Abstract
Most retinal ganglion cells respond only transiently, for ∼150 msec at the onset and termination of a light flash. The responses are transient because it has been shown that bipolar-to-ganglion cell transmission is truncated after 150 msec by a feedback inhibition to bipolar cell terminals. The feedback inhibition itself must be delayed by ∼150 msec to allow the initial bipolar–ganglion cell transmission. This study identifies a three-component serial synaptic pathway from glycinergic amacrine cells to GABAergic amacrine cells to bipolar cell terminals as one source of this delay. We used perforated and whole-cell patch-clamp recordings to measure the timing of light responses in amacrine, bipolar, and ganglion cells under control and glycine and GABA receptor-blocked conditions. Our results suggest that, after a light flash, a population of glycinergic amacrine cells responds first, inhibiting a population of GABAergic amacrine cells for ∼150 msec. The GABAergic amacrine cells feed back to bipolar terminals, but only after the 150 msec delay, allowing the bipolar terminals to excite ganglion cells for the first 150 msec. Blocking the glycinergic amacrine cell activity with strychnine allows the GABAergic system to become active earlier. GABAergic amacrine cells then inhibit release from bipolar cells earlier. Under these conditions, the ganglion cell response to change would be decreased.
Keywords: amacrine cell, glycine, GABA, disinhibition, reciprocal inhibition, retina, patch clamp
A majority of retinal ganglion cells in the salamander retina respond transiently, generating a brief burst of activity at the onset and termination of a light flash. Previous studies suggested that this transient response was mediated by a feedback inhibition from amacrine cells, truncating release from bipolar cell terminals (Dowling and Werblin, 1969; Burkhardt, 1972;Toyoda and Fujimoto, 1984; Tachibana and Kaneko, 1987; Werblin et al., 1988). Recently, Dong and Werblin (1997) identified a locally driven GABAC synapse at bipolar cell terminals that may underlie these transient responses. When this feedback synapse is blocked by picrotoxin, ganglion cell responses become much more sustained. To mediate transient activity, the inhibitory feedback must be delayed so that bipolar cells can transmit an initial brief burst of excitation to ganglion cells before being inhibited. This study identifies a mechanism mediating this delay in the feedback pathway.
It is well established from analysis of anatomical measurements that amacrine cells form both serial and reciprocal synapses throughout the inner plexiform layer in a variety of vertebrates (Dowling and Boycott, 1966; Dowling and Werblin, 1969; Boycott and Wassle, 1974; Wong-Riley, 1974; Vallerga, 1981). Except for the well-studied role of the AII amacrine cell in mammals (Vaney, 1985; Wassle et al., 1991, 1995;Strettoi et al., 1992; Mills and Massey, 1995; Smith and Vardi, 1995;Vardi and Smith, 1996), little is known about the functional role of these complex synaptic pathways. Recently, Zhang et al. (1997), recording from ganglion cells in salamander, inferred that serial interactions between glycinergic and GABAergic amacrine cells could mediate disinhibition and alter the timing of ganglion cell activity. Our study specifically identifies the response waveforms of each of the amacrine cell types and shows how the required delay is mediated by amacrine cell interactions.
Previous studies in salamander have identified two main amacrine cell classes, a narrow-field cell with a lateral spread of processes of ∼150 μm that contains GABA and a wide-field cell with a lateral spread up to 500 μm that contains glycine. Both cell types appear to have both glycine and GABA receptors. Both cell types make synaptic contact with other amacrine cells, but only GABAergic cells make feedback synaptic contact to bipolar cell terminals (Barnes and Werblin, 1987; Werblin et al., 1988; Maguire et al., 1989; Yang et al., 1991; Lukasiewicz and Werblin, 1994; Dong and Werblin, 1997). Both cell types also feed forward to ganglion cells, and it has been shown that the lateral extent of both glycine and GABA inhibition matches the spread of the processes for the amacrine cell types that contain these transmitters (Lukasiewicz and Werblin, 1990).
We show here that the wide-field amacrine cells respond first, inhibiting the narrow-field amacrine cells. The narrow-field amacrine cells respond only after a delay and make delayed inhibitory synaptic contact with bipolar cell terminals, generating a delayed feedback inhibition. This allows bipolar cells to excite ganglion cells briefly before being inhibited by the delayed feedback.
MATERIALS AND METHODS
Preparation. Experiments were performed on larval tiger salamander slices described by Werblin (1978).
Whole-cell patch-clamp recording. Whole-cell patch-clamp recordings (Hamill et al., 1981) were performed on amacrine cells as described by Barnes and Werblin (1986). Patch pipettes were pulled from borosilicate glass tubes (TW120F-4; World Precision Instruments, Sarasota, FL) on a Flaming-Brown micropipette puller (P-87; Sutter Instruments, Novato, CA). The pipette resistance was 5–10 MΩ in the control bath solution. The voltage- and current-clamp recordings were performed with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA). The signal was filtered at 1 kHz and digitized at 1 kHz (voltage-clamp mode) or 3 kHz (current-clamp mode) by a 100 kHz Lab Master DMA board (Scientific Solutions, Solon, OH) in a personal computer. The recording software Patchit was developed by George Grant (Grant and Werblin, 1994) in our laboratory. The recorded data were analyzed in Mathematica 3.0 (Wolfram Research, Champaign, IL) and Origin 3.5 (MicroCal Software, Northampton, MA). The junction potential was measured to be −12 mV and is corrected by a constant offset during the experiment.
Perforated patch-clamp recording. Nystatin-perforated patch (Horn and Marty, 1988) was used to eliminate the rundown of light response in ON bipolar cells and to keep intact the state of GABA and glycine receptors in both bipolar and amacrine cells. Briefly, a stock solution of 50 mg/ml nystatin (in DMSO) was diluted to a final concentration of 300 μg/ml in the intracellular solution. The tip of the pipette was filled with nystatin-free solution, and the pipette was backfilled with the nystatin-containing electrode solution. After obtaining a gigaohm seal, a capacitive transient appeared within 5 min and increased to its final, stable magnitude within 20 min. All the bipolar cells and ∼80% of the amacrine cells were measured by this method.
