Abstract
The lizard Iguana iguana when kept in constant ambient temperature displays endogenously generated circadian rhythms of body temperature and locomotor activity. Although surgical removal of the parietal eye has only slight effects on overt circadian rhythmicity, subsequent pinealectomy completely abolishes the rhythm of body temperature. However, the rhythm of locomotor activity is only slightly affected by parietalectomy plus pinealectomy. Our results demonstrate that the pineal complex is centrally involved in the generation and control of the circadian rhythm of body temperature but is only marginally involved in locomotor rhythmicity. Plasma melatonin levels are not significantly reduced by parietalectomy, whereas pinealectomy dramatically lowers the level and completely eliminates the circadian rhythm of melatonin in the circulation. Isolated parietal eye, pineal, and retina all synthesize melatonin with robust circadian rhythmicity when maintained for ≥4 d in culture, although in the intact animal all or almost all of the circulating melatonin comes from the pineal. The circadian system of I. iguana is composed of multiple circadian oscillators that reside in different tissues and have specific and different roles.
Keywords: circadian rhythms, body temperature, locomotor activity, lizards, melatonin, pineal, parietal eye, retina
Although a great deal of experimental evidence indicates that circadian systems of multicellular organisms are composed of several discrete circadian oscillators,circadian organization, by which is meant the interactions of these oscillators with the environment, with each other, and with the rest of the organism, is poorly understood. This is attributable in part to lack of experimental attention and in part to emphasis on the use of mammalian models in which such organization is particularly difficult to dissect. Nonmammalian vertebrates can provide useful alternatives with which to develop the principles that underlie circadian organization, and the green iguana, the circadian system of which is described here, appears to offer particular advantages.
The pineal complex forms part of the diencephalic roof of the brain of most vertebrates; it may be directly photosensitive, as in fish, amphibians, reptiles, and birds, or it may receive photic information via sympathetic neural pathways, as in mammals. Many reptile species possess several circadian photoreceptors: a photoreceptive pineal gland, an extracranial parapineal organ (parietal eye), and deep brain photoreceptors (Engbretson, 1992; Solessio and Engbretson, 1993; Grace et al., 1996).
The pineal gland is a central component in the circadian organization of many nonmammalian vertebrates. In some Agnatha (Lampetra japonica) pinealectomy abolishes the circadian rhythm of locomotor activity (Morita et al., 1992) and has the same effect in some species of lizards (Underwood, 1983; Molina-Borja, 1996); in others it produces marked changes in the free-running period and circadian activity time (Underwood 1977, 1981, 1992; Foà, 1991; Innocenti et al., 1996); however, in Dipsosauros dorsalis it has no obvious effects (Janik and Menaker, 1990). Pinealectomy abolishes circadian body temperature and locomotor activity rhythms in the house sparrowPasser domesticus and disrupts or abolishes rhythmicity in other passerine birds (Gaston and Menaker, 1968; Binkley et al., 1971;McMillan, 1972; Gwinner, 1978; Ebihara and Kawamura, 1981; Fuchs, 1983). In pigeons (Columbia livia) the circadian rhythms of locomotor activity and body temperature are abolished by removal of the pineal and the retinas, both sources of circulating melatonin (Ebihara et al., 1984), and can be restored by rhythmic infusions of exogenous melatonin (Chabot and Menaker, 1992).
The isolated pineals of some birds, lizards, and fish, when culturedin vitro, can maintain circadian rhythms of melatonin synthesis for many days in constant darkness (Takahashi et al., 1980;Menaker and Wisner, 1983; Falcon et al., 1987; Barrett and Takahashi, 1995; Bolliet et al., 1996). The lateral eyes of the frog Xenopus laevis, chicken, quail, fish (Esox lucius), and mammals also contain self-sustained circadian oscillators that regulate melatonin synthesis (for review, see Cahill and Besharse, 1995; also see Menaker and Tosini, 1996; Tosini and Menaker, 1996a). Although there is evidence suggesting that the parietal eye of some lizards may synthesize melatonin (Quay, 1965; Firth and Kennaway, 1987), this structure has never been studied in vitro to test for the capacity to synthesize melatonin and for the presence of circadian oscillators.
We have recently shown that the lizard Iguana iguana when kept in constant ambient temperature displays endogenously generated circadian rhythms of body temperature and locomotor activity, and that these two rhythms may free run with different periods. These data suggest that spontaneous internal desynchronization between these two rhythms may occur in this species under some laboratory conditions. This result, which is unique among circadian responses of animals (although a similar response has been observed in humans; Aschoff and Wever, 1976), suggests that the two rhythms may be generated by two separate circadian oscillators (Tosini and Menaker, 1995). We report here the results of experiments designed to clarify the roles played by the retina, pineal, and parietal eye in the circadian organization of this species.
MATERIALS AND METHODS
Animals
Seven adults (weight range, 973–1985 gm) and 25 juveniles (weight range, 105–150 gm) of I. iguana were obtained from an authorized dealer (Safari Pet and Supply, San Diego, CA). They were housed in groups of two or three in large enclosures (130 × 70 × 40 cm). Heat for thermoregulation was furnished by a thermal pad placed beneath the floor of the enclosures; the air temperature was 26°C, and the light cycle was 12 hr light/12 hr dark (light from 7 A.M.–7 P.M. Eastern Standard Time). Lizards were fed with a diet of lettuce and fruit combined with Zu/Preem marmoset food and multivitamin–protein complex (Vitalife). Water was available ad libitum.
Temperature and activity recording
For experiments lizards were housed individually in plastic cages (measuring 48 × 26 × 20 cm) inside an environmental chamber at 27 ± 0.2°C with no daily component (see Tosini and Menaker, 1995). Body temperature and level of activity were measured by telemetry using implanted transmitters (models VM-FH in adults and XM-FM in juveniles; Mini-Mitter Co., Sunriver, OR). A radio receiver (model RA-1010, Mini-Mitter Co.) coupled to a data acquisition system (Dataquest III; Data Sciences Inc., St. Paul, MN) was placed under the plastic cage inside the environmental chamber. Body temperature and activity (as number of movements) were recorded in 6 min bins and saved on disk for later analysis. Cages were cleaned once per week, and animals were fed three times per week. Because the experiments were conducted in constant darkness, lizards inside the environmental chamber were fed with the aid of an infrared viewer (FJW Optical Systems, Elgin, IL) to avoid exposing them to visible light.
Surgery
Surgery was performed during the animal’s day (6–9 hr after the onset of light). To implant a transmitter in the abdominal cavity, animals were first cooled in a refrigerator (1–4°C) until immobilized and then embedded in crushed ice. A small cut (1–2 cm) was made on the lateral–ventral surface, and a transmitter was implanted in the abdominal cavity. The operation required 10–15 min. Animals were allowed to recover for 24 hr before recording began. For parietalectomy, animals were first cooled until immobilized and then embedded in crushed ice, and the parietal eye was removed using a microcurette (diameter, 0.5 mm). Sham surgery (sham PAR-X) was performed using the same procedure, except that instead of removing the parietal eye, a scale behind it was removed. The whole procedure lasted <1 hr. For pinealectomy the lizards were cooled and immobilized, and a square flap of skin above the pineal was reflected to expose the skull. Using a dental drill, a small circle of bone was cut out of the skull above the pineal exposing the pigmented dura. The pigmented layer was removed, the meninges covering the pineal were cut, and the pineal was grasped with fine spring forceps. After the pineal was removed, the small circle of bone was replaced, the wound was packed with Gelfoam, and the flap of skin was fixed in place with cyanoacrylate glue. Sham operations (sham PIN-X) were performed in the same manner, except that the pineal was not grasped and removed. During the surgery it was evident that the pineal was completely removed, because it came out easily as an intact structure. In some animals we also performed postmortem histological analysis to confirm the absence of pineal tissue. In all the animals examined no pineal tissue was found. After the operation lizards were allowed to recover for 6–8 hr before returning them to the environmental chamber.
