressed soluble F11. Soluble F11 that was isolated from culture supernatants of transfected COS cells by immunoaffinity chromatography was characterized by SDS-PAGE followed by silver staining (a–c) or Western blot analysis with an F11-specific monoclonal antibody (d–f). F11 isolated from chick brain (a, 600 ng; d, 120 ng) is compared with recombinant soluble F11 (b, 1.6 μg;e, 320 ng). The latter contains a 53 kDa contaminant (b), which is unrelated to F11 (e) and which is also found in preparations of other recombinant proteins isolated from tissue culture supernatants, for instance, the 23 kDa DiFc protein (c, 3.2 μg;f, 640 ng), which consists of two IgG Fc domains (our unpublished data). Because preparations of the DiFc protein did not enhance neurite outgrowth of retinal neurons on FN (data not shown), suggesting that the 53 kDa protein does not interfere with aspects of this study, we did not attempt to separate the contaminant from recombinant F11.
Taken together, we showed that the addition of F11 to an FN substrate did not affect retinal cell attachment to FN but enhanced retinal neuron outgrowth on this substrate.
The enhancement of retinal neurite outgrowth is mediated by neuronal NrCAM
The enhancement of retinal neuron outgrowth, which is triggered by F11 in the FN–F11 substrate, is likely to be mediated by a receptor on the retinal neurons. The two similar IgSF cell surface molecules NgCAM and NrCAM are expressed on retinal neurons (Morales et al., 1996) (and on other neurons) and have been demonstrated in previous studies to interact with F11 (Brümmenorf et al., 1993;Morales et al., 1993). Whereas NrCAM has been shown to represent an axonal receptor mediating neurite outgrowth of tectal cells by interaction with F11 (Morales et al., 1993), the biological function of the F11–NgCAM interaction is currently unknown. We therefore investigated whether NgCAM or NrCAM is involved in the F11-mediated enhancement of FN-dependent neurite outgrowth. To this end, retinal cells were cultured in the presence of Fab fragments of NgCAM- or NrCAM-specific antibodies.
Fab fragments of NgCAM-specific antibodies were not found to interfere with attachment of retinal cells to FN–IgY or FN–F11 substrates (Fig.7a), indicating that they have no nonspecific toxic effect. Furthermore, addition of the Fab fragments to the culture medium of cells grown on an FN–F11 substrate did not neutralize the F11-mediated enhancement of neurite outgrowth (Fig.7b,c). We therefore conclude that NgCAM is unlikely to be involved in the enhancement effect.
Fig. 7.
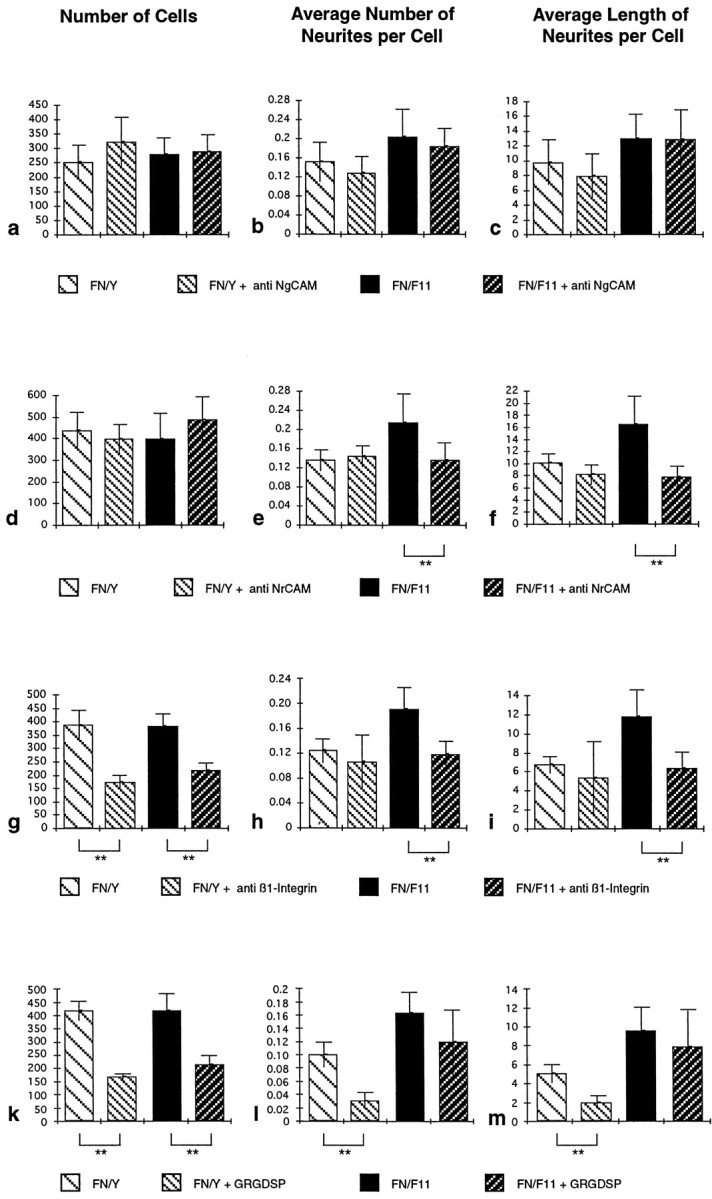
Perturbation of F11-induced enhancement of neurite outgrowth by different reagents. Retinal cells were cultured on an FN–IgY or an FN–F11 substrate in the presence or absence of polyclonal NgCAM-specific Fab fragments (a–c), NrCAM-specific Fab fragments (d–f), a β1-integrin-specific mAb (g–i), or GRGDSP peptides (k–m). Cultures were evaluated, and data are presented as outlined in the legend of Figure 2. Data from one representative experiment of at least three independent experiments are shown. **Statistical significance of the difference (Mann–WhitneyU test, p < 0.001). The number of substrate-bound cells was not influenced by application of the NrCAM or NgCAM antibodies (a, d). On the FN–IgY substratum, neither the NgCAM antibodies (b, c) nor the NrCAM antibodies (e, f) interfered with the average number of neurites per cell (b, e) or the average length of neurites per cell (c, f). On the FN–F11 substratum, the NgCAM antibodies also did not interfere with the F11-induced enhancement of outgrowth (b, c). By contrast, the NrCAM-specific antibodies reduced outgrowth to the FN-dependent level (e, f). The β1-integrin antibody (g) and the GRGDSP peptide (k) interfere significantly with cell attachment on both substrates. Although the antibody did not reduce FN-dependent basal neurite outgrowth (h, i), the peptide strongly decreased outgrowth on the FN substrate (l, m). In the presence of the antibody, the subpopulation of attached cells does not respond to F11 in the FN–F11 substrate but shows only FN-dependent basal outgrowth (h,i). By contrast, the cells that are attached in the presence of the peptide show F11-mediated enhancement of outgrowth, which is not significantly different (p > 0.001) from that in the absence of the peptide (l, m).
