Abstract
The senile plaques of Alzheimer’s disease are foci of local inflammatory responses, as evidenced by the presence of acute phase proteins and oxidative damage. Fibrillar forms of β-amyloid (Aβ), which are the primary constituents of senile plaques, have been shown to activate tyrosine kinase-dependent signal transduction cascades, resulting in inflammatory responses in microglia. However, the downstream signaling pathways mediating Aβ-induced inflammatory events are not well characterized.
We report that exposure of primary rat microglia and human THP1 monocytes to fibrillar Aβ results in the tyrosine kinase-dependent activation of two parallel signal transduction cascades involving members of the mitogen-activated protein kinase (MAPK) superfamily. Aβ stimulated the rapid, transient activation of extracellular signal-regulated kinase 1 (ERK1) and ERK2 in microglia and ERK2 in THP1 monocytes. A second superfamily member, p38 MAPK, was also activated with similar kinetics. Scavenger receptor and receptor for advanced glycated end products (RAGE) ligands failed to activate ERK and p38 MAPK in the absence of significant increases in protein tyrosine phosphorylation, demonstrating that scavenger receptors and RAGE are not linked to these pathways. Importantly, the stress-activated protein kinases (SAPKs) were not significantly activated in response to Aβ. Downstream effectors of the MAPK signal transduction cascades include MAPKAP kinases, such as RSK1 and RSK2, as well as transcription factors. Exposure of microglia and THP1 monocytes to Aβ resulted in the activation of RSK1 and RSK2 and phosphorylation of cAMP response element-binding protein at Ser133, providing a mechanism for Aβ-induced changes in gene expression.
Keywords: Alzheimer’s disease, β-amyloid, microglia, THP1 monocytes, signal transduction, tyrosine kinase, MAPK superfamily, piceatannol, inflammatory, RAGE, scavenger receptor
The senile plaques of Alzheimer’s disease (AD) are sites of inflammatory processes, as evidenced by the presence of degenerating neurons and numerous reactive microglia and astrocytes associated with the plaques (Itagaki et al., 1989). Moreover, the plaques contain acute phase proteins, such as cytokines, complement proteins, proteases, and protease inhibitors that are secreted by microglia (Abraham et al., 1988; Griffin et al., 1989;Itagaki et al., 1989; McGeer et al., 1989; Cataldo and Nixon, 1990;Bauer et al., 1991; Walker et al., 1995). The main protein component of senile plaques is the β-amyloid protein (Aβ), a 39–43 amino acid product of the larger β-amyloid precursor (Glenner and Wong, 1984;Masters et al., 1985). Although considerable genetic evidence implicates Aβ in the pathogenesis of AD (Selkoe, 1996), a direct causal link remains to be established.
We have recently demonstrated that exposure of primary cultures of rat microglia and human THP1 monocytes to fibrillar forms of Aβ resulted in the activation of protein tyrosine kinases that initiate the activation of complex signaling pathways in these cells. Exposure of these cells to Aβ led to the activation of Lyn, Syk, and the focal adhesion kinase (FAK), resulting in the generation of toxic superoxide radicals (McDonald et al., 1997). Exposure of microglia and monocytes to Aβ has also been shown to stimulate increased expression of interleukin 1β (IL-1β) (Walker et al., 1995; Lorton et al., 1996). Thus, Aβ is capable of directly activating inflammatory intracellular signaling pathways in microglia and monocytic cells. The downstream components of this Aβ-stimulated inflammatory signal transduction pathway have not been characterized. Recently, three receptors have been identified that can interact with Aβ: the scavenger receptor (El Khoury et al., 1996; Paresce et al., 1996), the receptor for glycated end products (RAGE) (Yan et al., 1996), and the serpin-enzyme complex receptor (Boland et al., 1996). However, these receptors are not linked to the rapid activation of tyrosine kinases, and we have been unable to detect the subsequent production of superoxide radicals after ligand binding (McDonald et al., 1997), thus it is unclear how each of these receptors is coupled to signal transduction pathways.
The mitogen-activated protein kinase (MAPK) cascade represents a prototypic signal transduction system through which extracellular stimuli are transduced. The MAPK superfamily comprises three distinct, but similarly organized, signaling pathways. The central elements of these cascades are extracellular signal-regulated kinases (ERKs), stress-activated protein kinases (SAPKs), also termed Jun N-terminal kinases, and p38 MAPK. Activation of the ERKs occurs in response to growth factor stimulation and also after activation of high-affinity IgG receptors (FcγRI) (Durden et al., 1995). Activation of the SAPKs and p38 MAPK occurs after exposure to environmental stresses, such as UV irradiation, hyperosmolarity, and endotoxin (Freshney et al., 1994;Han et al., 1994; Lee et al., 1994; Cano and Mahadevan, 1995; Raingeaud et al., 1995). Recently, colony-stimulating factor 1, granulocyte-macrophage colony-stimulating factor, and IL-3 were found to activate the ERKs and p38 MAPK in mast cells and macrophages, suggesting that the ERKs and p38 MAPK are involved in the regulation of development and function of immune cells (Foltz et al., 1997). The substrates of MAPK family members include MAPKAP kinases, such as RSK1, RSK2, MAPKAP kinase-2, MAPKAP kinase-3, and transcription factors, such as Elk1, Jun, CHOP, activating transcription factor 2, and MEFC2 (Pulverer et al., 1991; Stokoe et al., 1992a,b; Gille et al., 1992;Blenis, 1993; Grove et al., 1993; Raingeaud et al., 1995; McLaughlin et al., 1996; Wang and Ron, 1996; Han et al., 1997). Thus, MAPK signaling cascades are one of the major pathways linking extracellular stimuli to transcriptional activation and gene expression.
Exposure of microglia to Aβ results in the generation of superoxide radicals and elevated IL-1β expression (Walker et al., 1995; El Khoury et al., 1996; Lorton et al., 1996; McDonald et al., 1997). These observations led us to test whether MAPK family members are components of an Aβ-stimulated signal transduction cascade mediating these effects. We report that exposure of microglia and THP1 monocytes to fibrillar Aβ peptides resulted in the tyrosine kinase-dependent activation of the MAPK family members ERK1, ERK2, and p38 MAPK. SAPKs were not significantly activated by exposure to Aβ. Furthermore, stimulation of these cells with Aβ resulted in the activation of RSK1 and RSK2 and phosphorylation of the transcription factor cAMP response element-binding protein (CREB) at serine 133, a critical regulatory site for transcriptional activation (Gonzalez and Montminy, 1989; Ginty et al., 1994), providing a mechanistic link between Aβ and the regulation of gene expression. Importantly, exposure of THP1 monocytes to scavenger receptor and RAGE ligands did not lead to significant activation of the MAP kinases. These observations demonstrate that scavenger receptors and RAGE are linked to signal transduction cascades distinct from those activated by fibrillar Aβ and provide support for the existence of other Aβ-interactive receptors.
