Abstract
The integrin antagonist Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) was applied by local ejection to one of two recording sites in hippocampal slices at various times before and after long-term potentiation (LTP) was induced at both sites with theta burst stimulation. Applications 10 min before, immediately after, and 10 min after induction caused LTP at the experimental site to decay steadily relative to that at the within-slice control site. However, application at 25 min or more after induction had no detectable effect on potentiation. Similar results were obtained when the integrin antagonist was perfused into the slice rather than applied locally. The time period after induction during which GRGDSP interfered with LTP consolidation corresponds to that during which LTP is susceptible to reversal by low-frequency afferent stimulation and newly formed memories are vulnerable to various disruptive treatments. Comparable experiments using a peptide that blocks an extracellular binding site of neural cell adhesion molecules (NCAMs) did not yield time-dependent reversal of LTP; i.e., an antagonist that interacts with the fourth immunoglobulin-like domain reduced LTP when applied before induction but not afterward. Moreover, LTP formation occurred normally in the presence of an antibody against the fibronectin repeat domain of NCAM. These results suggest that integrin activation and signaling occurring over several minutes after LTP induction are necessary for stabilizing synaptic potentiation and by inference may be required for the conversion of new memories into a not readily disrupted state.
Keywords: LTP reversal, adhesion receptors, integrins, NCAMs, consolidation, memory, hippocampus, retrograde amnesia
Long-term potentiation (LTP) is vulnerable to disruption for several minutes after its induction but then becomes resistant to even extreme treatments. For example, episodes of hypoxia, too brief to more than transiently affect control synaptic responses, eliminate LTP if applied within 1–2 min of induction, but are without effect if administered 30 min later (Arai et al., 1990). Reversal of LTP by afferent stimulation (Barrionuevo et al., 1980; Stäubli and Lynch, 1990; Larson et al., 1993) is also time dependent: low-frequency stimulation erases potentiation when delivered within 1–2 min of theta burst stimulation (TBS) but has progressively less influence as the time after induction approaches 30 min (Stäubli and Chun, 1996a,b). These and other results indicate that although the neurochemical processes that consolidate LTP are set in motion by synaptic events in the millisecond range, they require many minutes to reach completion. In these features, the substrates of potentiation resemble those for the encoding of memory (Duncan, 1949;Riccio et al., 1968; Popik et al., 1994).
Neural cell adhesion molecules (NCAMs) and integrins, two classes of cell surface receptors involved in the assembly of pericellular matrices (Akiyama et al., 1989; Wu et al., 1995a,b) and maintenance of contact morphology (Horwitz et al., 1986; Burridge et al., 1988; Hynes, 1992) have been implicated in the formation of LTP (Stäubli et al., 1990; Xiao et al., 1991; Lüthi et al., 1994; Rønn et al., 1995; Bahr et al., 1997). Specifically, the processes triggered by the initiation of LTP are believed to involve activation of adhesion receptors that control configurational changes of the synaptic architecture and rearrange the membrane environment. It has been suggested that the two classes of adhesion receptors contribute to separate phases of LTP formation, with NCAMs being involved in the development of LTP (Lüthi et al., 1994; Rønn et al., 1995), a stage initiated and completed within 30 sec of induction (Gustafsson et al., 1989), and integrins contributing to later stabilization phases (Xiao et al., 1991; Bahr et al., 1997). Integrins commonly exist in a quiescent state, requiring an activation event, often triggered by other types of transmembrane receptors, for their adhesive properties to appear (Newton et al., 1997). The conversion from a latent to an active state can involve several minutes (van Willigen et al., 1996;Newton et al., 1997), and it is thus possible that the protracted consolidation period for LTP, and by inference possibly memory, reflects the time needed to mobilize integrin adhesion receptors.
The present studies tested predictions arising from the hypothesis that integrin, but not NCAM, chemistries dictate the protracted time period during which LTP is vulnerable to disruption. A technique was used in which LTP could be induced simultaneously at two sites in the same hippocampal slice, only one of which was exposed to adhesion receptor antagonists or control compounds. The use of within-slice and same time-frame comparisons reduced several sources of variance typically present in LTP experiments and thus increased the likelihood of accurately defining effects arising from the experimental manipulations. The compounds tested included (1) the peptide Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) (Pierschbacher and Ruoslahti, 1984), which competes with the recognition site for a large subclass of integrins, (2) the control peptide Gly-Arg-Ala-Asp-Ser-Pro (GRADSP), (3) the peptide MS2, which binds to the fourth Ig-like region in the extracellular domain of NCAMs, and (4) an antibody selective for the fibronectin type III repeat region, an extracellular segment of NCAMs closer to the membrane than the Ig-like domains. The specific question examined was whether the integrin antagonist, alone of the test compounds, would reverse already established LTP and do so with decreasing efficiency during the period beginning immediately after induction and ending 30 min later; i.e., during the same period that reversal by afferent activity becomes progressively less effective.
MATERIALS AND METHODS
Rat hippocampal slices were prepared from 2- to 3-month-old Sprague Dawley rats and maintained in an interface chamber using standard conditions, as described in earlier work (Stäubli and Chun, 1996a,b). The rats were decapitated, and their brains were removed rapidly and placed in 0°C oxygenated (95% O2/5% CO2) artificial CSF (aCSF) of the following composition (in mm): NaCl 124, KCl 3, KH2PO4 1.25, MgSO4 2.5, CaCl2 3.4, NaHCO3 36, d-glucose 10, and l-ascorbate 2. The hippocampi were quickly dissected free in ice-cold aCSF, placed on a McIlwain tissue chopper, cut into 400 μm sections, and collected in a petri dish containing ice-cold aCSF. The slices were then immediately placed on a nylon net in an interface chamber and maintained at a temperature of 31 ± 1°C. They were perfused continuously with preheated aCSF at a rate of 75 ml/hr while their upper surface was exposed to warm humidified 95% O2/5% CO2.