Bath solution. The control bath solution contained (in mm): 108 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES, and 10 glucose. The pH was adjusted to 7.8 with NaOH. The blockers were added to the control solution. The concentrations of the blockers were 10 μm strychnine, 100 μm bicuculline, and 100 μm picrotoxin. All drugs were purchased from Sigma (St. Louis, MO) unless otherwise indicated.
The solutions were changed by a gravity-driven perfusion setup with a 3–4 ml/min flow rate at room temperature.
Electrode solution. The composition of the patch electrode solution (in mm) was the following: 101 Kgluconate, 8.5 KCl, 0.0078 CaCl2, 1 MgCl2, 0.1 BAPTAK4, 10 HEPES, 4 ATPNa2, and 0.5 GTPNa3. The pH was adjusted to 7.4 with KOH. The calculated ECl was −60 mV.
Light stimulus. We used a red light-emitting diode set to its maximum intensity to stimulate the retinal slice and sufficient to elicit a maximal response from each cell type studied here.
Cell identification. Cells were identified by their light response and morphology. To reveal morphology, we patched clamped the measured cell after the experiments with another patch electrode containing 1% Lucifer yellow (Aldrich, Milwaukee, WI) attached to a PC 501-A patch-clamp amplifier (Warner Instrument, Hamden, CT). Cells were viewed using a Nikon mercury fluorescent epi-illuminator with an XF15 filter set (Omega Optical, Brattleboro, VT).
Classification of amacrine cells as ON–OFF or ON amacrine cells. We classified an amacrine cell as ON–OFF if the cell, voltage clamped to −60 mV (ECl), responded to light stimulus with an inward current at both light ON and OFF in the presence of strychnine plus bicuculline plus picrotoxin. It was classified to be ON if it responded only at light ON. Data are presented as mean ± SD.
RESULTS
Yang et al. (1991) correlated the pharmacology, physiology, and morphology of amacrine cells in the salamander. They found that there exist two major classes of amacrine cells in the salamander retina. The narrow-field amacrine cells respond with multiple spikes, contain GABA, and have processes that extend ∼150 μm across the inner plexiform layer (IPL). The wide-field cells respond with a single spike at ON and OFF, contain glycine, and have processes that extend up to 500 μm across the IPL. These two cell types also interact with each other (Barnes and Werblin, 1987; Zhang et al., 1997), and the measurements below suggest some of the functional consequences of these interactions.
Narrow-field amacrine cells are inhibited via both glycine and GABAA receptors
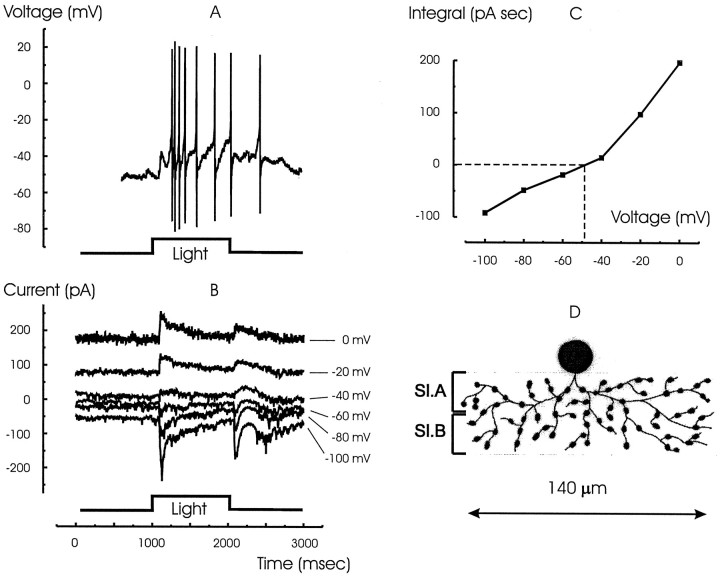
Figure 1A shows the voltage response typical of 13 of 18 narrow-field cells that generated a train of spikes at the onset and one or two spikes at the termination of the light step. The responses of the same cell under voltage clamp are shown in Figure 1B. The currents reversed at negative potentials near −50 mV at light ON and OFF (Fig.1C), suggesting the presence of strong transient inhibitory components. A sketch of the cell filled with Lucifer yellow after the experiment using a second electrode is shown in Figure1D. The processes ramified diffusely in both sublaminae in the IPL, consistent with the ON–OFF behavior of the cell. The spatial extent of this cell, typical of this class, was limited to only 140 μm, confirming that this cell belonged to the narrow-field amacrine cell family (Yang et al., 1991).
Fig. 1.
The light response, synaptic currents, and morphology of a narrow-field amacrine cell. A, The ON–OFF voltage response to a full-field red light step stimulus.B, Current responses of the same cell to the same stimulus voltage clamped to the potentials indicated at theright of each trace. The light-evoked current reversed near −50 mV, indicating strong inhibitory component.C, The integral of the light-evoked current (average current) from 1000 to 2000 msec (ON response) plotted as a function of the holding potential. The curve, created by joining the points with linear segments, passes through zero at −48 mV. D, Stylized sketch of the cell filled with 1% Lucifer yellow after the electrical measurement. The cell ramified in both ON and OFF sublaminae (Sl.A and Sl.B). The extent of its processes was 140 μm, consistent with its classification as a narrow-field (100–200 μm) amacrine cell (Yang et al., 1991).
We used the light responses when the cell was voltage clamped to 0 mV to isolate the inhibitory currents so that we could measure the dynamics of these responses. Subtracting the response in strychnine from the control response revealed the magnitude and dynamics of glycinergic inhibition to this cell (ON time to peak, 121 ± 36 msec; peak magnitude, 94 ± 34 pA; n = 9; OFF time to peak, 138 ± 82 msec; peak magnitude, 217 ± 140 pA;n = 9). Subtracting the response in the presence of bicuculline from the control response revealed the dynamics of the GABAergic inhibition via GABAA receptors (ON time to peak, 166 ± 79 msec; peak magnitude, 37 ± 44 pA;n = 4; OFF time to peak, 135 ± 50 msec; peak magnitude, 28 ± 35 pA; n = 4).
