Abstract
Preexposing subjects to visual stimuli is sufficient to establish a subsequent preference, even when previous exposure is subliminal, such that explicit recognition is at chance. This influence of previous exposure on preference judgments, known as the “mere exposure effect,” is a form of unconscious memory. The present functional neuroimaging study examines the mechanism of this effect. Nine volunteer subjects were studied using functional imaging while making forced choice judgments about abstract stimuli on the basis of either preference or memory. Each judgment type was made under two conditions: under one condition one or the other stimulus had previously been presented subliminally, whereas under the second condition both stimuli were novel. Memory judgments were associated with activation of left frontopolar cortex and parietal areas, whereas preference judgments were associated with activation of medial prefrontal cortex and regions of occipital cortex. The modulation of preference by objective familiarity (implicit memory) was associated with right lateral frontal activation. Significant activation of hippocampal gyrus was seen in response to objective stimulus novelty, regardless of judgment type required. Our data thus demonstrate activations of a memory system independent of recollective experience. Dissociable activations within this system implicate a frontopolar involvement in explicit retrieval attempt and right lateral prefrontal cortex involvement in implicit memory expressed in preference judgments. Furthermore, the results suggest that hippocampal response to stimulus novelty can be independent of conscious reportability of familiarity.
Keywords: episodic retrieval, implicit memory, subliminal presentation, hippocampal gyrus, prefrontal cortex, positron emission tomography
Functional imaging studies suggest that episodic memory retrieval is associated with activations in a distributed network of brain regions, including prefrontal and parietal cortices and, less consistently, medial temporal lobe structures (Shallice et al., 1994; Tulving et al., 1994; Buckner and Tulving, 1995; Fletcher et al., 1995; McCarthy, 1995). However, unresolved issues remain regarding the functional roles of different structures in aspects of memory retrieval.
A critical issue concerns the distinction between neural systems supporting explicit and implicit memory, and recent studies have attempted to dissociate these systems (Buckner et al., 1995; Schachter et al., 1996). Explicit, compared with implicit, recall was associated with more widespread prefrontal activation and more significant hippocampal activation. A related issue is the distinction between systems engaged by intentional and incidental paradigms. In a recent study of intentional compared with incidental retrieval, Rugg et al. (1997) found greater prefrontal activation during intentional memory, whereas hippocampal activation was independent of whether memory was intentional or incidental.
In many studies of explicit retrieval, a distinction is made between retrieval attempt or effort and retrieval success. It has been argued that prefrontal activation during retrieval reflects processes related to retrieval attempt (Nyberg et al., 1995; Schachter et al., 1996), although this view is not supported by other data (Rugg et al., 1996) indicating sensitivity to retrieval success. Another issue in studies of retrieval is the influence of item novelty (Stern et al., 1996;Tulving et al., 1996). These studies suggest roles for medial temporal lobe structures in processing stimulus novelty, an effect also seen at encoding (Dolan and Fletcher, 1997).
We examined these issues using a paradigm in which retrieval of nonverbal stimuli is more successful during an indirect rather than a direct task. This paradigm, based on the “mere exposure effect” (Zajonc, 1980), demonstrates that previous exposure to stimuli can increase subjects’ subsequent preference for those stimuli (Zajonc, 1968; Harrison, 1977). Critically, the effect has been demonstrated most strongly when the stimuli are subliminally presented such that subsequent recognition is at chance (Shevrin et al., 1971; Kunst-Wilson and Zajonc, 1980; Bornstein and d’Agostino, 1992). Our study used functional neuroimaging to examine the neural basis of this mere exposure effect. Subjects made forced choice judgments on pairs of stimuli based on preference or on memory under two conditions. Under one condition, one of each pair had previously been presented subliminally, whereas under the other both were novel. This study therefore provides a new approach to determining the neural substrates of implicit memory. It also examines the distinct effects of retrieval effort and success in explicit memory, because during recognition subjects were trying to remember while performing at chance and thus showing no retrieval success. Finally, the study allowed us to examine the effect of stimulus novelty in a context in which subjects have no explicit awareness of stimulus novelty.
Although retrieval in this study is implicit, we hypothesized that task performance would be mediated by mnemonic subprocesses that overlap with those supporting conventional episodic retrieval. On the basis of previous research, we expected activations within an extended memory system, including prefrontal, medial temporal, and parietal regions. Furthermore, we predicted a role for medial temporal lobe structures in response to stimulus novelty (Dolan and Fletcher, 1997).
MATERIALS AND METHODS
Subjects. Nine male volunteers between 22 and 47 years of age were recruited. Six of the subjects were right-handed, and three were left-handed. When the activations associated with handedness were explicitly compared, there were no significant differences, and therefore all of the subjects were included in a group analysis. Subjects with any neurological or psychiatric history were excluded. The study was approved by the local hospital ethics committee, and permission to administer radioactive substances was obtained from the Administration of Radioactive Substances Advisory Committee (ARSAC) UK. Informed written consent was obtained before the study.
