Abstract
GABA is the primary transmitter released by neurons of the suprachiasmatic nucleus (SCN), the circadian clock in the brain. Whereas GABAB receptor agonists exert a significant effect on circadian rhythms, the underlying mechanism by which GABAB receptors act in the SCN has remained a mystery. We found no GABAB receptor-mediated effect on slow potassium conductance, membrane potential, or input resistance in SCN neuronsin vitro using whole-cell patch-clamp recording. In contrast, the GABAB receptor agonist baclofen (1–100 μm) exerted a large and dose-dependent inhibition (up to 100%) of evoked IPSCs. Baclofen reduced the frequency of spontaneous IPSCs but showed little effect on the frequency or amplitude of miniature IPSCs in the presence of tetrodotoxin. The activation of GABAB receptors did not modulate postsynaptic GABAA receptor responses. The depression of GABA release by GABAB autoreceptors appeared to be mediated primarily through a modulation of presynaptic calcium channels. The baclofen inhibition of both calcium currents and evoked IPSCs was greatly reduced (up to 100%) by the P/Q-type calcium channel blocker agatoxin IVB, suggesting that P/Q-type calcium channels are the major targets involved in the modulation of GABA release. To a lesser degree, N-type calcium channels were also involved. The inhibition of GABA release by baclofen was abolished by a pretreatment with pertussis toxin (PTX), whereas the inhibition of whole-cell calcium currents by baclofen was only partially depressed by PTX, suggesting that G-protein mechanisms involved in GABAB receptor modulation at the soma and axon terminal may not be identical. We conclude that GABABreceptor activation exerts a strong presynaptic inhibition of GABA release in SCN neurons, primarily by modulating P/Q-type calcium channels at axon terminals.
Keywords: GABA, GABAB receptor, suprachiasmatic nucleus, circadian, neuromodulation, presynaptic, autapse, G-protein
The suprachiasmatic nucleus (SCN) of the hypothalamus acts as a circadian clock in the mammalian brain (Moore and Eichler, 1972; Stephan and Zucker, 1972; for review, see van den Pol and Dudek, 1993). The inhibitory amino acid transmitter GABA is particularly important for SCN function. Most neurons here use GABA as their primary transmitter (van den Pol and Tsujimoto, 1985; Moore and Speh, 1993). Half of all presynaptic boutons in the SCN contain GABA, and all neurons studied ultrastructurally were postsynaptic to GABAergic axons, partially arising from other SCN neurons (van den Pol, 1980; Decavel and van den Pol, 1990).
GABA receptors can be divided into two broad groups, one that is coupled to Cl− channels and is activated by GABA and muscimol and blocked in most cases by the GABAAantagonist bicuculline, and a second type that operates through G-proteins and is activated by GABA and baclofen and blocked by 2-hydroxysaclofen (Bormann, 1988). GABAB receptors have been studied in many other brain regions, most extensively in the hippocampus. There GABAB receptors have both presynaptic and postsynaptic actions (Dutar and Nicoll, 1988; Lambert and Wilson, 1993; Pitler and Alger, 1994). A substantial part of the mechanism of GABAB receptor action in hippocampus and most other brain regions is mediated by activation of K+ channels through pertussis toxin-sensitive G-proteins (for review, see Gage, 1992; Bowery, 1993; Misgeld et al., 1995). Postsynaptic GABAB activation of K+ currents, often leading to hyperpolarization, has been found in many parts of the brain (for review, see Misgeld et al., 1995). GABAB receptors can also modulate Ca2+ channels (Mintz and Bean, 1993;Pfrieger et al., 1994; Diversé-Pierluissi et al., 1995; Dittman and Regehr, 1996; Lambert and Wilson, 1996; Rhim et al., 1996, Obrietan and van den Pol, 1998) and thereby inhibit neurotransmission (Scholz and Miller, 1991; Doze et al., 1995; Huston et al., 1995; Isaacson and Hille, 1997).
Autoradiographic studies have shown GABAB receptor binding in the SCN (Francois-Bellan et al., 1989). In parallel, a number of studies have shown that GABA or GABAB receptor agonists applied to the SCN phase shift circadian rhythms (Ralph and Menaker, 1989; Gannon et al. 1995; Gillespie et al., 1997). Despite the large body of evidence for GABAB actions relating to circadian rhythms, all postsynaptic GABA responses could be completely blocked by the antagonist bicuculline, leading to the conclusion that GABAA receptors probably account for all of the postsynaptic actions of GABA in the SCN (Kim and Dudek, 1992). To address the mystery of what mechanism GABAB actions in the SCN might be operating through, we examined several questions in the present study. If no postsynaptic GABAB-mediated slow IPSPs can be detected, do presynaptic GABAB receptors act on axon terminals? What is the ion channel target of GABAB actions? Can G-protein blockers alter GABAB receptor actions?
To differentiate presynaptic from postsynaptic actions clearly, we used cultures of SCN neurons consisting of either single self-innervating neurons or multicellular synaptically coupled neurons. Previous work has shown that SCN neurons in vitro demonstrate many of the same actions that they do in vivo, including GABA release and response to GABA (Chen and van den Pol, 1996, 1997). Furthermore, neurons in isolated SCN slices in vitro show circadian rhythms of activity (Inouye and Kawamura, 1979), and even single SCN neurons in culture show circadian rhythms of electrical activity (Welsh et al., 1995). Because of the robust presence of GABA, we tested the hypothesis that GABA would have a potent presynaptic action on GABAergic axons of SCN neurons.
MATERIALS AND METHODS
Cell culture. High-density multicell culture and low-density microculture of SCN neurons were used and have been described previously in detail (Chen and van den Pol, 1996, 1997). Briefly, the SCNs were dissected from brain slices containing optic chiasm removed from postnatal pups (postnatal days 1 and 2) of Sprague Dawley rats. The tiny tissue blocks were incubated for 30 min in a enzyme solution containing 10 U/ml papain, 0.5 mm EDTA, 1.5 mm CaCl2, and 0.2 mg/mll-cysteine. After washing the tissue twice with culture medium, cells were dissociated by mechanical trituration and plated on 35 mm Petri dishes (Corning, Corning, NY). For multicell cultures, a high density of cells (>20,000 cells/cm2) was plated in the central area of culture dishes precoated with poly-d-lysine (0.3 mg/ml). For microcultures, a low density of glial cells (∼2000 cells/cm2) was first plated on 35 mm Petri dishes. After 2–3 d of culture, 5 μmcytosine arabinoside (ARA-C) was added into culture medium to arrest the proliferation of glial cells. One to 2 d later, a low density of neurons (3000 cells/cm2) was plated in dishes containing microislands of glial cells (Furshpan et al., 1986; Bekkers and Stevens, 1991; Johnson, 1994; Chen and van den Pol, 1996, 1997). After 1–2 weeks of culture, single neurons grown on microislands of glial cells form synapses with themselves, and autaptic IPSCs can be reliably evoked by depolarizing pulses (Chen and van den Pol, 1996,1997). The culture medium contained minimal essential medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum (Hyclone, Logan, UT) and serum extender (Collaborative Research, Bedford, MA), 100 U/ml penicillin and streptomycin, and 6 g/l glucose. Two micromolar ARA-C was added 2 d after plating neurons. The cultures were maintained in an incubator at 37°C and 5% CO2, and fed twice weekly.