To test whether NrCAM is the F11-binding molecule on the E6 retinal neurons, Fab fragments of NrCAM-specific antibodies were tested and were not found to interfere with the cell attachment to FN–IgY or FN–F11 substrate (Fig. 7d), which demonstrates that they are not toxic for the neurons. Interestingly and in contrast to the finding concerning NgCAM, the antibodies directed to NrCAM neutralized the F11-mediated enhancement of neurite outgrowth and reduced it to the level observed with FN alone (Fig. 7e,f).
Taken together, our data suggest that F11-triggered enhancement of retinal neuron outgrowth is mediated by the neuronal F11 receptor NrCAM but not by NgCAM, a related F11-binding molecule that is also expressed by these neurons.
Involvement of β1-integrins in retinal neurite outgrowth
It has been shown previously that FN is an outgrowth-promoting substratum for E6 retinal cells (Akers et al., 1981; Thompson and Pelto, 1982; Leifer et al., 1984). As we have shown above, FN promotes weak but significant outgrowth if offered alone as a substrate but promotes increased outgrowth if coated together with F11. Because FN is known to bind a large set of different integrins, it is very likely that attachment and basal neurite outgrowth is mediated by one of these adhesion receptors. We therefore studied a putative cooperation of integrin signaling with the NrCAM pathway in more detail. As a first step to analyze which integrin(s) might be involved, we examined the effect of an mAb that blocks β1-integrin function (Greve and Gottlieb, 1982), and we also tested a peptide that competitively blocks integrin binding to the RGD motif within FN (Main et al., 1992, and references therein).
Addition of functionally saturating amounts of mAb JG22, which interferes with β1-integrin binding (Greve and Gottlieb, 1982), leads to a substantial reduction of the number of attached cells, both on FN and on the FN–F11 substrate (Fig. 7g). The subpopulation of attached cells, which is supposed to bind to FN in a β1-integrin independent manner, shows FN-dependent basal neurite outgrowth. However, these cells did not respond to F11 in the FN–F11 substrate by enhancement of outgrowth (Fig.7h,i). This suggests that a β1-integrin may contribute to the F11-mediated enhancement of outgrowth in the mixed substrate.
To get a first clue to which β1-integrin may be involved, we took advantage of observations that FN binding of some β1-integrins is dependent on the RGD site within FN, and that of others is not. To inactivate all RGD-dependent integrins, functionally saturating amounts of the peptide GRGDSP were added to the culture medium. Similar to the effect of the JG22 antibody, this leads to a significantly decreased number of cells bound to both substrates (Fig. 7k). Although basal, FN-dependent neurite outgrowth on FN alone was almost abolished in the presence of the peptide, it reduced the enhanced outgrowth on the FN–F11 substrate only weakly and nonsignificantly (Fig.7l,m). Therefore, in contrast to the effect of mAb JG22, the RGD peptide does not appear to interfere significantly with the F11-mediated enhancement of neurite outgrowth.
One possible interpretation of these findings might be that a β1-integrin that seems to interact with FN at a site distinct of its RGD site (a potential candidate may be integrin α4β1; see Discussion) cooperates functionally with the NrCAM-dependent signaling pathway in neurite outgrowth promotion.