MATERIALS AND METHODS
Materials. Anti-phospho-ERK, anti-phospho-p38 MAPK, anti-phospho-CREB, anti-p38, and anti-CREB antibodies were obtained from New England Biolabs (Beverly, MA). Anti-ERK was obtained from Zymed (San Francisco, CA). Anti-JNK1, anti-ERK2, anti-RSK1, anti-RSK2, and protein G-agarose were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FcγRII [monoclonal antibody (mAb) IV.3)] was obtained from Medarex (West Lebanon, NH). Goat anti-mouse F(ab)2 was obtained from Cappel-Worthington (Durham, NC). Affinity-purified horseradish peroxidase-conjugated goat anti-mouse, goat anti-rabbit, and porcine anti-goat secondary antibodies, piceatannol, and protein A-agarose were obtained from Boehringer Mannheim (Indianapolis, IN). Piceatannol was prepared as a 12.3 mm solution in 30% DMSO. Anti-phosphotyrosine mAb PY20 was obtained from Transduction Laboratories (Lexington, KY). Anti-phosphotyrosine mAb 4G10 and S6 peptide were obtained from Upstate Biotechnology (Lake Placid, NY). The MEK inhibitor PD98059 was obtained from Calbiochem (La Jolla, CA). Glutathione-Sepharose was obtained from Pharmacia (Uppsala, Sweden). A peptide comprising amino acids 25–35 of β-amyloid (Aβ25–35) was obtained from American Peptide Co. (Sunnyvale, CA), and a peptide comprising amino acids 25–35 of β-amyloid in a scrambled sequence (SC25–35; NAMGKILSGIG) was a kind gift from Dr. Kurt Brunden (Gliatech, Inc., Cleveland, OH). Aβ25–35 and SC25–35 were resuspended in sterile, distilled H20 at a concentration of 2 mm and incubated for 1 hr at 37°C to allow fibril formation (Terzi et al., 1994). Aβ1–40 was a product of Bachem (King of Prussia, PA) and was resuspended in sterile, distilled H20 at a concentration of 2 mm and incubated for 1 week at 37°C to allow fibril formation. Nonfibrillar Aβ1–40 was prepared by dissolving the peptide in hexafluoroisopropanol, lyophilizing, and resuspending in sterile, distilled H20 at a concentration of 2 mm (Jao et al., 1997). Acetylated low-density lipoprotein (AcLDL) was a gift from Dr. Frederick DeBeer (University of Kentucky). Maleylated bovine serum albumin (mBSA) was prepared as previously described (Haberland and Fogelman, 1985). Glycated BSA (AGE) and iron-saturated lactoferrin (L) were obtained from Sigma (St. Louis, MO).
Tissue culture. Human THP1 monocytic cells were grown in RPMI-1640 (Whitaker Bioproducts, Walkersville, MD) containing 10% heat-inactivated fetal calf serum, 5 × 10−5m β-mercaptoethanol, 5 mm HEPES, and 1.5 μg/ml gentamicin in an atmosphere of 5% CO2. Microglia were derived from the brains of neonatal rats as previously described (Giulian and Baker, 1986; McDonald et al., 1997).
Cell stimulation. THP1 monocytes (5 × 106 cells) or microglia (2 × 106 cells) were suspended in 250 μl of RPMI-1640, and the cells were stimulated by addition of peptide for the indicated times. In some instances cells were pretreated with 50 μg/ml piceatannol in RPMI-1640 for 1 hr before stimulation or 25 μm PD98059 for 20 min before stimulation. Low-affinity (FcγRII) Ig receptors were cross-linked by incubation of 1 × 107 THP1 monocytes with 5 μg of anti-FcγRII in ice-cold RPMI for 15 min. Cells were pelleted and resuspended in RPMI (37°C) and 20 μg of goat anti-mouse F(ab)2 for the indicated time. The cells were collected by centrifugation and were lysed in 300 μl of ice-cold radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100, 20 mm Tris, pH 7.5, 100 mm NaCl, 1 mmNa3VO4, 40 mm NaF, 5 mm EGTA, 0.2% SDS, 0.5% deoxycholate, and 0.2 mm PMSF) for Western blotting or Triton X-100 buffer (1% Triton X-100, 20 mm Tris, pH 7.5, 100 mm NaCl, 1 mm Na3VO4, 40 mm NaF, 5 mm EGTA, and 0.2 mm PMSF) for in vitro kinase assays.
Western blotting. Protein concentrations of RIPA lysates were determined by the method of Bradford (1976) using BSA as a standard. Sample buffer was added to aliquots (50 μg of protein) of lysates, boiled for 5 min, and then resolved by SDS-PAGE under reducing conditions. To examine the effect of piceatannol on Aβ-stimulated activation of p38 MAPK, cells were lysed in 500 μl of RIPA buffer, immunoprecipitated with 1 μg of PY20, followed by Western blotting with anti-p38 MAPK antibody. The proteins were transferred to PolyScreen membranes (DuPont NEN, Boston, MA) and then blocked in TBS-T (10 mm Tris, pH 7.5, 100 mm NaCl, and 0.05% Tween 20) containing 3% BSA overnight at 4°C. The blots were incubated with primary antibodies overnight and then washed three times in TBS-T, followed by incubation for 1 hr with goat anti-rabbit, goat anti-mouse, or porcine anti-goat secondary antibodies conjugated to HRP in TBS-T containing 5% nonfat dried milk. The blots were washed three times in TBS-T followed by detection with an ECL detection system (DuPont NEN, Boston, MA). In some instances blots were stripped by incubation in stripping buffer (62.5 mm Tris, pH 6.8, 100 mm β-mercaptoethanol, and 2% SDS) for 30 min at 50°C and then reprobed with other antibodies.
Jun kinase assays. SAPKs were isolated by their ability to bind the N terminus of c-Jun. A glutathione S-transferase fusion protein including amino acids 5–89 of c-Jun (gst-Jun 5–89, a gift from Dr. J. Woodgett, University of Toronto) was bound to glutathione-Sepharose and used to isolate SAPKs from Triton X-100 lysates of THP1 monocytes (Pombo et al., 1994). Cells were stimulated with Aβ as described above and also by the addition of 300 mm NaCl for 30 min, followed by lysis in 300 μl ice-cold Triton X-100 buffer. Triton X-100-insoluble material was pelleted by centrifugation for 10 min at 4°C. We added 40 μg of lysate protein to 200 μl of wash buffer (0.1% Triton X-100, 10 mm Tris, pH 7.5, 150 mm NaCl, 1 mmNa3VO4, 10 mm NaF, and 5 mm EGTA) containing 40 μg of gst-Jun beads and incubated for 1 hr at 4°C. The gst-Jun beads were washed three times in wash buffer and once in kinase buffer (10 mm HEPES, pH 7.4, 10 mm MgCl2, 100 μm sodium orthovanadate, and 10 mm NaF). Kinase reactions were performed in duplicate by addition of 40 μl of kinase buffer containing 10 μm [γ-32P]ATP (55 dpm/fmol) for 30 min at room temperature. Reactions were terminated by the addition of sample buffer and boiled for 5 min. Samples were resolved by SDS-PAGE, dried, and visualized by autoradiography. The gst-Jun band was excised from the gel and incorporated radioactivity was measured by Cerenkov counting.