Electrophysiology. Experimental procedures for whole-cell voltage-clamp and current-clamp recordings have been detailed previously (Chen and van den Pol, 1996, 1997). In brief, a List EPC-7 amplifier interfaced with an Apple Macintosh computer was used to acquire data with AxoData software. Axograph and Igor Pro software were used to analyze data. Data were sampled at 2–5 kHz and filtered at 1 kHz by an eight pole Bessel filter (Frequency Devices). Micropipettes were pulled from thin-wall borosilicate glass (World Precision Instruments) using a Narishige vertical puller, with a tip diameter of ∼2 μm. The pipette resistance was ∼3 MΩ after filling with the pipette solution. The series resistance was monitored continuously by application of a negative voltage pulse (−10 mV, 30 msec), and compensated by 40–70% with the amplifier. A brief depolarizing pulse (70 mV, 2 msec) was used to evoke presynaptic GABA release in single autaptic neurons, with a holding potential usually at −60 mV. Postsynaptic GABA receptor responses were induced by a brief pressure ejection of GABA (50 μm, 2–4 psi, 10 msec) through a micropipette (2 μm tip diameter) under computer control with the use of a Picospritzer (General Valve, Fairfield, NJ). The recording chamber was perfused continuously (2 ml/min) with a bath solution containing (in mm): 150 NaCl, 2.5 KCl, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.3. The pipette solution contained (in mm): 145 KCl, 0.5 K4-EGTA, 10 HEPES, 4 Mg-ATP, and 0.5 Na2-GTP, pH 7.3. For recording of whole-cell Ca2+ currents, the bath solution contained (in mm): 110 NaCl, 40 TEA-Cl, 2.5 KCl, 5 BaCl2, 10 HEPES, 10 glucose, and 1 μmtetrodotoxin (TTX), pH 7.3; the pipette solution contained (in mm): 145 CsCl, 2 Cs4-EGTA, 10 HEPES, 4 Mg-ATP, and 0.5 Na2-GTP, pH 7.3. Drugs were applied through a series of glass flow pipes (400 μm inner diameter) fed by gravity. Experiments were done at room temperature (22°C).
Baclofen, GABA, (−)-bicuculline methiodide, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), TTX, ω-conotoxin GVIA, Mg-ATP, and Na2-GTP were purchased from Sigma (St. Louis, MO); 2-hydroxysaclofen and nimodipine were from Research Biochemical International (RBI, Natick, MA); agatoxin IVB (also known as agatoxin TK, similar to agatoxin IVA) was from Peptide International; pertussis toxin (PTX) was from Sigma and RBI. Nimodipine was dissolved in methanol to 10 mm. The final concentration was 4 μm; the 0.04% methanol vehicle had no effect on Ca2+ currents or transmitter release. Toxins were dissolved in distilled water, aliquoted, and stored at −80°C.
RESULTS
GABAB receptor activation inhibits evoked IPSCs
The SCN contains primarily GABAergic neurons. GABAAreceptors mediating GABA neurotransmission in the SCN have been well documented (for review, see van den Pol et al., 1996). However, the function of GABAB receptors remains poorly understood. We examined the possible function of GABAB receptors in SCN cultures by applying the specific GABAB receptor agonist baclofen. A profound inhibition of GABA neurotransmission by baclofen was observed in a dose-dependent manner. In some neurons a complete (100%) block was found with high concentrations (100 μm) of baclofen. Figure 1 shows the action of baclofen on evoked autaptic IPSCs. Figure 1, A andB, shows a typical neuron in which baclofen at concentrations of 1–100 μm potently inhibited the evoked IPSCs in a dose-dependent manner. Figure 1C is a bar graph showing the group data of baclofen reduction of evoked IPSCs at various concentrations. The IC50 of baclofen was 5–10 μm.
Fig. 1.
Baclofen dose-dependent inhibition of evoked GABA release in a single autaptic SCN neuron. A, Traces showing a dose-dependent inhibition of baclofen on evoked IPSCs.B, Line graph illustrating the time course of baclofen inhibition. C, Histogram showing the dose-dependent inhibition of IPSC amplitude by baclofen. IC50 is between 5 and 10 μm.
To ensure that the baclofen inhibition on IPSCs was induced specifically by activating GABAB receptors, a GABAB receptor antagonist, 2-hydroxysaclofen, was used to antagonize the baclofen effect. Figure2, A and B, demonstrates that the evoked IPSC was totally blocked by the GABAA receptor antagonist bicuculline (30 μm). The GABAB receptor agonist baclofen (10 μm) inhibited the amplitude of the IPSC by ∼50%. Bath application of the GABAB receptor antagonist 2-hydroxysaclofen (500 μm) increased the amplitude of the IPSC in this neuron. In the presence of 2-hydroxysaclofen, the baclofen inhibition of the IPSC amplitude was greatly reduced (from 50 to 12%). After washing off 2-hydroxysaclofen, application of baclofen again produced a large inhibition of IPSCs (50%). Similar experiments were repeated in six neurons. Baclofen inhibition before and during 2-hydroxysaclofen was 46.3 ± 5.8 and 8.0 ± 2.3% (n = 7), respectively, a difference that is statistically significant (p < 0.01,t test), suggesting that the inhibition of baclofen on evoked IPSCs was attributed to the specific activation of GABAB receptors. The fact that application of 2-hydroxysaclofen increased the mean basal amplitude of synaptically evoked IPSCs (9.3 ± 0.7%) suggests that GABABreceptors were activated by synaptically released GABA transmitter, and that the GABAB receptor may participate in a tonic depression of GABA release.
Fig. 2.
Baclofen inhibition of GABA release is antagonized by 2-hydroxysaclofen. A, Traces show a total elimination of IPSC by 30 μm bicuculline (BIC) and a great reduction of IPSC by 5 μm baclofen. 2-Hydroxysaclofen (SAC) at 500 μm slightly increased IPSCs and largely reduced the baclofen inhibition of IPSC. B, Line graph shows a repeatable inhibition of IPSCs by bicuculline and baclofen and the antagonism of baclofen (BACL) effect by 2-hydroxysaclofen.
