Abstract
Dopamine (DA) within the prefrontal cortex (PFC) plays an important role in modulating the short-term retention of information during working memory tasks. In contrast, little is known about the role of DA in modulating other executive aspects of working memory such as the use of short-term memory to guide action. The present study examined the effects of D1 and D2 receptor blockade in the PFC on foraging by rats on a radial arm maze under two task conditions: (1) a delayed task in which spatial information acquired during a training phase was used 30 min later to guide prospective responses, and (2) a nondelayed task that was identical to the test phase of the delayed task but lacked a training phase, thereby depriving rats of previous information about the location of food on the maze. In experiment 1, microinjections of the D1 antagonist SCH-23390 (0.05, 0.5, or 5 μg/μl), but not the D2anatagonist sulpiride (0.05, 0.5, or 5 μg/μl), into the prelimbic region of the PFC before the test phase disrupted performance of the delayed task without affecting response latencies. In contrast, neither drug affected performance of the nondelayed task. In the present study, we also investigated the role of D1 receptors in modulating activity in hippocampal–PFC circuits during delayed responding. Unilateral injections of SCH-23390 into the PFC in the hemisphere contralateral to a microinjection of lidocaine into the hippocampus severely disrupted performance of the delayed task. Thus, the ability to use previously acquired spatial information to guide responding 30 min later on a radial arm maze requires D1 receptor activation in the PFC and D1 receptor modulation of hippocampal inputs to the PFC. These data suggest that D1receptors in the PFC are involved in working memory processes other than just the short-term active retention of information and also provide direct evidence for DA modulation of limbic–PFC circuits during behavior.
Keywords: prefrontal cortex, hippocampal formation, dopamine, memory, foraging, radial maze
The prefrontal cortex is involved in the ability to retain and use mnemonic information to guide action (see, for example, Funahashi and Kubota, 1995; Goldman-Rakic, 1995;Seamans et al., 1995). Neurons recorded from the prefrontal cortex (PFC) of behaving primates (Fuster, 1995; Goldman-Rakic, 1995, 1990) and rats (Orlov et al., 1988; Batuev et al., 1990) show sustained firing throughout the brief delay period of a delayed-response task, which is thought to provide an internal representation of previously presented stimuli. Likewise, imaging data from human subjects suggest that the PFC is involved in the active maintenance of information in a short-term memory store (Cohen et al., 1997; Courtney et al., 1997). The integrity of the active short-term memory trace within the PFC appears to be regulated by the activity of a dopamine (DA) system, because destruction of DA terminals in the PFC disrupts performance on delayed-response or delayed-alternation tasks (Brozoski et al., 1979;Bubser and Schmidt, 1990), whereas the administration of high doses of D1, but not D2, antagonists into the PFC also impair performance on delayed-response tasks and decrease delay-period activity of PFC neurons (Sawaguchi et al., 1990b;Sawaguchi and Goldman-Rakic, 1994; Williams and Goldman-Rakic, 1995;Zahrt et al., 1997). Moreover, iontophoresis of low doses of D1 antagonists or DA into the PFC increases delay-correlated activity of PFC neurons relative to background activity (Sawaguchi et al., 1988; Sawaguchi et al., 1990a; Williams and Goldman-Rakic, 1995). Thus, too much or too little DA may be detrimental to cognition. These data clearly indicate a role for DA modulation of neural processes within the PFC related to the short-term active retention of information.
In addition to active retention of information over very short delays, the PFC, in collaboration with a variety cortical and subcortical regions, is also involved in the ability to use mnemonic information to plan a sequence of forthcoming responses (Shallice, 1982; Robbins, 1996; Shallice and Burgess, 1996; Floresco et al., 1997). Indeed,Fuster (1993) has stated that frontal lobes are involved primarily in memory for action. This type of memory for action embodies the concept of working memory as defined by Baddeley (1986) because it emphasizes the executive control of memory to guide action. We have used a modified delayed-response task, termed the delayed spatial win-shift task in rats, specifically to investigate this component of working memory. During the training phase of the delayed spatial win-shift task, the rat acquires trial-unique spatial information that must be retained for use at a later time. During the subsequent test phase, this information must be retrieved and integrated into a prospective search strategy if the rat is to retrieve four food pellets efficiently from eight possible locations on a radial-arm maze. Efficient performance on this task (i.e., visiting only arms that contain food) can be achieved only if the rat has acquired, and can use, spatial information regarding the probable location of food on the maze. Lidocaine inactivations of the medial PFC before the training phase of the delayed task did not affect training phase performance, or memory for the location of food in the subsequent test phase, at a time when the anesthetic effects of lidocaine had dissipated. In contrast, reversible inactivations just before the test phase severely impaired the rats’ ability to use mnemonic information about the probable location of food to plan forthcoming foraging behaviors (Seamans et al., 1995). Subsequent results suggested that spatial information may be retained in the hippocampus over the 30 min delay and accessed by the PFC when it is required to plan an efficient sequence of foraging responses (Floresco et al., 1997). At present, it is not known whether this component of working memory is also influenced by DA in the PFC.
DA has multiple actions on PFC neurons as studied in vivoand in vitro. Local application of DA inhibits spontaneous activity within the PFC (Ferron et al., 1984; Mantz et al., 1988; Pirot et al., 1992), and this effect appears to be mediated indirectly via the action of DA on local interneurons, because it is occluded by pretreatment of a GABA antagonist (Pirot et al., 1992). Direct application of DA (but not VTA stimulation) enhances both excitatory responses and synaptic plasticity in the hippocampal–PFC pathway (Jay et al., 1995, 1996). Moreover, as noted above, iontophoresis of DA into the PFC of behaving primates enhances delay-period activity significantly more than background or non-task-correlated activity (Sawaguchi et al., 1990a). Collectively, these data suggest that DA may act to enhance functional inputs to PFC neurons from regions such as the hippocampus. Anatomical data indicate that DA and hippocampal terminals often are found in close proximity to each another on layer V PFC neurons (Carr and Sesack, 1996). We hypothesize that local blockade of DA receptors will disrupt the selective augmentation of hippocampal inputs to PFC neurons and, as a consequence, impair behaviors dependent on the integrity of the hippocampal–PFC pathway.
In the present study, we examined the effects of D1 and D2 receptor blockade in the PFC on delayed and nondelayed radial-arm-maze foraging (Seamans and Phillips, 1994; Seamans et al., 1995). We also investigated whether endogenous DA activity within the PFC specifically modulated hippocampal afferents during the performance of the delayed task. To this end, we used a modified version of the transient disconnection procedure. In the standard transient disconnection procedure (Floresco et al., 1997), unilateral lidocaine injections were delivered to the origin of the hippocampal–PFC pathway in the ventral CA1/subiculum (vSub) and the termination of this pathway in the contralateral prelimbic (PL) region of the medial PFC (Jay and Witter, 1991; Condé et al., 1995). This procedure caused a selective disruption of delayed-response performance, whereas unilateral injections into either site had no effect on working memory (Floresco et al., 1997). In the present study, we substituted an injection of the D1 antagonist SCH-23390 for the nonspecific lidocaine injection into the PL. A critical role for D1 receptors in the PFC would be revealed if task performance was disrupted selectively by the combination of the D1 antagonist in the PL and lidocaine in the vSub.
