Abstract
Outward current modulation by cAMP was investigated in wild type (wt) and dunce (dnc)Drosophila larval neurons. dnc is deficient in a cAMP phosphodiesterase and has altered memory. Outward current modulation by cAMP was investigated by acute or chronic exposure to cAMP analogs. The analysis included a scrutiny of outward current modulation by cAMP in neurons from the mushroom bodies (mrb). In Drosophila, the mrb are the centers of olfactory acquisition and retention. Based on outward current patterns, neurons were classified into four types. Downmodulation of outward currents induced by acute application of cAMP analogs was reversible and found only in type I and type IV neurons. In the general wt neuron population, approximately half of neurons exhibited cAMP-modulated, 4-aminopyridine (4-AP)-sensitive currents. On the other hand, a significantly larger fraction of mrb neurons in wt (70%) was endowed with cAMP-modulated, 4-AP-sensitive currents. Only 30% of thednc neurons displayed outward currents modulated by cAMP. The deficit of cAMP-modulated outward currents was most severe in neurons derived from the mrb of dnc individuals. Only 4% of the mrb neurons of dnc were cAMP-modulated. Thednc defect can be induced by chronic exposure of wt neurons to cAMP analogs. These results document for the first time a well defined electrophysiological neuron phenotype in correlation with the dnc defect. Moreover, this study demonstrates that in dnc mutants such a deficiency affects most severely neurons in brain centers of acquisition and retention.
Keywords: Drosophila, neurons, outward currents, cAMP, downmodulation, mushroom bodies, dunce mutants
In Drosophila, there is solid evidence that the cAMP cascade, involving a Ca- and calmodulin-responsive adenylyl cyclase, protein kinase A, and a cAMP phosphodiesterase, is relevant for the events leading to the retention of olfactory information (Tully et al., 1994; Davis, 1996). Mutants altering the cAMP cascade, such as dunce (dnc) and rutabaga (rut), are deficient in short-term memory (Tully et al., 1994). The evidence indicates also thatDCO, a gene encoding protein kinase A, plays a role in retention and acquisition (Drain et al., 1991; Skoulakis et al., 1993). As in Drosophila, behavioral plasticity associated with cAMP is found in vertebrate organisms (Kandel and Schwartz, 1982; Frey et al., 1993; Huang et al., 1994; Weisskopf et al., 1994). Thednc, rut, and DCO genes express preferentially in the mushroom bodies (mrb) (Nighorn et al., 1991; Han et al., 1992; Skoulakis et al., 1993), brain structures crucial to acquisition and retention in Drosophila (Heisenberg et al., 1985; Debelle and Heisenberg, 1994; Davis, 1996). The memory mutants of the fruit fly, with an altered cAMP cascade, such as dnc andrut, exhibit defective plasticity in peripheral synapses (Corfas and Dudai, 1989; Zhong and Wu, 1991; Delgado et al., 1992), and cAMP analogs can abolish plasticity in the normal larval neuromuscular synapse (Delgado et al., 1992). One target of cAMP modulation is ion channels (Levitan, 1988; Moreno et al., 1995). Modulation of ion channels, via cAMP-associated mechanisms, has been implicated in changes in synaptic efficacy (Kandel and Schwartz, 1982). InDrosophila, cAMP modulation of K+conductances has been described (Delgado et al., 1991; Zhong and Wu, 1993; Wright and Zhong, 1995), and a hyperpolarized resting potential was reported in dnc larval muscle (Delgado et al., 1991). Moreover, in dnc, synaptic plasticity in motor end plates could be restored by K+ channel blockers (Delgado et al., 1992), suggesting that the effects of cAMP are mediated by K+ channel conductance. In support of this notion,Zhao and Wu (1997) reported recently that cytochalasin arrested embryonic neuroblasts (“giant neurons”) from dnc andrut exhibit abnormal spontaneous spikes and altered firing patterns in correlation with altered K+ conductance. Despite this, the fundamental question of whether mrb neurons fromDrosophila mutants deficient in cAMP cascade exhibit altered K+ conductances remains open. We have taken advantage of the availability of wild-type (wt) and dncenhancer detector lines to address the following questions: (1) do mrb neurons represent a particular set of neurons distinguishable electrophysiologically and pharmacologically from the general population of neurons; and (2) does the dnc defect gives rise to specific electrophysiological deficiencies in mrb neurons?
A preliminary account of this work was communicated previously (Delgado and Labarca, 1997).
MATERIALS AND METHODS
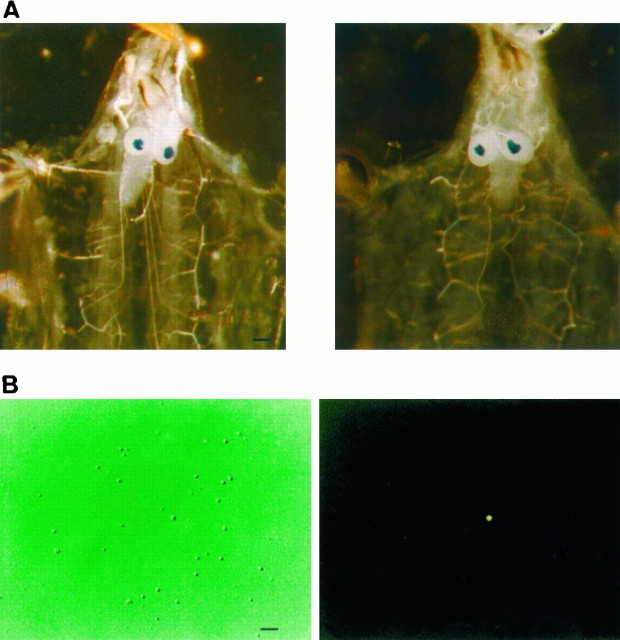
Fly stocks. In most experiments neurons from enhancer detector fly line 221 (derived from Canton-S), taken as wild type, and line dnc1,221, derived from dnc1, were used. Both enhancer detector lines express the lacZgene preferentially in the mrb (Fig.1A).
Fig. 1.
A, Staining of mushroom bodies in larval brain of enhancer detector lines. Left, Enhancer detector line 221 (wt); right, enhancer detector line 221,dnc1. Staining was achieved following the method of Han et al. (1992). Scale bar, 250 μm. B. FDG+ mrb neuron from enhancer detector line. Left, Neurons in culture under Hoffman optics; right, same under fluorescence microscope. FDG staining of mushroom body neurons was performed as described in Materials and Methods. Scale bar, 50 μm.