Recording and stimulating began after an incubation time of at least 1 hr. Experiments using local drug application via pressure ejection involved two extracellular recording sites (glass micropipettes filled with 2 mm NaCl) in the apical dendrites of fields CA1a and CA1c, with one of the two sites randomly selected for drug application and the other serving as control. Stimulation pulses were delivered to the Schaffer–commissural axons passing through stratum radiatum using a bipolar stimulating electrode (twisted nichrome wires, 65 μm) centered between the two recording electrodes, approximately 500 μm apart from each. The stimulus strength was adjusted to produce two field EPSPs with amplitudes that were ∼60% of the maximum spike-free response. After stable recording for at least 20 min, application of the various compounds to be tested for their effect on LTP began.
The peptide GRGDSP (Calbiochem, San Diego, CA), which blocks integrin binding to a diverse collection of ligands by competing with the Arg-Gly-Asp (RGD) consensus binding sequence, was diluted to 0.5 mm with aCSF and applied locally by pressure ejection (Picospritzer; General Valve, Fairfield, NJ) from a glass micropipette placed next to (within 150 μm), and at the same depth as (∼50–100 μm), the test recording electrode. Pipette ejection pressure was set at 8–12 psi (pulse duration 10 msec) to supply ∼3 nl of peptide every 5 sec throughout the experiment, starting at various time points before or after attempts to induce LTP and continuing throughout the experiment. A higher concentration of GRGDSP (2 mm) was tested in an additional set of experiments, with peptide ejection beginning 20 min before LTP induction. Control experiments involved local application of 0.5 mm GRADSP (Calbiochem), a peptide lacking the RGD sequence, with drug application starting 20 min before LTP induction.
The same arrangements were used to compare the effects on physiology of two compounds that interact with the extracellular domain of neural cell adhesion molecules (NCAMs), the second class of adhesion receptors. Specifically, the protein fragment MS2 (2 mg/ml), which contains the first 19 amino acids of the fourth Ig-like domain of NCAM, i.e., Ac-Glu-Ala-Ser-Gly-Asp-Pro-Ile-Pro-Ser-Ile-Thr-Trp-Arg-Thr-Ser-Thr-Arg-Asn-Ile-NH2(Horstkorte et al., 1993), and was synthesized by Dr. C. Glabe (University of California at Irvine), as well as an antibody against the fibronectin type III repeat domain of NCAM (4 mg/ml), were used [generous gift of Drs. E. Bock and M. Olsen (University of Copenhagen)].
LTP was induced by delivering TBS, consisting of ten theta bursts containing four pulses at 100 Hz each, separated by 200 msec. The stimulation intensity was not increased during TBS. Within-slice comparisons between potentiation at the site receiving the injection of the antagonist (“test response”) versus potentiation at the site that did not (“control response”), were used to test for blockade of LTP.
In experiments involving bath perfusion rather than local application of the integrin antagonist, GRGDSP was added directly to the chamber via perfusion pump, at a final concentration of 0.5 mm. Drug infusion started at various time points before and after attempts to induce LTP and lasted 60 min. Care was taken to keep the total rate of perfusion constant throughout the experiment (25 ml/hr) by adjusting the flow rate of the primary source of aCSF accordingly. One recording electrode in stratum radiatum of field CA1b and two stimulating electrodes (test and control), placed in equidistant positions in fields CA1a and CA1c to stimulate nonoverlapping Schaffer collateral/commissural projections, were used in the perfusion studies.
RESULTS
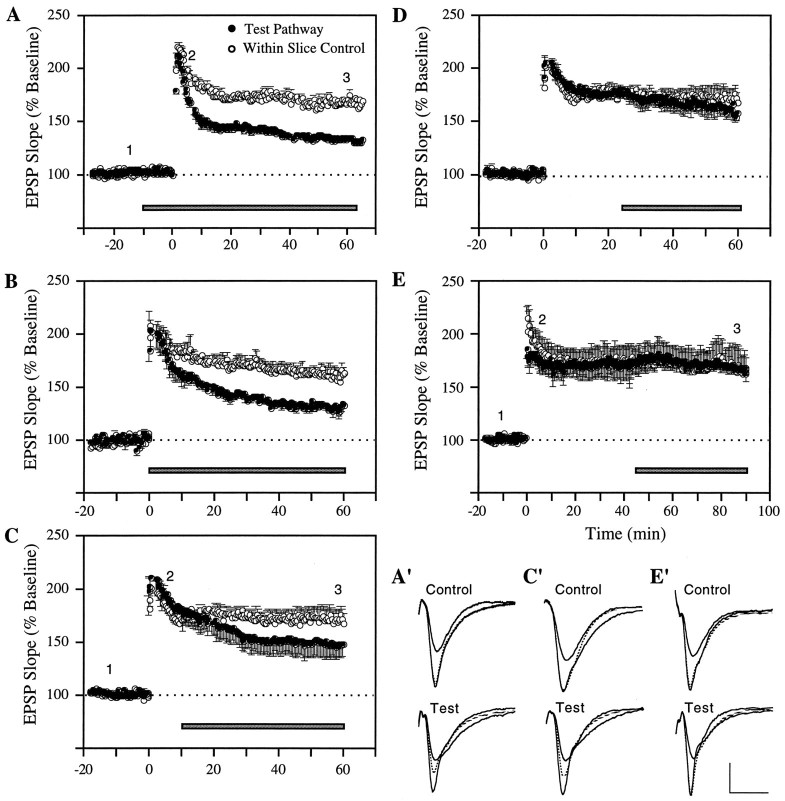
Local pressure ejection of the aCSF carrier vehicle used in these experiments had no detectable effect on slice physiology or potentiation. In accord with previous work involving bath perfusion (Stäubli et al., 1990; Xiao et al., 1991), GRGDSP did not alter the shape or size of baseline synaptic responses. Figure1 summarizes experiments in which local ejection of the peptide was initiated 10 min before (A), immediately after (B), 10 min after (C), 25 min after (D), or 45 min after the induction of LTP (E). As shown, when the infusion was started 10 min before TBS it resulted in an LTP that, in marked contrast to the potentiation elicited by the same stimulation at the control site, decayed steadily throughout the 60 min recording period after induction. Within-slice comparisons showed that the difference between control and experimental recording sites for the last 10 min were statistically significant (T(5)= 13.22; p < 0.001; two-tailed paired ttest). The initial potentiation (“short-term potentiation”) generated by TBS was left intact by GRGDSP (T(5)= 1.89 and 3.53; not significant, for comparisons of control versus test LTP during the first 5 and 10 min after induction). This finding is in agreement with earlier results (Bahr et al., 1997) and confirms that integrin antagonists interfere with neither the physiological events that induce LTP nor the development phase that occurs within the first 30 sec of LTP (Gustafsson et al., 1989). Infusions begun immediately after TBS were equally effective at destabilizing potentiation (T(3) = 7.46; p < 0.01, for comparison of the last 10 min of the LTP recording period). The infusion at 10 min after TBS, although it had no obvious immediate effect on the potentiated responses, also blocked stabilization to a significant degree (T(4) = 3.17;p < 0.05, for the last 10 min). Infusions at 25 and 45 min after TBS (Figs. 1D,E) caused no change in potentiated response compared with LTP at the within-slice control sites; i.e., responses recorded 50–60 min (25 min group) and 80–90 min (45 min group) after TBS exhibited virtually the same degree of potentiation at the GRGDSP and control sites [159.7 ± 9.4% vs 165.4 ± 7.1% (n = 6) for the 25 min group, and 165.2 ± 3.2% vs 166 ± 16.3% (n = 4) for the 45 min group].