MATERIALS AND METHODS
Subjects
The subjects were male Long-Evans rats weighing between 300 and 450 gm before surgery. All rats were given free access to water and were maintained at 85% of their free-feeding weight by providing 25 to 30 gm of Purina lab chow pellets once daily. Rats were tested 5 to 7 d/week.
Surgery
Rats were anesthetized with 100 mg/kg ketamine hydrochloride and 7 mg/kg xylazine. Twenty-three-gauge stainless steel guide cannulae were implanted into the brain regions using standard stereotaxic techniques. The stereotaxic coordinates (flat skull) were derived fromPaxinos and Watson (1986). Cannulae were implanted bilaterally into the PL (AP +2.6 mm, ML ±0.7 mm from bregma, DV −3.0 mm from dura) alone or in combination with bilateral cannulae implanted into the vSub region of the hippocampus (AP −6.0 mm from bregma, ML ±5.5 mm from midline, DV −5.3 ± 0.3 mm from dura). This region of the hippocampus was chosen because it sends dense projections to the PL region of the PFC in the rat (Jay et al., 1991; Condé et al., 1995). Thirty-gauge obdurators flush with the end of the guide cannulae remained in place until the injections were made. Each rat was given at least 7 d to recover from surgery before testing.
Microinfusion procedure
On injection days, the obdurators were removed and 30-gauge stainless steel injection cannulae were inserted 0.8 mm beyond the tip of the guide cannulae. In experiment 1, the D1 antagonist SCH-23390 (0.05, 0.5, or 5 μg in 0.5 μl dissolved in physiological saline), the D2 antagonist sulpiride (0.05, 0.5, or 5 μg in 0.5 μl dissolved with a drop of NaOH in PBS) (Research Biochemicals), or vehicle injections were delivered at a rate of 0.5 μl/1.2 min by a microsyringe pump (Sage Instruments Model 341). In experiment 2, SCH-23390 (0.5 μl/μg) or vehicle injections were made unilaterally into the PFC while lidocaine (20 μg in 0.5 μl of saline, Astra Pharmaceuticals or Research Biochemicals) or saline (0.5 μl) was delivered to the contralateral vSub. Injection cannulae were left in place for an additional 1 min after each injection to allow for diffusion. All SCH-23390, sulpiride, and corresponding vehicle injections were made 15 min before testing to ensure an optimal blockade of DA receptors (Sawaguchi and Goldman-Rakic, 1994). Lidocaine and corresponding vehicle injections were made 5 min before testing. Similar doses of D1 and D2 receptor antagonists have been used previously and are effective in disrupting working memory performance (Sawaguchi and Goldman-Rakic, 1994; Broersen et al., 1995a,b).
Apparatus
An eight-arm radial maze was used for all experiments. The maze had an octagonal center platform 40 cm in diameter connected to eight, equally spaced arms, each measuring 50 cm × 9 cm, with a cylindrical food cup at the end. Removable pieces of white opaque plastic (9 cm × 13 cm) were used to block the arms of the maze. The maze was elevated 40 cm from the floor and was surrounded by numerous extra maze cues (i.e., cupboards, posters, doors, the experimenter, etc.) in a room 4 m × 5 m × 3 m, which was illuminated with overhead fluorescent lights (100 W).
Foraging tasks
The two foraging tasks used in the present study were the delayed spatial win-shift (SWSh) and the nondelayed random foraging (RF) tasks.
Delayed SWSh task. This task was adapted from Packard et al. (1990) and has been described in detail elsewhere (Seamans and Phillips, 1994; Seamans et al., 1995). On the first 2 d of testing, rats were habituated to the maze environment. Subsequent training trials were given once daily. These trials consisted of a training phase and a test phase separated by a delay. Before the training phase, a set of four arms was chosen randomly and blocked. In both the delayed SWSh task and the RF task, a novel set of arms was chosen each day. Food pellets (Bioserv) were placed in the food cups of the four remaining open arms. During the training phase, each rat was given 5 min to retrieve the pellets from the four open arms and then was returned to its home cage for the delay period (see below). During the test phase of each daily trial, all arms were open but only the arms that were blocked previously contained food. Rats were allowed a maximum of 5 min to retrieve the four pellets during the test phase.
The initial delay between training and test phases was 5 min. After achieving criterion performance in which all four pellets were retrieved in five or fewer choices during the test phase for 2 consecutive days, the delay was increased to 30 min. A 30 min delay was used in previous studies because lidocaine injections could be delivered before the training phase or during the delay period while allowing sufficient time for the anesthestic effects to dissipate before the test phase (Seamans and Phillips, 1994; Seamans et al., 1995). In the present study, the first intracranial injections were administered after attaining 2 consecutive days of criterion performance at a 30 min delay. After the first injection day, animals were again retrained to the criterion performance. The next day, a second intracranial injection was administered. This procedure was repeated until an animal had received the designated sequence of injections, according to the protocols described below (see Design and Procedure).
On injection days, the number and order of arm entries were recorded. An arm entry was recorded when a rat moved down the entire length of an arm and reached the food cup at the end of the arm. Errors were scored as entries into nonbaited arms and further broken down into two error subtypes. An across-phase error was defined as any initial entry into an arm that had been visited previously during the training phase. A within-phase error was any reentry into an arm that had been entered earlier during the test phase. The latencies to reach the food cup of the first arm visited and to complete the phase also were recorded.
Nondelayed RF task. This task also has been described elsewhere (Seamans and Phillips, 1994; Seamans et al., 1995). Habituation to the maze during the first 2 days of training was identical to the delayed SWSh procedure described above. On subsequent daily trials, animals were required to forage for pellets placed at random in the food cups of four of the eight arms (hence the term “random foraging”). A novel set of arms was baited each day. Animals were trained to a criterion of no more than one reentry error per trial for 4 consecutive days. The day after criterion performance was achieved, the first intracranial injections were administered. After the first injection day, animals were retrained to criterion for 2 consecutive days. As with the delayed SWSh task, this procedure was repeated until each animal had received all of the designated injections.
Errors were scored as reentries into arms visited previously within a trial. These errors were broken down further into reentries into baited arms (arms that had been baited at the start of the trial) and reentries to nonbaited arms (arms that were not baited before the start of the trial). The number of reentries errors made on each of the injection days was recorded and used for data analysis. As with the delayed SWSh paradigm, the latencies to reach the first food cup (either baited or nonbaited) after being placed on the maze and the time required to retrieve all four pellets were also recorded.
