Abstract
The entire deletion of the cysteine string protein (CSP) gene causes a temperature-sensitive (ts) block of evoked neurotransmission in Drosophila. CSP has been found to interact in vitro with the clathrin-uncoating ATPase HSC70, suggesting a potential role of CSP in vesicle recycling. Using FM1-43 imaging, we analyzed whether the ts block of neurotransmission incsp mutants is caused by a defect in vesicle exocytosis or vesicle recycling. We determined that FM1-43-labeled synaptic boutons of csp mutant neuromuscular junctions fail to destain at 32°C after K+ depolarization, and that FM1-43 dye uptake cannot be evoked by K+ stimulation at 32°C. However, when we stimulated dye uptake independent of depolarization by using black widow spider venom (BWSV), we observed endocytotic uptake of FM1-43. This suggests that endocytosis exhibits no primary ts defect. In addition, we found no ts defect of vesicle recycling at 32°C that would correlate with the ts block of neurotransmission. We also discovered that BWSV and the calcium ionophore calcimycin stimulate FM1-43 destaining and quantal release incsp mutants at 32°C when depolarization fails to evoke any response. The wild-type-like, calcimycin-induced response incsp null mutants indicates that some aspect of the depolarization-dependent calcium signaling pathway must be impaired, either calcium entry, calcium action, or both. Collectively, our results indicate that the csp mutation affects calcium secretion coupling of evoked exocytosis but not vesicle recycling. This supports the hypothesis that CSP links synaptic vesicles to calcium secretion coupling.
Keywords: cysteine string protein, CSP, BWSV, calcimycin, synaptic vesicles, neuromuscular junction, exocytosis, endocytosis, vesicle recycling, synaptic transmission
The cysteine string proteins (CSPs) are conserved from invertebrates to mammals, including humans (for review, see Umbach et al., 1995; Buchner and Gundersen, 1997;Zinsmaier, 1997). The various CSP isoforms are associated with diverse vesicle membranes such as granules and synaptic, endocrine, and exocrine vesicles (Mastrogiacomo et al., 1994; Zinsmaier et al., 1994;Braun and Scheller, 1995; Kohan et al., 1995; Chamberlain and Burgoyne, 1996; Chamberlain et al., 1996; van deGoor and Kelly, 1996; Pupier et al., 1997). It has been suggested that the presence of the J domain in CSP mediates a cooperative interaction with proteins of the heat shock protein 70 family (Cyr et al., 1994). Recently, CSP has been shown to specifically stimulate in vitro the ATPase activity of the clathrin-uncoating ATPase heat shock coguate 70 (HSC70) (Braun et al., 1996; Chamberlain and Burgoyne, 1997) but not the ATPase activity of the Nethylmaleimide-sensitive fusion protein (Braun et al., 1996).
The precise role of CSP is still unknown, although the significance of CSP for neurotransmitter release has been demonstrated by the initial analysis of csp mutant Drosophila strains (Umbach et al., 1994; Zinsmaier et al., 1994). The complete loss of CSP causes a 50% reduction of evoked neurotransmitter release in mutant flies at 22°C. With gradually increasing temperatures, evoked release becomes increasingly impaired until it is completely blocked at >29°C. This has been demonstrated for adult first-order interneurons of the visual system (Zinsmaier et al., 1994) and for larval neuromuscular junctions (NMJs) (Umbach et al., 1994). However, spontaneous neurotransmitter release persists at high temperatures (Umbach et al., 1994). It has been shown independently that Torpedo CSP seems to act as a positive modulator of N-type calcium channels when coexpressed in frog oocytes (Gundersen and Umbach, 1992). Because CSP is apparently localized to secretory vesicles, this led to the proposal that CSP may link synaptic vesicles to presynaptic calcium channels (Mastrogiacomo et al., 1994). So far, however, no direct molecular interaction has been detected between CSP and presynaptic calcium channels (Martin-Moutot et al., 1996; Pupier et al., 1997).
Our initial electrophysiological studies of csp mutant flies did not determine whether the temperature-sensitive (ts) block of neurotransmitter release is caused by a defect of exocytosis or by a failure of vesicle recycling and genesis that would terminate neurotransmitter release by depleting the vesicle pool of mature synaptic vesicles. Such a potential defect of vesicle recycling, as suggested by Sudhof (1995), seems possible for two reasons. First, the interaction of CSP with HSC70 implies a potential role of CSP in vesicle recycling (Braun et al., 1996; Chamberlain and Burgoyne, 1997), because the only known function of HSC70 at the synaptic terminal is the uncoating of clathrin-coated vesicles (Sudhof, 1995). Second, the ts block of neurotransmitter release in csp mutants develops slowly with a lag phase of ∼2–10 min (Umbach et al., 1994), which could indicate a depleted vesicle pool. To resolve this issue, we determined whether vesicle recycling exhibits a ts block incsp mutant larvae that would correlate with the ts block of neurotransmitter release.
MATERIALS AND METHODS
Drosophila stocks and culture
Flies were cultured in standard medium at 20°C. The followingDrosophila strains were used: wild-type Berlin,shits1 (stock collection, Caltech),cspE16, and cspX1. Both csp mutant alleles represent null mutations (Zinsmaier et al., 1994). The strain shits1 was kept homozygous, whereas the csp alleles were kept heterozygous over the balancer chromosome TM6, Tubby (Tb). Absence of the dominant Tb marker allowed the selection ofcsp homozygous larvae.
Larval body wall muscle preparation
Climbing third instar larvae were dissected to expose their body wall muscles. For dissection, larvae were placed dorsal side up on a small dish with a thin layer of Sylgard resin in calcium-free HL-3 medium (Stewart et al., 1994). The larvae were pinned down anteriorly and posteriorly and cut along the dorsal midline, and the filleted larvae were pinned out. After removing the viscera, the segmentally repeated larval body wall muscles and the innervating nerve fibers were clearly visible. The muscles were identified as described previously (Johansen et al., 1989). For most of the toxin experiments we removed the CNS to reduce extensive muscle contraction.
FM1-43 assays
FM1-43 staining induced by K+stimulation. FM1-43 (Molecular Probes, Eugene, OR) staining was induced by K+ depolarization essentially as described previously (Ramaswami et al., 1994). However, instead of a saline solution, we used modified HL-3 media (Stewart et al., 1994), which prolonged the survival time of the preparation by several hours. In general, endocytosis was monitored by incubating the larval body wall muscle preparation for 5 min at the indicated temperature in 60 mm KCl, 4 μm FM1-43, and 1.5 mmCaCl2 in HL-k medium (in mm: 15 NaCl, 20 MgCl2, 10 NaHCO3, 115 sucrose, 5 trehalose, and 5 HEPES, pH 7.2). Then the preparation was washed extensively for up to 1 hr at 4°C with calcium-free (containing 0.5 mm EGTA) HL-n medium (in mm: 75 NaCl, 20 MgCl2, 10 NaHCO3, 115 sucrose, 5 trehalose, and 5 HEPES, pH 7.2) to remove surface bound FM1-43. For all experiments we used the NMJs of the muscle fibers 6, 7, 12, and 13 as described by Johansen et al. (1989).
