Abstract
Astrocytes swell during neuronal activity as they accumulate K+ to buffer the increase in external K+ released from neurons. This swelling activates volume-sensitive Cl− channels, which are thought to be important in regulatory volume decrease and in the response of the CNS to trauma and excitotoxicity. Mitogen-activated protein (MAP) kinases also are activated by cell volume changes, but their roles in volume regulation are unknown. We have investigated the role of tyrosine and MAP kinases in the activation of volume-activated Cl− channels in cultured astrocytes, using whole-cell patch-clamp recording and Western immunoblots. As previously described, hypo-osmotic solution induced an outwardly rectifying Cl− current, which was blocked by NPPB and SITS. This Cl− current did not depend on [Ca2+ ]i because it was still observed when 20 mm BAPTA was included in the pipette, but it did exhibit rundown when ATP was omitted. Inhibition of tyrosine kinases with genistein or tyrphostin A23 (but not the inactive agents daidzein and tyrphostin A1) blocked the Cl− current. The MAP kinase kinase (MEK) inhibitor PD 98059 reversibly inhibited activation of the Cl− current by hypo-osmotic solution. Western immunoblots showed that genistein or PD 98059 blocked activation of Erk-1 and Erk-2 by hypo-osmotic solution in astrocytes. Therefore, activation of tyrosine and MAP kinases by swelling is a critical step in the opening of volume-sensitive Cl− channels.
Keywords: volume-activated Cl− current, tyrosine kinase, MAP kinase, cell swelling, regulatory volume decrease, astrocyte
The swelling of astrocytes is an important response to neuronal activity in the CNS and occurs during normal and pathological conditions (for review, see Kimelberg, 1995). Astrocytes are thought to be the most labile in generating cellular volume changes. During neuronal activity there is an increase in extracellular K+, which is taken up into astrocytes via transport and passive ion flows. This, in turn, causes a net uptake of water and swelling in astrocytes and a reduction of the extracellular space (for review, see Kimelberg, 1995). Astrocytes and other cell types counteract volume increases by an efflux of Cl− and other anions, coupled with the coactivation of K+ channels (Pasantes-Morales et al., 1994) (for review, see Nilius et al., 1996; Strange et al., 1996). The Cl− channel involved in volume regulation is outwardly rectifying and is permeable to fairly large anions, including glutamate and taurine. However, the cellular mechanisms controlling the activation of the volume-sensitive Cl− channels in astrocytes are poorly understood.
The mitogen-activated protein (MAP) kinases Erk-1 and Erk-2 can be activated by hypo-osmotic solution and swelling (Tilly et al., 1993;Schliess et al., 1995, 1996; Noé et al., 1996) (but see Krause et al., 1996). Growth factors, cytokines, and some G-protein-coupled receptors activate MAP kinases, which are involved in the regulation of gene expression (for review, see Su and Karin, 1996; Robinson and Cobb, 1997). There are also several cytoplasmic targets that are modulated by MAP kinases (Campbell et al., 1995). However, the roles for MAP kinases in regulating ion channel activity are unknown. In contrast, it has been shown that tyrosine kinases are involved in the activation of the volume-sensitive Cl− channels in Intestine 407 and in cardiac cells (Tilly et al., 1993; Sorota, 1995). Because MAP kinases can be targets of tyrosine kinase cascades, we suggest that both tyrosine and MAP kinases may be involved in the activation of volume-sensitive Cl− channels in astrocytes.
In this paper we investigated the involvement of tyrosine kinase and the MAP kinases, Erk-1 and Erk-2, in the activation of a volume-sensitive Cl− current in cultured astrocytes, using whole-cell patch-clamp recording and Western immunoblots. Specific inhibitors of tyrosine kinases (genistein and tyrphostin A23; Negrescu et al., 1995) (for review, see Levitzki and Gazit, 1995) were used, and their specificity was tested by using the inactive structural analogs of these compounds (daidzein for genistein and tyrphostin A1 for tyrphostin A23). To prevent Erk-1 and Erk-2 activation, we used the specific MAP kinase kinase (MEK) inhibitor PD 98059 (Dudley et al., 1995). We report that hypo-osmotic solution induced an outwardly rectifying Cl− current, which was [Ca2+]i-independent and [ATP]i-dependent. Tyrosine kinase and MEK inhibitors reversibly blocked activation of volume-sensitive Cl− currents. In addition, Western immunoblots showed that activation of Erk-1 and Erk-2 by hypo-osmotic solution depended on the activation of tyrosine kinases and MEK. In conclusion, the volume-sensitive Cl− channel appears to be a cytoplasmic target for the MAP kinase signaling pathway.
MATERIALS AND METHODS
Cell culture. Astrocyte cultures were prepared from 1-d-old Sprague Dawley rats (University of Calgary, Alberta, Canada), using modifications of standard techniques (McCarthy and Devellis, 1980; Merrill et al., 1984). All procedures conformed to guidelines laid down by the Canadian Council on Animal Care. Briefly, neonates were decapitated and the brain exposed. The cortex was dissected free of the underlying brain tissue under sterile conditions, and the meninges and pia mater were removed. The tissue was dissociated by mechanical trituration, and the resulting cell suspension was plated onto glass coverslips and grown in DMEM and Ham’s F12 (1:1) with 10% fetal calf serum. Medium was changed twice per week. As previously described (MacVicar et al., 1991), immunocytochemical characterization of cultures indicated that >95% of cells stained positive for glial fibrillary acidic protein, confirming that the primary cell type present is astrocytes (data not shown).
Recording procedures. Coverslips with adherent glial cells were placed in a recording chamber (volume, ∼200 μl) and mounted on an inverted microscope (Zeiss, Oberkochen, Germany). Cells were observed via phase-contrast optics and superfused at 1–2 ml/min (20–22°C). Standard extracellular salt solution for recording anion currents was composed of (in mm): 70 Trizma-HCl, 100 sucrose, 1.5 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.3 with CsOH. TEA (5 mm) and BaCl2 (5 mm) were added to the extracellular solution to eliminate K+ currents. Solution osmolarity was determined by a vapor pressure osmometer (5120C, Wescor, Logan, UT). The extracellular solution was between 280 and 290 mOsm/l. In experiments testing hypo-osmotic stimulation (HOS), the sucrose was removed from the bathing solution (220 mOsm/l). We observed that, in this hypo-osmotic condition, astrocytes swelled as previously reported (Pasantes-Morales et al., 1994; Lascola and Kraig, 1996).
Ionic currents were recorded with the patch-clamp technique. Patch pipettes were pulled from borosilicate thin-wall glass capillaries (TW150F-4, World Precision Instruments, Sarasota, FL) with the Flaming/Brown micropipette puller (P-97, Sutter Instrument, Novato, CA) and had tip resistances of 3–7 MΩ when filled with electrode solution. The standard pipette solution was composed of (in mm): 60 Trizma-HCl, 70 Trizma-base, 70 aspartic acid, 15 HEPES, 0.4 CaCl2, 1 MgCl2, 1 ATP, 0.5 GTP, and 1 EGTA, adjusted to pH 7.25 with CsOH. In some other experiments the intracellular concentration of Cl−was decreased by replacing 30 mm Trizma-HCl by a 30 mm concentration of the less-permeant anion aspartate (Lewis et al., 1993) in the electrode solution. Free Ca2+ in the standard pipette solution was estimated to be ∼100 nm. In those experiments in which Ca2+ was chelated by using 20 mm BAPTA in the pipette to replace EGTA, free Ca2+ was calculated to be reduced to <10 nm. To investigate current decay, we performed some experiments in the absence of ATP and/or GTP in the electrode solution.