Fig. 1.
Time-dependent reversal of LTP by integrin antagonist GRGDSP. A–E, Experiments in which LTP in area CA1 was simultaneously monitored at a test (•) and control site (○) within the same slice. Local ejection of the integrin antagonist GRGDSP (0.5 mm) at the test site was initiated at various times before and after LTP induction, i.e., 10 min before (A) (n = 6), immediately after (B) (n = 4), 10 min after (C) (n = 5), 25 min after (D) (n = 6), and 45 min after TBS (E) (n = 4). Each data point represents the group mean of one response per animal (±SEM). A′, Superimposed representative responses from an individual experiment. The potentials were recorded from the control and within-slice test site at the times indicated by the numbers inA, i.e., 10–15 min before as well as 5 min and 45 min after TBS, with the dotted waveform representing the response recorded at 45 min. C′, E′, Same as inA′, except that the responses are taken from experiments included in the groups summarized in C andE. Calibration: 1 mV, 10 msec.
Figure 2A combines within-slice comparisons for all groups of slices and infusion periods. The percentage potentiation of the experimental response is expressed as a fraction of that in the paired (same slice) control response. ANOVA using paired differences at 35–45 min after application of the inhibitor, or at 35–45 min after TBS for the −10 min group, indicated that a time-dependent drug effect was present (F = 5.55; p < 0.01). As shown, the magnitude of LTP at sites exposed to the antagonist before (□) or immediately after (•) TBS was reduced to 50% of that in control synapses by the end of testing. Lesser but still substantial impairments were obtained with infusions begun at 10 min after induction (▵); in contrast, LTP at sites treated with the antagonist at or beyond the 25 min time point (▴) was not detectably different from the potentiation at the control sites. The within-slice comparisons for this last group were statistically different from the within-slice comparisons for the 10 min before TBS group (p < 0.01, Newman–Keuls), the immediate group (p < 0.05), and the 10 min after TBS group (p < 0.05).
Fig. 2.
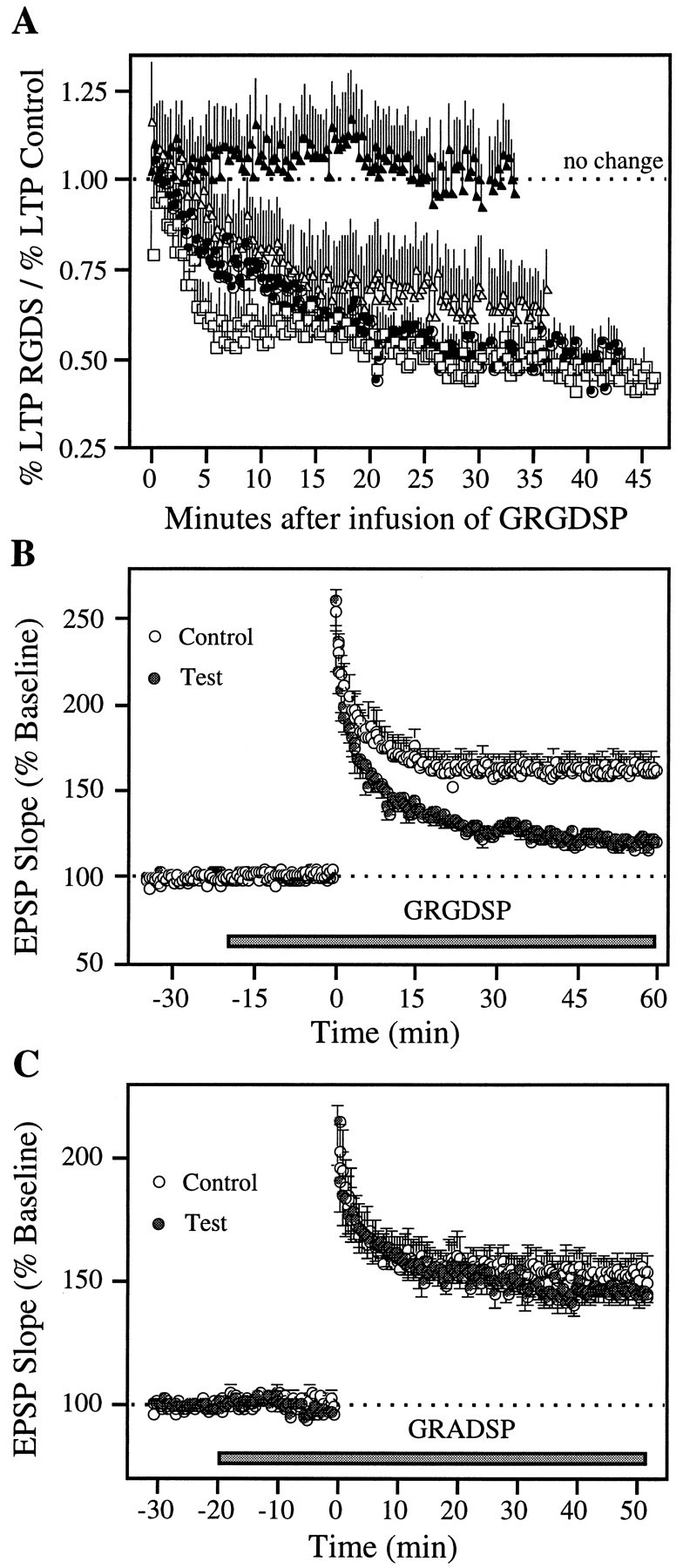
GRGDSP, but not the control peptide GRADSP, interferes with LTP stabilization. A, Same experiments as those illustrated in Figure 1, except that the data (mean ± SEM) for all groups are united in one graph and expressed as the ratio of the percent LTP at the test site divided by the percent LTP recorded simultaneously at the within-slice control site (□: start of infusion 10 min before TBS, n = 6; •: immediately after TBS, n = 4; ▵: 10 min after TBS,n = 5; ▴: >25 min after TBS,n = 10). B, GRGDSP was applied at a higher concentration (2 mm) and earlier, i.e., 20 min before LTP induction at both test and control site in a group of seven slices. C, Control experiments examining the effect of the non-RGD-containing peptide GRADSP (0.5 mm) in a group of seven slices, using the same experimental protocol as inB.
