Abstract
Configural theories of hippocampal function predict that hippocampal dysfunction should impair acquisition of the transverse patterning task, which involves the concurrent solution of three discrimination problems: A+ versus B−; B+ versus C−; and C+ versus A−. The present study tested this prediction in rats using computer-graphic stimuli presented on a touchscreen. Experiment 1 assessed the effects of fornix lesions when the three problems were introduced sequentially (phase 1: A+ vs B−; phase 2: A+ vs B−, B+ vs C−; phase 3: A+ vs B−, B+ vs C−, C+ vs A−). Fornix lesions significantly facilitated acquisition of the complete transverse patterning task (phase 3) but had no effect on the number of sessions or errors required to attain criterion during phase 1 or phase 2. In experiment 2, in which all three problems were presented concurrently from the outset of training, fornix-lesioned animals outperformed control animals during the seventh block of acquisition trials and were not impaired during any stage of acquisition. Importantly, these same animals were significantly impaired on two allocentric spatial tasks: T-maze alternation (experiments 1 and 2) and the Morris Swim Task (experiment 1). These results contradict the predictions of configural theories of hippocampal function and cast doubt on the popular notion that spatial learning is a special case of configural learning.
Keywords: hippocampus, fornix, learning, memory, discrimination, configural, transverse patterning, spatial learning, rat
General theories of hippocampal function need to explain how, in humans, hippocampal damage can disrupt episodic memory, whereas in rats such damage reliably produces allocentric spatial memory deficits. One attractive solution to this problem has been to posit a role for the hippocampal formation in “configural” or “relational” learning (see, for example,Wickelgren, 1979; Sutherland and Rudy, 1989; Eichenbaum et al., 1994;Rudy and Sutherland, 1995). One of the most influential of these theories has been “Configural Association Theory” (Sutherland and Rudy, 1989), which views the hippocampus as necessary for the formation of “configural” representations of stimuli. Careful testing of this theory, however, has revealed a growing number of contradictions (Saunders and Weizkrantz, 1989; Whishaw and Tomie, 1991; Gallagher and Holland, 1992; Davidson et al., 1993). In response, Rudy and Sutherland (1995) modified their original position by suggesting that configural representations lie in cortical regions outside the hippocampus, but that outputs from the hippocampus contribute to configural learning by increasing the discriminability between configural representations and the representations of the stimulus elements of which they are comprised. A configural task for which such a contribution is thought to be essential is the transverse patterning task (Rudy and Sutherland, 1995). In this task, animals must learn to solve three concurrent discrimination problems (A+ vs B−, B+ vs C−, and C+ vs A−). Because every individual stimulus is presented equally as an S+ or an S−, this task is thought to require the use of configural representations of stimulus compounds (e.g., AB) to guide the animals’ choice response (Rudy and Sutherland, 1995). In the present study, therefore, we examined the effects of fornix transection on the transverse patterning task to test the predictions of Rudy and Sutherland (1995).
Two other studies have used the transverse patterning task to test rats with hippocampal system damage (Alvarado and Rudy, 1995a,b). In these studies, rats discriminated between two patterns displayed on large stimulus cards suspended over a swimming pool. Swimming under the correct stimulus allowed the rat to escape from the water. The task was administered in three phases: phase 1, A+ versus B−; phase 2, A+ versus B−, B+ versus C−; phase 3, A+ versus B−, B+ versus C−, C+ versus A−. Only phase 3 represents the complete transverse patterning task, which requires a “configural” solution, and in both studies hippocampal damage impaired performance during this critical phase (Alvarado and Rudy, 1995a,b).
The recent development of a touchscreen apparatus for rats (Bussey et al., 1994) provides the opportunity to reassess these potentially important findings using an automated testing procedure. In this apparatus, rats are able to select a stimulus by nose-poking directly to the computer screen, thereby ensuring precise control over response parameters. Other key features of the present study include the following. (1) In experiment 1, animals were trained to criterion onall problems in a given phase before being moved on to a subsequent phase. This ensured that all animals were performing equally well on the simple discriminations before being tested in the critical configural phase 3. This is in contrast with the studies of Alvarado and Rudy (1995a,b), in which all animals were moved on to a subsequent phase after a predetermined number of sessions, irrespective of performance levels. (2) In experiment 2, the three problems were presented concurrently from the outset of training (“concurrent training”). This is in contrast with “sequential training” in experiment 1. (3) The experimental group received fornix lesions, which functionally disconnect the hippocampus and thus closely mimic the effects of hippocampectomy (Morris, 1983; Barnes, 1988; Aggleton et al., 1992; Wible et al., 1992; Whishaw and Jarrard, 1995) (but see alsoMcDonald et al., 1997). This surgery also ensures that cortical regions adjacent to the hippocampus are spared. This is potentially important because these same cortical regions may be involved in configural learning (Bunsey and Eichenbaum, 1993; Gluck and Myers, 1995). (4) The animals were also tested on two allocentric spatial tasks (T-maze alternation and Morris Swim Task) to confirm the effectiveness of the lesions and to test the proposal that the mechanisms necessary for configural learning are the same as those necessary for the acquisition of allocentric spatial tasks (Sutherland and Rudy, 1989; Rudy and Sutherland, 1995).
EXPERIMENT 1
Materials and Methods
Subjects
Sixteen male rats of the pigmented DA strain (Bantin and Kingman) weighing 220–250 gm were housed in pairs in a temperature-controlled room on a 14 hr light/10 hr dark cycle. Animals were provided with free access to water and by means of a restricted feeding regimen were maintained throughout the experiment at 85% of their free feeding weight.
Surgery
Each animal was anesthetized by intraperitoneal injection of pentobarbitone sodium (Sagatal, Rhône Mérieux) at a dose of 60 mg/kg. The animal was then placed in a stereotaxic frame (David Kopf Instruments), and the scalp was retracted to expose the skull. Radiofrequency lesions of the fornix (group FNX1, n = 8) were made using an RFG4-A Lesion Maker (Radionics). The electrode (0.3 mm tip length, 0.25 mm diameter) was lowered vertically, and at each site the temperature of the tip was raised to 75°C for 60 sec. The coordinates for lesions of the fornix were (AP, LM from ear bar zero; DV from top of cortex): (1) AP +5.3, LM ±0.7, DV −3.7; and (2) AP +5.3, LM ±1.7, DV −3.8. Animals of the Sham1 control group (n = 8) received the same surgical procedure except that the temperature of the tip of the electrode was not raised.
Histology
After the completion of the experiment, the animals were anesthetized with an intraperitoneal injection of pentobarbitone sodium (Euthatal, Rhone Merieux) and perfused transcardially with saline followed by 10% formol-saline. The brain was removed and post-fixed in formol-saline for a minimum of 2 hr before being transferred into 20% sucrose in 0.2 m phosphate buffer and left overnight. Coronal sections were cut at 60 μm on a freezing microtome and stained with cresyl violet Nissl stain.
Apparatus
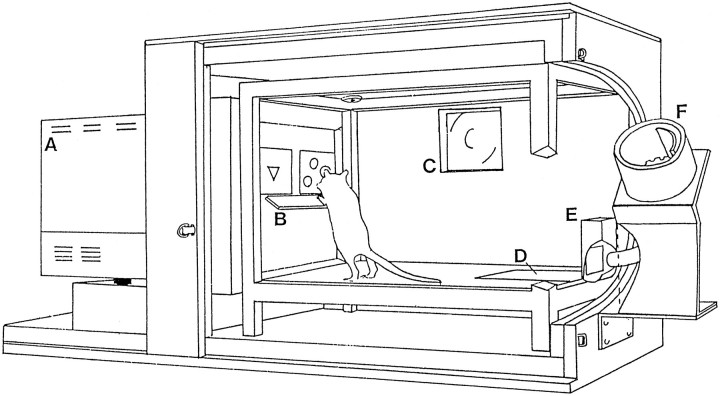
Touchscreen apparatus. A detailed description of the use of the touchscreen apparatus to administer a variety of tasks has been provided elsewhere (Bussey et al., 1994, 1997a,b). Four sets of apparatus were used (Fig. 1), each consisting of a test chamber and video display unit (VDU) housed within a wooden sound-attenuating box, fitted with a fan for ventilation and masking of extraneous noise. The inner chamber of each test unit consisted of a metal frame (48 cm × 30 cm × 30 cm) with clear Perspex walls and an aluminum floor. A 3 W houselight and a tone generator were attached to the ceiling of the chamber. Located centrally on the wall at the rear of the chamber was a food magazine attached to a pellet dispenser (Campden Instruments) situated outside the sound-attenuating box. The magazine could be illuminated by a standard 3 W bulb. Animals gained access to the magazine via a hinged Perspex panel monitored by a microswitch. A pressure-sensitive area of floor measuring 14 cm × 10 cm and located directly in front of the food magazine was attached to a microswitch to detect the presence of the rat when located in this area of the test chamber.