Membrane currents were recorded in whole-cell configuration in the voltage-clamp mode (VH = −60 mV) with an Axopatch-1D amplifier (Axon Instruments, Foster City, CA). Currents were filtered with a four-pole low-pass Bessel filter (−3 dB at 5 kHz). Offset potentials were nulled, using the amplifier circuitry before seals were made on cells. Liquid junction potentials (LJP) between the bath and patch-clamp electrodes were measured experimentally and defined as the potential of the bath with respect to the electrode solution (Barry and Lynch, 1991). For whole-cell recording the membrane potential of the cell,Vm, was calculated as:Vm = Ve −LJP. When the extracellular solution contained 88 mm Cl− and the intracellular solution contained 63 or 33 mm Cl−, the LJP was 1 or 2–3 mV, respectively. The membrane potential was not corrected for the LJP, because the variation of potential was minor.
Cell capacitance and series resistance values were measured and compensated by using the circuitry of the amplifier. The intrinsic membrane input resistance of the cell was monitored by the application of hyperpolarizing voltage steps of 10 mV (500 msec duration) within the initial 5–10 min of the experiment. The voltage dependence of ionic currents was studied by using voltage ramp commands: the membrane potential was stepped from VH = −60 to −120 mV, held at −120 mV for 50 msec, and then ramped to + 80 mV in 2 sec. The efficacy of the voltage clamp during the ramp command was tested by comparing the I/V curve obtained with the voltage ramp with that obtained with voltage steps (10 mV amplitude increments and 500 msec duration). Because no difference was found between I/Vcurves obtained by using either ramp or step commands over the voltage range tested (−120 to +80 mV), only I/V curves obtained with the voltage ramp commands are shown in this report.
Data analysis. Membrane currents were digitized every 20 sec with pClamp software (Axon Instruments). Continuous recordings of current and voltage were displayed on a chart recorder (RS 3200, Gould, Cleveland, OH). Data were analyzed off-line and are presented as mean ± SEM. Statistical analyses were performed with Student’s paired t test, and data were considered significantly different at p < 0.05.
Western immunoblotting. Confluent astrocyte cultures were starved in serum-free DMEM for 24–30 hr. After this period the cultures were transferred to a defined medium containing (in mm) 110 NaCl, 58 NaCO3, 25 glucose, 20 HEPES, 5 KCl, 0.6 MgSO4, 0.9 NaHPO4, and 1.8 CaCl2 for a further 8 hr. Hypo-osmotic treatment consisted of lowering the extracellular NaCl concentration from 110 to 33 mm by adding NaCl-free medium to the cultures. This represented a drop in osmolarity of ∼30%, as measured by vapor pressure osmometry. For the iso-osmotic condition, NaCl-free medium was supplemented with mannitol to maintain original osmolarity while still controlling for the drop in salt concentration. After stimulation with hypo-osmotic solution, cultures were washed with ice-cold PBS and harvested on ice with protein sample buffer (10% glycerol, 62.5 mm Tris-base, 2% SDS, and 2.5% β-mercaptoethanol). Then the samples were boiled, sonicated briefly, and subjected to SDS-Page (Laemmli, 1970) on 15% w/v acrylamide gels. The protein was transferred to polyvinylidene fluoride (PVDF) at 70 mA overnight. After transfer, the membranes were blocked in TTBS (0.05% Tween 20, 25 mm Tris-base, and 62.5 mm NaCl, solution pH-adjusted to 7.5) containing 5% w/v skim milk and then incubated in primary antibody. For MAP kinase blots, polyclonal anti-rat MAP kinase R2 (Erk-1-CT; 1:2000 dilution) was used in TTBS with 3% nonfat dry milk; the incubation time was ∼2 hr at room temperature. The monoclonal anti-phosphotyrosine antibody 4G10 was used for phosphotyrosine blots at a dilution of 1:1000 in 1% BSA in TTBS and incubated overnight at room temperature.
After being incubated with the primary antibodies, membranes were washed and incubated in secondary antibodies. For blots in which polyclonal primary antibodies were used, the secondary antibody was an anti-rabbit IgG, horseradish peroxidase-linked (H+L) whole antibody (1:3000). For monoclonal primary antibodies, the secondary antibody was goat anti-mouse IgG (H+L) diluted at 1:5000. All secondary antibodies were diluted in the same buffer as their respective primary antibodies, and membranes were incubated in the secondary antibodies for 2 hr at room temperature.
After secondary antibody incubation, membranes were washed and probed with the Supersignal Substrate Western Blotting ECL detection kit.
Materials. All culture reagents were purchased from Life Technologies (Burlington, Ontario). Genistein, daidzein, tyrphostin A23, tyrphostin A1, and PD 98059 were purchased from Calbiochem (La Jolla, CA). 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). 4-Acetamido-4"-isothiocyanatostilbene-2,2′-disulfonic acid (SITS) and all other drugs were purchased from SIGMA (Oakville, Ontario). Primary antibodies for Western blotting were obtained from Upstate Biotechnology (Lake Placid, NY). Secondary antibodies were obtained from Amersham (Oakville, Ontario; anti-rabbit IgG) and Bio-Rad laboratories (Mississauga, Ontario; goat anti-mouse IgG).
RESULTS
The recordings were performed on flat polygonal or stellate astrocytes. After the whole-cell recording configuration was established, the membrane current was recorded in voltage-clamp mode at a holding potential of −60 mV. Data presented here were selected from cells that had a mean membrane capacitance and input resistance of 40.4 ± 3.7 pF (n = 35) and 2.32 ± 0.4 GΩ (n = 35), respectively.
Hypo-osmotic stimulation activates a Cl−current in cultured astrocytes
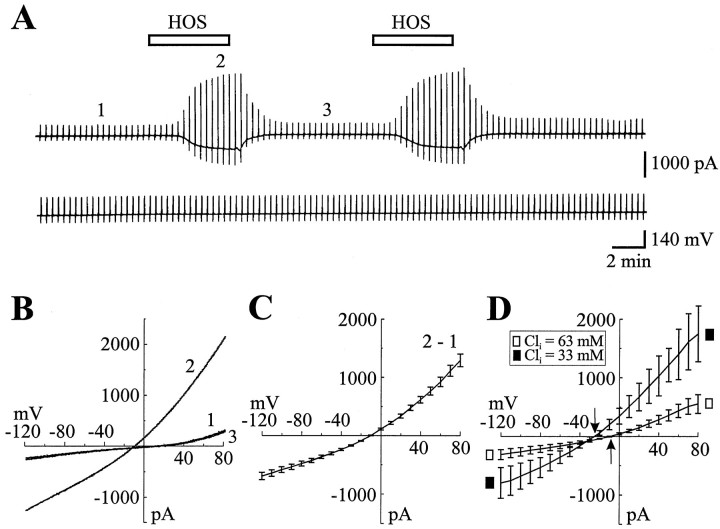
As shown in Figure1A, a short (3–5 min) superfusion of cells with HOS resulted in the reversible activation of an inward current at −60 mV. The voltage dependence of HOS-induced current was studied by plotting I/V relations that were derived with a 2 sec depolarizing ramp command from −120 to +80 mV. Figure 1B shows the I/V plots for currents recorded before (1), during (2), and after (3) recovery from HOS exposure. The I/V curve of HOS-induced current was obtained by subtracting the peak current recorded during HOS application from that recorded before HOS application (Fig. 1C). The HOS-induced current was outwardly rectifying and reversed at approximately −10 mV. The slope conductance calculated for two representative membrane potential intervals, −120 to −60 mV (referred to as negative conductance) and +20 to +80 mV (referred to as positive conductance), was 5.26 ± 0.52 nS (n = 35) and 15.3 ± 1.29 nS (n = 35), respectively.