DISCUSSION
Evaluation of neurite outgrowth by an automatic image analysis procedure
Analysis of multiple variables affecting neurite outgrowth and detection of subtle modulatory effects requires the quantification of outgrowth from a large number of neurons. In the present study, we introduce an automatic procedure to analyze hundreds of neuronsin vitro for each experimental condition to determine parameters reflecting the number of analyzed cells, the number of neurites, and their length. Neurite outgrowth was quantified by two criteria, the average number of neurites per cell and the average length of neurites per cell. Both criteria were found to be correlated and to be equally suited to describe neurite outgrowth in the present study under all experimental conditions. Comparison of this novel procedure with a manual method that we have used previously to quantify tectal neurite outgrowth (Morales et al., 1993) confirmed that application of both methods leads to the same conclusion, namely, that NgCAM is a more favorable substratum for tectal neurons than F11 (Fig.2).
In an attempt to analyze as many neurons as possible, we used a higher cell density of retinal neurons in the present study than we did previously with tectal neurons. Therefore, in our automatic procedure, we are more conservative and underestimate neurite outgrowth for two reasons. First, fasciculated neurites cannot be distinguished from single isolated neurites. This is attributable to the process of eroding to a single pixel width (Fig. 1), which is done before the neurites are measured and leads to an underestimation of the number of neurites. Second, growing neurites are more likely to encounter other neurons than in the low-density cultures used previously. Therefore the very long neurites are undetectable, which leads to an underestimation of neurite length. The cultures of retinal cells analyzed in this study contained a variable number of single cells and cell aggregates, which differed between distinct preparations of retinal cells and which were also dependent on the cell density at the time of plating. To avoid an influence of the total cell numbers and the size distribution of the aggregates to the outcome of the analyses, only data obtained in one single experiment were compared with each other, and data of consecutive experiments have not been pooled.
The main advantage of our approach is that any bias that might be introduced by the investigator (if neurites have to be chosen by eye and labeled manually for processing) can be excluded. Because a large number of neurons can be evaluated, subtle modulatory effects on neurite outgrowth can be revealed with this method, which therefore might facilitate future studies to elucidate signal transduction mechanisms leading to neurite extension.
NrCAM and NgCAM as receptors involved in neurite outgrowth
In the present study we provided strong evidence that NrCAM on retinal neurons mediates F11-triggered enhancement of FN-induced neurite outgrowth, either as a receptor or as component of a receptor complex. Therefore, the NrCAM molecule plays the role of a receptor in F11-induced neurite outgrowth of two distinct types of neurons, namely, retinal neurons (this study) and tectal neurons, as shown previously (Morales et al., 1993). Whereas in the tectal system, F11 alone is sufficient to promote significant outgrowth, this is not the case for the retinal neurons, which neither adhere significantly to F11 nor extend neurites under the same conditions (data not shown). Therefore, F11 seems to modulate outgrowth of different types of neurons differentially, suggesting that it may be a component of a complex system of neuronal cell surface molecules that regulate neuronal outgrowth during development.
Our approach does not allow us to distinguish between cisand trans interactions of F11 and NrCAM, because the F11 molecule is most likely bound to the substratum in random orientations. Therefore, concerning the retina, our model may reflect an in vivo interaction of NrCAM with F11 in the same cell membrane, with released F11 in the ECM or with F11 on the opposing cell membrane. A related situation has been analyzed recently in another cellular context, namely, outgrowth of dorsal root ganglion neurons. These cells extend neurites on immobilized NgCAM (Kuhn et al., 1991), and it has been demonstrated that this response is dependent on acis cooperation of neuronal NgCAM with neuronal axonin-1 (Buchstaller et al., 1996; Stoeckli et al., 1996). Because axonin-1 is related to F11 (Plagge and Brümmendorf, 1997), and NgCAM is similar to NrCAM (Grumet and Sakurai, 1996), F11 and NrCAM may also bind in a cis configuration. Accordingly, recent co-precipitation studies using transfected COS cells showed that F11 and NrCAM may form a cis complex at the cell surface (Sakurai et al., 1997), implicated in neurite outgrowth induced by receptor protein tyrosine phosphatase β/ζ on glia (Peles et al., 1995).
Binding to F11 is not the only heterophilic molecular interaction that has been characterized for the NrCAM protein, which also binds homophilically (Mauro et al., 1992). Recently, NrCAM has been demonstrated to bind to the L1-related molecule neurofascin (Volkmer et al., 1996) and to the F11-related protein axonin-1 (Suter et al., 1995). Whereas the NrCAM–neurofascin interaction has been identified in the context of tectal neurite outgrowth, the axonin-1 binding has been functionally implicated in axonal guidance in the developing spinal cord (Stoeckli and Landmesser, 1995).
In contrast to the functional role of the F11–NrCAM interaction in neurite outgrowth, the significance of the F11–NgCAM interaction is currently unknown. We therefore examined in this study whether NgCAM could play a role in F11-enhanced retinal neuron outgrowth, but we could not provide evidence for involvement of this molecule (Fig.7a–c). One possible reason for this may be that binding of NgCAM on the neuronal membrane to substrate-bound F11 does not elicit a signal or may be undetectable. Binding of F11 to NgCAM has been shown using NgCAM-coated covaspheres, on which the molecule is most likely immobilized in random orientations (Brümmendorf et al., 1993). It is conceivable that membrane-bound NgCAM may be in a conformation that is incompetent for F11 binding. In principle this would be similar to cell surface-anchored axonin-1, which has been shown to exist in a horseshoe-like conformation with a buried ligand-binding site (Rader et al., 1996). Further experiments are needed to understand the impact of molecular conformations on the F11–NgCAM interaction.
