Abstract
Vision in all vertebrates is dependent on an exchange of retinoids between the retinal pigment epithelium and the visual photoreceptors. It has been proposed that the interphotoreceptor retinoid-binding protein (IRBP) is essential for this intercellular exchange, and that it serves to prevent the potentially cytotoxic effects of retinoids. Although its precise function in vivo has yet to be defined, the early expression of IRBP suggests that it may also be required for normal photoreceptor development. To further assess the biological role of IRBP, we generated transgenic mice with targeted disruption of the IRBP gene (IRBP−/− mice). Specifically, homologous recombination was used to replace the first exon and promoter region of the IRBP gene with a phosphoglycerate kinase-promoted neomycin-resistant gene. Immunocytochemical and Western blot analyses demonstrated the absence of IRBP expression in theIRBP−/− mice. As early as postnatal day 11, histological examination of the retinas of IRBP−/− mice revealed a loss of photoreceptor nuclei and changes in the structural integrity of the receptor outer segments. At 30 d of age, the photoreceptor abnormalities in IRBP−/− mice were more severe, and electroretinographic recordings revealed a marked loss in photic sensitivity. In contrast, no morphological or electrophysiological changes were detected in age-matched heterozygotes. These observations indicate that normal photoreceptor development and function are highly dependent on the early expression of IRBP, and that in the absence of IRBP there is a slowly progressive degeneration of retinal photoreceptors.
Keywords: homologous recombination, interphotoreceptor retinoid-binding protein (IRBP), photoreceptor degeneration, retinal development, vitamin A deficiency, Electroretinography (ERG)
Vitamin A and its derivatives (retinoids) play an essential role in the development and maintenance of various tissues throughout the body. Because retinoids are typically insoluble in aqueous media and are in many cases cytotoxic, they are carried in the blood stream as well as intracellularly by a family of retinoid-binding proteins. One of the more unique members of this family is the interphotoreceptor retinoid-binding protein (IRBP), a large glycoprotein (Mr ∼140 kDa) synthesized by the visual cells and extruded into the interphotoreceptor matrix (IPM) of the vertebrate retina. IRBP is confined to the IPM by the permeability barriers formed at the retinal pigment epithelium (RPE) (Cohen, 1965) and the outer limiting membrane (Bunt-Milam et al., 1985). This localization, and the observation that the isomeric form of retinoid bound to IRBP varies with light and dark adaptation (Liou et al., 1982;Adler et al., 1985; Saari et al., 1985), led to the suggestion that IRBP plays a major role in the visual cycle, i.e., in the transport of retinoids between the photoreceptor outer segments and the RPE during the bleaching and regeneration of visual pigments (Lai et al., 1982;Liou et al., 1982; Adler and Spencer, 1991; Pepperberg et al., 1993).
Aside from its putative role in retinoid transport, it has been suggested that IRBP may be essential for normal retinal development. Evidence that IRBP gene expression occurs early in photoreceptor differentiation (Carter-Dawson et al., 1986; Gonzalez-Fernandez and Healy, 1990; Hauswirth et al., 1992; Liou et al., 1994) and that IRBP binds fatty acids required for the structural integrity of photoreceptor outer segments (Rodriguez et al., 1991; Chen et al., 1993) lends credence to this hypothesis. Also consistent with this notion is the observation that a reduction in IRBP may precede the loss of photoreceptors seen in some animal models of hereditary retinal degenerations (van Veen et al., 1988; Narfström et al., 1989;Wiggert et al., 1994). In the abyssinian cat, for example, there is a reduction in both IRBP message and protein before the onset of photoreceptor cell death (Narfström et al., 1989; Wiggert et al., 1994).
To assess further the role of IRBP in the development and maintenance of retinal structure, we used homologous recombination (gene targeting) to effectively disrupt the IRBP gene of transgenic mice. In the present study, we report (1) the procedures for achieving the “knock-out” of IRBP; (2) RT-PCR, Northern blot, Western blot, and immunocytochemical evidence of the absence of IRBP expression in the retinas of animals homozygous for the targeted allele; and (3) histological and electrophysiological findings that reveal the concomitant changes in retinal morphology and visual function.
MATERIALS AND METHODS
Construction of the targeting vector. The targeting vector of 11.6 kb was composed of two IRBP genomic fragments isolated from a genomic library of mouse strain 129 (no substrain name was designated; kindly provided by Dr. Hui Zheng, Merck Sharp & Dohme Research Laboratories; originally from Dr. Alan Bradley, Baylor College of Medicine), the bacterial neomycin resistant gene driven by a phosphoglycerate kinase promoter (pgk-neo;Colbere-Garapin et al., 1981; Adra et al., 1987), and the herpes simplex virus thymidine kinase (hsv-tk) gene (Thomas and Capecchi, 1987; Mansour et al., 1988). The 1.6 kb pgk-neogene was inversely placed downstream of an IRBP fragment spanning from −7200 to −1245, and upstream of another IRBP fragment spanning from +3152 to +5049. The 1.9 kb fragment of the hsv-tk gene was added to the upstream end of the vector. In the targeted allele, the promoter region (from −1246 to +106), 81% of the translated sequence of the IRBP gene (from +107 to +3151), and the hsv-tk gene were deleted.
Generation of IRBP−/− mice. Embryonic stem (ES) cells of strain 129/Ola (Komang et al., 1995; kindly provided by Drs. Peter Mombaerts and Richard Axel, Columbia University) were cocultured with STO-NEO and LIF (SNL) feeder cells (kindly provided by Dr. Hui Zheng) and were transfected and cultured under the conditions of positive selection for neomycin (G418) resistance (i.e., for the presence of the expressed neomycin gene) and negative selection for ganciclovir resistance (i.e., for the absence of hsv-tk gene expression). Appropriate targeting was confirmed by Southern analysis of genomic DNA using probes flanking the 5′ and 3′ ends of the targeting vector. The selected ES colonies were injected into the blastocysts of strain C57BL/6J mice to generate chimeric founder mice. Chimeric mice were bred with C57BL/6J mice to produce germ line-transmitted IRBP+/− mutants, which were identified by Southern analysis. Mice that were heterozygous and homozygous for the IRBP gene disruption were generated through inbreeding of germ line-transmitted mutants; wild-type littermates were used as controls. All animal procedures conformed to the Association for Research in Vision and Ophthalmology and National Institutes of Health statements on the use of animals in ophthalmic and vision research.
PCR (Saiki, 1990) was used to identify the genotypes resulting from this breeding protocol. The targeted (pgk-neo) allele was identified by amplification of the 530 bp fragment between −1273 of the IRBP gene and +592 of the pgk-neo gene. The wild-type IRBP allele was identified by amplification of the 403 bp fragment between +211 and +613 of the IRBP gene. IRBP gene expression was analyzed by RT-PCR (Kawasaki, 1990). The cDNA of the IRBP gene was identified by amplification of the same 403 bp fragment described above. Expression of the mouse β-actin gene was used as an internal control.