FM1-43 destaining induced by K+stimulation. After photodocumentation of FM1-43 staining, the preparation was destained by K+ depolarization to monitor depolarization-dependent exocytosis. FM1-43 destaining was stimulated at the indicated temperature for 10 min with 60 mm KCl and 1.5 mm CaCl2 in HL-k medium. For experiments at 32°C the larval preparation was preincubated for 15 min at 32°C, and all solutions were prewarmed.
FM1-43 staining induced by black widow spider venom stimulation. The larval body wall muscle preparation was incubated at the indicated temperature for 10 min in 4 μm FM1-43 and 1.5 mm CaCl2 in HL-n medium supplemented with 0.6 glands/ml crude black widow spider venom (BWSV). Then the preparation was extensively washed with Ca2+-free Hl-n medium at 4°C for 1 hr.
FM1-43 destaining induced by BWSV stimulation. BWSV induction of FM1-43 destaining was triggered by incubation with 0.6 glands/ml BWSV in Hl-n medium. BWSV stimulation was performed as indicated in the presence of external 1.5 mmCaCl2 or in the absence of Ca2+ (0.5 mm EGTA).
FM1-43 destaining induced by calcimycin stimulation. For the calcimycin (A23187; Molecular Probes) induction of FM1-43 destaining, we incubated the larval body wall muscle preparation for 10 min at the indicated temperature in 100 μm calcimycin, HL-n medium supplemented with 1.5 mm CaCl2 or with 0.5 mm EGTA (Ca2+-free).
FM1-43 pulse labeling during BWSV stimulation. Larval body wall preparations were equilibrated to 32°C for 15 min in Hl-n medium. In the presence of 1.5 mm CaCl2 in HL-n medium, exocytosis and endocytosis were stimulated with BWSV (0.6 glands/ml) at 32°C. Endocytosing vesicles were pulse-labeled by adding 4 μm FM1-43 for 5 min. The dye was removed by exchanging the solution twice with fresh BWSV solution (0.6 glands/ml BWSV, 1.5 mm CaCl2, HL-n medium). The BWSV stimulation was continued for 10 min at 32°C. Neurotransmission and BWSV were then inactivated by an incubation with formaldehyde (2%) in calcium-free HL-n medium for 5 min, and the preparation was washed extensively for up to 1 hr at 4°C in calcium-free HL-n medium before viewing. In contrast, in the control experiments the preparations were inactivated immediately after the FM1-43 pulse by the fixative incubation.
FM1-43 imaging. For photomicroscopy the stained preparation was kept in calcium-free HL-n medium, which ensured no spontaneous activity (Ramaswami et al., 1994). FM1-43-stained NMJs were visualized through fluorescein excitation and emission filters with a Reichert Polyvar 2 microscope. Images were acquired by standard microphotography using 400–1600 ASA Ektachrome film and a defined shutter-open time. Images of FM1-43 destaining were obtained either during or after the destaining protocol from the same NMJ that was used earlier for the FM1-43 uptake image. Identical shutter-open times were used for “staining” and “destaining” images. The temperature of the perfusion chamber (RC-20, Warner Instrument Corp.) was monitored and controlled using a stage-mountable heater platform (PH-5, Warner) connected to a TC-324A heater controller (Warner).
Preparation of black widow spider venom
Desiccated and frozen glands from Latrodectus mactans(black widow spider) were purchased from Sigma (St. Louis, MO) or collected by Hatari Invertebrates. The dissected spider glands were homogenized in 10 mm NaPO4, pH 7.2, with a glass homogenizer at 4°C. The homogenate was centrifuged at low speed for 5 min at 4°C to remove debris. We learned that it is essential to remove small molecular weight molecules; otherwise the classic slow-developing BWSV response is mixed with a fast depolarization-dependent induction of transmitter release (instantaneous). The slow response was sensitive to heat treatment, indicating the peptide nature of the agent. Therefore, the spider gland homogenate was gel-filtrated over a Sephadex G-50 spin column at 4°C to separate the interfering low molecular weight molecules from the high molecular weight latrotoxin proteins. The eluted proteins were pooled and stored at −80°C in small aliquots. Because this gel filtration only removes small molecules, we still refer to this homogenate as crude BWSV toxin.
Electrophysiology
All recordings were from muscle fibers 6 and 7 in the anterior abdomen of dissected third instar Drosophila larvae. Intracellular whole-cell recordings of miniature excitatory junction potentials (MEJPs) and evoked excitatory junction potentials (Jan and Jan, 1976) were made with a single microelectrode (20–40 MΩ) filled with 3 m KCl. Signals were amplified using an Axopatch-1D amplifier (Axon Instruments) and filtered at 1 kHz. The data were digitized at 10 kHz with a Digidata 1200 interface (Axon) and analyzed using the Axoscope application of pCLAMP 6.0 software. To stimulate evoked junction potentials (EJPs), nerve fibers were severed at the base of the ventral ganglion. EJPs were elicited with a suction electrode for 1 msec at one to two times threshold for maximum EJPs. All recordings of spontaneous or toxin-induced release were made in HL-3 medium (Stewart et al., 1994) supplemented with CaCl2as indicated (0 mm Ca2+ was supplemented with 0.5 mm EGTA). Calcimycin was added to a final concentration of 50 μm, tetrodotoxin to 1 μg/ml. The temperature of the recording chamber was monitored and controlled using a heater platform, a solution heater (PH-1 or SH-27A, Warner) connected to a TC-324A heater controller (Warner), or both. For the statistical analysis of MEJP frequencies, we determined the number of MEJPs with a minimal amplitude of 0.4 mV over several 15 sec intervals from a continuous recording.
RESULTS
The ts block of FM1-43 destaining at csp mutant NMJs is consistent with ts block of neurotransmitter release
We used the optical FM1-43 assay developed by Betz and Bewick (1992) to analyze synaptic vesicle endocytosis and exocytosis incsp mutants. This assay facilitates the direct visualization of endocytosis by the activity-dependent vesicular uptake of the lipophilic fluorescent dye FM1-43 (Betz and Bewick, 1992; Betz et al., 1992; Betz and Bewick, 1993). Further stimulation of the synapse causes activity-dependent FM1-43 destaining of the synaptic terminal attributable to exocytosis of dye-loaded synaptic vesicles (for review, see Betz et al., 1996). In Drosophila, this dye has been used successfully to monitor the dynamics of synaptic vesicle endocytosis at the larval NMJ (Ramaswami et al., 1994). For the analysis of the ts phenotype of csp null mutations, we chose the temperature of 32°C to ensure a complete block of CSP function, because our previous electrophysiological observations demonstrated that the ts block of neurotransmission is complete at >29°C (Umbach et al., 1994). In the following experiments we used shibire(shi) mutant flies as a positive control for a ts block of endocytosis, which slowly depletes the synaptic vesicle pool and terminates neurotransmitter release (Grigliatti et al., 1973; Ikeda et al., 1976; Poodry and Edgar, 1979; Kosaka and Ikeda, 1983a,b).