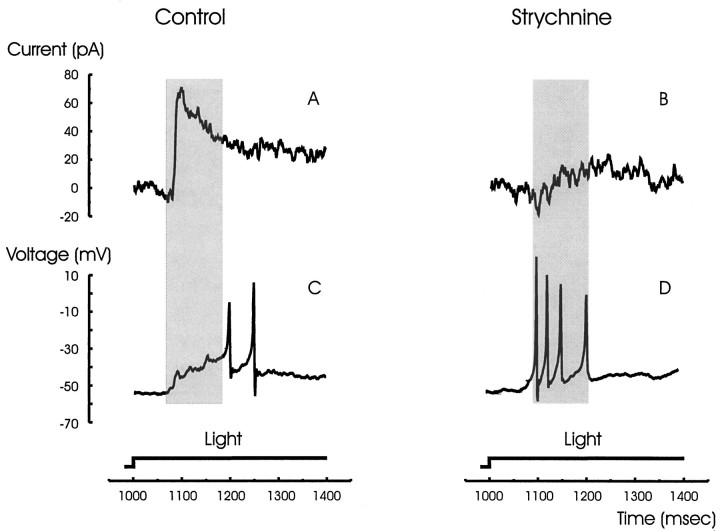
The effect of the transient glycinergic inhibition on the voltage response of a narrow-field amacrine cell is shown in Figure2. The transient glycinergic inhibition (Fig. 2A) seems to cause an early suppression of activity of the narrow-field amacrine cell. The timing of the delay in the onset of spiking (Fig. 2C, dark bar) matched the timing of the glycinergic inhibitory current (Fig.2A). Blocking glycine receptors with strychnine eliminated both the transient inhibitory current (Fig.2B) and the delay of spiking (Fig.2D).
Fig. 2.
Glycinergic inhibition causes a delay in the light-evoked activity of the narrow-field amacrine cells.A, A transient inhibitory current at light ON is shown.B, The inhibition is glycinergic and was blocked by strychnine. C, The normal light response of the cell was delayed by ∼150 msec at light ON. D, The delay was eliminated by blocking glycinergic receptors with strychnine. The duration of the inhibitory current (A) corresponds with the timing of the delay (C) as designated by the dark vertical bars.
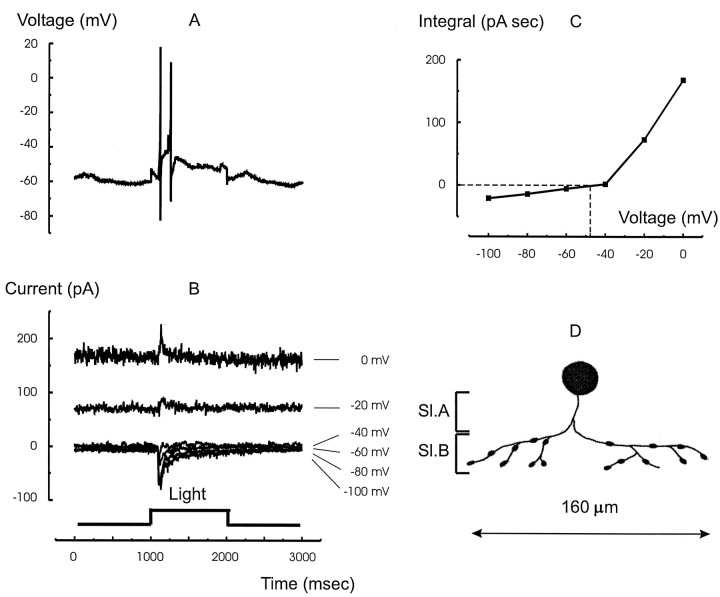
The light response under current clamp measured in one of the other five narrow-field amacrine cells studied is shown in Figure3A. These cells responded with a transient burst of spikes only at light ON that was not sustained throughout the duration of the light step. The responses of the same cell under voltage clamp are shown in Figure 3B. The currents were generated only at light ON but otherwise were similar to those measured in the ON–OFF cell shown in Figure 2; they reversed at negative potentials, near −48 mV (Fig. 3C), and received glycinergic inhibition with a time to peak of 124 ± 17 msec and a peak magnitude of 66 ± 18 pA (n = 3). The GABAA inhibition time to peak was 150 msec with a peak magnitude of 121 pA (n = 1). A sketch of the cell filled, using a second electrode, with Lucifer yellow after the experiment is shown in Figure 3D. The processes are constrained to a lateral spread of <160 μm and ramify in only sublamina B of the IPL, consistent with its ON behavior.
Fig. 3.
The light response, synaptic currents, and morphology of the narrow-field ON amacrine cells. A, The light response to a full-field red light stimulus. This cell type responds only at light ON. B, The current responses of the same cell to the same stimulus voltage clamped to the potentials indicated at the right of each trace. The inhibitory currents appear only at light ON. The light-evoked current reversed near −40 mV. C, The integral of the light-evoked current from 1000 to 2000 msec (ON response) plotted as a function of the holding potential. The curve, which was created by joining the points with linear segments, passes through zero at −48 mV, which indicates mixed excitatory and inhibitory inputs.D, Stylized sketch of the cell filled with 1% Lucifer yellow through a second electrode after electrical measurements. The cell ramified in sublamina B (Sl.B), consistent with its ON behavior. The extent of its processes was 160 μm, consistent with its classification as a narrow-field (100–200 μm) amacrine cell (Yang et al., 1991).
Activity in wide-field amacrine cells is truncated by GABAergic inhibition
A typical light response of a wide-field amacrine cell under current clamp is shown in Figure4A. These cells generate a single spike at light ON and another single spike at light OFF (Werblin, 1977; Barnes and Werblin, 1986; Eliasof et al., 1987). A sketch of the recorded cell filled with Lucifer yellow is shown in Figure 4B. The processes of this cell ramify broadly in both sublaminae over a diameter of 400 μm, consistent with the spread of processes of the cells from the wide-field class that were found to extend over a diameter of 300–500 μm (Yang et al., 1991).