Fig. 1.
Hypo-osmotic stimulation activates an outwardly rectifying Cl− current in cultured astrocytes.A, Membrane current recorded in response to voltage ramp commands (from −120 to + 80 mV, 2 sec duration, 0.05 Hz) before and during two successive hypo-osmotic (HOS) stimulations (5 min duration, VH = −60 mV) in a cultured astrocyte with (in mm) 63 Cl−, 2 ATP, and 0.5 GTP in the pipette solution. In this and the following figures the top and the bottom traces illustrate the current and the voltage traces, respectively. B, Individual current–voltage (I/V) relations obtained at the times indicated by the numbers inA. C, Mean I/V relations of HOS-induced current obtained by subtracting the peak current recorded during HOS application (2) from that recorded before HOS application (1) (n = 35). D, Mean I/Vrelations of HOS-induced current obtained with either 33 mmCl− (▪, n = 9) or 63 mm Cl− (□, n = 14) in the pipette solution (minus ATP and GTP). Note that HOS-induced current was outwardly rectifying and reversed at approximately −25 mV (downward arrow, D) or −10 mV (upward arrow, D) with either 33 or 63 mm Cl− in the pipette solution, respectively. In this and the following figures, the error bars represent the SEM.
To characterize the ionic selectivity of HOS-induced current, we examined the change of the reversal potential of this current with alterations in the transmembrane Cl− gradient. Cells were held at −60 mV and ramped from −120 to +80 mV in standard extracellular Ringer’s ([Cl]out = 88 mm) with either 33 or 63 mm [Cl−] in the electrode solution (and no ATP and GTP). A reduction in [Cl−]in shifted the reversal potential of HOS-induced current to more negative potentials. Figure1D shows that the reversal potential of the current was −9.5 ± 0.8 mV (n = 14) in 63 mmCl− and −24 ± 1.6 mV (n = 9) in 33 mm [Cl−]i. These values are nearly identical to those predicted by the Nernst equation (−8.6 and −25.4 with [Cl−]in = 63 and 33 mm, respectively) and confirm that the HOS-induced current is carried mainly by Cl− in our experimental conditions.
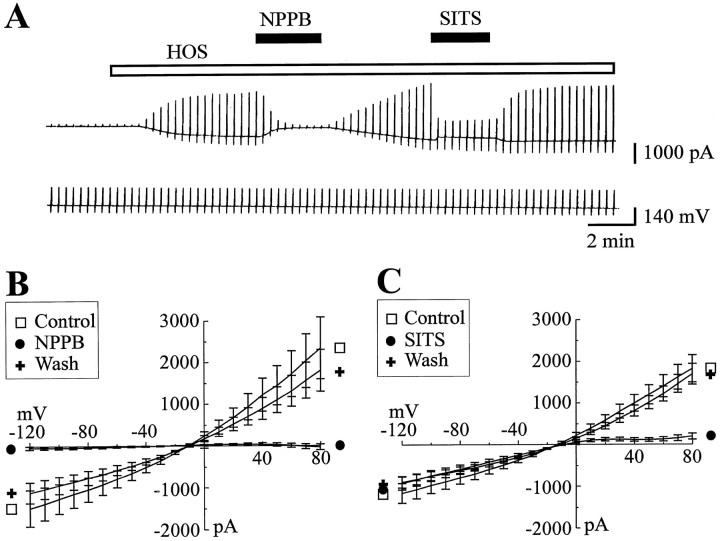
To characterize HOS-induced current further, we tested the effect of the Cl− channel blockers NPPB and SITS on this current. As shown in Figure2A, a 3 min external application of either NPPB (0.1 mm) or SITS (0.5 mm) rapidly and reversibly decreased HOS-induced current. Figure 2, B and C, shows the I/Vrelations recorded for HOS-induced current in the absence (Control) and presence of either NPBB or SITS. NPPB reduced HOS-induced current over the full range of potentials tested, with a similar decrease in both the negative conductance (by 89 ± 7.5%; p = 0.026; n = 5) and the positive conductance (by 93 ± 5.2%; p = 0.018;n = 5). The SITS block of the HOS-induced current, in contrast, was strongly voltage-dependent, as previously described (Kelly et al., 1994; Arreola et al., 1995). In the presence of SITS the positive HOS conductance was reduced by 94.3 ± 2.75% (p < 0.001; n = 11), but the negative conductance was not significantly modified (−0.5 ± 5.9%; p = 0.75; n = 11). Thus, under our experimental conditions, hypo-osmotic solution, which induces cell swelling, activates a Cl− current that will be referred to hereafter as volume-activated Cl−current or ICl,vol, as previously named in the literature (for review, see Nilius et al., 1996).
Fig. 2.
ICl,vol is blocked by NPPB and SITS. A, Membrane current and conductance changes evoked by a long HOS application (23 min in duration,VH = −60 mV) recorded in the absence or in the presence of either NPPB (0.1 mm) or SITS (0.5 mm) in the external media [and (in mm) 63 Cl−, 2 ATP, and 0.5 GTP in the pipette solution].B, C, Mean I/V relations of HOS-induced current obtained before (□), during (•), and after (+) bath application of either NPPB (B;n = 5) or SITS (C;n = 11). Note that HOS-induced current was strongly reduced by NPPB in a voltage-independent manner and by SITS in a voltage-dependent manner.
Activation of ICl,vol does not require a change in intracellular Ca2+
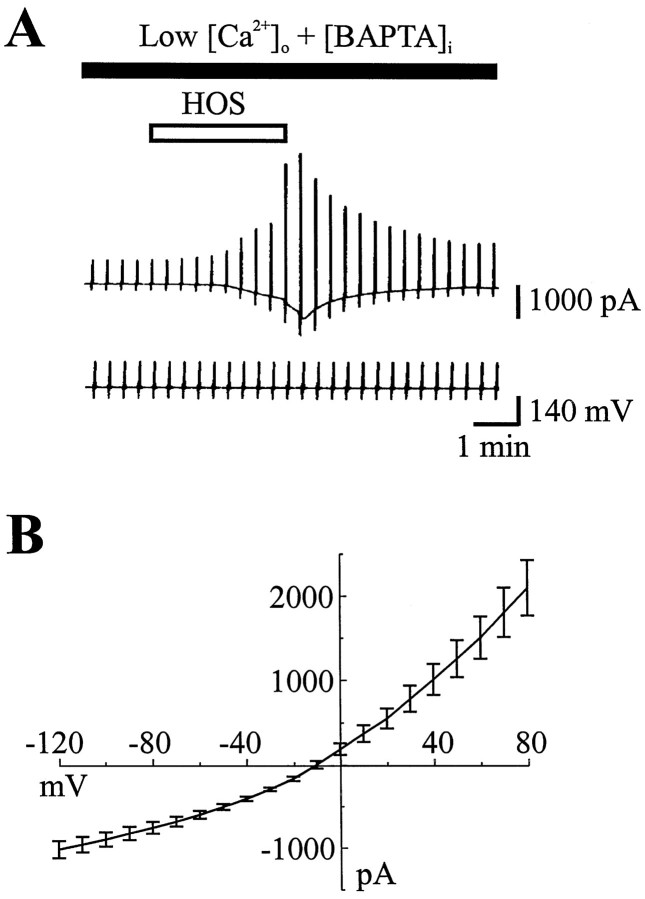
Because cell swelling induced by a hypo-osmotic shock is known to be associated with an increase of internal Ca2+([Ca2+]i) in some cell types (for review, see Hoffmann, 1992), we studied the dependence ofICl,vol on [Ca2+]i. For these experimentsICl,vol was recorded in the presence of the Ca2+ chelator BAPTA (20 mm) in the pipette solution and a low external concentration of Ca2+ (0.15 mm) to prevent a rise of [Ca2+]in during the cell swelling. Figure 3A shows whole-cell currents recorded from a representative cell exposed to a 3 min application of HOS in low [Ca2+]outwith 20 mm BAPTA/63 mm[Cl−] in the electrode. HOS still resulted in reversible activation of ICl,vol under these conditions. Figure 3B shows the mean I/V curve obtained for the peak current activated by HOS in five cells. Current amplitudes were comparable when recorded in either a low external concentration of Ca2+ with BAPTA in the pipette solution (67 ± 14.5 pA/pF at +80 mV, n = 5) or in standard Ca2+ containing external solutions with no BAPTA in the electrode solution (44 ± 6.4 pA/pF at +80 mV,n = 27). These data confirm that the activation ofICl,vol is independent of an increase in [Ca2+]i, as previously described in other systems (Hagiwara et al., 1992; Nilius et al., 1996) (for review, see Strange et al., 1996).
