Abstract
The rat glossopharyngeal nerve (GL), which innervates posterior tongue taste buds, contains several physiologically defined taste fiber types; at least one type is primarily responsive to certain alkaloids (such as quinine), and another is primarily responsive to acids and salts. In contrast, the chorda tympani (CT), which innervates anterior tongue taste buds, does not appear to contain fibers that differentially respond to quinine relative to salts and acids. It was therefore predicted that GL transection should disrupt behavioral discriminations between quinine and either acids or salts. Water-restricted rats were trained to press one of two levers if a sampled taste stimulus was quinine (0.1–1.0 mm) and the second lever if the sampled stimulus was KCl (0.1–1.0 m). Sham surgery, GL transection, and sublingual and submaxillary salivary gland extirpation were found to have no effect relative to presurgical performance. Both CT transection and combined GL and CT transection caused a substantial and approximately equal decrement in discrimination performance. Removal of the gustatory branches of the seventh cranial nerve [CT and greater superficial petrosal (GSP)] nearly eliminated the discrimination of the taste stimuli, and combined transection of the CT, GL, and GSP unequivocally reduced performance to chance levels. Although these findings were not presaged by the known electrophysiology, they nonetheless compare favorably with other studies reporting little effect of GL transection on behavioral responses to quinine. These results, in the context of other discrimination studies reported in the literature, suggest that, in rats, the neural coding of taste quality depends primarily on the input of the facial nerve.
Keywords: taste, chorda tympani nerve, glossopharyngeal nerve, greater superficial petrosal nerve, neural coding, animal psychophysics, quinine, KCl, nerve transection
Taste buds in the rat are innervated by four branches of three cranial nerves. Although all of these branches respond to all of the classes of prototypical taste compounds, they are differentially sensitive to these sapid stimuli. The chorda tympani (CT) branch of the seventh nerve, which innervates tastes buds on the anterior tongue, is noted for its sensitivity to sodium salts and acids, whereas this nerve responds poorly to sugars and alkaloids (Pfaffmann, 1955; Frank et al., 1983; Boudreau et al., 1985; Nejad, 1986; Dahl et al., 1997; Harada et al., 1997). The greater superficial petrosal branch of the seventh nerve (GSP), which innervates palatal taste buds, responds strongly to sugars but modestly to alkaloids (such as quinine) and salts (Nejad, 1986; Nejad and Beidler, 1987; Harada et al., 1997; Sollars and Hill, 1997). The lingual–tonsilar branch of the ninth nerve (GL) innervates the remaining lingual taste buds. Relative to the branches of the facial nerve, the GL has the strongest response to quinine, responds as well to acids, and has a somewhat weaker response to salts and sugars (Yamada, 1966; Oakley, 1967;Boudreau et al., 1987; Frank, 1991; Dahl et al., 1997). Only sparse data are available on the chemoresponsiveness of the superior laryngeal branch of the 10th nerve in the rat (Andrew, 1956; Shingai, 1980), which innervates a few taste buds in the laryngeal epithelium (Miller, 1977; Travers and Nicklas, 1990). On the whole, based on behavioral and electrophysiological data in rodents and sheep, as well as the anatomical position of its receptor field, this nerve has been hypothesized to play no major role in taste quality coding (Dickman and Smith, 1988; Smith and Hanamori, 1991; St. John et al., 1994; Spector et al., 1996b).
As might be expected from these electrophysiological observations, transection of the CT severely disrupts the behavioral discrimination of NaCl from water and other salt stimuli in rats (Spector et al., 1990b; Spector and Grill, 1992; Breslin et al., 1993). Surprisingly, however, GL transection has no effect on a variety of taste-guided behavioral responses to quinine. For example, GL transection has been found to have no effect on quinine avoidance in two-bottle preference tests (Akaike et al., 1965; Grill et al., 1992), lick rate of water-deprived rats to quinine in brief access tests (Yamamoto and Asai, 1986; St. John et al., 1994), and quinine detection thresholds assessed by a conditioned shock avoidance procedure (St. John and Spector, 1996). The failure of GL transection to compromise the behavioral expression of quinine responsiveness in these tasks is puzzling in light of the pronounced sensitivity of the ninth nerve to this alkaloid, as assessed electrophysiologically (Yamada, 1966;Boudreau et al., 1987; Frank, 1991). How is it possible that transection of a nerve that innervates >60% of all rat taste buds (Miller, 1977) and that is vigorously stimulated by quinine does not affect these taste-guided responses to this stimulus? One possible resolution of this paradox was suggested by St. John and Spector (1996), who noted that previous studies on behavioral responses to quinine merely required the rat to discriminate quinine from water. Although the quinine sensitivity of the GSP and CT is not remarkable, these nerves do, nonetheless, respond to quinine in a concentration-dependent manner and thus may be sufficient to maintain behaviors that do not require high resolution processing.