The 0.5 mm concentrations used in the above experiments are sufficient to block integrins (Cardwell and Rome, 1988; Haskel and Abendschein, 1989; Bahr and Lynch, 1992), but additional experiments (n = 7) were conducted to determine whether higher concentrations would result in a more rapid decrease in LTP. As shown in Figure 2B, application of the peptide at 2.0 mm beginning at 20 min before LTP induction resulted in a continuous and marked decay of potentiation at the test site (T(6) = 6.32; p < 0.001, for comparisons of control versus test LTP during the last 10 min). The average within-slice difference in potentiation between test and control sites during the last 10 min of recording was not obviously different for 0.5 mm versus 2 mm, i.e., 41.4 ± 6.5% for 0.5 mm versus 36.5 ± 6.8% for 2 mm. That GRGDSP, even when administered at 2 mm, did not influence the initial potentiation (T(6) = 1.13 and 1.85; not significant, for the first 5 and 10 min after induction), indicates that early LTP events are mediated by a mechanism other than integrins.
Figure 2C shows the results from experiments using GRADSP, a non-RGD-containing control peptide that was pressure-ejected at a concentration of 0.5 mm. This compound gave no evidence of interfering with LTP induction, development, or stabilization (T(6) = 1.30; not significant, for comparisons of control versus test LTP during the last 10 min of recording after induction).
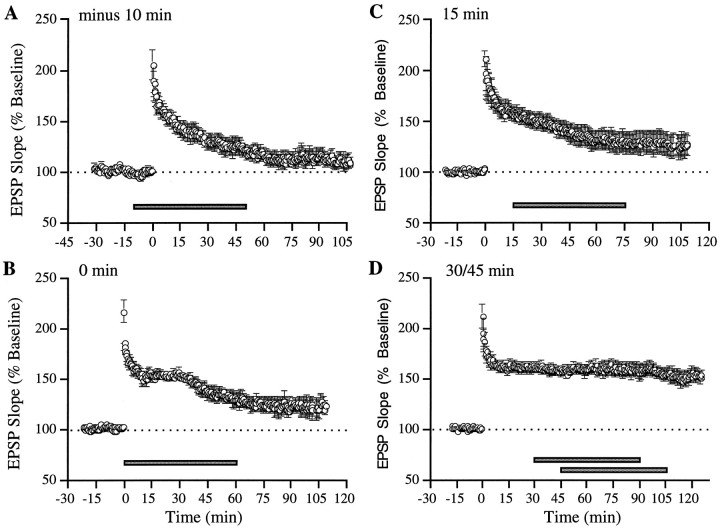
Results similar to those collected with local ejection of GRGDSP were obtained by bath application. As illustrated in Figure3A, adding GRGDSP before TBS resulted in LTP that decayed steadily throughout the subsequent recording period. Infusions beginning immediately or 15 min after induction (Figs. 3B,C) also interfered with LTP stabilization, whereas those begun at or after 30 min had no detectable influence on potentiation (Fig. 3D). ANOVA of the degree of LTP in place 60–70 min after the start of the peptide infusion revealed a significant effect of perfusion onset time (F = 7.46; p < 0.001). Post hoc comparisons indicated that LTP was greater in the long delay (30/45 min) group (155 ± 6%) than in the 10 min before TBS (118 ± 5%; p < 0.01, Neuman–Keuls), the 0 min (126 ± 5%; p < 0.05), or the 15 min after TBS groups (128 ± 9%; p < 0.01), despite being assessed at a greater interval after induction. An additional ANOVA comparing the degree of potentiation measured during the last 10 min of recording in each time group confirmed the presence of a significant effect of time (F = 6.6; p < 0.01). Specific comparisons indicated that the degree of LTP was significantly larger in the 30/45 min group than in the 10 min before TBS (p < 0.01; Neuman–Keuls), the 0 min (p < 0.05), and the 15 min after TBS groups (p < 0.05).
Fig. 3.
Whole-slice perfusion of integrin antagonist GRGDSP causes time-dependent reversal of LTP. A–D, Bath perfusion of the peptide (0.5 mm) was initiated at different times (horizontal bar) before and after LTP induction: A, 10 min before TBS (n = 5); B, immediately after TBS (n = 4); C, 10 min after TBS (n = 6); andD, 30 min (n = 5) and 45 min (n = 4) after TBS (data pooled for both time points). Each circle represents the group mean of one response per animal (±SEM).