Cooperativity between different outgrowth-mediating receptor systems
Because the size of the vertebrate genome is to small to encode all types of cellular interactions in neurohistogenesis by distinct receptor–ligand pairs, any combinatorial principle of cell surface labeling is of great conceptual interest. In the present experiments we provided evidence of cooperativity between two different receptor systems on retinal neurons, namely, integrins and IgSF molecules, with respect to neurite outgrowth. Our data suggest that the retinal neuron outgrowth response is based on stimulation of neuronal integrins by FN and enhanced by triggering of neuronal NrCAM by F11. Similar cooperative effects have been reported recently for other molecules involved in neurite outgrowth. Concerning retinal neurons, it has been demonstrated that purified NgCAM strongly promotes outgrowth, but purified NrCAM does not. However, if both are mixed, a synergistic growth-promoting effect is observed (Morales et al., 1996). A synergistic response has also been observed for NCAM and N-cadherin in a system in which neurite outgrowth of cerebellar neurons has been examined on a substrate of transfected fibroblasts (Doherty et al., 1991). Similarly, tectal neurons respond to F11 and not to the ECM protein tenascin-R (restrictin), but if both are combined, the F11 response is enhanced by tenascin-R (Nörenberg et al., 1995). One possible explanation for these observations may be that two distinct receptor systems are triggered in these experimental paradigms. This would be similar to a model proposed for the TAG-1-induced outgrowth of dorsal root ganglion cells, which implicated an L1-like molecule and β1-integrins as receptors (Felsenfeld et al., 1994).
Enhancement of β1-integrin-mediated outgrowth of retinal neurons by F11-binding to NrCAM
We have shown that F11-triggered enhancement of FN-dependent retinal neurite outgrowth can be blocked by an mAb that interferes with β1-integrin function. There are two interpretations that can be offered for these findings.
In our model, which is shown in Figure 8, a functional cooperation of the β1-integrin pathway and the NrCAM pathway is proposed. In the absence of any antibody, retinal neurons attach to an FN substrate via a β1-integrin and/or a non-β1-integrin, which promotes basal neurite outgrowth (Figs. 7g–i, 8a). If the cells are plated on an FN–F11 substrate, the mechanism of attachment is the same, but triggering of NrCAM by F11 leads to intracellular events that amplify the β1-integrin signal and increase the neurite outgrowth response (Figs. 7h,i, 8b). In the presence of NrCAM-specific antibodies, cell attachment is unchanged, and only the basal, FN-dependent, and integrin-mediated neurite outgrowth is observed (Figs. 7d–f, 8c). If β1-integrin function is blocked by an mAb, cell adhesion is decreased, and only a subpopulation of cells is attached (Fig.7g). These cells bind to FN in the FN–F11 substrate most likely via non-β1-integrins and show only the integrin-mediated basal outgrowth (Fig. 7h,i). This suggests that the non-β1-integrins are unable to cooperate functionally with the NrCAM pathway (Fig. 8d). It is unclear at present which β1-integrin might be involved in this model, but its identification might be guided by the observation that it seems to bind FN at a site distinct from the RGD motif (Fig. 7l,m). One plausible candidate might be integrin α4β1, because it is expressed in the E6 chick retina (Cann et al., 1996), and it interacts with a C-terminal fragment of FN, a property shared with retinal neurons (Reichardt et al., 1992). However, the involvement of the RGD motif within α4β1 ligands is only partially understood at present (Mould et al., 1991; Massia and Hubbell, 1992; Cardarelli et al., 1994; Sanchez Aparicio et al., 1994).
Fig. 8.