Tissue culture. Cell culture was achieved by following the method of Wu et al. (1983) with slight modifications. Fifteen to 30 larval brains were incubated for 15 min in 1 ml of PBS endowed with trypsin, (0.0125%, type III; Sigma, St. Louis, MO) at 37°C. The tissue was pelleted, washed twice in 1 ml of culture media made ofDrosophila Schneider (Life Technologies, Gaithersburg, MD), supplemented with 5% fetal bovine serum (Life Technologies) and gentamicin (50 μg/ml, Lab Astorga, Santiago, Chile), and resuspended in 1 ml of media. The suspension was gently passed repeatedly through a fire-polished Pasteur pipette until tissue dissociation was achieved. Cells were plated (25010; Corning, Corning, NY) to a final volume of 1.7 ml/plate and kept at room temperature (20°C).
Electrophysiology. Whole-cell recording was performed in 2- to 5-d-old cell cultures using an Axopatch 1-C amplifier (Axon Instruments Inc., Foster City, CA). The patch-clamp station included a Nikon Diaphot TMD microscope endowed with fluorescence and Hoffman optics. Pulse protocols were generated using pClamp 5.5 software (Axon Instruments). Before recording, the culture media was replaced with 2 ml of external solution made of (in mm) 140 NaCl, 2 KCl, 4 MgCl2, 2 CaCl2, and 5 HEPES, pH 7.2. Unless specified, 3–4 MΩ patch pipettes (Kimax-51; Fisher Scientific, Pittsburgh, PA) were filled with standard internal solution made of (in mm) 70 KF, 70 KCl, 2 MgCl2, 1 CaCl2, 11 EGTA, and 10 HEPES, pH 7.2. Cell capacitance averaged 1.9 ± 0.1 pF (n = 24) in wt and 1.8 ± 0.1 pF (n = 42) in dncneurons. Assuming a spherical shape, the average cell radius amounts to 3.9 μm, with an average surface area of 191 μm2/cell. A large fraction of dissociated neurons (50%) lacked or displayed very small outward currents (<10 pA) 2–5 d after plating. Such neurons were not considered in the analysis. Flow cytometry indicated that some 30% of freshly dissociated neurons were permeable to propidium iodide. Thus, it can be assumed that neurons void of outward currents in 2- to 5-d-old primary cultures represented damaged cells.
Voltage-gated currents, monitored 1 min after establishing whole-cell conditions, displayed steady amplitudes, and, in all cases, the effect of pharmacological agents on outward currents was tested only after showing that currents were of constant amplitude during three consecutive voltage jumps from the holding voltage (−120 mV) to 0 mV. The effects of acute application of cAMP analogs (100 μm8-bromo-cAMP or dibutyryl-cAMP; Sigma) and K+channel blockers were tested by release of the appropriate compound for 20–30 sec from a pipette located 10–20 μm from the cell surface by means of a picospitzer. Similar results were obtained with both cAMP analogs, and they were used indistinctly. For simplicity, in the text and figures cAMP analogs are referred to as X-cAMP. Control studies demonstrated that picospitzer application of external solution, lacking cAMP analogs or K+-channel blockers, from a pipette located 10–20 μm from the cell surface had no effect on outward current amplitude. Under such experimental conditions, high-resistance patches lasted, on average, 4.8 ± 0.7 min (n = 12).
Identification of mrb neurons. Neurons derived from larval mrb of enhancer detector fly lines were identified under the fluorescence microscope using fluorescein di-(β-d-galactopyranoside) (FDG; Molecular Probes, Eugene, OR), a substrate of β-galactosidase (Fig.1B). Cleavage of FDG releases fluorescein, which excites at 490 nm and emits at 514 nm. Loading of neurons with FDG was achieved by means of a 60 sec hyposmotic shock with diluted external solution enriched with 1 mm FDG. Analysis by flow cytometry revealed that ∼2.5% of freshly dissociated neurons were FDG+. This estimate was in good agreement with that obtained by an independent count of FDG+ neurons in 2- to 5-d-old culture plates, which yielded 1.2 ± 0.5% (n = 3) FDG+ cells, and with a previous estimate by Wright and Zhong (1995).
RESULTS
Outward currents in wt neurons
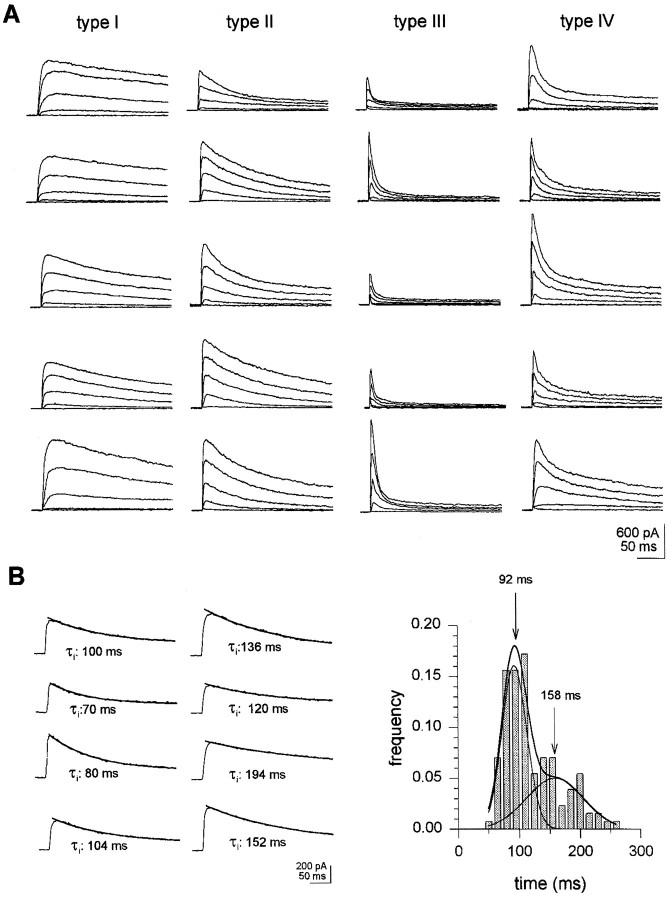
Outward currents properties were scrutinized in wt neurons (n = 502) from wt enhancer detector line 221, as described in Materials and Methods. This allowed us to define four cell types on the basis of outward current inactivation patterns (Fig.2A, Table1). Identical neuron types were observed in a sample (n = 60) from Canton-S larvae. Neuron classification was followed by a study of outward current sensitivity to cAMP and to K+ channel blockers.
Fig. 2.