Fig. 1.
The touchscreen apparatus. A, Video display unit; B, Perspex “mask” with response windows and “shelf”; C, fan; D, pressure-sensitive floor panel; E, magazine;F, pellet dispenser (from Bussey et al., 1994).
The VDU, on which the stimuli were presented, was located at the other end of the chamber. Surrounding the VDU was a touchscreen attachment (Touch-tec 501, Microvitec), an array of horizontally and vertically placed photocells which detected the location of nose pokes to the VDU screen. A black Perspex “mask” was attached to the face of the VDU, ∼2 cm from the surface of the display. The mask served to block access to the VDU display except through two response windows, each measuring 6 cm high × 8 cm wide. A shelf extending 7 cm from the surface of the Perspex mask was positioned just beneath the response windows, ∼15 cm from the floor of the chamber. The shelf was supported by springs to prevent attempts by animals to climb onto it. The combined effect of the response windows and the shelf was to force the animal to stop, rear up, and stretch toward the stimuli with a head-on approach, thus facilitating the rats’ attention to the stimuli. The stimuli used in the task consisted of colored shapes, each occupying a maximum area of 4 cm high × 5 cm wide (Fig.2). The apparatus was controlled and monitored by a BBC Master series computer using programs written in BBC BASIC.
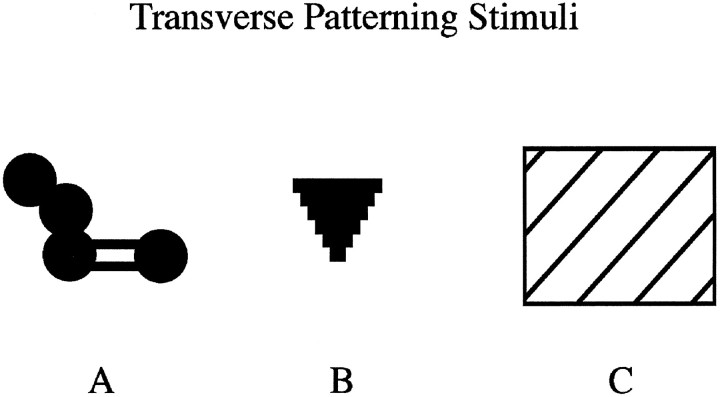
Fig. 2.
Computer-graphic stimuli used in the transverse patterning task. The figure provides the outline of the stimuli, which were drawn against a black background in blue (A), green (B), and white (C).
T-maze apparatus. The spatial delayed alternation task was performed in a modifiable T-maze. The floors of the maze were 10 cm wide and made of wood, and the walls were 17 cm high and made of clear Perspex. The stem was 70 cm long with a guillotine door located 25 cm from the end of the stem, thereby creating a start area. The cross piece was 140 cm long, and at each end there was a food well 2 cm in diameter and 0.75 cm deep. The entire maze was supported by two stands that were 94 cm high. Lighting was provided by a fluorescent light suspended 164 cm above the apparatus.
Morris Swim Task. The testing apparatus consisted of a white fiberglass pool, 2 m in diameter, 60 cm high, and mounted 58 cm above the floor. It was situated in a room containing posters on the wall that served as distal cues and a curtain that concealed the experimenter. Lighting was provided by four floor-mounted (500 W) lamps, one in each corner of the room. The water was made opaque by the addition of three pints of milk. An escape platform was placed 2 cm beneath the water surface and kept in a constant position in the pool during the acquisition trials. The temperature of the water was 25°C at the beginning of the daily testing period.
The paths of the rats were tracked using a video camera suspended centrally above the pool, and all sessions were recorded on video tape. Data were collected and analyzed on-line using an HVS image analyzer connected to an Archimedes RISC computer using Watermaze software (Edinburgh).
Behavioral procedures
Transverse patterning with sequential training.Pretraining. Pretraining consisted of six sessions. During the first 15 min session, the magazine panel was taped open to allow animals free access to food pellets placed in the magazine. On the second session, food pellets were delivered on a VI 40 schedule together with illumination of the magazine light and presentation of a tone. The next two sessions were identical to this except that the magazine panel was now untaped so that the animal was required to press the panel to gain access to food pellets. On the next two sessions, rats were trained to respond to the VDU display. During each of the 50 trials, a large yellow square was presented pseudorandomly in one of the response windows. The square remained on the screen until the rat responded to it, after which the rat was rewarded with magazine light, tone, and food pellet (45 mg, Bioserve, Inc.). Transverse patterning task. By the final phase of training, rats were presented with three discriminations concurrently: A+ versus B−, B+ versus C−, and C+ versus A−. During phase 1, animals were presented with the first of these discriminations (A+ vs B−). A trial began with the presentation of these stimuli, contingent upon the animal being located on the rear floor panel. The rat was then required to approach the VDU display and select a stimulus by responding to it directly via a nose poke. Correct responses were followed by the disappearance of the stimuli and the presentation of a 1 sec, 4 kHz tone, concomitant with the illumination of the magazine light and delivery of a food pellet into the magazine. Incorrect responses resulted in disappearance of the stimuli and the houselight being extinguished for a 5 sec time-out period. As part of a correction procedure, after an incorrect choice animals received the same stimulus configuration until the rat responded correctly. Animals received 90 trials (plus correction trials) in a session with the correct stimulus randomly presented in the left or right response window.
Once animals had achieved a criterion of 85% correct on 2 d consecutively, the second discrimination was introduced (B+ vs C−) and animals were then required to solve the two discriminations concurrently (phase 2). Again, animals received correction trials after an incorrect response and received 90 noncorrection trials in any one session (45 trials of each problem; the trial sequence was determined pseudorandomly). Once both discriminations were learned to a criterion of 80% correct across three consecutive sessions, the third discrimination (C+ vs A−) was introduced (phase 3) and animals received a total of 48 sessions with the 3 discriminations presented concurrently in each session of 90 noncorrection trials (30 trials of each problem; the trial sequence was determined pseudorandomly). In addition to percent correct scores, data from several behavioral measures were collected: (1) average magazine latency: the time from a correct nose-poke to the time the rat entered the magazine to collect reward; (2) average choice latency: the time from the onset of the choice stimuli to the time the rat made a nose-poke to one of the choice stimuli; and (3) percent bias: the absolute value of the difference between the number of responses to the left response window and 45 (half the number of noncorrection trials), expressed as a percent of total trials for that session.
T-maze alternation. Animals were given several days of pretraining so that they would run reliably down the stem of the maze to find food pellets in the food wells in both arms. This was immediately followed by a series of 10 acquisition sessions, each of six trials. Each trial was divided into two stages, a “sample run” followed by a “choice run.” At the start of each trial, three food pellets (45 mg, Sandown Instruments) were placed in each food well and a metal barrier was placed at the neck of the T-maze, thereby closing one arm. On the sample run, the animal was placed in the start area and the guillotine door was raised. Because of the metal barrier, the rat could enter only the one open arm, where it was confined for ∼10 sec while it ate the food. It was then picked up and confined to the start area for a delay of 15 sec while the barrier at the choice point was removed. The door to the start area was then raised, and the animal was allowed a free choice between the two arms of the T-maze.