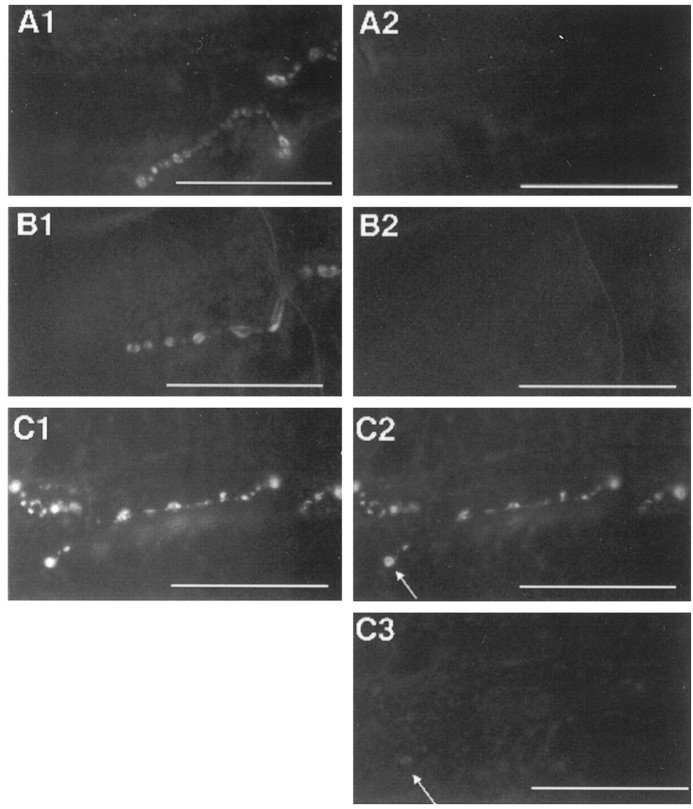
To test whether evoked synaptic vesicle exocytosis is indeed blocked incsp mutants at a nonpermissive temperature, we stained NMJs of dissected larval body wall preparations with FM1-43 by K+ stimulation at 22°C. After washing, we observed the typical punctate FM1-43 staining on wild-type muscle fibers, reminiscent of synaptic boutons at NMJs (Fig.1A1). This punctate staining corresponded well with type 1b and 1s terminals of muscle fibers 6 and 7 (Johansen et al., 1989; Atwood et al., 1993) when viewed by Nomarski optics (data not shown). After confirming the endocytotic uptake of FM1-43, we switched the temperature to 32°C to establish the complete ts block of evoked release in cspmutants and stimulated FM1-43 destaining by K+depolarization. Under these conditions, K+stimulation consistently failed to destain FM1-43 labeled synaptic boutons of csp mutant NMJs (Fig. 1C2). To demonstrate that this ts block of FM1-43 destaining is reversible, we cooled the preparation to 22°C, at which the FM1-43 labeled boutons of csp mutants were destained completely upon depolarization (Fig. 1C3). In control preparations the high temperature had no effect on the destaining of FM1-43-labeled synaptic boutons in wild-type or shi mutant larvae that were completely destained on K+ depolarization at 32°C (Fig.1A2,B2). The comparison of the csp andshi mutant phenotypes suggests that different mechanisms of vesicle trafficking are defective in each mutant. The ts block of depolarization-dependent FM1-43 destaining implies that synaptic vesicles are present in csp mutant terminals but unable to respond to nerve stimulation.
Fig. 1.
Depolarization fails to trigger FM1-43 destaining in csp mutants at 32°C. At 22°C K+ stimulation in the presence of FM1-43 induced a punctate FM1-43 staining of synaptic boutons at all NMJs of wild-type (A1), mutant homozygousshits1 (B1), and mutant homozygous cspE16 (C1) muscles. A subsequent K+ stimulation at 32°C completely destained the FM1-43-labeled synaptic boutons of wild-type (A2) and shits1 mutant (B2) NMJs. However, K+ stimulation at 32°C failed to destain the FM1-43-labeled synaptic boutons ofcspE16 mutant NMJs (C2). After cooling the cspE16 mutant preparation to 22°C, K+ depolarization destained the synaptic boutons (C3) that failed to destain with depolarization at 32°C (C2). Only minute traces of staining could be observed sometimes on large synaptic boutons (arrows). Each series of images (A–C) gives a partial view of the identical NMJ of muscle 6/7 after FM1-43 staining at 22°C (1), after FM1-43 destaining at 32°C (2), and after FM1-43 destaining at 22°C (3). Each series exemplifies at least 10 independent experiments (larvae) for each genotype. Scale bars, 50 μm.
Black widow spider venom bypasses the ts block of neurotransmitter release in csp mutants
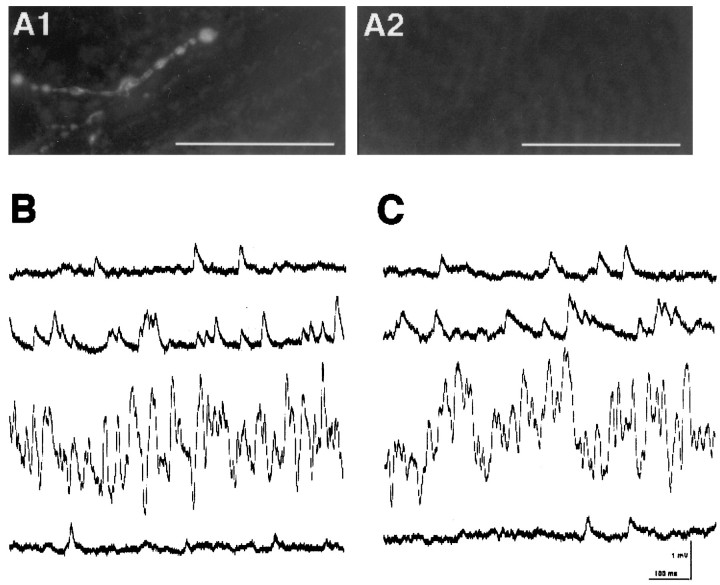
To characterize CSP function further, we tested whether the action of BWSV is impaired in csp mutants at 32°C when evoked release is blocked. Crude BWSV or its purified components, the latrotoxins, have been shown to induce a massive increase in the frequency of quantal transmitter release at vertebrate and invertebrate nerve endings, including Drosophila (Clark et al., 1970;Magazanik et al., 1992; Ramaswami et al., 1994; Storchak et al., 1994;Broadie et al., 1995; Linial et al., 1995). After staining NMJs with FM1-43 at 22°C, we switched the temperature to 32°C and tested whether ts block of evoked FM1-43 destaining had been established. No significant amount of FM1-43 staining was released from the boutons ofcsp mutants upon K+ stimulation (Fig.2A1). However, a subsequent stimulation with BWSV induced a complete destaining of FM1-43 at csp mutant NMJs in the presence of Ca2+ (Fig. 2A2). FM1-43 destaining could also be triggered by BWSV in the absence of external Ca2+; however, it required up to 60 min to destaincsp or wild-type NMJs (data not shown). These features are consistent with earlier reports demonstrating BWSV-induced release in the presence and absence of extracellular calcium (Clark et al., 1970;Magazanik et al., 1992; Ramaswami et al., 1994; Storchak et al., 1994;Broadie et al., 1995; Linial et al., 1995; Barnett et al., 1996). Independent recordings of MEJPs at 31°C consistently showed a dramatic increase in the frequency of quantal release after BWSV application, which was similar in wild-type andcspX1 mutant larvae (Fig.2B–C). Several minutes after the application of BWSV, the basal MEJP frequency of 3.7 ± 0.2 Hz (mean ± SEM;n = 3) for wild type and 3.7 ± 0.4 Hz (mean ± SEM; n = 3) for cspX1increased within 30 sec to a peak frequency of 61 ± 6 Hz (mean ± SEM; n = 3) for wild type and 70 ± 5 Hz (mean ± SEM; n = 3) forcspX1 before it steadily declined to the basal level. There was no significant difference between the peak frequency of BWSV-induced release for wild type and cspmutants (p = 0.27, Student’s ttest).