Unfortunately it has not been possible for us or, for that matter, for other researchers (Foà, 1991; Innocenti et al., 1993) to remove the pineal gland without seriously damaging the parietal eye. Because we thought that data from completely parietalectomized animals would be easier to interpret than data from animals with uncontrolled damage to the parietal eye, we first parietalectomized and then pinealectomized all of the animals in which the role of the pineal was to be tested (also see Discussion).
The eyes of iguanas are large and heavily vascularized. Although it might be possible to remove them, we chose to avoid this trauma in the present study, because we thought that our data would be clearly interpretable as they stand.
Plasma extraction and radioimmunoassay
Fifty microliters of plasma from each blood sample (see below) were extracted in 2 ml of chloroform and washed first with 0.1m sodium carbonate buffer, pH 10.25, and then with water. A 1 ml aliquot of extract was dried under nitrogen, resuspended in 0.5 ml of phosphate buffer with 0.9% NaCl and 1% gelatin, and washed with 2 ml of ethyl alcohol (100%). The extract was then assayed for melatonin according to a modification of the method of Rollag and Niswender (1976). This assay has been already validated for I. iguana(Tosini and Menaker, 1996b). The intra-assay coefficients of variation for pooled culture medium quality control samples containing low (15.6 pg), medium (31.2 pg), and high (125 pg) levels of melatonin were 12.2, 10.4, and 8.5%, respectively.
Flow-through culture technique
Parietal eyes, retinas, and pineals were maintained individually in flow-through perifusion chambers constructed from ELISA plates (seeTosini and Menaker, 1996a) and were placed inside a light-tight incubator at the constant temperature of 27°C. Culture medium [medium 199 (Life Technologies, Gaithersburg, MD) with HBSS andl-glutamine] was delivered (1 ml/hr) to the superfusion chambers from a multichannel syringe pump via Teflon tubing. Each organ was placed in a well of the ELISA plate and sealed by a plastic plug. Two stainless steel tubes penetrated the plastic plug into the well; one delivered culture medium to the well, and the other carried medium from the chamber to a fraction collector via flexible tubing. Samples were collected at 2 hr intervals and stored at 4°C until assayed at the end of the experiment.
Melatonin levels in the medium were measured by radioimmunoassay in a modification of the method of Rollag and Niswender (1976). Because we were primarily interested in relative differences in melatonin (or methoxyindole) concentrations rather than in absolute levels, we validated the assay without extraction of the samples. Melatonin (0, 10, 25, 50, 100, 250, and 500 pg/tube; four replicates at each level) added to pooled perifusate collected from each of the organs at night was quantitatively recovered. Dilution curves of pooled day and pooled night medium 199 perifusate samples from each of the organs were parallel to those of standard melatonin, indicating equivalent binding kinetics in unknowns and standards. The lower and upper limits of the assay were 2.5 and 500 pg/tube, respectively.
Data analysis
To determine the free-running period (τ) and robustness for both the circadian rhythm of activity (CRA) and the circadian rhythm of body temperature (CRT), we used the χ2 period periodogram procedure (Sokolove and Bushell, 1978). The recorded time series data for both body temperature and locomotor activity, which showed a probability level for circadian rhythmicity of p > 95%, were considered as statistically significant rhythms. The robustness of these rhythms was numerically defined as the value of the Sokolove–Bushell QP statistic (see Refinetti et al., 1994). To determine the effects of PAR-X and PIN-X on the CRT and CRA, statistical analysis (see Results) was performed on data from the 10 d before parietalectomy and the 10 d after parietalectomy and pinealectomy.
Analyses of the melatonin rhythms from cultured parietal eyes, pineals, and retinas were performed using an iterative, coupled Fourier transform–nonlinear least squares estimation procedure developed by Dr. Marty Straume (National Science Foundation Center for Biological Timing, University of Virginia) (see Tosini and Menaker, 1996a; Plautz et al., 1997). This method is capable of accommodating temporal nonstationarities in time series data (e.g., drifting observable and/or nonconstant oscillatory amplitudes) and maintaining an ability to detect underlying rhythmic behavior. This analysis provides a means for estimating amplitude, period, phase, and significance level of rhythms in time series data. This software is available from the National Science Foundation Center for Biological Timing.
Experimental design
CRA and CRT in constant darkness and effects of parietalectomy and pinealectomy. Lizards were moved from group enclosures to individual cages placed inside the environmental chamber at the constant temperature of 27°C and were allowed to free run in constant darkness (DD) for at least 12 d. After this initial period, 11 animals (four adults and seven juveniles) were parietalectomized, whereas five animals (three adults and two juveniles) were subjected to sham surgery. After the surgery the animals were returned to the environmental chamber and allowed to free run for at least 12 d. After this, lizards that had been parietalectomized previously were pinealectomized, whereas animals that had been sham parietalectomized were sham pinealectomized and were returned to the environmental chamber. Animals were then allowed to free run for an additional 12 d.
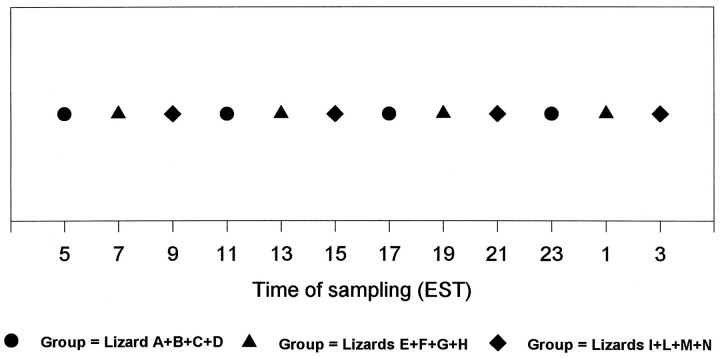
Circulating melatonin levels. Twelve juveniles were used in this experiment. Each lizard was kept in an individual plastic cage (48 × 26 × 20 cm), which was located inside a light-tight wooden box. The temperature inside the wooden box was maintained at a constant level of 27 ± 0.3°C. Food was provided three times per week, and water was available ad libitum. Lighting was provided by an overhead fluorescent bulb (Vita-lite 40 W), giving an average light intensity of 200 lux at the level of the lizard’s head. All lizards were held for 3 months under a 12 hr light/12 hr dark cycle (lights on at 7 A.M., lights off at 7 P.M.). Blood samples were taken from the lizards over a 24 hr period. The schedule of sampling is shown in Figure 1. Each test in DD started at 9 A.M. of the second day the lizard had spent in DD (39 hr after the light went off). The LD cycle was reinitiated with lights on at 7 A.M. of the fourth day. The short interval between the start of the DD treatment and the test was chosen to reduce variability (circadian oscillators are expected to free run with different periods in different individuals, thus increasing asynchrony among lizards as more time is spent in DD). Phase shift of the melatonin rhythm or suppression of melatonin synthesis by light was avoided by collecting the blood with the aid of an infrared viewer (FJW Optical Systems) when the collection interval fell during darkness. Sampling was accomplished by drawing ∼120 μl of blood from the blood vessel located on the midventral surface at the base of the tail with a 1 cc heparinized syringe. The blood was then transferred to heparinized tubes and centrifuged for 45 min at 4000 rpm, and the plasma was transferred to another tube and stored at −80°C until assayed.