Fig. 4.
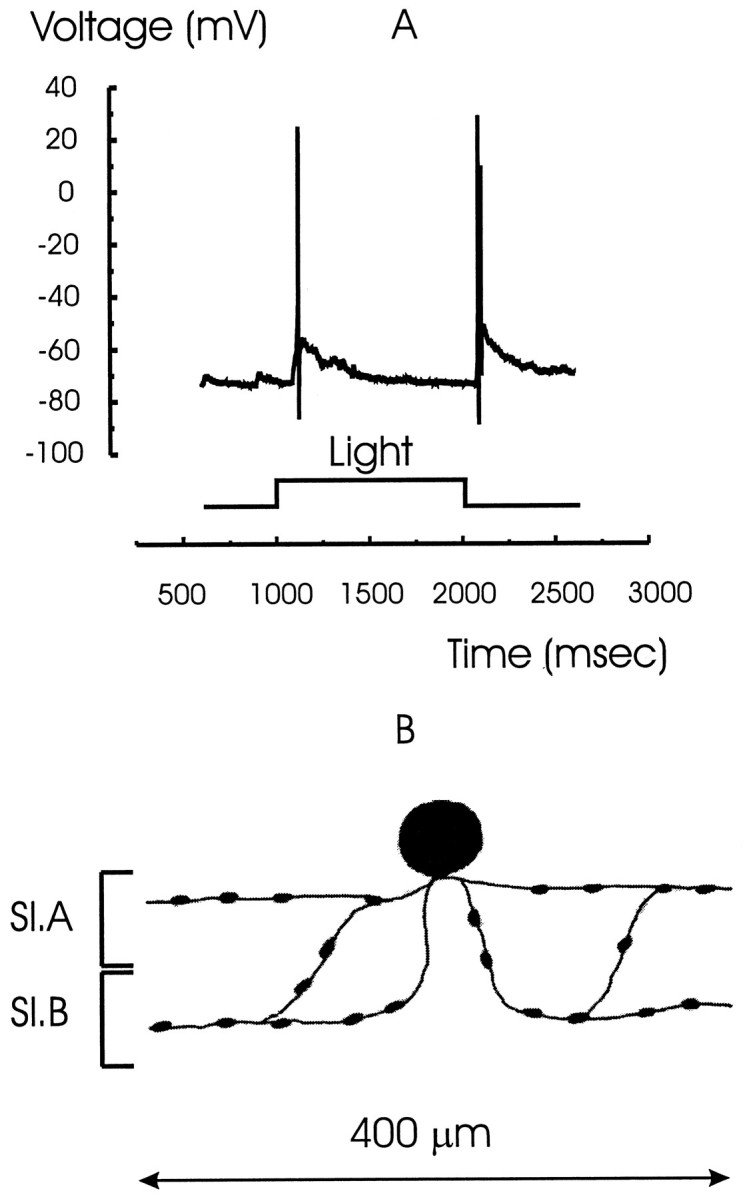
The light response and morphology of a wide-field amacrine cell. A, The light response of a wide-field amacrine cell that characteristically fires only one spike at light ON and OFF followed by a slow decay in response. B, Stylized sketch of the cell filled with Lucifer yellow. Processes ramify in both sublaminae A and B (Sl.A andSl.B) and spread laterally over ∼400 μm.
In three out of nine wide-field cells studied, no glycinergic inhibition could be measured. After perfusion with strychnine, the inhibitory response remained unchanged or increased slightly (compare Fig. 5A and B). Figure 5C shows that bath application of bicuculline together with strychnine blocked the inhibitory outward current at 0 mV, suggesting that the primary inhibitory input to these wide-field cells arrives via GABAA receptors. The increased GABAergic inhibition might be caused by the increased activity of GABAergic narrow-field amacrine cells when glycinergic inhibition to them was blocked. The remaining inward current is present probably because the membrane was not clamped to precisely the reversal potential for the excitatory inputs.
Fig. 5.
The effects of strychnine and bicuculline on the outward currents in wide-field amacrine cells. A, The current response of a wide-field amacrine cell voltage clamped to 0 mV and showing outward currents at light ON and OFF is shown.B, Strychnine had minor effects on the magnitude of the currents in the same wide-field amacrine cell. C, The outward currents were blocked by bicuculline, suggesting that they arrive at these cells via GABAA receptors. The remaining inward current is probably attributable to the fact that the cell was not clamped at the exact reversal potential for the excitatory currents.
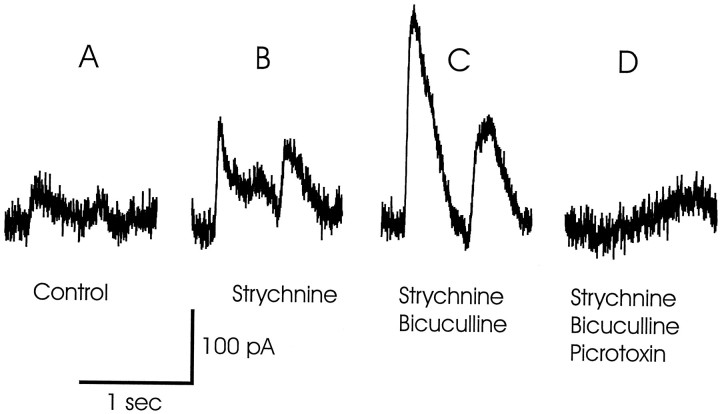
The GABAergic input to the wide-field glycinergic amacrine cells seems to be responsible for this characteristic single-spike activity. Figure6A shows the outward current in the presence of strychnine measured in a wide-field amacrine cell held at 0 mV. Figure 6B shows that this outward current was eliminated with the addition of bicuculline, thought to block GABAA receptors.
Fig. 6.
