Abstract
Previous work has shown that cysteine-string proteins (csps) are synaptic vesicle proteins that are important for evoked neurotransmitter release at Drosophila neuromuscular junctions. Indirect evidence has implicated csps in a regulatory link between synaptic vesicles and presynaptic calcium (Ca) channels. In this report, we use Ca Crimson to monitor stimulus-dependent changes of cytosolic Ca at motor nerve terminals of csp mutantDrosophila. These mutants display temperature-sensitive (TS) paralysis and a presynaptic failure of evoked synaptic transmission. We show that this TS inhibition of neuromuscular transmission is correlated with a block of Ca ion entry at nerve endings of csp mutants. These data support the hypothesis that csps mediate a regulatory interaction between synaptic vesicles and presynaptic Ca channels. Moreover, these results predict that if one depletes nerve endings of synaptic vesicles, one may see a reduction of presynaptic Ca ion entry. Defects of thedynamin gene in TS shibire mutantDrosophila interfere with synaptic vesicle recycling and lead to an activity-dependent depletion of these organelles. Our results show that Ca influx is blocked at nerve terminals ofshibire mutant larvae at the same time that synaptic transmission fails in these organisms. Thus, using two completely independent Drosophila mutants, we demonstrate that synaptic vesicles and csps are vital for the function of presynaptic Ca channels.
Keywords: Ca imaging, cysteine string proteins, Ca channels, presynaptic function, shibire, Drosophila
Cysteine-string proteins (csps) were first described in Drosophila as synapse-specific antigens (Zinsmaier et al., 1990). Independently, the cDNA encoding aTorpedo csp was identified using a suppression cloning strategy (Gundersen and Umbach, 1992). These latter investigations revealed that csp antisense RNA inhibited the expression of N-type calcium (Ca) channels in Xenopus oocytes that were co-injected with Torpedo electric lobe mRNA. Concomitantly, csp sense RNA augmented the expression of these same Ca channels without significantly altering their kinetics or voltage dependence (Gundersen and Umbach, 1992). These results led to the hypothesis that csps were either novel subunits or modulators of presynaptic Ca channels (Gundersen and Umbach, 1992).
Two developments have refined considerably our understanding of csps and their prospective function at synapses. First, studies of the subcellular distribution of csps revealed that these proteins were prominently associated with the P-face of synaptic vesicles from unstimulated Torpedo electric organ (Mastrogiacomo et al., 1994), and csps have since been reported to be membrane-associated components of several other types of secretory organelles (for review, see Buchner and Gundersen, 1997). These observations rendered it unlikely that csps were integral subunits of presynaptic Ca channels, and instead led us to propose that csps were participants in a regulatory interaction between docked (or docking) synaptic vesicles and presynaptic Ca channels (Mastrogiacomo et al., 1994; Umbach et al., 1995). However, the nature and mechanism of this interaction remain to be established.
The second important development is that Zinsmaier and colleagues (1994) isolated csp mutant alleles of Drosophila. These mutants developed slowly, showed sensory and motor deficits, and died prematurely (Zinsmaier et al., 1994). A particularly useful feature of these csp mutants was that they displayed temperature-sensitive (TS) paralysis (Zinsmaier et al., 1994). Subsequent investigations of the cellular basis of this TS paralytic phenotype revealed that stimulus-evoked neurotransmitter release ceased at temperatures above 30°C (Umbach et al., 1994). This block of synaptic transmission was not caused by changes in axonal conduction or in postsynaptic sensitivity to neurotransmitter. Instead, because spontaneous quantal transmitter secretion persisted in these mutants even when nerve impulses failed to evoke quantal transmitter release, we concluded that csp mutants were defective in excitation–secretion coupling (Umbach et al., 1994). These physiological results were consistent with the idea of a csp–Ca channel link, but they did not exclude other explanations.
To investigate further the cause of the secretory failure in TScsp mutants, we used ionic and pharmacological manipulations that either enhanced neurotransmitter secretion by augmenting Ca ion entry via presynaptic Ca channels or raised presynaptic Ca ion activity independently of Ca channels. Our results indicated that agents such as α-latrotoxin and ionomycin, which bypass presynaptic Ca channels and promote high frequency quantal discharges in wild-typeDrosophila, were equally effective at promoting quantal transmitter secretion in TS csp mutants (Umbach and Gundersen, 1997). However, other manipulations (e.g., depolarization using elevated KCl) that relied on the opening of presynaptic Ca channels failed to enhance quantal transmitter release incsp mutants (Umbach and Gundersen, 1997). These data provided further indirect support for the hypothesis that presynaptic Ca channels were blocked in csp mutants at the nonpermissive temperature. The current investigations examine this issue more directly. Here, we report the use of the fluorescent Ca-sensitive dye Ca Crimson to monitor presynaptic Ca dynamics of wild-type andcsp mutant Drosophila at permissive and nonpermissive temperatures. Moreover, we also monitored presynaptic Ca dynamics of TS shibire mutant Drosophila. Theshibire mutation produces a conditional blockade of synaptic vesicle recycling (Kosaka and Ikeda, 1983), and we hypothesized that the absence of synaptic vesicles might interfere with the function of presynaptic Ca channels. Our results argue that both synaptic vesicles and csps are important for Ca channel function at nerve endings.