Classification of neurons in wtDrosophila larvae. Whole-cell currents were recorded as described in Materials and Methods. A, Neuron types in wt larvae. Neurons were classified based on time constants of inactivation and relative amplitude of exponential components, at V = 0 mV, as explained in Results. Records show various examples of outward current patterns in different neurons types. Outward currents were elicited by 20 mV depolarizing steps, from −60 to 20 mV, in 20 mV intervals. Holding potential was −120 mV. B. Analysis of inactivation times in neurons with outward currents inactivating along a single exponential (n = 128).Left, Single exponential outward current inactivation patterns. Currents were triggered by depolarizing excursions from a −120 mV holding to 0 mV. Lines on top of current records are best fit of single exponential function to data.Right, Distribution of inactivation times. Thesolid line is best fit to data of two Gaussian curves, with peaks at 92 and 158 msec. The relative contribution of each Gaussian component is 0.58 and 0.42, respectively;n = 128. r2 = 0.96.
Table 1.
Outward current properties in Drosophila larval neurons
| Neuron type | N | I peak (pA) | τ1(msec) | τ2(msec) | A1 | A2 | Blocker | Amplitude of cAMP-sensitive currents (pA) |
|---|---|---|---|---|---|---|---|---|
| Wild type | ||||||||
| I | 54 | 397.8 ± (43.4) | 162.3 ± (7.5) | 4-AP | 212.5 ± 47 | |||
| II | 74 | 499.3 ± (58.4) | 88.9 ± (2.7) | TEA | ||||
| III | 23 | 346.3 ± (57.7) | 7.2 ± (0.5) | 98.5 ± (9.3) | 0.78 ± (0.01) | 0.22 ± (0.01) | 4-AP (>10 mm) | |
| IV | 351 | 522.0 ± (21.1) | 14.2 ± (0.4) | 110.1 ± (2.6) | 0.46 ± (0.01) | 0.54 ± (0.01) | 4-AP, TEA | 150 ± 17 |
| dunce | ||||||||
| I | 3 | 859.7 ± (260) | 158.0 ± (8.8) | 4-AP | 562, 257 | |||
| II | 35 | 326.2 ± (43.0) | 87.9 ± (4.8) | TEA | ||||
| III | 55 | 315.6 ± (35.5) | 10.7 ± (0.9) | 131.4 ± (17.7) | 0.77 ± (0.01) | 0.23 ± (0.01) | 4-AP (>10 mm) | |
| IV | 174 | 473.2 ± (54.0) | 14.1 ± (0.7) | 119.8 ± (6.1) | 0.46 ± (0.02) | 0.54 ± (0.02) | 4-AP, TEA | 130 ± 19 |
Outward current properties in different neuron types.I peak, Peak currents measured directly from experimental records at V = 0 mV. τ values are time constants obtained by single- or double-exponential fitting to experimental data. In type I and type II neurons outward currents inactivated along a single exponential, and τ1 represents the time constant for current decay. In type II and IV neurons outward currents inactivated double exponentially. Here, τ1 and τ2 are time constants; A1 and A2, relative amplitudes of fast and slow components, respectively, derived from fitting. Numbers in parentheses are SEM.
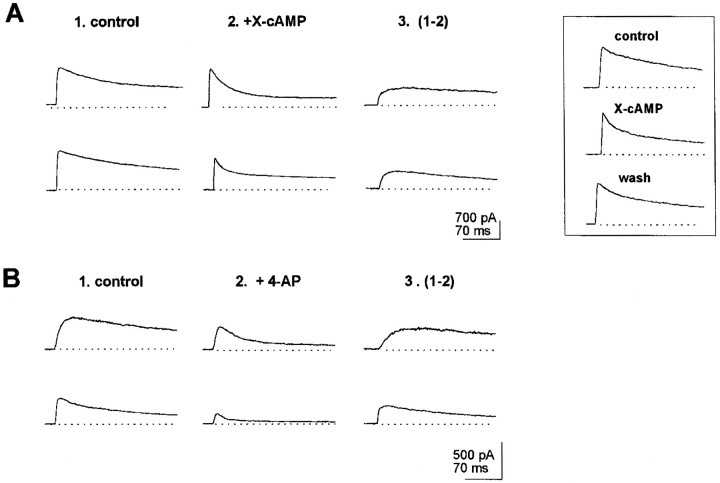
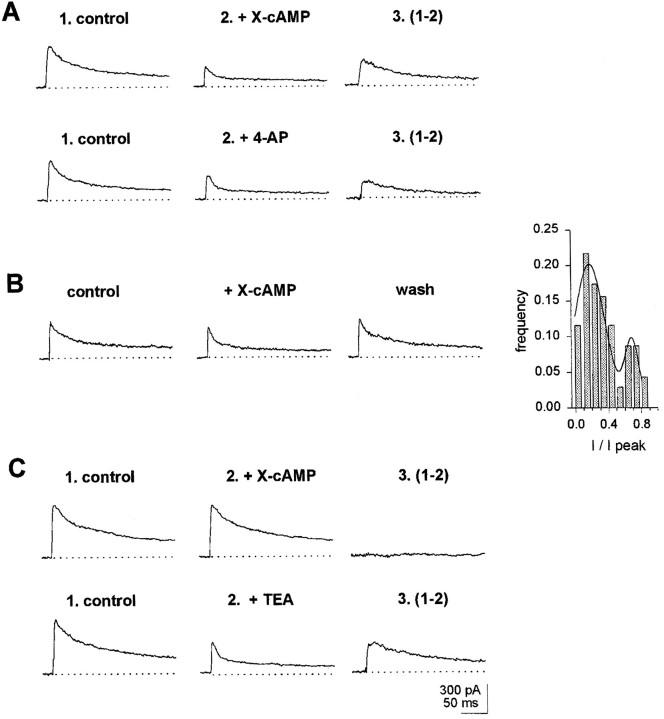
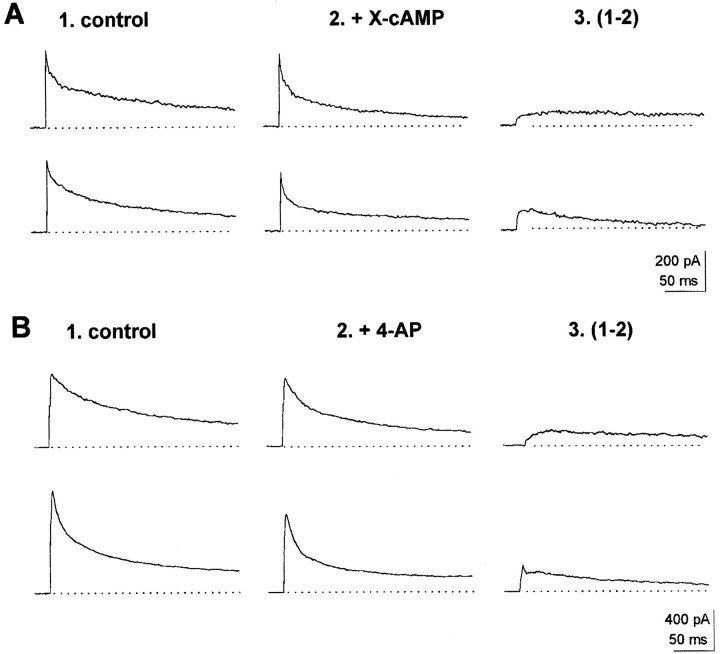
A fraction of wt neurons (25%) displayed slowly inactivating outward currents (τi > 50 msec) with an inactivation time course (at V = 0 mV) that could be accounted for with a single-component exponential fit (Fig. 2B). In these neurons, analysis of the distribution of outward current inactivation times, at V = 0 mV, yielded two Gaussian curves, with peaks at 92 and 158 msec, respectively (see histogram in Fig. 2B). In turn, acute exposure to cAMP analogs, applied via a picospitzer, to neurons exhibiting outward inactivation along a single exponential showed that a fraction of them responded to the X-cAMP with a decrement in outward current amplitude. In such cells, inactivation times at V = 0 mV averaged 162 ± 8 msec (n = 12) (Table 1). This is in coincidence with the peak at 158 msec in the histogram shown in Figure 2B. Thus, the Gaussian component with a maximum at 158 msec correlates with neurons exhibiting outward currents downregulated by cAMP. Such neurons were classified as type I (Fig.2A). In 6 of 12 type I neurons tested, the outward current component affected by X-cAMP was a maintained one (Fig.3A, top records). In six other type I neurons, cAMP analogs affected a slowly inactivating outward current component (Fig. 3A, bottom records). Diminishment in outward current amplitude reverted 1–2 min after acute application of cAMP analogs ceased (Fig. 3A, inset). In type I neurons, 4-AP (100 μm) blocked outward currents with properties similar to cAMP-sensitive ones (Fig.3B). Furthermore, when stimulation with X-cAMP was preceded by exposure to 4-AP, no effect of cAMP analogs on outward current amplitude was observed in type I neurons. In type I neurons outward currents insensitive to 4-AP inactivated with τi = 22 ± 4 msec.