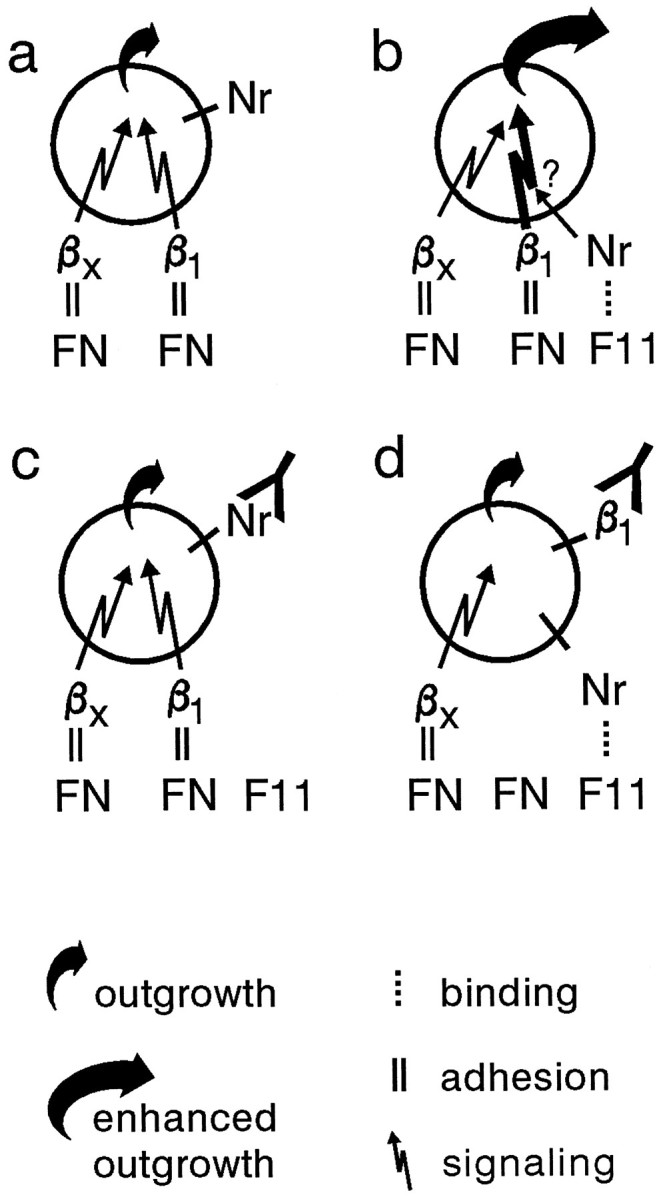
Model of NrCAM-mediated enhancement of β1-integrin-dependent neurite outgrowth. Retinal ganglion cells attach via non-β1-integrins (βx) and β1-integrins to the FN substrate, which promotes weak neurite outgrowth (a). If the cells attach via the same integrin(s) to an FN–F11 substrate, F11 binding to NrCAM triggers unknown intracellular events, which enhance the outgrowth-promoting effect mediated by β1-integrins (b). Blocking of NrCAM by an antibody neutralizes the NrCAM-mediated enhancement (c). In the presence of an anti-β1-mAb, cells attach via non-β1-integrins (βx), which stimulate outgrowth weakly. Because triggering of NrCAM by F11 does not enhance the outgrowth response in the presence of the mAb, it is suggested that the NrCAM pathway can only cooperate with β1-integrins, not with non-β1-integrins (d). Note that under these conditions, the NrCAM–F11 interaction does not contribute to cell adhesion and is therefore indicated by a dotted line.
Currently, we cannot formally exclude a second explanation for our data. We observed that even if a functionally saturating concentration of the β1-blocking mAb is applied, E6 retinal cell attachment is only reduced to ∼50% (Fig. 7g), which is consistent with the observation that approximately half of E6 retinal cells express β1-integrins (Cann et al., 1996). This suggests that there are two populations of cells, one with β1-integrins, which is not attached in the presence of mAb JG22, and one expressing non-β1 FN-binding integrins, which is attached. It is conceivable that only the former population can respond to F11, and therefore, F11-induced outgrowth enhancement cannot be observed in the presence of MAb JG22.
The molecular mechanisms of cooperation between the NrCAM and the integrin pathway remain uncharacterized. However, it may be important in this context that NrCAM and other members of the L1 subgroup that are involved in neurite outgrowth (for review, see Brümmendorf and Rathjen, 1995) bind ankyrin-like repeats within ankyrins (Davis and Bennett, 1994; Michaely and Bennett, 1995) and that an ankyrin-like molecule in Caenorhabditis elegans plays a role in axon guidance (Otsuka et al., 1995). Recently, β1-integrins have been demonstrated to associate with integrin-linked kinase, which contains four ankyrin-like repeats (Hannigan et al., 1996). It remains to be examined whether L1-like proteins might also associate with this serine/threonine protein kinase, which would provide a convergence point of the integrin and the L1 subgroup pathways of neurite outgrowth induction. However, detailed analyses of signal transduction downstream of L1 argue against an early convergence molecule of integrin- and L1-triggered neurite outgrowth, because the L1-dependent outgrowth can be inhibited by a set of agents that does not interfere with integrin-triggered outgrowth (for review, see Doherty and Walsh, 1996).
Because we used preparations of freshly dissociated whole E6 retinas, cells examined in our study are composed of at least two cell populations, undifferentiated neuroepithelial precursor cells and differentiated retinal ganglion cells. We therefore refer to the cells that extend neurites in our assays as early embryonic retinal cells, but we assume that most of them represent retinal ganglion cells (RGCs) for four reasons. First, RGCs are the first differentiating neurons in the retina at E6 (for review, see Mey and Thanos, 1992). Second, RGCs are the only retinal neurons that extend long neurites, a selection criterion implemented in our algorithm (Fig. 1g,h). Third, at this developmental stage of the retina, RGCs are the only cells expressing the NrCAM receptor (de la Rosa et al., 1990; Morales et al., 1996), which is involved in the outgrowth response (Fig.7e,f). Fourth, RGCs are the only E6 retina cells that express the NgCAM molecule (de la Rosa et al., 1990; Rager et al., 1996), and analysis of cultures stained with NgCAM-specific antibodies (rather than NCAM-specific antibodies) leads to essentially the same conclusions concerning F11-mediated enhancement of outgrowth (data not shown).