MATERIALS AND METHODS
Materials. Wild-type Drosophila were the Canton S strain. csp mutants were thecspu1null allele kindly supplied by Dr. E. Buchner (University of Würzburg, Germany), and theshibire mutants were theshits1 allele (Grigliatti et al., 1973). As before (Umbach et al., 1994), the csp mutant larvae were preselected for TS paralytic behavior. Ca Crimson AM ester was obtained from Molecular Probes (Eugene, OR). The de-esterified form of this dye has a KD for Ca ions of 205 nm(manufacturer’s specifications). Fetal bovine serum, dimethylsulfoxide, and cremophor were from Sigma (St. Louis, MO). Schneider’s medium was purchased from Life Technologies-BRL (Gaithersburg, MD), and ionomycin was from Calbiochem (San Diego, CA).
Ca imaging. The procedure we used to monitor presynaptic Ca dynamics in type 1b boutons of Drosophila larvae was modified from that described in Umbach et al. (1998). Ca Crimson AM ester was dissolved in dimethylsulfoxide (at 5 mm) and added to a final concentration of 10 μm in Schneider’s medium with 15% fetal bovine serum and 0.05% cremophor. Third instar larvae were dissected and immobilized in a small, Sylgard-lined chamber and incubated with dye-containing solution for 2–3 hr in the dark at 18–22°C. The preparation was rinsed with a conventionalDrosophila saline solution (Jan and Jan, 1976; Umbach et al., 1994) with 2 mm CaCl2 and mounted on the viewing stage of an upright Olympus (model BX50WI) fluorescence microscope. A suction electrode was used to stimulate the motor nerves innervating muscle fibers 6 and 7 of abdominal hemisegments 2, 3, or 4. Synaptic boutons were identified by their characteristic morphology, along with the fact that Ca Crimson accumulates in these elements and displays a detectable resting fluorescence (see Results) (Umbach et al., 1998). Excitation of Ca Crimson fluorescence was achieved using a single wavelength (580 nm) grating, and for individual images illumination was maintained for a duration of 270 msec. Photobleaching was minimized via a 25% neutral density filter (Olympus ND25). To capture fluorescence emissions, we used a wide band yellow filter set (Olympus WIY; dichroic mirror 600 nm; excitation filter 545–580 nm and barrier filter 610 nm), and images were captured and stored using a Hamamatsu cooled ccd camera (model C4880) and the Hamamatsu Argus 50 Ca imaging system. Image files of 8 bits (512 × 483 pixels in area) were later analyzed for fluorescence intensity on a 0–255 gray scale using the Axon Imaging Workbench 2.1 software from Axon Instruments (San Jose, CA). For each figure, the same pseudocolor scale (from blue low, to red high) was added to the images. The color palette used the central two-thirds of the 0–255 gray scale and was chosen to highlight changes of Ca Crimson fluorescence.
Our standard protocol was to record fluorescent images of nerve terminals immediately before and within 30 sec after a fixed stimulus train (10 Hz for 2 min). The time course of the decay of Ca Crimson fluorescence after this 10 Hz stimulus train is documented in Results. This stimulation protocol was chosen because pilot experiments showed that it yielded a reproducible and significant increase in the intensity of fluorescence emission from Ca Crimson-loaded nerve endings of wild-type preparations (see Fig. 1). Here, we need to emphasize two procedural constraints that led us to select this stimulus paradigm. First, for the experiments using csp and shibiremutant preparations, we used visual observation to confirm when nerve impulses were no longer eliciting muscle twitches at the nonpermissive temperature. Because of this requirement that we verify by visual observation the blockade of neuromuscular transmission, we could not use neuromuscular blocking agents (and we also reasoned that it was preferable to avoid the use of agents that might have indirect presynaptic effects). However, because our preparations (with the exception of those that became paralyzed) moved during the test stimulus train, we needed several seconds (usually, 5–20 sec) to refocus on the nerve terminal before capturing images for analysis of Ca Crimson fluorescence. This secondary constraint forced us to select a stimulation protocol that caused a prolonged and relatively stable increase of nerve ending Ca Crimson fluorescence. This stable change in turn allowed us to acquire suitable images for analysis. Moreover, because the critical issue for these experiments was the question of whether there was Ca entry at nerve endings of csp andshibire mutants when synaptic transmission was blocked, we also monitored Ca Crimson fluorescence in these paralyzed preparations during the stimulus train (as well as immediately after).
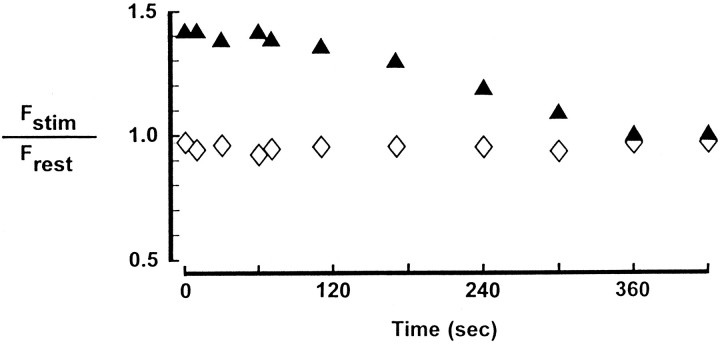
Fig. 1.