On this choice run, the animal was deemed to have selected an arm when it had placed a hindfoot down that arm; no retracing was allowed. If the rat had alternated, i.e., had entered the arm not previously visited on the sample run, it was allowed to eat the food reward and was then returned to the home cage. If the other arm was chosen, i.e., the same arm as visited on the sample run, the animal was confined to that arm for ∼10 sec and then returned to the home cage. The rats were tested in groups of ∼4, with each rat having one trial in turn, so that the intertrial interval was ∼3–4 min. Each animal received six trials per day, and each day contained a pseudorandom sequence of three correct left and three correct right choices between the two arms.
Morris Swim Task. Four equidistant start locations (North, South, East, and West) were allocated, thus delineating four quadrants (NE, SE, NW, and SW) of the pool. On each trial, a rat was placed in the pool facing the wall at one of the four start locations. To escape the cool water, the rat was required to swim to an invisible platform, which was positioned in the same quadrant throughout the acquisition sessions. The platform was located in the SW quadrant for half of the rats in each of the FNX1 and Sham1 groups and the NE quadrant for the other half. The relationship between start position and platform location was the same for the two groups of animals on each trial, i.e., if in one group the platform location was in the SW quadrant and the start position was at North, then in the other group the platform location was in the NE quadrant the start position was at South.
Animals were tested on four trials per day for 10 d. Each acquisition trial was terminated either when the animal located the hidden escape platform or after 120 sec had elapsed. If the rat located the platform, it was allowed to remain there for 30 sec. If the rat failed to find the platform after 120 sec, it was placed on the platform and allowed to remain on it for 60 sec. On the next trial, the rat was placed in the pool at the second start location, and so on for four trials.
The following performance measures were collected on each trial: (1) escape latency to find the hidden platform; (2) mean swim speed (m/sec); and (3) percentage of time spent within 15 cm of the side walls. This measure helped to assess whether lesion effects on escape latency were attributable to differences in the persistence in swimming around the edge of the pool.
Results
Histology
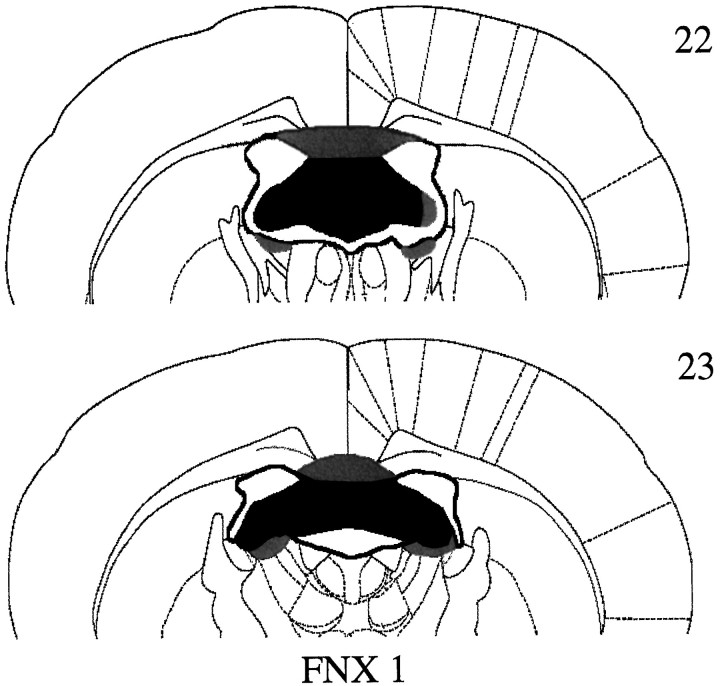
All animals in the FNX1 group, with a single exception, had complete bilateral transections of the fornix (Fig.3). In this one case, there was unilateral sparing of the most lateral tip of the fimbria (Fig. 3). In addition to lesions of the fornix, there was also very minor damage to the dorsal septum in four cases, and in all animals there was bilateral damage to the dorsal edge of the anterior dorsal and anterior ventral thalamic nuclei. The corpus callosum was cut in four cases.
Fig. 3.
Coronal sections illustrating the extent of the largest (gray) and smallest (black) lesions of the fornix in groupFNX1 (experiment 1) at −0.92 cm (section22) and −1.30 cm (section 23) from Bregma according to the atlas of Paxinos and Watson (1997).
Behavioral results
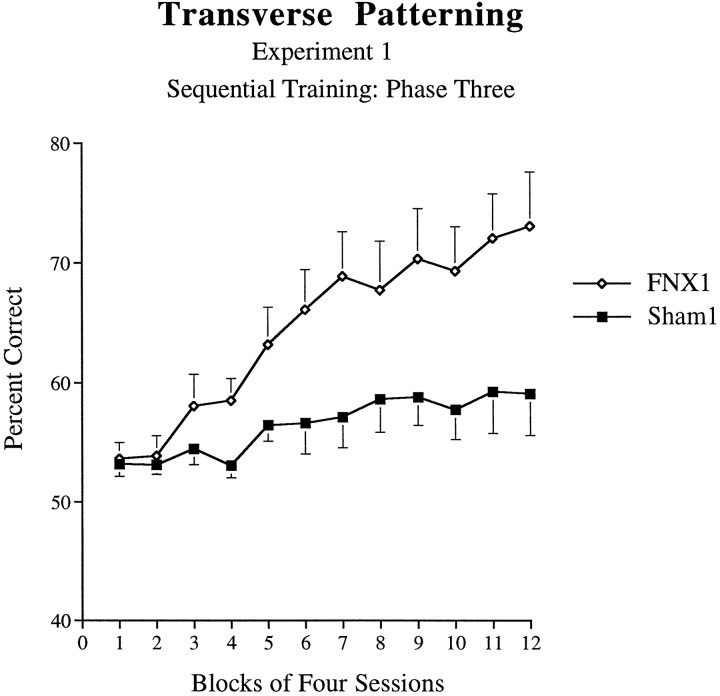
Transverse patterning.Phase 1. There was no significant difference between Sham1 control animals and those animals of the fornix lesion group (FNX1) during phase 1 in the number of sessions required to attain 85% criterion performance (excluding correction trials) on this problem: F1,14 = 1.80, p > 0.05 (Sham1 = 2.75; FNX1 = 3.13 sessions). There was similarly no significant difference between the groups in terms of errors to criterion: F1,14 = 3.30, p > 0.05 (Sham1 = 15.75; FNX1 = 24.6 errors). Phase 2. There was no significant difference between Sham1 control animals and animals of the fornix lesion group (FNX1) during phase 2 in the number of sessions required to attain 80% criterion performance (excluding correction trials) on problem 2 (problem 1 performance was already at criterion level after phase 1 training): F1,14 = 2.62, p > 0.05 (Sham1 = 11.25; FNX1 = 16.63 sessions). There was similarly no significant difference between the groups in terms of errors to criterion: F1,14 = 3.30,p > 0.05 (Sham1 = 56.25; FNX1 = 76.5 errors). Phase 3. The two groups of animals performed very differently during this final stage of the task. ANOVA of performance across 12 blocks of four sessions revealed a significant main effect of Lesion (F1,14 = 6.85, p = 0.02), with the FNX1 group responding more accurately than Sham1 controls on this task. As shown in Figure 4, there was also a significant Lesion × Session interaction effect (F11,154 = 4.23, p < 0.001). Although both groups initially responded at ∼55% correct during the first two blocks, after this point animals of the FNX1 group began to increase their level of performance, attaining a mean level of 78% accuracy by the end of 48 sessions. In contrast, animals of the Sham1 group only attained a mean of 60% correct by the end of the task. However, analysis of simple main effects revealed a significant effect of Block for the Sham1 group (p < 0.02), indicating significant learning in this group. Furthermore, a two-tailed t test performed on the mean of the last block of four sessions revealed that the performance of the Sham1 control group was significantly above chance level (50% correct;t7 = 2.36, p < 0.05).
Fig. 4.
Acquisition curves for animals of theFNX1 and Sham1 groups on the transverse patterning task (phase 3 of experiment 1: sequential training). Error bars indicate +/−SEM.