RSK kinase assays. Triton X-100 buffer lysates (500 μg) were precleared with 10 μl protein A-agarose for 30 min. RSK1 was immunoprecipitated by incubation with 1 μg of anti-RSK1 and 20 μl of protein A-agarose for 2 hr at 4°C. Immune complexes were washed three times in 1 ml of Triton X-100 buffer and once in TEV buffer (20 mm Tris, pH 7.4, 1 mm EGTA, and 100 μm Na3VO4). To isolate RSK2, 1 × 107 THP1 cells were lysed by sonication in TEV buffer plus 10 mm p-nitrophenylphosphate (PNP). Insoluble material was pelleted by centrifugation for 10 min at 4°C. Lysates (800 μg) were bound to 150 μl of Mono Q resin for 5 min and washed for 5 min in 1 ml of TEV buffer plus 50 mmNaCl, and RSK2 was eluted with two washes of 300 μl of TEV buffer plus 250 mm NaCl (Xing et al., 1996). RSK2 eluents were collected and immunoprecipitated by incubation with 1 μg of anti-RSK2 and 20 μl of protein G-agarose for 2 hr at 4°C. Immune complexes were washed three times in 1 ml of Triton X-100 buffer and once in TEV buffer. Washed immune complexes were assayed by phosphorylation of S6 peptide (Pelech and Krebs, 1987) in 50 μl of kinase buffer (20 mm Tris, pH 7.4, 10 mmMgCl2, 2 mm MnCl2, 10 μm [γ-32P]ATP (10 dpm/fmol), 10 μm S6 peptide, and 10 mm PNP). Reactions were performed at room temperature for 20 min and terminated by removal of supernatants from the immune complexes and addition of 35 μl of 10% trichloroacetic acid and 10 μl of BSA (1 mg/ml). Supernatants were incubated on ice for 15 min, and precipitated proteins were pelleted by centrifugation for 5 min. Aliquots (20 μl) of supernatants containing S6 peptide were spotted onto P81 phosphocellulose paper in triplicate and washed three times for 5 min each in 75 mm phosphoric acid. Incorporation of [32P]ATP into the S6 peptide was quantitated by Cerenkov counting. Sample buffer (30 μl) was added to immune complexes followed by boiling for 3 min for Western blot analysis of RSK1 levels.
RESULTS
Aβ peptides stimulate rapid, transient tyrosine phosphorylation in THP1 monocytes
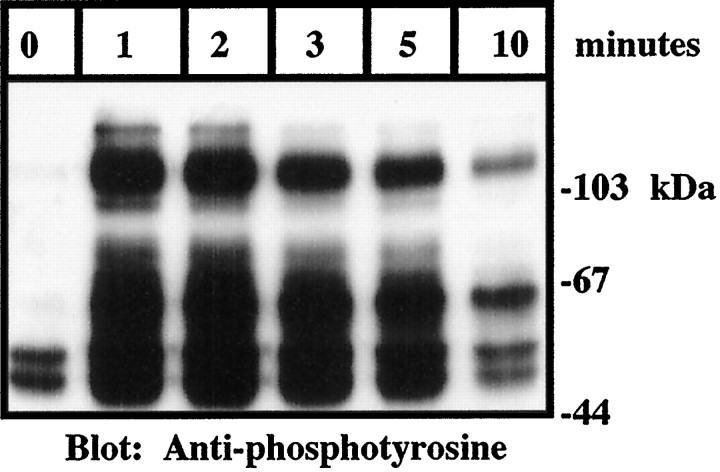
Signal transduction through tyrosine kinases characteristically leads to the activation of serine–threonine kinases that constitute downstream elements of signal transduction cascades. The interaction of microglia and THP1 monocytes with fibrillar forms of Aβ has been shown to stimulate production of superoxide and increased expression of IL-1β (Walker et al., 1995; El Khoury et al., 1996; Lorton et al., 1996; McDonald et al., 1997). The biologically active domain within Aβ was found to be restricted to amino acids 25–35 at the C terminus of Aβ (Yankner et al., 1990; Meda et al., 1995; Lorton et al., 1996;McDonald et al., 1997). This region possesses a β-pleated sheet structure and forms fibrils in aqueous solution (Terzi et al., 1994). Stimulation of THP1 monocytes with fibrillar Aβ25–35 rapidly elicited an increase in protein tyrosine phosphorylation that reached maximal levels within 1 min (Fig. 1).
Fig. 1.
Fibrillar Aβ stimulates rapid tyrosine phosphorylation in THP1 monocytes. THP1 monocytes were stimulated for the indicated times with 50 μm Aβ25–35. Western blot analysis of tyrosine phosphoproteins was performed on RIPA lysates (50 μg of protein) using monoclonal anti-phosphotyrosine antibodies (4G10). The positions of the molecular weight standards (in kilodaltons) are indicated.
Aβ peptides activate ERKs in THP1 monocytes and microglia
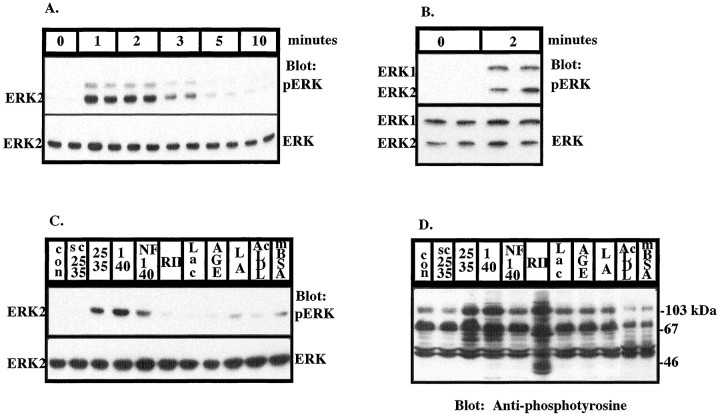
We tested whether Aβ-mediated stimulation of tyrosine kinases in microglia and THP1 monocytes led to the subsequent activation of members of the MAPK superfamily. Stimulation of THP1 monocytes with fibrillar Aβ1–40 and Aβ25–35 led to the rapid, transient activation of ERK2, which reached maximal levels within 1–2 min and returned to basal levels in 5–10 min, as detected by Western blot analysis with anti-phospho-ERK, an antibody that specifically recognizes the activated, tyrosine-phosphorylated form of ERK (Fig.2A,C). Nonfibrillar Aβ1–40 elicited modest activation of ERK, and this stimulation is likely to be attributable to the low levels of fibrils in this preparation caused by incomplete dissolution of fibrils in the peptide preparation and their subseqent formation after resuspension in water (Fig. 2C,D). This finding is similar to that previously observed with nonfibrillar preparations of Aβ25–35 (McDonald et al., 1997). THP1 monocytes express ERK1 at much lower levels than ERK2. Stimulation of primary cultures of rat microglia with Aβ25–35 led to the activation of both ERK1 and ERK2 with similar kinetics (Fig.2B). It is noteworthy that the Aβ-induced activation of these enzymes is consistently faster than that typically observed in growth factor-stimulated responses. A scrambled peptide consisting of amino acids 25–35 of Aβ (SC25–35) did not activate ERK, as demonstrated by a phospho-ERK Western blot (Fig.2C). Cross-linking of low-affinity Fcγ receptors (RII) for 5 min led to dramatic increases in tyrosine phosphorylation (Fig.2D); however, ERK was not activated. Scavenger receptors and RAGE have been shown to be capable of binding Aβ. Therefore, we tested the ability of scavenger receptor ligands, AcLDL and mBSA, and RAGE ligand, glycated BSA plus iron-saturated lactoferrin (AGE-L), to activate ERK. Acetylated LDL, maleylated BSA, and glycated BSA plus iron-saturated lactoferrin have previously been shown not to activate a tyrosine kinase-dependent pathway activated by Aβ fibrils (McDonald et al., 1997). Similarly, stimulation of THP1 monocytes with SC25–35, AcLDL, mBSA, AGE-L, AGE alone, and L alone failed to affect protein tyrosine phosphorylation (Fig. 2D) and did not significantly activate ERK (Fig. 2C). Simultaneous exposure of the cells to Aβ25–35 and AcLDL had no effect on the ability of Aβ to stimulate protein tyrosine phosphorylation (data not shown).