Fig. 3.
Properties of outward currents in type I neurons. In all experiments shown hereunder, currents were elicited by jumping the voltage from a −120 mV holding potential to 0 mV. Currents shown in records labeled 3 were obtained by subtraction of currents recorded after exposure to X-cAMP or 100 μm 4-AP (2) from control outward currents (1). A, top record, Maintained outward current abolished by cAMP analogs. Bottom record, Slowly inactivating cAMP-sensitive outward currents.Inset, Reversibility of effect of X-cAMP on outward currents in type I neurons. Reversibility was monitored 1–2 min after exposure to X-cAMP. B, Blockade by 100 μm4-AP of cAMP-sensitive currents in type I neurons.
Neurons displaying outward currents that inactivated along a single exponential at V = 0 mV and that did not respond to cAMP analogs (Fig. 4A) had inactivation times averaging 89 ± 3.6 msec, in correspondence with the major peak at 92 msec in the histogram in Figure2B. These neurons were classified as type II neurons (Table 1). In such neurons tetraethylammonium (TEA, 1 mm) but not 4-AP (100 μm) was effective in blocking outward currents, which, at V = 0 mV, inactivated with τi = 120 ± 13 msec (n = 6; Fig. 4B). Currents that remained after exposing type II neurons to 1 mm TEA inactivated with τi = 12 ± 2 msec.
Fig. 4.
Properties of outward currents in type II and type III neurons. Currents shown in records labeled 3 were obtained by subtraction of control outward currents in records labeled1 from currents recorded after exposure to X-cAMP, 1 mm TEA, or 100 μm 4-AP in records labeled2. A. Lack of effect of cAMP analog on outward currents in type II neurons. B, top record, Blockade by 1 mm TEA of outward currents in type II neurons. Bottom record, Lack of effect of 100 mm 4-AP on outward currents in type II neurons.C. Lack of effect of cAMP analogs on outward currents in type III neurons. D. Insensitivity of outward currents to TEA (1 mm, top record) and 100 4-AP (100 μm, bottom record) in type III neurons.
Type III neurons, which composed 4.6% of the sample, exhibited outward currents dominated by rapidly inactivating outward currents (Fig.2A ,Table 1). In all type III neurons tested (n = 10) outward currents were refractory to X-cAMP (Fig. 4C) and poorly sensitive to TEA (1 mm) and 4-AP (100 μm) (Fig. 4D).
Type IV neurons represented the largest subset of neurons (69.9%). At V = 0 mV they displayed outward currents with fast and slow inactivating components of about similar amplitudes (Table 1). From a sample of 46 type IV neurons, 28 exhibited currents that were diminished by exposure to X-cAMP. In all cases, cAMP analogs abolished a slowly inactivating outward current, blocked by 4-AP (100 μm; Fig. 5A). The effect of X-cAMP on outward currents reverted within 1–2 min after acute application ceased (Fig. 5B). The extent of inactivation of cAMP-modulated outward currents in wt neurons was investigated further during a long 400 msec depolarizing pulse. The analysis indicated the presence of two cAMP-sensitive currents, distinguishable by the extent of their inactivation at the end of a prolonged depolarization, as documented in Figure 5B, inset. The major peak in the histogram corresponds to cAMP-sensitive currents found in type IV and type I neurons, in which inactivation was almost complete at the end of the 400 msec pulse. The minor peak corresponds to cAMP-sensitive currents exhibiting little inactivation at the end of the 400 msec depolarizing pulse, found in type I neurons and in neurons from the mrb. In the fraction of type IV neurons that were sensitive to cAMP, outward currents that remained after exposure to cAMP analogs inactivated with τi = 8 ± 1 msec. In type IV neurons void of cAMP-sensitive currents, TEA (1 mm) blocked an outward component, which, at V = 0 mV, inactivated with τi = 86 ± 10 msec (Fig. 5C). Because in wt, all type I neurons and 60% type IV neurons displayed outward currents modulated by cAMP, it is expected that ∼50% of wt neurons exhibit such currents.
Fig. 5.