Our model of a functional cooperation of integrin- and IgSF-mediated neurite outgrowth is related to a similar model proposed previously for dorsal root ganglion (DRG) neurons. These neurons are known to extend neurites in response to axonin-1/TAG-1 (Kuhn et al., 1991; Stoeckli et al., 1996), a neural IgSF member structurally related to F11 (Plagge and Brümmendorf, 1997). Neurite outgrowth of these neurons can be inhibited independently by antibodies specific for β1-integrins or for L1, suggesting that a neuronal β1-integrin and neuronal L1 may cooperate in the induction of outgrowth (Felsenfeld et al., 1994). A striking similarity of the retinal neuron system reported in the present study and the situation in the DRGs lies in the nature of the molecules involved, namely NrCAM in the retinal neurons (Fig. 8) and L1 in the DRGs (Felsenfeld et al., 1994). Because both are members of the L1 subgroup of neural IgSF molecules, it is conceivable that other members of this subgroup, such as neurofascin and CHL1 in mammals, E587 in goldfish, and neuroglian in Drosophila (for review, seeBrümmendorf and Rathjen, 1995, 1996; Holm et al., 1996), might also cooperate with β1-integrins in the regulation of neurite outgrowth.
Footnotes
This study was partly supported by European Union Biotechnology Programme Project BIO4-CT96-0450, The Role of the Neural Cell Adhesion Molecule L1 and its Ligands in Normal Brain Development and Hereditary Brain Diseases. We thank Dr. A. Gierer for generous support and encouragement and Dr. F. G. Rathjen for stimulating discussions. The kind gift of antibodies by Drs. G. Morales, H. Volkmer, and G. E. Pollerberg is gratefully acknowledged. We thank Drs. H. Volkmer, F. G. Rathjen, and T. Lufkin for helpful comments on this manuscript, A. Schöffski for technical assistance, and C. Hug for secretarial help.
Correspondence should be addressed to Dr. Thomas Brümmendorf, Max-Delbrueck-Centrum für Molekulare Medizin, Robert-Rösslestrasse 10, D-13122 Berlin, Germany.
Dr. Treubert’s present address: Brookdale Center for Developmental and Molecular Biology, Mount Sinai School of Medicine, New York, NY 10029.
REFERENCES
- 1.Akers RM, Mosher DF, Lilien JE. Promotion of retinal neurite outgrowth by substratum-bound fibronectin. Dev Biol. 1981;86:179–188. doi: 10.1016/0012-1606(81)90328-6. [DOI] [PubMed] [Google Scholar]
- 2.Bixby JL. Diversity of axonal growth-promoting receptors and regulation of their function. Curr Opin Neurobiol. 1992;2:66–69. doi: 10.1016/0959-4388(92)90164-g. [DOI] [PubMed] [Google Scholar]
- 3.Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin α8 subunit: a new integrin β1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brümmendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Protein Profile. 1995;2:963–1108. [PubMed] [Google Scholar]
- 6.Brümmendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- 7.Brümmendorf T, Hubert M, Treubert U, Leuschner R, Tarnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- 8.Brümmendorf T, Plagge A, Treubert U. Epitope mapping on extracellular domains of cell-surface proteins using exonuclease III. In: Morris GE, editor. Methods in molecular biology: epitope mapping protocols. Humana; Totowa, NJ: 1996. pp. 319–342. [DOI] [PubMed] [Google Scholar]
- 9.Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, Sonderegger P. Cell adhesion molecules Ng-CAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buttiglione M, Revest JM, Rougon G, Faivre Sarrailh C. F3 neuronal adhesion molecule controls outgrowth and fasciculation of cerebellar granule cell neurites: a cell-type-specific effect mediated by the Ig-like domains. Mol Cell Neurosci. 1996;8:53–69. doi: 10.1006/mcne.1996.0043. [DOI] [PubMed] [Google Scholar]
- 11.Cann GM, Bradshaw AD, Gervin DB, Hunter AW, Clegg DO. Widespread expression of β1 integrins in the developing chick retina: evidence for a role in migration of retinal ganglion cells. Dev Biol. 1996;180:82–96. doi: 10.1006/dbio.1996.0286. [DOI] [PubMed] [Google Scholar]
- 12.Cardarelli PM, Cobb RR, Nowlin DM, Scholz W, Gorcsan F, Moscinski M, Yasuhara M, Chiang SL, Lobl TJ. Cyclic RGD peptide inhibits α4 β1 interaction with connecting segment 1 and vascular cell adhesion molecule. J Biol Chem. 1994;269:18668–18673. [PubMed] [Google Scholar]
- 13.Chien CB. Py in the fly: receptor-like tyrosine phosphatases in axonal pathfinding. Neuron. 1996;16:1065–1068. doi: 10.1016/s0896-6273(00)80131-2. [DOI] [PubMed] [Google Scholar]
- 14.Culotti JG, Kolodkin AL. Functions of netrins and semaphorins in axon guidance. Curr Opin Neurobiol. 1996;6:81–88. doi: 10.1016/s0959-4388(96)80012-2. [DOI] [PubMed] [Google Scholar]