A more definitive and rigorous test of the GL contribution to the neural code representing quinine would involve a task that required the discrimination of quinine from other taste stimuli. We therefore examined the performance of rats operantly trained to discriminate quinine from KCl, a salt that tastes salty–bitter to humans (van der Klaauw and Smith, 1995). This stimulus pair was chosen because single fibers in the CT do not appear to respond differentially to quinine and KCl, whereas GL fibers do (Ogawa et al., 1968; Boudreau et al., 1983,1987; Frank et al., 1983; Frank, 1991). Thus, we hypothesized that the GL would be critical in the behavioral discrimination between these stimuli, whereas the CT would not.
MATERIALS AND METHODS
Subjects
Forty naive, male, Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) that weighed 259–314 gm at the start of the experiment served as subjects. Two squads of 20 rats each were tested (see Surgery). The rats were housed individually in hanging, wire mesh cages in a room where temperature, humidity, and lighting (lights on from 6:00 A.M. to 6:00 P.M.) were automatically controlled. All manipulations were performed during the lights on phase. The rats always had access to Purina Chow (5001; Ralston–Purina, St. Louis, MO) while in the home cage. Distilled water was also available but was removed ∼24 hr before the first behavioral test session of the week and was replaced at the completion of the final session. In most cases, behavioral testing took place 5 d/week and the rats had ad libitum access to distilled water on the weekend (i.e., for 48 hr after last session).
Apparatus
The design of the apparatus was modified from the gustometer ofSpector et al. (1990a). This apparatus was essentially an operant chamber housed inside a sound attenuation enclosure. The operant chamber contained two levers equidistant (80 mm) from a narrow aperture through which the rat could lick one of two drinking spouts. The sample spout delivered 5 μl of a taste stimulus (quinine hydrochloride, KCl, or distilled water) per lick. The reinforcement spout delivered distilled water at the same rate. Either spout could be positioned opposite the aperture and was computer-controlled via a stepping motor. Between taste trials, the sample spout was moved over a drainage funnel, rinsed with distilled water, and blown dry with pressurized air. The chamber also contained a speaker that provided white noise during behavioral sessions. The house lights and two glass-covered cue lights (each located 50 mm above one lever) were illuminated at various phases of a taste trial.
Trial structure
The two-lever discrimination paradigm was modeled from earlier work by Morrison (1967). The trial structure was essentially the same as used in a previous taste discrimination study except for the stimuli used (St. John et al., 1997b). Basically, rats were trained to press one of the two levers if the sampled stimulus was quinine and the other lever if the sampled stimulus was KCl (Fig.1). The appropriate lever (left or right) for quinine and KCl was counterbalanced across rats, but was held constant for a given rat throughout the experiment. During a behavioral session, stimuli were delivered in discrete trials that were initiated when the rat made two licks on the dry sample spout. Briefly, the rat was allowed 10 licks (or 3 sec of licking after the second lick, whichever came first) of quinine (0.1, 0.3, or 1.0 mm) or KCl (0.1, 0.3, or 1.0 m). The spout was then withdrawn from the stimulus-access aperture, and a lever press was required. After a correct lever press, the reinforcement phase of the trial began, consisting of 40 licks from, or 10 sec access to, the reinforcement spout, whichever came first. After an incorrect response or the absence of a response 5 sec after the sample spout was withdrawn, a time-out period began during which the chamber remained dark and the spouts were removed from the stimulus-access aperture. During the training phase, the duration of the time-out period was systematically increased over sessions from 0 to 30 sec. All contingencies took effect immediately after a lever press or the end of the 5 sec period. At the end of the time-out punishment or reinforcement period, a 10 sec intertrial interval was initiated, and the sample spout was cleaned as described previously. Rats typically took >60 trials per 40 min behavioral session.