Properties of outward currents in type IV neurons. Currents shown in records labeled 3 were obtained by subtraction of control outward currents in records labeled1 from currents recorded after exposure to X-cAMP, 1 mm TEA, or 100 μm 4-AP in records labeled2. A, Block of X-cAMP-sensitive outward currents by 100 μm 4-AP. Top record, cAMP-dependent outward currents in a type IV neuron. Bottom record, In the same neuron as in top recordafter exposure to X-cAMP, outward currents were allowed to recover for 2 min. Then, 100 μm 4-AP was applied via the picospitzer.B, Further evidence for reversibility of X-cAMP modulation of outward currents in type IV neuron. Inset, Extent of inactivation of cAMP-sensitive currents at the end of a 400 msec depolarization to 0 mV in wt neurons. The histogram was built with data from 60 neurons. I, Amplitude of cAMP-sensitive currents at the end of the depolarizing pulse; I peak, peak amplitude of cAMP-sensitive currents at the beginning of the depolarization. The solid line represents the best fitting to two Gaussian components, with peaks at 0.7 and 0.18.C, Outward current block by 1 mm TEA in type IV neuron lacking cAMP-sensitive currents. Top record, Lack of effect of X-cAMP in the type IV neuron. Bottom record, 1 mm TEA blocks outward currents in same type IV neuron as in top record.
Outward currents in wt mrb neurons
A sample of 40 mrb neurons was tested to investigate cAMP modulation of outward currents. In 27 of these neurons X-cAMP was effective in downregulating outward currents. Current subtraction revealed that cAMP-sensitive outward currents were either maintained or slowly inactivating (Fig.6A) and were blocked by 4-AP (Fig. 6B). These observations indicate that ∼70% of mrb neurons display outward currents downregulated by cAMP. To a p ≤ 0.05 level of confidence, mrb neurons differ from the whole wt neuron population in that they contained a larger fraction of units exhibiting cAMP-sensitive outward currents. The presence of a small outward current component, displaying fast inactivation and blocked by 4-AP (100 μm; Fig.6B, bottom record), was also apparent in half of mrb neurons tested for 4-AP sensitivity. Both type II and type III neurons were detected in those mrb neurons that did not display outward currents sensitive to cAMP. Thus, the same neuron types seen in the general neuron population seemed to be present in the mrb.
Fig. 6.
Properties of cAMP-modulated outward currents in mushroom body neurons. Mushroom body neurons were identified under the fluorescence microscope as described in Materials and Methods. Currents shown in records labeled 3 were obtained by subtraction from control outward currents in records labeled 1 and outward currents recorded after exposure to X-cAMP or 100 μm 4-AP in records labeled 2.A, cAMP-modulated outward currents in mushroom body neuron. Top record, Maintained, cAMP-sensitive outward current component. Bottom record, Slowly inactivating, cAMP-sensitive outward current in mushroom body neuron.B, Blockade by 100 μm 4-AP of maintained and slowly inactivating outward currents in mushroom body neurons.Top record, 4-AP-sensitive, maintained outward current.Bottom record, 4-AP-sensitive, slowly inactivating outward current. Notice in record labeled 3 the presence of a fast-inactivating, 4-AP-sensitive outward current component.
Outward currents in dnc neurons
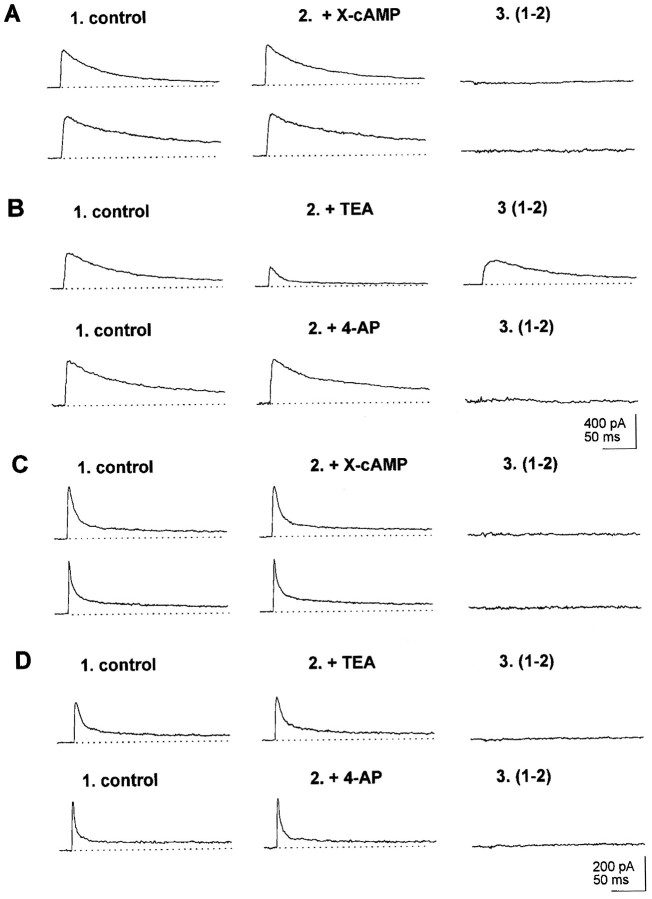
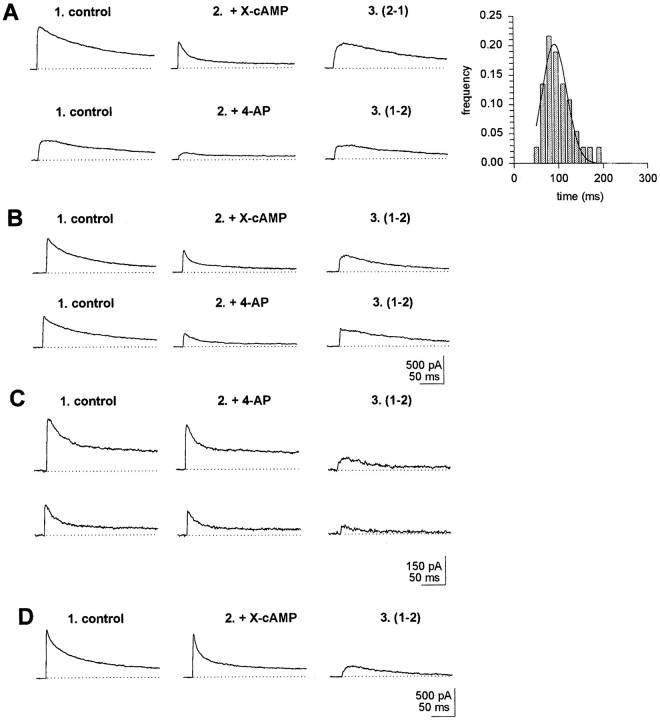
Outward currents were investigated also in a sample of neurons (n = 267) derived from larval brains of the 221,dnc line. It was found that, as a population,dnc neurons differed from wt. First, type I neurons were almost absent in dnc (Table 1, Fig.7A, inset). In fact, only three such neurons could be found in the sample. The effects of cAMP on outward currents could be tested in one of these neurons. X-cAMP caused decrements in outward current amplitude, owing to inhibition of a slowly inactivating current. In another type I neuron, 4-AP (100 μm) was effective in blocking a slowly inactivating outward current component, similar to that inhibited by X-cAMP (Fig.7A). In the dnc sample, type III neurons were fourfold more abundant than in wt. Similar results were obtained in a set of 60 neurons from dncM14 larvae (Table2). Otherwise, the electrophysiological and pharmacological properties of outward currents in dncneurons were similar to those found in wt (Table 1).