GABAergic inhibition truncates spiking in wide-field amacrine cells. A, When voltage clamped to 0 mV in the presence of strychnine, wide-field amacrine cells receive a strong inhibitory current. B, Bicuculline blocks this inhibitory current. C, The typical light response of the wide-field amacrine cell consisting of a single spike persists in strychnine. D, The addition of bicuculline, which eliminates the GABAA-mediated inhibition, makes the light response more sustained. Light now elicited a train of spikes. The delayed GABAergic inhibition (A) constrains the cell to fire only one spike at light ON (C). Thedark vertical bars show the time interval of ∼150 msec for GABAergic inhibition.
When the GABAergic inhibition was blocked, the normal single-spike response of this cell class (Fig. 6C) was extended to a train of spikes with decreasing amplitude (Fig. 6D). But even in the absence of the glycine-mediated delay (measured in the presence of strychnine), wide-field amacrine cells still generated only a single spike. This suggests that the GABAergic inhibitory signal is inherently delayed with respect to the excitatory input, even in the absence of a glycinergic input (Fig. 6C).
In six out of nine wide-field amacrine cells tested, we measured glycinergic as well as GABAergic inhibition, but blocking the glycinergic inhibition did not significantly change the form of the voltage response. The glycinergic input was apparently small enough that the cell membrane could still reach threshold to initiate the single-spike response. The blockage of GABAA receptors had effects similar to those measured in other wide-field cells described in Figure 6. The function of the glycinergic inhibition to these cells is unclear.
GABAC inhibition at ON bipolar cells was increased as glycine and GABAA receptors were blocked
Viewed from the perspective of the bipolar cell terminal, the inhibitory pathway comprises three neurons in a serial synaptic chain. One possible pathway includes wide-field amacrine to narrow-field amacrine to bipolar terminal. When recording from bipolar cells, we encountered measurements that may reflect the serial inhibitory action of this neuronal chain. We found an increase in inhibition at bipolar cells that was brought about by the bath application of inhibitory blockers.
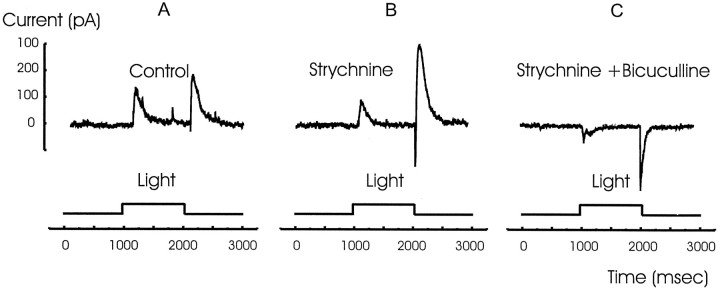
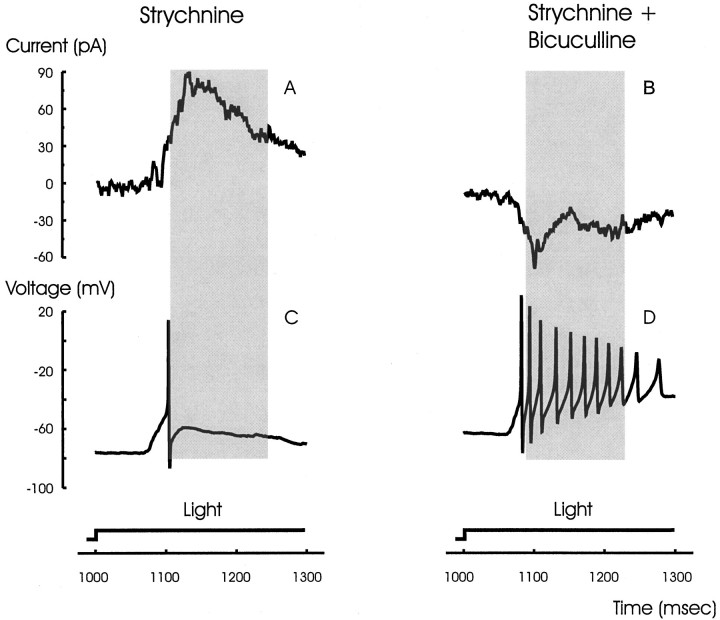
We isolated the inhibitory currents by voltage clamping bipolar cells to 0 mV. Figure 7A shows these inhibitory outward currents at light ON and OFF under control conditions in an ON bipolar cell. The inhibitory currents were augmented in the presence of strychnine in six out of nine cells (Fig.7B). The currents were further augmented by perfusion with strychnine plus bicuculline (Fig. 7C). However, the currents were completely blocked in the presence of strychnine, bicuculline, and picrotoxin (Fig. 7D). This suggests that the final synapse in this disinhibitory chain lies at the GABAC receptors at the bipolar cell synaptic terminals (Feigenspan et al., 1993;Lukasiewicz and Werblin, 1994; Lukasiewicz et al., 1994; Enz et al., 1996; Lukasiewicz, 1996; Dong and Werblin, 1997). Possible pathways for these events and their functional consequences are suggested in Discussion.
Fig. 7.
The inhibition recorded in an ON bipolar cell is enhanced in the presence of strychnine and further enhanced by bicuculline. A, ON bipolar cells normally generate small inhibitory currents when voltage clamped to 0 mV at both light ON and OFF. B, The inhibition was enhanced in the presence of strychnine. C, The addition of bicuculline further enhanced the inhibitory currents at both light ON and OFF.D, The inhibition was blocked by the addition of picrotoxin, suggesting that the inhibition is mediated via GABAC receptors.
Truncation of the bipolar cell response is delayed by a glycinergic signal
Dong and Werblin (1997) showed that responses in amacrine and ganglion cells were made more sustained in the presence of picrotoxin but not bicuculline and suggested that the feedback synapse from GABAergic amacrine cells to bipolar terminals at GABACreceptors was the site of signal truncation.