Fig. 1.
Flow chart of the trial structure. After 10 licks or 3 sec access to either quinine or KCl (SAMPLING PHASE), the sample spout was removed, and the rat was required to press one of two levers (DECISION PHASE). A correct lever press (e.g., the left-hand lever when KCl was sampled) produced the reinforcement spout (REINFORCEMENT), which was present for 40 licks or 10 sec access to distilled water. An incorrect press, or lack of a response for 5 sec, resulted in a 30 sec time-out (TIME OUT). In either event, the trial ended with a 10 sec interval during which the sample spout was rinsed with water (INTERTRIAL INTERVAL).
Training procedure
The only notable differences in the training procedure used in this study compared with the previously published study (St. John et al., 1997b) were the stimuli used and the number of sessions during each phase of training. During training, some of the parameters of the trial structure were relaxed, such as the 5 sec period allowed for a lever press. For a more detailed explanation of the training phases, see St. John et al. (1997b).
Responses were initially shaped for one concentration of one of the two stimuli (0.1 m KCl or 0.1 mm quinine). An experimenter observed the shaping sessions through a one-way mirror in the sound attenuation chamber and controlled reinforcement delivery remotely. The behavior of pressing the lever appropriate to the first stimulus was shaped by the reinforcement of successive approximations. Once all rats were performing appropriately, sessions included only the one concentration of the other stimulus and responses on the second lever were shaped. Two rats (one in each squad) did not learn to press the levers reliably and were removed from the experiment.
In the next phase of training, both 0.1 m KCl and 0.1 mm quinine were included in the stimulus array, and the stimuli were delivered in alternation such that the rat was required to make a fixed number of correct responses on one lever before receiving a block of trials with the second stimulus. The purpose of these sessions was to ensure that responses on both levers were reinforced within a single behavioral session.
For the remainder of training, solutions were delivered in randomized blocks. At first, the stimulus array included only the training concentrations but eventually included all three concentrations of each stimulus (0.1, 0.3, and 1.0 mm quinine hydrochloride; Sigma, St. Louis, MO; 0.1, 0.3, and 1 m KCl, Fisher Scientific, Houston, TX). It was important to use a range of concentrations for each stimulus so that responses could not reliably be reinforced merely on the basis of perceived intensity. Although we cannot be absolutely certain that these concentrations overlapped in perceived intensity, it seems unlikely that the broad ranges chosen did not at least partly overlap on the basis of other behavioral studies measuring avoidance behavior or detection thresholds of quinine and KCl (St. John et al., 1994; St. John and Spector, 1996; Geran et al., 1997). The high quinine concentration and the two lower KCl concentrations approximate those used in electrophysiological studies (Boudreau et al., 1987; Frank, 1991).
Testing
The presurgical assessment of taste discriminability occurred in five daily sessions that were identical to the final training sessions. The 5 d postsurgical assessment was begun after a surgical recovery period (see Postsurgical treatment). On the day after the final postsurgical test session, the rats were given a “water test” in which all six stimuli were replaced with distilled water; half were assigned as left-lever appropriate and half as right-lever appropriate. The water test was conducted to determine whether rats required a chemical cue to respond at better than chance levels in this paradigm. After the water test, sham rats were given five “retraining” sessions that were identical to the postsurgical behavioral sessions to return the rats to asymptotic performance levels. The rats were then given a 5 d test with a modified stimulus array; the high concentration of each stimulus was replaced with distilled water. Thus, one water stimulus was reinforced as if it were quinine, and the other was reinforced as if it were KCl. The stimulus array thus modeled the hypothetical case that a nerve section rendered the lowest of the three concentrations of each stimulus tasteless and reduced the perceived intensity of the higher concentrations.
The rats were tested in two separate squads. Presurgical and postsurgical testing was identical for both squads, and there were only minor differences in the training phases (some phases ended in fewer sessions for one squad compared with another). The greatest difference between the squads was in their treatment during the surgical recovery period, as noted below. It is important to note that both squads included subjects from the sham control group (squad 1,n = 4; squad 2, n = 3).