Fig. 1.
Blood-sampling schedule. Twelve lizards (A–N) were subdivided into three groups (circles, triangles, diamonds) of four lizards each. Blood samples were drawn at 12 times (every 2 hr) during a 24 hr period (4 times from each group during the 24 hr) following the time schedule and alternation among groups shown. This schedule of sampling and the same grouping of lizards was used in both LD and DD (for results, see Fig. 5).
Melatonin release from cultured parietal eyes, pineals, and retinas. Parietal eyes, pineals, and retinas were removed from the lizards under anesthesia (ketamine, 100 mg/kg body weight) 30 min before the offset of the light (7 P.M.) and within 10–20 min were cultured individually at a constant temperature of 27°C either in a 12 hr light/12 hr dark cycle or in DD.
A fiber-optic light powered by a 150 W tungsten–quartz halogen lamp was used in the experiments in which the cultured organs were exposed to LD cycles. The source of the light was located outside the incubator, whereas the end of the fiber-optic probe was located inside the incubator about 15 cm from the plate containing the cultures. Light intensities at the level of the cultured organs were estimated by placing the irradiance detector of a United Detector Technology (Hawthorne, CA) UDT 350 radiometer-photometer at the location of the culture chambers during the experiments. The irradiance level was estimated to be 800 μW/cm2.
RESULTS
CRT and CRA in DD and the effects of parietalectomy and pinealectomy
All 16 intact lizards (seven adults and nine juveniles) tested in DD displayed significant circadian rhythms of body temperature and activity (Tables 1,2). The mean amplitudes of CRTs were 0.8 ± 0.2 and 0.5 ± 0.1°C in adults and juveniles, respectively (Figs. 2, 3). The difference in amplitude with age (i.e., size) was significant, but there were no differences among animals in the same age class (one-way ANOVA,p > 0.1). Comparisons of CRT and CRA showed that the CRA is more robust (i.e., the QP values are greater) than the CRT (Tables 1, 2, Fig. 4).
Table 1.
Period estimates from periodogram analysis of I. iguana body temperature time series data recorded during 10 d in DD in intact, parietalectomized, and pinealectomized animals
| ID | Intact | PAR-X | PIN-X | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | QP | p(%) | Period | QP | p(%) | Period | QP | p (%) | |
| Experimentals | |||||||||
| IO2 | 24.1 | 365 | 96 | 24.41-a | 379 | 98 | NS | ||
| IO3 | 24.0 | 430 | 100 | 24.51-a | 478 | 100 | NS | ||
| IO4 | 23.8 | 663 | 100 | 24.11-a | 355 | 100 | NS | ||
| IO5 | 24.1 | 417 | 100 | 24.61-a | 410 | 100 | 23.11-b | 345 | 96 |
| IO7 | 24.0 | 459 | 100 | 23.31-a | 392 | 95 | NS | ||
| IO9 | 23.5 | 1150 | 100 | 23.81-a | 665 | 100 | NS | ||
| IO10 | 23.8 | 377 | 100 | 24.41-a | 339 | 95 | NS | ||
| IO11 | 23.9 | 398 | 100 | 24.31-a | 421 | 100 | NS | ||
| IO12 | 24.3 | 1005 | 100 | 24.1 | 452 | 100 | NS | ||
| IO13 | 23.6 | 1041 | 100 | 24.11-a | 396 | 100 | NS | ||
| IO14 | 24.1 | 487 | 100 | 24.51-a | 449 | 98 | NS | ||
| Controls | |||||||||
| IO1 | 23.9 | 438 | 100 | 23.8 | 456 | 100 | 23.9 | 361 | 97 |
| IO6 | 23.8 | 335 | 96 | 23.9 | 337 | 100 | 23.9 | 401 | 98 |
| IO8 | 23.9 | 1295 | 100 | 23.8 | 452 | 100 | 23.8 | 496 | 100 |
| IO15 | 24.1 | 530 | 100 | 23.9 | 512 | 100 | 23.8 | 652 | 100 |
| IO16 | 23.9 | 391 | 99 | 24.1 | 756 | 100 | 24.0 | 725 | 100 |
Adults, IO1–IO7; juveniles, IO8–IO16. Controls received sham surgeries.
Change (>0.2 hr) of the period after parietalectomy.
Change after pinealectomy.
Table 2.
Period estimates from periodogram analysis of I. iguana locomotor activity time series data recorded during 10 d in DD in intact, parietalectomized, and pinealectomized animals
| ID | Intact | PAR-X | PIN-X | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | QP | p(%) | Period | QP | p(%) | Period | QP | p (%) | |
| Experimentals | |||||||||
| IO2 | 23.9 | 675 | 100 | 24.1 | 550 | 100 | 23.22-a | 432 | 100 |
| IO3 | 23.8 | 888 | 100 | 24.32-b | 906 | 100 | 24.4 | 703 | 100 |
| IO4 | 23.6 | 682 | 100 | 23.5 | 750 | 100 | 23.92-a | 553 | 100 |
| IO5 | 24.0 | 622 | 100 | 24.2 | 645 | 100 | 23.02-a | 662 | 100 |
| IO7 | 24.1 | 499 | 100 | 24.2 | 501 | 100 | 24.52-a | 485 | 100 |
| IO9 | 23.5 | 750 | 100 | 23.6 | 790 | 100 | 23.92-a | 719 | 100 |
| IO10 | 23.8 | 555 | 100 | 24.22-b | 581 | 100 | 24.52-a | 542 | 100 |
| IO11 | 24.1 | 722 | 100 | 24.1 | 503 | 100 | 23.62-a | 504 | 100 |
| IO12 | 24.6 | 900 | 100 | 24.1 | 576 | 100 | 23.9 | 705 | 100 |
| IO13 | 24.2 | 975 | 100 | 23.82-b | 631 | 100 | 23.9 | 590 | 100 |
| IO14 | 23.9 | 805 | 100 | 23.9 | 906 | 100 | 23.12-a | 752 | 100 |
| Controls | |||||||||
| IO1 | 24.1 | 470 | 100 | 24.0 | 381 | 100 | 24.1 | 412 | 100 |
| IO6 | 23.9 | 501 | 100 | 24.1 | 556 | 100 | 23.9 | 587 | 100 |
| IO8 | 23.7 | 812 | 100 | 23.8 | 615 | 100 | 23.8 | 623 | 100 |
| IO15 | 23.9 | 435 | 100 | 23.7 | 498 | 100 | 23.8 | 502 | 100 |
| IO16 | 24.1 | 986 | 100 | 23.9 | 856 | 100 | 24.1 | 866 | 100 |
Adults, IO1–IO7; juveniles, IO8–IO16. Controls received sham surgeries.
Change after pinealectomy.
Change (>0.2 hr) of the period after parietalectomy.
Fig. 2.