Baclofen inhibits GABA release through a presynaptic mechanism
The potent inhibition of baclofen on neurotransmission could possibly be mediated at either presynaptic or postsynaptic sites. In brain slices, GABAB receptor-mediated slow IPSPs were not detected in the SCN (Kim and Dudek, 1992). To examine whether there is any GABAB receptor-mediated slow IPSPs or IPSCs in our SCN cultures, antagonists for both GABAA and GABABreceptors were used to differentiate fast and slow components. Figure3 demonstrates the absence of postsynaptic GABAB receptor-mediated slow responses in cultured SCN neurons, in agreement with brain slice experiments (Kim and Dudek, 1992). Figure 3A illustrates that the GABAA receptor antagonist bicuculline (30 μm) totally blocked the evoked IPSC. Additional application of GABAB receptor antagonist 2-hydroxysaclofen (500 μm) together with bicuculline had no further effect (n = 4). The holding potential was maintained at −45 mV to facilitate the detection of GABAB receptor-mediated slow responses by increasing the driving force. The effect of GABAB receptor activation on membrane potential and membrane conductance was further examined. Figure 3, B andC, demonstrates that application of baclofen (10 μm) under both voltage-clamp and current-clamp conditions potently inhibited spontaneous IPSCs and IPSPs but had no effect on the membrane current (n = 12) or membrane potential (n = 4). The downward current injection-induced hyperpolarizing potentials in Figure 3C remained constant before and during baclofen application, indicating no membrane conductance change induced by baclofen (n = 4). Together, these results suggest that baclofen inhibits GABA neurotransmission not through a postsynaptic mechanism but solely through activation of presynaptic GABAB autoreceptors.
Fig. 3.
No slow postsynaptic GABAB responses in SCN neurons. A, Evoked autaptic IPSC was totally abolished by 30 μm bicuculline (BIC). Applied together with bicuculline, 2-hydroxysaclofen (SACL, 500 μm) induced no further change.B, In voltage clamp, baclofen (20 μm) greatly reduced spontaneous IPSCs but induced no change in baseline.C, In current-clamp condition, baclofen (20 μm) had no effect on resting membrane potential. Current injection-induced hyperpolarizing potentials were not affected by baclofen, indicating no change in cell membrane conductance after baclofen application.
For a direct demonstration of a presynaptic action of GABABreceptors, we compared the effect of baclofen on presynaptically evoked IPSCs with postsynaptic flow pipe GABA application-induced responses in single self-innervating neurons. The box in Figure4A illustrates the experimental arrangement. Figure 4, A–C, demonstrates that baclofen (5 μm) strongly inhibited the presynaptically evoked IPSC but had little effect on the postsynaptic GABA response in the same neuron. Figure 4D is a line graph showing the differential effect of baclofen on presynaptic and postsynaptic responses of a single neuron. Grouped data from six neurons are summarized in Figure 4E, demonstrating a significant reduction of presynaptically evoked IPSCs (p < 0.001) by baclofen but no effect on postsynaptic GABA application-induced responses (p > 0.5), suggesting a specific action of baclofen on presynaptic GABA release instead of a modulation of postsynaptic GABA receptors.
Fig. 4.
GABAB receptors presynaptically modulate GABA release as negative feedback autoreceptors.A, Control trace showing presynaptically evoked IPSC and micropipette GABA application induced postsynaptic response. Thebox illustrates that a brief depolarizing pulse of the recorded single autaptic neuron evoked presynaptic axon release, and that a brief flow pipe GABA application onto the cell induced a postsynaptic response. B, Responses in the presence of baclofen (20 μm). C, Superimposed traces showing a reduction in IPSC by baclofen but little effect on postsynaptic GABA receptor responses. D, Line graph illustrating differential effects of baclofen on presynaptic evoked IPSC and postsynaptic GABA response. E, Bar graph of group data showing specific baclofen inhibition of evoked IPSCs but no inhibition of postsynaptic GABA responses.
To address further the mechanism of baclofen actions on GABA neurotransmission, spontaneous IPSCs in the absence and presence of TTX (1 μm) were examined. The glutamate receptor antagonist CNQX (10 μm) was routinely added to the bath solution, and this eliminated glutamatergic EPSCs. Figure5A1 illustrates that in the normal bath solution without TTX there were many large IPSCs attributed to the spontaneous firing of action potentials. Baclofen (10 μm) eliminated almost all of the large IPSCs, whereas small IPSCs persisted. Figure 5A2 is an amplitude distribution histogram (n = 4) demonstrating a significant inhibition of large-amplitude IPSCs by baclofen (white bar). The median amplitude of IPSCs before baclofen was 59.2 pA (range, 5.2–4920 pA) and after baclofen was 35.6 pA (range, 5.4–3343 pA). There is a significant difference between the two amplitude distribution histograms before and after baclofen application (p < 0.001, Kolmogorov–Smirnov test). Figure 5B1 illustrates that in the presence of TTX (1 μm), baclofen (10 μm) had no remarkable effect on miniature IPSCs (mIPSCs). This is supported by the amplitude distribution histogram (n = 5) shown in Figure5B2. Baclofen did not significantly alter the amplitude distribution histogram of mIPSCs (p > 0.2, Kolmogorov– Smirnov test). The median mIPSC amplitude before baclofen was 33.0 pA (range, 5.1–130.9 pA) and after baclofen was 32.8 pA (range, 5.2–122.8 pA). The absence of an effect of baclofen on the amplitude of mIPSCs provides further evidence that baclofen does not modulate postsynaptic GABAA receptors. The substantial inhibition of baclofen on action potential evoked-large IPSCs but little effect on TTX resistant mIPSCs suggests that the baclofen inhibition may be related to Ca2+ influx, possibly through modulation of voltage-dependent Ca2+channels.
Fig. 5.
Differential inhibition of baclofen on action potential-dependent large IPSCs and action potential-resistant miniature IPSCs. A1,A2, Consecutive traces (A1) and amplitude distribution histogram (A2) illustrating substantial baclofen inhibition of large-amplitude IPSCs. B1,B2, Consecutive traces (B1) and amplitude distribution histogram (B2) showing little effect of baclofen on the amplitude of mIPSCs in the presence of TTX (no significant difference by Kolmogorov–Smirnov test). Note the different vertical scale bars between A1 and B1.
P/Q-type calcium channels are the major target of GABABreceptor modulation
Whole-cell Ca2+ currents, using Ba2+ as the carrier, were evoked by a depolarizing pulse of 40 msec, 90 mV at a holding potential of −90 mV. Figure6A illustrates that the whole-cell Ca2+ currents were almost completely blocked by 100 μm Cd (n = 3). In the same neuron, baclofen (1 μm) also reversibly inhibited the whole-cell Ca2+ current. Figure 6Bshows a dose-dependent inhibition of baclofen on whole-cell Ca2+ currents. The IC50 of baclofen was between 1 and 10 μm, the same range of the IC50 of baclofen on evoked IPSCs (see Fig. 1).