Ca Crimson fluorescence in larval neuromuscular preparations with and without nerve stimulation. Separate nerve terminals in a Ca Crimson-loaded preparation were analyzed for the relative intensity of fluorescence emission in the absence of nerve stimulation (⋄) or immediately after (at the times indicated) 2 min of 10 Hz stimulation of the motor nerve (▴). In the control preparation (⋄) the motor nerve of the adjacent hemisegment was stimulated (10 Hz, 2 min) to mimic the movement seen with stimulation of the correct motor nerve (▴). Frest was obtained from images captured 30 sec before the stimulus train.Fstim is mean bouton fluorescence assessed at the indicated times after stimulation. Data are mean values for at least 10 boutons per terminal. The SD did not exceed 20% of the mean.
To quantitate changes of Ca Crimson fluorescence for individual synaptic boutons, we measured the intensity of Ca Crimson fluorescence at each bouton before stimulation and after nerve stimulation. We performed a background subtraction for each bouton by obtaining the mean fluorescence intensity from at least 10 different background regions (in the muscle), each the equivalent size of individual boutons. This background value varied among preparations (ranging from 40 to 80 units on the 0–255 gray scale) and was obtained both at rest and after nerve stimulation. Background was subtracted from the resting or poststimulation fluorescence intensity associated with single boutons to yield Frest andFstim, respectively. These parameters were measured for a minimum of 10 boutons per neuromuscular junction and are reported in Table 2. The ratio ofFstim/Frest was determined separately for each bouton to quantitate the change in fluorescence of individual boutons, and the meanFstim/Frest value was then determined for each neuromuscular junction by averaging the individual bouton ratios. By computing this ratio one minimizes differences among preparations that are caused by variable dye loading. The stimulus-induced changes in Ca Crimson fluorescence were then compared for the same boutons at permissive (22°C) and nonpermissive (32°C) temperatures. These data comprise Table1. To assess possible changes in the basal, bouton fluorescence of Ca Crimson between 22°C and 32°C, we computed the ratio of Frest values for individual boutons at these two temperatures and calculated the meanFrest32/Frest22for each neuromuscular junction. Frest at 32°C was measured 15 min after heating to 32°C for wild-type andcsp mutants, and unless indicated otherwise forshibire mutants, Frest at 32°C was measured after a total 16–17 min incubation at 32°C, which included the 5 min rest period after nerve stimulation that was used to deplete synaptic vesicles.
Table 2.
Frest andFstim intensity values
| Preparation | Temperature (°C) | Frest | Fstim |
|---|---|---|---|
| Wild-type control (n = 4) | 22 | 35 ± 8.5 | 54 ± 8.3 |
| 32 | 36 ± 9.2 | 53 ± 14.0 | |
| csp mutant (n = 6) | 22 | 27 ± 7.2 | 33 ± 6.7 |
| 32 | 27 ± 6.6 | 26 ± 6.0 | |
| 22 | 25 ± 4.9 | 30 ± 4.7 | |
| shibire mutant (n = 4) | 22 | 22 ± 5.0 | 32 ± 9.5 |
| 32 | 23 ± 3.8 | 22 ± 3.5 | |
| 22 | 21 ± 2.2 | 25 ± 2.4 |
These are the mean (±SD) intensity measurements (on a 0–255 gray scale) for all boutons either at rest or after stimulation in the number of preparations indicated in parentheses.
Table 1.
Relative fluorescence of Ca Crimson at nerve terminals before and after stimulation of the motor nerve
| Preparation | Temperature (°C) | Fstim/Frest |
|---|---|---|
| Wild-type control (4) | 20 | 1.52 ± 0.23 |
| 32 | 1.43 ± 0.20 | |
| csp mutant (6) | 20 | 1.26 ± 0.20 |
| 32 | 0.95 ± 0.07 | |
| 20 | 1.18 ± 0.11 | |
| shibire mutant (4) | 20 | 1.51 ± 0.27 |
| 32 | 0.99 ± 0.03 | |
| 20 | 1.21 ± 0.10 |
For each nerve terminal we obtained a meanFstim/Frest from individual boutons (≥10 boutons per terminal). For nterminals (in separate preparations) a population mean (±SD) was computed and is presented here asFstim/Frest.
To assess the maximum detectable increase in Ca Crimson fluorescence intensity in larval boutons, we used the calcium ionophore ionomycin (10 μm). This reagent was applied in the presence of 5 mm CaCl2, and fluorescent images were captured after 10 min in ionomycin and compared with resting fluorescence before ionophore application.