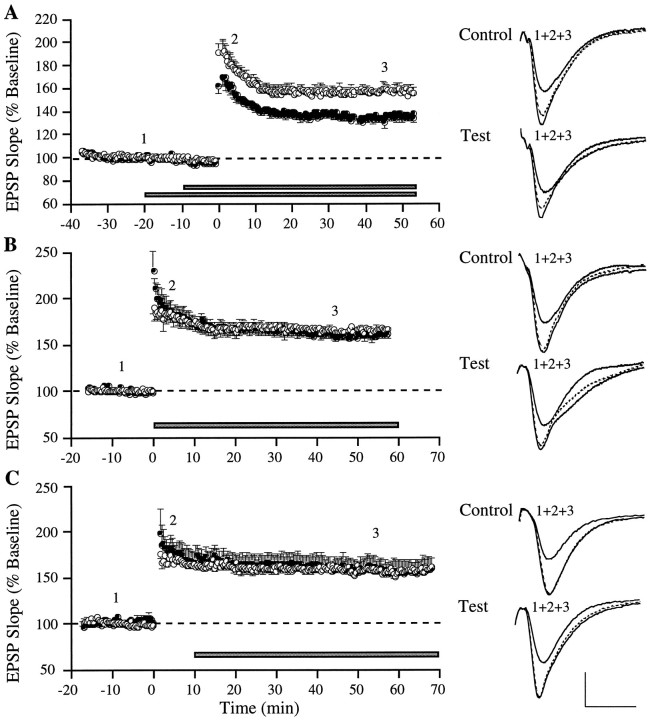
Figures 4 and5 summarize results obtained with local application of two compounds that interact with the extracellular domain of NCAMs, the second class of cell surface adhesion molecules implicated in the production of LTP (Lüthi et al., 1994; Muller et al., 1996). Time-dependent reversal was not obtained with local infusion of either compound. As shown in Figure 4A, local ejection of MS2 in advance of TBS caused a distinct reduction in immediate potentiation compared with LTP at the within-slice control site, an effect that was evident from the first response after TBS. The potentiation in both pathways stabilized within 15 min, but the initial gap between control and test LTP persisted throughout the rest of the experiment. Average responses were 153.7 ± 3.8% (test) versus 174.8 ± 5.9% (control) for the first 10 min after TBS (T(9) = 6.84; p < 0.001) and 134.7 ± 3.7% (test) versus 157.0 ± 3.4% (control) for 45–55 min after TBS (T(9) = 6.42;p < 0.001). It thus appears that MS2 reduces the initial magnitude of LTP but has no impact on its stabilization, suggesting that the NCAM antagonist interferes with LTP induction and the LTP development phase, in agreement with findings by others (Lüthi et al., 1994; Rønn et al., 1995). This mode of operation distinguishes MS2 from integrin antagonists, which leave immediate potentiation intact but interfere with a later step in the sequence leading to LTP stabilization (Stäubli et al., 1990; Bahr et al., 1997; present study). Microejection of MS2 initiated immediately and 10 min after TBS (Figs. 4B,C) had no initial or delayed impact on test LTP compared with that at the control site, an observation that differs from the postinduction time course over which integrin antagonists were found to be effective at destabilizing LTP in the present study. The lack of effect of MS2 when applied after LTP induction demonstrates that microejection of bioactive compounds is readily accomplished without retroactive changes in recently induced potentiation.
Fig. 4.
MS2, an NCAM antagonist, reduces the amount of initial LTP when applied before, but not after, induction.A–C, Experiments in which LTP was induced simultaneously at control (○) and test (•) sites within the same slice. The polypeptide MS2, which binds to the fourth immunoglobulin domain of NCAM, was pressure-ejected at 2 μg/μl at the test site, starting at various times (horizontal bar) before and after LTP induction: A, 10 min before (n = 10); B, immediately after (n = 7); or C, 10 min after TBS (n = 4). Each data point represents the group mean of one response per animal (±SEM). Superimposed waveforms on the right of each graph illustrate representative recordings from individual experiments taken at the times indicated by the numbers in the graphs.Dotted waveform is the response collected 45 min after TBS (A, B) or start of peptide application (C). Calibration: 1 mV, 10 msec.
Fig. 5.
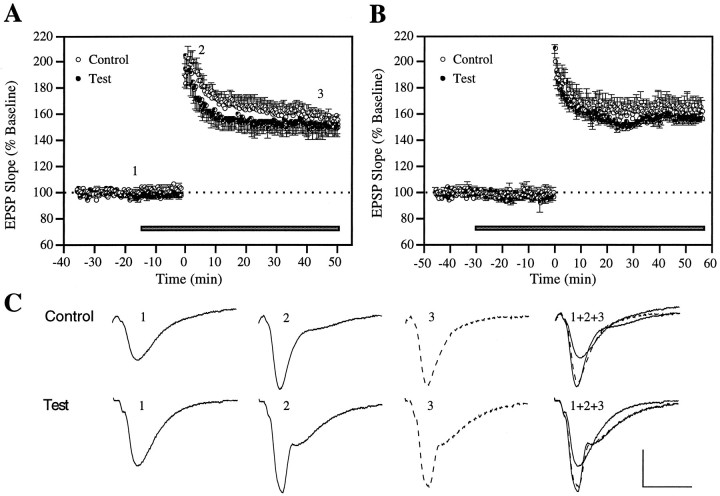
NCAM antibody does not affect LTP induction, development, or stabilization. A, An antibody against the fibronectin type III repeat domain of NCAM was pressure-ejected at 4 μg/μl, starting 10 min (n = 4) and 20 min (n = 5) before LTP induction (data pooled for both time points). B, Same as in A, except that antibody application was initiated 30 min before LTP induction (n = 5). C, Representative waveforms from an individual experiment taken at the times indicated by thenumbers in A. Calibration: 1 mV, 10 msec.
Finally, as illustrated in Figure 5A, antibodies against the fibronectin type III repeat region of NCAM had no effect on LTP when pressure-ejected 10 or 20 min before induction, a result that remained unchanged when the drug application time before TBS was increased to 30 min (Fig. 5B). A previous study testing NCAM antibodies against the fourth Ig-like binding domain found a significant LTP impairment, although the concentration ejected was 80 times lower (Lüthi et al., 1994), suggesting that our negative observation is not likely attributable to an insufficient antibody level but rather that the fibronectin site of NCAM is not essential for LTP.
