Abstract
To examine the influence of activity-dependent cues on differentiation of primary afferent neurons, we investigated the short- and long-term effects of depolarization and calcium influx on expression of transmitter traits in sensory ganglion cell cultures. We focused on expression of tyrosine hydroxylase (TH), a marker for dopaminergic neurons, in developing petrosal ganglion (PG), nodose ganglion, and dorsal root ganglion neurons grown in the presence or absence of depolarizing concentrations of KCl. Exposure to 40 mm KCl increased the proportion of TH-immunoreactive neurons in all three ganglia in a developmentally regulated manner that corresponded to the temporal pattern of dopaminergic expressionin vivo. PG neurons, for example, were most responsive to elevated KCl on embryonic day 16.5 (E16.5), the age at which the dopaminergic phenotype is first detectable in vivo. However, KCl was relatively ineffective at increasing TH expression in neonatal PG, indicating a critical period for induction of this phenotype by depolarization. Detailed analysis of TH induction in PG neurons demonstrated that, although N-type calcium channels carried the majority of the high voltage-activated calcium current, only L-type calcium channel blockade inhibited the effect of elevated KCl. Further studies revealed that after removal of high KCl, neurons remained sensitized to subsequent stimulation for >1 week. Specifically, cultures exposed to KCl beginning on E16.5 (the conditioning stimulus), then returned to control medium, and subsequently re-exposed to elevated KCl after 9 d (the test stimulus) contained fourfold more TH-positive neurons than did cultures exposed to the test stimulus alone. Moreover, blockade of L-type calcium channels during the conditioning stimulus completely abolished long-term potentiation of the TH response to elevated KCl. These findings demonstrate a novel role for L-type calcium channels in activity-dependent plasticity of transmitter expression in sensory neurons and indicate that exposure to depolarizing stimuli during early development may alter neuronal response properties at later ages.
Keywords: Bay K-8644, ω-conotoxin GVIA, depolarization, dopaminergic, long-term potentiation, nimodipine, petrosal ganglion, primary sensory neurons, transmitter plasticity, tyrosine hydroxylase, voltage-dependent calcium channels
Activity of developing primary sensory neurons plays a critical role in regulating maturation of postsynaptic target cells in the CNS. For example, blocking the activity of vestibulocochlear afferents alters gene transcription, protein synthesis, and the size of neurons in the brainstem cochlear nucleus (Sie and Rubel, 1992; Garden et al., 1995), and the activity of primary olfactory afferents is thought to regulate developmental expression of dopaminergic transmitter properties in the olfactory bulb (Baker and Farbman, 1993). Although the importance of afferent activity in the development of second-order sensory neurons seems clear, it is unknown whether maturation of primary afferents themselves is also regulated by activity-dependent cues.
In mature primary sensory neurons, activity regulates quantitative expression of transmitter properties, such as preprotachykinin mRNA (Noguchi et al., 1988). Therefore, to explore the possibility that activity may also play a role in sensory neuron differentiation, we have been examining the influence of depolarizing stimuli on development of sensory transmitter phenotypes (Fan, 1995; Hertzberg et al., 1995). We have focused in particular on dopaminergic traits, which are expressed by subpopulations of sensory neurons in cranial and spinal ganglia (Katz et al., 1983; Price and Mudge, 1983). In vivo, the magnitude and time course of dopaminergic phenotypic expression vary widely among different sensory ganglia. In adult dorsal root ganglia (DRG), for example, only 1–4% of the total neuronal population is dopaminergic, depending on axial level (Price and Mudge, 1983; Vega et al., 1991). Tyrosine hydroxylase (TH) immunoreactivity and dopamine histofluorescence are first detectable in lumbar DRG neurons 1–2 weeks after birth (Price and Mudge, 1983). In contrast, dopaminergic afferents in cranial sensory ganglia are more abundant and differentiate earlier. In the adult glossopharyngeal petrosal ganglion (PG), for example, at least 10–20% of the neuron population is dopaminergic (Katz and Black, 1986), and TH immunoreactivity is first detectable in these cells on embryonic day 16.5 (E16.5) (Katz and Erb, 1990).
Recently, we found that expression of dopaminergic properties by developing cranial and spinal sensory neurons is highly regulated by depolarizing stimuli in vitro, indicating that activity-dependent mechanisms may influence the number of sensory neurons that express a dopaminergic phenotype (Fan, 1995; Hertzberg et al., 1995). Specifically, treatment of fetal cranial sensory neurons with depolarizing concentrations of veratridine (10 μm) or KCl (40 mm) increased the proportion of ganglion cells that expressed TH and elevated dopamine synthesis by up to 10-fold (Hertzberg et al., 1995). Thus, although dopaminergic properties are normally expressed by a relatively small subset of primary sensory neurons in vivo (Katz et al., 1983), exposure to depolarizing stimuli can unmask a widespread potential for dopaminergic phenotypic expression in these cells, raising the possibility that activity plays an important role in sensory neuron differentiation.
The present study was designed to examine the relationship between sensory neuron responsiveness to depolarizing stimuli in vitro and dopaminergic development in vivo and to begin defining mechanisms that underlie depolarization-mediated TH expression. We found that KCl-induced depolarization increases the proportion of dopaminergic neurons in sensory ganglia in a developmentally regulated manner that corresponds to the temporal pattern of dopaminergic expression in vivo. In addition, we made the unexpected discovery that transient exposure of sensory neurons to depolarizing stimuli during fetal development leads to long-term changes in the regulation of TH expression. Moreover, calcium influx through L-type channels is required for both short-term regulation and long-term potentiation to occur.
MATERIALS AND METHODS
Cell culture
Pregnant dams (Sprague Dawley rats; Zivic-Miller, Zelienople, PA) were rapidly killed by exposure to carbon dioxide. The uterine horns were removed and placed in PBS containing 10% glucose, and the embryos were excised. To assign gestational ages, we designated the day after mating E0.5. Newborn (P0) and 1-week-old (P7) pups were killed with an overdose of sodium pentobarbital (6.0 gm/kg, i.p.). Dissociate cultures of E13.5, E14.5, E16.5, E19.5, P0, and P7 PG, nodose ganglia (NG), and cervical DRG were grown in Leibovitz’s L-15/CO2medium containing 10% NuSerum (Collaborative Biomedical Products, Bedford, MA), 5% heat-inactivated rat serum, fresh vitamin mixture (Mains and Patterson, 1973), penicillin (50 IU/ml; GIBCO BRL, Gaithersburg, MD), and streptomycin (50 μg/ml; GIBCO BRL). Embryonic ganglia were digested in Dispase (Collaborative Biomedical Products; diluted 1:1 in PBS) for 1 hr at 37°C followed by trituration through fire-polished Pasteur pipettes; P0 and P7 ganglia were digested in 0.5% trypsin (Worthington, Freehold, NJ) for 20 min at 37°C followed by trituration. Embryonic and newborn cells were plated onto glass coverslips coated with poly-d-lysine (0.1 mg/ml) and laminin (0.3 mg/ml), whereas P7 cells were grown on growth factor-reduced Matrigel matrix (Collaborative Biomedical Products; diluted 1:5). All cultures were supplemented with recombinant human brain-derived neurotrophic factor (BDNF; Regeneron Pharmaceuticals, Inc., Tarrytown, NY) at a concentration of 10 ng/ml. DRG cultures were supplemented with, in addition to BDNF, 10 ng/ml nerve growth factor (NGF; Dr. Kenneth Neet, Chicago Medical College) and 10 ng/ml neurotrophin-3 (NT-3; Regeneron Pharmaceuticals, Inc.). Depolarizing conditions were produced by supplementing cultures with 34 mm KCl to achieve a final concentration of 40 mm. Control cultures were not adjusted to isosmolarity because we found previously that TH expression in these cells was unaffected when the concentration of NaCl, rather than KCl, was raised to 40 mm (Hertzberg et al., 1995). In most experiments, neurons were cultured for a total of 3 d in the presence or absence of depolarizing concentrations of KCl. In some experiments, neurons were cultured for 2 d in control medium containing 6 mm KCl and then transferred for 24 hr to medium containing 40 mm KCl either with or without a selective calcium channel antagonist. Specifically, the N-type calcium channel antagonist ω-conotoxin GVIA (1 μm; Sigma, St. Louis, MO) and the L-type calcium channel antagonists nifedipine (1 μm; Calbiochem, San Diego, CA), nimodipine (2 μm; a gift from Miles Pharmaceuticals, West Haven, CT), and verapamil (10 μm; Sigma) were used. In other experiments, an L-type calcium channel agonist, (±)-Bay K-8644 (1 μm; Calbiochem), was added at the beginning of the 3 day culture period. In long-term experiments (15 d), neurons were cultured on growth factor-reduced Matrigel. During the initial 3 d of culture, cytosine β-d-arabinofuranoside (10 μm; Sigma) was added to the culture medium to eliminate non-neuronal cells.