Design and procedures
Experiment 1: bilateral injections of SCH-23390 or sulpiride into the PL. A within-subjects design was used for all four parts of experiment 1. Four groups of rats with cannulae implanted bilaterally into the PL were trained on either the delayed SWSh task or the RF task. Rats in group 1 received bilateral infusions of either SCH-23390 or vehicle into the PL in a counterbalanced order before the test phase of the delayed SWSh task. Rats in group 2 received bilateral infusions of either sulpiride or vehicle into the PL in a counterbalanced order before the test phase of the delayed SWSh task. Rats in group 3 received bilateral infusions of either SCH-23390 or vehicle into the PL in a counterbalanced order before a daily trial of the nondelayed RF task. Rats in group 4 received bilateral infusions of either sulpiride or vehicle into the PL in a counterbalanced order before a daily trial of the nondelayed RF task. After the first infusion, each subsequent infusion was administered when the rats reattained criterion performance for 2 consecutive days. The order of injections was counterbalanced between rats using a quasi-Latin square design.
Experiment 2: unilateral injections of SCH-23390 into the PL combined with lidocaine inactivation of the contralateral vSub. In experiment 2, we used a modified version of a transient disconnection procedure (Floresco et al., 1997), in which unilateral injections of the D1 antagonist SCH-23390 were substituted for the nonspecific neural blocker lidocaine. A within-subjects design was used for this experiment. Well trained rats received a total of four injections before the test phase of the delayed SWSh task. We used the following combinations of asymmetrical unilateral injections: (1) a unilateral inactivation of the vSub in combination with a contralateral injection of SCH-23390 into the PL; (2) a unilateral inactivation of the vSub in combination with a vehicle injection into the contralateral PL; (3) a unilateral injection of SCH-23390 into the PL and a vehicle injection into the contralateral vSub; and (4) injections of vehicle into the vSub and contralateral PL. The order of injections was counterbalanced between animals using a quasi-Latin square design. The counterbalancing ensured that a given sequence of injections was not repeated. The hemisphere (left or right) used for the first injection was also counterbalanced and was alternated for subsequent injections. The injection procedure was repeated until the animal had received all four combinations.
Histology
After completion of behavioral testing, the rats were killed in a CO2 chamber. Brains were removed and fixed in a 10% formalin solution. The brains were frozen and sliced in 50 μm sections before being mounted. Placements were verified with reference to the neuroanatomical findings of Jay and Witter (1991), Condéet al. (1995), and Paxinos and Watson (1986).
Data analysis
The number and type of errors made on the day before the first injection sequence and for all injection days for each experiment were analyzed using separate two-way, between/within, mixed design ANOVAs with the injection order as a between-subjects factor and treatment day as a within-subjects factor. Main effects of treatment were analyzed further using Tukey’s post hoc tests for repeated measures. Whenever a significant main effect of treatment was observed, a planned comparison was performed that analyzed the number of each type of error made on drug injection days.
The latency data to reach the first food cup and the average time per subsequent choice on injection days were analyzed with a one-way repeated-measures ANOVA. The average time per subsequent choice was calculated using the following formula: [(time to complete trial − time to initiate trial) ÷ number of choices for trial].
Whenever a significant main effect of treatment was observed in experiment 2, three one-way ANOVAs were conducted assessing the number of errors made on injection days, with the side of the injection as a between-subjects factor. This analysis was conducted to rule out the possibility that unilateral inactivations in one hemisphere would lead to a greater increase in errors than inactivations of the other hemisphere.
RESULTS
Experiment 1: bilateral injections of SCH-23390 or sulpiride into the PL
Histology
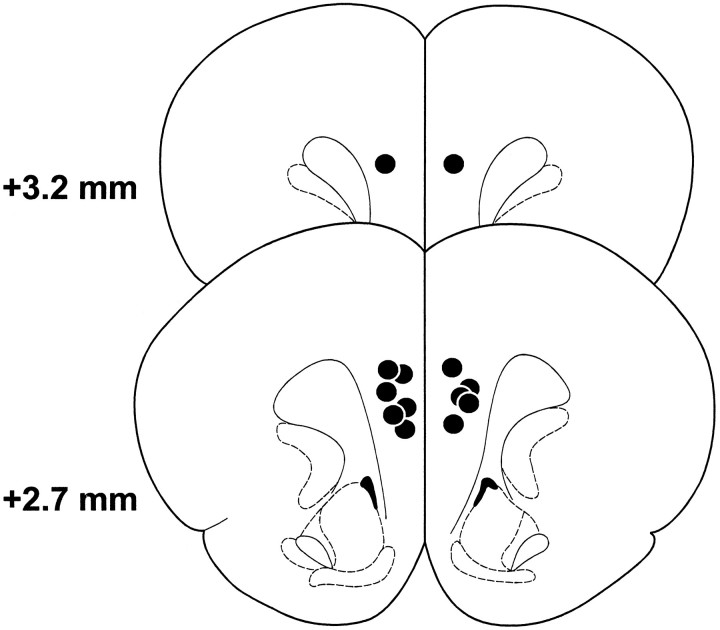
The location of the cannulae tips for all animals receiving bilateral SCH-23390 injections into the PL before the delayed SWSh task of experiment 1 are shown in Figure 1. Placements were similar for the other groups in experiments 1 and 2. Data from animals the placements of which were not located in the PL region of the PFC were not included in the data analysis.
Fig. 1.
Schematic representation of the SCH-23390 injection sites for rats in experiment 1. Black dotsrepresent the location of cannula tips. Illustrated brain sections are computer-generated adaptations from Paxinos and Watson (1997).
Delayed SWSh task.SCH-23390. Before the test phase of the delayed SWSh task, seven rats received bilateral counterbalanced infusions into the PL of vehicle and three doses of SCH-23390 (0.05, 0.5, or 5 μg in 0.5 μl of vehicle) on separate days. Analyses of the number of errors made on vehicle and all drug injection days revealed a significant main effect of Treatment (F3,18 = 4.96, p < 0.05; see Fig. 2A). Tukey’spost hoc analysis for repeated measures showed that rats made significantly more errors after injections of 0.5 and 5.0 μg of SCH-23390 compared with vehicle and 0.05 μg SCH-23390 treatments (p < 0.05). Subsequent planned comparisons on the type of errors made on SCH-23390 injection days revealed that, after injections of either 0.5 or 5.0 μg of SCH-23390 into the PL, an equal number of across- and within-phase errors were made [allF < 2.0, not significant (n.s.)]. There were no significant effects of Injection Order or Treatment × Order interactions (all F < 1.8, n.s.).Sulpiride. Before the test phase of the delayed SWSh task, seven rats received bilateral counterbalanced infusions of either vehicle or three doses of sulpiride (0.05, 0.5, or 5 μg in 0.5 μl of vehicle) into the PL on separate days. Analyses of the number of errors made on vehicle and all drug injection days revealed no significant main effect of Treatment (F3,18 = 1.62, n.s; see Fig. 2B). There were also no significant effects of Injection Order or Treatment × Order interactions (all F < 1.8, n.s.).
Fig. 2.