RESULTS
The fluorescent, Ca-sensitive dye Ca Crimson was loaded into nerve endings of wild-type Drosophila larvae. As reported (Umbach et al., 1998), this dye loads preferentially into presynaptic boutons. Thus, our first goal was to establish conditions of nerve stimulation that lead reproducibly to an increase of Ca Crimson fluorescence in these nerve terminals. The protocol that we adopted (10 Hz stimulation for 2 min) yields a significant increase (p < 0.01 by the paired Student’s t test) in the mean intensity of fluorescence emission from nerve terminal boutons immediately after the stimulus train (Figs. 1,2a,b). The ratio ofFstim/Frestremains elevated for several tens of seconds before decaying exponentially to the resting fluorescence level ∼5 min after the stimulus train (Fig. 1). We have not investigated systematically the processes that contribute to this relatively sustained increase of Ca Crimson fluorescence at motor endings of Drosophila larvae under these conditions. However, four additional sets of observations are important for interpreting these results. First, as shown in Figure1, basal nerve terminal fluorescence (without stimulation of the nerve) is very stable. In other words, the increase of Ca Crimson fluorescence emission in the stimulated preparation of Figure 1 is a response to nerve stimulation. Second, this stimulus-dependent change of Ca Crimson fluorescence (Figs. 1, 2) is eliminated in solutions devoid of Ca (containing 10 mm MgCl2 and 0.1 mmEGTA with no added CaCl2) or when CdCl2(1 mm) is added to the extracellular medium (data not shown). These latter results indicate that the observed change of Ca Crimson fluorescence is contingent on entry of extracellular Ca ions, presumably via presynaptic Ca channels. Third, we have detected a smaller (Fstim/Frest < 1.1) and more rapid decay of fluorescence (a return to basal fluorescence intensity occurs in <5 sec) in immobilized preparations subjected to 10 Hz stimulation for 5 sec. However, the more robust signal-to-noise ratio obtained with 2 min of 10 Hz stimulation led us to select this paradigm for subsequent experiments. Fourth, we assessed the maximum increase of Ca Crimson fluorescence that could be detected in these preparations using the calcium ionophore ionomycin (10 μm). Ionomycin treatment produces a nearly fourfold increase of Ca Crimson fluorescence (3.7 ± 0.7 forn = 5), which indicates that the 1.4- to 1.5-fold changes (Fig. 1, Table 1) we detect with nerve stimulation are subsaturating.
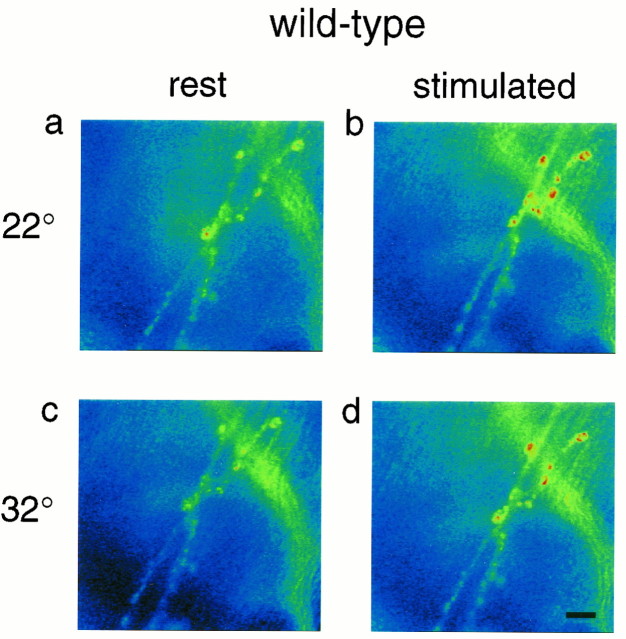
Fig. 2.
Stimulus-dependent changes of Ca Crimson fluorescence at a wild-type neuromuscular junction. Ca Crimson fluorescence increases at presynaptic boutons as a function of nerve stimulation at both 22 and 32°C (a, c: rest; b, d: stimulated). Scale bar, 17 μm. The same magnification is used in Figures 3 and 4.
Images typical of those analyzed to produce Figure 1 are presented in Figure 2a,b. Here, a wild-type larval preparation is shown before (Fig. 2a) and ∼15 sec after (Fig.2b) an episode of 10 Hz stimulation for 2 min. As indicated by the pseudocolor scale (Fig. 2a,b), Ca Crimson fluorescence emission increases appreciably in response to nerve stimulation in this preparation at 22°C. Data summarized in Table 1 show that the average value forFstim/Frest is 1.52 for four separate control preparations at 22°C. Using the paired Student’s t test, this mean increase of Ca Crimson fluorescence (relative to rest) is significant at p < 0.01. Next, we warmed these preparations, and after 15 min at 32°C, a stimulus train (10 Hz for 2 min) was delivered. This resulted in a similar (to 22°C) stimulus-dependent increase of Ca Crimson fluorescence (Fig. 2c,d, Table 1). Again, this stimulus-dependent increase of Ca Crimson fluorescence at 32°C was statistically significant (p < 0.01 by the paired Student’s t test). Mean boutonFrest and mean boutonFstim intensities are in Table2. In addition to confirming the trend in Table 1, the data in Table 2 and the ratios in Table3 are important because they show that there is no significant change in the resting fluorescence intensity of Ca Crimson at 32°C versus 22°C. This stability ofFrest is necessary to maintain the fidelity of detection of stimulus-dependent changes of presynaptic Ca.
Table 3.