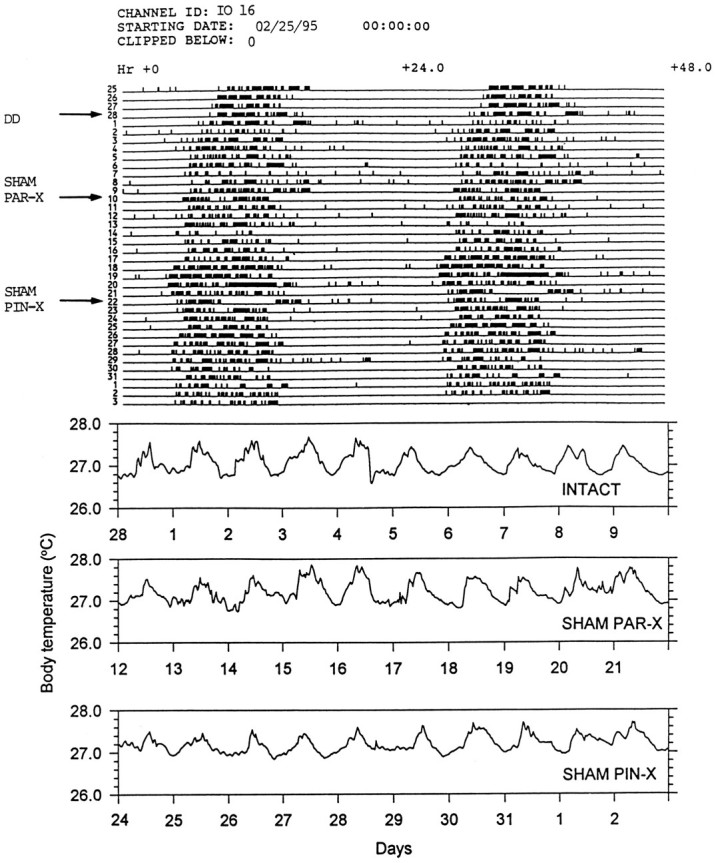
Locomotor activity (top) and body temperature (bottom) records of an individual ofI. iguana (IO 16) showing effects on these rhythms of sham PAR-X and sham PIN-X in constant temperature and darkness. The arrows indicate the days on which the animal was released in DD, sham PAR-X, and sham PIN-X. Estimates of the free-running period for these data sets are reported in Tables 1 and2.
Fig. 3.
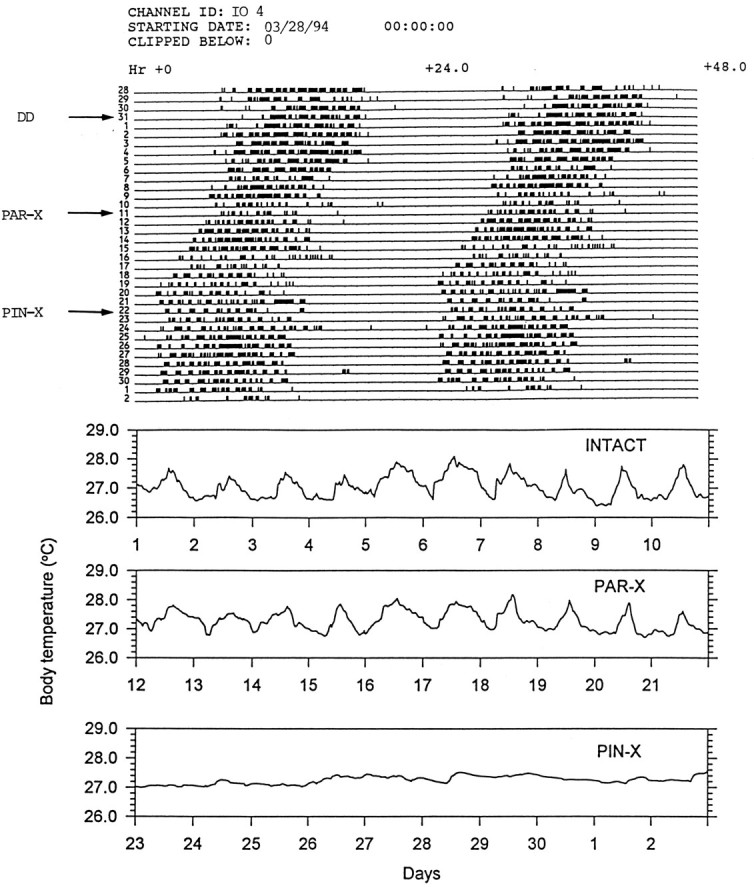
Locomotor activity (top) and body temperature (bottom) records of an individual ofI. iguana (IO 4) showing effects on these rhythms of PAR-X and PIN-X in constant temperature and darkness. The arrows indicate the days on which the animal was released into DD, PAR-X, and PIN-X. Estimates of the free-running period for these data sets are reported in Tables 1 and2.
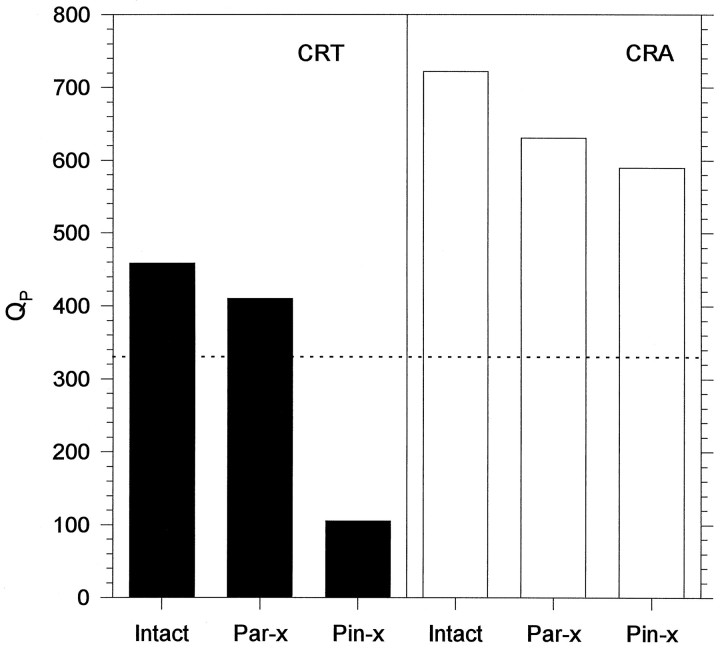
Fig. 4.
Effects of PAR-X and PIN-X on the robustness of the body temperature and locomotor activity rhythms of I. iguana. Each bar corresponds to the median of 11 animals. The line indicates the 0.05 level of significance, values below this line indicate arrhythmicity.
Eleven of the sixteen lizards free running in DD were PAR-X and five were sham PAR-X. All of these animals continued to display CRT and CRA (Tables 1, 2). Although parietalectomy did not affect the amplitude of the CRT (Fig. 3), 10 of 11 lizards showed changes in the free-running period (the free-running period lengthened between 0.3 and 0.6 hr), and the robustness of this rhythm was somewhat reduced (Table 1, Fig. 4). After parietalectomy, three lizards showed changes of CRA free-running period; however, the robustness of the CRA was not affected (Table 2, Fig. 4). Sham PAR-X lizards did not show any differences with respect to intact animals (Tables 1, 2, Fig. 3). After parietalectomy, but not before, QP values of the CRA were higher thanQP values of the CRT (Fig. 4).
All of the lizards survived parietalectomy and sham parietalectomy and were then PIN-X or sham PIN-X. Pinealectomy produced a dramatic effect on the CRT (Table 1, Fig. 3). In 10 (91%) of 11 lizards the CRT was completely abolished. CRA was not abolished by pinealectomy. However, in eight lizards significant changes in τ (three shortened and five lengthened τ; Table 2) were observed. There was no effect on the robustness of the CRA with respect either to the intact animals or to the PAR-X ones (Kruskal–Wallis test, p > 0.1) (Fig.4). Sham pinealectomy did not produce any significant effects either on the CRT or on the CRA (Kruskal–Wallis tests, p > 0.1) (Tables 1, 2, Fig. 2).