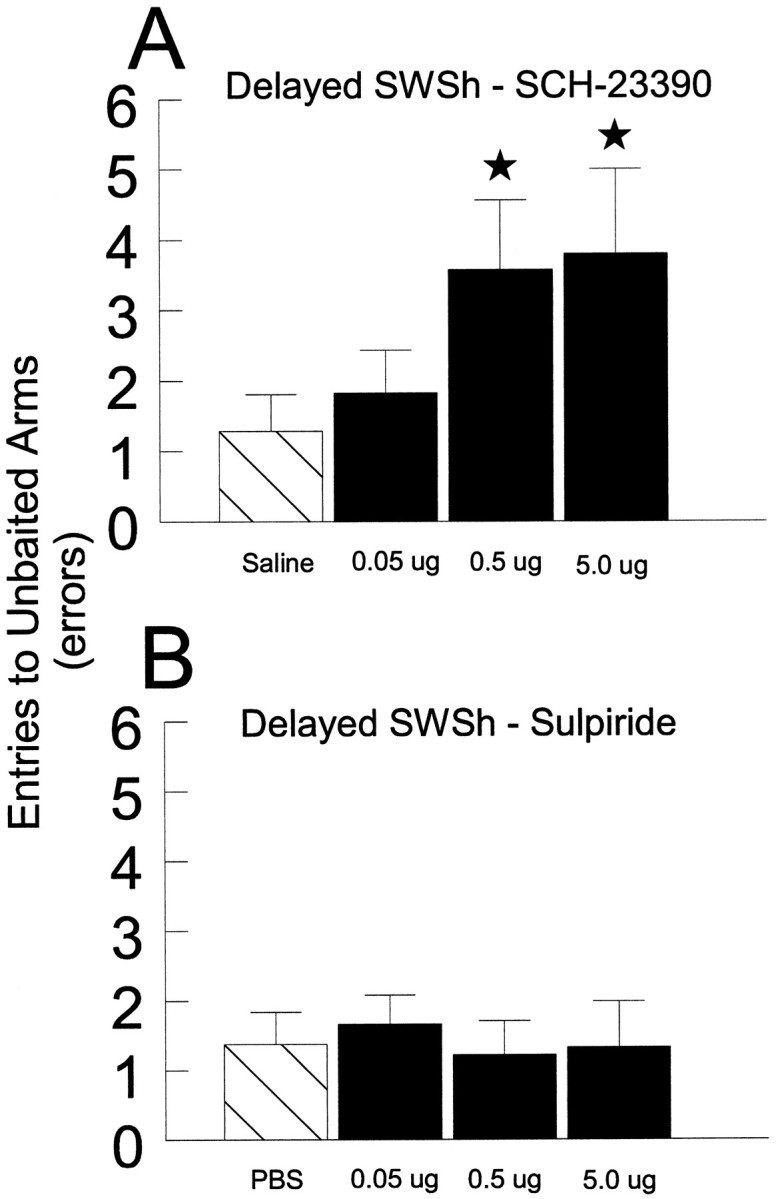
The effects of bilateral injections of D1 or D2 antagonists into the PL on performance of the delayed SWSh task. A, Number of errors (mean ± SEM) made during the test phase by rats receiving saline (hatched bar): 0.05, 0.5, and 5 μg of SCH-23390 (black bars) into the PL. B, Number of errors (mean ± SEM) made during the test phase by rats receiving PBS (hatched bar): 0.05, 0.5, and 5 μg of sulpiride (black bars) into the PL. *p < 0.05 compared with saline injections.
Nondelayed RF task.SCH-23390. Before a daily trial of the nondelayed RF task, seven rats received counterbalanced bilateral infusions of either vehicle or SCH-23390 (0.05, 0.5, or 5 μg in 0.5 μl of vehicle) into the PL on separate days. Analysis of these data revealed no significant main effect of Treatment (F3,18 = 2,27, n.s.; see Fig.3A). There also were no significant effects of Order of injection or Order × Treatment interactions (all F < 2.3, n.s.).Sulpiride. Before a daily trial of the nondelayed RF task, seven rats received bilateral counterbalanced infusions of either vehicle or sulpiride (0.05, 0.5, or 5 μg in 0.5 μl of vehicle) into the PL on separate days. Analysis of these data revealed no significant main effect of Treatment (F3,18 = 0.56, n.s.; see Fig. 3B). There also were no significant effects of Order of injection or Order × Treatment interactions (allF < 0.7, n.s.).
Fig. 3.
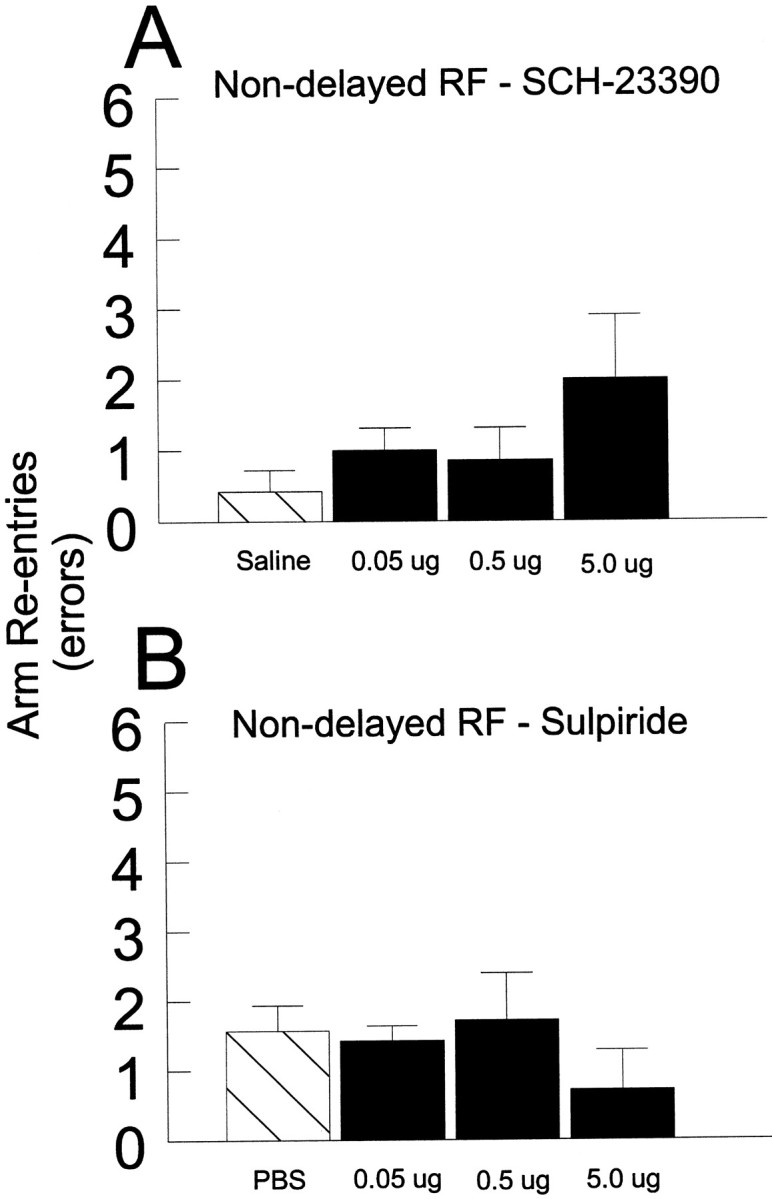
The effects of bilateral injections of D1 or D2 antagonists into the PL on performance of the nondelayed RF task. A, Number of errors (mean ± SEM) made during the nondelayed RF task by rats receiving saline (hatched bar): 0.05, 0.5, and 5 μg of SCH-23390 (black bars) into the PL. B, Number of errors (mean ± SEM) made during the nondelayed RF task by rats receiving PBS (hatched bar): 0.05, 0.5, and 5 μg of sulpiride (black bars) into the PL.