Fig. 2.
BWSV stimulates exocytosis incsp mutant NMJs at 32°C. A, Larval NMJs of muscle 6/7 from homozygous cspE16larvae were labeled by FM1-43 K+-induced depolarization at 22°C (data not shown). The ts block of K+-induced FM1-43 destaining at 32°C was confirmed, and no significant amounts of FM1-43 stain were released (A1). Subsequent FM1-43 destaining was stimulated with BWSV in the presence of external Ca2+ at 32°C. This completely destained the cspE16synaptic boutons of the same NMJ (A2) that failed to destain with the previous K+ depolarization (A1). The images represent eight independent experiments (larvae). B, C, Recordings of BWSV-induced quantal release from wild-type NMJs (B) andcspX1 homozygous NMJs (C) at 31°C. MEJPs were recorded continuously from muscle fiber 6 in the presence of external Ca2+at 31°C. The first trace (B, C) shows a typical example of MEJPs during continuous recording before the application of BWSV. After the application of BWSV (15–180 sec) a rapid increase of up to 50 Hz in MEJP frequency was observed within a 30 sec interval (trace 2, B, C). The peak frequencies (highest 15 sec interval observed per preparation) of this irregular response were 71 Hz for wild type and 77 Hz forcspX1 mutants and lasted for ∼1–2 min (trace 3, B, C). Within the next minute the MEJP frequency of the BWSV response declined steadily to its original level (trace 4, B, C). After this rather silent “depletion phase,” the BWSV response recovered and kept oscillating repeatedly, but with a significantly lower peak frequency (data not shown). With the onset of the high-frequency release induced by BWSV, a dramatic depolarization of the muscle was observed. The muscle potentials for these particular recordings dropped from −65 mV to −35 mV (wild type,B) and from −70 mV to −50 mV (cspX1, C). Both potentials recovered as soon as the frequency of release declined to its original value. Only recordings that recovered to the original muscle potential during the depletion phase were used for the analysis. Scale bars, 50 μm.
Calcimycin triggers calcium-dependent release at cspmutant NMJ at 32°C at a time when evoked release is blocked
Because the wild-type-like BWSV response in csp mutant larvae indicated that the mutation does not affect aspects of quantal release, we speculated that the csp mutation may indeed interrupt calcium signaling with depolarization at 32°C. Our previous work demonstrated that the csp mutation interferes with the calcium sensitivity of evoked release at 22°C, suggesting that CSP is involved in calcium secretion coupling (Umbach et al., 1994). However, because a morphological change of the NMJ could not be ruled out as an alternative cause for this phenotype, we gathered further evidence for this speculation. Calcium ionophores, such as calcimycin and ionomycin, have been shown to bypass voltage-gated calcium channels, to elevate intracellular calcium concentrations, and to trigger neurotransmitter exocytosis in synaptosomes (Verhage et al., 1991; Alder et al., 1992) and in hippocampal or bipolar cell synaptic terminals (von Gersdorff and Matthews, 1994; Capogna et al., 1996). However, calcium ionophores appear less effective than calcium channels in activating exocytosis (Verhage et al., 1991; von Gersdorff and Matthews, 1994; Capogna et al., 1996).
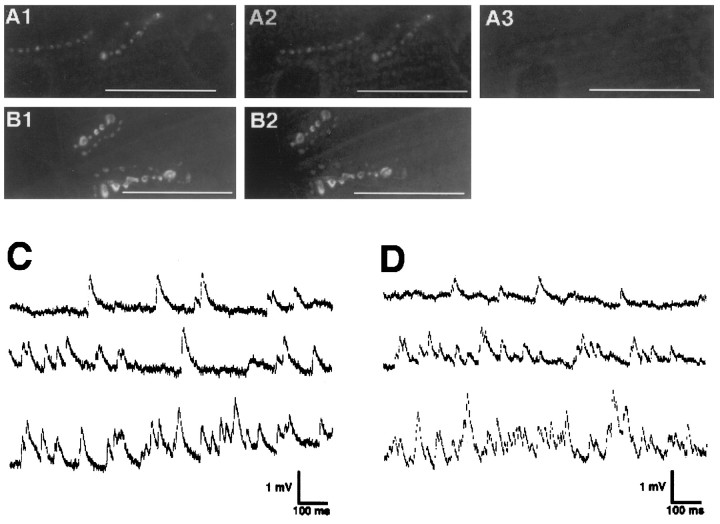
To test whether the calcium signaling pathway is affected by thecsp mutation at 32°C, we tested calcimycin (A-23187) for its ability to trigger exocytosis in csp mutants. After K+ depolarization failed to induce FM1-43 destaining at 32°C (Fig. 3A1), we stimulated the preparation with calcimycin in the absence of external Ca2+ at 32°C, which induced no significant destaining the synaptic boutons (Fig. 3A2). However, subsequent incubation at 32°C with calcimycin and external Ca2+ induced a complete destaining of the FM1-43-labeled csp mutant synaptic boutons (Fig.3A3). The calcimycin-induced FM1-43 destaining in the presence of external calcium could be blocked by preincubation of the preparation with BAPTA-AM ester (Fig. 3B2). Electrophysiological recordings from NMJs confirmed the ability of calcimycin to increase the frequency of quantal release significantly in wild type and in csp mutants at 32°C (Fig.3C,D). The frequency of quantal release increased from the basal spontaneous level of 3.5 ± 1.4 Hz (mean ± SEM;n = 3) for wild type and 2.9 ± 1.2 Hz (mean ± SEM; n = 3) for cspX1 to a mean peak frequency of 42 ± 3 Hz (mean ± SEM;n = 3) for wild type and 41 ± 5 Hz (mean ± SEM; n = 3) for csp mutants. Because there is no significant difference between the calcimycin-induced quantal release in csp mutants and the calcimycin response in wild type (p = 0.92, Student’s t test), this suggests that there is no effect of the csp mutation on the calcimycin response.
Fig. 3.