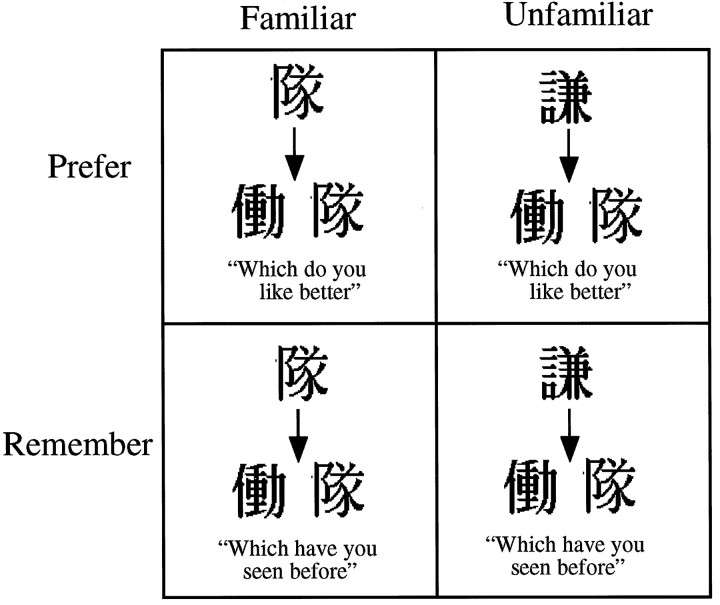
Cognitive activation paradigm. This experiment was a 2 × 2 factorial design. The two factors were stimulus familiarity (familiar compared with unfamiliar) and type of judgment subjects were required to make (preference compared with memory). Before each scan, subjects were presented with 20 black and white stimuli (Japanese ideograms), 10 times each. These presentations were very brief, with stimuli appearing on the screen for only 50 msec. Presentations were masked using a black and white checkerboard, which was presented for 450 msec between each stimulus. These parameters were established in a behavioral pilot study of 14 subjects to set an appropriate threshold where the stimuli were not subjectively identifiable. The total presentation time was thus 100 sec, and the subjective percept during this time was of a flickering checkerboard. All ideograms presented during the prescanning exposure sequences were unique.
During each scan, subjects were presented with 20 pairs of ideograms and asked to make one of two judgments about each pair: either which of the stimuli had been seen in the prescan presentation phase (“memory” judgment) or which of the stimuli was more pleasant to look at (“preference” judgment). For each of these two judgment conditions, there were two familiarity conditions: “familiar,” which was when one of each pair had been seen in the presentation stage, and “unfamiliar,” which was when neither had been seen (in this condition, the 20 stimuli the subjects saw during prescanning exposure were never seen again) (Fig.1).
Fig. 1.
Schematic representation of the four conditions in the 2 × 2 factorial design.
Positron emission tomography scanning technique. Regional cerebral blood flow was measured with an ECAT HR+ scanning system (CTI Siemens, Knoxville, TN) in three-dimensional mode with septa retracted. For each scan, 330 MBq of H215O were flushed through a venous cannula in the left antecubital vein with normal saline over 20 sec at a rate of 10 ml/min by an automatic pump. After a delay of ∼35 sec, a rise in counts could be detected at the head, peaking 30–40 sec later, which varied for individual subjects. The data were acquired during one 90 sec frame, beginning 5 sec before the rising phase of the head curve. A total of 12 scans were performed at intervals of 8 min. Correction for attenuation was made by performing a transmission scan with an exposed 68Ge/68Ga external ring source before each session. Images were reconstructed by filtered-back projection to give a resolution of ∼6 mm at full width half maximum and displayed in a 128 × 128 pixel format, with 43 planes rendering the voxels approximately cubic.
Data analysis. Data were analyzed using Statistical Parametric Mapping (SPM96, Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks Inc., Sherborn, MA) and run on a SPARC workstation (Sun Microsystems Inc., Surrey, UK). Scans were realigned using the first as a reference and were subsequently transformed into a standard space corresponding to the stereotactic atlas of Talairach and Tournoux (1988) using MNI templates (Montreal Neurological Institute). These normalized images were smoothed with a 16 mm FWHM isotropic Gaussian kernel.
Analysis of this factorial experiment was performed using the general linear model. The conditions for each subject were specified in the appropriate design matrix, which also included global activity as a confounding covariate and can therefore be considered an ANCOVA. Effects at each and every voxel were estimated according to the general linear model, and regionally specific effects were compared using linear contrasts. The resulting set of voxel values for each contrast constituted a statistical parametric map of the t statistic [SPM(t)], which was then transformed to the unit normal distribution SPM(Z). Statistical inferences were based on the theory of random Gaussian fields (Friston et al., 1995).
Furthermore, we attempted to examine interregional patterns of activity for critical brain activations using the concept of “psychophysiological interactions” (Friston et al., 1997). This term has been coined to refer to the interaction between psychological context and physiological activity in the brain. The aim of such an analysis is to attempt to explain the neural response in one brain region in terms of an interaction between input from a different region and experimental cognitive conditions. Specifically, in this study, because the right lateral prefrontal cortex plays a role in retrieval related processes, the activation in this region (x = 46, y = 12, z = 28), derived from the interaction term assessing modulation of judgment type by familiarity, was used as a covariate of interest to determine condition-specific regressions at every voxel. The resulting SPM(t) demonstrates the presence of significant psychophysiological interactions, that is, context-specific changes in the contribution of right prefrontal cortex activity to other brain regions.
We report activations at a significance level of p < 0.001, in view of the fact that many of the activations reported were in predicted brain regions. The stereotactic coordinates of Talairach and Tournoux (1988) are used to report the observed activation foci. However, the descriptions of the anatomical localization of these foci were determined using the averaged structural magnetic resonance imaging (MRI) of the group and the atlas of Duvernoy (1991). We have found that this method provides a more accurate localization than the Talairach and Tournoux atlas (1988).