- 15.Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- 16.de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor α6 β1 and its isoforms. Development. 1993;118:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Curtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: sequence analysis of the chick integrin α6 subunit. Evidence for transcriptional and posttranslational regulation. J Cell Biol. 1991;113:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Rosa EJ, Kayyem JF, Roman JM, Stierhof YD, Dreyer WJ, Schwarz U. Topologically restricted appearance in the developing chick retinotectal system of Bravo, a neural surface protein: experimental modulation by environmental cues. J Cell Biol. 1990;111:3087–3096. doi: 10.1083/jcb.111.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd J, Schuchardt A. Axon guidance: a compelling case for repelling growth. Cell. 1995;81:471–474. doi: 10.1016/0092-8674(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 20.Doherty P, Walsh FS. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994;4:49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 21.Doherty P, Walsh FS. CAM-FGF receptor interactions: a model for axonal growth. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- 22.Doherty P, Rowett LH, Moore SE, Mann DA, Walsh FS. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991;6:247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- 23.Duband JL, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of α1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992;116:585–600. doi: 10.1242/dev.116.3.585. [DOI] [PubMed] [Google Scholar]
- 24.Felsenfeld DP, Hynes MA, Skoler KM, Furley AJ, Jessell TM. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires an L1-like molecule and β1 integrins. Neuron. 1994;12:675–690. doi: 10.1016/0896-6273(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GC, O’Leary DDM. EPH receptor tyrosine kinases and their ligands in neural development. Curr Opin Neurobiol. 1996;6:127–133. doi: 10.1016/s0959-4388(96)80018-3. [DOI] [PubMed] [Google Scholar]
- 26.Gervin DB, Cann GM, Clegg DO. Temporal and spatial regulation of integrin vitronectin receptor mRNAs in the embryonic chick retina. Invest Ophthalmol Vis Sci. 1996;37:1084–1096. [PubMed] [Google Scholar]
- 27.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 28.Greve JM, Gottlieb DI. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J Cell Biochem. 1982;18:221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- 29.Grumet M, Sakurai T. Heterophilic interactions of the neural cell adhesion molecules Ng-CAM and Nr-CAM with neural receptors and extracellular matrix proteins. Semin Neurosci. 1996;8:379–389. [Google Scholar]
- 30.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 31.Hall H, Walsh FS, Doherty P. Review: a role for the FGF receptor in the axonal growth response stimulated by cell adhesion molecules? Cell Adhes Commun. 1996;3:441–450. doi: 10.3109/15419069609081021. [DOI] [PubMed] [Google Scholar]
- 32.Hannigan GE, Leung Hagesteijn C, Fitz Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 33.Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, Montag D, Schachner M. Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur J Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 34.Keynes R, Cook GM. Axon guidance molecules. Cell. 1995;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4). J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leifer D, Lipton SA, Barnstable CJ, Masland RH. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984;224:303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- 37.Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- 38.Massia SP, Hubbell JA. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin α4 β1. J Biol Chem. 1992;267:14019–14026. [PubMed] [Google Scholar]
- 39.Mauro VP, Krushel LA, Cunningham BA, Edelman GM. Homophilic and heterophilic binding activities of Nr-CAM, a nervous system cell adhesion molecule. J Cell Biol. 1992;119:191–202. doi: 10.1083/jcb.119.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mey J, Thanos S. Development of the visual system of the chick: a review. J Hirnforsch. 1992;33:673–702. [PubMed] [Google Scholar]
- 41.Michaely P, Bennett V. Mechanism for binding site diversity on ankyrin: comparison of binding sites on ankyrin for neurofascin and the Cl−/HCO3- anion exchanger. J Biol Chem. 1995;270:31298–31302. doi: 10.1074/jbc.270.52.31298. [DOI] [PubMed] [Google Scholar]
- 42.Morales G, Hubert M, Brümmendorf T, Treubert U, Tarnok A, Schwarz U, Rathjen FG. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and NrCAM/Bravo. Neuron. 1993;11:1113–1122. doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- 43.Morales G, Sanchez Puelles JM, Schwarz U, de la Rosa EJ. Synergistic neurite-outgrowth promoting activity of two related axonal proteins, Bravo/Nr-CAM and G4/Ng-CAM in chicken retinal explants. Eur J Neurosci. 1996;8:1098–1105. doi: 10.1111/j.1460-9568.1996.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 44.Mould AP, Komoriya A, Yamada KM, Humphries MJ. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin α4β1: inhibition of α4β1 function by RGD peptide homologues. J Biol Chem. 1991;266:3579–3585. [PubMed] [Google Scholar]
- 45.Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller BK, Bonhoeffer F, Drescher U. Novel gene families involved in neural pathfinding. Curr Opin Genet Dev. 1996;6:469–474. doi: 10.1016/s0959-437x(96)80069-4. [DOI] [PubMed] [Google Scholar]