Immunocytochemistry
All cultures were fixed with 4% paraformaldehyde in 0.1m sodium phosphate buffer, pH 7.4, overnight at 4°C. Double immunostaining was performed using polyclonal anti-TH (Pel-Freeze Biologicals, Rogers, AR), polyclonal anti-substance P (SP) (Incstar Corporation, Stillwater, MN), monoclonal anti-neurofilament protein (NF160,68; Sigma), goat anti-rabbit IgG-FITC (for TH staining, Boehringer Mannheim, Indianapolis, IN; for SP staining, Cappel, West Chester, PA), and goat anti-mouse IgG-rhodamine (Cappel) antibodies.
TH immunostaining. Cells were (1) incubated overnight at room temperature in anti-TH (1:200) and anti-NF (1:100) diluted in PBS containing 0.5% Triton X-100, (2) washed three times in PBS, (3) incubated for 1 hr at room temperature in goat anti-rabbit IgG-FITC (1:200) plus goat anti-mouse IgG-rhodamine (1:200) diluted in PBS–Triton containing 10% goat serum and 10% rat serum, (4) washed in PBS, (5) incubated in ρ-phenylenediamine (1 mg/ml) for 1 min, (6) washed in PBS, and (7) coverslipped with glycerol gel.
Substance P (SP) immunostaining. The same protocol described for TH immunostaining was used except that the primary incubation was performed in anti-SP (1:2000) and anti-NF (1:50) diluted in PBS containing 0.5% Triton X-100, 2% rat serum, and 2% goat serum.
Cell counts and statistical analysis. The two fluorophores were distinguished using an Olympus fluorescence microscope (model BH-2) with rhodamine and FITC filter cubes. The number of TH- and NF-immunoreactive neurons in each culture was estimated by counting all cells in a measured area of each coverslip. All experiments were performed at least three times with three cultures per experimental group. Percentages were normalized (arcsine transformation), and values were compared using ANOVA followed by Scheffé’s multiple comparison procedure (Kleinbaum and Kupper, 1978). p < 0.05 was considered significant.
Electrophysiology
Calcium currents in dissociated E16.5 PG neurons were examined using conventional whole-cell recording techniques (Hamill et al., 1981). Experiments were performed at room temperature (25°C), ∼20 hr after dissociation. Currents were amplified (80 dB/decade low-pass Bessel filter set at a −3 dB frequency of 10 kHz; Axopatch-1C; Axon Instruments, Inc., Foster City, CA), digitized (100 μsec/point), and analyzed using Clampfit (pClamp v. 6.0; Axon Instruments, Inc.), whereas whole-cell current measurements were made in response to voltage protocols controlled by pClamp v. 5.6 (Axon Instruments, Inc.).
Solutions. The compositions of solutions used to isolate calcium currents were as follows (in mm): for the pipette solution, 124.0 CsCl, 11.0 EGTA, 1.0 CaCl2, 2.0 MgCl2, and 10.0 HEPES, pH 7.4, and for the bath solution, 140.0 TEA (tetraethylammonium chloride), 5.0 4-AP (4-aminopyridine), 15.0 HEPES, 2.0 CaCl2, and 20.0 glucose, pH 7.4. In one series of experiments, membrane potentials were examined in a bath solution containing L-15 serum-free media and varying concentrations of KCl (6, 15, and 40 mm). In these experiments, the pipette solution contained (in mm): 145 K-Asp, 5.0 HEPES, 1.3 CaCl2, 2.2 EGTA, 2.0 MgCl2, 5.0 NaCl, and 10.0 glucose, pH 7.2. The membrane potential was defined as the voltage (in millivolts) inside, relative to outside, the cell and was measured at zero current in current-clamp mode.
Calcium channel modulators. For electrophysiological measurements, S(−)-Bay K-8644 (Research Biochemicals, Natick, MA) was dissolved in ethanol and stored in a 1 mmstock solution, nimodipine was dissolved in methanol and stored at a concentration of 0.5 mm, and ω-conotoxin GVIA was dissolved in distilled water and stored at a concentration of 100 μm.
RESULTS
Sensory neuron responsiveness to depolarizing stimuli is developmentally regulated
We found previously that chronic exposure of E16.5 PG, NG, or DRG neurons to depolarizing stimuli in vitro can markedly increase both the percentage of ganglion cells that express TH and dopamine synthesis (Hertzberg et al., 1995). However, the proportion of neurons in which TH expression could be evoked varied from ∼8% in the DRG to almost 100% in the PG (Hertzberg et al., 1995). These differences could reflect an underlying heterogeneity in phenotypic potential among different sensory neuron populations. Alternatively, responsiveness to depolarizing stimuli may be temporally regulated and exhibit a different time course in each ganglion. To explore this issue further, we compared the effect of KCl-mediated depolarization on TH expression in PG, NG, and DRG neurons cultured at various ages between E13.5 and P7. Neurons were grown for 3 d in the presence of control medium (6 mm KCl) or medium containing a depolarizing concentration of KCl (40 mm) and subsequently were processed for TH and NF immunostaining. An exposure time of 3 d was chosen because preliminary experiments demonstrated that, although 6 hr of KCl treatment was sufficient to induce TH expression, a 3 day exposure produced the greatest induction. The membrane potential of neurons grown for 3 d in control medium was between −62 and −66 mV (n = 6), compared with −18 to −20 mV (n = 6) in neurons grown for the same period of time in 40 mm KCl.
The percentage of TH-positive (TH+) neurons in all three ganglia was significantly increased by KCl treatment; however, the magnitude and time course of the response were different in each population (Fig. 1). PG neurons exhibited the largest and earliest peak response (92% TH+neurons on E16.5), followed by the NG (55% TH+neurons on E19.5) and the DRG (22% TH+ neurons on P0). In addition, KCl treatment seemed to define a window of responsiveness in TH regulation; in the PG, for example, KCl treatment was 2.5- and 5-fold less effective at inducing TH expression in E13.5 and P7 cultures, respectively, than in E16.5 cultures.
Fig. 1.