Because the degree of LTP resulting from TBS is known to be closely tied to the amount and duration of the postsynaptic depolarization occurring during each burst (Arai and Lynch, 1992b), it was of interest to determine whether the reduction in initial LTP observed with MS2 was caused by the presence of the peptide during TBS, thereby causing interference with induction mechanisms. Comparisons were made of the degree of individual burst facilitation between control and test pathways in experiments involving drug application before TBS. Typically, and as confirmed in Figure6, bursts 2–10 are markedly facilitated under control conditions, with the effect being greater in the early rather than the late segments of the train (Arai and Lynch, 1992b). There were no obvious differences in burst facilitation between control and test pathways of slices treated with GRGDSP (Fig.6A). In contrast, MS2 dramatically reduced the area of test relative to control burst responses across the entire train (Fig. 6B). Comparisons of the degree of test burst facilitation obtained in presence of MS2 with that measured during application of GRGDSP, GRADSP, or the NCAM antibody revealed a significant suppressive action of MS2 in all cases (Fig.6C,D,F). This pattern of results strongly suggests that the reduction in immediate LTP seen with MS2 reflects an interaction with the induction rather than the development or stabilization of LTP.
Fig. 6.
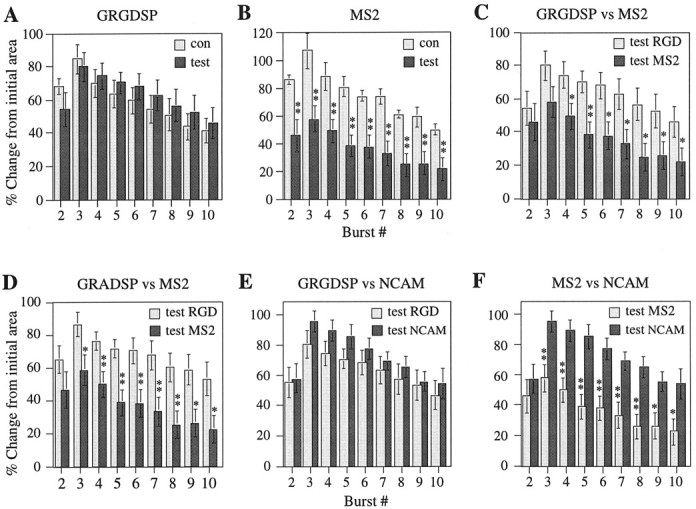
MS2 reduces facilitation of postsynaptic theta burst responses during LTP-inducing afferent stimulation, whereas GRGDSP, GRADSP, and the NCAM antibody have no effect. A, Increase in burst area (mean ± SEM) across a train of 10 bursts expressed relative to the initial burst response for both control (n = 5) and test (n = 5) pathways of slices in which GRGDSP (2 mm) was applied locally at the test site starting 20 min before LTP induction.B, Same as in A, but showing comparisons between control (n = 5) and test (n = 7) pathways of slices involving local ejection of MS2 (2 μg/μl) at the test site starting 20 min before TBS.C, Data adapted from A andB, comparing results between the two groups of test pathways. D, Comparisons of the amount of burst facilitation between test pathways of slices exposed to the control peptide GRADSP (n = 8) or MS2 (n = 7), with drug application beginning 20 min before TBS in both cases. E, Comparisons of burst facilitation between test pathways of slices in which GRGDSP (2 mm; n = 5) or NCAM antibodies (4 μg/μl; n = 5) were applied locally, starting 20 min before TBS. F, Data adapted from Cand E showing comparisons between test pathways treated with MS2 (n = 7) or NCAM antibody (n = 5). Significance levels: *p < 0.05, **p < 0.01;t test.
DISCUSSION
Proteins with integrin epitopes that bind to appropriate ligands via the consensus RGD sequence are concentrated in forebrain synapses (Bahr and Lynch, 1992; Grooms et al., 1993; Paulus et al., 1993; Einheber et al., 1996; Bahr et al., 1997). Previous work implicated the synaptic integrins in LTP consolidation by showing that diverse peptide antagonists of RGD binding prevent the formation of stable potentiation without affecting synaptic potentials or the complex physiological responses to theta bursts (Xiao et al., 1991;Bahr et al., 1997). The dose dependency of these effects corresponded to that for peptide suppression of integrin-mediated adhesion in various tissues (Cardwell and Rome, 1988; Haskel and Abendschein, 1989). Similar sized peptides with no relationship to the RGD site did not interact with LTP (Xiao et al., 1991; Bahr et al., 1997). The present experiments significantly extended this control by showing that a single amino acid substitution in the RGD segment of infused peptide (i.e., GRADSP) was sufficient to remove any effect on LTP. Assuming from these observations that integrins contribute to consolidation, it was expected that the peptide antagonists (1) would reverse long-term potentiation in a time-dependent manner and (2) be effective over the same time period as low-frequency synaptic activity, i.e., if administered within ∼15–30 min of induction (Stäubli and Chun, 1996a,b). The results from the present study confirm both predictions and in addition specify that integrin-binding events, rather than participating in early consolidation steps, contribute to delayed stabilization events beginning between 5 and 10 min after TBS.
The LTP blocking effects obtained with agents that interfere with NCAMs, the second class of adhesion receptors, differed from those found with integrin antagonists. Previous work by others using antibodies against the fourth Ig-like domain implicated NCAMs in LTP induction (Rønn et al., 1995) and early stabilization processes occurring in the first few minutes after induction (Lüthi et al., 1994). The present results modify and extend these findings. (1) The peptide MS2, which binds to the fourth Ig-like domain, reduced the magnitude of LTP by a constant amount, from the beginning to the end of the recording period after TBS, but only if it was present during TBS. Despite this reduction in LTP amount, the potentiation stabilized normally. A subsequent analysis of burst responses revealed that MS2 significantly suppressed response facilitation across the entire TBS train, a result consistent with an impairment in induction, but not excluding an additional deficit in LTP development. (2) LTP induction, development, and stabilization remained unaffected by the application of an antibody against the fibronectin type III repeat region of NCAMs, suggesting that this binding site, in contrast to the fourth Ig-like domain, does not contribute to LTP.
Burst response facilitation during LTP induction was not affected by any of the other agents involved in this study, as would be expected from compounds that selectively interfere with consolidation as opposed to induction (i.e., the integrin antagonist GRGDSP) or have no impact on LTP at all (i.e., the fibronectin antibody and the integrin control peptide GRADSP). In all, this pattern of results suggests that the two classes of cell surface adhesion receptors, NCAMs and integrins, participate in distinctly different stages of LTP, with the former playing a role in induction and perhaps also development, and the latter contributing to stabilization processes taking place between 5 and 30 min after induction of potentiation.