Fig. 3.
Activation of ICl,volis independent of a change in intracellular Ca2+.A, Membrane current evoked by an HOS application (3 min duration, VH = −60 mV) recorded in the presence of the Ca2+ chelator BAPTA (20 mm) with (in mm) 63 Cl−, 2 ATP, and 0.5 GTP in the pipette solution. The external concentration of Ca2+ was reduced to 0.15 mm.B, Mean I/V relations ofICl,vol recorded in five separate cells under the same conditions as for currents shown in A(n = 5). Note that, in the presence of the Ca2+ chelator BAPTA in the pipette solution and a low external concentration of Ca2+, the HOS stimulation still evoked an outwardly rectifying current that reversed at approximately −10 mV.
Activation of ICl,vol depends on the cellular metabolic state
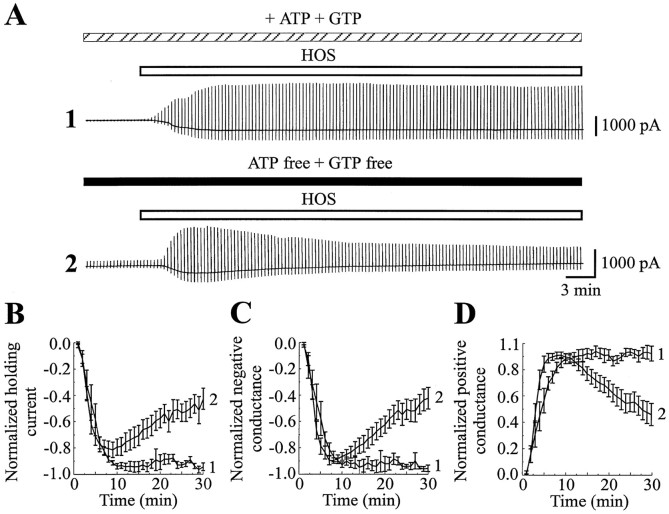
We next investigated other possible mechanisms involved in the regulation of ICl,vol. Studies performed in kidney, T lymphocytes, and endothelial cells have shown that activation of volume-sensitive anion currents depends on the metabolic state of the cell and is linked to cellular nucleotide levels (Lewis et al., 1993; Jackson et al., 1994; Oike et al., 1994; Strange et al., 1996). To test this hypothesis in astrocytes, we comparedICl,vol in the presence and the absence of ATP and GTP in the pipette solution (Fig. 4). We showed that under control conditions, with 2 mm ATP and 0.5 mm GTP in the pipette solution (Fig.4A, 1), a long exposure to HOS (at least 30 min) resulted in the activation of a stableICl,vol, which reached a maximal amplitude of 45.4 ± 9.2 pA/pF (at +80 mV, n = 6) in 16 ± 3.5 min (n = 6) and showed no decline over the period of HOS exposure. In contrast,ICl,vol, recorded during 30 min of HOS in the absence of ATP and GTP in the pipette solution (Fig.4A, 2), reached a maximal amplitude of 16.3 ± 2.9 pA/pF (at +80 mV, n = 10) in 7.8 ± 0.72 min (n = 10) but then progressively declined at a rate of 2.25 ± 0.2% per minute (n = 10). In the absence of ATP and GTP the current amplitude ran down by one-half in 16.5 ± 2.4 min (n = 10), and the maximal amplitude of ICl,vol (16.33 ± 2.9 pA/pF at +80 mV, n = 10) was significantly smaller than that recorded under control conditions (45.4 ± 9.2 pA/pF at +80 mV;n = 6; p < 0.0025). To discriminate between ATP binding and hydrolysis, we recorded the rundown ofICl,vol while including AMP–PNP, a nonhydrolyzable form of ATP, in the pipette solution. GTP also was included in the pipette. In these conditions the rundown ofICl,vol still occurred, indicating that hydrolysis of ATP was necessary (n = 10; data not shown). Therefore, the activation of ICl,volapparently involves phosphorylation processes.
Fig. 4.
ICl,vol is regulated by the cellular nucleotides. A, Membrane current evoked by a long HOS application (30 min in duration,VH = −60 mV) recorded in the presence (1) or in the absence (2) of ATP (2 mm) and GTP (0.5 mm) in the pipette solution. B–D, Graphs showing the changes of the normalized holding current (B), negative conductance (C), and positive conductance (D) of ICl,vol versus time during the HOS application (time 0 corresponds to the beginning of the HOS application), either in the presence (1; n = 6) or in the absence (2; n = 10) of ATP and GTP in the pipette solution. The positive and negative conductances were calculated for potentials ranging from +20 to +80 mV and −120 to −60 mV, respectively. The holding current and the negative and positive conductances have been normalized to 1, using their maximal values obtained during the HOS application. Note that activation ofICl,vol was stable in the presence of ATP and GTP. In contrast, when ATP and GTP were omitted from the pipette solution, ICl,vol progressively declined.