Ratio of Ca Crimson fluorescence intensity at resting presynaptic terminals at 32°C versus 22°C
| Preparation | Frest(32)/Frest (22) |
|---|---|
| Wild-type control (4) | 0.96 ± 0.13 |
| cspmutant (6) | 0.98 ± 0.08 |
| shibire mutant (4) | 1.09 ± 0.13 |
Results are the mean ± SD of the number of experiments in parentheses.
Our next step was to investigate the effect of nerve stimulation on presynaptic Ca ion activity in TS csp mutant larvae. As shown (Fig. 3a,b, Table1), there was a significant increase (p < 0.05 by the paired Student’s t test) in the intensity of Ca Crimson fluorescence using the standard stimulation paradigm at 22°C (again, significance was assessed by comparingFstim vs Frest at individual boutons in these preparations, and as before the mean intensity data are in Table 2). Interestingly, at 22°C the ratioFstim/Frest was consistently lower in these mutants relative to wild-type controls (Table 1), which indicated that presynaptic Ca ion activity was reduced in these organisms even at room temperature (note that quantal content was also lower in csp mutants relative to controls at 22°C) (Umbach et al., 1994).
Fig. 3.
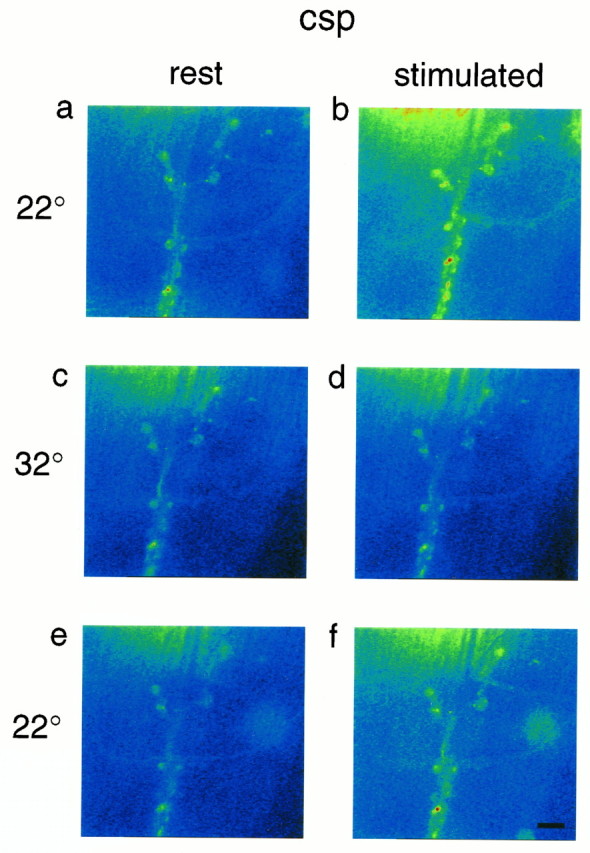
Stimulus-dependence of Ca Crimson fluorescence at a csp mutant neuromuscular junction. Nerve impulse-dependent increases of Ca Crimson fluorescence are seen at nerve endings at 22°C before (a, b) and after (e, f) a 32°C challenge. At 32°C, nerve impulses fail to alter Ca Crimson fluorescence (c, d).
Previous work had shown that when TS csp mutant larvae were warmed to 30–32°C, there was a progressive decline of evoked transmitter release that eventually gave way to a complete failure of evoked responses and muscle twitch (Umbach et al., 1994; Umbach and Gundersen, 1997). For the current experiments, we warmed the preparations to 32°C and waited (10–15 min) until single nerve impulses failed to elicit muscle contraction before recording the impact of a stimulus train on Ca Crimson fluorescence. As illustrated (Fig. 3c,d, Table 2), a standard episode of nerve stimulation produced no increase in the intensity of Ca Crimson fluorescence at motor nerve terminals of this csp mutant preparation at 32°C. In six separate preparations,Fstim/Frest was 0.95 ± 0.07 (Table 1). Because these preparations did not move, we were able to capture images within 1 sec after the completion of the stimulus train. Moreover, we recorded separate images during the test stimulus train at 32°C and observed no increase of Ca Crimson fluorescence in the synaptic boutons (data not shown). Thus, at no time during or after the test stimulus train did we detect any increase of Ca Crimson fluorescence at nerve endings of csp mutants at the nonpermissive temperature. These results suggest that Ca entry is severely compromised in these mutants at elevated temperature (compared with room temperature or wild-type controls at 32°C) (Table 1). Finally, the results in Table 3 show that resting Ca Crimson fluorescence is essentially unchanged between 22°C and 32°C (the ratio is close to 1), which indicates that there was no temperature-dependent change of resting Ca ion homeostasis in these mutants.