Calcimycin induces FM1-43 destaining and elevated quantal release in csp mutants at >30°C. A, NMJs of homozygouscspE16 mutant larvae were stained with FM1-43 by K+ depolarization at 22°C (data not shown), and the ts block of FM1-43 destaining was confirmed. At 32°C K+ stimulation failed to destain the labeled synaptic boutons (A1). Calcimycin (A23187) stimulation of FM1-43 destaining in calcium-free medium at 32°C released little, if any, FM1-43 dye from the synaptic boutons (A2). However, calcimycin stimulation in the presence of external Ca2+ at 32°C destained the same NMJ (A3) that failed to destain with the previous two stimuli (A1, A2). The images represent five independent preparations. B, After FM1-43 staining at 22°C (data not shown) and confirming the ts block of evoked FM1-43 destaining at 32°C (B1), NMJs of homozygouscspE16 mutants were treated for 5 min with 10 μm BAPTA-AM ester in calcium-free medium. After removal of the residual external BAPTA ester, subsequent calcimycin stimulation failed to destain the FM1-43-labeled and BAPTA-loaded synaptic boutons at 32°C (B2). The images represent five preparations. C, D, MEJPs were recorded contin-uously at 31°C in the presence of 0.5 mm Ca2+ and 1 μg/ml TTX from muscle fiber 6 of wild-type (C) andcspX1 mutant (D) larvae. The first trace (C, D) represents MEJP recordings before the application of calcimycin. Trace 2 shows the onset of the response (C, D) shortly after the application (30 sec) of calcimycin. Trace 3shows the highest activity, which was on average 42 ± 3 Hz (mean ± SEM; n = 3) for wild-type and 41 ± 5 Hz (mean ± SEM; n = 3) forcspX1 mutant larvae recorded before fast muscle depolarizations hindered any further electrophysiological analysis. Muscle potentials were −67 mV for wild-type (C) and −57 mV forcspX1 mutant (D) larvae. Scale bars, 50 μm.
csp mutant flies exhibit no ts defect of vesicle recycling that would correlate with the complete ts block of neurotransmitter release
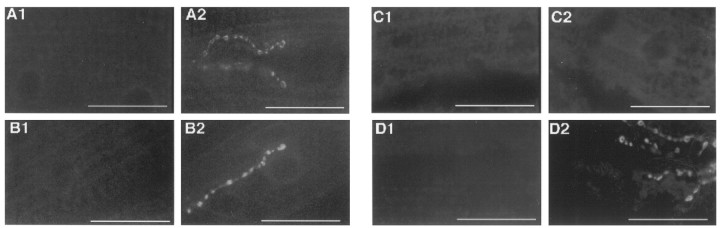
The wild-type-like BWSV-mediated increase of quantal release incsp mutants (Fig. 2B,C) provided some indirect evidence that the synaptic vesicle pool at larval NMJs is not affected by the csp mutation. To gather direct evidence for this speculation, we assayed evoked endocytosis at 32°C incsp mutants. At this temperature, we never observed the typical punctate FM1-43 staining of synaptic boutons at cspor shi mutant synaptic boutons on K+stimulation (Fig.4A1,B1). However, wild-type muscle fibers consistently showed endocytotic uptake of FM1-43 (data not shown). Because both shi and cspphenotypes are temperature-sensitive, we cooled the same preparations to 22°C and repeated the FM1-43-staining protocol. This stainedcsp and shi mutant NMJs with FM1-43 (Fig.4A2,B2). The ts block of FM1-43 uptake in theshi control flies at 32°C is consistent with similar observations of FM1-43 uptake (Ramaswami et al., 1994). The lack of FM1-43 uptake in csp mutant NMJs normally would be suggestive of a ts defect of endocytosis. However, because evoked exocytosis is blocked in csp mutants at 32°C (Fig.1C2), but not in shi mutant flies (Fig.1B2), we considered an alternative explanation that the observed ts block of evoked endocytosis may be secondary and attributable to a ts block of evoked exocytosis. In this case, the stimulation of exocytosis by K+ depolarization simply would not generate any membranes to be recycled. To test this alternative explanation, we stimulated endocytosis with BWSV and observed FM1-43 dye uptake at csp mutant boutons at 32°C (Fig. 4D2). In contrast, we never detected any FM1-43 uptake in shi mutant terminals with BWSV stimulation at 32°C (Fig. 4C2). This indicates that the ts block of K+-induced FM1-43 uptake in csp mutants at 32°C is caused by a defect of depolarization-dependent exocytosis. However, an alternative possibility may be that BWSV activates a parallel route of endocytosis, which is blocked in shi but not in csp mutant flies.
Fig. 4.
Depolarization-induced, but not BWSV-induced, FM1-43 staining is blocked at csp mutant NMJs at 32°C. At 32°C K+ depolarization failed to induce a significant FM1-43 uptake in synaptic boutons of homozygousshits1 NMJs (A1) and homozygous cspE16 NMJs (B1). After cooling to 22°C, K+ stimulation stained synaptic boutons of shits1 NMJs (A2) with FM1-43 andcspE16 NMJs (B2). For the next series of experiments (C, D), the mutant ts block of FM1-43 uptake at 32°C was confirmed with K+depolarization for homozygous shits1 NMJs (C1) and homozygous cspE16NMJs (D1). No staining was observed in either case. The subsequent BWSV stimulation in the presence of external Ca2+ at 32°C failed to induce any uptake of FM1-43 dye at shits1 mutant synaptic boutons (C2). However, BWSV stimulation at 32°C induced a strong uptake of FM1-43 dye at cspE16mutant NMJs (D2). Each series of images represents at least five independent experiments and shows partial NMJs of muscle 6/7 except in D. Scale bars, 50 μm.
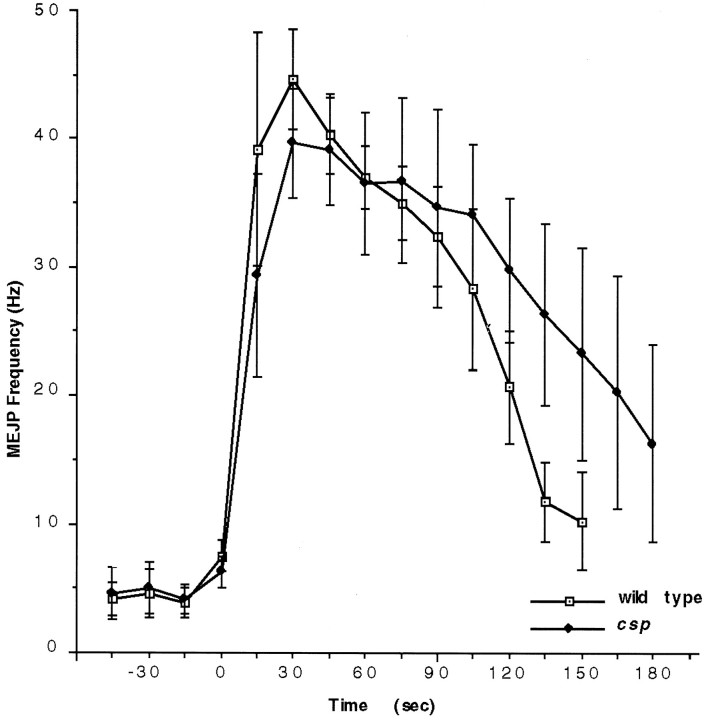
An interesting general feature of BWSV-poisoned nerve terminals in the absence of external Ca2+ is that they quickly become depleted of synaptic vesicles, because the endocytosis of BWSV-secreted vesicles is blocked (Ceccarelli et al., 1979; Fritz et al., 1980). These electron microscopic studies correlate well with the minute quantity of FM1-43 dye uptake in the absence of Ca2+(Ramaswami et al., 1994; Henkel and Betz, 1995). The ability to stimulate exocytosis and to block endocytosis simultaneously allows for, in principle, an electrophysiological estimate of the amount of the BWSV-releasable synaptic vesicle pool. Thus, we compared the frequency of BWSV-induced quantal release in the absence of Ca2+ in wild type and csp mutants. We found that the mean MEJP frequencies and the time course of the BWSV-induced response in csp mutants at 32°C are similar to those of wild type at 32°C (Fig. 5). Although the BWSV responses are rather variable, even in wild type, there is almost certainly no correlation of the BWSV response with the ts block of evoked release in csp mutants. The wild-type-like features of BWSV-induced quantal release imply that the pool of BWSV-releasable vesicles in csp mutants is similar to that of wild type at 32°C.