Fig. 6.
Baclofen dose-dependent inhibition of calcium currents. A, Recording traces and line graph showing the abolition of ICa by Cd and strong inhibition of ICa by baclofen (10 μm) in the same neuron. B, Traces and histogram showing dose-dependent inhibition of ICa by baclofen. IC50 is between 1 and 10 μm.
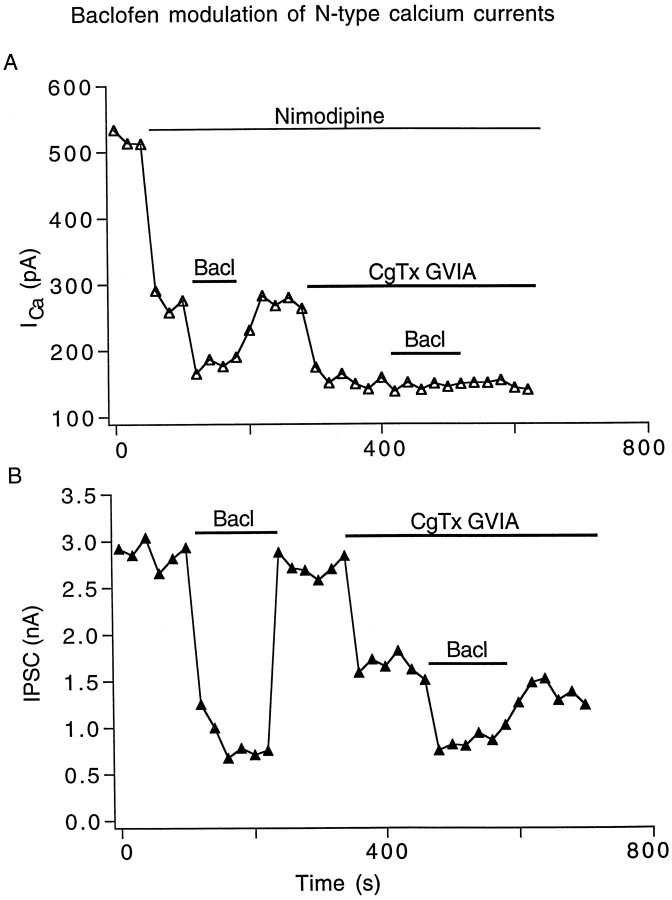
Multiple types of Ca2+ channels, including L-, N-, P-, and Q-type, have been characterized in central and peripheral neurons (Tsien et al., 1988; Bean, 1989; Llinas et al., 1992). To test the hypothesis that baclofen inhibits evoked IPSCs in SCN neurons by modulating specific subtypes of Ca2+ channels, selective Ca2+ channel blockers were used. Figure7A illustrates that after application of the L-type Ca2+ channel blocker nimodipine (4 μm) and N-type Ca2+channel blocker conotoxin GVIA (2 μm), additional application of baclofen (10 μm) inhibited the remaining non-L/N Ca2+ currents. Similar results were found in four of six neurons. In another two neurons, baclofen inhibition disappeared after blocking N-type Ca2+ currents (see Fig. 9). Figure 7B shows a parallel experiment testing the effect of baclofen on evoked IPSCs in the presence of L- or N-type Ca2+ channel blockers. Blocking L-type channels by nimodipine (4 μm) had no effect on evoked IPSCs (n = 3), indicating no participation of L-type channels in synaptic GABA release in SCN neurons. The N-type channel blocker conotoxin GVIA (2 μm) significantly reduced the evoked IPSC, suggesting that N-type channels mediate GABA release in SCN axons. After the reduction of IPSC amplitude by conotoxin GVIA (2 μm), application of baclofen (10 μm) further inhibited the remaining IPSC, suggesting a target other than N-type channels being involved in baclofen inhibition.
Fig. 7.
Baclofen modulation of non-L-, non-N-type calcium currents. A, In the presence of 4 μmnimodipine (blocking L-type ICa) and 2 μm conotoxin GVIA (CgTx, blocking N-typeICa), baclofen (20 μm) inhibited the remaining non-L, non-N ICa.B, Baclofen (20 μm) inhibited IPSCs in the presence of nimodipine or conotoxin GVIA. Nimodipine had no effect on IPSCs.
Fig. 9.
Baclofen modulation of N-type calcium currents.A, In the presence of 4 μm nimodipine, baclofen (20 μm) inhibition of the remaining non-LICa disappeared after 2 μmconotoxin GVIA blocking of N-type ICa.B, Baclofen (20 μm) inhibition of IPSCs was greatly reduced in the presence of 2 μm conotoxin GVIA.
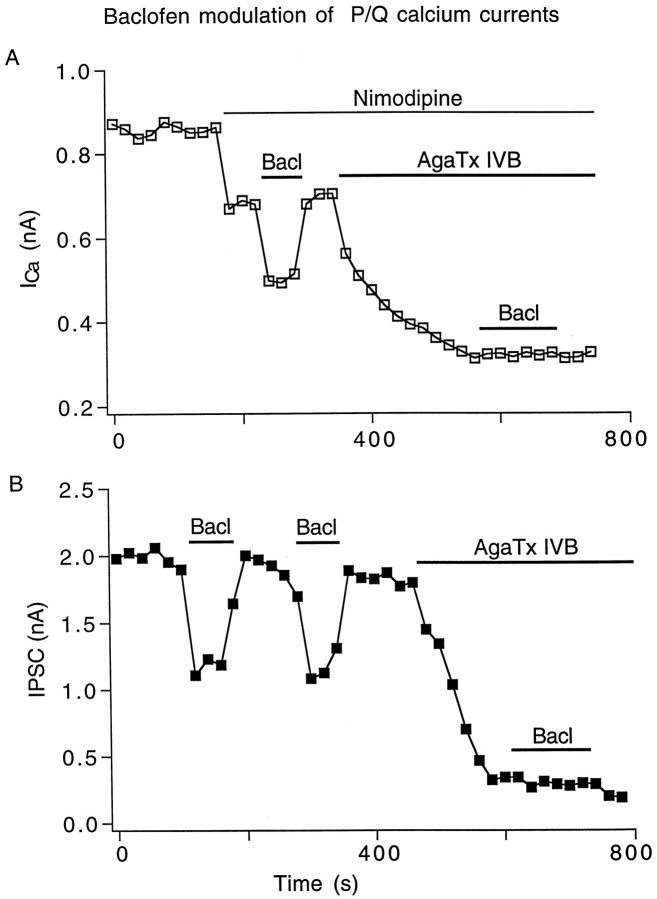
Figure 8A demonstrates that the remaining non-L/N Ca2+ current modulated by baclofen in Figure 7A may be mediated by P/Q-type Ca2+ channels. In this representative neuron of five tested, the baclofen inhibition of Ca2+ currents disappeared after treatment with agatoxin IVB (500 nm, also known as agatoxin TK), a specific blocker of P/Q-type channels (Teramoto et al., 1993), suggesting that the Ca2+current component modulated by baclofen was a P/Q-type current. Figure8B shows a parallel experiment of baclofen combined with agatoxin IVB on evoked IPSCs (n = 5). Repeated application of baclofen (10 μm) reversibly inhibited IPSCs. After application of agatoxin IVB (500 nm), the application of baclofen induced no inhibition, suggesting that the agatoxin IVB sensitive P/Q-type Ca2+ channels are the target of baclofen modulation.