Application of strychnine had two effects on cells presynaptic to the ganglion cells. Strychnine decreased the delay in GABAergic amacrine cell activity (Fig. 2D) and increased the outward currents measured in bipolar cells (Fig. 7C). When we measured from ganglion cells, the application of strychnine should cause excitation to occur later (because early release is blocked) or to be diminished (because inhibition is increased at bipolar cells). We found evidence of both of these effects in recordings from ganglion cells held at −60 mV to isolate the excitatory currents. Figure8A shows that the excitatory currents in ganglion cells were reduced by ∼40% in the presence of strychnine. In this cell the timing of the currents was not strongly affected by the strychnine block. Figure 8B, recorded in another ganglion cell, shows that the current, which normally began at ∼50 msec, reaching (in this cell) approximately −50 pA, was delayed by an additional 50 msec in the presence of strychnine and that the magnitude of the current was reduced by ∼40%. The two cells in Figure 8 are representative of the seven ganglion cells studied. The magnitude of the currents was consistently reduced during the first 150 msec, but the timing of the suppression varied. In some cells, the response was delayed; in others, the response was truncated. We cannot yet explain these differences.
Fig. 8.
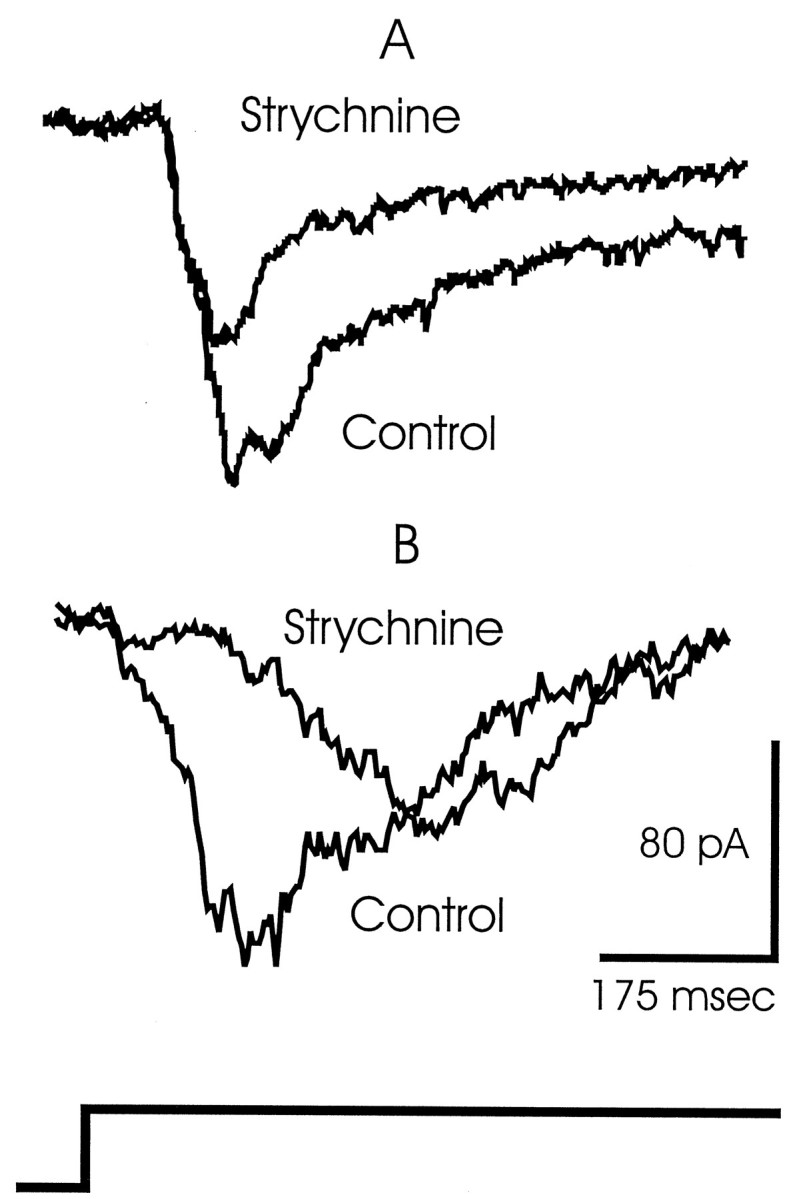
The strychnine-mediated changes in excitatory currents measured in two ganglion cells. Cells were clamped to −60 mV to isolate the excitatory currents. A, The currents were reduced by ∼40% in this cell without any significant effect on the timing of the response. B, In another ganglion cell, the excitatory current was delayed by ∼50 msec in the presence of strychnine, and the response magnitude was reduced by ∼40%.
DISCUSSION
Numerous authors have proposed that transient responses in ganglion cells are mediated by feedback inhibition at bipolar terminals from amacrine cells (Dowling, 1968; Burkhardt, 1972; Toyoda and Fujimoto, 1984; Werblin et al., 1988). Recently, Dong and Werblin (1997) have identified a local feedback loop between individual bipolar cells and their GABAergic amacrine cell counterparts, acting via GABAC receptors at the bipolar terminal, that may mediate signal truncation contributing to transient activity. For feedback to generate a transient release from bipolar cells, the feedback signal itself must be suppressed during the first 150 msec, allowing an initial transient burst of excitatory activity to reach the ganglion cells. This study addresses the mechanisms underlying this delay.
Timing of the two components of the reciprocal relation
Our measurements suggest that reciprocal inhibition between the two main amacrine cell classes, the narrow- and wide-field cells (Yang et al., 1991), generates complimentary dynamics in the two cell types. Figure 2 shows that the effect of the early single-spike response activity of the wide-field amacrine cells can be measured in the narrow-field cells as an early outward current lasting ∼150 msec (Fig. 2A). Its association with the glycinergic wide-field amacrine cell is supported by the observation that this current was blocked in strychnine (Fig. 2B). This current serves to delay the activity in the narrow-field amacrine cell (Fig. 2C). When the glycine-elicited current was blocked, the narrow-field cell responded within the initial 150 msec time interval in which it had normally been silent (Fig.2D).