Tyrosine and MAP kinases are involved in the regulation ofICl,vol
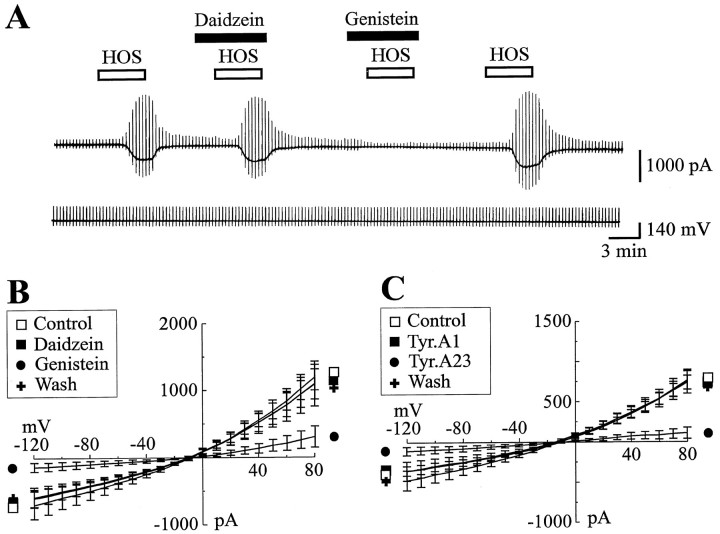
Previous studies on other cell types have shown that volume-activated Cl− currents can be modulated by tyrosine kinase-dependent phosphorylation (Tilly et al., 1993; Sorota, 1995; Strange and Jackson, 1995). To investigate the role of tyrosine kinases in the modulation of ICl,vol in cultured astrocytes, we performed successive short (3–5 min) applications of HOS in the presence or absence of different tyrosine kinase inhibitors (with 2 mm ATP and 0.5 mm GTP in the pipette solution). First, we established that repeated applications (up to five) of HOS consistently activated ICl,vol with no decrement in current amplitude (data not shown). Second, as illustrated in Figure 5, we activatedICl,vol with HOS in the absence or presence of the tyrosine kinase inhibitor genistein (50 μm) and its inactive analog, daidzein (50 μm), at concentrations according to previous studies (for review, see Levitzki and Gazit, 1995). Figure 5A shows that, in the presence of daidzein, HOS-activated ICl,vol was comparable to that seen in absence of the drug, whereas concurrent application of HOS and genistein not only prevented activation ofICl,vol but also further decreased the control current. The effects of genistein were reversible because HOS activatedICl,vol within 7 min of genistein washout. Figure 5B shows I/V relations forICl,vol measured from ramp commands in the absence (Control) or presence of daidzein and genistein and after drug washout. Genistein decreased the positive and negative conductance of ICl,vol by 68 ± 14% (p = 0.015; n = 5) and by 69.9 ± 11.5% (p = 0.022;n = 5), respectively.
Fig. 5.
Tyrosine kinases are involved in the activation ofICl,vol. A, Membrane current evoked by four successive HOS stimulations (5 min in duration,VH = −60 mV) recorded in the presence or in the absence of either daidzein (50 μm) or genistein (50 μm) in the external media [and (in mm) 63 Cl−, 2 ATP, and 0.5 GTP in the pipette solution].B, Mean I/V relations ofICl,vol obtained in five cells before (□), during, and after (+) bath application of either daidzein (50 μm, ▪) or genistein (50 μm, •).C, Mean I/V relations ofICl,vol obtained in seven cells before (□), during, and after (+) bath application of either tyrphostin A1 (50 μm, ▪) or tyrphostin A23 (50 μm, •). Note that genistein and tyrphostin A23 strongly and reversibly reduced ICl,vol, in contrast to their inactive analogs, daidzein and tyrphostin A1, which had no significant effect on ICl,vol.
Similar experiments also were performed with another tyrosine kinase inhibitor, tyrphostin A23 (50 μm), and its inactive analog, tyrphostin A1 (50 μm), at concentrations according to previous studies (for review, see Gazit et al., 1989) (Fig. 5C). Tyrphostin A23 decreased the positive and negative conductance of ICl,vol by 82.1 ± 6.3% (p = 0.001; n = 7) and by 76 ± 9.5% (p = 0.0026; n= 7), respectively. Interestingly, like genistein (see Fig.5A), tyrphostin A23 acted very rapidly onICl,vol (within 3 min of preincubation), and, like genistein, the effects of this drug were fully reversible. The inhibitory action of genistein and tyrphostin A23 onICl,vol was specific to activation of a tyrosine kinase, because we did not obtain a significant change ofICl,vol in the presence of the inactive analogs of either genistein (daidzein) or tyrphostin A23 (tyrphostin A1). Daidzein (50 μm) changed the positive and the negative conductance of ICl,vol by −5.5 ± 10.5% (p = 0.41; n = 5) and by −5.4 ± 14.3% (p = 0.65;n = 5), respectively, whereas tyrphostin A1 (50 μm) changed the positive and negative conductance ofICl,vol by −7.3 ± 17.7% (p = 0.27; n = 5) and by −12.0 ± 5.2% (p = 0.11;n = 5), respectively.
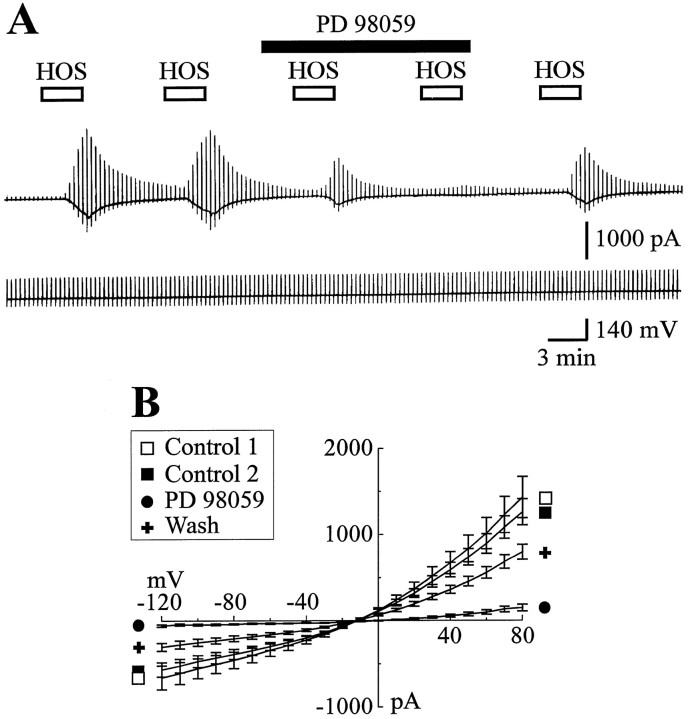
Some tyrosine kinase signaling pathways can lead to MAP kinase activation (Malarkey et al., 1995). Recent studies performed in different cell types have shown that hypotonic stress activates MAP kinases (Schliess et al., 1995, 1996; Noé et al., 1996; Tilly et al., 1996). To test whether MAP kinase activation is integral to the activation of ICl,vol, we exposed astrocytes to successive short (3–5 min) applications of HOS in the presence or in the absence of the MEK inhibitor PD 98059 (50 μm) at a concentration according to previous studies (Alessi et al., 1995; Dudley et al., 1995). As shown in Figure6A, 50 μmPD 98059 strongly inhibited ICl,vol, and continuous exposure (10–15 min) to PD 98059 prevented the activation of HOS current. In contrast to the tyrosine kinase inhibitors, only a partial recovery of ICl,vol was obtained after PD 98059 exposure. The slow effect and recovery from the actions of the MEK inhibitor are consistent with previous reports of the pretreatment time necessary for inhibitory actions (Alessi et al., 1995; Dudley et al., 1995). Figure 6B shows I/V relations for the maximal current activated during two control applications of HOS (in the absence of drug), during HOS stimulation after an initial 12–15 min exposure to PD 98059, and after washout of the MEK inhibitor. Compared with controls, PD 98050 decreased the positive and negative conductance of ICl,vol by 83.7 ± 6.2% (p = 0.005; n = 6) and by 88 ± 4.7% (p = 0.009; n = 6), respectively.
Fig. 6.