Fig. 2.
Fibrillar Aβ stimulates rapid, transient activation of ERK1 and ERK2 in THP1 monocytes and microglia.A, Exposure of THP1 monocytes to 50 μmAβ25–35 for the indicated times leads to activation of ERK2. Activation of ERK (A–C) was determined by Western blot analysis of RIPA lysates (50 μg of protein) using anti-phospho-ERK polyclonal antibodies (top panels). The blots were reprobed with anti-ERK antibody (bottom panels). B, Exposure of primary cultures of rat microglia to 50 μm Aβ25–35 for 2 min leads to activation of ERK1 and ERK2. C, Ligand specificity of ERK activation in THP1 monocytes. THP1 monocytes were incubated alone (control, con) or stimulated for 2 min with 50 μm scrambled Aβ25–35 (SC25–35; NAMGKILSGIG), Aβ25–35 (25–35), fibrillar Aβ1–40 (1–40), and nonfibrillar Aβ1–40 (NF1–40) or with anti-FcγRII cross-linked with goat anti-mouse F(ab)2 for 5 min (RII). The cells were also incubated with the RAGE ligands: either 20 μg/ml L, AGE, or AGE-L. The scavenger receptor ligands AcLDL and mBSA were incubated with the cells for 2 min. D, Ligand specificity of tyrosine phosphorylation in THP1 monocytes. THP1 monocytes were stimulated with ligands, as described in C, for 2 min. Western blot analysis of tyrosine phosphoproteins was performed on RIPA lysates (50 μg of protein) using monoclonal anti-phosphotyrosine antibodies (4G10).
Aβ peptides activate p38 MAPK in THP1 monocytes and microglia
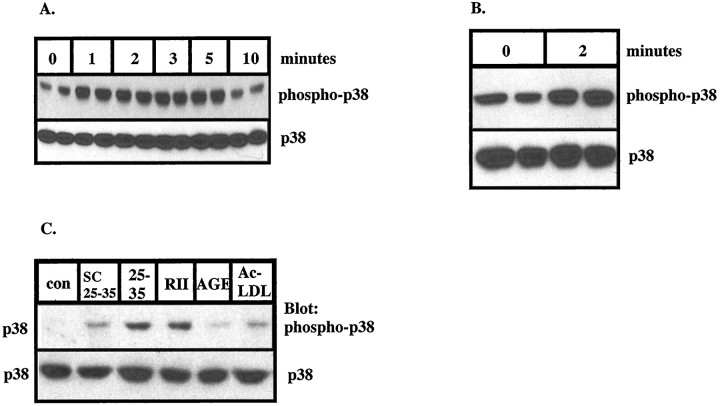
The responses of microglia and THP1 monocytes to Aβ are consistent with those observed after exposure to cellular stressors. Therefore, we examined whether Aβ activated p38 MAPK in microglia and THP1 monocytes. Stimulation of THP1 monocytes with Aβ25–35 led to the activation of p38 MAPK with a time course similar to that observed with ERK2, as determined by Western blot analysis using anti-phospho-p38 MAPK, an antibody that specifically recognizes the activated, tyrosine-phosphorylated form of p38 MAPK (Fig.3A,C). Aβ25–35 stimulated maximal levels of p38 MAPK activation in 1–2 min that returned to basal levels within 10 min. In addition, stimulation of microglia with Aβ25–35 led to activation of p38 MAPK (Fig. 3B) with similar kinetics. RAGE and scavenger ligands failed to activate p38 MAPK, as demonstrated by a phospho-p38 MAPK Western blot (Fig.3C). The Aβ-induced activation of p38 MAPK, like the ERKs, was very rapid; however, the magnitude of p38 phosphorylation was not as great as that observed in the ERKs.
Fig. 3.
Fibrillar Aβ stimulates the rapid, transient activation of p38 MAPK in THP1 monocytes and microglia.A, THP1 monocytes were exposed to 50 μmAβ25–35 for the indicated times, and the activation of p38 MAPK was detected using anti-phospho-p38 antibody (top panel). The blots were reprobed with anti-p38 antibody (bottom panel). B, Exposure of primary cultures of rat microglia to 50 μm Aβ25–35 for 2 min leads to activation of p38 MAPK. C, Ligand specificity of p38 MAPK activation in THP1 monocytes. THP1 monocytes were stimulated with 50 μm Aβ25–35 or 20 μg/ml AcLDL, mBSA, and AGE-L for 2 min. Activation of p38 MAPK inA–C was determined by Western blot analysis on RIPA lysates (50 μg of protein) using anti-phospho-p38 MAPK polyclonal antibodies.
Aβ peptides do not significantly activate SAPKs
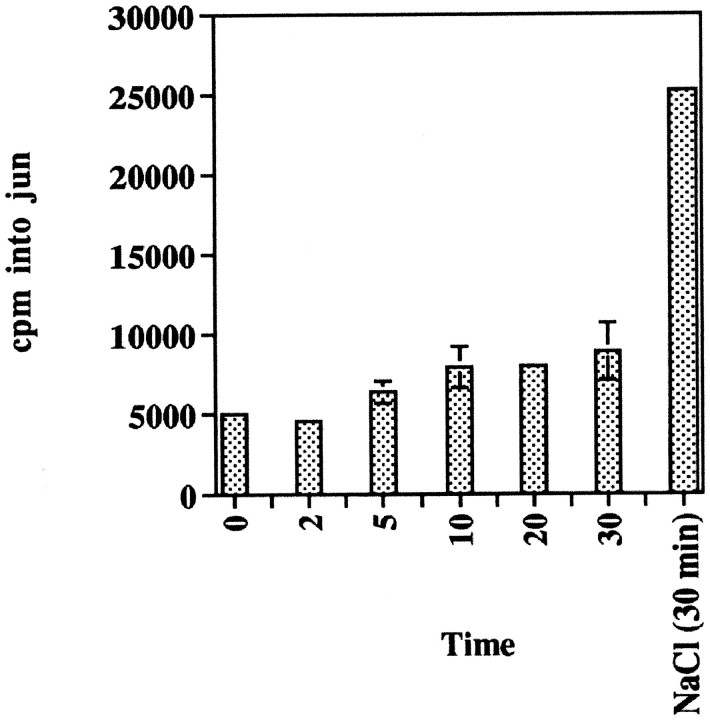
The SAPKs are other MAPK superfamily members that are activated by stress, such as hyperosmolarity, UV irradiation, and protein synthesis inhibitors (Freshney et al., 1994; Han et al., 1994; Lee et al., 1994;Cano and Mahadevan, 1995; Raingeaud et al., 1995). SAPK activity was assayed by use of a jun capture assay and phosphorylation of the SAPK substrate, gst-Jun 5–89 (Pombo et al., 1994). THP1 monocytes were exposed to Aβ25–35 or hyperosmolar conditions (300 mmNaCl). Stimulation of THP1 monocytes with Aβ25–35 did not significantly alter the activity of SAPKs, suggesting that these enzymes are not linked to the Aβ-stimulated signal transduction pathway (Fig. 4). Exposure of THP1 monocytes to hyperosmolar conditions for 30 min led to a greater than fourfold increase in SAPK activity.