The TS failure of evoked neurotransmitter release in cspmutants was reversible with cooling (Umbach et al., 1994; Umbach and Gundersen, 1997) and so was the effect on stimulus-dependent Ca ion entry. Thus, when the same preparation of Figure 3a–d was cooled to 22°C, we observed a recovery of the Ca Crimson response to nerve stimulation (Fig. 3e,f, Table 2). As summarized in Table 1,Fstim/Frest of preparations after a challenge at 32°C was similar to the ratio obtained before warming (1.26 ± 0.20 vs 1.18 ± 0.11). These results show that csp mutant preparations retained the ability to respond to nerve stimulation and that the absence of a change of cytosolic Ca at 32°C reflected a physiological deficit in these organisms.
shibire mutant Drosophila display a TS failure of stimulus-evoked neurotransmitter release that has been correlated with a loss of synaptic vesicles (Poodry and Edgar, 1979; Kosaka and Ikeda, 1983). We reasoned that if a regulatory link between synaptic vesicles and presynaptic Ca channels normally existed (Mastrogiacomo et al., 1994; Umbach et al., 1995), this link might cease to function when synaptic vesicles were depleted. To assess this possibility, we monitored presynaptic Ca ion activity of shibire mutant larvae at permissive and nonpermissive temperatures. As indicated (Fig.4a,b, Table 2),shibire mutants displayed an enhanced fluorescence intensity of Ca Crimson in response to nerve impulses that was reminiscent of wild-type controls at 22°C (Figs. 1, 2). Overall,Fstim/Frest forshibire larvae at 22°C was indistinguishable from controls (Table 1). These results imply that Ca entry was unaffected in these mutants at the permissive temperature.
Fig. 4.
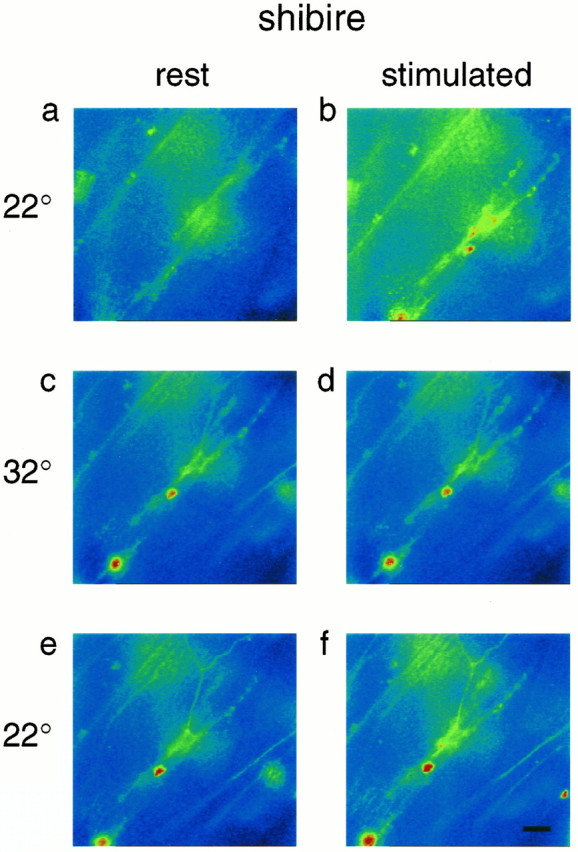
Stimulus dependence of Ca Crimson fluorescence at a shibire mutant neuromuscular junction. Nerve impulses evoke an increase of Ca Crimson fluorescence at 22°C (a, b). After warming to 32°C and depleting the nerve terminals of releasable quanta, the same stimulation protocol fails to raise Ca Crimson fluorescence (c, d). Note that the 5 min recovery period that we used after the initial stimulus train was insufficient for the resting fluorescence of Ca Crimson to return to the same level as in a. Partial recovery of the stimulus-dependent increase of Ca Crimson fluorescence is detected 10 min after the preparation was cooled from 32 to 22°C (e, f).
We next warmed the shibire mutant preparations to 32°C for 10 min and stimulated the motor nerve (at 10 Hz for 1–2 min) until paralysis (failure of muscle twitch) was complete. This conditioning train of impulses elicited a significant increase (relative to resting terminals) in the intensity of Ca Crimson fluorescence measured at the onset of paralysis (Fstim/Frest = 1.29 ± 0.10 for the same four preparations of Table 1). Thus, we were able to detect changes of presynaptic Ca ion activity during the period of time when shibire nerve terminals were being depleted of synaptic vesicles. This was an important observation, because we were concerned that as vesicle depletion proceeded, it might have increased the volume of shibire nerve terminals (thereby diluting the Ca Crimson), and this could have compromised our ability to detect changes of Ca ion activity. Instead, our data argue that nerve terminal volume did not change appreciably inshibire mutants under these conditions. A similar conclusion was drawn by Koenig and Ikeda (1989), who showed that there was no detectable expansion of the plasma membrane of shibire nerve terminals at the nonpermissive temperature.
After we induced paralysis in the shibire mutant larvae at 32°C, we were then interested in the response of the nerve terminals to nerve stimulation. Thus, after a rest period (5 min) to allow cytosolic Ca to recover to the basal level, we delivered the standard train of nerve impulses (10 Hz for 2 min) and recorded presynaptic Ca Crimson fluorescence. In this situation, we detected no increase of Ca Crimson fluorescence in images taken within 1 sec after the stimulation protocol (Fig. 4c,d, Tables 1, 2) (moreover, images captured during this stimulus train also showed no significant change of Ca Crimson fluorescence; data not shown). The difference betweenFstim/Frest at 22°C and 32°C (Table 1) was highly significant for these mutants (p < 0.01 by the paired Student’s ttest). These data suggest that Ca ion entry was inhibited, and that presynaptic Ca channels failed to operate in shibiremutants, after they were paralyzed at 32°C. As before, we also verified that the resting fluorescence of Ca Crimson at 32°C was not abnormally high relative to 22°C (Table 3), because potential dye saturation would have compromised our ability to detect changes of cytosolic Ca. Instead, all of our results (Fig. 4, Tables 1, 2) point to a block of the stimulus-dependent change of cytosolic Ca ion activity in paralyzed shibire mutants.