Experiment 2: unilateral injection of SCH-23390 into the PL combined with inactivation of the contralateral vSub
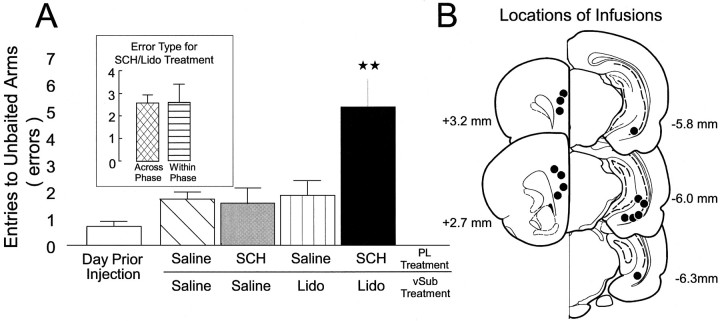
In experiment 2, a group of 7 rats with two sets of bilateral cannulae implanted into the PL and the vSub received the injection protocol described above, before the test phase of the delayed SWSh task, on four occasions. Statistical analyses revealed a highly significant main effect of Treatment (F3,18 = 9.720, p < 0.001; see Fig.4A). Tukey’spost hoc analysis for repeated measures revealed that rats made significantly more errors when unilateral infusions of SCH-23390 into the PL were paired with contralateral infusions of lidocaine into the vSub (p < 0.001). There were no other significant differences in the number of errors made on any of the other injection days. Subsequent planned comparisons on the type of errors made after unilateral PL infusion of SCH-23390 and contralateral vSub inactivations showed that rats made an identical number of across- and within-phase errors (F1,6 = 0.0, n.s.; see Fig. 4A, inset). Furthermore, there were no significant effects of Injection Order, Error type, or any significant interactions (all F < 1.4, n.s.). Collectively, these results showed that D1 receptors selectively modulate hippocampal afferents to the PL during the performance of a long-delay SWSh task.
Fig. 4.
The effects of unilateral inactivation of the vSub in combination with unilateral injections of SCH-23390 into the PL of the contralateral hemisphere. A, Number of errors (mean ± SEM) made during the delayed SWSh task by rats on the day before the first injection (open bar), after unilateral infusions of saline into both the PL and vSub (hatched bar), unilateral infusions of SCH-23390 (SCH, 0.5 μg) into the PL and contralateral infusions of saline into the vSub (gray bar), unilateral infusions of saline into the PL and contralateral infusions of lidocaine into the vSub (vertical stripe bar), and unilateral injections of SCH-23390 (SCH, 0.5 μg) into the PL and contralateral injections of lidocaine into the vSub (black bar). **p < 0.01 versus all other treatment conditions.Inset shows the number of across-phase (cross-hatched bar) and within-phase (horizontal-striped bar) errors made by rats after the SCH/lidocaine injection condition. B, Schematic representation of the SCH-23390 injection sites into the PL and lidocaine injection sites into the vSub for rats in the SCH/lidocaine condition. Black dots represent the location of cannula tips. Illustrated brain sections are computer-generated adaptations from Paxinos and Watson (1997).
A separate series of tests revealed no evidence of hemispheric biases on the number of errors made after unilateral vSub inactivations or unilateral SCH-23390 injections into the PL (all F < 0.7, n.s.). However, rats that received unilateral inactivations of the right vSub made significantly more errors than those rats that received left vSub inactivations (F1,5 = 7.10,p < 0.05). This effect was not observed previously after identical unilateral vSub lidocaine injections (Floresco et al., 1997). Given the large number of comparisons made in the present study, this increase in errors after right unilateral vSub inactivations is likely to be a spurious finding. Moreover, the total number of errors made by rats after unilateral vSub inactivations, combined for both hemispheres, did not differ significantly from other control treatments.
Histology
The locations of the cannulae tips for all animals in experiment 2 are represented in Figure 4B. Bilateral placements in the vSub were similar to those observed by Floresco et al. (1997). Similarly, bilateral placements in the PL were within the same region of the PFC as those observed in experiment 1.
Analysis of response latencies
The latency data for both experiments 1 and 2 are presented in Table 1. The latency data for each phase of experiments 1 and 2 were analyzed separately. These analyses revealed that none of the drug treatments in experiment 1 significantly affected the latency to initiate the trial or the average time per subsequent choice (all F < 1.5, n.s.). In experiment 2, SCH-23390 PFC microinjections paired with vehicle injections into the vSub significantly reduced initiation times (p < 0.05). Because neither bilateral SCH-23390 injections nor unilateral vSub vehicle injections affected response latencies in the other conditions, this anomolous result may not be a real effect.
Table 1.
Latency to initiate and average time per subsequent choice(s)
| Experiment 1 | |||||
| Delayed SWSh | SCH-23390 | Saline | 0.05 μg | 0.5 μg | 5.0 μg |
| Initiate | 31.6 (3.2) | 29.7 (13.1) | 42.1 (10.0) | 25.6 (5.1) | |
| Average time per choice | 28.1 (2.6) | 21.8 (2.8) | 18.9 (2.4) | 20.0 (2.3) | |
| Sulpiride | Saline | 0.05 μg | 0.5 μg | 5.0 μg | |
| Initiate | 18.3 (2.0) | 10.2 (2.7) | 8.7 (4.0) | 16.8 (5.2) | |
| Average time per choice | 20.1 (2.3) | 17.8 (1.9) | 11.8 (1.1) | 11.8 (2.9) | |
| Non-delayed RF | SCH-23390 | Saline | 0.05 μg | 0.5 μg | 5.0 μg |
| Initiate | 23.8 (4.8) | 25.5 (8.0) | 20.6 (4.8) | 23.3 (4.6) | |
| Average time per choice | 19.4 (3.8) | 24.1 (7.2) | 19.1 (3.5) | 24.3 (4.8) | |
| Sulpiride | Saline | 0.05 μg | 0.5 μg | 5.0 μg | |
| Initiate | 16.3 (8.0) | 22.1 (8.1) | 21.4 (3.0) | 26.0 (10.1) | |
| Average time per choice | 13.1 (2.1) | 29.1 (10.9) | 17.9 (1.3) | 37.9 (18.1) | |
| Experiment 2 | Saline (PL) and saline (vSub) | Saline (PL) and Lido (vSub) | SCH (PL) and saline (vSub) | SCH (PL) and Lido (vSub) | |
| Initiate | 43.2 (8.4) | 46.4 (18.7) | 26.8 (8.8)* | 65.0 (16.2) | |
| Average time per choice | 22.7 (8.8) | 28.5 (11.2) | 21.7 (5.1) | 32.7 (10.0) |
Values are means ± SEM. * p < 0.05.