Northern blot analysis. To isolate total RNA, individual retinas were homogenized in treated sand (Liou and Matragoon, 1992). RNA was isolated using the selective binding properties of silica gel-based membrane with the microspin technique RNeasy kit (Qiagen, Chatsworth, CA). Equal amounts (10 μg) of total RNA, determined by absorbance at 260 nm, were electrophoresed in 1% agarose gel in formaldehyde. RNA was blotted to Zeta-Probe GT blotting membrane (Bio-Rad, Richmond, CA) by capillary transfer and hybridized with probes for the mouse IRBP gene (a cDNA fragment extending from +3075 to +3796), the pgk-neo gene, or the mouse β-actin gene. Probes were labeled with [α-32P]dCTP with a Klenow fragment of DNA polymerase I using random oligomers (Pharmacia, Piscataway, NJ). Blotting, hybridization, and washing were all performed according to the manufacturer’s instructions.
RNase protection analysis (RPA). The protection assay has been described previously (Liou et al., 1994). Briefly, sense and antisense RNA probes for the IRBP gene were synthesized using T3 or T7 polymerase on a cDNA template for the IRBP gene spanning from +3075 to +3796 cloned in pBluescript. The coding strand probe will protect a 259 bp fragment of the noncoding strand IRBP transcript from +3333 to +3075. The noncoding strand probe will protect a 214 bp fragment of the coding strand IRBP transcript from +3583 to +3796. Linearized templates were labeled with [α-32P]UTP and purified as described previously (Liou et al., 1994). One microgram of total RNA was hybridized with each probe present in excess (500–800 pg or 4.0 × 105 cpm). Single-stranded RNA was removed with RNase, purified, and subjected to electrophoresis on a denaturing 5% polyacrylamide and urea gel. Gels were dried and exposed to x-ray film with an intensifying screen.
Immunocytochemistry. Localization of IRBP was visualized by immunocytochemical labeling with a monoclonal antibody (mAbH3-B5, kindly provided by Dr. Larry Donoso; Donoso et al., 1990) and two polyclonal antibodies: a rabbit anti-monkey (Redmond et al., 1985) and a goat anti-bovine (van Veen et al., 1988), both kindly provided by Dr. Barbara Wiggert. Eyes were fixed in 4% paraformaldehyde in 0.1m sodium phosphate buffer, pH 7.4, and then rinsed twice (30 min each) in cold buffer. After cryoprotection by overnight immersion in cold 30% sucrose, the tissue was mounted with Tissue-Tek OCT in a cryostat (−20°C), sectioned at 8 μm, and picked up on gelatin-coated slides. The sections were washed in PBS at room temperature, and nonspecific binding sites were saturated by immersion in antiserum diluent (0.3% Triton X-100 and 0.02% sodium azide in PBS) containing either 2.5% normal (nonimmune) serum or 3% nonfat dry milk. The primary antibodies were applied to the sections in various concentrations and allowed to incubate overnight at 4°C. The sections were then exposed to an FITC-labeled secondary antibody for 1 hr at room temperature, washed twice (10 min each) in PBS, coverslipped with Vectashield, and photographed under incident light fluorescence. Controls were prepared in the same manner, except that the primary antibody was eliminated from the sequence; no fluorescent signal was detected in control preparations, and they will not be considered further.
Western immunoblot analysis. After removing the lens, individual eyes were homogenized by an Omni International (Waterbury, CT) 2000 Polytron homogenizer in 500 μl of a buffer containing (in mm): 10 HEPES, 1 EDTA, 500 NaCl, and 1 phenylmethylsulonylfluoride, pH 7.5, and centrifuged at 14,000 rpm in a microcentrifuge at 4°C for 30 min. Ten micrograms of Protein, determined by Bio-Rad Protein Assay (Bio-Rad, Richmond, CA) from the supernatant fraction were analyzed by SDS-PAGE on 10% acrylamide gels and electrophoretically transferred to a Trans-Blot transfer medium polyvinylidene difluoride membrane (Bio-Rad). The antibodies used included a monoclonal antibody for mouse β-actin (Chemicon, Temecula, CA) (Herman and Pollard, 1979) and two of the antibodies that were used for immunocytochemistry, namely, the mouse mAb H3B5 directed against a seven-amino acid sequence of the native molecule (Donoso et al., 1990) and the polyclonal rabbit anti-monkey IRBP (Redmond et al., 1985). Antibody–antigen reactions and their detection by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL) were performed according to the manufacturer’s instructions. A recombinantXenopus IRBP fusion protein of 37 kDa consisting of all but the first eight amino acids of the corresponding fourth repeat of human IRBP was used as a positive control in some of the Western analyses (Baer et al., 1994; kindly provided by Dr. Federico Gonzalez-Fernandez, University of Virginia).
Histology and electroretinography. Structural studies were conducted on mice at postnatal day 11 (P11), the age at which the mice first opened their eyelids, and on 1-month-old animals. Enucleated eyes were opened at the ora serrata and placed in 0.1 mphosphate buffer, pH 7.4, containing 2% formaldehyde and 2.5% glutaraldehyde, at 4°C. After overnight fixation, the anterior segments were removed, and after three rinses in phosphate buffer, the eyecups were post-fixed in 1% OsO4 in buffer for 90 min. After dehydration through a graded ethanol series, the tissue was infiltrated ultimately with a 1:1 mixture of Epon/Araldite, and 1 μm sections were stained with azure II–methylene blue. Sections were cut through the optic nerve head approximately along the horizontal meridian of the eye, and micrographs were taken at ∼300 μm from the edge of the optic disk.
To determine whether the photoreceptors of IRBP−/− mice were capable of generating an electrical signal, we obtained recordings of the electroretinogram (ERG) from several 1-month-old mice of each genotype; recordings from P11 mice were difficult to obtain, owing to the size of the eye and partial lid closure. After an overnight period of dark adaptation, the animals were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg), and the ERG was recorded in response to a high-intensity stimulus (0.3 log cd sec/m2). The procedure has been described in detail in an earlier paper (Goto et al., 1995).