Fig. 7.
cAMP-sensitive outward currents indnc neurons and wt neurons exposed chronically to X-cAMP. cAMP-sensitive currents in records labeled 3were obtained by subtraction from control record in 1and record obtained after exposure to X-cAMP in 2.A, Top record, Abolishment of slowly inactivating outward current by X-cAMP in type I neuron fromdnc. Bottom record, blockade of outward currents by 100 μm 4-AP in type I dncneuron. Inset, Distribution of inactivation times indnc neurons exhibiting single exponential inactivation. The distribution displays a single peak and is well fitted to a single Gaussian component and peaks at 91.6 msec (n = 38; r2 = 0.97). B, Properties of cAMP-modulated currents in dnc neurons. Top record, Abolishment of slowly inactivating outward current by X-cAMP in type IV neurons from dnc. Bottom record, Blockade by 100 μm 4-AP of slowly inactivating outward current in type IV neuron from dnc. C, 4-AP-sensitive outward currents in wt neuron after chronic exposure to X-cAMP. D, cAMP-sensitive, slowly inactivating outward current component in dnc mrb neuron.
Table 2.
Percentage of different larval neuron types
| Strain | Type | |||
|---|---|---|---|---|
| I | II | III | IV | |
| wt | 11.0 | 14.8 | 4.6 | 69.9 |
| wt + X-cAMP | 13.0 | 22.0 | 65.0 | |
| dncf; 221 | 1.1 | 13.1 | 20.6 | 65.2 |
| dncM14 | 15.0 | 18.0 | 67.0 | |
From 27 type IV dnc neurons tested, 12 displayed slowly inactivating cAMP-sensitive outward currents, blocked by 4-AP (Fig.7B). Thus, as a population, ∼30% of dncneurons display cAMP-sensitive outward currents. This percentage, to ap ≤ 0.05 level of confidence, is significantly lower than the fraction of neurons exhibiting cAMP-sensitive currents observed in the wt population. The extent of inactivation of cAMP-sensitive currents at the end of a prolonged, 400 msec depolarization in dnc averaged 84 ± 6% (n = 30), relative to peak currents measured at the beginning of the depolarizing pulse. This percentage of inactivation at the end of a 400 msec depolarization is similar to that of cAMP-sensitive currents in type IV in wt individuals, as documented in Figure 5, inset.
Effect of chronic exposure of wt neurons to cAMP
Further studies were performed to determine whether a chronic increase in intracellular levels of cAMP suffices to account for the deficit of dnc neurons exhibiting cAMP-sensitive outward currents. wt neurons were exposed chronically (12 hr) to a 100 μm concentration of either cAMP analog before recording. As shown in Table 2, in a sample of 60 such neurons, the presence of type I cells could not be documented. Given that type I neurons made 11% of the wt population, one would predict in this sample, to ap = 0.05 level of confidence, the occurrence of at least two type I neurons. Thus, chronic exposure to X-cAMP causes a significant drop in the number of type I neurons in the wt population. Thirty wt neurons chronically exposed to cAMP analogs were tested also for 4-AP sensitivity, to asses the fraction of wt neurons exhibiting cAMP-sensitive outward currents. Outward currents blocked by 4-AP could be documented in seven of these neurons (Fig. 7C). This number is significantly less than that expected for wt neurons not exposed chronically to cAMP analogs but is similar to that ofdnc (p = 0.05 level of significance). Moreover, the amplitude of 4-AP-sensitive currents in wt neurons exposed chronically to cAMP analogs (37 ± 3 pA; n= 7) was significantly smaller than in type IV neurons not exposed chronically to the cAMP analog (150 ± 17; n = 46) (Table 1). In addition to causing a significant decrement of type I neurons, chronic exposure of wt neurons to cAMP analogs produced an increase in the fraction of type III neurons to levels similar to those found in the dnc population (Table 2).
The reversibility of the decrement in amplitude of cAMP-modulated current seen in wt neurons after chronic incubation in X-cAMP was also investigated. To this purpose, outward currents were recorded from these neurons for a 3 min period after whole-cell recording conditions had been established. During this period, in which they were exposed to cAMP-free solution applied via the picospitzer, no increase in outward current amplitude was observed. In a second protocol, designed to investigate reversibility of the effects of chronic application of cAMP analogs on outward currents, whole-cell recording was performed in 60 wt neurons that had been exposed for 12 hr to X-cAMP after they were incubated for 1 hr in a cAMP-free solution. In this sample, no type I neuron was detected, and the fraction of type III neurons was still significantly larger than that expected for wt neurons not incubated chronically with cAMP analogs. The amplitude of slowly inactivating, cAMP-modulated currents in these neurons (62 ± 12 pA;n = 6) was larger (p ≤ 0.1 level of significance) than the amplitude of cAMP-sensitive currents recorded from wt neurons exposed chronically to cAMP but that were not subjected to incubation for 1 hr in a medium free of cAMP analogs before recording (37 ± 3 pA; n = 7). This result suggests a slow reversal of the effect of chronic exposure to cAMP on the slowly inactivating cAMP-sensitive current.
mrb neurons from dnc
From a sample of 37 dnc mrb neurons tested, only two (5%) were found to exhibit outward currents downmodulated by cAMP. In both cases, exposure to cAMP analogs abolished a slowly inactivating outward current component (Fig. 7D). Thus, in dncthere is a significant drop in the fraction of mrb neurons displaying cAMP-sensitive currents, compared with the dnc neuron population as a whole. This difference is still more dramatic when mrb neurons from dnc are compared with mrb body neurons from wt larvae. Such a comparison reveals that in dnc, the fraction of mrb neurons exhibiting outward currents down modulated by cAMP is 14-fold smaller than in mrb neurons from normal individuals.