DISCUSSION
Our research showed that microinjection of a D1 but not a D2 antagonist into the PL region of the PFC produced a dose-dependent impairment on a delayed-foraging task. This indicated that D1 receptor blockade in the PFC impaired the process by which spatial information acquired 30 min earlier is used to guide forthcoming approach responses. From the results of experiment 2, it can be inferred that D1 receptors modulate hippocampal inputs to the PFC during performance of a spatially mediated working-memory task with a relatively long delay. These data complement and extend previous reports that application of a D1antagonist into the PFC in primates disrupts the active retention of information during a brief delay (<6 sec) in an occulomotor delayed-response task (Sawaguchi and Goldman-Rakic, 1994; Williams and Goldman-Rakic, 1995). Taken together, these studies indicate that D1 receptors modulate several different aspects of working memory function in the PFC.
Our results demonstrate that D1 but not D2receptors in the PL modulate delayed responding on the delayed task. These data are consistent with those of Bubser and Schmidt (1990), who showed that 6-OHDA lesions of the rat PL selectively impaired delayed but not spontaneous alternation on a T-maze. Furthermore, D1 receptor blockade in the PFC of primates or rats produces deficits on delayed-response tasks but typically not those without a delay component (Sawaguchi et al., 1990b; Sawaguchi and Goldman-Rakic, 1994; Broersen et al., 1995b; Williams and Goldman-Rakic, 1995). Enhanced DA activity in the PFC also disrupts delayed responding in rats, as shown by impaired performance of delayed alternation on a T-maze after pharmacologically induced high rates of DA turnover in the PFC or administration of D1 agonists into the PFC (Murphy et al., 1996a,b; Arnsten, 1997; Zahrt et al., 1997). These data indicate that maintenance of D1 activity in the PFC within an optimal range is essential for working memory. Although D2 receptor blockade in the PFC has been reported to affect certain memory tasks in rats (Bushnell and Levin, 1983), most studies have not found an effect of D2 antagonists on delayed responding (Sawaguchi et al., 1990b; Sawaguchi and Goldman-Rakic, 1994; Broersen et al., 1995b). There are a greater number of D1 receptors compared with D2receptors in the PFC (Farde et al., 1987; Gaspar et al., 1995), and D1 receptor agonists have more clearly observed effects on the firing properties of PFC neurons recorded in vitro (Yang and Seamans, 1996). However, it has been suggested that D2receptor modulation may play a more prominent role in older animals (Arnsten et al., 1995).
Microinjections of the D1 receptor antagonist had no effect on response initiation or on the average time per choice. This is consistent with the observation of Broensen et al. (1995a,b) that response latencies on the choice component of a delayed nonmatching-to-position task were unaffected after bilateral injections of similar doses of SCH-23390 into the rat PL. The fact that D1 antagonists produced dissociable effects on the use of previously acquired spatial information to guide subsequent responding as opposed to any direct effects on response generation is consistent with the suggestion that a planned series of responses generated in the PFC must be relayed to other brain regions, such as the striatum for translation into action (Robbins, 1991; Seamans et al., 1995; Floresco et al., 1997). Furthermore, the deficit observed in this study after microinjections of a D1 antagonist into the PL was specific to performance of the delayed task, because similar injections had no effect on foraging during the nondelayed single-trial procedure. This indicated that SCH-23390 injections did not affect either the ability of the rats to navigate in a spatial environment or basic motivational processes.
Spatially mediated foraging appears to be critically dependent on the PFC and reciprocal interactions with the hippocampus (Floresco et al., 1997). Experiment 2 demonstrated that D1 receptor blockade in the PL coupled with inactivation of the vSub in the contralateral hemisphere disrupted performance on the delayed foraging task. In this context, it is important to emphasize that unilateral injection of SCH-23390 in the PL in combination with vehicle injections into the vSub had no effect on memory for the location of food. In our previous “disconnection” study, we used asymmetrical injections of lidocaine into both the PL and the vSub to demonstrate a critical role for a hippocampal–PFC circuit in the working-memory function that enabled rats to anticipate the location of food in complex environment (Floresco et al., 1997). The logic underlying the use of this disconnection procedure to identify components of a functional neural circuit is based on the assumption that information is transferred serially from one structure to an efferent region, on both sides of the brain in parallel. Furthermore, the design assumes that dysfunction will result from blockade of neural activity at the origin of a pathway in one hemisphere and the termination of the efferent pathway in the contralateral hemisphere. The fact that blockade of D1receptors in the PL in one hemisphere disrupted delayed foraging when combined with a reversible lesion of the vSub is consistent with an important gating function for D1 receptors during the transmission of information in a hippocampal–PFC circuit.
A circuit for working memory
It has been proposed that short-term retention of visuo-spatial information and executive functions are components of working memory (Baddeley and Hitch, 1974; Baddeley and Della Sala, 1996). Evidence for a role of the PFC in the former component of working memory comes from recording studies in nonhuman primates showing that PFC neurons encode and retain actively information about previously presented visuo-spatial information over brief delays (Funahashi et al., 1989;Goldman-Rakic, 1995; Miller et al., 1996). In humans, PFC activation is also observed during the active retention of information on a working-memory task (Cohen et al., 1997). The executive component of working memory is hypothesized to coordinate and manipulate visuo-spatial information (Baddeley and Hitch, 1974; Baddeley, 1986;Baddeley and Della Sala, 1996) and is necessary for the performance of tasks requiring the planning and selection of new and appropriate response strategies (Shallice and Burgess, 1993; Robbins, 1996). Patients with PFC damage perform poorly on tasks such as the Tower of London task that depend on planning a sequence of responses (Shallice, 1982; Owen et al., 1990; Robbins, 1996). In normal subjects, PFC activation is observed during performance of the Tower of London task (Baker et al., 1996) as well as during tasks requiring the generation of novel response sequences (Jueptner et al., 1996). Thus, both the short-term retention of visuo-spatial information and the central executive components of working memory have been linked to the PFC.
The delayed task used in the present study does not require the active retention of information within the PFC because lidocaine-induced inactivation of the PFC before or during the delay period does not disrupt performance (Seamans et al., 1995). Likewise, lidocaine-induced inactivations of the PFC do not affect performance of the nondelayed task even though this task requires the short-term retention of spatial information. It has been postulated that imposing a delay in a radial-arm maze task biases rats to forage prospectively, whereas nondelayed tasks bias rats to forage retrospectively (Cook et al., 1985; Floresco et al., 1997). On the delayed task, foraging is severely disrupted after transient lidocaine inactivations of the PFC at a time when previously acquired information must be accessed and integrated into a prospective foraging strategy (Seamans et al., 1995). These data, therefore, are consistent with a role of the rat PFC in executive functions related to planning.