Circulating melatonin levels
There was a robust daily rhythm of melatonin in the plasma of intact lizards in the LD cycle (Fig.5A); melatonin concentration increased rapidly after the offset of the lights (7 P.M.), reached a peak at 1 A.M., and began to decrease before the onset of the lights. After parietalectomy lizards did not show any significant differences in the levels of circulating melatonin with respect to intact lizards (t tests, p > 0.1). Pinealectomy abolished the normal circadian rhythm in melatonin level; levels throughout the 24 hr period were relatively constant and were not significantly different from levels measured during the day for intact animals (t test, p > 0.1).
Fig. 5.
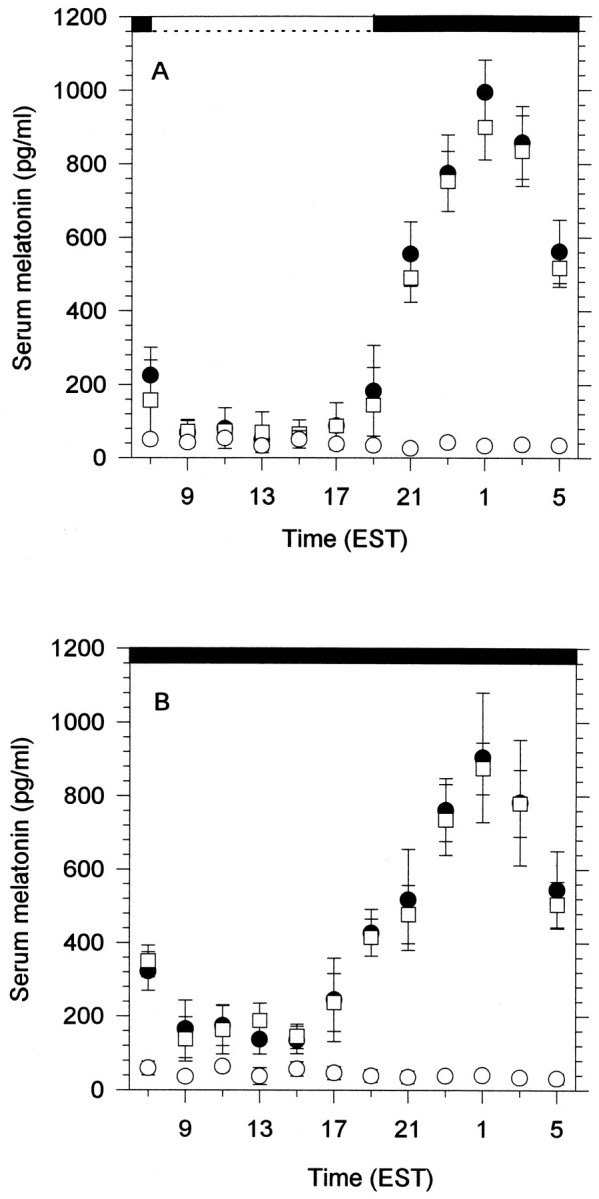
Mean serum melatonin profiles for 12 individuals of I. iguana under a 12 hr light/12 hr dark cycle (A) and mean serum melatonin profiles in constant darkness (B). Each pointrepresents the mean value of serum melatonin of four individuals. Each individual was sampled every 6 hr (Fig. 1). Error bars represent SEM. If error bars are lacking, the SEM is encompassed by the symbol representing the mean values of intact (•), PAR-X (□), PIN-X (○) lizards. Each of the 12 lizards was subjected to sampling under six conditions: as intact in LD and DD, as parietalectomized in LD and DD, and finally as pinealectomized (and parietalectomized) in LD and DD. See Figure 1 for sampling schedule.
In constant darkness, both intact and PAR-X lizards showed clear rhythms in plasma melatonin level (Fig. 5B), with a somewhat broader peak than in the 12 hr LD cycle (Fig. 5, compare A, B). Melatonin levels of PIN-X lizards in DD were low throughout the 24 hr and were not different from those recorded in PIN-X lizards in the LD cycle (t test, p > 0.1).
Melatonin synthesis in cultured parietal eyes, pineals, and retinas
Individually cultured parietal eyes, pineals, and retinas “released” melatonin into the perifusate rhythmically and were entrained to the LD cycles with the normal phase relationship; i.e., melatonin was high during the dark phase of the cycle and low during the light phase (Fig. 6). (Release is used here in the general rather than the technical sense, because all available evidence indicates that melatonin diffuses out of cells as it is synthesized and is not subject to controlled release). Although there are quantitative differences in the amplitude of the rhythms within and between the different structures, the general pattern is remarkably stable and uniform. In all cases the decrease in melatonin release preceded the lights-on transition, suggesting that the rhythm is not directly driven by the light itself but rather shows the anticipatory behavior that may occur when a self-sustained oscillator is entrained. To test for the influence of self-sustained circadian oscillators on melatonin rhythmicity in the parietal eye, the pineal, and the retina, the release of melatonin by these structures was examined in DD for 4 d. Figure 7shows 4 d of melatonin release in DD from simultaneously but separately cultured parietal eye, pineal gland, and retina from the same animal. In all three structures the circadian rhythms of melatonin release free ran with a period close to but different from 24 hr (Table3).
Fig. 6.
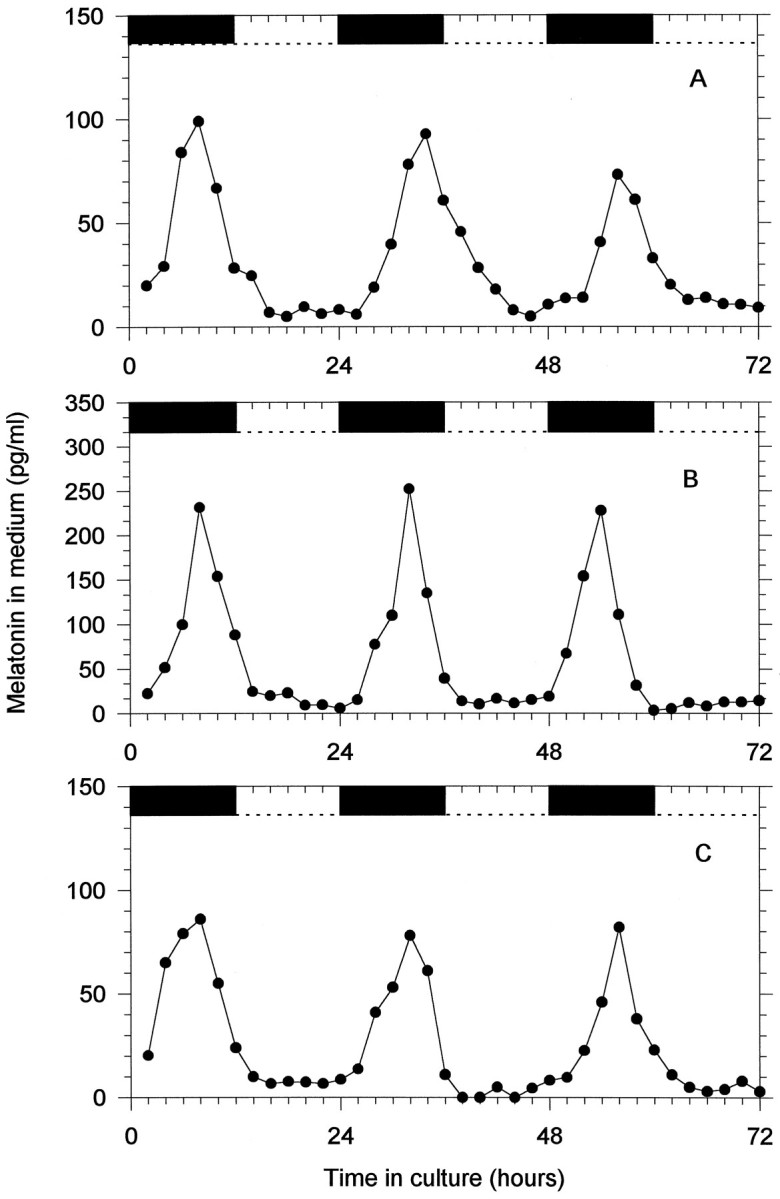
In vitro melatonin rhythms recorded under a 12 hr light/12 hr dark cycle from parietal eye (A), pineal (B), and retina (C) collected from the same individual and placed simultaneously in the culture apparatus. The black barsat the top represent periods of darkness.