RESULTS
Performance data
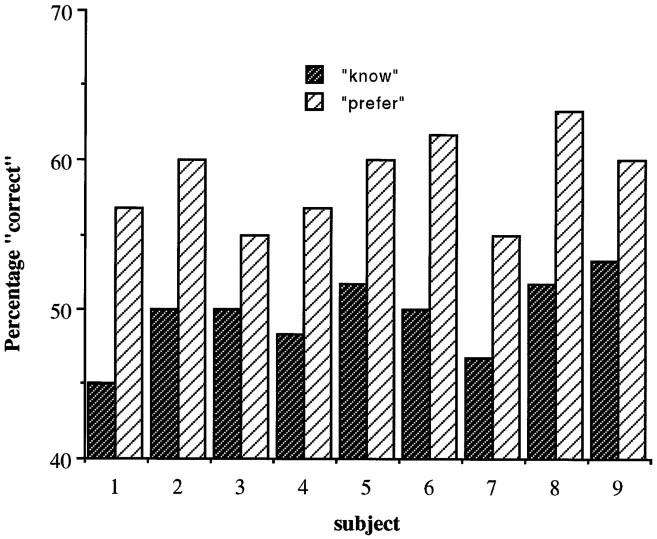
All nine subjects showed the preexposure effect. In the memory condition, the percentage correct forced choice recognition did not differ significantly from chance (50%). In the preference condition, subjects were significantly more likely to select the stimulus they had seen before (mean percent recognized 49.6%, mean percent preferred 59.3%; t = 11.5; p < 0.001). (Fig.2). Thus, subjects were preexposed to stimuli under such degraded conditions that they performed at chance levels when tested explicitly. However, by asking subjects which stimulus they prefer, it was shown that behavior is influenced by implicit information about the stimuli.
Fig. 2.
Performance data for the nine individual subjects, all of whom clearly show the mere preexposure effect.
Activations associated with memory compared with preference (retrieval attempt)
These comparisons represent the main effects of the type of judgment required (memory compared with preference), regardless of objective familiarity (Table 1). Significantly greater activations associated with memory, compared with preference, were seen in the left posterior (BA 7) and left inferior parietal cortex (BA 40) and left medial frontal gyrus (BA 10) (Fig.3a). Significantly greater activations associated with preference compared with memory were seen in left anterior frontal cortex (BA 8) (Fig. 3b), left premotor cortex (BA 6), left caudate nucleus and left pulvinar, right superior temporal gyrus (BA 22) and right fusiform gyrus (BA 20), and bilateral cerebellum and bilateral medial occipital gyrus (BA 18/19).
Table 1.
Coordinates of significant rCBF change associated with memory compared with preference
| Region of activation | Left/right | Brodmann’s area | Talairach coordinates | Zvalue (3.09 for p < 0.001) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Relative activations for memory | ||||||
| Inferior parietal cortex | L | 7 | −36 | −70 | 42 | 3.81 |
| 40 | −44 | −48 | 44 | 3.43 | ||
| Anterior frontal cortex | L | 10 | −26 | 58 | 8 | 3.49 |
| Relative activations for preference | ||||||
| Medial occipital gyrus | L | 19 | −38 | −68 | 0 | 3.93 |
| R | 19 | 50 | −74 | 4 | 3.75 | |
| Anterior prefrontal cortex | L | 8 | −8 | 60 | 38 | 4.07 |
| Superior temporal gyrus | R | 22 | 70 | −16 | 14 | 3.92 |
| Premotor cortex | L | 6 | −18 | 26 | 68 | 3.91 |
| Fusiform gyrus | R | 20 | 40 | −16 | −10 | 3.77 |
| Caudate nucleus | L | −22 | −2 | 22 | 3.77 | |
| Pulvinar | L | −18 | −36 | 8 | 3.66 | |
| Cerebellum | L | −28 | −80 | −24 | 4.30 | |
| R | 32 | −78 | −30 | 4.23 | ||
Fig. 3.
a, Adjusted blood flow in the left frontopolar cortex, activated in memory compared with preference.b, Adjusted blood flow in the left anterior frontal cortex, activated in preference compared with memory. The adjusted response, indicative of regional cerebral blood flow, is based on normalized counts, and these graphs show the relativecounts associated with each condition; therefore there are no associated units.
Activations associated with objective stimulus familiarity/novelty
These comparisons represent the main effects of whether the stimulus had been presented in the preexposure phase, regardless of the type of judgment required (Table 2). There were no significant activations associated with familiarity compared with unfamiliarity. Unfamiliar, or novel, compared with familiar stimuli were associated with significant activations in the left mediodorsal thalamus, left fusiform gyrus (BA 19), left superior temporal gyrus (BA 38), right cuneus (BA 19), and right hippocampal gyrus (Fig. 4).
Table 2.