Fig. 4.
Stimulation of THP1 monocytes with fibrillar Aβ does not lead to significant activation of SAPKs. THP1 monocytes were stimulated for the indicated times with 50 μm Aβ25–35 or 300 mm NaCl. SAPK activity was measured using c-Jun5–89 as a substrate. Proteins were resolved by SDS-PAGE, the gel was dried, and c-Jun protein was excised and subjected to Cerenkov counting.
Activation of the ERK and p38 MAPK pathways by Aβ peptides is blocked by the Syk-selective tyrosine kinase inhibitor piceatannol
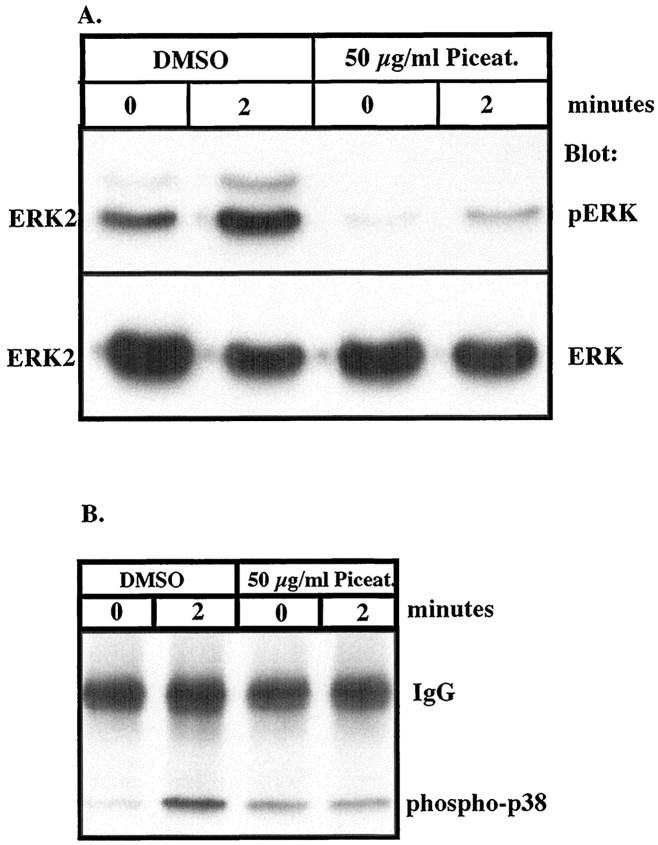
Stimulation of microglia and THP1 monocytes with Aβ leads to activation of parallel MAP kinase pathways including ERKs and p38 MAPK. Because protein tyrosine phosphorylation and activation of ERKs and p38 MAPK occur over similar intervals, we examined whether the MAPK pathways were downstream effectors of the Aβ-activated tyrosine kinases. Exposure of THP1 monocytes pretreated with DMSO vehicle followed by stimulation with Aβ25–35 led to dramatic activation of both ERK2 and p38 MAPK (Fig.5A,B). We noted that pretreatment of cells with DMSO led to small, nonspecific elevations in the basal levels of protein tyrosine phosphorylation (compare Figs. 1,7E). However, pretreatment of the cells with piceatannol, a Syk-selective tyrosine kinase inhibitor (Oliver et al., 1994), significantly reduced Aβ-induced activation of both ERK2 and p38 MAPK (Fig. 5A,B). These data demonstrate that the activation of both MAPK pathways in THP1 monocytes by Aβ is mechanistically linked to the activation of the tyrosine kinase Syk.
Fig. 5.
The tyrosine kinase inhibitor piceatannol inhibits Aβ-induced activation of ERK and p38 MAPK. THP1 monocytes were pretreated for 1 hr with 50 μg/ml piceatannol or an equal amount of DMSO (final concentration, 70 mm) and then exposed to 50 μm Aβ25–35 for 2 min. A, Activation of ERK2 was demonstrated by Western blot analysis of RIPA lysates (50 μg of protein) using anti-phospho-ERK polyclonal antibodies (top panel). The blots were reprobed with anti-ERK antibody (bottom panel). B, Activation of p38 MAPK was assessed by immunoprecipitation of tyrosine phosphoproteins using monoclonal anti-phosphotyrosine antibodies (PY20) and Western blot analysis using anti-phospho-p38 MAPK antibodies.
Fig. 7.
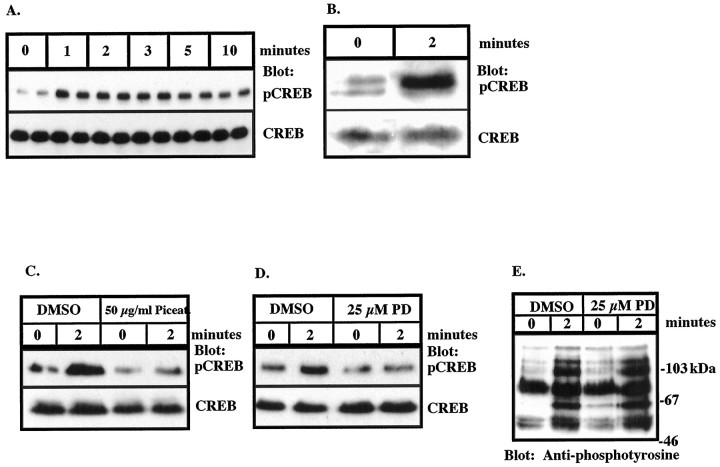
Fibrillar Aβ stimulates rapid, transient phosphorylation of CREB on serine 133 in THP1 monocytes and microglia that is blocked by piceatannol and PD98059. A, THP1 monocytes were stimulated with 50 μm Aβ25–35 for the indicated times. B, Primary cultures of rat microglia were stimulated with 50 μm Aβ25–35 for 2 min.C, Aβ-induced phosphorylation of CREB is blocked by pretreatment of THP1 monocytes with piceatannol. THP1 monocytes were pretreated with 50 μg/ml piceatannol or an equal amount of DMSO (final concentration, 70 mm) for 1 hr, followed by stimulation with 50 μm Aβ25–35 for 2 min.D, Aβ-induced phosphorylation of CREB is blocked by pretreatment of THP1 monocytes with PD98059. THP1 monocytes were pretreated with 25 μm PD98059 or an equal amount of DMSO for 20 min, followed by stimulation with 50 μm Aβ25–35 for 2 min. Phosphorylation of CREB in A–D was determined by Western blot analysis on RIPA lysates (100 μg of protein) using anti-phospho-CREB polyclonal antibodies (top panel). The blots were stripped and reprobed using anti-CREB antibody (bottom panel).E, Pretreatment of THP1 monocytes with PD98059 does not inhibit tyrosine phosphorylation. THP1 monocytes were pretreated with 25 μm PD98059 or an equal amount of DMSO for 20 min, followed by stimulation with 50 μm Aβ25–35 for 2 min. Western blot analysis of tyrosine phosphoproteins was performed on RIPA lysates (100 μg of protein) using monoclonal anti-phosphotyrosine antibodies (4G10).