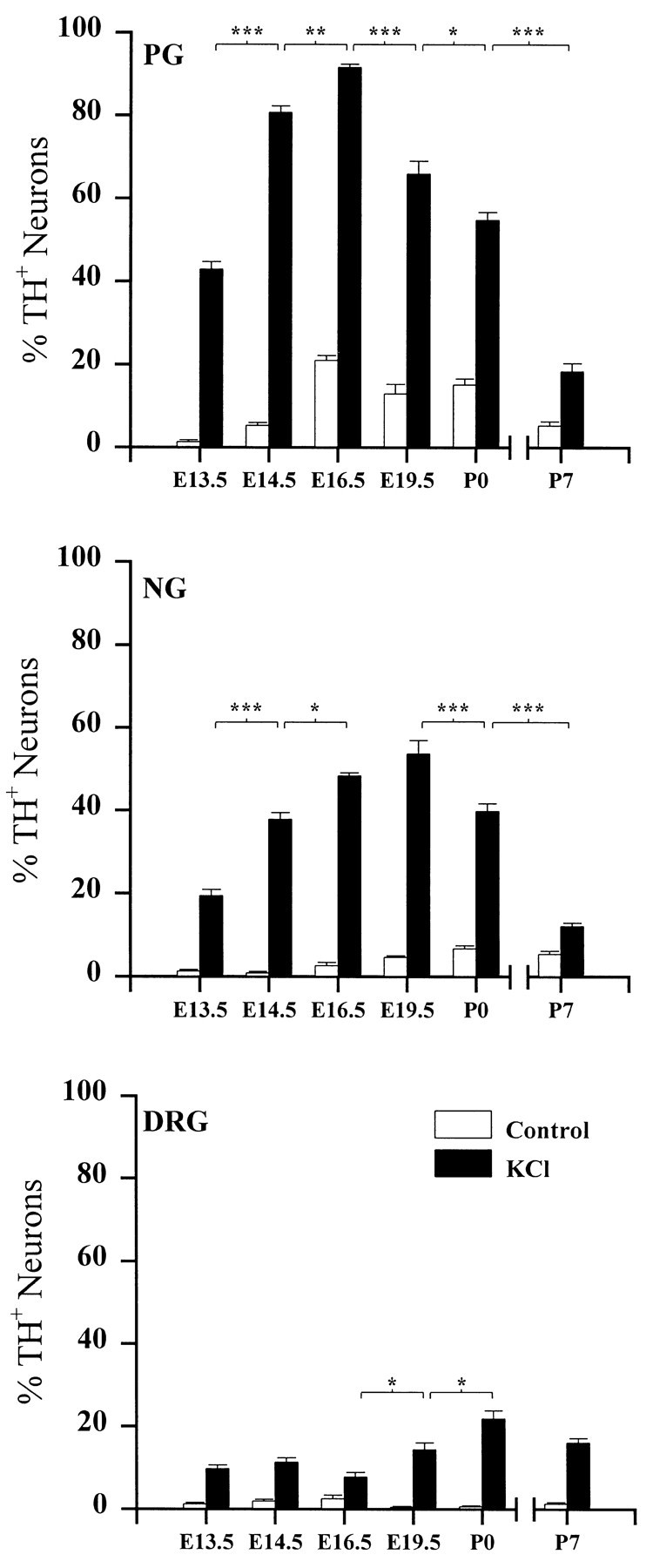
The developmental time course of depolarization-induced TH expression in PG, NG, and DRG neurons. Ganglia were removed at the ages indicated and grown in dissociate cell culture for 3 d in the absence (Control) or presence (KCl) of 40 mm KCl. Eachbar indicates the percentage of neurons exhibiting TH immunoreactivity. Data are presented as the mean ± SEM. P7 ganglia were grown on Matrigel, whereas all others were grown on laminin (see Materials and Methods). Each KCl-treated group is significantly different from its corresponding control. Comparisons between ages were made using ANOVA followed by Scheffé’s test; *p < 0.05; **p < 0.01; ***p < 0.001.
To determine whether depolarization increased the percentage of TH+ cells by raising TH expression per cell or by selectively increasing either proliferation or survival of TH-immunoreactive neurons, we compared total neuron numbers in the absence or presence of depolarizing concentrations of KCl. In NG and DRG cultures, neuronal survival was unaffected by KCl treatment at all ages examined (Fig. 2). In the PG, KCl treatment had no effect on neuronal survival at E13.5, E14.5, E16.5, and P7 but significantly increased survival in E19.5 and P0 cultures (Fig. 2). However, when P0 cultures were grown on Matrigel, a basement membrane-rich substrate that augments neuronal attachment and survival, KCl evoked a comparable increase in the percentage of TH+ neurons without altering neuronal survival [%TH+ neurons, 16.1 ± 1.7 (control) vs 51.2 ± 4.1 (KCl-treated); number of NF-positive (NF+) neurons, 235 ± 36 (control) vs 247 ± 50 (KCl-treated)]. Thus, altered survival or proliferation of TH-immunoreactive neurons cannot explain the increase in the percentage of TH+ cells in KCl-treated cultures compared with age-matched controls. Moreover, PG neurons are postmitotic by E13–E14 (Altman and Bayer, 1982), i.e., before the age at which we observed the greatest percentage of TH+ neurons in the presence of high KCl.
Fig. 2.
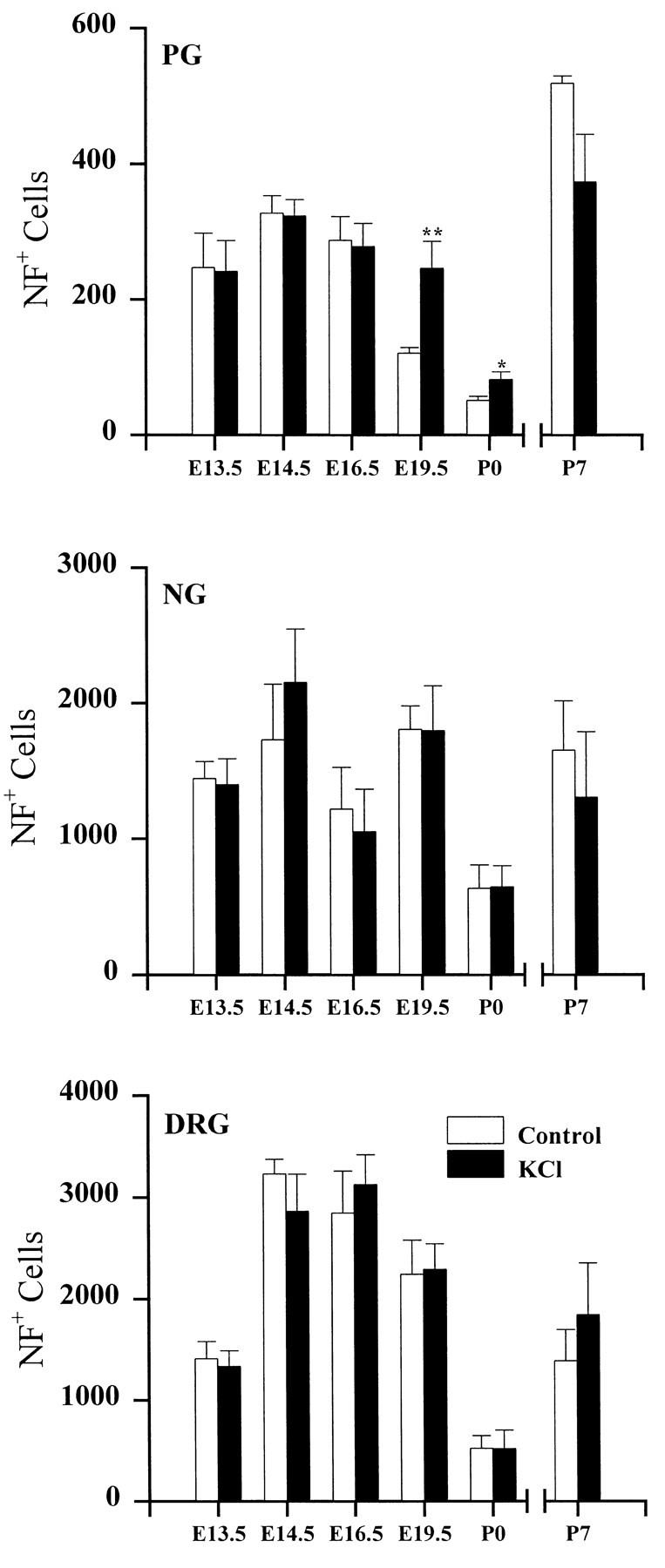
Neuron survival in PG, NG, and DRG. Cultures were grown for 3 d in the absence (Control) or presence (KCl) of 40 mm KCl.Bars represent the mean number (± SEM) of neurofilament-positive cells per ganglion. Significant differences between KCl and corresponding control values were detected using ANOVA followed by Scheffé’s multiple comparison; *p < 0.05; **p < 0.01.
Substance P expression in NG and PG neurons is unaffected by depolarization
To determine whether depolarizing stimuli act selectively to upregulate dopaminergic traits, we examined expression of another neurotransmitter, the neuropeptide SP in PG, NG, and DRG cultures. SP is normally expressed by a subset of neurons in all three ganglia; however, TH and SP are not localized in the same cells (Price, 1985;Kummer et al., 1990; Finley et al., 1992). In marked contrast to TH, the percentage of PG and NG neurons exhibiting SP immunoreactivity (SP+) was unchanged by KCl treatment at all ages examined (Fig. 3), indicating that depolarization does not increase transmitter traits in these ganglia in a nonselective manner. KCl treatment did, however, increase the percentage of SP neurons in E16.5 DRG cultures.
Fig. 3.