Coordinates of significant rCBF change associated with objectively unfamiliar/novel stimuli
| Region of activation | Left/right | Brodmann’s area | Talairach coordinates | Zvalue (3.09 for p < 0.001) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Fusiform gyrus | L | 19 | −30 | −68 | 6 | 3.27 |
| Cuneus | R | 19 | 18 | −94 | 32 | 3.24 |
| Superior temporal gyrus | L | 38 | −36 | 2 | −32 | 3.78 |
| Hippocampal gyrus | R | 35 | 26 | −38 | −12 | 3.09 |
| Thalamus | L | −12 | −18 | 16 | 3.33 | |
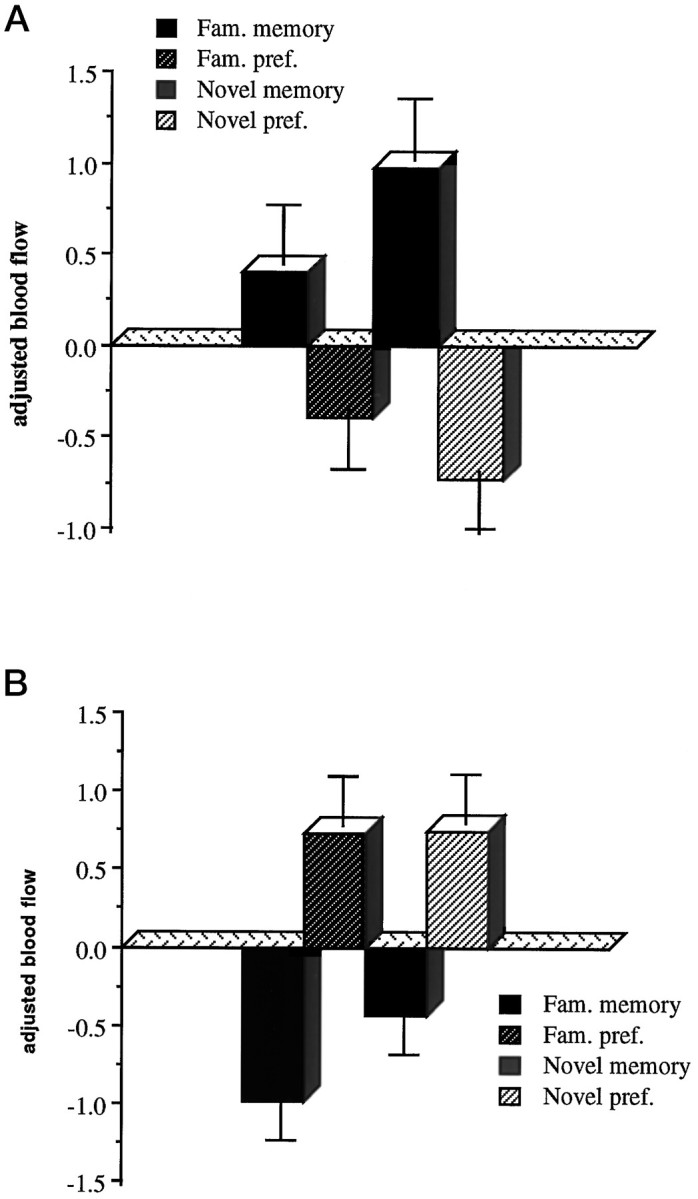
Fig. 4.
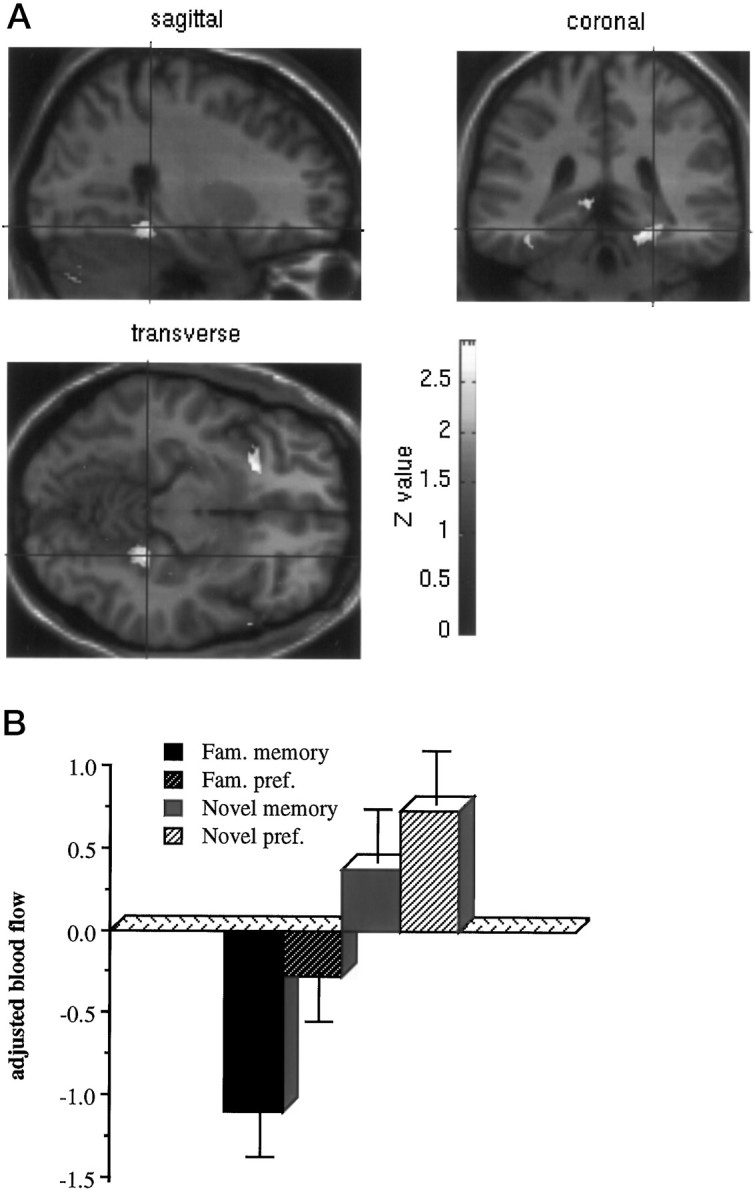
Activation of the right hippocampal gyrus associated with the main effect of objective novelty compared with familiarity. a shows the statistical parametric map of the t statistic [after transformation to a SPM(Z)] thresholded at p < 0.01 and rendered onto a standard MRI template. b shows adjusted blood flow in this region under the four conditions.