RESULTS
Generation of IRBP−/− mice
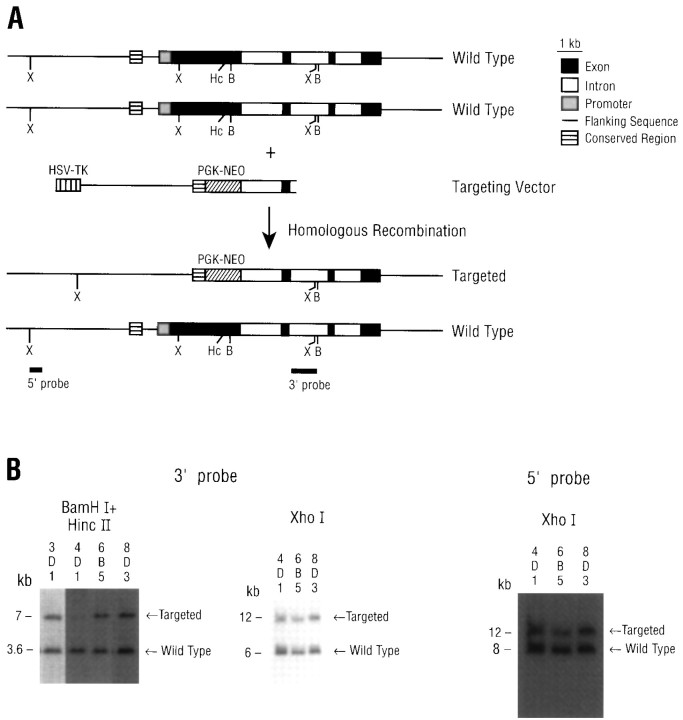
IRBP−/− mice were generated by gene targeting in ES cells. Figure 1Aillustrates the two wild-type IRBP alleles (top), the targeting vector (middle), and the result of homologous recombination of the targeting vector with one of the wild-type alleles (bottom). From 200 independent ES colonies that were G418- and ganciclovir-resistant, a total of four colonies yielded the predicted Southern hybridization pattern (Fig. 1B). The variation in intensity of the bands identifying the wild-type and the targeted alleles reflects differences in the amount of feeder cell DNA. Of the four positive ES colonies, three were used for blastocyst injection and generated chimeras that were 60–100% Agouti in coat color.
Fig. 1.
Targeted disruption of the IRBP gene in ES cells.A, Restriction maps of the mouse IRBP genomic locus, the targeting vector, and the predicted structure of the targeted IRBP gene. The locations of the 5′ and 3′ flanking probes used for Southern analysis are indicated. B, BamHI;Hc, HincII; X,XhoI. B, Southern analysis of clones positive for the targeting vector.
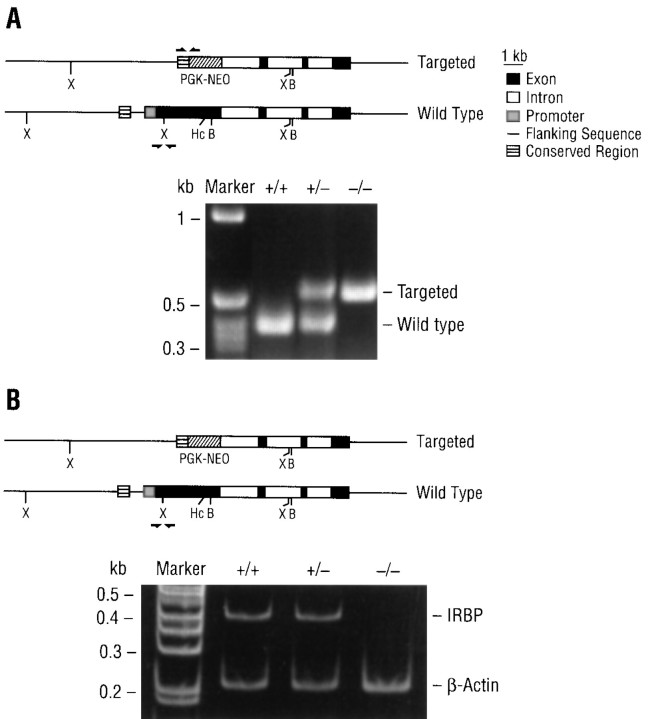
Figure 2A shows the PCR identification of the genotypes of animals derived from inbreeding heterozygous mice. The PCR-amplified fragments are of the expected sizes and clearly distinguish the three genotypes. Expression of the IRBP gene was determined by RT-PCR; as shown in Figure2B, the 403 bp marker for the wild-type IRBP mRNA was seen in wild-type mice (IRBP+/+), heterozygotes (IRBP+/−) but not in homozygotes (IRBP−/−) in which both IRBP alleles were disrupted.
Fig. 2.
Identification of the genotype and IRBP gene expression. A, PCR identification of the genotypes ofIRBP+/+, IRBP+/−, andIRBP−/− mice; amplified sequences are indicated byarrows. The 530 bp marker for the targeted (pgk-neo) allele represents a region between −1273 of the IRBP gene and +592 of the pgk-neo gene. For the wild-type allele, the 403 bp marker represents a region between +211 and +613 of the IRBP gene. B, RT-PCR analysis of wild-type IRBP gene expression. Mouse β-actin was used as an internal control.
Northern blot analysis
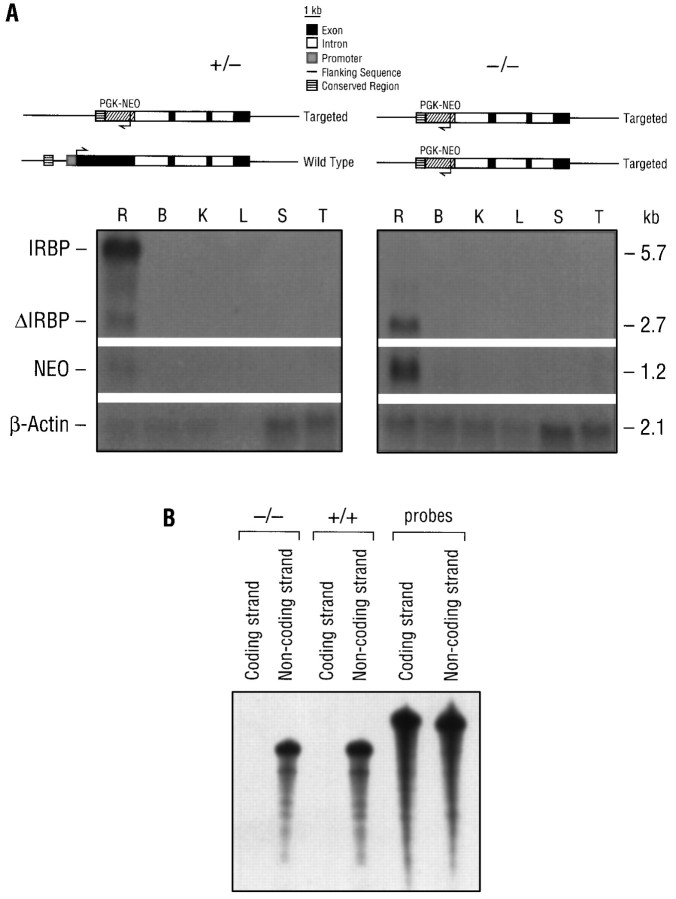
In the targeted IRBP allele, the IRBP gene segment from −1246 to +3151 was deleted (Fig. 1). The remaining segment includes an upstream conserved region between −1526 and −1245 (Liou et al., 1991), 24 bp of exon 1, all of the introns, and exons 2–4. Expression of the IRBP gene, the inserted pgk-neo gene, and the remaining segment of the IRBP gene in IRBP+/− and IRBP−/− mice was determined by Northern blot analysis. As shown in Figure3A, a probe spanning from +3075 to +3796 of the IRBP gene detected a retina-specific transcript of 5.7 kb in IRBP+/− mice but not in IRBP−/− mice. The same probe also detected a retina-specific transcript of 2.7 kb in both IRBP+/− and IRBP−/− mice. A probe for pgk-neo detected a retina-specific transcript of 1.2 kb in both IRBP+/− and IRBP−/− mice. This result indicates that although the upstream region of the IRBP gene including its promoter was selectively deleted, the inserted pgk-neogene and the remaining exons of the IRBP gene continue to be transcribed in the retina.