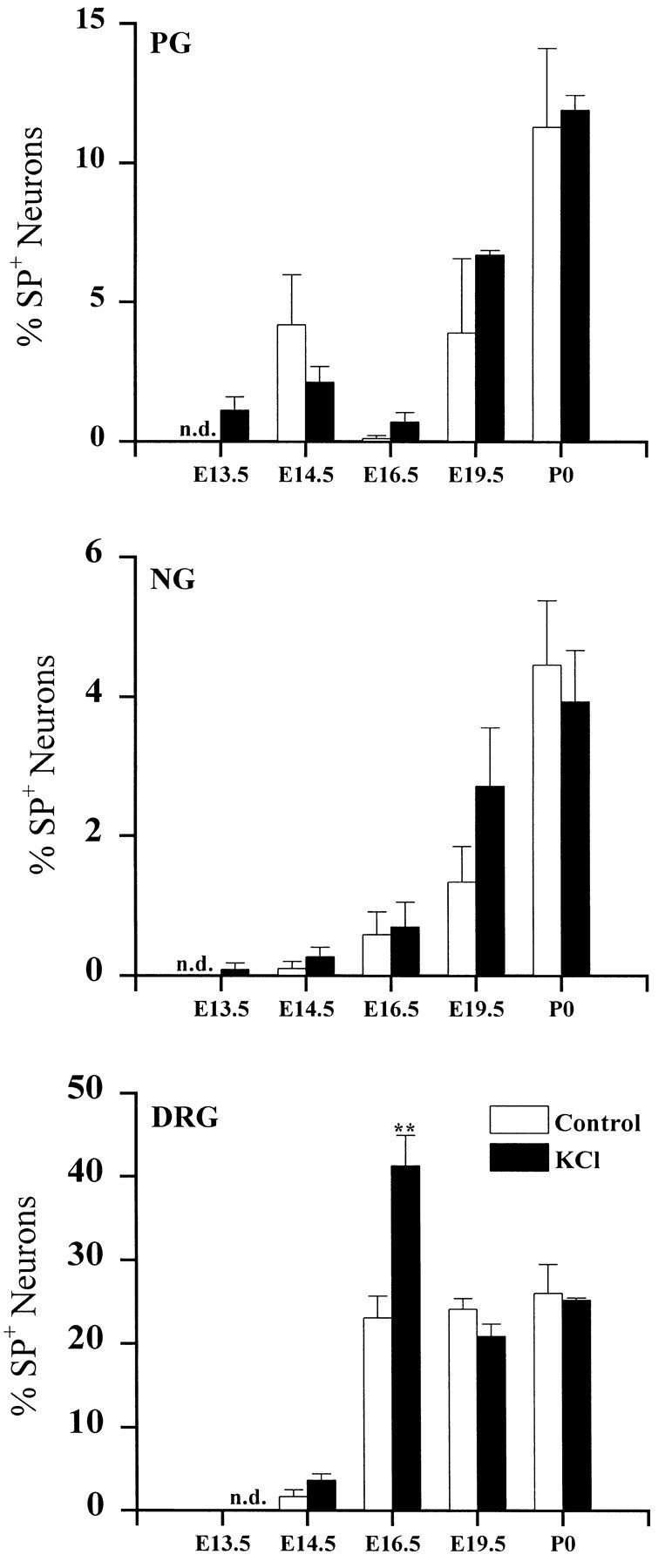
The developmental time course of depolarization-induced SP expression in PG, NG, and DRG neurons. Ganglia were removed at the ages indicated and grown in dissociate cell culture for 3 d in the absence (Control) or presence (KCl) of 40 mm KCl. Eachbar indicates the mean percentage (± SEM) of neurons exhibiting SP immunoreactivity; n.d., not detectable. Comparisons with corresponding controls were made using Student’st test; **p < 0.01.
Transient exposure to high KCl leads to long-term potentiation of TH expression
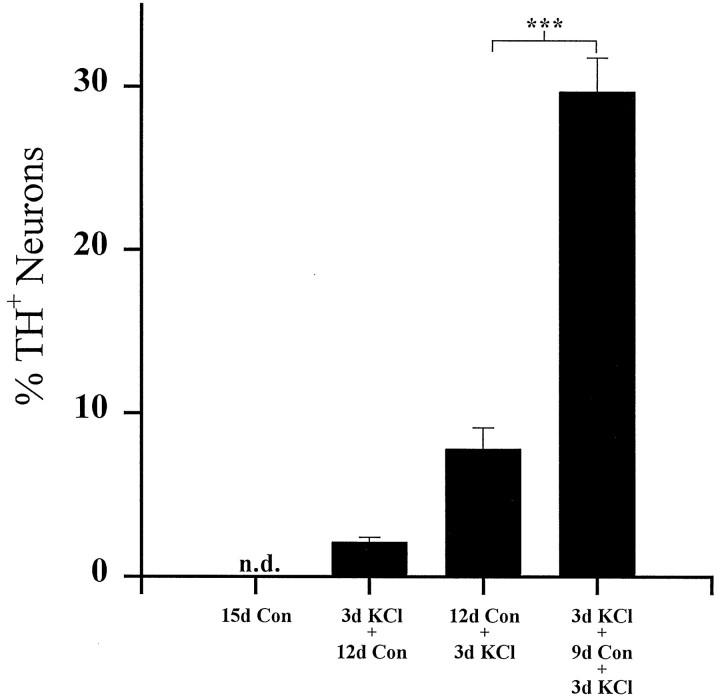
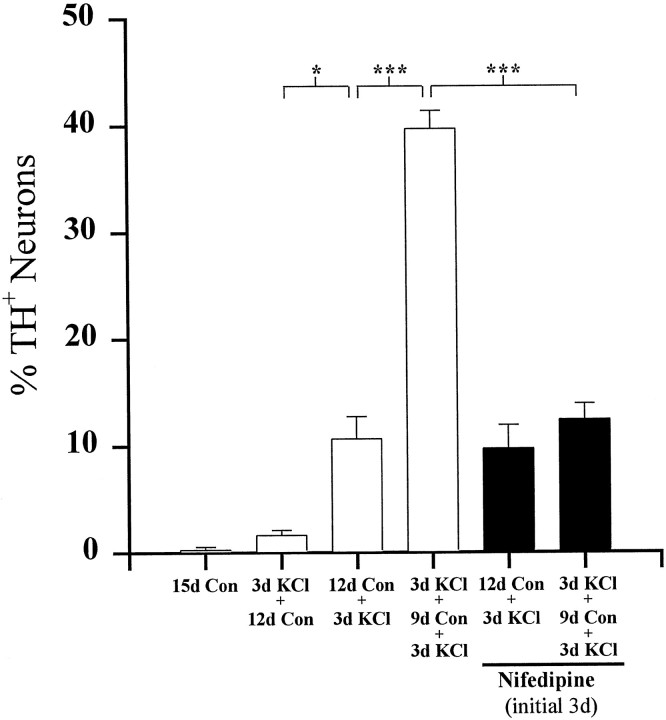
The KCl-mediated increase in TH expression in E16.5 PG cultures is completely reversed after a return to control medium for 12 d [compare Figs. 1 and 4, 91.6 ± 0.9% (3d KCl) vs 2.1 ± 0.3% (3d KCl + 12d Con) TH+ neurons]. To determine whether transient depolarization altered neuronal responsiveness to subsequent stimulation, E16.5 PG cultures were initially depolarized for 3 d (conditioning stimulus), returned to control medium for 9 d, and then depolarized a second time for 3 d (test stimulus). TH expression in these cultures was compared with that in cultures that received only the test stimulus during the last 3 d of the 15 d culture period. Controls included neurons cultured for the entire 15 d in medium without additional KCl, as well as neurons that received only the initial conditioning stimulus. Cultures grown in either of these control conditions contained few TH-immunoreactive neurons at the end of the culture period (<2.5% of the total population; Fig. 4, 15d Con and 3d KCl + 12d Con). Conversely, cultures that received the conditioning stimulus contained 29.7 ± 2.1% TH-positive neurons, a fourfold increase compared with cultures exposed to the test stimulus alone (Fig. 4, 7.8 ± 1.3%, 12d Con + 3d KCl). Total neuronal survival was equivalent in all experimental groups [NF+ cells/ganglion, 114 ± 6.5 (15d Con), 132 ± 10.3 (3d KCl + 12d Con), 124 ± 17.2 (12d Con + 3d KCl), and 130 ± 16.1 (3d KCl + 9d Con + 3d KCl); p > 0.05]. Therefore, although the increase in TH expression induced by the conditioning stimulus alone was reversible, this initial period of depolarization led to a long-term potentiation of the TH response to a subsequent depolarizing stimulus. However, conditioned cultures, after re-exposure to elevated KCl, did not contain as high a percentage of TH+ neurons as did E16.5 cultures examined immediately after 3 d of KCl treatment (Fig. 1). These data indicate that at least some neurons became refractory to the effect of KCl depolarization with time.