MAP kinase is involved in the activation ofICl,vol. A, Membrane current evoked by five successive HOS stimulations (3 min and 30 sec in duration, VH = −60 mV) recorded in the presence or in the absence of 50 μm PD 98059 in the external media [and (in mm) 63 Cl−, 2 ATP, and 0.5 GTP in the pipette solution]. B, MeanI/V relations of ICl,volobtained in six cells before (Control 1, □;Control 2, ▪), during (•), and after (+) bath application of PD 98059. Note that PD 98059 strongly inhibitedICl,vol and that this inhibition was partially reversible during wash.
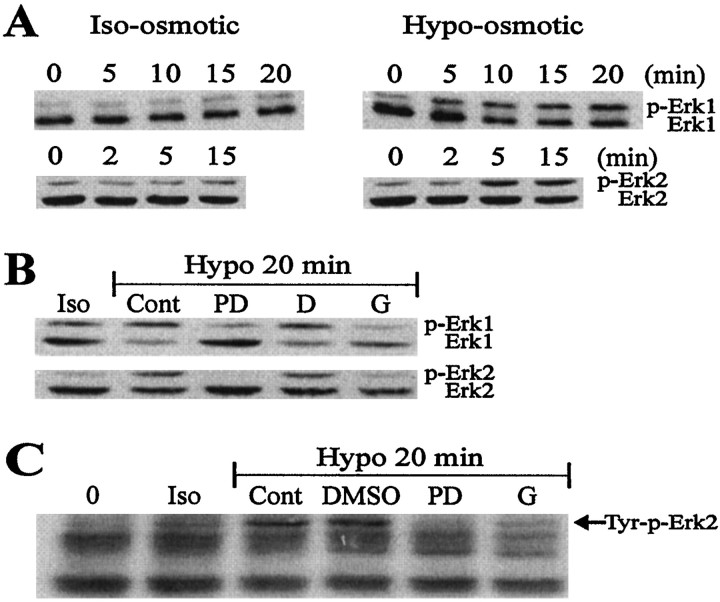
To characterize the link between astrocyte swelling and the activity of MAP kinase further, we sought to determine whether treatments affectingICl,vol similarly affected MAP kinase activation. To this end we used a gel shift assay to monitor the activation of MAP kinases. Activation of Erk-1 and Erk-2 by phosphorylation decreases their electrophoretic mobilities; this “shift” can be visualized in Western blots because the activated forms run at an increased apparent molecular weight. Figure7A depicts the response of Erk-1 and Erk-2 to hypo-osmolarity. In untreated, starved cultures (at time 0), both Erk-1 and Erk-2 appeared as distinct bands with approximate molecular weights of 44 and 42 kDa, respectively. Hypo-osmotic solution induced an evident shift in mobility of both Erk-1 and Erk-2 by 5 min, with maximal effect by 15–20 min. This is denoted by the appearance of the upper bands, representing the activated forms of these proteins. Under isotonic conditions there was a very slight increase in activated (phosphorylated) Erk-1 and Erk-2. This possibly resulted from the cellular stress associated with the addition of new iso-osmotic medium (Schliess et al., 1996). We next examined the role of tyrosine kinases and MEK on Erk activation by hypo-osmotic treatment. The tyrosine kinase inhibitor genistein (50 μm) markedly depressed the mobility shift of Erk-1 and Erk-2 when it was applied 15 min before and during stimulation with hypo-osmotic solution (Fig. 7B), whereas the inactive analog, daidzein, had no effect. Similarly, the MEK inhibitor PD 98059 (50 μm) also inhibited Erk-1 and Erk-2 activation in hypo-osmotic solution (Fig. 7B).
Fig. 7.
Activation of MAP kinase in response to hypo-osmotic conditions is sensitive to tyrosine kinase and MEK inhibition. Astrocyte cultures were exposed to either iso-osmotic conditions (Iso) or hypo-osmotic conditions (Hypo) with or without other treatments for the time periods indicated. Whole-cell extracts were separated by SDS-Page, transferred to PVDF, and immunoblotted with either an anti-MAP kinase antibody (A and B) or an anti-phosphotyrosine antibody (C).A indicates that the gel shift of p44 Erk-1 and p42 Erk-2 was greater in the Hypo condition, indicating activation. In B and C, the cultures were subjected to a number of different treatments as indicated: PD 98059 (PD, 50 μm), 30 min preincubation followed by stimulation; DMSO 0.1%, 30 min preincubation followed by stimulation; and daidzein (D, 50 μm) and genistein (G, 50 μm), 15 min preincubation followed by stimulation. The decrease in the intensity of theupper bands representing activated Erk-1 and Erk-2 (B) indicates that MAP kinase activity was attenuated in these treatments. In C, the increased tyrosine phosphorylation of Erk-2 in hypo-osmotic solution is apparent at 20 min. PD 98059 and genistein dramatically reduced tyrosine phosphorylation of Erk-2. Other apparently immunoreactive proteins were not affected by these treatments (C).
Because tyrosine phosphorylation of MAP kinase is a prerequisite for activity, the presence of enhanced tyrosine phosphorylation of the Erks has been used as an indicator of kinase activation. Anti-phosphotyrosine Western blotting confirmed the activation of Erk-2 in response to hypo-osmotic conditions, as well as the inhibitory effect of genistein and PD 98059 (Fig. 7C). The Western blots of Erk-1 were too faint to give a clear indication of activity changes.
DISCUSSION
The present report describes the second messenger systems modulating an outwardly rectifying Cl− current activated by hypo-osmotic solution in cultured astrocytes. Current activation was correlated with phosphorylation processes but did not require changes in intracellular Ca2+. This study demonstrates that activation of tyrosine and MAP kinases plays a crucial role in the activation of the swelling-activated Cl− current. Western immunoblots confirmed that MAP kinases were activated by swelling and showed that activation of MAP kinases depended on the activation of tyrosine kinases and MEK. This study provides the first demonstration that MAP kinases and tyrosine kinases are involved in the activation of the volume-activated Cl− current in astrocytes.
Properties of ICl,vol in cultured astrocytes
Activation of ion channels appears to be a primary step in the regulatory volume decrease (RVD) that follows an increase in cell volume. In many cells RVD requires coactivation of K+ and Cl− channels and a net efflux of KCl (Roy and Banderali, 1994) (for review, see Nilius et al., 1996; Strange et al., 1996). Activation of volume-activated Cl− channels has been described in a variety of cell types in response to challenge with extracellular hypotonic solution (for review, see Nilius et al., 1996; Strange et al., 1996), increased pressure applied to the cell membrane (Hagiwara et al., 1992), or morphological changes (Lascola and Kraig, 1996). The characteristics of the volume-activated Cl− current vary among cells, but, in general, the current is outwardly rectifying and does not appear to be mediated by intracellular Ca2+ (for review, see Nilius et al., 1996; Strange et al., 1996). Volume-activated anion currents have been described in astrocytes cultures, astrocytomas, and glioma cells (Pasantes-Morales et al., 1994; Bakhramov et al., 1995; Strange and Jackson, 1995;Lascola and Kraig, 1996). In the present study we characterized the Cl− current activated by swelling with HOS in cultured cortical astrocytes. All experiments were performed in the absence of Na+ and K+ in the internal and external media and in the presence of K+ channel blockers in the internal (Cs+) and the external media (TEA and Ba2+). We showed that bath application of HOS solution activated an outwardly rectifying current, which was blocked by Cl− channel inhibitors (NPPB and SITS) and displayed a reversal potential close to the predicted chloride equilibrium potential calculated with the Nernst equation. Current activation was independent of intracellular [Ca2+] changes, because its activation also occurred in the presence of a Ca2+ chelator (BAPTA) in the internal pipette solution.