- 47.Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- 48.Nörenberg U, Hubert M, Brümmendorf T, Tarnok A, Rathjen FG. Characterization of functional domains of the tenascin-R (restrictin) polypeptide-cell attachment site, binding with F11, and enhancement of F11-mediated neurite outgrowth by tenascin-R. J Cell Biol. 1995;130:473–484. doi: 10.1083/jcb.130.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otsuka AJ, Franco R, Yang B, Shim KH, Tang LZ, Zhang YY, Boontrakulpoontawee P, Jeyaprakash A, Hedgecock E, Wheaton VI. An ankyrin-related gene (unc-44) is necessary for proper axonal guidance in Caenorhabditis elegans. J Cell Biol. 1995;129:1081–1092. doi: 10.1083/jcb.129.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 51.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plagge A, Brümmendorf T. The gene of the neural cell recognition molecule F11: conserved exon-intron arrangement in genes of neural members of the immunoglobulin superfamily. Gene. 1997;192:215–225. doi: 10.1016/s0378-1119(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 53.Pollerberg GE, Beck Sickinger A. A functional role for the middle extracellular region of the neural cell adhesion molecule (NCAM) in axonal fasciculation and orientation. Dev Biol. 1993;156:324–340. doi: 10.1006/dbio.1993.1081. [DOI] [PubMed] [Google Scholar]
- 54.Rader C, Kunz B, Lierheimer R, Giger RJ, Berger P, Tittmann P, Gross H, Sonderegger P. Implications for the domain arrangement of axonin-1 derived from the mapping of its NgCAM binding site. EMBO J. 1996;15:2056–2068. [PMC free article] [PubMed] [Google Scholar]
- 55.Rager G, Morino P, Schnitzer J, Sonderegger P. Expression of the axonal cell adhesion molecules axonin-1 and Ng-CAM during the development of the chick retinotectal system. J Comp Neurol. 1996;365:594–609. doi: 10.1002/(SICI)1096-9861(19960219)365:4<594::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichardt LF, Bossy B, de Curtis I, Neugebauer KM, Venstrom K, Sretavan D. Adhesive interactions that regulate development of the retina and primary visual projection. Cold Spring Harb Symp Quant Biol. 1992;57:419–429. doi: 10.1101/sqb.1992.057.01.047. [DOI] [PubMed] [Google Scholar]
- 59.Sakurai T, Lustig M, Nativ M, Hemperly JJ, Schlessinger J, Peles E, Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase β. J Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez Aparicio P, Dominguez Jimenez C, Garcia Pardo A. Activation of the α4β1 integrin through the β1 subunit induces recognition of the RGDS sequence in fibronectin. J Cell Biol. 1994;126:271–279. doi: 10.1083/jcb.126.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schachner M, Martini R. Glycans and the modulation of neural-recognition molecule function. Trends Neurosci. 1995;18:183–191. doi: 10.1016/0166-2236(95)93899-9. [DOI] [PubMed] [Google Scholar]
- 62.Sonderegger P, Rathjen FG. Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J Cell Biol. 1992;119:1387–1394. doi: 10.1083/jcb.119.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spaltmann F, Brümmendorf T. CEPU-1, a novel immunoglobulin superfamily molecule is expressed by developing cerebellar Purkinje cells. J Neurosci. 1996;16:1770–1779. doi: 10.1523/JNEUROSCI.16-05-01770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoeckli ET, Landmesser L. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- 65.Stoeckli ET, Ziegler U, Bleiker AJ, Groscurth P, Sonderegger P. Clustering and functional cooperation of Ng-CAM and axonin-1 in the substratum-contact area of growth cones. Dev Biol. 1996;177:15–29. doi: 10.1006/dbio.1996.0141. [DOI] [PubMed] [Google Scholar]
- 66.Stoker AW. Axon guidance: motor-way madness. Curr Biol. 1996;6:794–797. doi: 10.1016/s0960-9822(02)00597-3. [DOI] [PubMed] [Google Scholar]
- 67.Suter DM, Pollerberg GE, Buchstaller A, Giger RJ, Dreyer WJ, Sonderegger P. Binding between the neural cell adhesion molecules axonin-1 and Nr-CAM/Bravo is involved in neuron-glia interaction. J Cell Biol. 1995;131:1067–1081. doi: 10.1083/jcb.131.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 69.Thompson JM, Pelto DJ. Attachment, survival and neurite extension of chick embryo retinal neurons on various culture substrates. Dev Neurosci. 1982;5:447–457. doi: 10.1159/000112705. [DOI] [PubMed] [Google Scholar]
- 70.Volkmer H, Leuschner R, Zacharias U, Rathjen FG. Neurofascin induces neurites by heterophilic interactions with axonal NrCAM while NrCAM requires F11 on the axonal surface to extend neurites. J Cell Biol. 1996;135:1059–1069. doi: 10.1083/jcb.135.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wadsworth WG, Hedgecock EM. Hierarchical guidance cues in the developing nervous system of C. elegans. BioEssays. 1996;18:355–362. doi: 10.1002/bies.950180505. [DOI] [PubMed] [Google Scholar]
- 72.Warr GW, Magor KE, Higgins DA. IgY: Clues to the origins of modern antibodies. Immunol Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 73.Wolff JM, Rathjen FG, Frank R, Roth S. Biochemical characterization of polypeptide components involved in neurite fasciculation and elongation. Eur J Biochem. 1987;168:551–561. doi: 10.1111/j.1432-1033.1987.tb13453.x. [DOI] [PubMed] [Google Scholar]