Surgery
All rats received a prophylactic dose of penicillin the day before (squad 1) or the day of (squad 2) surgery (30,000 U, s.c.) and were anesthetized intramuscularly with a mixture of ketamine hydrochloride (125 mg/kg of body weight) and xylazine hydrochloride (5 mg/kg of body weight). All nerve transections were performed bilaterally.
Squad 1. Five rats received CT transection (CTX). The auditory meatus was widened with five blunted and curved hypodermic needles. The tympanic membrane and ossicles were removed, and the CT was avulsed with number 7 microforceps.
Five rats had the GL transected alone (GLX), and five rats had the GL transected along with the CT (GLX + CTX). To transect the GL, an incision was made in the skin of the ventral neck, and the sublingual and submaxillary glands, the sternohyoid, omohyoid, and digastric muscles were retracted to expose the GL. A large section of the nerve (5–10 mm) was removed, and the wound was closed with a nylon suture.
Finally, four rats received sham surgery, in which the tympanic membrane was punctured, and the GL was exposed but not manipulated.
Squad 2. Five rats had the GSP avulsed in addition to the CT (7TH), which removed the combined gustatory input of the facial nerve, and six rats had the GL sectioned along with the GSP and CT (TRIPLE). Transection of the GSP involved an incision around the dorsal side of the pinna, which was then retracted. The auditory meatus was cut and widened by careful dissection of the fascia and retraction of the surrounding musculature. The bony meatus was enlarged with a pneumatic dental drill. The tympanic membrane, ossicles, and CT were removed. The tensor tympani and a small piece of temporal bone were removed, exposing the GSP, which was avulsed with microforceps. The incision was closed with wound clips.
Five rats were partially desalivated (DSAL). An incision was made in the ventral neck, and fascia was dissected to expose the sublingual and submaxillary salivary glands. The duct was ligated with a 4–0 silk suture, and the glands were then removed distal to the ligature. The wound was closed with a nylon suture.
Finally, three rats received sham surgery (CON). The procedure was basically identical to the sham surgery in squad 1, except that an incision was made around the dorsal side of the pinna, and the auditory meatus was retracted before cutting the tympanic membrane.
Postsurgical treatment
Squad 1. The rats in squad 1 were allowed at least 12 d to recover before postsurgical testing. These rats were not given any special diets during the recovery period.
Squad 2. As in previous studies in which water-restricted, partially desalivated rats were tested (St. John et al., 1994, 1997b), we gave the rats a moist “corn oil diet” (five parts Purina Chow to two parts 100% corn oil) (Catalanotto and Sweeney, 1973) to facilitate food intake in the home cage. The corn oil diet was given to all rats in squad 2 beginning the last 10 sessions of training before surgery and continuing throughout presurgical testing, postsurgical recovery, postsurgical testing, and the water test.
In addition, some rats (especially in the 7TH and TRIPLE groups) were hypophagic and dropped to ∼80% of their presurgical body weight during the first week of recovery. Two palatable diets were introduced in an effort to stimulate feeding in these rats (Jacquin, 1983). First, wet mash was supplemented with Nutri-Cal (Evsco Pharmaceuticals, Buena, NJ) for 6 d. Then, for the next 4 d, all rats were given a palatable liquid diet (354 ml of Borden condensed milk, 354 ml of distilled water, and 1 ml of multiple vitamins with iron; Poly Visol, Mead–Johnson). Approximately 50 ml of the milk diet was available each night.
Histology
Rats were deeply anesthetized with sodium pentobarbital and then perfused transcardially with saline followed by 10% formalin. Sham rats were perfused at the conclusion of the additional behavioral testing, whereas the other rats were perfused after the water test. The tongue, nasoincisal papilla, and soft palate were removed and post-fixed for at least 1 week in 10% formalin. Because taste buds degenerate after gustatory nerve transection, inspection of the appropriate taste bud receptor field allowed confirmation of the efficacy of nerve transection.
The tongue anterior to the intermolar eminence was soaked in distilled water for at least 30 min and was then dipped briefly in 0.5% methylene blue and rinsed with distilled water. The epithelium was removed and pressed between two glass slides. The number of taste pores and fungiform papillae were quantified in tissue from all groups except the GLX. After such staining, taste pores appear as dark blue dots on the background of pale staining fungiform papillae and reliably indicate the presence of morphologically intact taste buds (St. John et al., 1995).