Fig. 5.
The BWSV-induced increase in quantal release ofcsp mutants appears similar in frequency and duration to wild type when endocytosis is blocked. MEJPs were recorded continuously from muscle fiber 6 in the absence of calcium at 31°C. We compared the BWSV-induced increase of the MEJP frequency for wild-type and forcspX1 mutant larvae. The MEJP frequency was determined in 15 sec intervals. Because the onset of the toxin response differed significantly from trial to trial, we adjusted the curves for the onset of the BWSV response, which was identified as the first interval showing approximately twice the spontaneous frequency observed before drug application [4.2 ± 1.5 Hz (mean ± SEM; n = 3) for wild type and 4.6 ± 1.6 Hz (mean ± SEM; n = 3) forcspX1]. The responses of three independent recordings (3 larvae for each genotype) were averaged and plotted. The curves appear similar for their peak frequencies, the decline in MEJP frequency, and the duration of the response.
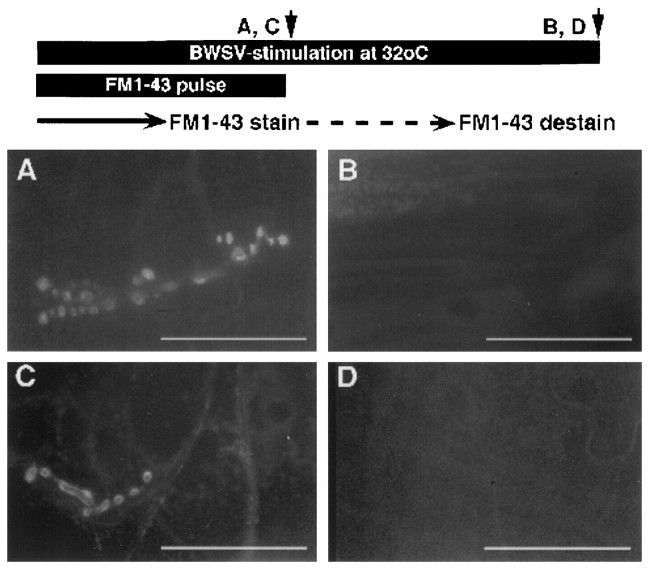
To gather further evidence for a potential ts defect in a late step of vesicle recycling, such as the uncoating of clathrin-coated vesicles, we tested the “functionality” of vesicles by asking whethercsp mutant vesicles were able to exocytose if they were recycled at 32°C. To address this question experimentally, we used an FM1-43 pulse–chase protocol and stimulated exocytosis and endocytosis with BWSV. We pulsed the preparation with FM1-43 dye at 32°C to label newly endocytosed vesicles. After the dye had been omitted, the BWSV stimulation was continued at 32°C to trigger the release of the stained vesicles. We always observed FM1-43 destaining ofcsp mutant or wild-type synaptic boutons with this protocol (Fig. 6B,D). Because the controls studied in parallel always generated FM1-43-stained NMJs in wild type (Fig. 6A) and in csp mutants (Fig. 6C), we assume that the unstained synaptic boutons (Fig. 6B,D) were stained during the FM1-43 pulse and became destained after the dye had been omitted. Thus, FM1-43 stain that has been taken up at 32°C with BWSV stimulation can be released properly at 32°C in wild type and in csp mutants. This suggests that synaptic vesicles of csp mutants that were endocytosed at 32°C are able to exocytose at 32°C with BWSV stimulation.
Fig. 6.
FM1-43 dye endocytosed at 32°C by BWSV stimulation can be released immediately at 32°C at cspmutant NMJs. At 32°C, exocytosis and subsequent endocytosis were induced with BWSV in the presence of external Ca2+. To label freshly endocytosed vesicles (FM1-43 staining), the preparation was pulsed with FM1-43 dye. After omitting the dye the BWSV stimulation was continued at 32°C (FM1-43 destaining). Using this protocol, neither wild-type (B) norcspE16 mutant (D) NMJs were stained with FM1-43 dye. However, control experiments, in which BWSV and neurotransmission were inactivated after the FM1-43 pulse, always showed FM1-43-stained synaptic boutons as exemplified for wild type (A) andcspE16 mutants (C). The images represent six independent trials using three larvae for each genotype per experiment (A–D).
DISCUSSION
Two alternative models of CSP function have been suggested. The first suggested that CSP may link synaptic vesicles to presynaptic calcium channels and thereby may modulate neurotransmission (Mastrogiacomo et al., 1994). This hypothesis was supported primarily by the positive modulation of N-type calcium channels whenTorpedo CSP cDNA was coexpressed in frog oocytes (Gundersen and Umbach, 1992). Our previous phenotypic analysis of cspnull mutations in Drosophila revealed a ts block of neurotransmission at >29°C (Umbach et al., 1994; Zinsmaier et al., 1994). This observation and the reduced calcium sensitivity of evoked release at 22°C (Umbach et al., 1994) were consistent with the “channel hypothesis.” Alternatively, it has been suggested that CSP may function at a step in the clathrin-dependent recycling of synaptic vesicles by cooperating with the clathrin-uncoating ATPase HSC70 (Sudhof, 1995). The slowly developing ts phenotype of cspmutant flies (Umbach et al., 1994) and the discovery that mammalian CSP interacts in vitro with HSC70 (Braun et al., 1996;Chamberlain and Burgoyne, 1997) strengthened the “recycling hypothesis.”
Two assumptions were critical for our analysis of a potential vesicle-recycling phenotype. First, a potential genetic defect in recycling should be most severe at >30°C and should correlate with the complete ts block of evoked neurotransmitter release incsp deletion mutants. Second, a ts-recycling defect should deplete the releasable synaptic vesicle pool in csp mutants, as seen in the shi mutation. Therefore, we compared the dynamics of synaptic vesicle exocytosis and endocytosis ofcsp mutants with those of shi mutant flies using the optical FM1-43 assay developed by Betz and Bewick (1992). First, we tested whether evoked exocytosis, endocytosis, or both are blocked incsp mutants at 32°C. We demonstrated that at 32°C, FM1-43 destaining with K+ stimulation is blocked atcsp but not at shi mutant synaptic boutons (Fig.1). The persistence of FM1-43 staining at csp mutant boutons indicates a primary block of evoked exocytosis at 32°C and argues against the theory of a depleted vesicle pool. However, when we monitored endocytosis, we observed that at 32°C both cspand shi mutant NMJs exhibited a depolarization-dependent block of FM1-43 dye uptake (Fig. 4). Because evoked exocytosis is blocked in csp mutant larvae, but not in shimutant larvae (Fig. 1), we considered the possibility that the depolarization-dependent block of endocytosis may be secondary and may be caused by the primary block of evoked exocytosis. This possibility is consistent with the observation that endocytotic activity is coupled tightly to exocytosis (Heuser and Reese, 1973; Betz and Wu, 1995; Ryan and Smith, 1995). We tested our speculation by stimulating endocytosis with BWSV, the exocytotic action of which is not impaired by thecsp mutation (Fig. 3). The BWSV stimulation labeled synaptic boutons of csp mutant NMJs at 32°C but not ofshi mutant NMJs, which exhibited a complete block of dye uptake (Fig. 4). A similar result, FM1-43 staining in cspand no staining in shi mutants, has been obtained when endocytosis was triggered with calcimycin (data not shown), which makes it less likely that BWSV activates a parallel pathway of endocytosis that does not require CSP function. The possibility of a maverick route of endocytosis stimulated by BWSV is also diminished by the result that FM1-43 staining with BWSV stimulation is readily releasable (Fig. 6). Therefore, our results provide compelling evidence that the ts block of evoked endocytosis is a secondary effect of the primary ts block of depolarization-dependent exocytosis in csp mutants.