Fig. 3.
Transcription analysis of the targeted IRBP gene.A, Northern blot analysis of the total RNA isolated fromIRBP+/− and IRBP−/− mice. Ten micrograms of total RNA were run on a formaldehyde–agarose gel and transferred to a Zeta Probe membrane. The top paneldepicts the structure of the wild-type and targeted IRBP alleles in each genotype. The blot was hybridized with the following probes in sequence: mouse IRBP cDNA, the pgk-neofragment, and a mouse β-actin gene. R, Retina;B, brain; K, kidney; L, liver; S, spleen; T, testis; ΔIRBP, the remaining segment of the IRBP gene.B, RPA of total retinal RNA from IRBP+/+ and IRBP−/− mice. One microgram of total RNA was hybridized with 32P-labeled RNA probes from the coding (sense) or noncoding (antisense) strand of the mouse IRBP cDNA.
RNase protection analysis (RPA)
In the absence of the upstream promoter region and exon 1, the retina-specific RNA transcript of 2.7 kb from the remaining IRBP gene could be from either the upstream or downstream regions of the gene. This cannot be determined by the DNA probe used in the Northern analysis. Accordingly, we determined the orientation of this transcript with RPA using sense and antisense RNA probes. Total RNA (1 μg) from the retinas of IRBP−/− mice was hybridized with labeled probes, digested with RNase, and subjected to gel electrophoresis and autoradiography. As shown in Figure 3B, only the antisense RNA probe protected a band of bases from the total retinal RNA. This result indicates that the 2.7 kb RNA that was transcribed from the remaining IRBP gene was in the same orientation as the endogenous gene.
Immunocytochemistry
The lack of IRBP gene expression in IRBP−/− mice was demonstrated by immunocytochemistry. Figure4 shows IRBP immunoreactivity in sections taken from 1-month-old IRBP+/+, IRBP+/−, andIRBP−/− mice using a rabbit anti-monkey polyclonal antibody (Redmond et al., 1985). The corresponding Nomarski images demonstrate that the immunofluorescence was confined exclusively to the IPM in IRBP+/+ and IRBP+/− animals; no fluorescent signal was detected in IRBP−/− mice. Similar results (data not shown) were obtained with mAb H3B5 (Donoso et al., 1990) and a goat anti-bovine polyclonal antibody (van Veen et al., 1988). In addition, it is obvious that there is a thinning of the outer nuclear layer and outer segment abnormalities in theIRBP−/− retina, which are more apparent in the histological sections presented in Figure 6.
Fig. 4.
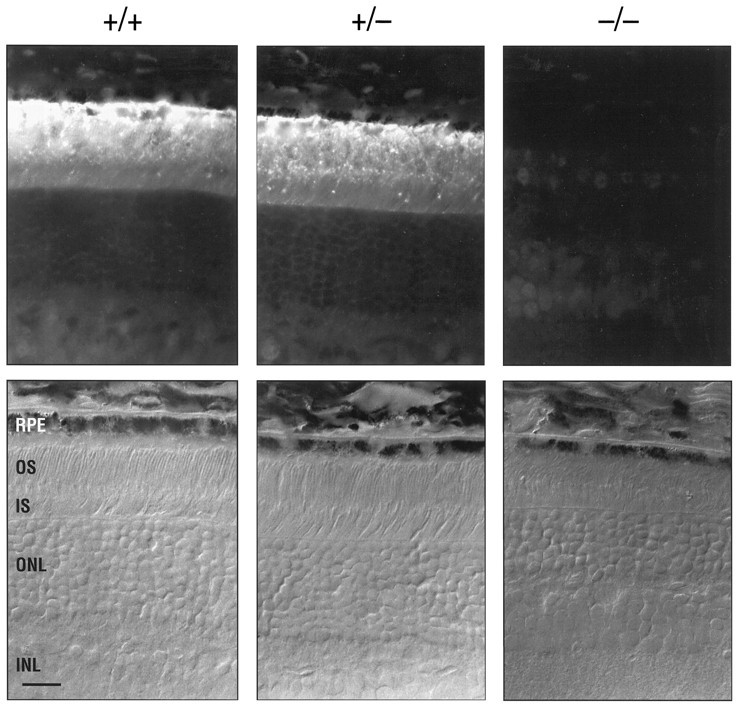
IRBP immunocytochemistry. Retinal sections of 1-month-old IRBP+/+, IRBP+/−, andIRBP−/− mice. The antigen is visualized with a 1:200 dilution of the primary (rabbit anti-monkey) polyclonal antibody (Redmond et al., 1985) and FITC-labeled goat anti-rabbit IgG. The corresponding Nomarski interference contrast images of the sections are shown in the bottom panels. Scale bar, 20 μm.
Fig. 6.
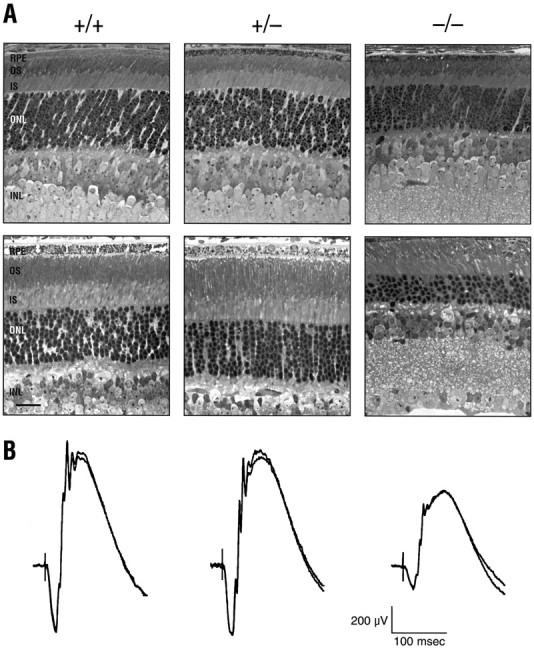
Retinal histology and electroretinography.A, Light micrographs of the midperipheral retina fromIRBP+/+, IRBP+/−, andIRBP−/− mice; the sections are from P11 (top row) and P30 (bottom row) animals. Scale bar, 20 μm. B, Electroretinographic recordings from 1-month-old littermates. The ERG traces were in response to a high-intensity (0.3 log cd sec/m2) flash stimulus delivered to the dark-adapted retina; two successive traces obtained for each mouse are superimposed.