Finally, to verify the integrity of the shibire preparations subsequent to the 32°C challenge, we cooled the larvae back to 22°C and saw a partial recovery of stimulus-dependent changes of presynaptic Ca ion activity (Fig. 4e,f, Table 2). We consistently found that the ratio,Fstim/Frest, after recovery from the 32°C challenge in shibire, was less than the ratio before 32°C (Table 1). Concomitantly, we observed that the rate of failure of excitatory junctional potentials (in response to 10 Hz stimulation for 2 min), before 32°C challenge was <4%, whereas afterward in two of three preparations it exceeded 50%. Thus, within the time period that we were testing for recovery of responses in shibire mutant larvae, there was a parallel decline of both the Ca signal and quantal transmitter release elicited by 10 Hz stimulation.
DISCUSSION
These investigations were undertaken to test the hypothesis that the TS abolition of neurotransmitter release in cspmutant Drosophila reflects a failure of stimulus-dependent Ca ion entry at nerve endings. Previous work had shown that evoked, but not spontaneous, quantal secretion of neurotransmitter ceased in these mutants at the nonpermissive temperature (Umbach et al., 1994; Umbach and Gundersen, 1997). Moreover, because action potential conduction and postsynaptic sensitivity to glutamate remained unaltered incsp mutants at elevated temperatures, we had concluded that there was a failure in the process that couples action potential-dependent depolarization to the synchronous release of neurotransmitter at csp nerve endings (Umbach et al., 1994). A recent extension of these studies revealed that agents that raised cytosolic Ca ion activity independently of voltage-gated Ca channels were effective at promoting quantal transmitter release incsp mutants at the nonpermissive temperature (Umbach and Gundersen, 1997). However, treatments that relied on the opening of presynaptic Ca channels were ineffective at overcoming the secretory block in csp mutants (Umbach and Gundersen, 1997). Although these latter results were compatible with the hypothesis that there was a defect in the entry of Ca ions at nerve endings of cspmutant Drosophila, we could not formally exclude other explanations (for instance, our data did not eliminate the possibility that there was a shift in the affinity for Ca ions of the Ca sensor that regulates exocytosis at nerve endings (Umbach and Gundersen, 1997). The current investigations provide independent support for the idea that presynaptic Ca channels fail to operate normally incsp mutant Drosophila. Thus, our results indicate that there is a parallel blockade of evoked neurotransmitter release and stimulus-dependent changes of presynaptic ionized Ca in paralyzedcsp and shibire mutant Drosophila at 32°C. Because there is no precedent for the involvement of thecsp and shibire gene products in presynaptic Ca buffering (and there are significant, stimulus-dependent changes of presynaptic Ca in these mutants at permissive temperatures), we interpret the lack of change of nerve terminal Ca in the mutant organisms at 32°C as reflecting a decline of Ca entry. These observations have important implications for nerve terminal and csp function.
Among the implications of this study is that csps participate in a regulatory interaction involving presynaptic Ca channels. Because csps are synaptic vesicle proteins (Mastrogiacomo et al., 1994; for review, see Buchner and Gundersen, 1997), we infer that this reaction occurs during or subsequent to the docking of a synaptic vesicle in the vicinity of presynaptic Ca channels. Obviously, many questions remain to be answered about the biochemical and biophysical mechanisms of this csp–Ca channel link. However, one conclusion, which is inescapable, is that this interaction is not essential for the survival ofDrosophila. Thus, we know that mutants lacking thecsp gene are viable; however, they die prematurely (Zinsmaier et al., 1994). Hence, it appears that this csp–Ca channel link helps to sustain normal presynaptic function but that compensatory mechanisms can supplant the loss of csps in csp mutantDrosophila. We propose that it is the temperature-dependent failure of this “compensatory machinery” that underlies the TS blockade of transmitter release in the csp mutants. Future investigations of the mechanism of the csp–Ca channel link should provide insights into these compensatory pathways and their thermal sensitivity in the csp mutants.