Fig. 4.
Long-term potentiation of TH expression by KCl depolarization. Dissociated E16.5 PG neurons were cultured for a total of 15 d as indicated. Each bar shows the mean percentage (± SEM) of neurons exhibiting TH immunoreactivity at the end of the culture period. Con, Control;n.d., not detectable. Comparisons were made using ANOVA followed by Scheffé’s test; ***p < 0.001.
N-type channels carry a majority of the high voltage-activated calcium current in embryonic PG neurons
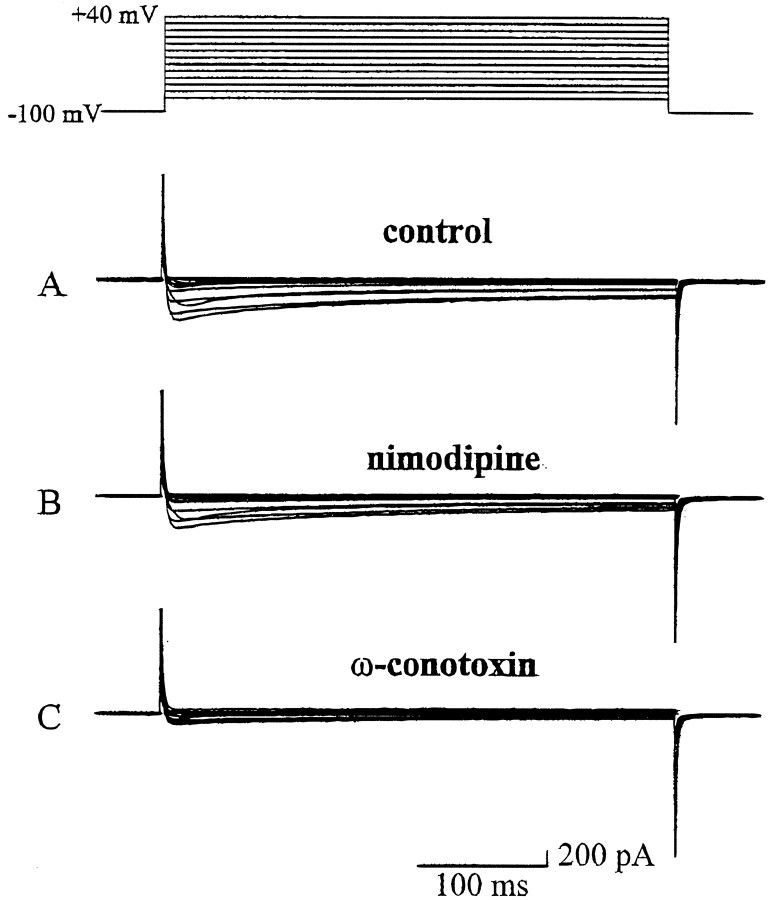
Previous studies using the pheochromocytoma cell line PC12 demonstrated that calcium entry into the cell is required for depolarization-mediated gene expression, including regulation of TH (Kilbourne and Sabban, 1990; Menezes et al., 1996; Bito et al., 1997). To determine whether calcium entry is also important for short-term regulation and long-term potentiation of TH expression in developing sensory neurons, we first characterized the calcium currents present in E16.5-dissociated PG neurons using whole-cell recording techniques. We focused on PG neurons because these cells exhibited the greatest potential to express dopaminergic traits in response to depolarization (Fig. 1). Depolarizing voltage steps (400 msec in duration) were applied from a holding potential of −100 mV to potentials ranging from −80 to +40 mV. The current–voltage relationship consisted of two major components: a small, low threshold (−50 mV) rapidly activating and inactivating T-type current and a larger, high threshold (−30 mV) partially inactivating current. The average peak calcium current evoked at 0 mV from the holding potential of −100 mV was 648 ± 169 pA (Fig. 5A). To further characterize the calcium currents, we repeated these experiments in the presence of selective calcium channel antagonists. ω-Conotoxin (1 μm), an N-type calcium channel antagonist, reduced the average peak current by 68% to 196 ± 36 pA (Fig. 5C). In contrast, nimodipine (2 μm), which blocks L-type calcium currents, reduced the average peak current by only 16% to 539 ± 140 pA (Fig. 5B).
Fig. 5.
Representative recordings of inward calcium currents from an E16.5 PG neuron. Depolarizing voltage steps from −80 to +40 mV were applied in 10 mV increments from a holding potential of −100 mV. A, A recording from a neuron in the control bath solution. B, A recording from the same neuron in the presence of nimodipine (2 μm). C, A recording from the same neuron after washing with control bath solution to remove nimodipine and superfusing with ω-conotoxin (1 μm).
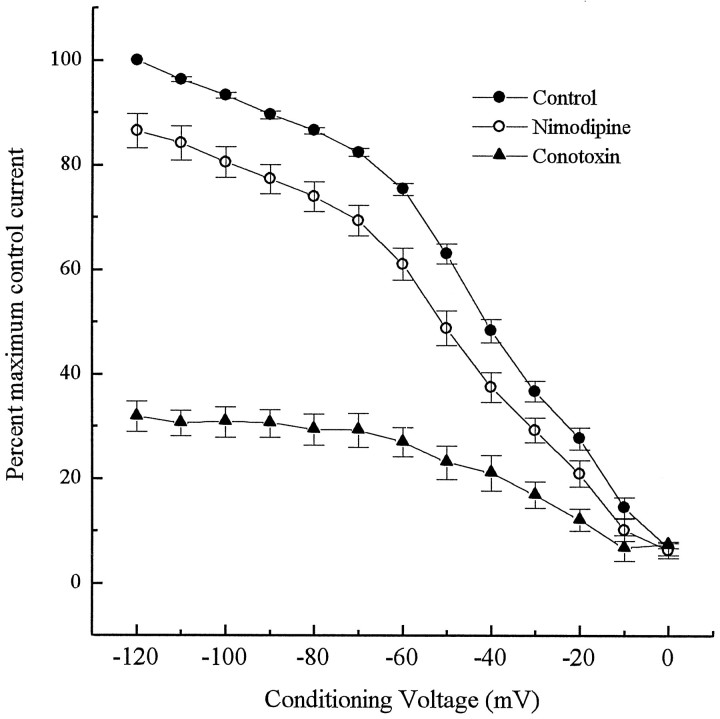
To examine the voltage-dependent inactivation of calcium currents in PG neurons, we applied conditioning voltage steps ranging from −120 to 0 mV for 2 sec, before pulsing the neurons with a test voltage of 0 mV. The holding potential in these experiments was −100 mV. Inactivation of the calcium current began gradually but became steeply voltage dependent at conditioning voltages between −70 and −10 mV (Fig.6). At the 0 mV step, most of the current was inactivated with only 6.7% of the peak current remaining. In the presence of ω-conotoxin (1 μm), a profound inhibition was observed at all conditioning voltages, whereas nimodipine (2 μm) had only a small inhibitory effect on total current at each step (Fig. 6).
Fig. 6.
Voltage-dependent inactivation of high threshold calcium current in E16.5 PG neurons. Conditioning pulses ranging from −120 to 0 mV were applied for 2 sec before applying a test voltage of 0 mV. Points represent normalized data of percentages of maximum control current (± SEM; n = 5).Closed circles, Control inactivation curve;closed triangles, inactivation in the presence of ω-conotoxin (1 μm); open circles, inactivation in the presence of nimodipine (2 μm).