Western blot analysis
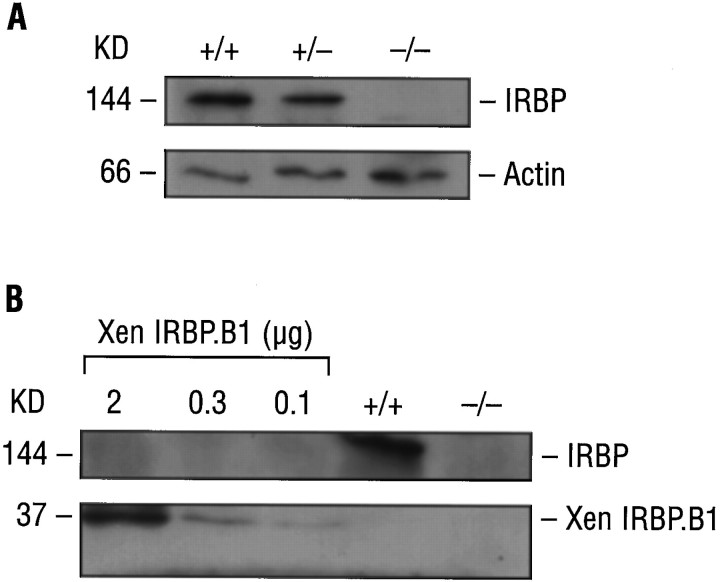
The absence of IRBP in homozygous animals was confirmed by Western blot analysis using monoclonal and polyclonal antibodies (Fig.5). Figure 5A shows that a 144 kDa protein band was detected in IRBP+/+ andIRBP+/− mice but not in IRBP−/− animals. Moreover, after normalization with mouse β-actin, the amount of IRBP expressed in heterozygous mice was approximately one-half of that in normal littermates.
Fig. 5.
Translation analysis of the targeted IRBP gene.A, Western blot analysis of IRBP inIRBP+/+, IRBP+/−, andIRBP−/− mice. Ten micrograms of protein from the supernatant fraction were analyzed. IRBP was identified with a monoclonal antibody for IRBP (mAbH3B5, diluted 1:500; Donoso et al., 1990). Internal standard, mouse β-actin. Antibody–antigen reactions and their detection are according to ECL (Amersham). B, Western blot analysis of IRBP and a recombinant XenopusIRBP fusion protein of the fourth repeat (Baer et al., 1994). Antigen detection is with a polyclonal rabbit anti-monkey antibody for IRBP (diluted 1:100; Redmond et al., 1985).
In the targeted allele, 24 bp of exon 1 and exons 2–4 were spared. If these exons were expressed and translated in-frame, the predicted protein would consist of 230 amino acids, from position 1000 (Met) to position 1231 (stop codon), and would have a molecular weight of ∼23 kDa. Moreover, this protein would include ∼75% of the fourth repeat of IRBP, including all three conserved hydrophobic domains (Rajendran et al., 1996). To test this possibility, we used a polyclonal antibody (Redmond et al., 1985) that detects a recombinant XenopusIRBP fusion protein consisting of 298 amino acids, which correspond to the fourth repeat of human IRBP, Xen IRBP.B1 (Baer et al., 1994). As shown in Figure 5B, the polyclonal antibody detected IRBP of the appropriate molecular weight (144 kDa) in IRBP+/+ but not in IRBP−/− mice. In addition, the antibody detected the 37 kDa recombinant Xenopus fusion protein at levels as low as 0.1 μg, whereas no signal was seen from IRBP+/+ mice (as expected) or from IRBP−/− animals. These results indicate that there was no protein that corresponded to the residual portion of the IRBP gene in IRBP−/− animals.
Histology and Electroretinography
Figure 6A shows light micrographs of retinal sections from 11- and 30-d-oldIRBP+/+, IRBP+/−, and IRBP−/− mice. No significant differences were seen between wild-type andIRBP+/− mice in the two age groups. However,IRBP−/− mice in both age groups exhibited photoreceptor abnormalities, but the changes were more severe in the older animals. In the P11 mice, there was clear evidence of the loss of photoreceptor cells and a thinning of the outer nuclear layer, i.e., a reduction in thickness from ∼10–11 cell nuclei (in IRBP+/+ mice) to ∼6–8 cells deep in the IRBP−/− mice. In addition, the photoreceptor outer segments were poorly oriented and significantly shorter than normal (∼60% of the normal length). By contrast, there were no detectable changes in the pigment epithelial cells or in the more proximal layers of the IRBP−/− retina; the cell content of the inner nuclear and ganglion cell layers appeared normal, and the thickness of the inner synaptic (plexiform) layer was equivalent to that of wild-type animals. However, the loss of photoreceptor cell perikarya and the shortened outer segments of the remaining cells resulted in a significant thinning of the neural retina.
At 1 month of age, retinal sections from IRBP−/− mice revealed a greater deterioration of retinal structure. The outer nuclear layer showed a further reduction in thickness; the outer segments were shorter and even more disoriented than at the P11 stage; and there were signs that many of the densely staining membraneous disks were lost or disrupted. The retinas of 1-month-oldIRBP+/− mice, on the other hand, still presented a normal appearance.
Despite these structural abnormalities, the IRBP−/− retina retained the ability to generate a light-evoked electrical signal. As shown in Figure 6B, the ERG response of the 1-month-old IRBP−/− retina was significantly reduced in amplitude compared with the recordings from either IRBP+/+ or IRBP+/− littermates. All components of the ERG waveform were diminished, but it is particularly noteworthy that the ERG a-wave, an index of photoreceptor activity (Penn and Hagins, 1969; Pugh and Lamb, 1993), was retained in the 1-month-old IRBP−/− mouse. Although the a-wave amplitude was less than half of that recorded from wild-type and IRBP+/− mice, it is evident that even at this age the IRBP−/− receptor outer segments contain the light-sensitive pigments required to initiate the electrical response.