As outlined above (and previously) (Umbach et al., 1995), it is reasonable to propose that csps interact with Ca channels during or after the docking of a synaptic vesicle at the plasma membrane. Assuming that this is the case, it is also plausible that this interaction ceases to operate at nerve endings that are depleted of synaptic vesicles. It is in this context that shibire mutantDrosophila offer a unique opportunity to test the hypothesis that synaptic vesicles (and vesicle-associated proteins, such as csps) participate in modulating nerve ending Ca channels. This is because point mutations in the shibire gene, which encodes dynamin (Chen et al., 1991; van der Bliek and Meyerowitz, 1991), lead to a temperature-dependent block of synaptic vesicle recycling in these organisms. This failure of vesicle recycling produces a TS block of synaptic transmission and a depletion of synaptic vesicles (Poodry and Edgar, 1979; Kosaka and Ikeda, 1983). Thus, we tested the status of Ca ion influx at nerve endings ofshibire mutant Drosophila at a time when synaptic transmission was completely blocked. Our results indicate that Ca ion influx at nerve endings is conditionally attenuated in theseshibire mutants. Ca influx is normal at the permissive temperature, but it is blocked at elevated temperatures. These observations support the hypothesis that synaptic vesicles participate in a physiological modulation of presynaptic Ca channels. On the basis of the results with the csp mutants, we infer that csps are part of this vesicle-mediated signaling process. Presumably, the purpose of this vesicle-channel link is to confine Ca ion entry to sites at the nerve ending where a vesicle is poised to discharge its contents. In other words, our results predict that Ca ion entry at nerve endings should be restricted to those sites where synaptic vesicles (with their associated csps) are appropriately docked and primed for exocytosis.
An important goal for the future is to relate the current observations to work of others that has documented a considerable degree of variation among cell types in the size of the Ca domains that regulate secretion (for review, see Schweizer et al., 1995; Stanley, 1997). For instance, work of Borst and Sakmann (1996) led to the conclusion that an influx of >10,000 Ca ions from as many as 60 Ca channels contributes to the triggering of individual exocytotic events at a calyciform synapse in the rat auditory system. At the other end of the spectrum, work of Stanley (1993) has suggested that single exocytotic events may be induced by the influx of far fewer Ca ions (∼200). Because we have estimated that a single synaptic vesicle harbors appreciably fewer than 60 csp molecules (Mastrogiacomo et al., 1994), the stoichiometry of the csp–Ca channel link becomes an issue. In other words, to reconcile our results with those of Borst and Sakmann (1996), it is necessary to assume that a single csp can alter the function of more than one presynaptic Ca channel (or that there is cooperation among vesicles in the modulation of these channels). At the same time, it is easier to accommodate a link between csps and the smaller number of release-triggering Ca channels that is suggested by the work of Stanley (1993). Resolution of these issues should considerably improve our understanding of presynaptic secretory dynamics.
Footnotes
This work was supported by National Institutes of Health Grant NS31934 (J.A.U.) and a Grant-in-aid and a research travel grant from the Japanese Ministry of Education (Y.K.). We thank Drs. H. Kuromi and A. Ueda for help with the Ca imaging and Dr. A. Grinnell for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Joy A. Umbach, The Department of Molecular and Medical Pharmacology and The Crump Institute for Biological Imaging, University of California Los Angeles School of Medicine, Los Angeles, CA 90095.
REFERENCES
- 1.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 2.Buchner E, Gundersen CB. The DNA-J-like cysteine string protein and exocytotic neurotransmitter release. Trends Neurosci. 1997;20:223–227. doi: 10.1016/s0166-2236(96)10082-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 4.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster XIV. Selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 5.Gundersen CB, Umbach JA. Suppression cloning of the cDNA encoding a candidate presynaptic calcium channel subunit of Torpedo. Neuron. 1992;9:527–537. doi: 10.1016/0896-6273(92)90190-o. [DOI] [PubMed] [Google Scholar]
- 6.Jan L, Jan YN. Properties of the larval neuromuscular junction of Drosophila melanogaster. J Physiol (Lond) 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockade of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- 9.Mastrogiacomo A, Parsons SM, Zampighi GA, Jenden DJ, Umbach JA, Gundersen CB. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic calcium channels. Science. 1994;263:981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- 10.Poodry CA, Edgar L. Reversible alterations in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweizer FE, Betz H, Augustine GJ. From vesicle docking to endocytosis: intermediate reactions of exocytosis. Neuron. 1995;14:689–696. doi: 10.1016/0896-6273(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 12.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 13.Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- 14.Umbach JA, Gundersen CB. Evidence that cysteine string proteins regulate an early step in the Ca2+-dependent secretion of neurotransmitter at Drosophila neuromuscular junctions. J Neurosci. 1997;17:7203–7209. doi: 10.1523/JNEUROSCI.17-19-07203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13:899–908. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 16.Umbach JA, Mastrogiacomo A, Gundersen CB. Cysteine string proteins and presynaptic function. J Physiol (Paris) 1995;89:95–101. doi: 10.1016/0928-4257(96)80556-0. [DOI] [PubMed] [Google Scholar]
- 17.Umbach JA, Grasso A, Zurcher SD, Kornblum HI, Mastrogiacomo A, Gundersen CB (1998) Electrical and optical monitoring of α-latrotoxin action at Drosophila neuromuscular junctions. Neuroscience, in press. [DOI] [PubMed]
- 18.van der Bliek A, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 19.Zinsmaier KE, Hofbauer A, Heimbeck G, Pflugfelder GO, Buchner S, Buchner E. A cysteine-string protein is expressed in retina and brain of Drosophila. J Neurogenet. 1990;7:15–29. doi: 10.3109/01677069009084150. [DOI] [PubMed] [Google Scholar]
- 20.Zinsmaier KE, Eberle KK, Buchner E, Walter K, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]