Therefore, the Cl− current activated by hypo-osmotic-induced swelling in cultured astrocytes has similar features to Cl− currents previously described in astrocytes and other cell types (Lascola and Kraig, 1996) (for review, see Nilius et al., 1996; Strange et al., 1996).
The activation of ICl,vol involves phosphorylation processes and the MAP kinases signaling pathway
The stable activation of volume-sensitive Cl−currents in many, but not all, cell types requires intracellular ATP (but see Kelly et al., 1994; Leaney et al., 1997). For example, in cardiac and cortical-collecting duct cells, the hydrolyzable form of ATP is required (Sorota, 1995; Meyer and Korbmacher, 1996), but in other cells types nonhydrolyzable ATP analogs can substitute for ATP (Jackson et al., 1994; Oike et al., 1994; Oiki et al., 1994). It has been suggested that ATP is linked to a nonhydrolytic binding site (for review, see Strange et al., 1996). To clarify the role of ATP in the activation of ICl,vol in cultured astrocytes, we performed a set of experiments either in the presence or in the absence of ATP or in the presence of the nonhydrolyzable ATP analog, AMP–PNP. We report that, in cultured astrocytes, ICl,volactivation depends on the hydrolyzable form of ATP in the internal solution, because a stable activation of ICl,volwas obtained in the presence of ATP, but not in the absence of ATP or in the presence of AMP–PNP, a nonhydrolyzable ATP analog. This clearly suggests that in cultured astrocytes the activation ofICl,vol involved phosphorylation-dependent processes.
The role of phosphorylation processes in the activation of the volume-activated Cl− current has been studied extensively. Although it is well established in many cells that PKC and PKA signaling pathways are not involved in the activation of this current (Hagiwara et al., 1992; Kelly et al., 1994; Nilius et al., 1994; Gosling et al., 1995; Szucs et al., 1996; Leaney et al., 1997) (but see Schwiebert et al., 1994; Kinard and Satin, 1995; Verdon et al., 1995), they may be involved in its modulation (McCann et al., 1989; Hardy et al., 1994; Du and Sorota, 1997). The role of tyrosine kinase signaling pathways also has been tested, and it was shown that tyrosine kinase activation is a key step in the activation of the volume-sensitive Cl− conductance in Intestine 407 and cardiac cells (Tilly et al., 1993; Sorota, 1995), but not in other cell types (Gosling et al., 1995; Szucs et al., 1996). In astrocytes the role of tyrosine kinases in the activation ofICl,vol has not been studied. However, because biochemical studies have demonstrated clearly that MAP kinase is activated by swelling (Tilly et al., 1993; Schliess et al., 1995, 1996;Noé et al., 1996) and because MAP kinases are linked to some tyrosine kinase signaling pathways (for review, see Graves et al., 1995), we hypothesized that both tyrosine and MAP kinase activation are necessary for the activation of ICl,vol in astrocytes.
In the present study we show that, as in cardiac cells (Sorota, 1995),ICl,vol activation in astrocytes depends on the activation of tyrosine kinases. In the presence of specific inhibitors of tyrosine kinases (genistein and tyrphostin A23),ICl,vol was reversibly depressed while the respective inactive structural analogs of these compounds, daidzein and tyrphostin A1 (Negrescu et al., 1995), were ineffective. Furthermore, we demonstrate that MAP kinases also are involved in the activation ofICl,vol, because the specific MEK inhibitor PD 98059 (Dudley et al., 1995) reversibly inhibited the current. Finally, we confirmed, using Western immunoblots, that MAP kinase phosphorylation was induced by the hypo-osmotic solution and that the activation of MAP kinases depended on tyrosine kinase and MEK activity, because it was blocked by the tyrosine kinase inhibitor genistein (and not by its structural inactive analog daidzein) and a specific inhibitor of MEK, PD 98059. These results point as well to the relationship of tyrosine kinases to MAP kinases. The tyrosine kinase inhibitors used in this study do not act directly on MEK (Cox et al., 1996); however, they inhibited the activation of MAP kinase, suggesting that they act upstream of MEK.
Several studies have shown that the swelling of astrocytes also induces changes in the cytoskeleton. Recent studies indicate that reorganization of the F-actin cytoskeleton may play an important role in the activation of ICl,vol (Schwiebert et al., 1994; Lascola and Kraig, 1996; Tilly et al., 1996), possibly via the regulatory protein plCln (Krapivinsky et al., 1994). It also has been shown that the p21rho signaling cascade, implicated in tyrosine kinase-mediated F-actin reorganization, is involved in the activation of ICl,vol(Lascola and Kraig, 1996). Although it is premature to speculate that the dependence of ICl,vol on tyrosine kinases may be linked to these events, the cytoskeleton does present an attractive future route for further study into signaling andICl,vol.
Concluding remarks
The Cl− permeability triggered by osmotic swelling is a requisite for RVD in astrocytes (Pasantes-Morales et al., 1994) as well as in other cell types (Nilius et al., 1996; Strange et al., 1996). Delineating the signaling pathways in the activation ofICl,vol may be important in understanding the mechanisms involved in RVD. In the present study we demonstrate that both tyrosine and MAP kinases play a crucial role in the activation ofICl,vol, and we show for the first time that the MAP kinase cascade is involved in the activation of an ionic channel. We also demonstrate that tyrosine kinases are likely upstream in the signaling pathway of the MAP kinase-dependent activation ofICl,vol in astrocytes. Further investigations will be required to determine the specific target of the tyrosine and MAP kinases involved in the activation ofICl,vol, as well as the sensor leading to the activation of these second messenger cascades during the cell swelling.
Footnotes
This work was supported financially by the Alberta Heritage Foundation for Medical Research (AHFMR), the Institut National de la Santéet de la Recherche Médicale (INSERM), and the Medical Research Council of Canada (MRC). B.A.M. is an MRC Senior Scientist and an AHFMR Scientist. M.E.M.K. was an AHFMR visiting scientist. We thank D. Feighan for technical assistance and for preparing astrocyte cultures. We also thank Drs. S. Williams and M. Hollenberg for helpful discussions.
Correspondence should be addressed to Dr. Brian A. MacVicar at the above address.