The extended period over which LTP was found vulnerable to GRGDSP presumably reflects the time needed to engage latent integrins. Integrins are activated by various bioactive molecules, one of the most prominent of which, the platelet activating factor (PAF), is rapidly generated in the brain and has receptors concentrated in synapses (Marcheselli et al., 1990; Mori et al., 1996). The mechanisms whereby PAF operates on integrins are not well understood, although recent work points to kinase activation and Ser-Thr phosphorylation of the β subunit of the integrin dimer as being critical, at least for platelets (van Willigen et al., 1996). Other studies using endothelial cells suggest that tyrosine phosphorylation of the focal adhesion kinase closely associated with the adhesion molecules is involved (Soldi et al., 1996). In any event, bursts of afferent activity are likely to generate at least one activation signal (stimulation of PAF receptors) in the immediate vicinity of latent synaptic integrins. Studies showing that inhibitors of PAF receptors block LTP are of interest with regard to this idea (del Cerro et al., 1990; Arai and Lynch, 1992a; Bazan et al., 1997).
Once activated, integrins can be expected to produce, over time, two types of changes pertinent to consolidation. First, by cross-linking the membrane cytoskeleton with extracellular matrix components (Horwitz et al., 1986), newly functional integrins will shape and stabilize morphological changes caused by high-frequency stimulation. Numerous electron microscopic studies have shown that LTP occurs in association with rapidly appearing, persistent modifications in synaptic anatomy (Lee et al., 1980; Desmond and Levy, 1983; Chang and Greenough, 1984). Second, integrin engagement triggers a mitogen-activated protein (MAP) kinase cascade (Chen et al., 1994) that interacts with other signal transduction pathways to modify gene expression. Although integrin effects on adhesion and cell morphology can occur independently of these events (Clark and Hynes, 1996; Lin et al., 1997), the link to MAP kinases could account for the gene induction reported to occur with high-frequency synaptic activity (Isackson et al., 1991; Andreasson and Worley, 1995; Link et al., 1995) and potentially could add a genomic contribution to the later stages of LTP consolidation.
Links between integrin activation and the phenomenon of LTP reversal remain to be explored. An intriguing possibility is suggested by experiments showing that stimulation of adenosine receptors within minutes after TBS selectively erases potentiation (Arai et al., 1990) and that antagonists of the receptors prevent LTP reversal by repetitive stimulation (Larson et al., 1993; Stäubli and Chun, 1996b; Abraham and Huggett, 1997). Related to these results is the finding that repetitive stimulation at frequencies well suited for reversal causes an efflux of adenosine at synaptic sites (Cunha et al., 1996). These observations are of interest in the present context because of evidence that adenosine receptors inhibit activation of integrins and that endogenously formed adenosine regulates adhesion in leukocytes (Thiel et al., 1996). In all, the adenosine–integrin connection, if present in the brain, provides a possible route whereby low-frequency afferent activity could disrupt the stabilization of recently induced LTP.
A final and critical issue concerns the behavioral relevance of the present findings. The observation that two very different manipulations, i.e., RGD peptides and low-frequency synaptic activity, are effective at causing depotentiation over the same time frame is intriguing not only because it supports the notion that the stabilization of LTP requires ∼15–30 min to reach completion, but also because the estimated consolidation time during which newly acquired memories are susceptible to disruption by temporary inactivation of hippocampal processes (electroconvulsive shock, hypothermia, etc.) is typically on the order of 15 min to <1 hr (Duncan, 1949; Riccio et al., 1968; Popik et al., 1994). Although these numbers are based on animal models of memory consolidation, studies on the duration of retrograde amnesia in humans after accidental head injury (excluding lesions) have provided similar estimates (Russell, 1959). In contrast, permanent memory loss of events that occurred at longer intervals is associated with lesions or extreme trauma, such as severe concussion or coma, that caused damage of the medial temporal lobe (Moscovitch, 1994; Nadel and Moscovitch, 1997).
Establishment of a link between the role of integrins in LTP stabilization and possible contributions to memory consolidation will require comparisons of how intracerebral injections of antagonists into freely moving rats affect recently induced potentiation and recently encoded memories. Chronic recording studies have established that thein vivo time course for LTP erasure with low-frequency stimulation is about the same as that observed in slices (U. Stäubli and J. Scafidi, unpublished observations), but this point remains to be tested for integrin antagonists. With regard to memory, it has been reported that agents that interfere with NCAM interactions disrupt spatial learning in rodents (Arami et al., 1996; Becker et al., 1996), but behavioral results of any type for RGD peptides are lacking.
Footnotes
This work was supported in part by the Whitehall Foundation Grant M97R05 (U.S.) and the Air Force Office of Scientific Research (AFOSR F49620-95-1-0304) (G.L.).
Correspondence should be addressed to Dr. Ursula Stäubli, New York University, Center for Neural Science, New York, NY 10003.