Fig. 8.
Baclofen modulation of P/Q type calcium currents.A, In the presence of 4 μm nimodipine, baclofen (20 μm) inhibition of the remaining non-LICa disappeared after agatoxin IVB (AgaTx, 500 nm) blockade of P/Q typeICa. B, Repeatable inhibition of IPSCs by baclofen (20 μm) disappeared after agatoxin IVB (500 nm), blocking most of the IPSC.
Although P/Q-type Ca2+ channels appear to be the major targets of baclofen modulation in many SCN neurons, N-type Ca2+ channels may also be modulated. Figure9A shows one example of two neurons in which baclofen (10 μm) inhibition of Ca2+ currents did not occur after blocking N-type currents by conotoxin GVIA (2 μm), suggesting a direct modulation of N-type channels by baclofen. In a parallel experiment of evoked IPSCs combining baclofen with conotoxin GVIA as shown in Figure9B, baclofen (10 μm) in the control condition greatly reduced the IPSC amplitude from 2.9 to 0.7 nA, whereas after application of conotoxin GVIA (2 μm), baclofen (10 μm) only reduced the IPSC from 1.5 to 0.7 nA, suggesting that N-type channels are part of the target of baclofen modulation.
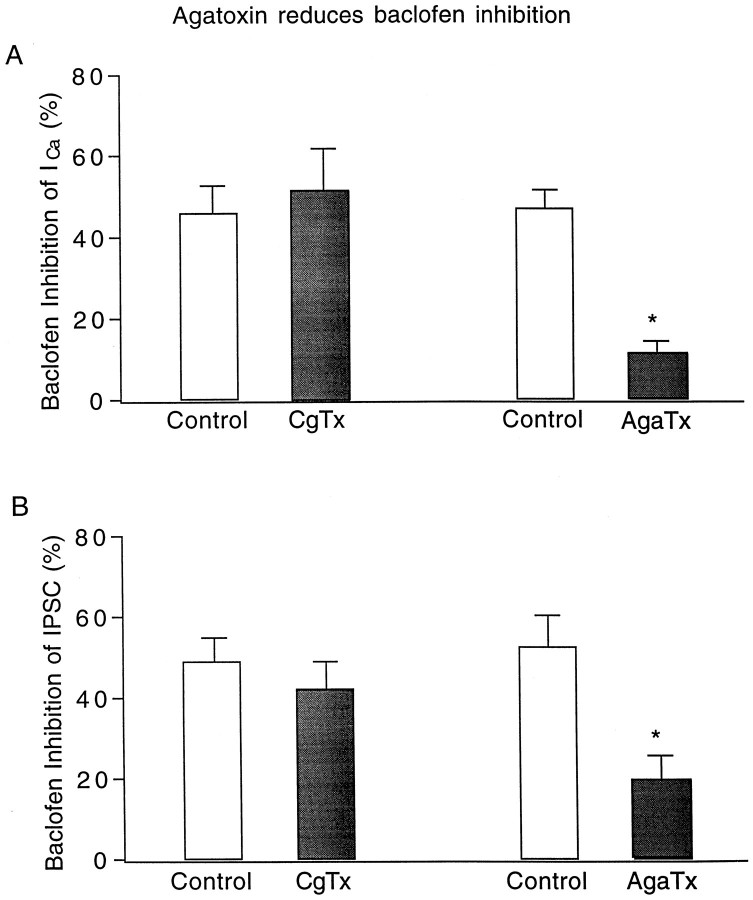
Figure 10 summarizes the change of baclofen inhibition of both Ca2+ currents and evoked IPSCs before and after application of conotoxin GVIA and agatoxin IVB. The average (n = 6) baclofen inhibition of Ca2+ currents before conotoxin GVIA was 46.0 ± 6.2% and after conotoxin GVIA was 51.4 ± 10.4%, showing no significant difference (paired t test, p > 0.3). Whereas no significant conotoxin GVIA inhibition of baclofen actions was found in the group mean, two of the six neurons tested did show a conotoxin GVIA-mediated block of the baclofen inhibition (Fig.9A). In contrast to the N-type Ca2+channel blocker, the P/Q channel blocker showed a substantial block of the baclofen effect. The baclofen inhibition of Ca2+currents before agatoxin IVB was 47.2 ± 4.1% and after agatoxin IVB was 11.4 ± 2.5%, showing a significant reduction (n = 4; paired t test, p < 0.001). In parallel with the action on Ca2+currents, the mean baclofen inhibition of evoked IPSCs before conotoxin GVIA was 49.1 ± 6.1% and after conotoxin GVIA was 42.3 ± 6.6%, having no statistical significance (n = 5, paired t test, p > 0.2). However, the baclofen inhibition of IPSCs before agatoxin IVB was 52.7 ± 7.9% and after agatoxin IVB was 19.6 ± 5.8%, indicating that the P/Q channel blocker mediated a significant reduction in the baclofen inhibition of IPSCs (n = 7; paired t test,p < 0.01).
Fig. 10.
Reduction of baclofen inhibition after blocking P/Q-type calcium channels. A, Comparison of baclofen inhibition of calcium currents before and after conotoxin GVIA and agatoxin IVB. Baclofen inhibition was significantly reduced by application of agatoxin IVB (p < 0.001).B, Comparison of baclofen inhibition of evoked IPSCs before and after conotoxin GVIA and agatoxin IVB. Similar to calcium currents, baclofen inhibition of IPSCs was also reduced by application of agatoxin IVB (p < 0.01).
Together, these results demonstrate that baclofen strongly modulates P/Q-type and in some cells N-type Ca2+ channels. This inhibitory modulation may underlie the mechanism of baclofen inhibition on GABA neurotransmission in the SCN.