In the rat, the short-term retention of spatial information may be mediated by the hippocampal formation and overlying cortices. Rats with lesions of the hippocampus are impaired in memory for spatial location (Morris et al., 1991; Jarrard, 1993), whereas transient lesions of the ventral hippocampus, similar to those reported here, are effective in disrupting the short-term acquisition and retention of spatial information (Poucet et al., 1991; Floreso et al., 1996, 1997). The importance of the hippocampal afferents in the present study was likely attributable to this task being spatially cued and, therefore, biased the use of circuits involving the hippocampal formation. D1receptors conceivably could modulate functional inputs from other brain regions, depending on the nature of the task. Asymetric “disconnections” of the ventral hippocampus and PFC selectively disrupt the delayed-working-memory task (Floresco et al., 1997), possibly by disconnecting the spatial memory buffer in the temporal lobe from the central executive in the PFC. The present results indicated that D1 receptors in the PFC may modulate the transfer of spatial information from the hippocampus to the PFC at a time when a prospective series of response must be organized and executed.
Potential mechanisms for D1 receptor modulation of PFC function
Insight into the mechanisms by which D1 receptor activation modulates both the active retention of information in the PFC and the processing of information accessed from a spatial memory buffer in the temporal lobe is provided by recent electrophysiological studies. It is important to note that the effect of DA within the PFC is determined by the activity level of PFC neurons and the specific inputs that are driving this activity. Although DA inhibits spontaneous activity within the PFC of the anethesized rat (Ferron et al., 1984;Mantz et al., 1988; Pirot et al., 1992), it enhances task-related single-unit activity more than background activity in the behaving primate (Sawaguchi et al., 1988, 1990a). DA (but not VTA stimulation) also enhances responses evoked by hippocampal stimulation in the anesthetized rat and use-dependent changes in synaptic efficacy in the hippocampal–PFC pathway that may be associated with learning (Dòyere et al., 1993; Jay et al., 1995, 1996). Thus, DA may enhance task- or learning-related activity relative to spontaneous or background activity within the PFC.
In vitro studies suggest that DA influences the behavior of PFC neurons in a way that is consistent with this hypothesis. DA directly depolarizes interneurons in the PFC and enhances spontaneous and evoked IPSPs recorded in pyramidal neurons (Penit-Soria et al., 1987; Yang et al., 1997; Zheng et al., 1997). Accordingly, the suppressive action of DA on spontaneous firing of PFC neurons in vivo is blocked by previous application of a GABA antagonist (Pirot et al., 1992), suggesting that DA acts through interneurons in the PFC to reduce spontaneous activity of pyramidal neurons. In contrast, the effects of strong depolarizing inputs that bring the neuron to spike threshold are enhanced directly by DA via the D1 receptor (Yang and Seamans, 1996). In this way, DA acting through the D1 receptor may enhance selectively the effects of the most salient inputs to PFC neurons relative to background or spontaneous inputs. Supranormal stimulation of the DA system in the PFC may strongly activate inhibitory mechanisms so as to severely restrict the number of inputs that potentially could evoke firing of PFC neurons. Response perseveration may be one consequence of restricting afferent input in this manner. In fact, Zahrt et al. (1997)reported recently that D1 receptor agonists microinjected into the PFC of rats did impair delayed responding by increasing response perservation.
If D1 receptors act as a gating mechanism to control the responses of layer V PFC output neurons, in the manner described above, it follows that D1 antagonists would attenuate functional signals in afferent pathways including the hippocampal–PFC projection relative to spontaneous firing in PFC output neurons. Indeed, Sawaguchi (1997) has demonstrated recently that iontophoresis of SCH-23390 into the premotor cortex of primates decreased delay-period activity significantly more than background activity during a delayed-response task. In the present delayed-foraging task, blockade of D1receptors would be predicted to decrease the effectiveness of functional inputs from the hippocampus relative to spontaneous activity, possibly resulting in random modes of behavior. This was the pattern of results observed here after bilateral injections of SCH-23390 into the PL, or unilateral D1 receptor blockade in the PL, in combination with a transient lesion of the contralateral vSub.
Conclusions
D1 receptors appear to play an important role in at least two different aspects of working memory function in the PFC: (1) the ability to hold information in an active state for a short time, as shown previously (Sawaguchi and Goldman-Rakic, 1994; Williams and Goldman-Rakic, 1995), and (2) the recall of information from a spatial memory buffer via a hippocampal–PFC circuit and the integration of spatial memory into a prospective response strategy. The recall of spatial information from a buffer in the hippocampal formation may involve “top-down” feedback inputs from the PFC to the temporal cortex in a manner analogous to that proposed by Desimone and colleagues (Desimone et al., 1994; Miller et al., 1996) for visual recognition memory but over much greater temporal intervals. Our research is consistent with a neural model in which the executive system and the spatial memory buffer are located in separate regions of the rat cortex, namely the PFC and temporal lobe, respectively, and that DA modulates the flow of information between these two regions via the D1 receptor.
Footnotes
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to A.G.P. S.B.F. is the recipient of a Natural Sciences and Engineering Research Council scholarship, and J.K.S. holds a University of British Coloumbia Graduate Fellowship. We thank Glen Wundelich and Tony Drew for their assistance with behavioral testing.
Correspondence should be addressed to Anthony G. Phillips, Department of Psychology, University of British Columbia, 2136 West Mall, Vancouver, British Columbia, Canada V6T 1Z4.