Interaction of psychological context and familiarity: explicit and implicit retrieval
These comparisons represent the interaction terms in the factorial design (Table3). Significant differences between activation associated with objective familiarity in the memory compared with the preference condition was seen in the right cerebellum. Significant differences between activations associated with objective familiarity in the preference compared with the memory condition were seen in the left premotor cortex (BA 6), right inferior frontal gyrus (BA 44) extending into middle frontal gyrus (BA 9), right precentral gyrus (BA 4), right lateral orbitofrontal cortex (BA 11), and right medial occipital gyrus (BA 19) (Fig. 5).
Table 3.
Coordinates of significant rCBF change associated with modulation of judgment type by objective familiarity
| Region of activation | Left/right | Brodmann’s area | Talairach coordinates | Zvalue (3.09 for p < 0.001) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Modulation: memory compared with preference | ||||||
| Cerebellum | R | 14 | −50 | −34 | 3.20 | |
| Modulation: preference compared with memory | ||||||
| Premotor cortex | L | 6 | −32 | 4 | 70 | 3.90 |
| Inferior frontal gyrus | R | 44 | 46 | 12 | 28 | 3.54 |
| Middle frontal gyrus | R | 19 | 46 | 18 | 38 | 3.13 |
| Precentral gyrus | R | 4 | 4 | −24 | 54 | 3.40 |
| Lateral orbitofrontal cortex | R | 11 | 42 | 38 | −16 | 3.29 |
| Medial occipital gyrus | R | 19 | 42 | −76 | 38 | 3.16 |
Fig. 5.
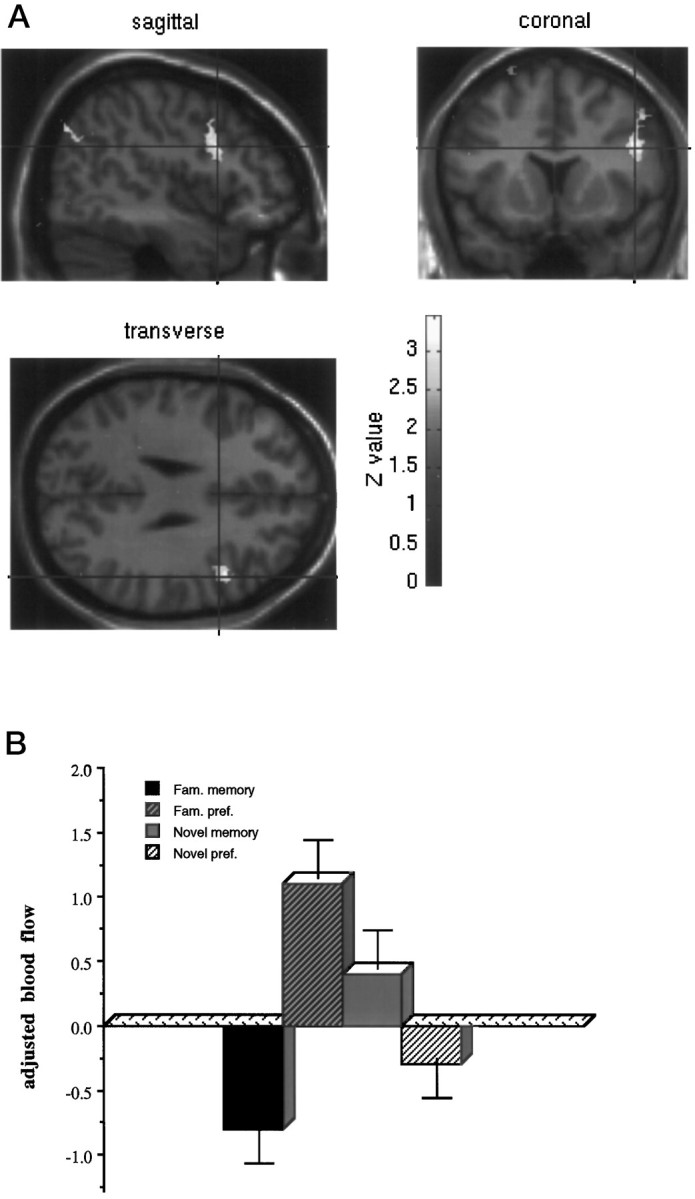
Activation of the right lateral prefrontal associated with the modulation of preference compared with guessing by objective familiarity. a shows the statistical parametric map of the t statistic [after transformation to a SPM(Z)] thresholded atp < 0.01 and rendered onto a standard MRI template. b shows adjusted blood flow in this region under the four conditions.
Effect of judgment for familiar stimuli
This comparison represents the simple main effects of judgment in the familiarity conditions alone and reflects implicit compared with explicit retrieval (Table 4). There were no significant activations associated with memory compared with preference for familiar stimuli. Preference compared with memory for familiar stimuli was associated with significant activations in left fusiform gyrus (BA 19), left premotor cortex (BA 6), right inferior frontal gyrus (BA 44) extending to middle frontal gyrus (BA 9), right superior temporal cortex (BA 42), bilateral anterior frontal cortex (BA 10), and posterior cingulate gyrus (BA 23).
Table 4.