Involvement of PTX-sensitive and -insensitive G-proteins
GABAB receptors may be coupled to G-proteins in many central neurons (Gage, 1992; Misgeld et al., 1995). To determine whether GABAB receptors in SCN neurons are linked to PTX-sensitive G-proteins, SCN cultures were treated with PTX (300 ng/ml) for 48 hr before baclofen was tested. Surprisingly, we found a differential effect of baclofen on evoked IPSCs and Ca2+ currents after treatment of PTX. Figure11, A–C, illustrates an example in which, in the same autaptic neuron, baclofen (10 μm) had no effect on evoked IPSCs but produced a remarkable inhibition (45%) of Ca2+ currents. This phenomenon was confirmed by using PTX from two different sources (Sigma and RBI), and repeated in different cultures. Figure11D summarizes data from 14 neurons after PTX pretreatment, examining the differential baclofen effect on IPSCs and Ca2+ currents. The inhibition of baclofen on evoked IPSCs was clearly blocked by PTX pretreatment, suggesting that baclofen modulation of synaptic GABA release, possibly by modulation of presynaptic N- and P/Q-type Ca2+ channels, is dependent on PTX-sensitive G-proteins. The baclofen inhibition of whole-cell Ca2+ currents was reduced (from 50 to 25%) but not fully blocked after PTX pretreatment, indicating that part of the baclofen modulation of Ca2+ currents may be mediated by PTX-insensitive G-proteins.
Fig. 11.
Differential G-protein mediation of baclofen inhibition of GABA release and calcium currents. A–C, Line graph (A) and recording traces (B, C) illustrating a differential effect of baclofen (20 μm) on IPSCs and ICa in the same autaptic neuron after treatment PTX. D, Bar graph showing pooled data that PTX abolished the baclofen inhibition of IPSCs but only partially reduced the baclofen inhibition ofICa.
DISCUSSION
Although substantial effects of GABAB receptors on phase shifts of circadian rhythms, general neuronal activity levels, and c-Fos gene expression are found in the SCN (Ralph and Menaker, 1989; Colwell et al., 1993; Gannon et al., 1995; Gillespie et al., 1997), specific actions of GABAB receptors were not detected in electrophysiological studies (Kim and Dudek, 1992). Our data suggest that this apparent mystery is not a real discrepancy but instead is caused by a substantial block of transmitter release from presynaptic GABAB autoreceptors, rather than a postsynaptic GABAB receptor-mediated slow hyperpolarizing response found in many other brain regions. The mechanism underlying the presynaptic modulation of GABA neurotransmission in SCN neurons appears to be mediated by a strong inhibition of Ca2+ channels, specifically the P/Q- and N-type channels.
Presynaptic action of GABAB receptors
In most other regions of the brain, GABAB receptors at postsynaptic sites inhibit activity by increasing K+conductance and thus hyperpolarizing the membrane potential, resulting in slow IPSPs (Newberry and Nicoll, 1984; Gahwiler and Brown, 1985;Stevens et al., 1985; Crunelli et al., 1988; Lacey et al., 1988;Osmanovic and Shefner, 1988). In previous work examining the actions of GABAB receptors in SCN slices, no slow IPSPs were found (Kim and Dudek, 1992). Similarly, we detected no evidence of GABAB-mediated membrane potential changes in SCN neurons. However, in striking contrast to the lack of GABABinhibition of GABAA responses at the soma, we found substantial GABAB-mediated inhibition in presynaptic SCN axons that released GABA. Phase shifts of circadian rhythms regulated by the SCN can be generated by GABAB agonists (Ralph and Menaker, 1989; Gannon et al., 1995; Gillespie et al., 1997). Our data suggest that the primary action of GABAB receptors in SCN neurons may be to modulate GABA release from axon terminals; this inhibiton of GABA release may be one possible cellular substrate that plays a role in phase shifting.
Inhibition of calcium channels
GABAB receptors reduced Ca2+currents in SCN neurons. The channel that showed the greatest response to baclofen was the voltage-activated P/Q-type Ca2+channel. This channel appeared to account for a substantial Ca2+ current. This is the first demonstration of the relative importance of the P/Q channel in SCN neurons. N-type channels were also modulated by GABAB receptor activation. These channels (P/Q and N) may mediate the primary Ca2+influx in presynaptic axons required to trigger transmitter release. Blocking their actions with specific blockers substantially reduced the actions of GABAB receptors, suggesting that Ca2+ channels are the major effector of GABAB receptor modulation.
GABAB modulation of SCN neurons can be compared with hippocampal neurons, in which GABAB receptors have been studied more extensively. The SCN primarily contains GABAergic neurons, whereas the hippocampus contains both glutamatergic and GABAergic neurons. In the hippocampus, GABAB receptors modulate neurotransmission both presynaptically and postsynaptically and modulate both K+ and Ca2+channels (Dutar and Nicoll, 1988; Lambert and Wilson, 1993;Pfrieger et al., 1994; Doze et al., 1995). In contrast in the SCN, GABAB receptors modulate neurotransmission in a specific presynaptic way and only modulate Ca2+ channels, with little detectable effect on K+ channels (Kim and Dudek, 1992; this study). In hippocampal glutamatergic neurons, the miniature EPSCs in the presence of TTX were significantly affected by GABAB receptors (Scanziani et al., 1992;Dittman and Regehr, 1996). However, in both hippocampal and SCN GABAergic neurons, the miniature IPSCs were not modulated by GABAB receptors (Doze et al., 1995; this study). Furthermore, in both hippocampal and SCN inhibitory neurons, GABAB receptors modulate both P/Q-type and N-type Ca2+ channels, with a larger effect on P/Q-type Ca2+ channels. Whether these shared common properties of inhibitory hippocampal and SCN neurons can be extended to other GABAergic central neurons needs further work.
Modulation of calcium channels underlying the inhibition of GABA release
The present study demonstrates that the activation of GABAB receptors inhibited both whole-cell Ca2+ currents and presynaptic GABA release in SCN neurons. GABAB receptors can influence Ca2+ currents at the cell body and may modulate GABA release from the presynaptic axon in a similar manner. This is supported by several lines of evidence. First, baclofen inhibited both evoked IPSCs and Ca2+ currents in a dose-dependent manner. Second, baclofen strongly inhibited action potential-dependent IPSCs in the absence of TTX but had little effect on miniature IPSCs in the presence of TTX, suggesting that the release apparatus down-stream of Ca2+ influx is not modulated (Dittman and Regehr, 1996). More direct evidence comes from the parallel experiments using a combination of selective Ca2+ channel blockers together with baclofen. Baclofen inhibition of IPSCs was largely eliminated in the presence of Ca2+ channel blockers ω-conotoxin GVIA and agatoxin IVB, indicating that Ca2+ channels are probable mediators of GABAB receptor modulation in presynaptic nerve terminals.