Fig. 7.
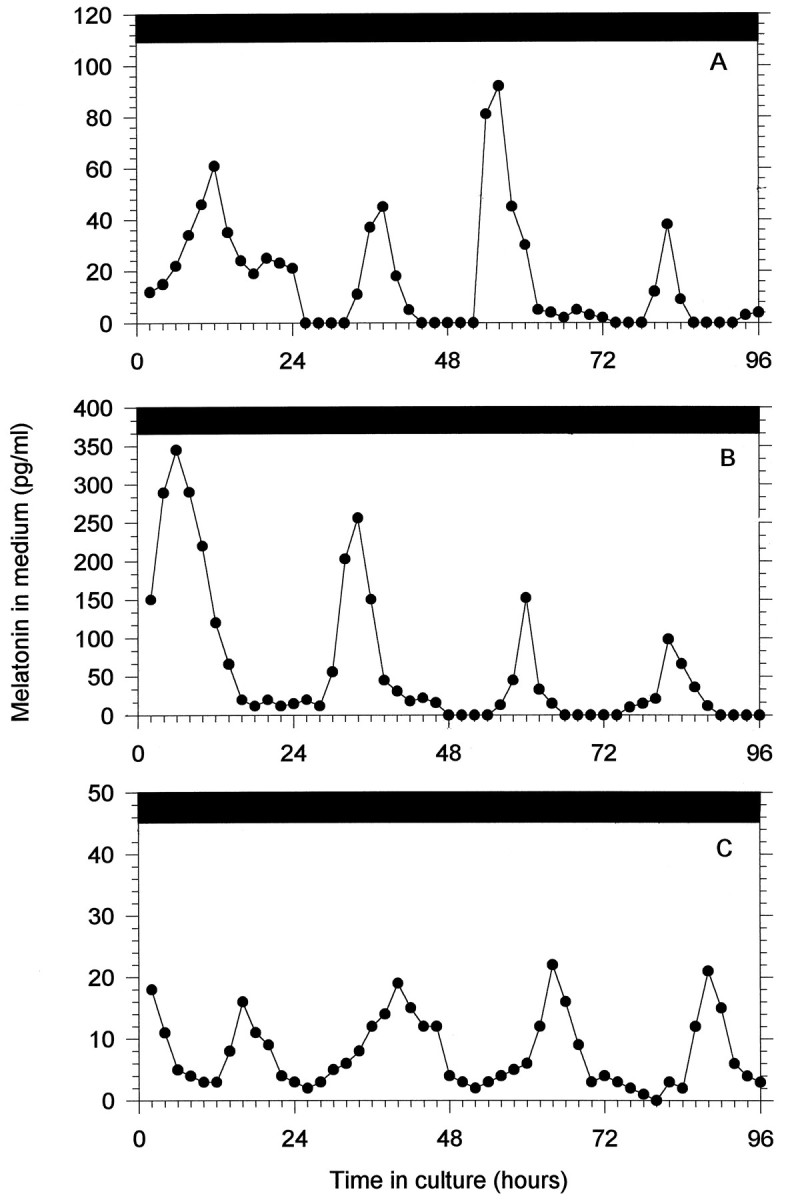
In vitro circadian rhythms of melatonin release during 4 d in DD from parietal eye (A), pineal (B), and retina (C) collected from the same individual and placed simultaneously in the culture apparatus.
Table 3.
Average free-running periods (see Materials and Methods) of circadian rhythms of melatonin release by cultured parietal eye, pineal, and retina of I. iguana held in constant darkness for 4 d and mean melatonin release (i.e., the total amount of melatonin released during the entire experiment divided by the number of samples) and averaged peak level
| n | τ ± SEM (hr) | Mean release (pg/ml) | Peak ± SEM (pg/ml) | |
|---|---|---|---|---|
| Parietal eye | 5 | 24.8 ± 1.3 | 28.2 | 70.1 ± 5.1 |
| Pineal | 5 | 25.5 ± 1.2 | 70.1 | 249.8 ± 25.7 |
| Retina | 5 | 25.1 ± 1.1 | 19.4 | 38.2 ± 14.9 |
DISCUSSION
We have demonstrated directly that an individual I. iguana possesses four self-sustained circadian oscillators (one in the pineal organ, one in the parietal eye, and one in each lateral eye). Furthermore, it is likely that each of these organs actually contains a large number of individual cellular circadian oscillators, as has been demonstrated for the chicken pineal (Takahashi and Menaker, 1984), and that the locomotor rhythm is driven by a fifth set of self-sustained circadian oscillators in the hypothalamus [suprachiasmatic nucleus (SCN)] as in D. dorsalis andPodarcis sicula (Janik et al., 1990; Minutini et al., 1995; see also Underwood and Edmonds, 1995). Each of the structures that we have demonstrated to contain circadian oscillators is also photoreceptive and therefore has direct access to environmental light cycles. In addition, deep brain photoreceptors with input to the circadian system exist in most nonmammalian vertebrates (for review, see Foster et al., 1994) and have been tentatively identified inI. iguana (Grace et al., 1996).
I. iguana is perhaps the best example of what has by now become a generalization to which there are no clearly documented exceptions; circadian rhythms in behavior and physiology of all vertebrates (including mammals) are generated and controlled, not by a single biological clock, but by a circadian system composed of several photoreceptors, several self-sustained oscillators, and their interconnections. These complexities raise questions on at least three levels: (1) how circadian systems are organized (i.e., how the environment affects the components of the system, how the components affect each other, and how the system regulates its outputs); (2) how the circadian organization of a particular species relates to the environmental niche that it occupies; and (3) how vertebrate circadian systems have evolved, in particular, what selection pressures have produced the variability that we observe (Menaker and Tosini, 1996). This formidable set of questions cannot be approached other than by detailed analysis of the circadian systems of several different vertebrate species. For reasons that are clear from the data reported above, I. iguana is an excellent candidate for one such model species.