DISCUSSION
There is convincing evidence that acquisition and retention of olfactory clues in the fruit fly depend critically on the mrb. InDrosophila, these bilateral integrative brain centers are made of a few thousand neurons that receive inputs from the antennal and optical lobes, as well as from other sensory centers. Their axons establish connections with other structures in the brain (Nassel, 1987;Davis, 1996). Altered mrb structure, or chemical ablation of the mrb, causes loss of acquisition and retention (Heisenberg et al., 1985;Debelle and Heisenberg, 1994). Moreover, genes engaged in acquisition and retention, such as dnc, are expressed preferentially in the mrb. Thus, mrb neurons are a logical locus to search for electrophysiological correlates to altered acquisition and retention in neurological mutants of the fruit fly. The establishment of such correlates in the brain of Drosophila should provide important clues on the functional aspects of acquisition and retention in the nervous system. From the present study it can be concluded that, in Drosophila larval neurons, outward currents downmodulated by cAMP segregate to specific neurons, namely type I and type IV neurons. Therefore, downregulation by cAMP of K+conductances is not restricted to neurons from the mrb. Indeed, the fraction of wt neurons exhibiting outward current modulation by the cyclic nucleotide in the whole neuron sample (∼50%) is well above the fraction of neurons expected to be contributed by the mrb (1–5%). Our results indicate also that mrb neurons can display a 4-AP-sensitive, fast-inactivating outward current component refractory to cAMP. This outward current differs from fast-inactivating outward currents found in type III neurons, in which significant blockade by 4-AP requires concentrations well above 1 mm. As a whole, our results point to a differential functional expression of K+ channels in larval neurons. Differential expression of K+ channels has been reported previously by various groups in the brain of Drosophila. For example, Shaker, which yields 4-AP-sensitive A-type currents (Covarrubias et al., 1991), distributes nonuniformly, expressing preferentially in the mrb (Schwarz et al., 1990). In turn, Baker and Salkoff (1990) found that only a fraction of neurons derived from the thoracic ganglia of late-stage Drosophila pupae exhibitedShaker currents. More recently, Zhao and Wu (1997) reported a differential expression of K+ currents in “giant” neurons derived from cytokinesis-arrestedDrosophila embryonic neuroblasts.
cAMP-modulated K+ conductances in mrb neurons exhibited properties similar to those monitored in type I and type IV neurons. However, a significantly larger fraction of wt mrb neurons possessed cAMP-sensitive currents. On the other hand, the fraction of mrb neurons exhibiting cAMP-modulated currents in dnc was 14-fold smaller than in normal individuals. These observations provide the first direct clue that deficiencies in a cAMP phosphodiesterase, expressing preferentially in the mrb, correlates with altered electrophysiology in neurons derived from these brain structures attributable to a significant decrement of cAMP-modulated K+ conductances.
The comparison of neuron populations evidenced significant differences between wt and dnc. First, in the mutant, type I neurons were almost absent, whereas type III neurons were four times more abundant. Chronic exposure of wt neurons to cAMP analogs (12 hr) caused a loss of type I neurons and an increase in the number of type III neurons, which mimics the properties of the dnc neuronal population. This result, to a first approximation, reveals that in wt, chronic increases in cAMP suffice to yield the dnc neuronal population. cAMP-modulated outward currents in larval neurons were of two types: maintained or slowly inactivating. The experimental evidence suggests that slowly inactivating and maintained cAMP-sensitive currents are two functionally different outward components. In wt, maintained currents were found exclusively in type I neurons and in neurons from the mrb. Only the maintained cAMP-sensitive component was absent in dnc. On the other hand, both cAMP-sensitive currents were blocked by 100 μ 4-AP and had similar activation properties. Recent studies in our laboratory indicate that cAMP-sensitive outward currents such as those monitored in larval neurons are present in adult neurons (P. Labarca and R. Delgado, unpublished results). Chronic exposure to cAMP analogs of wt larval neurons abolished the maintained component and caused a 6-fold diminishment in amplitude of the slowly inactivating cAMP-sensitive current. Thus, the two currents might differ in their susceptibility to downmodulation by cAMP. Perhaps these two currents correspond to splicing variants, or mRNA editions, of a same K+channel. Alternatively, it could be that the different inactivation properties of cAMP-sensitive currents result from regulatory units that control the extent of channel inactivation during long depolarizing episodes.
The effects of transient, acute increases in cAMP differ from those caused by chronic exposure to the cyclic nucleotide. Thus, although the effects on cAMP-sensitive outward currents induced by acute application of cAMP analogs reversed within 1–2 min, full reversal of the effects of chronic exposure could not be documented for up to 1 hr. Perhaps chronic increases in cAMP, lasting many hours, hinders expression of cAMP-sensitive maintained currents, for example, by interfering with the channel-forming protein synthesis or by blocking protein maturation. According to the results obtained here, reversal of the effects of chronic increases in cAMP in Drosophila neurons would require a longer time span than the maximal experimental time span tested.
In neurons from Drosophila embryos, outward currents seemed to be accounted for mainly by three K+ channel genes, namely Shal, Shaw, and Shab(Tsunoda and Salkoff, 1995). Shal is, by and large, the major constituent of A-type currents in such neurons. Shawwould associate with noninactivating, weakly voltage-dependent outward currents, and Shab would build delayed rectifier K+ currents. Further studies would be necessary to establish to what extent outward current patterns in larval and adult neurons coincide with those found in neurons from Drosophilaembryos and in “giant” neurons, derived from cytokinesis-arrested embryonic neuroblasts (Zhao and Wu, 1997). Based on available evidence, it would be expected that A-type currents present in type III larval neurons associate mostly with Shal and not Shaker(Solc et al., 1987; Baker and Salkoff, 1990; Tsunoda and Salkoff, 1995). Fast-inactivating outward currents, insensitive to cAMP and 4-AP, such as those recorded in type III neurons, were apparent in type I and type IV neurons. Similarly, type II neurons were found to posses a TEA-insensitive outward current with fast inactivation times. Therefore, A currents, insensitive to 4-AP (0.1 μm) and TEA (1 mm), would be present in all neuron types. Lacking their pharmacological profiles, it seems imprudent at this moment to attempt to associate Shaw and ShabK+ currents reported in embryonic neurons (Tsunoda and Salkoff, 1995) with K+ conductances found in larval and adult neurons, in particular with 4-AP sensitive currents downmodulated by cAMP. It is worth pointing out, however, that when expressed in Xenopus oocytes, Shabchannels are insensitive to 4-AP (Covarrubias et al., 1991). Thus, a suitable candidate to account for the maintained, 4-AP-sensitive outward currents downmodulated by cAMP is Shaw. Shaw is sensitive to 4-AP but not to TEA (Wei et al., 1990). Kv3.1, aShaw homolog expressing preferentially in fast-spiking neurons from mice (Messengill et al., 1997), is sensitive also to 4-AP (Yokoyama et al., 1989), and another Shaw homolog, Kv3, is amenable to downmodulation by cAMP through cAMP-dependent protein kinase (Moreno et al., 1995).