The circumvallate papilla was examined in all rats except those in the CTX and 7TH groups, and both the geschmacksstreifen (a taste bud-rich stripe of soft palate bordering the hard palate) and nasoincisal papilla were examined in all rats except those in the CTX, GLX, and GLX + CTX groups. These tissues were embedded in paraffin and cut (10 μm) on a rotary microtome. Sections were mounted on glass slides and were stained with hematoxylin and eosin. Taste pores were then visualized under a light microscope and counted.
Data analysis
The primary dependent measure was the percentage of trials on which a correct response was made. Because trials in which the rat did not make a response were discarded, 50% represented chance performance. Group means were analyzed using ANOVAs.
Rats in this experiment received 175–415 trials in which the rat made a lever press during the 5 d presurgical or postsurgical test. In the tradition of animal psychophysics, large group sizes were sacrificed in preference to obtaining a large number of trials for each individual subject. To capitalize on this feature of the data set, performance was also analyzed within individual subjects such that each trial was considered an independent Bernoulli trial resulting in a correct or incorrect response. The postsurgical percentage correct across all trials, or as a function of concentration, was compared with the presurgical percentage correct using the normal approximation to the binomial distribution (Brown and Hollander, 1977).
For all analyses, two-tailed tests were conducted, and the statistical rejection criterion (i.e., α) was set at p = 0.05.
RESULTS
Histology
Examination of oral tissue confirmed the efficacy of all nerve sections (Table 1). No rat in the CTX, GLX + CTX, 7TH, or TRIPLE groups had >21 pores in the fungiform papillae, whereas sham rats always had at least 141. The persistence of a few taste pores after CT transection is consistent with previous reports (Whitehead et al., 1987; Ganchrow and Ganchrow, 1989; St. John et al., 1994). Although an occasional taste bud was seen in palatal fields in GSP-sectioned rats, no rat in the 7TH or TRIPLE group had more than nine buds in the nasoincisal papilla or four buds in the geschmacksstreifen compared with at least 97 and 93 in control tissue, respectively. Therefore, it does not appear that any significant regeneration occurred during the postsurgical recovery and testing phases of the experiment. Separate one-way ANOVAs confirmed that the groups differed in the number of taste pores counted in the four receptor fields analyzed (all p < 0.05), with rats in the control and DSAL groups always having more taste pores than experimental rats (Tukey’s honestly significant difference test,p < 0.05). The post hoc test also revealed that the DSAL group had statistically fewer taste pores in the vallate papilla and geschmacksstreifen relative to controls (p < 0.05). Other researchers have shown that removal of the major salivary glands compromises the integrity of vallate taste buds (Cano and Rodriguez-Echandia, 1980; Nanda and Catalanotto, 1981), but decreases in taste bud number were not reported. In a previous behavioral experiment with a larger sample size, we found no effect of desalivation on the number of vallate taste buds (Markison et al., 1995). Therefore, considering the small sample in this study, as well as the lack of a consistent effect across studies in vallate taste bud number, we recommend caution in concluding that desalivation results in a loss of taste buds in these fields. In any event, both CON and DSAL rats had substantially more taste buds than rats in the other groups (Table 1).
Table 1.
Histological results
| Group | Mean number of taste buds (± SE) | |||
|---|---|---|---|---|
| Anterior tongue | Vallate papilla | Nasoicisal papilla | Geschmacksstreifen | |
| Control1-a | 159.7 (5.2) | 437.3 (10.4) | 103.7 (4.4) | 98.0 (4.5) |
| DSAL1-a | 156.4 (8.6) | 378.0 (6.6) | 91.7 (10.3) | 75.7 (5.2) |
| CTX | 3.8 (0.9) | |||
| GLX | 0.01-1002 | |||
| GLX + CTX | 8.0 (3.6) | 0.01-1002 | ||
| 7TH | 8.2 (2.4) | 1.6 (0.5) | 1.6 (0.7) | |
| TRIPLE | 7.5 (2.1) | 0.01-1002 | 2.7 (1.3) | 1.3 (0.6) |
Sub-sample only; n = 3.