Coordinates of significant rCBF change associated with preference compared with memory for familiar stimuli only (implicit compared with explicit memory)
| Region of activation | Left/right | Brodmann’s area | Talairach coordinates | Zvalue (3.09 for p < 0.001) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Premotor cortex | L | 6 | −18 | 24 | 68 | 4.01 |
| Inferior frontal gyrus | R | 44 | 44 | 8 | 32 | 3.85 |
| Middle frontal gyrus | R | 9 | 50 | 22 | 18 | 3.60 |
| Anterior frontal cortex | R/L | 8 | −2 | 56 | 42 | 3.74 |
| Fusiform gyrus | L | 19 | −28 | −62 | −6 | 4.23 |
| Superior temporal cortex | R | 42 | 72 | −24 | 10 | 3.45 |
| Posterior cingulate | R | 23 | 6 | −20 | 34 | 3.45 |
Regression analysis [psychophysiological interaction (Friston et al., 1997)]
A critical region of activation identified in many functional imaging studies of memory for both verbal and visual stimuli is the right prefrontal cortex. We identified a region of right prefrontal cortex extending from inferior (BA 44) to middle frontal gyrus (BA 9), where there was differential activation in preference compared with memory for stimulus familiarity. Consequently, using a regression analysis based on activity in the maximally activated voxel within this region (x = 46, y = 14,z = 28), we sought to determine the contribution of this region to activity in other brain regions under different task conditions (i.e., preference vs memory judgment crossed with stimulus familiarity).
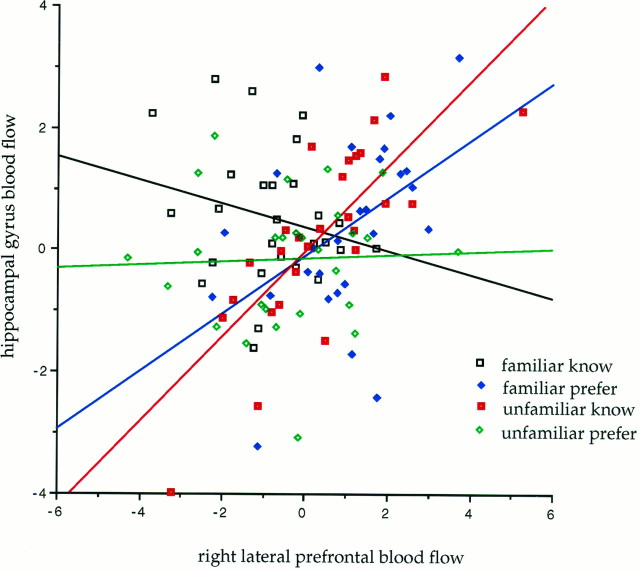
This analysis identified a number of significant interactions in regions previously associated with episodic memory. A significantly greater contribution of right lateral prefrontal cortex to activity in the right hippocampal gyrus (x = 18, y= −26, z = −6) was expressed when preference, compared with memory, was modulated by objective familiarity (Fig.6). Two effects contributed to this interaction. Within the objectively familiar condition, a significantly greater contribution to hippocampal activity was seen in the context of preference compared with memory (implicit compared with explicit memory). For the memory (i.e., attempting to remember) as opposed to the preference condition, a significantly greater contribution to hippocampal activity was seen in the context of novelty compared with familiarity. The other striking effect revealed by this regression analysis was a significant contribution of right prefrontal cortex activity to inferior parietal activation, extending into medial parietal cortex (precuneus) on the left, in the explicit memory condition for familiar stimuli.
Fig. 6.
Plot of the regression between prefrontal blood flow (at the voxel x = 46, y = 14, z = 28) and blood flow of right hippocampal gyrus (at the voxel x = 18, y = −26, z = −6) under the four different cognitive conditions. The figure shows that prefrontal contribution to hippocampal activation is maximally expressed under two conditions: preference judgments for familiar stimuli and memory judgment for unfamiliar stimuli.
DISCUSSION
The key findings of this study were activations in distinct regions of prefrontal cortex in association with hippocampal regions and lateral and medial parietal cortex, all of which are implicated in memory (Buckner et al., 1995; Fletcher et al., 1997). These activations were dissociable with respect to both psychological context and stimulus familiarity. Attempting to remember was associated with activation in left medial frontal gyrus. Objective stimulus novelty was associated with activation of right parahippocampal gyrus. The interaction between preference compared with memory and stimulus familiarity, which can be interpreted in terms of implicit compared with explicit memory, was associated with modulation of activity in right lateral prefrontal cortex. This prefrontal focus exerted a significant context-dependent influence on right hippocampal gyrus and on bilateral inferior parietal cortex. Therefore, the novel aspect of our findings is a demonstration of activations in memory systems, characterized in previous studies, in a context in which there is no subjective recognition of stimuli. Furthermore, processing objectively novel compared with familiar stimuli resulted in activation of right hippocampal gyrus despite the subjects’ lack of conscious awareness of this distinction.
Prefrontal cortex
Three regions of prefrontal cortex were activated under different experimental contexts. A region of right lateral prefrontal cortex was significantly more activated in preference than memory conditions for objectively familiar stimuli. This region therefore responds to implicit retrieval in the absence of subjective reportability. These observations speak to the controversy concerning the distinction between retrieval attempt or effort and success in explicit memory tasks. The retrieval attempt and effort hypotheses suggests that lateral prefrontal activation is associated with trying to retrieve information (Kapur et al., 1995; Nyberg et al., 1995; Schachter et al., 1996). The retrieval success hypothesis suggests that it is the successful retrieval of items from episodic memory that elicit lateral prefrontal activation (Tulving et al., 1994, 1996; Rugg et al., 1996). In the present study, retrieval attempt and effort are expressed in the memory but not the preference condition. Our observation of right lateral prefrontal activation in the preference, but not memory, condition for familiar stimuli challenges a retrieval effort or attempt account.