To exclude the possibility of a severe ts defect in cspmutants during late steps of vesicle recycling, we estimated the size of the BWSV-releasable vesicle pool. This was possible because BWSV triggers vesicle fusion in the absence of calcium when endocytosis is blocked (Ceccarelli et al., 1979; Fritz et al., 1980; Ramaswami et al., 1994; Henkel and Betz, 1995). As we show in Figure 5, the Ca2+-independent BWSV response of quantal release in wild-type larvae is similar to that of csp mutant larvae in its time course and frequency. This is inconsistent with the speculation that the csp mutation may cause a ts block of vesicle recycling and may deplete the vesicle pool. In addition, we tested the functionality of csp mutant vesicles that were recycled at 32°C. Here, we assume that immature vesicles cannot exocytose, because most steps of vesicle genesis, such as clathrin uncoating, are thought to be essential for vesicles to exocytose. We pulse-labeled synaptic vesicles with FM1-43 as they were endocytosed at 32°C and tested whether these vesicles were immediately able to exocytose. If the CSP protein is required for vesicle genesis, theoretically we should observe a significant deficit of FM1-43 destaining in this experiment. We found no evidence of any ts impairment of FM1-43 destaining in csp mutants in this experiment (Fig. 6), suggesting that essential steps of vesicle genesis are not affected by the ts-csp mutation. However, a less penetrant non-ts defect of vesicle recycling cannot be ruled out. The experiment also indicates that BWSV stimulates a normal route of endocytosis, leading to readily releasable vesicles, which supports the physiological significance of our results. In summary, our analysis of vesicle recycling shows that the csp mutation does not cause a ts block of synaptic vesicle recycling that would correlate with the ts block of evoked neurotransmission.
Consistent with the assumption that the csp mutation blocks evoked exocytosis, we demonstrated that depolarization-dependent FM1-43 destaining of csp mutant synaptic boutons is blocked reversibly at 32°C (Fig. 1), which correlates well with earlier recordings of the ts block of evoked release (Umbach et al., 1994). Interestingly, we discovered that the csp mutation does not affect the stimulation of neurotransmitter release induced by BWSV or calcimycin (Figs. 2, 3). Similar results with a purified component of BWSV, α-latrotoxin, have been obtained for csp mutant larvae (Umbach et al., 1995). The ability of BWSV to induce quantal release and FM1-43 destaining in csp mutants at nonpermissive temperatures (Fig. 2) implies that the CSP-dependent step of evoked release must be upstream of the quantal release step stimulated by BWSV. This is interesting because the BWSV response is inhibited in synaptobrevin and syntaxin mutant embryos of Drosophila (Broadie et al., 1995). There, the failure of BWSV to induce quantal release, together with the observed docking of synaptic vesicles, led to the conclusion that synaptobrevin and syntaxin proteins function downstream of vesicle docking (Broadie et al., 1995). Consequently, the action of BWSV in cspmutants argues that CSP is required upstream of synaptobrevin and syntaxin function.
All of our results are consistent with the conclusion that thecsp mutation may interfere with evoked exocytosis. To obtain further evidence, we tested the calcium ionophore calcimycin to trigger release in csp mutant larvae at 32°C when the ts block of evoked release is most severe. As speculated, calcimycin requires extracellular calcium to induce the destaining of FM1-43-labeled synaptic boutons in csp mutants that failed to destain with depolarization at 32°C (Fig. 3A1–3). These FM1-43 results are supported strongly by recordings from mutant NMJs that demonstrate the ability of calcimycin to increase quantal release in cspmutants at 32°C as in wild type (Fig. 3C–D). Because calcium ionophores trigger neurotransmitter release by bypassing voltage-gated calcium channels (Alder et al., 1992; Capogna et al., 1996), the calcimycin stimulation of exocytosis in cspmutant larvae at 32°C suggests that the ts mutant defect must be upstream of, or within, the calcium signaling cascade mediating evoked exocytosis. Because we demonstrated previously that the propagation of action potentials is normal in csp mutants (Umbach et al., 1994), we conclude that the depolarization-dependent calcium signaling pathway of evoked release is mutated. Thus, either calcium entry (by voltage-gated calcium channels), its release-triggering activity (calcium receptors and downstream signaling pathway), or both mechanisms must be defective in csp mutants. This conclusion is consistent with the recent observation that the time constant of the evoked current decay is increased in csp null mutations at 16–18°C, implying that CSP helps to synchronize evoked release (Heckmann et al., 1997).
Our electrophysiological recordings of the calcimycin response incsp mutants are not able to differentiate conclusively between defects of calcium entry and calcium action. However, they point toward a defect of calcium entry, because a defect of calcium action should reduce significantly, if not abolish, the calcimycin response for a given calcimycin or calcium concentration. Because this has not been observed (Fig. 3), CSP may be required to modulate the kinetics of calcium entry, as has been proposed previously (Mastrogiacomo et al., 1994). Alternatively, in cooperation with HSC70, the CSP protein may cluster calcium entry sites in close proximity to neurotransmitter release sites. The significance of clustering calcium channels with vesicle release sites has been described by the single calcium domain model (Stanley, 1993) and is supported by recent findings that severing the physical interaction between presynaptic calcium channels and several synaptic proteins makes evoked release less efficient and less synchronous (Mochida et al., 1996; Rettig et al., 1997).
Footnotes
This work was supported by the Whitehall Foundation, the March of Dimes Birth Defects Foundation, and the National Science Foundation by grants to K.E.Z. We acknowledge the excellent technical support provided by S. Hong.
While this paper was under review, a paper by Umbach and Gundersen (1997) reported a similar stimulation of neurotransmission incsp mutants with latrotoxin and ionomycin.
Correspondence should be addressed to Dr. Konrad E. Zinsmaier, Department of Neuroscience, University of Pennsylvania School of Medicine, 232a Stemmler Hall, Philadelphia, PA 19104-6074.