Calcium entry via L-type channels mimics depolarization-dependent induction of TH
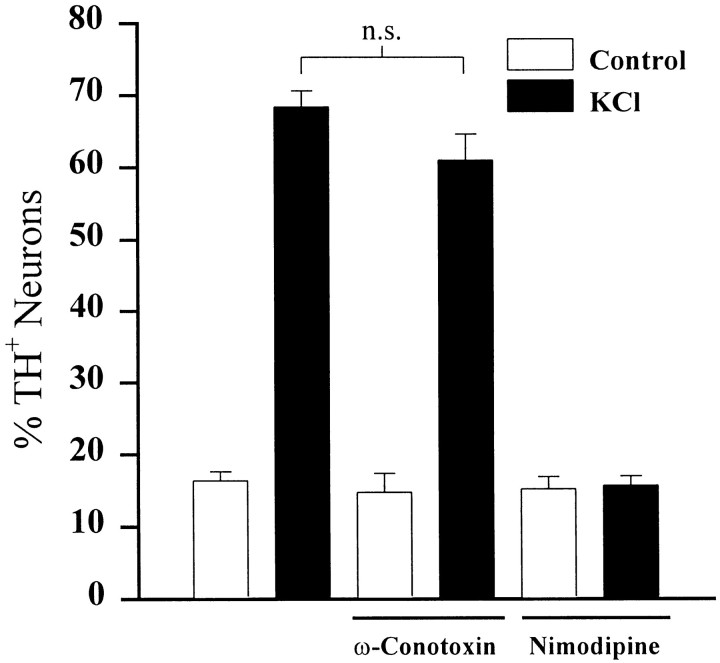
To determine whether calcium entry through a specific channel type is important for short-term regulation of TH expression, we exposed E16.5 PG neurons to KCl for 24 hr in the presence or absence of specific calcium channel antagonists. Application of ω-conotoxin (1 μm) had no effect on TH induction by KCl (Fig.7). However, application of nimodipine (2 μm), nifedipine (1 μm), or verapamil (10 μm), L-type calcium channel antagonists, significantly inhibited depolarization-dependent induction of TH. Nimodipine completely abolished the TH response to elevated KCl (Fig. 7), whereas nifedipine and verapamil resulted in 37 and 51% inhibition, respectively (data not shown).
Fig. 7.
The effect of selective calcium channel antagonists on depolarization-induced TH expression. Dissociated E16.5 PG neurons were cultured for 2 d and subsequently exposed (KCl) or not exposed (Control) to 40 mm KCl in the presence or absence of either ω-conotoxin (1 μm) or nimodipine (2 μm) for 24 hr. Each barindicates the percentage (± SEM) of neurons exhibiting TH immunoreactivity; n.s., not significantly different; ANOVA followed by Scheffé’s test.
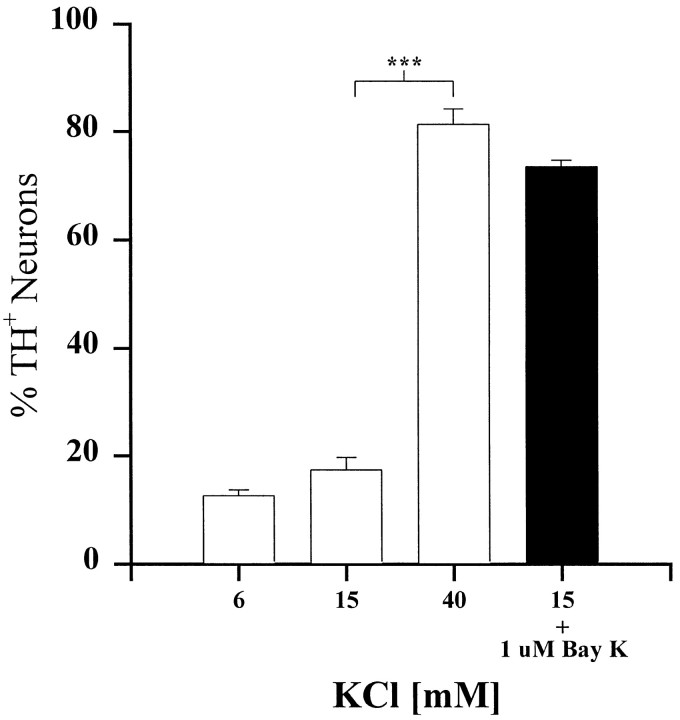
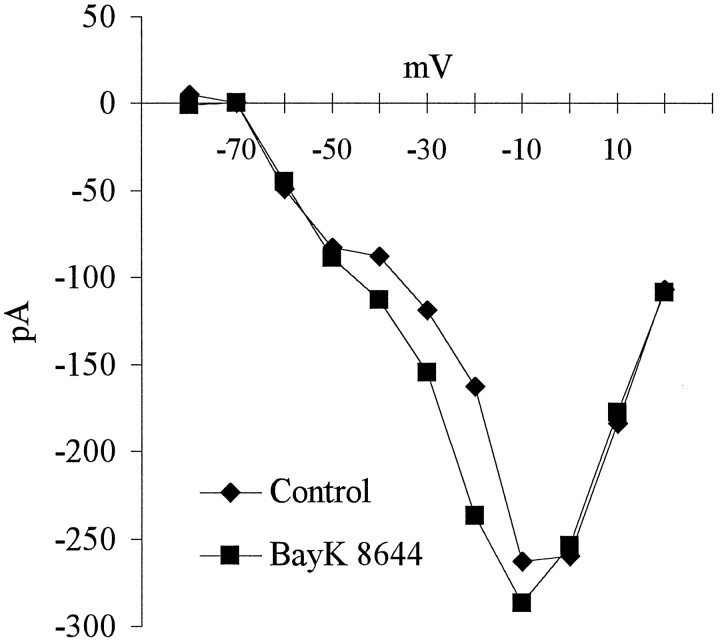
To examine whether activation of L-type channels wassufficient to increase TH expression, we exposed E16.5 PG neurons to Bay K-8644, a selective L-type channel agonist (Nilius et al., 1985; Nowycky et al., 1985). Because Bay K-8644 is most effective when neurons are slightly depolarized, we first determined a KCl concentration that depolarized the neurons without altering TH expression. In the presence of 15 mm KCl, neurons were depolarized to approximately −42 mV (range between −41 and −43 mV;n = 4), but TH expression remained unchanged (Fig.8). However, Bay K-8644, in the presence of 15 mm KCl, induced an increase in the percentage of TH+ neurons that was similar in magnitude to that produced by 40 mm KCl alone (Fig. 8). Moreover, at −40 mV, Bay K-8644 produced a substantial increase in calcium current (Fig.9), indicating that the increase in TH expression observed in the presence of Bay K-8644 was indeed associated with an increase in calcium entry.
Fig. 8.
Selective activation of L-type calcium channels mimics the effect of 40 mm KCl. E16.5-dissociated PG cultures were grown for 3 d in medium containing 6 mm KCl (control), 15 mm KCl, 40 mmKCl, or 15 mm KCl plus 1 μm Bay K-8644 (black bar). Each bar indicates the mean percentage (± SEM) of neurons exhibiting TH immunoreactivity. Comparisons were made using ANOVA followed by Scheffé’s test; ***p < 0.001
Fig. 9.
Current–voltage relationship derived from recordings from an E16.5 PG neuron in the presence of Bay K-8644. Depolarizing voltage steps from −80 to +40 mV were applied in 10 mV increments from a holding potential of −100 mV in the absence (diamonds) or presence (squares) of 1 μm Bay K-8644. The effect of Bay K-8644 is greatest between −40 and −20 mV.
Long-term potentiation of TH regulation requires calcium entry through L-type channels
Because activation of L-type channels can mimic depolarization-dependent induction of TH expression in PG neurons, we wondered whether calcium entry was required for KCl-induced long-term potentiation of TH expression. Therefore, we examined whether blockade of L-type channels by nifedipine during the conditioning stimulus would alter the TH response to a subsequent KCl test stimulus. These experiments followed the same protocol as the conditioning experiments described above. At the end of the 15-day culture period, the percentage of TH neurons in cultures exposed to nifedipine (1 μm) during the conditioning stimulus alone was not significantly different from that in cultures exposed to only the test stimulus [Fig. 10, 12.4 ± 1.5% (nifedipine plus 3d KCl + 9d Con + 3d KCl) vs 10.6 ± 2.1% (12d Con + 3d KCl) TH+ neurons; p = 0.96], indicating that L-channel blockade completely abolished the effect of the conditioning stimulus. Moreover, exposure to nifedipine alone during the initial 3 d in vitro did not significantly change the percentage of TH+ neurons in cultures that received only the test stimulus (Fig. 10, compare 12d Con + 3d KCl with nifedipine plus 12d Con + 3d KCl), indicating that the nifedipine treatment did not alter the ability of PG neurons to respond to high KCl at the end of the culture period.