Because it is difficult to measure Ca2+ currents directly at the small SCN axon terminals, we measured Ca2+ currents at the cell body. Although both the cell body and axon terminals may express similar Ca2+ channels, Ca2+ influx at the terminal may not be the same as at the cell body. The channel density and relative proportion of each specific type (L-, N-, P/Q-, and R-type) may be different in the soma and axon terminals of the same cell.
GABAB-related G-proteins
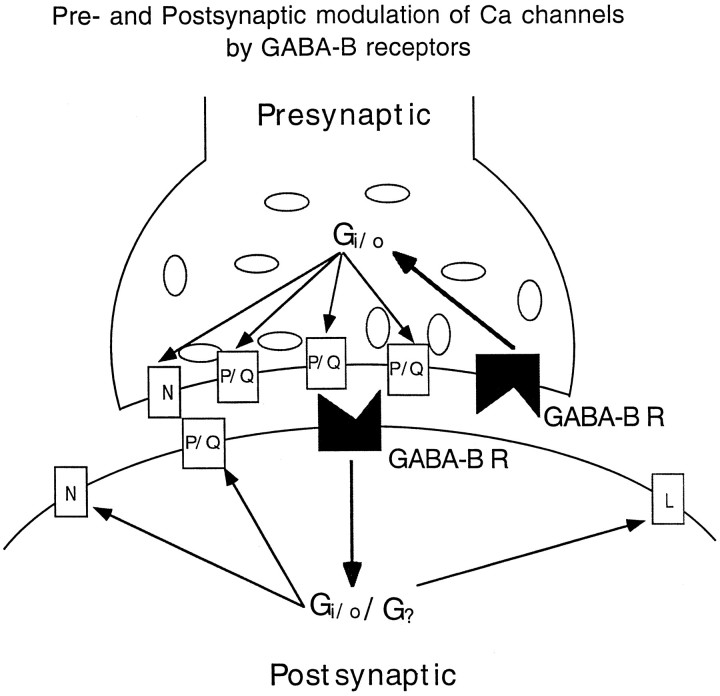
The actions of GABAB receptors on the presynaptic GABA release were substantially blocked by PTX. In contrast, the actions of GABAB receptors on the somatic Ca2+channels were only partially blocked by PTX. This suggests that although somatic Ca2+ channels in the SCN can be modulated by a PTX-sensitive GABAB action, there may be additional mechanisms whereby PTX-insensitive G-proteins also play a modulatory role in Ca2+ channels. Another possibility is that a different response to PTX exists in somata and presynaptic axons. A simplified scheme illustrating a differential modulation of GABAB receptors on presynaptic and postsynaptic Ca2+ channels is shown in Figure12, in which presynaptic P/Q-type and N-type channels are modulated by PTX-sensitive Gi and G0 proteins, but postsynaptic Ca2+channels (including P/Q-, N-, and L-type) are modulated by both PTX-sensitive and -insensitive G-proteins.
Fig. 12.
Simplified diagram illustrating G-protein-mediated presynaptic and postsynaptic GABABreceptor (GABAB R) modulation of multiple-type calcium channels.
Our data indicate that the GABAB autoreceptors modulate presynaptic GABA release by activating PTX-sensitive G-proteins. This is in contrast to one group of reports that found little effect of PTX on GABAB actions in presynaptic axons in hippocampal preparations (Dutar and Nicoll, 1988; Thompson and Gahwiler, 1992) but similar to another group that found that PTX blocked presynaptic effects of GABAB receptors on axon terminals (Scholz and Miller, 1991; Pfrieger et al., 1994; Pitler and Alger, 1994). These differences could be caused by multiple mechanisms of GABAB actions in the hippocampus or to different activational states of the GABAB receptor–second messenger system in different preparations. One advantage of the single neuron culture approach we used is that PTX has full access to all parts of the neuron plasma membrane. The same may not be true when PTX or related agents are injected into the brain or applied to brain slices in which diffusion may be impeded by multiple layers of astrocytes that surround axon terminals.
Functional implications
The modulation of intracellular Ca2+ may be generally relevant to phase shifting and clock mechanisms. For instance, in bulla, phase shifts of the circadian clock in the ocular circadian pacemaker are blocked by Ca2+ channel antagonists (Khalsa and Block, 1988). Similarly, Ca2+ can modulate the circadian variation of melatonin secretion in chick pineal cells (Nikaido and Takahashi, 1996). In previous work on the rat SCN in vitro, intracellular Ca2+ was found to play a crucial role in the maintenance of circadian rhythms of 2-deoxyglucose utilization (Shibata et al., 1987). The participation of GABAB receptors in modulating Ca2+influx may allow GABA to modulate cytoplasmic Ca2+levels, thereby potentially influence clock timing.
Previous studies have shown that GABAA and GABAB receptors may both play important roles in the phase-shifting actions of light on circadian rhythms (Ralph and Menaker, 1989; Gannon et al., 1995; Gillespie et al., 1997). The present study is the first to demonstrate a widespread and profound action of GABAB receptors on SCN neurons themselves, and that GABA released by axons of SCN neurons can act autosynaptically, depressing further GABA release from the same axon terminal. Golgi impregnation studies of SCN axons show substantial axon collaterals within the nuclei (van den Pol, 1980). Although many SCN synapses are surrounded by astrocytes that may inhibit free diffusion to other axon terminals (van den Pol et al., 1992), in some cases GABA can diffuse from an active axon to another nearby (Isaacson et al., 1993). Although the immediate action of GABA is inhibitory, the GABABreceptor-mediated inhibition of GABA release could have a net positive effect on local synaptic circuits by reducing inhibition.
A primary action of GABAB receptors in SCN neurons appears to be the modulation of GABA release from axons, and this inhibition of GABA release may be one cellular substrate influencing circadian phase shifts.
Footnotes
This work was supported by National Institutes of Health Grants NS34887 and NS10174, the National Science Foundation, and the Air Force Office of Scientific Research. We thank Drs. Anne Williamson, Karl Deisseroth, and Erika Piedras-Renteria for helpful suggestions on this manuscript and Dr. Y. Yang for technical help.
Correspondence should be addressed to Anthony van den Pol, Department of Neurosurgery, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520.
Gong Chen’s present address: Department of Molecular and Cellular Physiology, Beckman Center, Stanford University Medical School, Stanford, CA 94305.