REFERENCES
- 1.Alder J, Lu B, Valtorta F, Greengard P, Poo M. Calcium-dependent transmitter secretion reconstituted in Xenopus oocytes: requirement for synaptophysin. Science. 1992;257:657–661. doi: 10.1126/science.1353905. [DOI] [PubMed] [Google Scholar]
- 2.Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- 3.Barnett DW, Liu J, Misler S. Single-cell measurements of quantal secretion induced by alpha-latrotoxin from rat adrenal chromaffin cells: dependence on extracellular Ca2+. Pflügers Arch. 1996;432:1039–1046. doi: 10.1007/s004240050232. [DOI] [PubMed] [Google Scholar]
- 4.Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- 5.Betz WJ, Bewick GS. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol (Lond) 1993;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betz WJ, Wu LG. Synaptic transmission: kinetics of synaptic-vesicle recycling. Curr Biol. 1995;5:1098–1101. doi: 10.1016/s0960-9822(95)00220-x. [DOI] [PubMed] [Google Scholar]
- 7.Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betz WJ, Mao F, Smith CB. Imaging exocytosis and endocytosis. Curr Opin Neurobiol. 1996;6:365–371. doi: 10.1016/s0959-4388(96)80121-8. [DOI] [PubMed] [Google Scholar]
- 9.Braun JEA, Scheller RA. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology. 1995;34:1361–1369. doi: 10.1016/0028-3908(95)00114-l. [DOI] [PubMed] [Google Scholar]
- 10.Braun J, Wilbanks SM, Scheller RH. The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem. 1996;271:25989–25993. doi: 10.1074/jbc.271.42.25989. [DOI] [PubMed] [Google Scholar]
- 11.Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 12.Buchner E, Gundersen CB. The DnaJ-like cysteine string protein and exocytotic neurotransmitter release. Trends Neurosci. 1997;20:223–227. doi: 10.1016/s0166-2236(96)10082-5. [DOI] [PubMed] [Google Scholar]
- 13.Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of calcium-dependent and -independent release elicited with ionomycin, gadolinium, and alpha-latrotoxin in the hippocampus. J Neurophysiol. 1996;75:2017–2028. doi: 10.1152/jn.1996.75.5.2017. [DOI] [PubMed] [Google Scholar]
- 14.Ceccarelli B, Grohovaz F, Hurlbut WP. Freeze-fracture studies of frog NMJs during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J Cell Biol. 1979;81:163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain LH, Burgoyne RD. Identification of a novel cysteine string protein variant and expression of cysteine string proteins in non-neuronal cells. J Biol Chem. 1996;271:7320–7323. doi: 10.1074/jbc.271.13.7320. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain LH, Burgoyne RD. Activation of the ATPase activity of heat-shock proteins Hsc70/Hsp70 by cysteine-string protein. Biochem J. 1997;322:853–858. doi: 10.1042/bj3220853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain LH, Henry J, Burgoyne RD. Cysteine string proteins are associated with chromaffin granules. J Biol Chem. 1996;271:19514–19517. doi: 10.1074/jbc.271.32.19514. [DOI] [PubMed] [Google Scholar]
- 18.Clark AW, Mauro A, Longenecker HJ, Hurlbut WP. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970;225:703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- 19.Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 20.Fritz LC, Atwood HL, Jahromi SS. Lobster neuromuscular junctions treated with black widow spider venom: correlation between ultrastructure and physiology. J Neurocytol. 1980;9:699–721. doi: 10.1007/BF01205034. [DOI] [PubMed] [Google Scholar]
- 21.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 22.Gundersen CB, Umbach JA. Suppression cloning of the cDNA for a candidate subunit of a presynaptic calcium channel. Neuron. 1992;9:527–537. doi: 10.1016/0896-6273(92)90190-o. [DOI] [PubMed] [Google Scholar]
- 23.Heckmann M, Adelsberger H, Dudel J. Evoked transmitter release at neuromuscular junctions in wild-type and cysteine string protein null mutant larvae of Drosophila. Neurosci Lett. 1997;228:167–170. doi: 10.1016/s0304-3940(97)00390-x. [DOI] [PubMed] [Google Scholar]
- 24.Henkel AW, Betz WJ. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995;34:1397–1406. doi: 10.1016/0028-3908(95)00126-q. [DOI] [PubMed] [Google Scholar]
- 25.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda K, Ozawa S, Hagiwara S. Synaptic transmission reversibly conditioned by a single gene mutation in Drosophila melanogaster. Nature. 1976;259:489–491. doi: 10.1038/259489a0. [DOI] [PubMed] [Google Scholar]
- 27.Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol (Lond) 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutaminergic synapses in identified muscle cells of Drosophila larvae. J Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohan SA, Pescatori M, Brecha NC, Mastrogiacomo A, Umbach JA, Gundersen CB. Cysteine string protein immunoreactivity in the nervous system and adrenal gland of rat. J Neurosci. 1995;15:6230–6238. doi: 10.1523/JNEUROSCI.15-09-06230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosaka I, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983a;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- 31.Kosaka I, Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983b;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linial M, Ilouz N, Feinstein N. Alpha-latrotoxin is a potent inducer of neurotransmitter release in Torpedo electric organ–functional and morphological characterization. Eur J Neurosci. 1995;7:742–752. doi: 10.1111/j.1460-9568.1995.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 33.Magazanik LG, Fedorova IM, Kovalevskaya GI, Pashkov VN, Bulgakov OV, Grishin EV. Selective presynaptic insectotoxin (alpha-latroinsectotoxin) isolated from black widow spider venom. Neuroscience. 1992;46:181–188. doi: 10.1016/0306-4522(92)90017-v. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seager M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J Biol Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 35.Mastrogiacomo A, Parsons SM, Zampighi GA, Jenden DJ, Umbach JA, Gundersen CB. Cysteine string proteins—a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994;263:981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- 36.Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 37.Poodry CA, Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pupier S, Leveque C, Marqueze B, Kataoka M, Takahashi M, Seagar MJ. Cysteine string proteins associated with secretory granules of the rat neurohypophysis. J Neurosci. 1997;17:2722–2727. doi: 10.1523/JNEUROSCI.17-08-02722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 40.Rettig J, Heinemann C, Ashery U, Sheng Z, Yokoyama CT, Catterall WA, Neher E. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel–syntaxin interaction. J Neurosci. 1997;17:6647–6656. doi: 10.1523/JNEUROSCI.17-17-06647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 42.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 43.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 44.Storchak LG, Pashkov VN, Pozdnyakova NG, Himmelreich NH, Grishin EV. Alpha-latrotoxin-stimulated GABA release can occur in Ca(2+)-free, Na(+)-free medium. FEBS Lett. 1994;351:267–270. doi: 10.1016/0014-5793(94)00790-x. [DOI] [PubMed] [Google Scholar]
- 45.Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 46.Umbach JA, Gundersen CB. Evidence that cysteine string proteins regulate an early step in the Ca2+-dependent secretion of neurotransmitter at Drosophila neuromuscular junctions. J Neurosci. 1997;17:7203–7209. doi: 10.1523/JNEUROSCI.17-19-07203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13:899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 48.Umbach JA, Mastrogiacomo A, Gundersen CB. Cysteine string proteins and presynaptic function. J Physiol (Paris) 1995;89:95–101. doi: 10.1016/0928-4257(96)80556-0. [DOI] [PubMed] [Google Scholar]
- 49.Umbach JA, Grasso A, Mastrogiacomo A, Pescatori M, Buchner E, Güudeisen CB (1995) Soc Neurosci Abstr 21:329.
- 50.van deGoor J, Kelly RB. Association of Drosophila cysteine string proteins with membranes. FEBS Lett. 1996;380:251–256. doi: 10.1016/0014-5793(96)00026-9. [DOI] [PubMed] [Google Scholar]
- 51.Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- 52.von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994;367:735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- 53.Zinsmaier KE. Cysteine string proteins. In: Gething MJ, editor. Guidebook to molecular chaperones and protein-folding catalysts. Oxford UP; New York: 1997. pp. 115–117. [Google Scholar]
- 54.Zinsmaier KE, Eberle KK, Buchner E, Walter N, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]