Right lateral prefrontal activation is associated with implicit retrieval, which is psychologically distinct from explicit retrieval success. However, it is possible that implicit retrieval may involve overlapping processes with explicit retrieval. An elaboration of the retrieval success hypothesis suggests that lateral prefrontal activation is associated with postretrieval processing of stimulus information. Rugg et al. (1997) suggested that one function of this region was the “use of retrieved information to guide behavior.” Retrieval in the present study was implicit and unconscious, yet it influenced behavior as expressed in preference judgments. We suggest that this region of right lateral prefrontal cortex may subsume behavioral guidance functions, even in the absence of conscious awareness. This proposal is consistent with recent findings of Berns et al. (1997), who reported right lateral prefrontal activation during implicit behavioral guidance without awareness.
The findings of the present study also provide evidence for a functional dissociation between lateral frontal and frontopolar regions. A region of the left frontopolar cortex (BA 10) was activated in the context of memory compared with preference judgments. This is a region that has been activated in previous studies of episodic retrieval, although activations are either bilateral (Rugg et al., 1996; Schachter et al., 1996; Tulving et al., 1996) or right-sided (Buckner et al., 1995). In the majority of these studies, frontopolar activation has been coincidental with dorsolateral activation. However, in our study, frontopolar activation was preferentially associated with attempting to retrieve stimulus information, whereas implicit retrieval was associated with right lateral activity. Therefore, by dissociating retrieval effort from success in the direct memory task, the present results implicate this frontopolar region in retrieval effort rather than success.
An additional anterior medial prefrontal region was activated in the preference condition, regardless of objective familiarity. This activation is therefore unlikely to be specifically memory-related but may represent a neural correlate of processing related to affective judgment. This region is also implicated in conditional learning tasks (Petrides, 1982, 1990; Petrides et al., 1993), which depend on establishing stimulus preferences. There is also evidence that a region close to the focus that we identified is associated with emotional compared with nonemotional responses to pictorial stimuli (Lane et al., 1997). Our results also suggest a role for this medial anterior frontal region in affective stimulus processing.
Medial temporal lobe
Processing of objectively novel stimuli was associated with activation of right hippocampal gyrus. One interpretation is that activation is decreased by previous exposure to the stimuli because of perceptual priming. Previous studies have suggested deactivations of medial temporal lobe in association with perceptual priming (Squire et al., 1992). An alternative, and not mutually exclusive, explanation is that activation in this region reflects a response to stimulus novelty. Such an activation for subliminal stimulus presentation would imply that processing of stimulus novelty by medial temporal cortex is independent of subjective reportability. Strikingly, this novelty-related activation was not influenced by psychological context: it occurred in both the preference and memory conditions. This second interpretation is consistent with previous findings of novelty-related activations of hippocampal regions under both encoding and retrieval conditions (Stern et al., 1996; Tulving et al., 1996; Dolan and Fletcher, 1997; Gabrieli et al., 1997). Electrophysiological studies in animals have demonstrated responsiveness of medial temporal units to stimulus novelty, both in animals (Fahy et al., 1993;) and in humans (Fried et al., 1997).
A striking aspect of our data is the observation that activation in right lateral prefrontal cortex showed condition-dependent covariation with activity in right hippocampal gyrus. Prefrontal contributions to hippocampal activity were maximally expressed in the interaction between judgment type and objective familiarity. Greater hippocampal activation was seen in the unfamiliar compared with the familiar condition for memory judgments and in the preference compared with the memory condition for familiar stimuli. When stimuli are objectively familiar, hippocampal activity is associated with implicit rather than attempted retrieval. This interpretation accords with previous data demonstrating hippocampal activation during retrieval, even without intention to retrieve (Squire et al., 1992; Schachter et al., 1996; Rugg et al., 1997).
Neuropsychological studies have found that Korsakoff’s amnesics, with medial temporal lobe damage, showed the mere exposure effect (Johnson et al., 1985). It thus seems unlikely that the prefrontal contribution to hippocampal activity observed in our study is a necessary substrate for this effect. One possibility is that rather than being necessary for implicit retrieval, this contribution reflects some form of unconscious postretrieval processing.
Parietal cortex
The psychophysiological interaction analysis also showed greater prefrontal contribution to bilateral parietal regions, extending to precuneus on the left, when a memory judgment was required for familiar stimuli. A role for parietal cortices, and particularly medial parietal cortex in episodic retrieval, is well established (Shallice et al., 1994; Tulving et al., 1994; Fletcher et al., 1995). The present findings suggest that prefrontal contributions to parietal activation are specifically associated with an explicit intention to retrieve.
Activations associated with priming
Previous neuroimaging studies of implicit retrieval using various priming paradigms typically report that perceptual priming is associated with reductions of blood flow in occipital regions (Squire et al., 1992; Buckner et al., 1995, 1998; Schachter et al., 1996), whereas conceptual priming is associated with reductions of blood flow in left prefrontal regions (Raichle et al., 1994; Demb et al., 1995;Wagner et al., 1997; Buckner et al., 1998). The task used in the present study involves a form of perceptual priming, but we failed to see reductions in blood flow. Instead, we observed that priming was associated with increases of blood flow in regions including right lateral prefrontal cortex. There are several possible reasons for this discrepancy. First, priming in our study was based on subliminal previous exposure allowing only fleeting, and unconscious, perceptual processing of the stimuli. Second, and perhaps critically, priming was expressed during affective preference judgments, raising the possibility that patterns of activation associated with priming may be influenced by psychological, including affective, context. Finally, we note that preference compared with memory for both familiar and unfamiliar stimuli was associated with increased blood flow in visual areas that may “mask” priming-related reductions.