F1-1002: No standard error (all subjects had zero taste pores).
Quinine–KCl Discrimination Performance
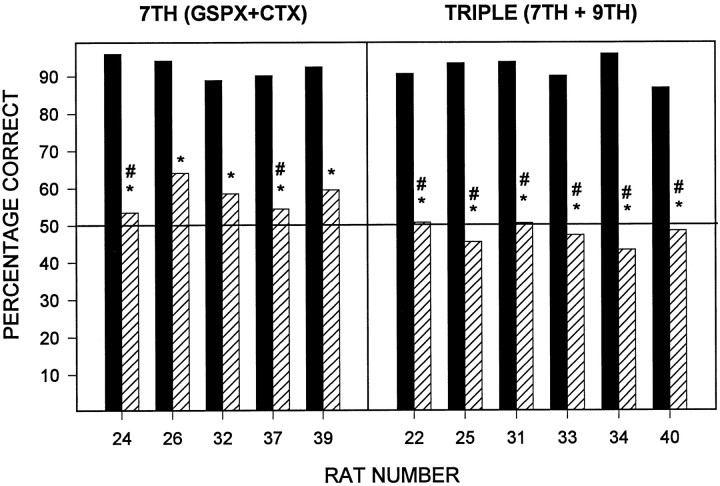
Surprisingly, transection of the GL had no effect on postsurgical performance, whereas CTX significantly degraded performance on this task (Fig. 2). Combined transection of the GL and CT (GLX+CTX) appeared to have the same effect on performance as CTX alone, whereas combined facial nerve transection (7TH) had a statistically more pronounced effect than CTX alone. Finally, combined transection of the GSP, CT, and GL (TRIPLE) reduced postsurgical performance to 50%, a result consistent with the conclusion that these rats could not discriminate quinine from KCl. Matched t tests for each group (presurgical vs postsurgical percentage correct) revealed statistically significant decreases in performance in the CTX, GLX + CTX, 7TH, and TRIPLE groups (all p < 0.05). Separate one-sample t tests indicated that the postsurgical percentage correct in all groups differed from chance (50%) with one exception: there was not evidence to conclude that the postsurgical performance of the TRIPLE group was different from 50%.
Fig. 2.
Mean + SE percentage correct before (filled bars) and after (hatched bars) sham surgery (CON), glossopharyngeal transection (GLX), removal of the sublingual and submaxillary salivary glands (DSAL), chorda tympani transection (CTX), combined GLX + CTX (GLX+CTX), combined CTX and transection of the greater superficial petrosal (7TH), and combined 7TH and GLX (TRIPLE). Because only trials on which a lever press was made were included, 50% represents chance responding (solid line). Asterisksindicate a significant difference from presurgical percent correct (matched t test, p < 0.05);pound sign indicates statistically indistinguishable from chance (Student’s t test vs 50%,p > 0.05).
Additionally, a one-way ANOVA on the postsurgical percentage correct revealed a significant main effect of surgical group (F(6,31) = 47.1; p < 0.0001).Post hoc analysis with Tukey’s honestly significant difference test, with the statistical rejection criterion set at the 0.05 level, revealed three clusters of groups: the sham, GLX, and DSAL groups formed one cluster; the CTX and GLX + CTX groups formed a second; and the 7TH and TRIPLE groups formed a third cluster. The groups within these statistically defined clusters did not differ from one another but did differ from all groups in the other clusters.
When each rat was treated as a separate experiment, the same conclusions could be drawn. These results are shown in Figures3-5. Although two rats in the control group had significantly lower postsurgical performance (Fig. 3, rats 14 and 35), the magnitude of those differences were relatively small. Likewise, rat 30 in the DSAL group had a slight decrease in postsurgical performance after surgery (Fig. 4). Another rat in that group, however, showed a more substantial decrease in postsurgical performance that appears to be atypical compared with the other subjects in the DSAL group (rat 27). Note that no rat in the GLX group showed any evidence of decreased performance after neurotomy (Fig. 3).
Fig. 3.
Percentage correct before (filled bars) and after (hatched bars) surgery for each rat in the CONTROL (left panel) and glossopharyngeal transection (GLX; right panel) groups. Because only trials on which a lever press was made were included, 50% represents chance responding (solid line). Asterisks indicate a significant difference from presurgical percent correct (normal approximation to the binomial distribution, p < 0.05).