DISCUSSION
The study of transgenic mice with targeted disruption of the IRBP gene has made it possible to demonstrate the fundamental importance of IRBP in preserving the structural and functional integrity of the retina. The characterization of this in vivo model system is still in its early stages, but the present results provide a clear indication that IRBP−/− mice exhibit a significant loss of photoreceptors, as well as outer segment abnormalities, even before their eyes have opened. Although it is highly unlikely that the damage is related directly to photic exposure, the relatively transparent eyelid of a newborn mouse does not preclude the possibility that the photoreceptor abnormalities were exacerbated by light. Dark-rearing experiments are currently being conducted to examine this possibility.
In most other respects, the absence of IRBP appears to have had no deleterious effects, and we could not detect any signs indicating that the protein is essential for normal prenatal development.IRBP−/− mice appeared to be healthy, and there were no obvious cellular abnormalities in the postreceptoral layers of the neural retina. Interestingly, the results obtained fromIRBP+/− mice suggest that IRBP may be produced in overabundance in the normal retina. In the heterozygous animals, in which only ∼50% of the full complement of IRBP is present, the photoreceptors have developed normally, and the electrical responses of 1-month-old mice are comparable to that of the normal. However, we have yet to determine whether the decreased amounts of IRBP compromise other aspects of photoreceptor function, e.g., the bleaching and regeneration cycle of rhodopsin, or lead to photoreceptor damage at later ages.
Although the IRBP promoter was deleted by the transgene, the insertedpgk-neo gene and the remaining exons of the IRBP gene were transcribed in the retina. This transcription may have been initiated by the bidirectional activity of the pgk-neo promoter (Johnson and Friedman, 1990; Abeliovich et al., 1992). The explanation for the retina-specific nature of this transcription is not known but may be related to the retina-specific hypomethylation of the mouse IRBP allele (Liou et al., 1994). It may also reflect a retina-specific regulatory element within the remaining upstream conserved region of the targeted IRBP gene (i.e., between −1526 and −1245; Liou et al., 1991). Nevertheless, immunocytochemistry and Western blot analysis indicated that these transcripts were not expressed. This failure is likely to reflect the lack of a translation initiation codon at the beginning of the last IRBP repeat. In eukaryotic cells, the efficient use of AUG as a translation initiation codon is critically dependent on the purines at positions −3 and +4 (the A in the AUG is designated +1;Kozak, 1987; McBratney and Sarnow, 1996). The methionine encoded by the sequence CCT ATGC (+3167 to +3173) at the beginning of the last IRBP repeat does not have purine at either −3 or +4.
Rhodopsin constitutes ∼95% of the protein content of the rod outer segment membranes (Smith et al., 1975; Krebs and Kühn, 1977). The light-sensitive form requires the 11-cis isomer of vitamin A aldehyde (11-cis retinal) to be linked to the apoprotein opsin. Because 11-cis retinal is not synthesized within the rod photoreceptor, it must be transferred from its source in the RPE (Bernstein et al., 1987) through the IPM to the outer segment membranes. There is mounting evidence that IRBP is essential for the removal of 11-cis retinal from the RPE (Flannery et al., 1990; Okajima et al., 1990; Carlson and Bok, 1992) and for its delivery to the receptor outer segments (Okajima et al., 1990; Duffy et al., 1993). Thus, in the absence of IRBP, the photoreceptors would suffer essentially a local vitamin A deficiency.
Vitamin A deficiency is a well known clinical entity, and as Dowling and Wald (1958, 1960) have shown in their classical studies of vitamin A-deprived rats, animals reared on a vitamin A-deficient diet exhibit a profound loss of visual sensitivity, widespread destruction of all retinal cell layers, severe weight loss, and a broad range of defects in other parts of the body. The pathology they describe is very different from that encountered in the IRBP−/− mouse; mice lacking IRBP appeared to be healthy in all respects, and the only abnormalities that we could detect were confined to the photoreceptor layer.
However, it is important to recognize the fundamental difference in the type of vitamin A deficiency that is seen under these different experimental conditions. Vitamin A deficiency induced by dietary means deprives all tissues of this essential substance, and despite the enormous stores of retinol housed in the liver, prolonged deprivation eventually leads to the extensive tissue damage reported by Dowling and Wald (1958, 1960). On the other hand, the genetically induced depletion of a retina-specific, extracellular binding protein such as IRBP has no effect on the circulating levels of vitamin A within the blood stream. Thus, the vitamin A requirements of the RPE, the cells of the inner retina, and virtually all tissues throughout the body are met. The fact that IRBP is confined to the extracellular matrix of the subretinal space and serves primarily to shuttle retinoids between the photoreceptors and the RPE makes the photoreceptors the only cells susceptible to damage in the IRBP−/− mouse.
Despite the absence of IRBP, evidence from 1-month-oldIRBP−/− mice indicates that light-evoked electrical potentials can be elicited from the dark-adapted retina. Although the histological abnormalities in the photoreceptor layers are more extensive than in P11 IRBP−/− mice, and the electroretinographic responses are greatly reduced in amplitude, it is evident that photopigments are present in visual cells of theIRBP−/− mice. As already indicated, the visual cells require a source of 11-cis retinal to synthesize their light-sensitive pigments, and it seems likely that some degree of retinoid exchange between the RPE and photoreceptors has occurred in the absence of IRBP. Whether this takes place entirely via aqueous transfer through the IPM (Rando and Bangerter, 1982; Fex and Johannesson, 1987; Ho et al., 1989) or whether another retinoid-binding protein not normally expressed in the IPM is derived from other cellular compartments that border the IPM (e.g., RPE and Müller cells) has yet to be determined. Nevertheless, the early onset of photoreceptor deterioration and the progressive loss of visual cells are strong indications that whatever system is used, it is inadequate to the task.
It is difficult to identify with certainty the cause of the degenerative changes in the retinas of IRBP−/− mice. The observations that IRBP gene expression occurs well before the appearance of opsin (Hauswirth et al., 1992; Liou et al., 1994) and that IRBP binds fatty acids that are an essential component of the photoreceptor outer segments (Rodriguez et al., 1991; Chen et al., 1993) indicate that IRBP may be a sine qua non for normal photoreceptor development. On this view, IRBP is essential to support the normal synthesis of outer segment membranes, and its absence would lead to outer segment malformation and ultimately to the death of visual cells.
As mentioned earlier, it has been postulated that aberrant expression of IRBP may be implicated in some genetically mediated retinal degenerations seen in cat (Narfström et al., 1989; Wiggert et al., 1994) and in mouse (van Veen et al., 1988). Abyssinian cats homozygous for a slowly progressive form of hereditary rod and cone degeneration show a 50% reduction in the levels of IRBP mRNA and protein as early as 4 weeks of age, well before the onset of significant changes in retinal structure or the appearance of ERG abnormalities. The present findings are consistent with these observations. The 1-month-old IRBP+/− mice we have studied show a similar reduction in IRBP levels and do not exhibit early signs of photoreceptor abnormalities; as already indicated, we have yet to determine whether pathological changes develop in older animals. Alternatively, the reduced level of IRBP may be only one of several factors contributing to the degenerative changes encountered in the abyssinian cat (Applebury, 1992; Naash et al., 1996).