A complimentary scenario exists in the wide-field glycinergic amacrine cells shown in Figure 6. These cells receive a GABAergic input via GABAA receptors as shown by the blockade of this inhibition with bicuculline in Figure 6B. The delayed activity of the narrow-field cells seems to truncate spike activity in these cells, because when the GABAergic inhibition is blocked, the cells, which normally generate only a single spike (Fig.6C), continue to spike throughout the stimulus duration (Fig. 6D). The “control” traces of Figure 6 were measured in the presence of strychnine.
Figure 2 shows that strychnine removed a significant delay in the GABAergic amacrine cell response. Figure 6 shows that even with this delay removed, the glycinergic amacrine cells still respond with an initial single spike. This comparison suggests that there is an inherent delay in the GABAergic neurons, even without the influence of the glycinergic inhibition shown in Figure 2.
An important consequence of this interaction is that the activity of the narrow-field GABAergic amacrine cells is actively delayed by an early inhibition from the wide-field amacrine cells (Fig.2C). The narrow-field amacrine cells inhibit the bipolar cells, so the delayed inhibition of the narrow-field cells allows an early release from the bipolar terminals. This is manifest as an early excitation in ganglion cells (Fig. 8A) and may be a crucial component for the generation of a transient response.
Possible mechanisms underlying the timing of reciprocal inhibition in amacrine cells
The relative timing of the activity of the two classes of amacrine cells seems to be dominated at early times by the robust spiking mechanism in the wide-field amacrine cell. Wide-field amacrine cells show a very low threshold for spiking (Werblin, 1977) that is initiated by a strong regenerative sodium current (Eliasof et al., 1987). Both wide- and narrow-field amacrine cells may be driven by the same population of bipolar cells, but the wide-field cells dominate the reciprocal inhibitory interaction during the first 150 msec because their regenerative current enhances activity at early times after the onset of light. The transition in the direction of inhibition from the wide-field cells inhibiting narrow-field cells at early times (Fig. 2) to the narrow-field amacrine cells inhibiting the wide-field cells at later times (Fig. 6) may be attributable to the inherent decay of wide-field amacrine cell activity after ∼150 msec (Fig.6D).
Possible circuitry for disinhibition at bipolar cells
The results of Figure 7, showing that the inhibitory input to the bipolar cells is enhanced in the presence of strychnine and bicuculline, suggest the presence of at least two disinhibitory pathways that are expressed in the GABAC-mediated inhibition at bipolar cells. One involves wide-field amacrine cells acting via a glycinergic pathway that is blocked by strychnine (Fig.7B). The other involves a class of GABAergic amacrine cells acting via a GABAA pathway (Fig. 7C). A possible circuitry underlying these findings is outlined in Figure9.
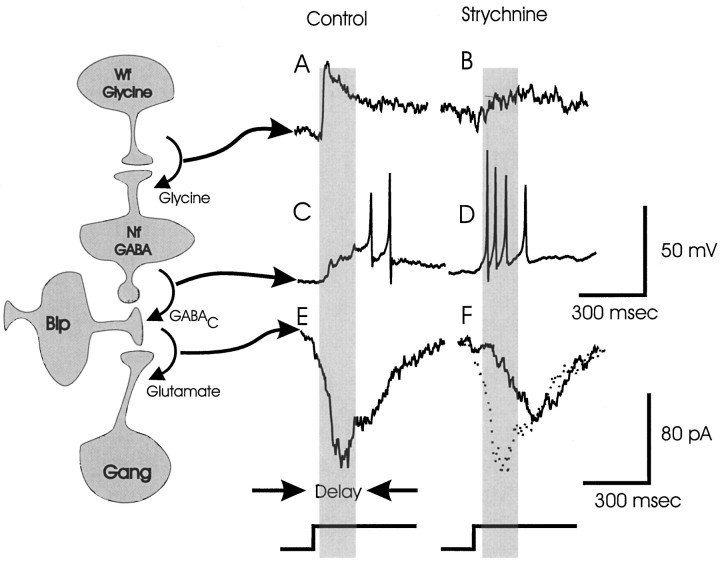
Fig. 9.
A proposed circuitry and time courses of cellular responses for serial synaptic connections mediating the delayed inhibition at bipolar cell terminals. Each cell iconrepresents a local population of cells. After a light flash (bottom stepped horizontal lines), wide-field amacrine cells (Wf) are active first (A). The wide-field cells inhibit narrow-field amacrine cells (Nf), delaying their activity (C). This allows bipolar cells (Bip) to excite ganglion cells (Gang) at early times (E). In the presence of strychnine, the glycinergic input to the narrow-field amacrine cell is lost (B). This allows the narrow-field cells to fire sooner (D), delaying the activity in the ganglion cells (F). The dotted linein F was copied from E to show the difference in timing. The dark vertical bars indicate the time interval during which the glycine-mediated delay is present.
A possibility for both wide-field and narrow-field glycinergic amacrine cell types
In their original classifications, Yang et al. (1991) established a broad dichotomy; wide-field amacrine cells were associated with glycine, and narrow-field amacrine cells were associated with GABA. We have relied on this general classification throughout this paper to identify the cell types that serve as sources of different pharmacological inputs. We were unable to distinguish between the cell types providing GABAergic input at GABAC receptors at the bipolar cell terminal and those providing input at the GABAA receptors on narrow-field amacrine cells mediating disinhibition in Figure 7.
Yang et al. (1991) found one class of narrow-field cell that contained both GABA and glycine but did not clearly define the time course of its response. This cell type introduces an additional ambiguity in our classifications. It is possible that the source of glycinergic disinhibition arises not only from wide-field cells but also from a separate class of narrow-field glycinergic amacrine cells as tentatively suggested by Yang et al. (1991).
Explanation for the discrepancy between our results and those ofYang et al. (1991)
Yang et al. (1991) classified most of the GABAergic narrow-field amacrine cells as ON cells. But we classified 13 out of 18 narrow-field amacrine cells as ON–OFF. It is possible that −60 mV, the potential to which these cells were clamped to observe excitatory currents in both studies, was slightly more positive than the actual chloride reversal potential. Under these conditions, the outward inhibitory currents would “mask” the relatively smaller excitatory currents of the OFF response. The excitatory OFF response would be augmented in our measurements in the presence of strychnine plus bicuculline and further augmented by the addition of picrotoxin, but Yang et al. (1991) did not use these blockers and therefore might have missed the excitatory currents at light off.