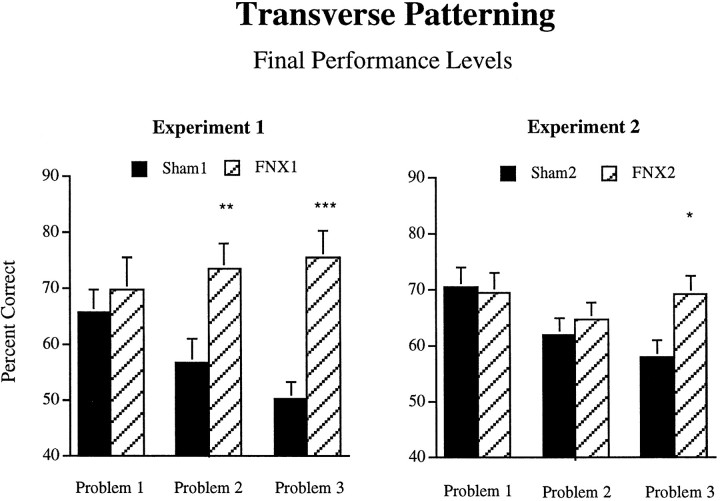
Finally, we compared performance levels of the FNX1 and Sham1 groups on each of the three problems separately (see Fig.5). ANOVAs performed on the final four-session block of acquisition for each problem revealed that the groups did not differ in final performance levels on problem 1 (F < 1), but that the FNX1 group significantly outperformed the Sham1 group during the final acquisition block on both problem 2 (F1,14 = 8.49, p = 0.01) and problem 3 (F1,14 = 23.3,p < 0.001).
Fig. 5.
Performance levels of the FNX1,Sham1, FNX2, and Sham2groups on each of the three problems of the full transverse patterning problem in experiments 1 and 2. Data are mean percent correct scores on the final four-session block of acquisition for each problem.Asterisks indicate significant differences; ***p < 0.001; **p = 0.01; *p < 0.05.
Two-tailed t tests performed on the final acquisition block for each of the three problems separately revealed that the FNX1 group performed significantly above chance on all three problems (allp < 0.01). The Sham1 group performed significantly above chance on problems 1 and 2 (both p < 0.01) but not on problem 3 (t7 = 0.14).
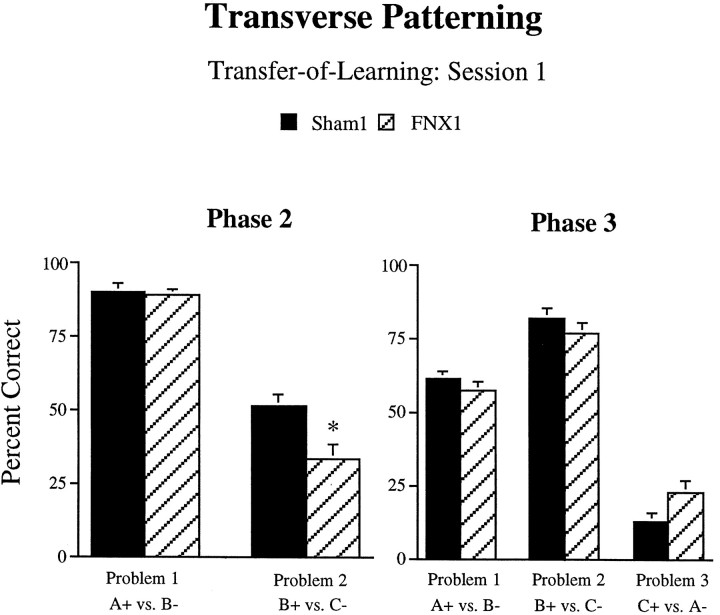
Transfer-of-learning. By examining performance on the first session of each phase, additional information is provided concerning the associations learned during the individual discrimination problems. First, data from session 1 of phase 2 were examined to investigate the possibility of transfer of learning from phase 1. As shown in the left panel of Figure 6, there was a difference between the groups in performance during the first session of phase 2. ANOVA performed on the percent correct scores from the two problems in this session revealed a significant main effect of Lesion (F1,14 = 9.33, p = 0.01) and a significant Lesion × Problem interaction (F1,14 = 6.06, p = 0.03). Analysis of simple interaction effects revealed that for both Sham1 animals and animals of the FNX1 lesion group, accuracy of performance on problem 2 of the task (B+ vs C−) was significantly poorer during this session than for problem 1 (A+ vs B−). In addition, although performance on problem 1 was not significantly different for the two groups, performance on problem 2 was significantly poorer in the FNX1-lesioned group than for Sham1 controls (p< 0.05). This difference suggests that FNX1 animals had learned more about the B− stimulus during phase 1 than did Sham1 control animals.
Fig. 6.
Percent correct scores of animals of theFNX1 and Sham1 groups on the first sessions of phase 2 (left) and phase 3 (right) in experiment 1. Animals of theFNX1 group obtained significantly lower scores than did control animals during the first session of problem 2 in phase 2, suggesting differential transfer of learning from phase 1. Note also the pattern of results during phase 3, in which all animals solve one problem very well, another at approximately chance level performance, and the third at well below chance, a pattern that may indicate an attempt at an “elemental” solution. Error bars indicate +/−SEM.Asterisk denotes a significant difference fromSham1 animals at p < 0.05.
To assess the nature of any transfer of learning from phase 2, we compared the accuracy of performance of the two groups during the first session of phase 3 (right panel of Fig. 6). There was no significant main effect of Lesion (F1,14 = 0.07, p > 0.05). Although the Lesion × Problem interaction was also not significant (F2,28 = 2.79, p = 0.07), there was a strong trend for animals of the FNX1 lesion group to show better performance on problem 3 during this session than Sham1 animals (p = 0.054). In contrast, performance of both groups was very similar on problems 1 and 2 during this first session of phase 3.
Additional performance measures. Several additional performance measures were collected during phase 3. These were analyzed across the 12 blocks of four sessions with Lesion and Block as factors.
There was no significant effect of Lesion on magazine latency (F < 1) and no significant Lesion × Block interaction (F11,154 = 1.61, p> 0.01). There was, however, a significant effect of Block (F11,154 = 4.25, p < 0.001), indicating that animals of both groups became faster to approach the magazine as training continued.
Analysis of choice latency revealed that fornix-lesioned animals were faster to respond to the stimuli than control animals (F1,14 = 14.6, p = 0.002). There was, however, no significant effect of Block (F < 1) and no significant Lesion × Block interaction (F< 1). Analysis of simple main effects revealed that the effect of Lesion on choice latency was present at each of the 12 training blocks (all comparisons p < 0.05).
Analysis of percent bias revealed no significant effect of Lesion (F < 1) or Block (F < 1) and no Lesion × Block interaction (F11,154 = 1.23, p > 0.05).