REFERENCES
- 1.Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten AFT, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddeley AD. Working memory. Clarendon; Oxford, UK: 1986. [Google Scholar]
- 4.Baddeley A, Della Sala S. Working memory and executive control. Phil Trans R Soc (Lond) 1996;351:397–1404. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- 5.Baddeley AD, Hitch G. Working memory. In: Bower IGA, editor. The psychology of learning and motivation. Academic; New York: 1974. pp. 47–90. [Google Scholar]
- 6.Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 7.Batuev AS, Kurina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res. 1990;41:95–102. doi: 10.1016/0166-4328(90)90145-5. [DOI] [PubMed] [Google Scholar]
- 8.Broersen LM, Heinsbroek RP, deBruin JPC, Joosten RNJMA, van Hest A, Oliver B. Effects of local application of dopaminergic drugs into the dorsal part of the medial prefrontal cortex of rats in a delayed matching to position task: comparison with cholinergic blockade. Brain Res. 1995a;645:113–122. doi: 10.1016/0006-8993(94)91644-6. [DOI] [PubMed] [Google Scholar]
- 9.Broersen LM, Heinsbroek RP, deBruin JP, Uylings HB, Oliver B. The role of the medial prefrontal cortex of rats in short-term memory functioning: further support for involvement of cholinergic, rather than dopaminergic, mechanisms. Brain Res. 1995b;674:221–229. doi: 10.1016/0006-8993(95)00025-l. [DOI] [PubMed] [Google Scholar]
- 10.Brozowski TS, Brown RM, Rosvold HE, Goldman PS. Cognitive deficits caused by regional depletion of dopamine in prefrontal cortex of Rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 11.Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- 12.Bushnell PJ, Levin ED. Effects of dopaminergic drugs on working and reference memory in rats. Pharmacol Biochem Behav. 1993;45:756–776. doi: 10.1016/0091-3057(93)90119-e. [DOI] [PubMed] [Google Scholar]
- 13.Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 15.Condé F, Marie-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 16.Cook RG, Brown RF, Riley DA. Flexible memory processing by rats: use of prospective and retrospective information in the radial arm maze. Anim Behav Proc. 1985;11:453–469. [PubMed] [Google Scholar]
- 17.Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 18.Desimone R, Miller EK, Chelazzi L. The interaction of neural systems for attention and memory. In: Koch C, Davis JL, editors. Large-scale theories of the brain. MIT; Cambridge, MA: 1994. pp. 75–91. [Google Scholar]
- 19.Doyère V, Burette F, Negro CR, Laroche S. Long-term potentiation of hippocampal afferents and efferents to prefrontal cortex: implications for associative learning. Neuropsychologia. 1993;31:1031–1053. doi: 10.1016/0028-3932(93)90031-t. [DOI] [PubMed] [Google Scholar]
- 20.Farde L, Haldin C, Stone-Elander S, Sedvall G. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology. 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- 21.Ferron A, Thierry AM, Le Douarin C, Glowinski J. Inhibitory influence of the mesocortical dopaminergic system on spontaneous activity or excitatory response induced from the thalamic mediodorsal nucleus in the rat medial prefrontal cortex. Brain Res. 1984;302:257–265. doi: 10.1016/0006-8993(84)90238-5. [DOI] [PubMed] [Google Scholar]
- 22.Floresco SB, Seamans JK, Phillips AG. Differential effects of lidocaine infusions into the ventral CA1/subiculum or the nucleus accumbens on the acquisition and retention of spatial information. Behav Brain Res. 1996;81:163–172. doi: 10.1016/s0166-4328(96)00058-7. [DOI] [PubMed] [Google Scholar]
- 23.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 25.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the primate dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 26.Fuster JM. Frontal Lobes. Curr Opin Neurobiol. 1993;3:160–165. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- 27.Fuster JM. Memory in the cerebral cortex: an empirical approach to neural networks in the human and nonhuman primate. MIT; Cambridge, MA: 1995. [Google Scholar]
- 28.Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Neurobiol. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- 30.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 31.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 32.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of the anterograde transport of Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 33.Jay TM, Glowinski J, Thierry AM. Inhibition of hippocampo-prefrontal cortex excitatory responses by the mescocortical DA system. NeuroReport. 1995;6:1845–1848. doi: 10.1097/00001756-199510020-00006. [DOI] [PubMed] [Google Scholar]
- 34.Jay TM, Burette F, Laroche S. Dopaminergic modulation of long-term potentiation in the hippocampal-prefrontal cortex pathway. Soc Neurosci Abstr. 1996;22:332. [Google Scholar]
- 35.Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE. The anatomy of motor learning. I. The frontal cortex and attention to action. J Neurophysiol. 1996;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 36.Mantz J, Milla C, Glowinski J, Thierry AM. Differential effects of ascending neurons containing dopamine and noradrenaline in the control of spontaneous activity and of evoked responses in the rat prefrontal cortex. Neuroscience. 1988;27:517–526. doi: 10.1016/0306-4522(88)90285-0. [DOI] [PubMed] [Google Scholar]
- 37.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris RGM, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of the hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1991;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 39.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996a;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy BL, Arnsten AFT, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996b;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 42.Orlov AA, Kurzina NP, Shutov AP. Activity of medial wall neurons in frontal cortex of rat brain during delayed response reactions. Neurosci Behav Physiol. 1988;18:31–37. doi: 10.1007/BF01186902. [DOI] [PubMed] [Google Scholar]
- 43.Packard MG, Regenold W, Quirion R, White NM. Post-training injection of the acetylcholine M2 receptor antagonist AF-DX 116 improves memory. Brain Res. 1990;524:72–76. doi: 10.1016/0006-8993(90)90493-u. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd Ed. Academic; New York: 1986. [Google Scholar]
- 45.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 3rd Ed. Academic; San Diego: 1997. [Google Scholar]
- 46.Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- 47.Pirot S, Godbout R, Mantz J, Tassin J-P, Glowinski J, Thierry A-M. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evience for involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- 48.Poucet B, Herrmann T, Buhot MC. Effects of short lasting inactivations of the ventral hippocampus and medial septum on long term and short term acquisition of spatial information in rats. Behav Brain Res. 1991;4:453–465. doi: 10.1016/s0166-4328(05)80239-6. [DOI] [PubMed] [Google Scholar]
- 49.Robbins TW. Cognitive deficits in schizophrenia and parkinson’s disease: neural basis and the role of dopamine. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: from motivation to action. Wiley; New York: 1991. pp. 497–528. [Google Scholar]
- 50.Robbins TW. Dissociating executive functions of the prefrontal cortex. Phil Trans R Soc (Lond) 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 51.Sawaguchi T. Attenuation of preparatory activity for reaching movements by a D1-dopamine antagonist in the monkey premotor cortex. J Neurophysiol. 1997;78:1769–1774. doi: 10.1152/jn.1997.78.4.1769. [DOI] [PubMed] [Google Scholar]
- 52.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 53.Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory performance in the primate prefrontal cortex. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 54.Sawaguchi T, Matsumura M, Kubota K. Catecholamine effects on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990a;63:1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- 55.Sawaguchi T, Matsumura M, Kubota K. Effects of dopamine antagonists on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990b;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- 56.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–468. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- 57.Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- 58.Shallice T. Specific impairments of planning. Phil Trans R Soc (Lond) 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 59.Shallice T, Burgess P. Supervisory control of action and thought selection. In: Baddeley A, Weiskrantz L, editors. Attention: selection, awareness and control. Clarendon; Oxford, UK: 1993. pp. 171–187. [Google Scholar]
- 60.Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Phil Trans R Soc (Lond) 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- 61.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 62.Yang CR, Seamans JK. Dopamine D1 receptor actions in layer V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang CR, Seamans JK, Gorelova N. Mechanisms of dopamine (DA) modulation of GABAergic inputs to rat layer V-VI pyramidal prefrontal cortical (PFC) neurons in vitro. Soc Neurosci Abstr. 1997;692.2:1771. [Google Scholar]
- 64.Zahrt J, Taylor JR, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng P, Bunney BS, Shi WX. Electrophysiological characterization and effects of dopamine on visually identified non-pyramidal neurons in the prefrontal cortex. Soc Neurosci Abstr. 1997;481.13:1212. [Google Scholar]