In double homozygous (rd/rd,rds/rds) mutant mice, on the other hand, the loss of IRBP from the extracellular photoreceptor matrix is thought to result from an abnormality in the secretory mechanism responsible for extruding IRBP; i.e., the available IRBP is retained within the photoreceptor cell soma (van Veen et al., 1988). Whether the intracellular accumulation of IRBP is cytotoxic is not known, but the profound structural changes seen in these animals at very early ages are far greater than we have observed in 1-month-oldIRBP−/− mice. Once again, the aberrant localization of IRBP and its absence from the IPM are probably not the sole factors contributing to the degenerative process. However, in both of these animal models, the reduced levels of IRBP and the resultant membranolytic effects of unbound retinoids may have contributed to the course of the degeneration (Meeks et al., 1981). In any event, these observations suggest that IRBP should be considered a potential candidate gene for some forms of inherited retinal degeneration (Dryja, 1997).
Footnotes
This work was supported by National Institutes of Health Grants EY03829 (G.I.L.) and EY06516 (H.R.), the Department of Veterans Affairs, and unrestricted awards from Research to Prevent Blindness, Inc. to the Departments of Ophthalmology at the Medical College of Georgia and the University of Illinois College of Medicine. We are grateful to Jane Zakevicius for histology and immunocytochemistry, Cecilia Artacho for providing animal care, and Brenda Sheppard for secretarial assistance. We thank Drs. Hui Zheng and Howard Chen for helpful suggestions and gifts of the mouse genomic library, plasmid clones, and SNL feeder cells, Drs. Peter Mombaerts and Richard Axel for the ES cells, Dr. Larry Donoso for the monoclonal antibody, Dr. Barbara Wiggert for the polyclonal antibodies, and Dr. Federico Gonzalez-Fernandez for the recombinant Xenopus IRBP fusion protein. We also thank Dr. Keith Green for comments and suggestions on this manuscript.
Correspondence should be addressed to Dr. Gregory I. Liou, Department of Ophthalmology, Medical College of Georgia, Augusta, GA 30912.
REFERENCES
- 1.Abeliovich A, Gerber D, Tanaka O, Katsuki M, Graybiel AM, Tonegawa S. On somatic recombination in the CNS of transgenic mice. Science. 1992;257:404–408. doi: 10.1126/science.1631561. [DOI] [PubMed] [Google Scholar]
- 2.Adler AJ, Spencer SA. Effect of light on endogenous ligands carried by interphotoreceptor retinoid-binding protein. Exp Eye Res. 1991;53:337–346. doi: 10.1016/0014-4835(91)90239-b. [DOI] [PubMed] [Google Scholar]
- 3.Adler AJ, Evans CD, Stafford WF. Molecular properties of bovine interphotoreceptor retinoid-binding protein. J Biol Chem. 1985;260:4850–4855. [PubMed] [Google Scholar]
- 4.Adra CN, Boer PH, McBurney MW. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- 5.Applebury ML. Variations in retinal degeneration. Curr Biol. 1992;2:113–115. doi: 10.1016/0960-9822(92)90238-6. [DOI] [PubMed] [Google Scholar]
- 6.Baer CA, Kittredge KL, Klinger AL, Briercheck DM, Braiman M, Gonzalez-Fernandez F. Expression and characterization of the fourth repeat of Xenopus IRBP in E. coli. Curr Eye Res. 1994;13:391–400. doi: 10.3109/02713689408999866. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci USA. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunt-Milam AH, Saari JC, Klock IB, Garwin GG. Zonulae adherents pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985;26:1477–1380. [PubMed] [Google Scholar]
- 9.Carlson A, Bok D. Promotion of the release of 11-cis retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992;31:9056–9062. doi: 10.1021/bi00152a049. [DOI] [PubMed] [Google Scholar]
- 10.Carter-Dawson L, Alvarez RA, Fong S-L, Liou GI, Sperling HG, Bridges CDB. Rhodopsin, 11-cis vitamin A, and interstitial retinol-binding protein (IRBP) during retinal development in normal and rd mutant mice. Dev Biol. 1986;116:431–438. doi: 10.1016/0012-1606(86)90144-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Saari JC, Noy N. Interactions of all trans-retinol and long-chain fatty acids with IRBP. Biochemistry. 1993;32:11311–11318. doi: 10.1021/bi00093a007. [DOI] [PubMed] [Google Scholar]
- 12.Cohen AI. A possible cytological basis for the “R” membrane in the vertebrate eye. Nature. 1965;205:1222–1223. [Google Scholar]
- 13.Colbere-Garapin F, Horodniceanu F, Kourilsky P, Garapin A. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981;150:1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- 14.Donoso LA, Rodrigues M, Vrabec TR, Sery TW, Fong SL. IRBP: preparation and characterization of site-specific monoclonal antibodies. Curr Eye Res. 1990;9:357–362. doi: 10.3109/02713689008999623. [DOI] [PubMed] [Google Scholar]
- 15.Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci USA. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowling JE, Wald G. The biological function of vitamin A acid. Proc Natl Acad Sci USA. 1960;46:587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dryja TP. Gene-based approach to human gene-phenotype correlations. Proc Natl Acad Sci USA. 1997;94:12117–12121. doi: 10.1073/pnas.94.22.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy M, Sun Y, Wiggert B, Duncan T, Chader GJ, Ripps H. IRBP enhances rhodopsin regeneration in the experimentally detached retina. Exp Eye Res. 1993;57:771–782. doi: 10.1006/exer.1993.1185. [DOI] [PubMed] [Google Scholar]
- 19.Fex G, Johannesson G. Studies of the spontaneous transfer of retinol from the retinol:retinol-binding protein complex to unilamellar liposomes. Biochim Biophys Acta. 1987;901:255–264. doi: 10.1016/0005-2736(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 20.Flannery JG, O’Day W, Pfeffer BA, Horwitz J, Bok D. Uptake, processing and release of retinoids by cultured human retinal pigment epithelium. Exp Eye Res. 1990;51:717–728. doi: 10.1016/0014-4835(90)90057-2. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Fernandez F, Healy JI. Early expression of the gene for IRBP during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. J Cell Biol. 1990;111:2775–2784. doi: 10.1083/jcb.111.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goto Y, Peachey NS, Ripps H, Naash MI. Functional abnormalities in transgenic mice expressing a mutant rhodopsin gene. Invest Ophthalmol Vis Sci. 1995;36:62–71. [PubMed] [Google Scholar]
- 23.Hauswirth WW, Langerijt AVD, Timmers AM, Adamus G, Ulshafer RJ. Early expression and localization of rhodopsin and IRBP in the developing fetal bovine retina. Exp Eye Res. 1992;54:661–670. doi: 10.1016/0014-4835(92)90021-j. [DOI] [PubMed] [Google Scholar]