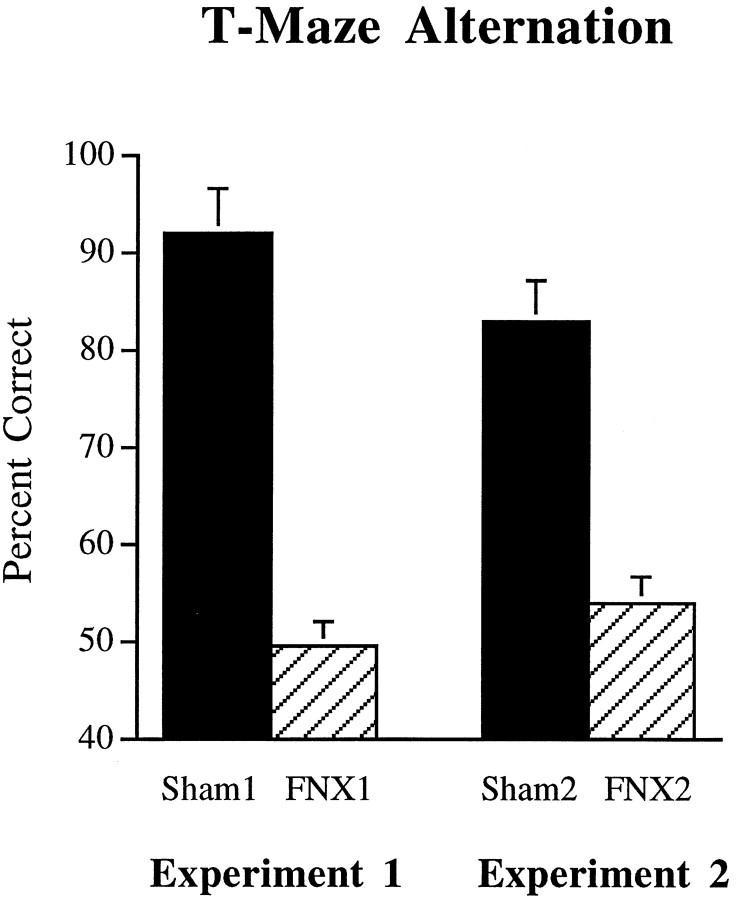
T-maze alternation. Animals with lesions of the fornix were severely impaired on spatial alternation in the T-maze (Fig.7). ANOVA performed on the mean percent correct scores across the 6 d of training revealed a significant main effect of Lesion (F1,14 = 225.5,p < 0.001), with animals of the FNX1 group performing at no better than chance level.
Fig. 7.
Mean percent correct scores of animals of theFNX1, Sham1, FNX2, andSham2 groups on T-maze alternation in experiments 1 and 2. Error bars indicate +/−SEM.
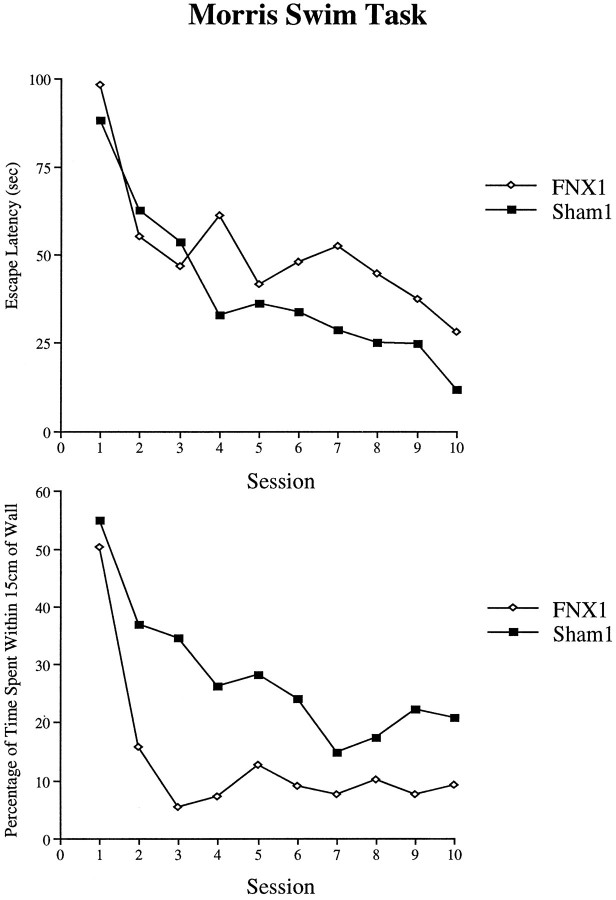
Morris Swim Task. Animals with fornix lesions were significantly impaired in the Morris Swim Task. The escape latencies of the FNX1 and Sham1 groups (Fig. 8, top panel) revealed a significant main effect of Lesion (F1,14 = 4.88, p = 0.04), although the Lesion × Session interaction failed to reach significance (F9,126 = 1.76, p = 0.08). Analysis of the swim speeds revealed that there was no significant group difference (F < 1). Comparisons of the percentage of time spent within 15 cm of the side walls did reveal, however, a clear group difference with animals of the FNX1 group spending less time swimming close to the side walls than animals of the Sham1 group (F1,14 = 13.8, p = 0.002). Evidence that the FNX1 group learned more rapidly to swim away from the side walls is shown in the highly significant Lesion × Session interaction (F9,126 = 3.26,p = 0.001) (Fig. 8, bottom panel). These results suggest that the spatial navigation impairment in the fornix group was actually more severe than that indicated by the group difference in escape latencies. Thus, although the fornix-lesioned animals spent less time swimming close to the pool walls, they still required more time to find the platform that was located in the central portion of the pool.
Fig. 8.
Performance of animals of the FNX1and Sham1 groups on the Morris Swim Task. The top panel shows mean escape latencies, and the bottom panel shows the percentage of time spent swimming within 15 cm from the walls of the pool.
EXPERIMENT 2
In experiment 1, it was found that fornix lesions facilitated the acquisition of the transverse patterning task. This result contradicts the predictions made by a class of theories that propose that an intact hippocampal formation is necessary for the solution of certain “configural” problems (Wickelgren, 1979; Sutherland and Rudy, 1989;Rudy and Sutherland, 1995). Although there was no effect of fornix lesions on the number of sessions required to learn the discriminations in phases 1 and 2, fornix-lesioned animals obtained significantly lower percent correct scores than did control animals on session 1 of problem 1 in phase 2. This suggests that fornix-lesioned animals learned problem 1 in a different way than did control animals. It is conceivable that such differences in learning during phases 1 and 2 may underlie the facilitation observed in phase 3 and that this facilitation “masked” a deficit in configural learning as predicted by Rudy and Sutherland (1995). To test this possibility, a new cohort of animals was tested on the transverse patterning task but without the preceding phases 1 and 2, i.e., with all three problems presented concurrently from the outset of training.
Materials and Methods
Subjects
Sixteen male rats of the pigmented DA strain (Bantin and Kingman) weighing 220–250 gm were housed in pairs in a temperature-controlled room on a 14 hr light/10 hr dark cycle. Animals were provided with free access to water and by means of a restricted feeding regimen were maintained throughout the experiment at 85% of their free feeding weight.
Surgery
Surgical procedures were the same as in experiment 1. Two groups of animals were prepared: FNX2 (n = 9) and surgical controls (Sham2; n = 9).
Apparatus
The apparatus was the same as in experiment 1.
Behavioral procedures
Transverse patterning with “concurrent” training.Pretraining. Pretraining proceeded in the same manner as in experiment 1. Transverse patterning task. Training proceeded as in experiment 1 with the important difference that phases 1 and 2 were omitted. Thus, immediately after pretraining, rats were presented with the three discriminations concurrently: A+ versus B−, B+ versus C−, and C+ versus A−, as in phase 3 of experiment 1. Animals received a total of 96 sessions consisting of 90 trials (30 trials of each problem; the trial sequence was determined pseudorandomly). Additional correction trials were provided as in experiment 1. T-maze alternation. Testing on T-maze alternation proceeded in the same manner as in experiment 1. Pretraining was followed by a series of 10 acquisition sessions of six trials each.