REFERENCES
- 1.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Arreola J, Melvin JE, Begenisich T. Volume-activated chloride channels in rat parotid acinar cells. J Physiol (Lond) 1995;484:677–687. doi: 10.1113/jphysiol.1995.sp020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhramov A, Fenech C, Bolton TB. Chloride current activated by hypotonicity in cultured human astrocytoma cells. Exp Physiol. 1995;80:373–389. doi: 10.1113/expphysiol.1995.sp003854. [DOI] [PubMed] [Google Scholar]
- 4.Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JS, Seger R, Graves JD, Graves LM, Jensen AM, Krebs EG. The MAP kinase cascade. Recent Prog Horm Res. 1995;50:131–159. doi: 10.1016/b978-0-12-571150-0.50011-1. [DOI] [PubMed] [Google Scholar]
- 6.Cox ME, Ely CM, Catling AD, Weber MJ, Parsons SJ. Tyrosine kinases are required for catecholamine secretion and mitogen-activated protein kinase activation in bovine adrenal chromaffin cells. J Neurochem. 1996;66:1103–1112. doi: 10.1046/j.1471-4159.1996.66031103.x. [DOI] [PubMed] [Google Scholar]
- 7.Du XY, Sorota S. Modulation of dog atrial swelling-induced chloride current by cAMP: protein kinase A-dependent and -independent pathways. J Physiol (Lond) 1997;500:111–122. doi: 10.1113/jphysiol.1997.sp022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 10.Gosling M, Smith JW, Poyner DR. Characterization of a volume-sensitive chloride current in rat osteoblast-like (ROS 17/2.8) cells. J Physiol (Lond) 1995;485:671–682. doi: 10.1113/jphysiol.1995.sp020761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves JD, Campbell JS, Krebs EG. Protein serine/threonine kinases of the MAPK cascade. Ann NY Acad Sci. 1995;766:320–343. doi: 10.1111/j.1749-6632.1995.tb26684.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara N, Masuda H, Shoda M, Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol (Lond) 1992;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy SP, Valverde MA, Goodfellow HR, Higgins CF, Sepulveda FV. Regulation of volume-activated chloride channels by protein kinase C-mediated phosphorylation of p-glycoprotein. Jpn J Physiol. 1994;44:S9–S15. [PubMed] [Google Scholar]
- 14.Hoffmann EK. Cell swelling and volume regulation. Can J Physiol Pharmacol. 1992;70:S310–S313. doi: 10.1139/y92-277. [DOI] [PubMed] [Google Scholar]
- 15.Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MEM, Dixon SJ, Sims SM. Outwardly rectifying chloride current in rabbit osteoclasts is activated by hypoosmotic stimulation. J Physiol (Lond) 1994;475:377–389. doi: 10.1113/jphysiol.1994.sp020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimelberg HK. Brain edema. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford UP; Oxford: 1995. pp. 919–935. [Google Scholar]
- 18.Kinard TA, Satin LS. An ATP-sensitive Cl− channel current that is activated by cell swelling, cAMP, and glyburide in insulin-secreting cells. Diabetes. 1995;44:1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- 19.Krapivinsky GB, Ackerman MJ, Gordon EA, Krapivinsky LD, Clapham DE. Molecular characterization of a swelling-induced chloride conductance regulatory protein, plCln. Cell. 1994;76:439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 20.Krause U, Rider MH, Hue L. Protein kinase signaling pathway triggered by cell swelling and involved in the activation of glycogen synthase and acetyl-CoA carboxylase in isolated rat hepatocytes. J Biol Chem. 1996;271:16668–16673. doi: 10.1074/jbc.271.28.16668. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lascola CD, Kraig RP. Whole-cell chloride currents in rat astrocytes accompany changes in cell morphology. J Neurosci. 1996;16:2532–2545. doi: 10.1523/JNEUROSCI.16-08-02532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaney JL, Marsh SJ, Brown DA. A swelling-activated chloride current in rat sympathetic neurones. J Physiol (Lond) 1997;501:555–564. doi: 10.1111/j.1469-7793.1997.555bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 25.Lewis RS, Ross PE, Calahan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacVicar BA, Hochman D, Delay MJ, Weiss S. Modulation of intracellular Ca2+ in cultured astrocytes by influx through voltage-activated Ca2+ channels. Glia. 1991;4:448–455. doi: 10.1002/glia.440040504. [DOI] [PubMed] [Google Scholar]
- 27.Malarkey K, Chilvers ER, Lawson MF, Plevin R. Stimulation by endothelin-1 of mitogen-activated protein kinases and DNA synthesis in bovine tracheal smooth muscle cells. Br J Pharmacol. 1995;116:2267–2273. doi: 10.1111/j.1476-5381.1995.tb15063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCann JD, Li M, Welsh J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989;94:1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrill JE, Kutsunai S, Mohlstrom C, Hofman F, Groopman J, Golde D. Astroglia and oligodendroglia proliferate in response to human T cell-derived factors. Science. 1984;224:1428–1430. doi: 10.1126/science.6610212. [DOI] [PubMed] [Google Scholar]
- 31.Meyer K, Korbmacher C. Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. J Gen Physiol. 1996;108:177–193. doi: 10.1085/jgp.108.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negrescu EV, de Quintana KL, Siess W. Platelet shape change induced by thrombin receptor activation. J Biol Chem. 1995;270:1057–1061. doi: 10.1074/jbc.270.3.1057. [DOI] [PubMed] [Google Scholar]
- 33.Nilius B, Eggermont J, Voets T, Droogmans G. Volume-activated Cl− channels [review]. Gen Pharmacol. 1996;27:1131–1140. doi: 10.1016/s0306-3623(96)00061-4. [DOI] [PubMed] [Google Scholar]
- 34.Noé B, Schliess F, Wettstein M, Heinrich S, Haussinger D. Regulation of taurocholate excretion by a hypo-osmolarity-activated signal transduction pathway in rat liver. Gastroenterology. 1996;110:858–865. doi: 10.1053/gast.1996.v110.pm8608896. [DOI] [PubMed] [Google Scholar]
- 35.Oike M, Droogmans G, Nilius B. The volume-activated chloride current in human endothelial cells depends on intracellular ATP. Pflügers Arch. 1994;427:184–186. doi: 10.1007/BF00585960. [DOI] [PubMed] [Google Scholar]
- 36.Oiki S, Kubo M, Okada Y. Mg2+ and ATP dependence of volume-sensitive Cl− channels in human epithelial cells. Jpn J Physiol. 1994;44[Suppl 2]:S77–S79. [PubMed] [Google Scholar]
- 37.Pasantes-Morales H, Murray RA, Lilja L, Moran J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. Am J Physiol. 1994;266:C165–C171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 39.Roy G, Banderali U. Channels for ions and amino acids in kidney cultured cells (MDCK) during volume regulation. J Exp Zool. 1994;268:121–126. doi: 10.1002/jez.1402680208. [DOI] [PubMed] [Google Scholar]
- 40.Schliess F, Schreiber R, Haussinger D. Activation of extracellular signal-regulated kinase Erk-1 and Erk-2 by cell swelling in H4IIE hepatoma cells. Biochem J. 1995;309:13–17. doi: 10.1042/bj3090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schliess F, Sinning R, Fisher R, Schmalenbach C, Haussinger D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem J. 1996;320:167–171. doi: 10.1042/bj3200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwiebert EM, Mills JW, Stanton BA. Actin-based cytoskeleton regulates a chloride channel and cell volume in a retinal cortical collecting duct cell line. J Biol Chem. 1994;269:7081–7089. [PubMed] [Google Scholar]
- 43.Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflügers Arch. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- 44.Strange K, Jackson PS. Swelling-activated organic osmolyte efflux: a new role for anion channels. Kidney Int. 1995;48:994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- 45.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 46.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 47.Szucs G, Heinke S, De greef C, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. The volume-activated Cl− current in endothelial cells from bovine pulmonary artery is not modulated by phosphorylation. Pflügers Arch. 1996;431:540–548. doi: 10.1007/BF02191901. [DOI] [PubMed] [Google Scholar]
- 48.Tilly BC, Van den Berghe N, Tertoolen LGJ, Edixhoven MJ, De Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993;268:19919–19922. [PubMed] [Google Scholar]
- 49.Tilly BC, Edixhoven MJ, Tertoolen LGJ, Morii N, Saitoh Y, Narumiya S, de Jonge HR. Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol Biol Cell. 1996;7:1419–1427. doi: 10.1091/mbc.7.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdon B, Winpenny JP, Whitfield KJ, Argent BE, Gray MA. Volume-activated chloride currents in pancreatic duct cells. J Membr Biol. 1995;147:173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]