REFERENCES
- 1.Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasson K, Worley PF. Induction of beta-A activin expression by synaptic activity and during neocortical development. Neuroscience. 1995;69:781–796. doi: 10.1016/0306-4522(95)00245-e. [DOI] [PubMed] [Google Scholar]
- 4.Arai A, Lynch G. Antagonists of the platelet activating factor receptor block long term potentiation in hippocampal slices. Eur J Neurosci. 1992a;4:411–419. doi: 10.1111/j.1460-9568.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Arai A, Lynch G. Factors regulating the magnitude of LTP induced by theta pattern stimulation. Brain Res. 1992b;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 6.Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- 7.Arami S, Jucker M, Schachner M, Welzl H. The effect of continuous intraventricular infusion of L1 and NCAM antibodies on spatial learning in rats. Behav Brain Res. 1996;81:81–87. doi: 10.1016/s0166-4328(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 8.Bahr BA, Lynch G. Purification of an Arg-Gly-Asp selective matrix receptor from brain synaptic plasma membranes. Biochem J. 1992;281:137–142. doi: 10.1042/bj2810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahr BA, Stäubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 11.Bazan NG, Packard MG, Teather L, Allan G. Bioactive lipids in excitatory neurotransmission and neuronal plasticity. Neurochem Int. 1997;30:225–231. doi: 10.1016/s0197-0186(96)00020-4. [DOI] [PubMed] [Google Scholar]
- 12.Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:478–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 14.Cardwell MC, Rome LH. RGD-containing peptides inhibit the synthesis of myelin-like membrane by cultured oligodendrocytes. J Cell Biol. 1988;107:1551–1559. doi: 10.1083/jcb.107.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang F-LF, Greenough WT. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984;309:35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 17.Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- 18.Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 19.del Cerro S, Arai A, Lynch G. Inhibition of long-term potentiation by an antagonist of platelet-activating factor receptors. Behav Neural Biol. 1990;54:213–217. doi: 10.1016/0163-1047(90)90595-w. [DOI] [PubMed] [Google Scholar]
- 20.Desmond NL, Levy WB. Synaptic correlates of associative potentiation/depression: an ultrastructural study in the hippocampus. Brain Res. 1983;265:21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- 21.Duncan CP. The retroactive effect of electroshock on learning. J Comp Physiol Psychol. 1949;42:32–44. doi: 10.1037/h0058173. [DOI] [PubMed] [Google Scholar]
- 22.Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Grooms SY, Terracio Jones LS. Anatomical localization of beta 1 integrin-like immunoreactivity in rat brain. Exp Neurol. 1993;122:253–259. doi: 10.1006/exnr.1993.1125. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson B, Asztely F, Hanse E, Wigström H. Onset characteristics of long-term potentiation in the guinea-pig hippocampal CA1 region in vitro. Eur J Neurosci. 1989;1:382–394. doi: 10.1111/j.1460-9568.1989.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 25.Haskel EJ, Abendschein DR. Deaggregation of human platelets in vitro by an RGD analog antagonist of platelet glycoprotein IIb/IIIa receptors. Thromb Res. 1989;56:687–695. doi: 10.1016/0049-3848(89)90286-7. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz A, Duggan E, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin: a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 27.Horstkorte R, Schachner M, Magyar JP, Vorherr T, Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J Cell Biol. 1993;121:1409–1421. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynes RO. Integrin versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 29.Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 30.Larson J, Xiao P, Lynch G. Reversal of LTP by theta frequency stimulation. Brain Res. 1993;600:97–102. doi: 10.1016/0006-8993(93)90406-d. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Schottler F, Oliver M, Lynch G. Brief bursts of high-frequency stimulation produce two types of structural changes in rat hippocampus. J Neurophysiol. 1980;44:247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- 32.Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signalling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Nat Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lüthi A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 35.Marcheselli V, Rossowska MJ, Domingo MT, Baquet P, Bazan NG. Distinct platelet-activating factor binding sites in synaptic endings and in intracellular membranes of rat cerebral cortex. J Biol Chem. 1990;265:9140–9145. [PubMed] [Google Scholar]
- 36.Mori M, Aihara M, Kume K, Hamanoue M, Kohsaka S, Shimizu T. Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci. 1996;16:3590–3600. doi: 10.1523/JNEUROSCI.16-11-03590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscovitch M. Memory and working with memory: evaluation of a component process model and comparisons with other models. In: Schacter DL, Tulving E, editors. Memory systems. MIT; Cambridge, MA: 1994. pp. 269–310. [Google Scholar]
- 38.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-dependent synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 39.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 40.Newton RA, Thiel M, Hogg N. Signalling mechanisms and the activation of leukocyte integrins. J Leukoc Biol. 1997;61:422–426. doi: 10.1002/jlb.61.4.422. [DOI] [PubMed] [Google Scholar]
- 41.Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- 42.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 43.Popik P, Mamczarz J, Vetulani J. The effect of electroconvulsive shock and nifedipine on spatial learning and memory in rats. Biol Psychiatry. 1994;35:864–869. doi: 10.1016/0006-3223(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 44.Riccio DC, Hodges LA, Randall PK. Retrograde amnesia produced by hypothermia in rats. J Comp Physiol Psychol. 1968;66:618–622. doi: 10.1037/h0026530. [DOI] [PubMed] [Google Scholar]
- 45.Rønn LCB, Bock E, Linnemann D, Jahnsen H. NCAM-antibodies modulate induction of long-term potentiation in rat hippocampal CA1. Brain Res. 1995;677:145–151. doi: 10.1016/0006-8993(95)00147-i. [DOI] [PubMed] [Google Scholar]
- 46.Russell RW. Brain, memory, learning. Clarendon; Oxford: 1959. [Google Scholar]
- 47.Soldi R, Sanavio F, Primo L, Defilippi, Marchiso PC, Bussolino F. Platelet-activating factor (PAF) induces the early tyrosine phosphorylation of focal adhesion kinase (p125FAK) in human endothelial cells. Oncogene. 1996;13:515–525. [PubMed] [Google Scholar]
- 48.Stäubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996a;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stäubli U, Chun D. Proactive and retrograde effects on LTP produced by theta pulse stimulation: mechanisms and characteristics of LTP reversal. Learn Memory. 1996b;3:96–105. doi: 10.1101/lm.3.2-3.96. [DOI] [PubMed] [Google Scholar]
- 50.Stäubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- 51.Stäubli U, Vanderklish P, Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behav Neural Biol. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- 52.Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, Arfors KE, Peter K. Effect of adenosine on the expression of beta (2) integrins and L-selectin of human polymorphonuclear leukocytes in vitro. J Leukoc Biol. 1996;59:671–682. doi: 10.1002/jlb.59.5.671. [DOI] [PubMed] [Google Scholar]
- 53.van Willigen G, Hers I, Gorter G, Akkerman N. Exposure of ligand-binding sites on platelet integrin AIIb/B3 by phosphorylation of the B3 subunit. Biochem J. 1996;314:769–779. [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, Chung AE, McDonald JA. A novel role in α3β1 integrins in extracellular matrix assembly. J Cell Sci. 1995a;108:2511–2523. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]
- 55.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995b;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 56.Xiao P, Bahr BA, Stäubli U, Vanderklish PW, Lynch G. Evidence that matrix recognition contributes to stabilization but not induction of LTP. NeuroReport. 1991;2:461–464. doi: 10.1097/00001756-199108000-00013. [DOI] [PubMed] [Google Scholar]