Conclusions
The critical observation in this study is that activation of memory systems does not depend on subjective reportability of previous occurrence of target stimuli. The subliminal stimulus presentation meant that there was no subjective recollection of the stimuli. When a preference judgment was required, however, subjects reliably showed implicit retrieval. Retrieval effort and implicit retrieval were thus dissociated and found to depend on distinct prefrontal activations. A region of right hippocampal gyrus was activated in association with objective stimulus novelty in the absence of awareness of novelty. An analysis of condition-dependent interactions between right prefrontal cortex and other brain regions revealed a complex role for a more anterior right hippocampal region in memory. The right prefrontal contribution to this hippocampal activation reflects two interacting factors: stimulus novelty and implicit retrieval. By contrast, bilateral parietal cortex was activated in association with right lateral prefrontal cortex when stimuli were objectively familiar and retrieval effort was required. Thus, use of this paradigm has allowed us not only to demonstrate activations of regions previously described as subserving episodic memory in the absence of conscious retrieval, but also to dissociate different functional roles for structures within this system.
Footnotes
R.J.D. is supported by the Wellcome Trust. We are grateful to Paul Fletcher and Mick Rugg for their helpful suggestions and discussion.
Correspondence should be addressed to Dr. Rebecca Elliott, Wellcome Department of Cognitive Neurology, 12 Queen Square, London, WC1N 3BG, UK.
REFERENCES
- 1.Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein RF, d’Agostino PR. Stimulus recognition and the mere exposure effect. J Pers Soc Psychol. 1992;63:545–552. doi: 10.1037//0022-3514.63.4.545. [DOI] [PubMed] [Google Scholar]
- 3.Buckner RL, Tulving ET. Neuroimaging studies of memory: theory and recent PET results. In: Boller F, Grafman J, editors. Handbook of neuropsychology, Vol 10. Elsevier; Amsterdam: 1995. pp. 439–466. [Google Scholar]
- 4.Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL, Goodman J, Burock M, Rotte M, Koutsaal W, Schachter DL, Rosen B, Dale A. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 6.Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 8.Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy and MRI. Springer; New York: 1991. [Google Scholar]
- 9.Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;93:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RSJ, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends Neurosci. 1997;20:213–218. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- 12.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 13.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 14.Friston KJ, Buechel C, Fink G, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 15.Gabrieli JSE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 16.Harrison AA. Mere exposure. In: Berkowitz L, editor. Advances in experimental social psychology, Vol 10. Academic; New York: 1977. [Google Scholar]
- 17.Johnson MK, Kim JK, Risse G. Do alcoholic Korsakoff’s syndrome patients acquire affective reactions? J Exp Psychol Learn Mem Cogn. 1985;11:27–36. doi: 10.1037//0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- 18.Kapur S, Craik FIM, Jones C, Brown GM, Houlse S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 19.Kunst-Wilson MR, Zajonc B. Affective discrimination of stimuli that cannot be recognized. Science. 1980;207:557–558. doi: 10.1126/science.7352271. [DOI] [PubMed] [Google Scholar]
- 20.Lane RD, Fink GR, Chua P, Dolan RJ. Neural activation during selective attention to subjective emotional responses. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy G. Functional neuroimaging of memory. Neuroscientist. 1995;1:155–163. [Google Scholar]
- 22.Nyberg L, Tulving E, Habib R, Nilsson L-G, Kapur S, Houle S. Functional brain maps of retrieval mode and recovery of episodic information. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 23.Petrides M. Motor conditional associative learning after selective prefrontal lesions in the monkey. Behav Brain Res. 1982;5:407–413. doi: 10.1016/0166-4328(82)90044-4. [DOI] [PubMed] [Google Scholar]
- 24.Petrides M. Non-spatial conditional learning impaired in patients with unilateral frontal but not unilateral temporal lobe excisions. Neuropsychologia. 1990;28:137–149. doi: 10.1016/0028-3932(90)90096-7. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raichle ME, Fiez JA, Videen TO, Macleod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during non-motor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 28.Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Brain regions supporting intentional and incidental memory: a PET study. NeuroReport. 1997;8:1283–1287. doi: 10.1097/00001756-199703240-00045. [DOI] [PubMed] [Google Scholar]
- 29.Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shallice T, Fletcher PC, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 31.Shevrin H, Smith WH, Fritzler DE. Average evoked response and verbal correlates of unconscious mental processes. Psychophysiology. 1971;8:149–162. doi: 10.1111/j.1469-8986.1971.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 32.Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern CE, Corkin S, Gonzales RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen VR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 35.Tulving E, Kapur S, Craik GFIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge: a functional MRI study of left inferior prefrontal cortex. J Cognit Neurosci. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- 38.Zajonc RB. Attitudinal effects of mere exposure. J Pers Soc Psychol. 1968;9:1–28. [Google Scholar]
- 39.Zajonc RB. Feeling and thinking: preferences need no inferences. Am Psychol. 1980;35:151–175. [Google Scholar]