- 24.Herman IM, Pollard TD. Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol. 1979;80:509–520. doi: 10.1083/jcb.80.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho MTP, Massey JB, Pownall HJ, Anderson RE, Hollyfield JG. Mechanisms of vitamin A movement between rod outer segments, IRBP, and liposomes. J Biol Chem. 1989;264:928–935. [PubMed] [Google Scholar]
- 26.Johnson P, Friedmann T. Limited bidirectional activity of two housekeeping gene promoters: human HPRT and PGK. Gene. 1990;88:207–213. doi: 10.1016/0378-1119(90)90033-n. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki ES. Amplification of RNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic; San Diego: 1990. pp. 21–27. [Google Scholar]
- 28.Komang H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Mombaerts P, Tonegawa S, Yamamoto H, Itohara S, Nanno M, Ishikawa H. Homeostatic regulation of intestinal epithelia by intraepithelial T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak M. An analysis of 5′-noncoding sequences upstream from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs W, Kühn H. Structure of isolated bovine rod outer segment membranes. Exp Eye Res. 1977;25:511–526. doi: 10.1016/0014-4835(77)90180-4. [DOI] [PubMed] [Google Scholar]
- 31.Lai YL, Wiggert B, Liu YP, Chader GJ. Interphotoreceptor retinoid-binding proteins: possible transport vehicles between compartments of the retina. Nature. 1982;298:848–849. doi: 10.1038/298848a0. [DOI] [PubMed] [Google Scholar]
- 32.Liou GI, Matragoon S. A simple method to homogenize multiple tissue samples in small sizes without cross contamination. Biotechniques. 1992;13:719. [PubMed] [Google Scholar]
- 33.Liou GI, Bridges CDB, Fong S-L, Alvarez RA, Gonzalez-Fernandez F. Vitamin A transport between retina and pigment epithelium-an interstitial protein carrying endogenous retinol (IRBP). Vision Res. 1982;22:1457–1467. doi: 10.1016/0042-6989(82)90210-3. [DOI] [PubMed] [Google Scholar]
- 34.Liou GI, Matragoon S, Yang J, Geng L, Overbeek PA, Ma D-P. Retina-specific expression from the IRBP promoter in transgenic mice is conferred by 212 bp of the 5′-flanking region. Biochem Biophys Res Commun. 1991;181:159–165. doi: 10.1016/s0006-291x(05)81395-6. [DOI] [PubMed] [Google Scholar]
- 35.Liou GI, Wang M, Matragoon S. Timing of interphotoreceptor retinoid-binding protein (IRBP) gene expression and hypomethylation in developing mouse retina. Dev Biol. 1994;161:345–356. doi: 10.1006/dbio.1994.1036. [DOI] [PubMed] [Google Scholar]
- 36.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to nonselectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 37.McBratney S, Sarnow P. Evidence for involvement of trans-acting factors in selection of the AUG start codon during eukaryotic translation initiation. Mol Cell Biol. 1996;16:3523–3534. doi: 10.1128/mcb.16.7.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeks RG, Zaharevitz D, Chen RF. Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys. 1981;207:141–147. doi: 10.1016/0003-9861(81)90019-9. [DOI] [PubMed] [Google Scholar]
- 39.Naash MI, Ripps H, Li S, Goto Y, Peachey NS. Polygenic disease and retinitis pigmentosa: albinism exacerbates photoreceptor degeneration induced by the expression of a mutant opsin in transgenic mice. J Neurosci. 1996;16:7853–7858. doi: 10.1523/JNEUROSCI.16-24-07853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narfström K, Nilsson SE, Wiggert B, Lee L, Chader GJ, van Veen T. Reduced level of interphotoreceptor retinoid-binding protein (IRBP), a possible cause for retinal degeneration in the abyssinian cat. Cell Tissue Res. 1989;257:631–639. doi: 10.1007/BF00221474. [DOI] [PubMed] [Google Scholar]
- 41.Okajima T-IL, Pepperberg DR, Ripps H, Wiggert B, Chader GJ. Interphotoreceptor retinoid-binding protein promotes rhodopsin regeneration in toad photoreceptors. Proc Natl Acad Sci USA. 1990;87:6907–6911. doi: 10.1073/pnas.87.17.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–205. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- 43.Pepperberg DR, Okajima T-IL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP): molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;7:61–84. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- 44.Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 45.Rajendran RR, van Niel EE, Stenkamp DL, Cunningham LL, Raymond PA, Gonzalez-Fernandez F. Zebrafish IRBP: differential circadian expression among cone subtypes. J Exp Biol. 1996;199:2775–2787. doi: 10.1242/jeb.199.12.2775. [DOI] [PubMed] [Google Scholar]
- 46.Rando RR, Bangerter FW. The rapid intermembraneous transfer of retinoids. Biochem Biophys Res Commun. 1982;104:430–436. doi: 10.1016/0006-291x(82)90655-6. [DOI] [PubMed] [Google Scholar]
- 47.Redmond TM, Wiggert B, Robey FA, Nguyen NY, Lewis MS, Lee L, Chader GJ. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein–a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez de Turco EB, Gordon WC, Bazan NG. Rapid and selective uptake, metabolism, and cellular distribution of docosahexaenoic acid among rod and cone photoreceptor cells in the frog retina. Curr Eye Res. 1991;13:21–28. doi: 10.1523/JNEUROSCI.11-11-03667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saari JC, Teller DC, Crabb JW, Bredberg L. Properties of an interphotoreceptor retinoid-binding protein from bovine retina. J Biol Chem. 1985;260:195–201. [PubMed] [Google Scholar]
- 50.Saiki RK. Amplification of genomic DNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic; San Diego: 1990. pp. 13–20. [Google Scholar]
- 51.Smith HG, Jr, Stubbs GW, Litman BJ. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975;20:211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- 52.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 53.van Veen T, Ekstrom P, Wiggert B, Lee L, Hirose Y, Sanyal S, Chader GJ. A developmental study of interphotoreceptor retinoid-binding protein (IRBP) in single and double homozygous rd and rds mutant mouse retinae. Exp Eye Res. 1988;47:291–305. doi: 10.1016/0014-4835(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 54.Wiggert B, van Veen T, Kutty G, Lee L, Nickerson J, Si J, Nilsson SEG, Chader GJ, Narfström K. An early decrease in IRBP gene expression in abyssinian cats homozygous for hereditary rod-cone degeneration. Cell Tissue Res. 1994;278:291–298. doi: 10.1007/BF00414173. [DOI] [PubMed] [Google Scholar]