REFERENCES
- 1.Bean BP. Multiple types of calcium channels in heart muscle and neurons. Modulation by drugs and neurotransmitters. Ann NY Acad Sci. 1989;560:334–345. doi: 10.1111/j.1749-6632.1989.tb24113.x. [DOI] [PubMed] [Google Scholar]
- 2.Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 4.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, van den Pol AN. Multiple NPY receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, van den Pol AN. Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. J Neurophysiol. 1997;77:3035–3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- 7.Colwell CS, Kaufman CM, Menaker M. Photic induction of Fos in the hamster suprachiasmatic nucleus is inhibited by baclofen but not by diazepam or bicucullin. Neurosci Lett. 1993;163:177–181. doi: 10.1016/0304-3940(93)90376-v. [DOI] [PubMed] [Google Scholar]
- 8.Crunelli V, Haby M, Jassik-Gerschenfeld D, Leresche N, Pirchio M. Cl−- and K+-dependent inhibitory postsynaptic potentials evoked by. J Physiol (Lond) 1988;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decavel C, van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 10.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diversé-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G-protein and tyrosine kinase pathways. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doze VA, Cohen GA, Madison DV. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol. 1995;74:43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Dutar P, Nicoll RA. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- 14.Francois-Bellan AM, Segu L, Hery M. Regulation by estradiol of GABAA and GABAB binding sites in the diencephalon of the rat: an autoradiographic study. Brain Res. 1989;503:144–147. doi: 10.1016/0006-8993(89)91715-0. [DOI] [PubMed] [Google Scholar]
- 15.Furshpan EJ, Landis SC, Matsumoto SG, Potter DD. Synaptic functions in rat sympathetic neurons in microcultures. I. Secretion of norepinephrine and acetylcholine. J Neurosci. 1986;6:1061–1079. doi: 10.1523/JNEUROSCI.06-04-01061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage PW. Activation and modulation of neuronal K+ channels by GABA. Trends Neurosci. 1992;15:46–51. doi: 10.1016/0166-2236(92)90025-4. [DOI] [PubMed] [Google Scholar]
- 17.Gahwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gannon RL, Cato MJ, Kelley KH, Armstrong DL, Rea MA. GABAergic modulation of optic nerve-evoked field potentials in the rat suprachiasmatic nucleus. Brain Res. 1995;694:264–270. doi: 10.1016/0006-8993(95)00854-j. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABAA and GABAB agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- 20.Huston E, Cullen GP, Burley JR, Dolphin AC. The involvement of multiple calcium channel sub-types in glutamate release from cerebellar granule cells and its modulation by GABAB receptor activation. Neuroscience. 1995;68:465–478. doi: 10.1016/0306-4522(95)00172-f. [DOI] [PubMed] [Google Scholar]
- 21.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacson JS, Hille B. GABAB-mediated presynaptic inhibition of excitatory transmission and synaptic vesicle dynamics in cultured hippocampal neurons. Neuron. 1997;18:143–152. doi: 10.1016/s0896-6273(01)80053-2. [DOI] [PubMed] [Google Scholar]
- 23.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12:433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 25.Khalsa SB, Block GD. Calcium channels mediates phase shifts of Bulla circadian pacemaker. J Comp Physiol [A] 1988;164:195–206. doi: 10.1007/BF00603950. [DOI] [PubMed] [Google Scholar]
- 26.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J Physiol (Lond) 1992;458:247–260. doi: 10.1113/jphysiol.1992.sp019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacey MG, Mercuri NB, North RA. On the K+ conductance increase activated by GABAB and dopamine D2. J Physiol (Lond) 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert NA, Wilson WA. Discrimination of post- and presynaptic GABAB receptor-mediated responses by tetrahydroaminoacridine in area CA3 of the rat hippocampus. J Neurophysiol. 1993;69:630–635. doi: 10.1152/jn.1993.69.2.630. [DOI] [PubMed] [Google Scholar]
- 29.Lambert NA, Wilson WA. High-threshold Ca2+ currents in rat hippocampal interneurones and their selective inhibition by activation of GABAB receptors. J Physiol (Lond) 1996;492:115–127. doi: 10.1113/jphysiol.1996.sp021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llinas R, Sugimori M, Hillman DE, Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian CNS. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 31.Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 32.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian CNS. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 33.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 34.Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- 35.Newberry NR, Nicoll RA. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. Nature. 1984;308:450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- 36.Nikaido SS, Takahashi JS. Calcium modulates circadian variation in cAMP-stimulated melatonin in chick pineal cells. Brain Res. 1996;716:1–10. doi: 10.1016/0006-8993(95)01521-3. [DOI] [PubMed] [Google Scholar]
- 37.Obrietan K, van den Pol AN (1998) GABAB receptor mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J Neurophysiol, in press. [DOI] [PubMed]
- 38.Osmanovic SS, Shefner SA. Baclofen increases the potassium conductance of rat locus coeruleus. Brain Res. 1988;438:124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- 39.Pfrieger FW, Gottmann K, Lux HD. Kinetics of GABAB receptor-mediated inhibition of calcium currents and excitatory synaptic transmission in hippocampal neurons in vitro. Neuron. 1994;12:97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 40.Pitler TA, Alger BE. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. J Neurophysiol. 1994;72:2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- 41.Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhim H, Toth PT, Miller RJ. Mechanism of inhibition of calcium channels in rat nucleus tractus solitarius by neurotransmitters. Br J Pharmacol. 1996;118:1341–1350. doi: 10.1111/j.1476-5381.1996.tb15543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 44.Scholz KP, Miller RJ. GABAB receptor-mediated inhibition of Ca currents and synaptic transmission in cultured rat hippocampal neurones. J Physiol (Lond) 1991;444:669–686. doi: 10.1113/jphysiol.1991.sp018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata S, Newman GC, Moore RY. Effects of calcium ions on glucose utilization in the rat suprachiasmatic nucleus in vitro. Brain Res. 1987;426:332–338. doi: 10.1016/0006-8993(87)90886-9. [DOI] [PubMed] [Google Scholar]
- 46.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens DR, Gallagher JP, Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal. Brain Res. 1985;358:360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- 48.Teramoto T, Kuwada M, Niidome T, Sawada K, Nishizawa Y, Katayama K. A novel peptide from funnel web spider venom, omega-Aga-TK, selectively blocks P-type calcium channels. Biochem Biophys Res Commun. 1993;196:134–140. doi: 10.1006/bbrc.1993.2225. [DOI] [PubMed] [Google Scholar]
- 49.Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol (Lond) 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 51.van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 52.van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- 53.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 54.van den Pol AN, Finkbeiner SM, Cornell-Bell A. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci. 1992;12:2648–2664. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Pol AN, Strecker GJ, Dudek FE. Excitatory and inhibitory amino acids and synaptic transmission in the suprachiasmatic nucleus. Prog Brain Res. 1996;111:41–56. doi: 10.1016/s0079-6123(08)60399-4. [DOI] [PubMed] [Google Scholar]
- 56.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]