Fig. 10.
Inhibition of long-term potentiation of TH expression by L-type calcium channel blockade. Dissociated E16.5 PG neurons were grown for a total of 15 d as indicated. Eachbar shows the mean percentage (± SEM) of neurons exhibiting TH immunoreactivity at the end of the culture period.Black bars represent the percentage of TH-positive neurons in cultures exposed to 1 μm nifedipine during the initial 3 d of culture only. Comparisons were made using ANOVA followed by Scheffé’s test; *p< 0.05; ***p < 0.001.
DISCUSSION
Our data demonstrate that depolarizing stimuli and subsequent activation of L-type calcium channels can produce both short- and long-term changes in dopaminergic phenotypic expression in developing primary sensory neurons in vitro. These observations raise the possibility that activity-dependent mechanisms and transmitter differentiation may be linked. In particular, our findings indicate a critical period during which exposure to depolarizing stimuli can unmask a relatively widespread potential for dopaminergic expression, most notably in developing nodose and petrosal cranial sensory neurons. Moreover, the time course of neuronal responsiveness to depolarizing stimuli in vitro correlates well with developmental expression of dopaminergic traits in vivo. For example, TH expression in PG neurons was most sensitive to the effect of elevated KCl when neurons were cultured on E16.5, the same age at which a stable dopaminergic phenotype is first detectable in vivo (Katz and Erb, 1990; dopaminergic expression in NG neurons also begins prenatally; however, the time course of this expression has not been studied in detail). Similarly, DRG neurons, in which dopaminergic traits do not appear in vivo until after birth, were most sensitive to elevated KCl in culture at P0 (Fig. 1). These correlations, therefore, are consistent with a role for neuronal activity in potentiating dopaminergic expression in vivo. This possibility is further supported by our finding that, in the absence of depolarizing stimuli in vitro, PG neurons did not express TH (Figs. 4, 10, 15d Con). In addition, these cells lost the ability to express TH in response to depolarization after 2 weeks in culture (Figs. 4, 10, 12d Con + 3d KCl). In contrast, cultures conditioned by an initial exposure to elevated KCl (Figs. 4, 10, 3d KCl + 9d Con + 3d KCl) exhibited fourfold more TH+ neurons after a second depolarizing stimulus compared with unconditioned cultures. This result demonstrates that depolarization is required for fetal PG neurons to maintain their ability to express TH, at least in vitro. Moreover, we found previously that target contact alone, unlike depolarization, does not induce expression of dopaminergic traits (Hertzberg et al., 1994). On the basis of these findings, we hypothesize that acquisition of the dopaminergic phenotype in vivo requires that neurons become electrically active during the critical period of responsiveness to depolarizing influences defined by our in vitro studies.
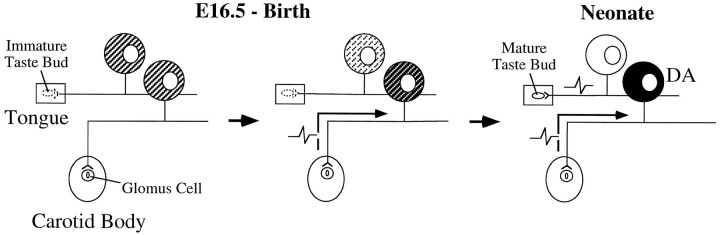
In fact, several lines of evidence indicate that in vivo the dopaminergic subset of PG neurons undergoes transmitter differentiation coincident with the onset of tonic electrical activity. The timing of these events is related to the selective projection of dopaminergic PG neurons to the carotid body (Katz and Black, 1986), a chemoreceptive organ that responds to changes in arterial pO2, pCO2, and pH; tonic activation of chemoafferent PG neurons is mediated by excitatory transmitter release from presynaptic glomus cell receptors in the carotid body (Gonzalez et al., 1992). First, synapse formation between PG neurons and their glomus cell targets, which is required for trans-synaptic activation of afferent terminals, increases rapidly beginning on E16.5 (Kondo, 1975), the same time at which a stable dopaminergic phenotype is first detectable in PG neurons (Katz and Erb, 1990). Carotid body receptors exhibit differentiated properties at this age (Kondo, 1975) and are capable of responding to changes in arterial pO2 in utero(Blanco et al., 1984; Dawes, 1984). The number of dopaminergic neurons in the PG then gradually increases (Katz and Erb, 1990), coincident with proliferation of synaptic contacts in the carotid body (Kondo, 1975). Indeed, recordings of action potentials from carotid body afferents in fetal sheep demonstrated that these neurons are active by the third trimester of gestation (Biscoe et al., 1969). Together, these data indicate that the carotid body afferent pathway is functional in the fetus at the time when the dopaminergic phenotype first begins to appear. In contrast, most other PG neurons innervate taste buds in the tongue, which do not undergo final differentiation until the second week after birth (Torrey, 1940; Hosley and Oakley, 1987). TH expression in PG neurons is relatively insensitive to depolarizing influences by this time (Fig. 1), and gustatory afferents fail to develop a dopaminergic phenotype in vivo [a subset of carotid body afferents is also nondopaminergic (Finley et al., 1992); however, at least some, such as those that express SP, do not form synapses with chemoreceptor cells (Chen et al., 1986; Kummer et al., 1989)]. We hypothesize, therefore, that selective expression of dopaminergic traits by carotid body afferents, and not gustatory afferents, is related to the asynchronous functional maturation of these two populations of cells (Fig. 11). In particular, we propose that the early, tonic activity of carotid body afferents in vivo potentiates TH expression in a manner analogous to the effect of chronic depolarization in vitro. However, depolarization alone is unlikely to account completely for neuronal commitment to the dopaminergic phenotype, because the increase in TH expression induced by high KCl in vitro is reversible after return to control medium. Recent experiments in our laboratory support a role for activity-dependent mechanisms in regulating development of the sensory dopaminergic phenotype in vivo. Specifically, exposure of newborn rats to mild hypoxia, which increases carotid body chemoafferent impulse activity (Bisgard and Neubauer, 1995), significantly increases the number of PG neurons that express TH (J. T. Erickson, T. Hertzberg, and D. M. Katz, unpublished observations).
Fig. 11.
Proposed model for activity-dependent development of the dopaminergic (DA) phenotype in carotid body afferent neurons. On E16.5, all PG neurons have the potential to express the DA phenotype (shading). However, during late fetal development (middle panel), this potential is gradually lost (light shading), except in those neurons that become sufficiently depolarized (dark shading). We hypothesize that a subpopulation of PG neurons that innervate the carotid body become active during this window of transmitter plasticity and, as a consequence, develop a stable DA phenotype. In contrast, gustatory afferents, whose receptors are not terminally differentiated until after birth, do not become sufficiently depolarized during this critical period to induce DA phenotypic traits.
Our data indicate that activation of L-type calcium channels is sufficient to reproduce the effects of KCl depolarization on TH expression in developing sensory neurons. However, we cannot exclude a potential role for other channel subtypes as well. For example, the inability of ω-conotoxin to block depolarization-dependent induction of TH in PG neurons may reflect an inactivation of N-type channels during long-term exposure to high KCl. Specifically, neurons treated with 40 mm KCl exhibited membrane potentials of approximately −20 mV, and after a 2 sec conditioning pulse of −20 mV, only 28% of the total calcium current remained (Fig. 6). Alternatively, the induction of TH expression by depolarization might require calcium entry through specific channel subtypes, such as the L-type, and not through others, because of activation of channel-specific intracellular signaling cascades. Greenberg and colleagues have proposed such a mechanism based on studies of hippocampal neurons (Gallin and Greenberg, 1995; Ghosh and Greenberg, 1995). In these cells, calcium entry through either NMDA or L-type channels increases expression of the immediate-early response gene c-fos (Bading et al., 1993). However, in each case distinct signaling cascades are recruited to induce this expression; calcium/calmodulin-dependent (CaM) kinase is required for c-fos induction after activation of L-type channels but not after stimulation of NMDA receptors (Bading et al., 1993). In addition, unique promoter elements in the c-fos gene are required for calcium-induced c-fos expression, depending on whether calcium enters through L-type channels or NMDA receptors (Bading et al., 1993).