Results
Histology
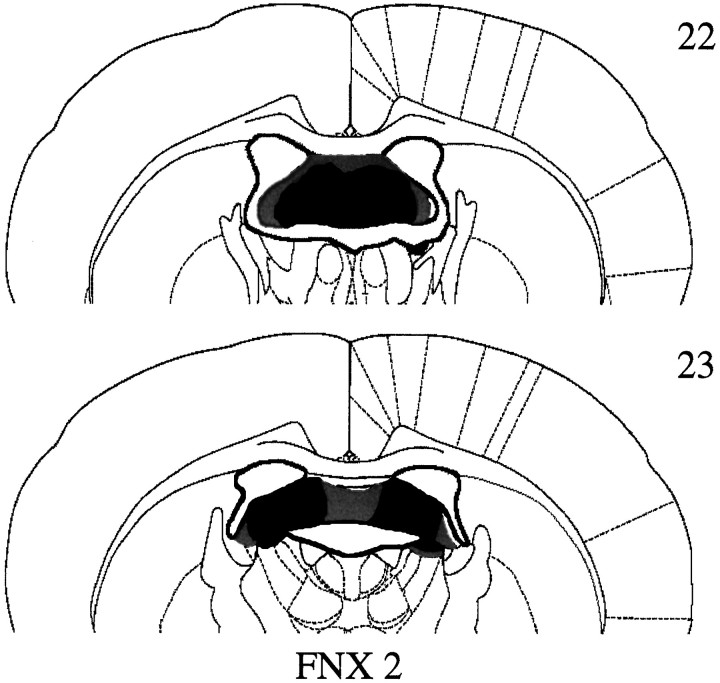
All animals in the FNX2 group had extensive bilateral lesions of the fornix (Fig. 9), with the exception of one animal. This animal had substantially less damage to the fornix, but more septal and thalamic damage, than the other animals in this group and, therefore, was excluded from all subsequent behavioral analyses. Final group membership, therefore, was FNX2 (n = 8) and Sham2 (n = 9). In these remaining FNX2 cases, the fornix received considerable bilateral damage, but there was often bilateral sparing of the most lateral tips of the fimbria (typically ∼10% of the fimbria/fornix). In six cases, the dorsal limit of the anterior ventral nucleus of the thalamus was also involved. There was no other consistent damage. Overall, lesions in the FNX2 group were slightly smaller than those in the FNX1 group (experiment 1).
Fig. 9.
Coronal sections illustrating the extent of the largest (gray) and smallest (white) lesions of the fornix in group FNX 2 (experiment 2) at −0.92 cm (section 22) and −1.30 cm (section 23) from Bregma according to the atlas of Paxinos and Watson (1997).
Behavioral results
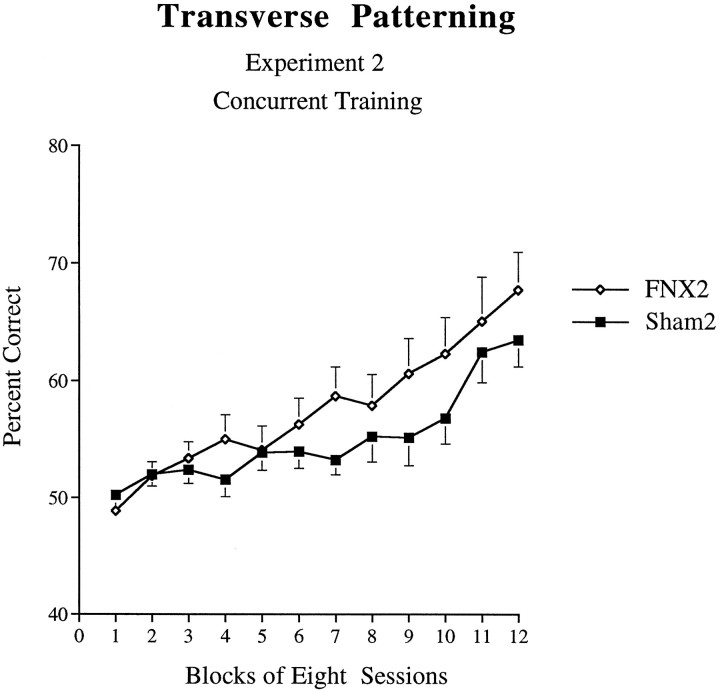
Transverse patterning task. There was no evidence that the FNX2 group was impaired; indeed, these animals tended to learn the task more rapidly than did controls (Fig. 10). An ANOVA performed on percent correct scores across 12 blocks, each of eight sessions, revealed that in this experiment this trend did not reach statistical significance (F1,15 = 1.35, p > 0.05). There was also no significant Lesion × Block interaction (F11,165 = 1.63, p > 0.05), but there was a significant effect of Block (F11,165= 2.12, p < 0.001). Because the interaction was close to significance, analysis of simple main effects was performed, which revealed a significant effect of Lesion on the seventh block (F1,15 = 4.60, p < 0.05).
Fig. 10.
Acquisition curves for animals of theFNX2 and Sham2 groups on the transverse patterning task (experiment 2: concurrent training). Error bars indicate +/−SEM.
Analysis of simple main effects of Block confirmed that animals of the Sham2 group improved across sessions (F11,165 = 10.84, p < 0.001), eventually attaining a mean of 64% correct. Animals of the FNX2 group also improved across sessions (F11,165 = 17.5, p < 0.001). A two-tailed t test performed on the mean of the last block of eight sessions revealed that the performance of the Sham2 control group was significantly above the chance-level performance of 50% (t8 = 6.35, p < 0.001).
To compare final performance levels of the two groups on each of the three problems separately, ANOVAs were performed on the final eight-session block of acquisition for each problem (see Fig. 5). This analysis revealed that the groups did not differ in final performance levels on problems 1 or 2 (F < 1), but that animals of the FNX2 group significantly outperformed controls during the final acquisition block of problem 3 (F1,15 = 5.30,p = 0.036).
Finally, two-tailed t tests performed on the final acquisition block for each of the three problems separately revealed that both the FNX2 and Sham2 groups performed significantly above chance on all three problems (all p < 0.05).
Additional performance measures. Additional performance measures were analyzed across the 12 blocks of eight sessions with Lesion and Block as factors. There was no significant effect of Lesion on magazine latency (F < 1) and no significant Lesion × Block interaction (F11,165 = 1.15, p > 0.05). There was, however, a significant effect of Block (F11,165 = 12.09,p < 0.001), indicating that animals of both groups were faster to approach the magazine as training continued.
There was no significant effect of Lesion on magazine latency (F1,15 = 2.7, p < 0.05) and no significant Lesion × Block interaction (F < 1). There was, however, a significant effect of Block (F11,165 = 13.1, p < 0.001), indicating that animals of both groups were faster to respond to the stimuli on choice as training continued.
There was no significant effect of Lesion on percent bias (F < 1) and no significant Lesion × Block interaction (F < 1). There was, however, a significant effect of Block (F11,165 = 3.7,p < 0.001), indicating that animals of both groups became less biased to respond to one side or another as training continued.
T-maze alternation. As in experiment 1, animals with lesions of the fornix were severely impaired on spatial alternation in the T-maze (Fig. 7). ANOVA performed on the mean percent correct scores across the six sessions of training revealed a significant main effect of lesion group (F1,14 = 67.7, p< 0.001), with animals of the FNX2 group again performing at no better than chance level.
DISCUSSION
Several influential theories have suggested that the hippocampal formation participates in the solution of a class of problems that require “configural” or “relational” representations for their solution (see, for example, Wickelgren, 1979; Sutherland and Rudy, 1989; Eichenbaum et al., 1994; Rudy and Sutherland, 1995). These theories view allocentric spatial learning, which is disrupted by lesions of the hippocampus (O’Keefe and Nadel, 1978; Morris et al., 1982; Aggleton et al., 1986), as a special case of “configural” or “relational” learning. Such a suggestion is potentially of great importance for understanding why damage to the hippocampal formation leads to amnesia in humans. Indeed, the mnemonic deficits seen in human amnesia have been described in terms of a deficit in configural or relational learning (Wickelgren, 1979; Sutherland and Rudy, 1989; Rudy and Sutherland, 1992, 1995; Eichenbaum et al., 1994).
The results of the present study, however, suggest that this view of hippocampal function is unlikely to be correct. Radiofrequency lesions of the fornix, which functionally disconnect the hippocampus and thereby typically mimic the effects of hippocampectomy, led to a significant facilitation of learning of the transverse patterning task, a test of configural learning (Rudy and Sutherland, 1995). That fornix lesions produced a facilitation of acquisition shows that the task was not insensitive to the effects of lesions and that the lesions were functionally effective. The same lesions severely impaired T-maze alternation and performance on the Morris Swim Task. This double dissociation is particularly problematic for a recent theory of hippocampal function that regards the transverse patterning task as one that should be especially sensitive to hippocampal dysfunction (Rudy and Sutherland, 1995).