The endogenously generated circadian rhythm of body temperature change (in itself remarkable in such a small ectotherm; Bartholomew, 1982) depends on the presence of the pineal gland, although our experimental design does not rigorously exclude the possibility that the effects that we are attributing to pinealectomy are in fact the results of pinealectomy plus parietalectomy. By implication (although not by direct demonstration) the body temperature rhythm is a consequence of the circadian rhythm of circulating melatonin for which the pineal is solely responsible. Melatonin levels are normally high in the circulation at night when the body temperature is low; therefore, it is likely that melatonin suppresses heat production (i.e., basal metabolic rate), increases heat loss, or both. Its effect on body temperature is not an indirect result of an effect on activity, because pinealectomy does not abolish the circadian activity rhythm or markedly reduce the level of activity (Fig. 3). However, pinealectomy did have small but significant effects on the free-running period of the activity rhythms of 8 of 11 lizards, suggesting that the pineal oscillator is weakly coupled to whatever circadian oscillator (SCN?) is driving the activity rhythm. The observation that an identified circadian oscillator (in this case the pineal) drives one circadian output rhythm but not another is unique in the circadian literature and suggests the need to reevaluate the interpretation of experiments in which lesion of a suspected circadian oscillator has no effect on a single endpoint (e.g., our own studies of pinealectomy in D. dorsalis; Janik and Menaker, 1990).
Very little is known about the function of the parietal eye of lizards. Although it has previously been suspected of producing melatonin (Quay, 1965; Firth and Kennaway, 1987), ours is the first unequivocal demonstration of this capacity. The observation that melatonin is synthesized under the control of circadian oscillators within the parietal eye is also unique. Rhythmic synthesis of melatonin by cultured retinas has now been shown in amphibians (for review, seeCahill and Besharse, 1995), reptiles (this paper), and mammals (Tosini and Menaker, 1996a), and the photoreceptive pineals of all classes of nonmammalian vertebrates also contain circadian oscillators that regulate melatonin synthesis (Underwood, 1990; Takahashi et al., 1989). Therefore, it appears that all vertebrate photoreceptors synthesize melatonin rhythmically (although the deep brain photoreceptors possessed by most nonmammalian vertebrates have not yet been tested either for rhythmicity or for the capacity to synthesize melatonin). This generalization supports the suggestion (Gern, 1982) that melatonin must fulfill unknown but critically important functions in photoreception itself, perhaps in light and/or dark adaptation. Elsewhere (Menaker and Tosini, 1996) we have speculated that its role in photoreception may have been its original function and that it was converted into a circulating hormone by a simple amplification of the synthetic capacity of the pineal and/or suppression of the enzymatic pathway for its rapid degradation. These speculations suggest an explanation for the role of melatonin rhythmicity in the parietal and lateral eyes of I. iguana; it may fulfill a local function involved in some way with photoreceptor adaptation to the environmental light/dark cycle and might play either no role or only a small one in circadian regulation at the level of the whole organism. However, even if correct, this explanation is far from complete, because it does not deal with the several ways in which the retina and parietal eye might be coupled to the rest of the circadian system, either as sources of photic information or as recipients of circadian signals. Nor is such an explanation applicable to all nonmammalian vertebrates, because there are at least two well documented cases among birds in which the retinas exert circadian influence at the organismal level (Konishi et al., 1985; Oshima et al., 1989; Underwood et al., 1990). Viewed in this way, the circadian system of I. iguana may conform rather closely to the general neuroendocrine loop model proposed by Cassone and Menaker (1984) if one assumes that the locomotor activity rhythm is driven by the SCN. Revisions of this model required by our new information are limited to (1) removal of the uncertainty about the existence of a retinal circadian oscillator, (2) addition of a parietal eye circadian oscillator, and (3) recognition that each oscillator in the system may regulate different rhythmic outputs. Although the model of Cassone and Menaker (1984) remains a useful framework on which to hang new facts about vertebrate circadian organization, it is well to remember that it has been assembled using data from several different organisms and contains important untested assumptions.
Footnotes
This work was supported by International Human Frontier Science Program Fellowship LT-145/93 to G.T. and by Air Force Office of Scientific Research Grant F49620-97-1-0012 to M.M.
Correspondence should be addressed to Michael Menaker, Department of Biology, Gilmer Hall, University of Virginia, Charlottesville, VA 22903.
REFERENCES
- 1.Aschoff J, Wever R. Human circadian rhythms: a multioscillatory system. Fed Proc. 1976;35:2326–2332. [PubMed] [Google Scholar]
- 2.Barrett RK, Takahashi JF. Temperature compensation and temperature entrainment of the chick pineal cell circadian clock. J Neurosci. 1995;15:5681–5692. doi: 10.1523/JNEUROSCI.15-08-05681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew GA. Physiological control of body temperature. In: Gans C, Pough FH, editors. Biology of the reptilia 12, physiology C, physiological ecology. Academic; London: 1982. pp. 167–211. [Google Scholar]
- 4.Binkley S, Kluth E, Menaker M. Pineal function in sparrows: circadian rhythms and body temperature. Science. 1971;174:311–314. doi: 10.1126/science.174.4006.311. [DOI] [PubMed] [Google Scholar]
- 5.Bolliet V, Ali MR, Lapointe FJ, Falcon J. Rhythmic melatonin secretion in different teleost species: an in vitro study. J Comp Physiol [B] 1996;165:677–683. doi: 10.1007/BF00301136. [DOI] [PubMed] [Google Scholar]
- 6.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retina: regulation by a photoreceptor oscillators. Prog Retinal Res. 1995;14:267–291. [Google Scholar]
- 7.Cassone VM, Menaker M. Is the avian circadian system a neuroendocrine loop? J Exp Zool. 1984;232:539–549. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- 8.Chabot CC, Menaker M. Effects of physiological cycles of infused melatonin on circadian rhythmicity in pigeons. J Comp Physiol [A] 1992;170:615–622. doi: 10.1007/BF00199337. [DOI] [PubMed] [Google Scholar]
- 9.Ebihara S, Kawamura H. The role of the pineal and suprachiasmatic nucleus in the control of circadian locomotor activity rhythms in the Java sparrow, Padda oryzivora. J Comp Physiol [A] 1981;141:207–214. [Google Scholar]
- 10.Ebihara S, Uchiyama K, Oshima I. Circadian organization in the pigeon, Columbia livia: the role of the pineal organ and the eye. J Comp Physiol [A] 1984;154:59–69. [Google Scholar]
- 11.Engbretson GA. Neurobiology of the lacertilian parietal eye system. Ethol Ecol Evol. 1992;4:89–107. [Google Scholar]
- 12.Falcon J, Guerlotte JF, Voisin P, Collin J-P. Rhythmic melatonin biosynthesis in a photoreceptive pineal organ: a study in the pike. Neuroendocrinology. 1987;45:479–486. doi: 10.1159/000124778. [DOI] [PubMed] [Google Scholar]