Our finding that calcium entry through L-type channels is required for the long-term effect of transient depolarization on TH induction suggests possible mechanisms by which this potentiation may occur. Early depolarization could change the sensitivity of second messenger pathways to subsequent calcium influx, a mechanism proposed by Schwartz and Greenberg (1987) as a molecular substrate for memory. CaM kinase II, which is required for some forms of long-term potentiation in the hippocampus (Malinow et al., 1989; Silva et al., 1992), has been proposed as a memory store because it can remain active in the absence of external stimuli (Lisman, 1994). Alternatively, depolarization of PG neurons may produce a long-lasting change in the transcription rate of the TH gene and thereby potentiate TH induction by subsequent stimulation. Experiments in progress are designed to differentiate among these potential mechanisms.
Our finding that exposure to depolarizing stimuli can produce long-term phenotypic changes in developing sensory neurons is reminiscent of the sensitization observed in mature nociceptive afferents. During nociceptor sensitization, neuronal excitability is enhanced for many days after an intense stimulus (Levine et al., 1993). Coincident with this change in excitability, transmitter expression and release are increased (Levine et al., 1993). The mechanisms underlying these long-term changes are not well understood, although there is evidence that alterations in ion channels may be involved. For example, agents that sensitize sensory neurons increase Na+ current in DRG cells (Gold et al., 1996) and inhibit calcium-dependent K+ current in nodose neurons (Weinreich and Wonderlin, 1987). Our finding that L-type calcium channels are important for long-term potentiation of TH expression in developing sensory neurons raises the possibility that these channels may contribute to sensitization of transmitter expression in adult sensory neurons as well.
Footnotes
This work was supported by National Institutes of Health Grants HL-25830 (Project 4, D.M.K.; Project 5, D.L.K.), T32NS07118 (T.A.B.), and American Heart Association of Northeast Ohio 233F (D.S.C.).
Correspondence should be addressed to Dr. David M. Katz, Department of Neurosciences, Case Western Reserve University, School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106.
REFERENCES
- 1.Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–87. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- 2.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 3.Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993;52:115–134. doi: 10.1016/0306-4522(93)90187-k. [DOI] [PubMed] [Google Scholar]
- 4.Biscoe TJ, Purves MJ, Sampson SR. Types of nervous activity which may be recorded from the carotid sinus nerve in the sheep foetus. J Physiol (Lond) 1969;202:1–23. doi: 10.1113/jphysiol.1969.sp008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Lung biology in health and disease: regulation of breathing. Dekker; New York: 1995. pp. 617–668. [Google Scholar]
- 6.Bito J, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7:419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- 7.Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol (Lond) 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Yates RD, Hansen JT. Substance P-like immunoreactivity in rat and cat carotid bodies: light and electron microscopic studies. Histol Histopathol. 1986;1:203–212. [PubMed] [Google Scholar]
- 9.Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol (Lond) 1984;346:1–18. doi: 10.1113/jphysiol.1984.sp015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol (Lond) 1984;346:1–18. doi: 10.1113/jphysiol.1984.sp015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan G (1995) Developmental regulation of catecholaminergic phenotypic expression in primary sensory neurons. PhD thesis, Case Western Reserve University, School of Medicine, Department of Neurosciences.
- 12.Gallin WJ, Greenberg ME. Calcium regulation of gene expression in neurons: the mode of entry matters. Curr Opin Neurobiol. 1995;5:367–374. doi: 10.1016/0959-4388(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 13.Garden GA, Redeker-DeWulf V, Rubel EW. Afferent influences on brainstem auditory nuclei of the chicken: regulation of transcriptional activity following cochlea removal. J Comp Neurol. 1995;359:412–423. doi: 10.1002/cne.903590305. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 15.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez C, Almaraz L, Obeso A, Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992;15:146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- 17.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 18.Hertzberg T, Finley JC, Katz DM. BDNF supports mammalian chemoafferent neurons in vitro and following peripheral target removal in vivo. Dev Biol. 1994;166:801–811. doi: 10.1006/dbio.1994.1358. [DOI] [PubMed] [Google Scholar]
- 19.Hertzberg T, Brosenitsch T, Katz DM. Depolarizing stimuli induce high levels of dopamine synthesis in fetal rat sensory neurons. NeuroReport. 1995;7:233–237. [PubMed] [Google Scholar]
- 20.Hosley MA, Oakley B. Postnatal development of the vallate papilla and taste buds in rats. Anat Rec. 1987;218:216–222. doi: 10.1002/ar.1092180217. [DOI] [PubMed] [Google Scholar]
- 21.Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz DM, Erb MJ. Developmental regulation of tyrosine hydroxylase expression in primary sensory neurons of the rat. Dev Biol. 1990;137:233–242. doi: 10.1016/0012-1606(90)90250-m. [DOI] [PubMed] [Google Scholar]
- 23.Katz DM, Markey KA, Goldstein M, Black IB. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proc Natl Acad Sci USA. 1983;80:3526–3530. doi: 10.1073/pnas.80.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilbourne EJ, Sabban EL. Differential effect of membrane depolarization on levels of tyrosine hydroxylase and dopamine beta-hydroxylase mRNAs in PC12 pheochromocytoma cells. Brain Res Mol Brain Res. 1990;8:121–127. doi: 10.1016/0169-328x(90)90056-j. [DOI] [PubMed] [Google Scholar]
- 25.Kleinbaum DG, Kupper LL. Applied regression analysis and other multivariable methods, pp 244–288. Duxbury; Boston: 1978. One-way analysis of variance. [Google Scholar]
- 26.Kondo H. A light and electron microscopic study on the embryonic development of the rat carotid body. Am J Anat. 1975;144:275–293. doi: 10.1002/aja.1001440303. [DOI] [PubMed] [Google Scholar]
- 27.Kummer W, Fischer A, Heym C. Ultrastructure of calcitonin gene-related peptide- and substance P-like immunoreactive nerve fibres in the carotid body and carotid sinus of the guinea pig. Histochemistry. 1989;92:433–439. doi: 10.1007/BF00492501. [DOI] [PubMed] [Google Scholar]
- 28.Kummer W, Gibbins IL, Stefan P, Kapoor V. Catecholamines and catecholamine-synthesizing enzymes in guinea-pig sensory ganglia. Cell Tissue Res. 1990;261:595–606. doi: 10.1007/BF00313540. [DOI] [PubMed] [Google Scholar]
- 29.Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994;17:406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 31.Mains RE, Patterson PH. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973;59:329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 33.Menezes A, Zeman R, Sabban E. Involvement of intracellular or extracellular calcium in activation of tyrosine hydroxylase gene expression in PC12 cells. J Neurochem. 1996;67:2316–2324. doi: 10.1046/j.1471-4159.1996.67062316.x. [DOI] [PubMed] [Google Scholar]
- 34.Nilius B, Hess P, Lansman JB, Tsien RW. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi K, Morita Y, Kiyama H, Ono K, Tohyama M. A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry. Brain Res. 1988;464:31–35. doi: 10.1016/0169-328x(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 36.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 37.Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price J, Mudge AW. A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature. 1983;301:241–243. doi: 10.1038/301241a0. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz JH, Greenberg SM. Molecular mechanisms for memory: second messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- 40.Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol. 1992;320:501–508. doi: 10.1002/cne.903200407. [DOI] [PubMed] [Google Scholar]
- 41.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 42.Torrey TW. The influence of nerve fibers upon taste buds during embryonic development. Proc Natl Acad Sci USA. 1940;26:627–634. doi: 10.1073/pnas.26.11.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vega JA, Amenta F, Hernandez LC, del Valle ME. Presence of catecholamine-related enzymes in a subpopulation of primary sensory neurons in dorsal root ganglia of the rat. Cell Mol Biol. 1991;37:519–530. [PubMed] [Google Scholar]
- 44.Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol (Lond) 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]