RSK1 and RSK2 are activated by Aβ peptides
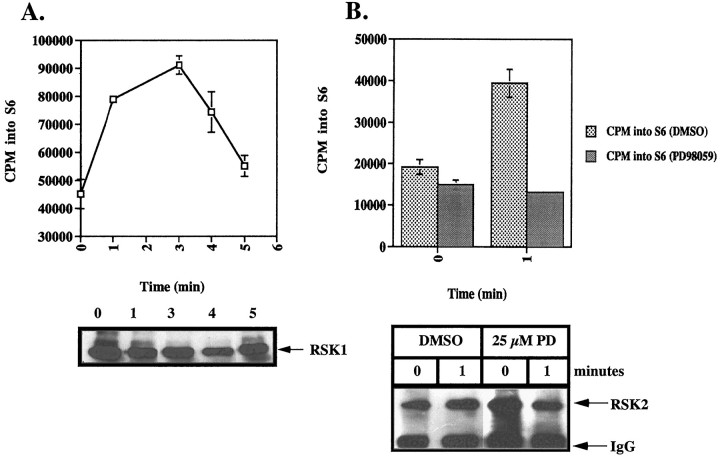
RSK1 and RSK2 are effectors of the ERK signal transduction pathway. THP1 monocytes were stimulated with Aβ25–35, and subsequent RSK activation was assayed by phosphorylation of S6 peptide, an RSK substrate (Pelech and Krebs, 1987). Maximal activation of RSK1 occurred within 3 min (Fig. 6A). RSK2 was also activated after Aβ treatment, and its stimulation was dependent on previous ERK activation, as demonstrated by the inhibition of RSK2 activation after pretreatment of the cells with PD98059 (Fig.6B,C). PD98059 is a specific inhibitor of the ERK kinase MEK (Dudley et al., 1995).
Fig. 6.
Fibrillar Aβ stimulates rapid, transient activation of RSK1 and RSK2 in THP1 monocytes, which is blocked by the MEK inhibitor PD98059. THP1 monocytes were stimulated with 50 μm Aβ25–35 for the indicated times. A, RSK1 was isolated by immunoprecipitation with anti-RSK1 polyclonal antibodies, and immune kinase activity was measured using the S6 peptide substrate. The data shown represent the mean ± SD of triplicate determinations. B, THP1 monocytes were pretreated for 20 min in the absence or presence of 25 μmPD98059 followed by stimulation for 2 min with 50 μmAβ25–35. RSK2 was isolated by immunoprecipitation with anti-RSK2 polyclonal antibodies. Kinase activity was measured using S6 peptide as a substrate, and incorporation of 32P was determined by Cerenkov counting. Immune complexes were resolved by SDS-PAGE and analyzed by Western blot analysis using anti-RSK1 or anti-RSK2 antibodies (bottom panel).
Aβ peptides stimulate phosphorylation of CREB on serine 133 in THP1 monocytes
The activation of MAP kinase family members, either directly or indirectly, leads to the phosphorylation of transcriptional factors and activation of transcription (Ginty et al., 1994). The transcription factor CREB has been shown to be phosphorylated and activated by RSK2 (Sheng et al., 1991; Xing et al., 1996). RSK1 and RSK2 are phosphorylated and activated by ERK1 and ERK2 (Blenis, 1993; Xing et al., 1996) and, thus, are regulated through activation of the MAPK cascade. We examined whether stimulation of THP1 monocytes and microglia with Aβ25–35 led to phosphorylation of CREB by Western blot analysis with anti-phospho-CREB, an antibody that specifically recognizes CREB that has been phosphorylated on serine 133, a key regulatory site required for the activation of transcription mediated by this protein (Gonzalez and Montminy, 1989; Sheng et al., 1991). Stimulation of THP1 monocytes with Aβ25–35 led to a rapid increase in CREB phosphorylation that was detectable within 1 min and remained elevated through 10 min (Fig.7A). Similarly, stimulation of microglia with Aβ25–35 led to phosphorylation of CREB at serine 133 (Fig. 7B). Interestingly, we consistently have observed that the anti-phospho-CREB antibody recognizes a doublet in Western blots of proteins from unstimulated rat microglia, but not from human THP1 monocytes. This is likely to be the result of a species-specific difference in CREB protein or may represent an elevated basal level of activation in cultures of primary microglia. To examine the dependence of CREB phosphorylation on activation of tyrosine kinases in Aβ-stimulated THP1 monocytes, cells were pretreated with piceatannol. Pretreatment of THP1 monocytes with piceatannol completely blocked Aβ-induced phosphorylation of CREB, linking activation of Syk to subsequent phosphorylation of CREB (Fig. 7C). Similarly, CREB phosphorylation was dependent on MEK and ERK activation as pretreatment of the cells with PD98059 abolished the Aβ-stimulated phosphorylation of CREB on serine 133 (Fig. 7D). Importantly, Aβ-stimulated protein tyrosine phosphorylation was not inhibited by PD98059, demonstrating the specificity of the drug action (Fig. 7E). These data provide a mechanism through which Aβ-induced, tyrosine kinase-dependent activation of MAPK pathways in THP1 monocytes and microglia can regulate gene expression.
DISCUSSION
The events leading to neuronal death and gliosis in Alzheimer’s disease are incompletely understood. Gliosis has been shown to be associated with mature senile plaques comprising fibrillar Aβ (Ohgami et al., 1991). Fibrillar Aβ, which forms the core of senile plaques, is capable of directly activating microglia, resulting in the production of superoxide radicals (El Khoury et al., 1996; McDonald et al., 1997). Indeed, examination of AD-afflicted brain tissue has revealed evidence of oxidative damage associated with senile plaques that colocalizes with reactive microglia (Hensley et al., 1995; Good et al., 1996; Smith et al., 1997). However, examination of senile plaques has also revealed the presence of cytokines, such as IL-1β and IL-6, proteases, protease inhibitors, and complement proteins (Abraham et al., 1988; Griffin et al., 1989; McGeer et al., 1989; Bauer et al., 1991). The senile plaque, therefore, is the focus of complex local inflammatory processes. Microglia, the main immune effector cells within the brain, are capable of secreting acute phase proteins detected in the AD brain (Leong and Ling, 1992). The detection of microglial-derived acute phase proteins in senile plaques is consistent with the ability of microglia to detect fibrillar Aβ, leading to activation of signal transduction pathways, altered gene expression, and acquisition of a reactive phenotype. We have previously shown that exposure of microglia and THP1 monocytes to fibrillar forms of Aβ leads to the activation of the tyrosine kinases Lyn, Syk, and FAK and production of superoxide. The present studies were initiated to identify the downstream intracellular elements mediating the effects of Aβ on these cells.