The mechanisms by which cAMP downmodulates outward currents inDrosophila neurons remain to be established. Downmodulation of K+ currents by the cyclic nucleotide has been reported to operate indirectly through protein kinase A (Levitan, 1988,Drain et al., 1994; Moreno et al., 1995). Probably a similar mechanism accounts for downmodulation of K+ currents inDrosophila. Up to now, direct modulation of K+ channels by cyclic nucleotides, including modulation of K+ channels in Drosophila(Delgado et al., 1991; Gomez and Nassi, 1995; Jorquera et al., 1995;Labarca et al., 1996), has been reported to give place to increases in channel open probability.
Footnotes
This work was supported by Fondo Nacional de Investigación Grant 1950457 and a Presidential Chair in Science to P.L. R.L. holds a Presidential Chair in Science. R.D. held a C.A.I. fellowship. Institutional support of a group of Chilean companies (Empresas CMPC, CGE, Codelco, COPEC, Minera Escondida, Novagas, Business Design Associates, and Xerox Chile) is recognized. Part of this work was performed during the tenure of a John Simon Guggenheim Fellowship to P.L. P.L. is an International Scholar of the Howard Hughes Medical Institute.
Correspondence should be addressed to Pedro Labarca, Centro de Estudios Cientificos de Santiago, Casilla 16443, Santiago 9, Chile.
REFERENCES
- 1.Baker K, Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ current in a subset of neurons. Neuron. 1990;2:129–140. doi: 10.1016/0896-6273(90)90449-p. [DOI] [PubMed] [Google Scholar]
- 2.Corfas G, Dudai Y. Habituation and deshabituation of a cleaning reflex in normal and mutant Drosophila. J Neurosci. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covarrubias M, Wei A, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- 4.Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 5.Debelle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 6.Delgado R, Labarca P. Larval neurons from dunce learning and memory Drosophila mutant lacks an outward current component down regulated by cAMP. Biophys J. 1997;72:A265. [Google Scholar]
- 7.Delgado R, Hidalgo P, Diaz F, Latorre R, Labarca P. A cyclic AMP-activated K+ channel is persistently activated in dunce. Proc Natl Acad Sci USA. 1991;88:557–560. doi: 10.1073/pnas.88.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado R, Latorre R, Labarca P. K+ channel blockers restore synaptic plasticity in the neuromuscular junction of dunce, a Drosophila learning and memory mutant. Proc R Soc Lond B Biol Sci. 1992;250:181–185. doi: 10.1098/rspb.1992.0147. [DOI] [PubMed] [Google Scholar]
- 9.Drain PE, Folkes E, Quinn WG. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron. 1991;6:711–782. doi: 10.1016/0896-6273(91)90123-h. [DOI] [PubMed] [Google Scholar]
- 10.Drain P, Dubin AE, Aldrich RW. Regulation of Shaker K+ channel inactivation gating by the cAMP-dependent protein kinase. Neuron. 1994;12:1097–1109. doi: 10.1016/0896-6273(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 11.Frey U, Huang Y-Y, Kandel ER. Effects of cAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 12.Gomez MP, Nassi E. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca cascade. Neuron. 1995;15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 13.Han P-L, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 14.Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y-Y, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 16.Jorquera O, Latorre R, Labarca P. Ion channel classes in purified olfactory cilia membranes: planar lipid bilayer studies. Am J Physiol. 1995;269:C1235–C1244. doi: 10.1152/ajpcell.1995.269.5.C1235. [DOI] [PubMed] [Google Scholar]
- 17.Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 18.Labarca P, Santi C, Zapata O, Morales E, Beltran C, Darszon A. A cAMP-regulated K+-selective channel from the sea urchin sperm plasma membrane. Dev Biol. 1996;174:271–280. doi: 10.1006/dbio.1996.0072. [DOI] [PubMed] [Google Scholar]
- 19.Levitan IB. Modulation of ion channels in neurons and other cells. Annu Rev Neurosci. 1988;11:119–136. doi: 10.1146/annurev.ne.11.030188.001003. [DOI] [PubMed] [Google Scholar]
- 20.Messengill JL, Smith MA, Son DI, O’Dowd DK. Differential expression of K+ currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. J Neurosci. 1997;17:3136–3147. doi: 10.1523/JNEUROSCI.17-09-03136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno M, Kentros C, Bueno E, Weiser M, Hernandez A, Vega-Saenz E, Ponce A, Thornhill W, Rudy B. Thalamocortical projections have a K+ channel that is phosphorylated and modulated by cAMP-dependent protein kinase. J Neurosci. 1995;15:5486–5501. doi: 10.1523/JNEUROSCI.15-08-05486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassel DR. Aspects of the functional and chemical anatomy of the insect brain. In: Ali MA, editor. Nervous systems of invertebrates. Plenum; New York: 1987. pp. 378–386. [Google Scholar]
- 23.Nighorn A, Healy JM, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz TL, Papazian DM, Carretto RC, Jan YN, Jan LY. Immunological characterization of K+ channel components from the Shaker locus and differential distribution of splicing variants in Drosophila. Neuron. 1990;4:119–127. doi: 10.1016/0896-6273(90)90448-o. [DOI] [PubMed] [Google Scholar]
- 25.Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalitic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- 26.Solc CK, Zagotta WN, Aldrich RW. Single channel and genetic analysis reveal two distinct A-type potassium channels in Drosophila. Science. 1987;236:1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- 27.Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci. 1995;15:1741–1754. doi: 10.1523/JNEUROSCI.15-03-01741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tully T, Preat SC, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 29.Wei A, Covarrubias M, Butler A, Baker K, Pak M, Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990;248:599–605. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- 30.Weisskopf MG, Castillo PE, Zalutzky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 31.Wright NJD, Zhong Y. Characterization of K+ currents and the cAMP dependent modulation in cultured Drosophila mushroom body neurons identified by lacZ expression. J Neurosci. 1995;15:1025–1034. doi: 10.1523/JNEUROSCI.15-02-01025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C-F, Suzuki N, Poo M-M. Dissociated neuron from normal and mutant Drosophila larval central nervous system in cell culture. J Neurosci. 1983;3:1888–1899. doi: 10.1523/JNEUROSCI.03-09-01888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. Potassium channels from NG108–15 neuroblastoma-glioma hybrid cells: Primary structure and functional expression from cDNAs. FEBS Lett. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M-L, Wu C-F. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Y, Wu C-F. Altered synaptic plasticity in Drosophila memory mutant with altered cAMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 36.Zhong Y, Wu C-F. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila. J Neurogenet. 1993;9:15–27. doi: 10.3109/01677069309167273. [DOI] [PubMed] [Google Scholar]