The effects of reciprocal inhibition and disinhibition on ganglion cells
Zhang et al. (1997), recording from ganglion cells, found that GABAergic inhibition was increased by blocking glycine receptors and that glycinergic inhibition was increased by blocking GABAAreceptors in some of these ganglion cells. From their measurements, they suggested serial inhibitory pathways between different pharmacological classes of amacrine cells. They also demonstrated a disinhibitory pathway involving GABAA receptors by showing that the light-evoked excitatory currents in a group of ganglion cells became more transient under the influence of SR95531. In Figure 7, we show that the magnitude of outward currents measured in bipolar cells increased in strychnine and bicuculline, consistent with the results ofZhang et al. (1997). The reduction in ganglion cell excitation in the presence of strychnine (Fig. 8) is also consistent with their results.
Footnotes
This work was supported by National Institutes of Health Grant NEI-00561. B.R. is supported by a Fulbright Fellowship.
Correspondence should be addressed to Dr. Frank Werblin, 145 Life Sciences Addition, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA 94720.
REFERENCES
- 1.Barnes S, Werblin F. Gated currents generate single spike activity in amacrine cells of the tiger salamander retina. Proc Natl Acad Sci USA. 1986;83:1509–1512. doi: 10.1073/pnas.83.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes S, Werblin F. Direct excitatory and lateral inhibitory synaptic inputs to amacrine cells in the tiger salamander retina. Brain Res. 1987;406:233–237. doi: 10.1016/0006-8993(87)90787-6. [DOI] [PubMed] [Google Scholar]
- 3.Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol (Lond) 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhardt DA. Effects of picrotoxin and strychnine upon electrical activity of the proximal retina. Brain Res. 1972;43:246–249. doi: 10.1016/0006-8993(72)90289-2. [DOI] [PubMed] [Google Scholar]
- 5.Dong CJ, Werblin F (1997) GABAergic feedback mediates temporal change enhancement in the tiger salamander retina. ARVO.
- 6.Dowling JE. Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc R Soc Lond [Biol] 1968;170:205–228. doi: 10.1098/rspb.1968.0034. [DOI] [PubMed] [Google Scholar]
- 7.Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond [Biol] 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- 8.Dowling JE, Werblin FS. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969;32:315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Eliasof S, Barnes S, Werblin F. The interaction of ionic currents mediating single spike activity in retinal amacrine cells of the tiger salamander. J Neurosci. 1987;7:3512–3524. doi: 10.1523/JNEUROSCI.07-11-03512.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enz R, Brandstatter JH, Wassle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigenspan A, Wassle H, Bormann J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- 12.Grant GB, Werblin FS. Low-cost data acquisition and analysis programs for electrophysiology. J Neurosci Methods. 1994;55:89–98. doi: 10.1016/0165-0270(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukasiewicz PD. GABAC receptors in the vertebrate retina. Mol Neurobiol. 1996;12:181–194. doi: 10.1007/BF02755587. [DOI] [PubMed] [Google Scholar]
- 16.Lukasiewicz PD, Werblin FS. The spatial distribution of excitatory and inhibitory inputs to ganglion cell dendrites in the tiger salamander retina. J Neurosci. 1990;10:210–221. doi: 10.1523/JNEUROSCI.10-01-00210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire G, Lukasiewicz P, Werblin F. Amacrine cell interactions underlying the response to change in the tiger salamander retina. J Neurosci. 1989;9:726–735. doi: 10.1523/JNEUROSCI.09-02-00726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cell. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Vis Neurosci. 1995;12:851–860. doi: 10.1017/s095252380000941x. [DOI] [PubMed] [Google Scholar]
- 22.Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- 23.Tachibana M, Kaneko A. gamma-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci USA. 1987;84:3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyoda J, Fujimoto M. Application of transretinal current stimulation for the study of bipolar-amacrine transmission. J Gen Physiol. 1984;84:915–925. doi: 10.1085/jgp.84.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallerga S. Physiological and morphological identification of amacrine cells in the retina of the larval tiger salamander. Vision Res. 1981;21:1307–1317. doi: 10.1016/0042-6989(81)90236-4. [DOI] [PubMed] [Google Scholar]
- 26.Vaney DI. The morphology and topographic distribution of AII amacrine cells in the cat retina. Proc R Soc Lond [Biol] 1985;224:475–488. doi: 10.1098/rspb.1985.0045. [DOI] [PubMed] [Google Scholar]
- 27.Vardi N, Smith RG. The AII amacrine network: coupling can increase correlated activity. Vision Res. 1996;36:3743–3757. doi: 10.1016/0042-6989(96)00098-3. [DOI] [PubMed] [Google Scholar]
- 28.Wassle H, Yamashita M, Greferath U, Grunert U, Muller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- 29.Wassle H, Grunert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- 30.Werblin FS. Regenerative amacrine cell depolarization and formation of on–off ganglion cell response. J Physiol (Lond) 1977;264:767–786. doi: 10.1113/jphysiol.1977.sp011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol (Lond) 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werblin F, Maguire G, Lukasiewicz P, Eliasof S, Wu SM. Neural interactions mediating the detection of motion in the retina of the tiger salamander. Vis Neurosci. 1988;1:317–329. doi: 10.1017/s0952523800001978. [DOI] [PubMed] [Google Scholar]
- 33.Witkovsky P, Dowling JE. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch. 1969;100:60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- 34.Wong-Riley MT. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974;3:1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- 35.Yang CY, Lukasiewicz P, Maguire G, Werblin FS, Yazulla S. Amacrine cells in the tiger salamander retina: morphology, physiology, and neurotransmitter identification. J Comp Neurol. 1991;312:19–32. doi: 10.1002/cne.903120103. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci. 1997;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]