Importantly, the facilitatory effect of fornix lesions on acquisition of the complete transverse patterning problem (phase 3, experiment 1) was not accompanied by changes in the number of sessions or errors required to attain criterion in phases 1 or 2. Furthermore, direct evidence that the task had been solved configurally came from the finding that performance of the fornix group was significantly above chance on each of the three problems in phase 3. Indeed, control animals solved only two of the three problems, showing that fornix-lesioned animals were able to learn configurally under conditions in which control animals could not. Because of this, floor effects in the control group may have masked, a facilitation of acquisition even greater than that observed.
Although there was no effect of fornix lesions on the number of sessions or errors required to attain criterion in phases 1 or 2, detailed inspection of the data revealed a subtle difference between groups in the transfer of learning between phase 1 and phase 2. This suggests that differences between the groups in the nature of learning during these initial phases could underlie the facilitation of acquisition observed in phase 3. For this reason, the training procedure was altered in experiment 2 such that phases 1 and 2 were omitted. Thus, all three problems of the transverse patterning task were presented concurrently from the outset of training. In this experiment, care was taken to ensure sufficient trials for control animals to perform consistently above chance level, and thereby provide the opportunity for a lesion-induced deficit to emerge. Although both groups were able to acquire all three problems of the task, the fornix-lesioned animals tended to learn more rapidly than controls. Indeed, comparisons of final performance levels revealed that fornix-lesioned animals outperformed control animals on one of the three problems. This experiment thus provides further evidence that lesions of the fornix do not impair acquisition of the transverse patterning task.
The results of the present study appear to conflict with Alvarado and Rudy (1995a,b). In their studies, the transverse patterning task involved as discriminative stimuli large stimulus cards suspended over a swimming pool, and rats were trained sequentially in three phases as in experiment 1 of the present study. In both of their studies, hippocampal damage impaired performance during phase 3. Unlike the present study, all animals were moved from phase 1 to 2 and from phase 2 to 3 after predetermined numbers of sessions, irrespective of performance levels. Because hippocampal lesions produced impairments in these initial phases, the lesion groups had performed at significantly lower levels than control animals before being moved on to the critical phase 3. This difficulty in matching performance levels may help to explain the discrepancy between the results of Alvarado and Rudy (1995a,b) and those of the present study.
An additional factor concerns the requirement for animals to swim toward large stimulus cards suspended above a swimming pool (Alvarado and Rudy, 1995a,b). It is conceivable that such large, distal stimuli may have been processed as spatial cues by the rats, possibly forming “scenes” that the animal could use in navigating to the platform, thus engaging a spatial processing system requiring an intact hippocampal system. Alternatively, stimulus size alone may be the critical factor. For example, Cassaday and Rawlins (1995) showed that fornix lesions impaired performance on a delayed nonmatching-to-sample task only when large, simple stimuli were used, possibly because such stimuli engage a spatial processing system. The idea that stimulus parameters, rather than “configural” task demands, may be responsible for the hippocampal deficits in the studies of Alvarado and Rudy (1995a,b) is consistent with the observation that hippocampal lesions can produce deficits in phase 1 and 2 of the transverse patterning task, when the stimulus parameters of the task are the same as in the critical phase 3, but the configural demands are not.
Although the lesion-induced facilitation reported in the present study appears paradoxical, such facilitations have been reported in other studies. For example, Eichenbaum and Bunsey (1995) reported that animals with lesions of the hippocampus were superior to control animals in distinguishing “paired-associates” (e.g., A→B+, C→D+) from “mispairs” (e.g., A→C−, B→D−). The similarity of this kind of discrimination to the transverse patterning task is apparent, although it is uncertain whether such paired-associate tasks require discrimination of configural representations. This is because such tasks could be solved using stimulus–stimulus associations or “conditional” rules of the type “If A is present, then B is rewarded; if D is present, then B is not rewarded.” Indeed, the transverse patterning task is a form of conditional task, in which animals must learn a rule of the type “If compound AB is present, then chose A” and thus may have both configural and conditional characteristics.
One possible reason for the rapid learning by fornix-lesioned animals is that the fornix is part of an “elemental” system and that removal of this system facilitates learning by a competing “configural” system. Indeed, Eichenbaum and Bunsey (1995) have suggested that the hippocampus may compute “relationships” between stimulus elements, whereas extrahippocampal cortical regions “fuse” stimuli into configural-like representations. A similar position has been forwarded by Gluck and Myers (1995), who suggest that the hippocampus is necessary for “predictive differentiation” of stimulus elements, but that extrahippocampal cortical regions are required for “redundancy compression,” a concept not unlike the configural processing hypothesized by Rudy and Sutherland (1995). Close inspection of data from the present study does not support, however, the view that the fornix is a critical component of an “elemental” learning system. When the third problem is first introduced (seeright panel of Fig. 6), control animals initially perform very well on one problem, at approximately chance on another, and well below chance on the third, a pattern that may indicate an attempt at an “elemental” solution (and which has been observed in the transverse patterning task previously; see Couvillon and Bitterman, 1996; Wynne, 1996). The performance of fornix-lesioned animals conforms to this same pattern. It thus appears that animals with fornix lesions, like control animals, may attempt initially to solve the problem “elementally” but may be better than controls at abandoning that strategy in favor of a configural one.
Other relevant evidence is found when the second problem (B+ vs C−) is added to the first (A+ vs B−, first session of phase 2; left panel of Fig. 6). Control animals behave as though they had learned to approach A, rather than to avoid B. If these animals had learned to avoid B, then performance on the new problem B+ versus C− would have been below chance; instead, performance was approximately at chance level. The fornix animals, in contrast, performed at a level below that of controls on problem 2, suggesting that these animals learned more about B− in phase 1 than did control animals. This suggests that whereas control animals do not attend to and learn about B, because this is not necessary for correct performance of the task (approaching A on each trial is sufficient), fornix animals “over-attend” to stimuli that provide redundant information. This interpretation is consistent with the view that the hippocampus mediates decrements in attention (see, for example, Solomon and Moore, 1975; Kaye and Pearce, 1987a,b; Han et al., 1995).
Importantly, the fornix lesions made in the present study were shown to be functionally effective in that they produced a devastating effect on spatial alternation in a T-maze, in both cohorts of animals used in this study (experiments 1B and 2B). Previous studies have shown that rats of the same strain and tested in the same apparatus as in the present study use allocentric cues to solve the task (Aggleton et al., 1996; Neave et al., 1997). Furthermore, fornix lesions produced a significant impairment in perfor- mance on the Morris Swim Task. Thus, the same animals that were impaired in allocentric spatial tasks were facilitated on a “configural” task. This double dissociation casts doubt on the notion that allocentric spatial learning is a special case of configural learning (Rudy and Sutherland, 1995).
An important consideration for the implications of the present study is the functional equivalence of fornix and hippocampal lesions in rats. In fact, those studies that have directly compared the effects of damage in these two sites have stressed the similarities between the effects of the lesions (Morris, 1983; Barnes, 1988; Aggleton et al., 1992; Wible et al., 1992) and, indeed, there is some evidence that fornix lesions may be more disruptive than neurotoxic hippocampal lesions on certain tasks, including the Morris Swim Task (Whishaw and Jarrard, 1995). Importantly, however, McDonald et al. (1997) have reported that neurotoxic kainate/colchicine lesions of the hippocampus can have effects on configural learning that are not observed after lesions of the fornix. These authors suggest that retrohippocampal connections, and not connections involving the fornix, may be necessary for certain types of learning. Kainate, however, is capable of producing widespread extrahippocampal cell loss (Jarrard and Meldrum, 1993), which may have contributed to the observed deficits. By using fornix lesions, the present study does not suffer from this potential confound. Furthermore, the high level of responding in the hippocampal animals may have prevented the expression of discrimination learning caused by the animals responding at a behavioral ceiling. Despite these problems, McDonald et al. (1997) raise an important possibility with respect to the equivalence of fornix and hippocampal lesions that should be tested in future studies.