The MAPK family of protein kinases is an important link in the transduction of extracellular signals from the membrane into the nucleus. ERK1 and ERK2 are positioned downstream of growth factor receptors in ras-dependent signal transduction cascades, which include raf family members and MEK (Blenis, 1993). On phosphorylation and activation, ERKs phosphorylate other cytoplasmic effectors and are translocated into the nucleus in which they phosphorylate transcription factors, such as Myc, Fos, Jun, and Elk1 (Blenis, 1993; Chen et al., 1993). Direct substrates of the ERKs include two members of the RSK family of protein serine–threonine kinases, RSK1 and RSK2. The transcription factor CREB is phosphorylated on serine 133 in vivo by RSK2 in NGF-stimulated PC12 cells (Xing et al., 1996). The stimulation of microglia and THP1 monocytes with fibrillar Aβ peptides led to activation of ERK1, ERK2, RSK1, and RSK2 and phosphorylation of CREB on serine 133. Importantly, Aβ-induced phosphorylation of CREB was inhibited by piceatannol and the MEK inhibitor PD98059, demonstrating the dependence of CREB phosphorylation on activation of the ERK pathway. It is possible that other kinases, such as protein kinase A or CAM kinases (Gonzalez and Montminy, 1989;Sheng et al., 1991) may also contribute to phosphorylation of CREB in response to Aβ and other ligands. The complete inhibition of CREB phosphorylation by PD98059, however, suggests that the ERK pathway is the main signaling pathway leading to transcriptional activation through CREB in Aβ-stimulated monocytic cells. The present data provide a mechanism by which Aβ alters gene expression through the transcription factor CREB. Importantly, these data indicate that compounds that block Aβ-stimulated intracellular signaling cascades in microglia may block changes in gene expression and, hence, the acquisition of an activated phenotype. Stimulation of B lymphocytes through surface Ig has also been shown to result in phosphorylation of CREB, demonstrating a role for activation of CREB in response to immune stimuli (Xie and Rothstein, 1995; Xie et al., 1996).
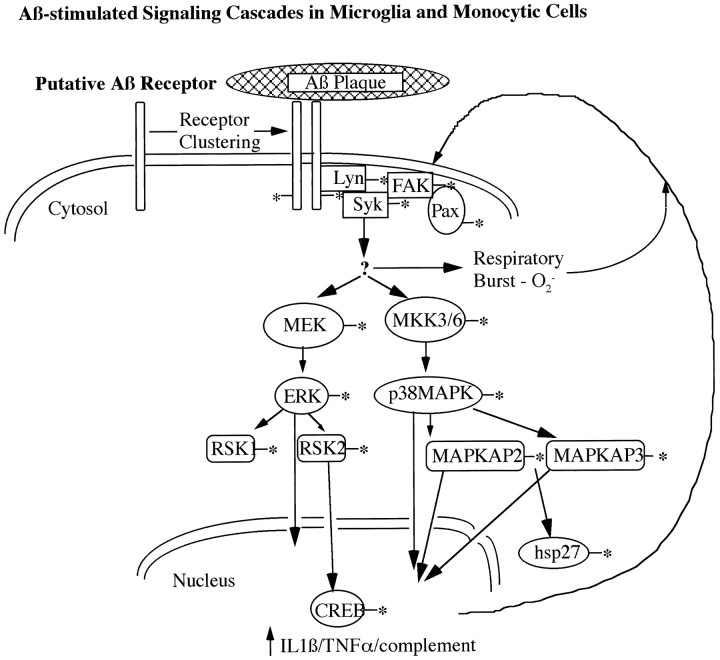
Cytokines, such as IL-1β and IL-6, have been detected within senile plaques, and total IL-1β levels were shown to be elevated in the AD brain (Griffin et al., 1989; Bauer et al., 1991). Stimulation of microglia and THP1 monocytes with Aβ resulted in increased expression of IL-1β (Walker et al., 1995; Lorton et al., 1996). Recently, activation of p38 MAPK was shown to be essential for the LPS-induced production of IL-1β in monocytes, as well as activation of the transcription factor MEFC2 (Lee et al., 1994; Han et al., 1997). Additionally, exposure of cells to IL-1β, stress, and heat shock leads to activation of MAPKAP kinase-2 and MAPKAP kinase-3, which are direct effectors of p38 MAPK (Rouse et al., 1994; McLaughlin et al., 1996). MAPKAP kinase-2 and MAPKAP kinase-3 then phosphorylate the small heat shock protein HSP27 in vivo (McLaughlin et al., 1996;Stokoe et al., 1992b). We have now shown that stimulation of microglia and THP1 monocytes with Aβ leads to activation of p38 MAPK, providing a mechanistic link between the interaction of microglia with Aβ and inflammatory responses, including activation of downstream kinases and transcription factors and the elevated levels of cytokines found in the AD brain. IL-1β, a proinflammatory cytokine that primes macrophage inflammatory responses and enhances macrophage cytotoxicity, recently has been shown to activate p38 MAPK (Erbe et al., 1990; Simms et al., 1991; Raingeaud et al., 1995). Therefore, activation of p38 MAPK in microglia by Aβ may be an initial step in a feedforward inflammatory process, resulting in the production of superoxide radicals and cytokines, which further stimulates the inflammatory responses of microglia to Aβ. A working model of the intracellular processes activated on interaction of microglia with Aβ plaques is diagrammed in Figure 8.
Fig. 8.
Working model of Aβ-induced intracellular signal transduction cascades in microglia and monocytic cells. On interaction of microglia with fibrillar aggregates of Aβ, a complex signal transduction cascade comprising tyrosine kinases and parallel MAP kinase pathways is activated, leading to respiratory burst and activation of transcription. The generation of superoxide radicals, cytokines, and complement components is likely to further stimulate inflammatory responses in microglia. An asterisk denotes phosphorylation and/or activation of enzymatic activity.
We were unable to detect significant increases in SAPK activity from Aβ-stimulated THP1 monocytes. The activation of p38 MAPK and SAPKs by environmental stressors and immune stimuli is thought to occur in parallel, and previous studies have demonstrated that SAPK and p38 MAPK are activated simulta-neously (Raingeaud et al., 1995). The present data clearly document that the activation of p38 MAPK and SAPK activation are not mechanistically linked, and Aβ stimulation selectively activates the p38 MAPK pathway. The upstream elements mediating these responses have not yet been defined and are presently controversial. Identification of the upstream regulators of ERKs and p38 MAPK activated by Aβ will allow further elucidation of the components of MAPK signal transduction pathways and provide potential therapeutic targets.
Recent data have shown that Aβ can bind RAGE and scavenger receptors (El Khoury et al., 1996; Paresce et al., 1996; Yan et al., 1996). RAGE is believed to act as a tether that binds Aβ to cell surfaces in which Aβ-generated oxygen radicals then produce cellular damage (Hensley et al., 1994). There is presently no compelling evidence that RAGE is directly linked to intracellular signaling pathways. Scavenger receptors were shown to mediate adhesion of microglia to Aβ, resulting in production of oxygen radicals by microglial cell lines (El Khoury et al., 1996). The cellular mechanisms used by these receptors to stimulate the generation of reactive oxygen species are unknown. We have shown that specific, saturating quantities of RAGE and scavenger receptor ligands do not stimulate the increased protein tyrosine phosphorylation and respiratory burst observed after exposure of THP1 monocytes to Aβ (McDonald et al., 1997). The present data demonstrate that RAGE and scavenger receptor ligands do not activate ERK or p38 MAPK in THP1 monocytes. The lack of activation of tyrosine kinases, ERK, and p38 MAPK by scavenger receptor ligands is not surprising, because undifferentiated THP1 monocytes express only low levels of scavenger receptors (Palkama, 1991). The observations that Aβ fibrils activate tyrosine kinases, MAP kinase pathways, respiratory burst, and transcription factors demonstrate that Aβ stimulates cellular responses through a receptor complex distinct from scavenger receptors and RAGE. Furthermore, characterization of the Aβ-stimulated signaling pathways in microglia and THP1 monocytes may provide more molecular targets for therapeutic interventions as well as specific, rapid assays for testing the efficacy of therapeutic agents.