- 13.Firth BT, Kennaway DJ. Melatonin content of pineal, parietal eye and blood plasma of the lizard, Thachydosaurus rugosus: effect of constant and fluctuating temperature. Brain Res. 1987;404:313–318. doi: 10.1016/0006-8993(87)91385-0. [DOI] [PubMed] [Google Scholar]
- 14.Foà A. The role of pineal and the retinae in the expression of circadian locomotor rhythmicity in the ruin lizard, Podarcis sicula. J Comp Physiol [A] 1991;169:201–207. [Google Scholar]
- 15.Foster RG, Grace MS, Provencio I, DeGrip WJ, Garcia-Fernandez JM. Identification of vertebrate deep brain photoreceptors. Neurosci Biobehav Rev. 1994;18:541–546. doi: 10.1016/0149-7634(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs JL. Effects of pinealectomy and subsequent melatonin implants on activity rhythms in the house finch (Carpodacus mexicanus). J Comp Physiol [A] 1983;153:413–419. [Google Scholar]
- 17.Gaston S, Menaker M. Pineal function: the biological clock in the sparrow? Science. 1968;160:1125–1127. doi: 10.1126/science.160.3832.1125. [DOI] [PubMed] [Google Scholar]
- 18.Gern WA. The evolution of melatonin function: a hypothesis. Adv Biosci. 1982;29:85–88. [Google Scholar]
- 19.Grace MS, Alones V, Menaker M, Foster RG. Light perception in the vertebrate brain: an ultrastructural analysis of opsin- and vasoactive intestinal polypeptide immunoreactive neurons in iguanid lizards. J Comp Neurol. 1996;367:575–594. doi: 10.1002/(SICI)1096-9861(19960415)367:4<575::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Gwinner E. Effects of pinealectomy on circadian locomotor activity rhythms in European starling, Sturnus vulgaris. J Comp Physiol [A] 1978;126:123–129. [Google Scholar]
- 21.Innocenti A, Minutini L, Foà A. The pineal and circadian rhythms of temperature selection and locomotion in lizards. Physiol Behav. 1993;53:911–915. doi: 10.1016/0031-9384(93)90268-k. [DOI] [PubMed] [Google Scholar]
- 22.Innocenti A, Bertolucci C, Minutini L, Foà A. Seasonal variations of pineal involvement in the circadian organization of the ruin lizard Podarcis sicula. J Exp Biol. 1996;199:1189–1194. doi: 10.1242/jeb.199.5.1189. [DOI] [PubMed] [Google Scholar]
- 23.Janik DS, Menaker M. Circadian locomotor rhythms in the desert iguana. I: the role of the eyes and the pineal. J Comp Physiol [A] 1990;166:803–810. doi: 10.1007/BF00187326. [DOI] [PubMed] [Google Scholar]
- 24.Janik DS, Pickard GE, Menaker M. Circadian locomotor rhythms in the desert iguana. II: effects of electrolytic lesions to the hypothalamus. J Comp Physiol [A] 1990;166:811–816. [PubMed] [Google Scholar]
- 25.Konishi H, Ohta M, Honma K. Important role of the eyes controlling the locomotor rhythm in quail. J Interdiscip Cycle Res. 1985;16:217–226. [Google Scholar]
- 26.McMillan JP. Pinealectomy abolishes the circadian rhythm of migratory restlessness. J Comp Physiol [A] 1972;79:105–112. [Google Scholar]
- 27.Menaker M, Tosini G. The evolution of vertebrate circadian system. In: Honma K, Honma S, editors. Circadian organization and oscillatory coupling. Hokkaido UP; Sapporo, Japan: 1996. pp. 39–52. [Google Scholar]
- 28.Menaker M, Wisner S. Temperature-compensated circadian clock in the pineal of Anolis. Proc Natl Acad Sci USA. 1983;80:6119–6121. doi: 10.1073/pnas.80.19.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minutini L, Innocenti A, Bertolucci C, Foà A. Circadian organization in the ruin lizard Podarcis sicula: the role of suprachiasmatic nuclei of the hypothalamus. J Comp Physiol [A] 1995;176:281–288. [Google Scholar]
- 30.Molina-Borja M. Pineal-gland and circadian locomotor-activity rhythm in the lacertid Gallotia galloti eisentrauti, pinealectomy induces arrhythmicity. Biol Rhythm Res. 1996;27:1–11. [Google Scholar]
- 31.Morita Y, Tabata M, Uchida K, Samejima M. Pineal-dependent locomotor activity of lamprey, Lampetra japonica, measured in relation to LD cycle and circadian rhythmicity. J Comp Physiol [A] 1992;171:555–562. [Google Scholar]
- 32.Oshima I, Yamada H, Goto M, Sato K, Ebihara S. Pineal and retinal melatonin is involved in the control of circadian locomotor activity and body temperature rhythms in the pigeon. J Comp Physiol [A] 1989;166:217–226. [Google Scholar]
- 33.Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay S. Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- 34.Quay WB. Retinal and pineal hydroxyindole-O-methyltransferase activity in vertebrates. Life Sci. 1965;4:983–991. doi: 10.1016/0024-3205(65)90202-x. [DOI] [PubMed] [Google Scholar]
- 35.Refinetti R, Kaufman CM, Menaker M. Complete suprachiasmatic lesions eliminate circadian rhythmicity of body temperature and locomotor activity in golden hamsters. J Comp Physiol [A] 1994;175:223–232. doi: 10.1007/BF00215118. [DOI] [PubMed] [Google Scholar]
- 36.Rollag MR, Niswender GD. Radioimmunoassay of serum concentration of melatonin in sheep exposed to different lighting conditions. Endocrinology. 1976;98:482–489. doi: 10.1210/endo-98-2-482. [DOI] [PubMed] [Google Scholar]
- 37.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 38.Solessio E, Engbretson GA. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature. 1993;364:442–445. doi: 10.1038/364442a0. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi JS, Hamm H, Menaker M. Circadian rhythms of melatonin release from individual superfused chicken pineal gland in vitro. Proc Natl Acad Sci USA. 1980;77:2319–2322. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi JS, Menaker M. Multiple redundant circadian oscillators within the isolated avian pineal gland. J Comp Physiol [A] 1984;154:435–440. [Google Scholar]
- 41.Takahashi JS, Murakami N, Nikaido SS, Pratt BL, Robertson LM. The avian pineal, a vertebrate model system of the circadian oscillator: circadian regulation of circadian rhythms by light, second messengers, and macromolecular synthesis. Recent Prog Horm Res. 1989;45:279–348. doi: 10.1016/b978-0-12-571145-6.50010-8. [DOI] [PubMed] [Google Scholar]
- 42.Tosini G, Menaker M. Circadian rhythm of body temperature in an ectotherm (Iguana iguana) J Biol Rhythms. 1995;10:248–255. doi: 10.1177/074873049501000307. [DOI] [PubMed] [Google Scholar]
- 43.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996a;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 44.Tosini G, Menaker M. Pineal complex and melatonin affect the daily rhythm of temperature selection in the green iguana. J Comp Physiol [A] 1996b;179:135–142. doi: 10.1007/BF00193441. [DOI] [PubMed] [Google Scholar]
- 45.Underwood H. Circadian organization in lizards: the role of the pineal organ. Science. 1977;195:587–589. doi: 10.1126/science.835015. [DOI] [PubMed] [Google Scholar]
- 46.Underwood H. Circadian organization in the lizard Sceloporus occidentalis: the effects of pinealectomy, blinding and melatonin. J Comp Physiol [A] 1981;141:537–547. [Google Scholar]
- 47.Underwood H. Circadian organization in the lizard Anolis carolinensis: a multioscillatory system. J Comp Physiol [A] 1983;152:265–274. [Google Scholar]
- 48.Underwood H. The pineal and melatonin: regulators of circadian function in lower vertebrates. Experientia. 1990;46:120–128. doi: 10.1007/BF01955437. [DOI] [PubMed] [Google Scholar]
- 49.Underwood H. Endogenous rhythms. In: Gans C, Crew D, editors. Biology of the reptilia 18, hormones, brain, and behavior. University of Chicago; Chicago: 1992. pp. 229–297. [Google Scholar]
- 50.Underwood H, Edmonds K. The circadian rhythm of thermoregulation in Japanese quail. II. Multioscillatory control. J Biol Rhythms. 1995;10:234–247. doi: 10.1177/074873049501000306. [DOI] [PubMed] [Google Scholar]
- 51.Underwood H, Barrett RK, Siopes T. The quail’s eye: a biological clock. J Biol Rhythms. 1990;5:257–265. doi: 10.1177/074873049000500307. [DOI] [PubMed] [Google Scholar]