Irrespective of these considerations, evidence from the present study that the same lesions that facilitate configural learning can impair spatial learning constitutes a problem for the idea that spatial learning is a special case of configural learning. Furthermore, Gallagher and Holland (1992) have shown that neurotoxic hippocampal lesions can produce a pattern of effects strikingly similar to those in the present study, namely, a facilitation of some aspects of performance on a “configural” task (the “feature-neutral” problem), concomitant with deficits in spatial learning. Thus, the present study confirms and extends these findings by providing a double dissociation between tests of spatial and configural learning, using a very different test of configural learning and an alternative method of damaging the hippocampal system. Furthermore, although Gallagher and Holland’s (1992) results were problematic for Configural Association Theory in its original form (Sutherland and Rudy, 1989), the present study has tested the predictions of the most recent version (Rudy and Sutherland, 1995) and has found striking contradictory evidence.
In summary, the present study provides evidence that is highly problematic for configural theories of hippocampal function. It also casts doubt on the popular notion that allocentric spatial learning is a special case of configural learning. Although it is conceivable that other brain regions may be necessary for “configural,” but not “elemental,” learning, so far no convincing evidence for such a dissociation exists. These considerations lead us to suggest, consistent with recent “configural” theories of associative learning (Pearce, 1987, 1994), that the “elemental” versus “configural”/“spatial” distinction is a false dichotomy and that an “elemental”/“configural” versus “spatial” distinction is a more likely principle of brain organization.
Footnotes
This research was supported by a project grant from the Wellcome Trust. We thank Angie Morgan and Angela McCarthy for invaluable assistance with behavioral testing and histology.
Correspondence should be addressed to John P. Aggleton, School of Psychology, University of Wales Cardiff, P.O. Box 901, Cardiff CF1 3YG, UK.
Dr. Bussey’s current address: Laboratory of Neuropsychology, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892.
REFERENCES
- 1.Aggleton JP, Hunt PR, Rawlins JNP. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- 2.Aggleton JP, Keith AB, Rawlins JNP, Hunt PR, Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behav Brain Res. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- 3.Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- 4.Alvarado MC, Rudy JW. A comparison of kainic acid plus colchicine and ibotenic acid-induced hippocampal formation damage on four configural tasks in rats. Behav Neurosci. 1995a;109:1052–1062. doi: 10.1037//0735-7044.109.6.1052. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado MC, Rudy JW. Rats with damage to the hippocampal formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behav Neurosci. 1995b;109:204–211. doi: 10.1037//0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- 6.Barnes CA. Spatial learning and memory processes: the search for their neurobiological mechanisms in the rat. Trends Neurosci. 1988;11:163–169. doi: 10.1016/0166-2236(88)90143-9. [DOI] [PubMed] [Google Scholar]
- 7.Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behav Neurosci. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- 8.Bussey TJ, Muir JL, Robbins TW. A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neurosci Res Commun. 1994;15:103–110. [Google Scholar]
- 9.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortex on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997a;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 10.Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of anterior cingulate, posterior cingulate and medial frontal cortex lesions on stimulus-reward learning studied using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997b;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- 11.Cassaday HJ, Rawlins JNP. Fornix-fimbria section and working memory deficits in rats: stimulus complexity and stimulus size. Behav Neurosci. 1995;109:594–606. doi: 10.1037//0735-7044.109.4.594. [DOI] [PubMed] [Google Scholar]
- 12.Couvillon PA, Bitterman ME. Transverse patterning in pigeons. Anim Learn Behav. 1996;24:410–422. [Google Scholar]
- 13.Davidson TL, McKernan MG, Jarrard LE. Hippocampal lesions do not impair negative patterning: a challenge to configural association theory. Behav Neurosci. 1993;107:227–234. doi: 10.1037//0735-7044.107.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H, Bunsey M. On the binding of associations in memory: clues from studies on the role of the hippocampal region in paired-associate learning. Curr Dir Psychol Sci. 1995;4:19–23. [Google Scholar]
- 15.Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–518. [Google Scholar]
- 16.Gallagher M, Holland PC. Preserved configural learning and spatial learning impairment in rats with hippocampal damage. Hippocampus. 1992;2:81–88. doi: 10.1002/hipo.450020111. [DOI] [PubMed] [Google Scholar]
- 17.Gluck MA, Myers CE. Representation and association in memory: a neurocomputational view of hippocampal function. Curr Dir Psychol Sci. 1995;4:23–29. [Google Scholar]
- 18.Han JS, Gallagher M, Holland P. Hippocampal lesions disrupt decrements but not increments in conditioned stimulus processing. J Neurosci. 1995;15:7323–7329. doi: 10.1523/JNEUROSCI.15-11-07323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarrard LE, Meldrum BS. Selective excitotoxic pathology in the rat hippocampus. Neuropathol Appl Neurobiol. 1993;19:381–389. doi: 10.1111/j.1365-2990.1993.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaye H, Pearce JM. Hippocampal lesions attenuate latent inhibition and the decline of the orienting response in rats. Q J Exp Psychol. 1987a;39B:107–125. [PubMed] [Google Scholar]
- 21.Kaye H, Pearce JM. Hippocampal lesions attenuate latent inhibition of a CS and of a neutral stimulus. Psychobiology. 1987b;15:293–299. [Google Scholar]
- 22.Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- 23.McDonald RJ, Murphy RA, Guarraci FA, Gortler JR, White NM, Baker AG. Systematic comparison of the effects of hippocampal and fornix-fimbria lesions on acquisition of three configural discriminations. Hippocampus. 1997;7:371–388. doi: 10.1002/(SICI)1098-1063(1997)7:4<371::AID-HIPO3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Morris RGM. An attempt to dissociate “spatial mapping” and “working memory” theories of hippocampal function. In: Seifert W, editor. Neurobiology of the hippocampus. Academic; New York: 1983. pp. 405–432. [Google Scholar]
- 25.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 26.Neave N, Nagle S, Aggleton JP. Evidence for the involvement of the mamillary bodies and cingulum bundle in allocentric spatial processing by rats. Eur J Neurosci. 1997;9:101–115. doi: 10.1111/j.1460-9568.1997.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon; Oxford, UK: 1978. [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 29.Pearce JM. A model of stimulus generalization for Pavlovian conditioning. Psychol Rev. 1987;94:61–73. [PubMed] [Google Scholar]
- 30.Pearce JM. Similarity and discrimination: a selective review and a connectionist model. Psychol Rev. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- 31.Rudy JW, Sutherland RJ. Configural and elemental associations and the memory coherence problem. J Cog Neurosci. 1992;4:208–216. doi: 10.1162/jocn.1992.4.3.208. [DOI] [PubMed] [Google Scholar]
- 32.Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5:375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- 33.Saunders RC, Weiskrantz L. The effects of fornix transection and combined fornix transection, mamillary body lesions, and hippocampal ablations on object-pair association memory in the rhesus monkey. Behav Brain Res. 1995;35:85–94. doi: 10.1016/s0166-4328(89)80109-3. [DOI] [PubMed] [Google Scholar]
- 34.Solomon P, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland RJ, Rudy JW. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- 36.Whishaw IQ, Jarrard LE. Similarities vs differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- 37.Whishaw IQ, Tomie JA. Acquisition and retention by hippocampal rats of simple, conditional, and configural tasks using tactile and olfactory cues: implications for hippocampal function. Behav Neurosci. 1991;105:787–797. doi: 10.1037//0735-7044.105.6.787. [DOI] [PubMed] [Google Scholar]
- 38.Whishaw IQ, Cassel JC, Jarrard LE. Rats with fimbria-fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wible CG, Shiber JR, Olton DS. Hippocampus, fimbria-fornix, amygdala, and memory: object discrimination in rats. Behav Neurosci. 1992;106:751–761. doi: 10.1037//0735-7044.106.5.751. [DOI] [PubMed] [Google Scholar]
- 40.Wickelgren WA. Chunking and consolidation: a theoretical synthesis of semantic networks, configuring in conditioning, S-R versus cognitive learning, normal forgetting, the amnesic syndrome, and the hippocampal arousal system. Psychol Rev. 1979;86:44–60. [PubMed] [Google Scholar]
- 41.Wynne CDL. Transverse patterning in pigeons. Behav Proc. 1996;38:119–130. doi: 10.1016/s0376-6357(96)00032-0. [DOI] [PubMed] [Google Scholar]