Footnotes
This work was supported by National Institute on Aging Grant AG08012.
Correspondence should be addressed to Dr. Gary Landreth, Alzheimer Research Laboratory, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106-4928.
REFERENCES
- 1.Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- 2.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Yolk B, Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute phase in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 3.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boland K, Behrens M, Choi D, Manias K, Perlmutter DH. The serpin enzyme complex receptor recognizes soluble, nontoxic amyloid-β peptide but not aggregated, cytotoxic amyloid-β peptide. J Biol Chem. 1996;271:18032–18044. doi: 10.1074/jbc.271.30.18032. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 7.Cataldo AM, Nixon RA. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci USA. 1990;87:3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Abate C, Blenis J. Phosphorylation of the c-Fos transrepressive domain by mitogen activated kinase and p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durden DL, Kim HM, Calore B, Liu Y. The FcγRI receptor signal through the activation of hck and MAP kinase. J Immunol. 1995;154:4039–4047. [PubMed] [Google Scholar]
- 11.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 12.Erbe DV, Collins JE, Shen L, Graziano RF, Fanger MW. The effect of cytokines on the expression of and function of Fc receptors for IgG on human myeloid cells. Mol Immunol. 1990;27:57–67. doi: 10.1016/0161-5890(90)90060-d. [DOI] [PubMed] [Google Scholar]
- 13.Foltz IN, Lee JC, Young PR, Schrader JW. Hematopoietic growth factors with the exception of IL-4 activate the p38 Mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:3296–3301. doi: 10.1074/jbc.272.6.3296. [DOI] [PubMed] [Google Scholar]
- 14.Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 15.Gille H, Sharrocks A, Shaw P. Phosphorylation of transcription factor p62tcf by MAP kinase stimulates ternary complex formation at the c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 16.Ginty DD, Bonni A, Greenberg MA. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 17.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenner GC, Wong CW. Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 20.Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence for neuronal oxidative damage in Alzheimer’s disease. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin W, Stanley L, Ling C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down’s syndrome and Alzheimer’s disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grove JR, Price DJ, Banerjee P, Balaubramanyam A, Ahmad MF, Avruch J. Regulation of an epitope-tagged recombinant Rsk-1 S6 kinase by phorbol ester and erk/MAP kinase. Biochemistry. 1993;32:7727–7738. doi: 10.1021/bi00081a018. [DOI] [PubMed] [Google Scholar]
- 23.Haberland ME, Fogelman AM. Scavenger receptor-mediated recognition of maleylbovine plasma albumin and the demaleylated protein in human monocyte macrophages. Proc Natl Acad Sci USA. 1985;82:2693–2697. doi: 10.1073/pnas.82.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J, Lee J-D, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 808–811. [DOI] [PubMed]
- 25.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 26.Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for β-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer’s disease. Proc Natl Acad Sci USA. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensley K, Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 28.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer’s disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 29.Jao S-C, Ma J, Talafous J, Orlando R, Zagorski MG. Trifluoroacetic acid pretreatment reproducibly disaggregates the amyloid β-peptide. Amyloid Int J Exp Clin Invest. 1997;4:240–252. [Google Scholar]
- 30.Lee JC, Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gobbita SP, Wu JF, Carney JM, Lovell M, Marksbery WR, Butterfield DA. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 31.Leong SK, Ling E-A. Ameboid and ramified microglia: their interrelationship and response to brain injury. Glia. 1992;7:39–47. doi: 10.1002/glia.440060106. [DOI] [PubMed] [Google Scholar]
- 32.Lorton D, Kocsis J-M, King L, Madden K, Brunden KR. β-amyloid induces increased release of Interleukin1β from Lipopolysaccharide-activated human monocytes. J Neuroimmunol. 1996;67:21–29. doi: 10.1016/0165-5728(96)00030-6. [DOI] [PubMed] [Google Scholar]
- 33.Masters CL, Simms G, weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid core protein in Alzheimer’s disease and Down’s Syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer’s disease. Can J Neurol Sci. 1989;16:516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin MM, Kumar S, McDonnell PC, Van Horn S, Lee JC, Livi GP, Young PR. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 37.Meda L, Cassatella MA, Szendrei GI, Otovos L, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon γ. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 38.Ohgami T, Kitamoto T, Shin R-W, Kaneko Y, Ogomori K, Tateishi J. Increased senile plaques with microglia in Alzheimer’s disease. Acta Neuropathol. 1991;81:242–247. doi: 10.1007/BF00305864. [DOI] [PubMed] [Google Scholar]
- 39.Oliver JM, Burg DL, Wilson BS, McLaughlin JM, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- 40.Palkama T. Induction of interleukin-1 production by ligands binding to the scavenger receptor in human monocytes and the THP1 cell line. Immunology. 1991;74:432–438. [PMC free article] [PubMed] [Google Scholar]
- 41.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalized aggregates of the Alzheimer’s disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 42.Pelech SL, Krebs EG. Mitogen-activated S6 kinase is stimulated via protein kinase C-dependent and independent pathways in Swiss 3T3 cells. J Biol Chem. 1987;262:11598–11606. [PubMed] [Google Scholar]
- 43.Pombo CM, Bonventre JV, Avurch J, Woodgett JR, Kyriakis JM, Force T. The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. J Biol Chem. 1994;269:26546–26551. [PubMed] [Google Scholar]
- 44.Pulverer B, Kyriakis J, Avuruch J, Nikolakaki E, Woodget J. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:760–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 45.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 Mitogen-activated Protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 46.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 47.Selkoe DJ. Amyloid β-Protein and the Genetics of Alzheimer’s Disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 48.Sheng M, Thompson MA, Greenberg ME. CREB: A Ca2+-regulated transcription factor phosphorylated by Calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 49.Simms HH, Gaither TA, Fries LF, Frank MM. Monokines released during short-term Fc gamma receptor phagocytosis upregulate polymorphonuclear leukocytes and monocyte-phagocytic function. J Immunol. 1991;147:265–272. [PubMed] [Google Scholar]
- 50.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s Disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992a;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase-2 as a major enzyme responsible for the phosphorylation of the small heat shock proteins. FEBS Lett. 1992b;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 53.Terzi E, Holzemann G, Seelig J. Reversible random coil-β-sheet transition of the Alzheimer β-amyloid fragment (25–35). Biochemistry. 1994;33:1345–1350. doi: 10.1021/bi00172a009. [DOI] [PubMed] [Google Scholar]
- 54.Walker DG, Kim SU, McGeer PL. Complement and cytokine expression in cultured microglia derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- 55.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 56.Xie H, Rothstein TL. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J Immunol. 1995;154:1717–1723. [PubMed] [Google Scholar]
- 57.Xie H, Wang Z, Rothstein TL. Signaling pathways for antigen receptor-mediated induction of transcription factor CREB in B lymphocytes. Cell Immunol. 1996;169:264–270. doi: 10.1006/cimm.1996.0117. [DOI] [PubMed] [Google Scholar]
- 58.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–962. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 59.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 60.Yankner BA, Duffy LK, Kirschner DA. Neurotropic and neurotoxic of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]