Fig. 4.
Percentage correct before (filled bars) and after (hatched bars) surgery for each rat in the partially desalivated (DSAL, left panel), chorda tympani transected (CTX, center panel) and combined CTX and glossopharyngeal transection (GLX+CTX, right panel) groups. Because only trials on which a lever press was made were included, 50% represents chance responding (solid line).Asterisks indicate a significant difference from presurgical percent correct (normal approximation to the binomial distribution, p < 0.05).
Fig. 5.
Percentage correct before (filled bars) and after (hatched bars) surgery for each rat in the combined greater superficial petrosal and chorda tympani transected (7TH, left panel) and combined 7TH and glossopharyngeal transection (TRIPLE) groups. Because only trials on which a lever press was made were included, 50% represents chance responding (solid line).Asterisks indicate a significant difference from presurgical percent correct (normal approximation to the binomial distribution, p < 0.05); pound signs indicate indistinguishable from chance (p > 0.05, postsurgical percent correct vs 50%).
Every single rat in the CTX and GLX + CTX groups demonstrated a statistically significant deficit after neurotomy (Fig. 4), and the decrease in percentage correct was substantial in each rat. Even more pronounced decreases were seen in rats in the 7TH and TRIPLE groups (Fig. 5). No rat in the TRIPLE group performed at >50.8% correct after neurotomy; in this task, rats would be expected to do no <50% because random lever pressing would still result in a correct choice on half of the trials. Indeed, there was not statistical evidence to reject the null hypothesis that any rat in the TRIPLE group (as well as rats 24 and 37 in the 7TH group) performed different from 50% after surgery.
It should be noted that, across rats, there did not seem to be systematically greater deficits in responding to one of the taste stimuli relative to the other (data not shown). In a previous discrimination experiment, we found that high doses of amiloride (>10 μm) added to NaCl and KCl test stimuli (0.05–0.2m) severely impaired the ability of rats to discriminate these two salts (Spector et al., 1996a). Interestingly, rats routinely pressed the lever associated with KCl regardless of whether the stimulus was NaCl or KCl adulterated with amiloride. We concluded that amiloride was predominantly affecting the taste quality of NaCl. In the current study, however, the effective nerve transections did not systematically disrupt responding to one stimulus over the other, so it is not possible to conclude that the taste quality of quinine or KCl was preferentially altered.
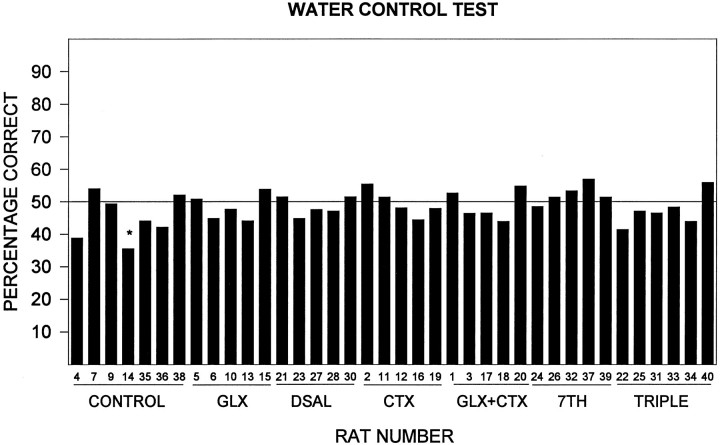
Water test
During the water test, the rats averaged 48.3% ± 0.79 correct responses (Fig. 6), and an analysis of each rat’s performance indicated that no rat performed at >50%. Thus, there was no evidence to conclude that any rat could perform appropriately in this task in the absence of chemical cues.
Fig. 6.
Percentage correct for each subject in the water control test (abbreviations as in Fig. 2). Asterisksrepresent a significant difference from 50% (normal approximation to a binomial distribution, p < 0.05).
Additional testing
After the water test, sham rats were given five behavioral sessions with the standard stimulus array. At first, the water test appeared to disturb performance, but by the fifth day, all rats were performing as well as they were during the postsurgical test. Over the next 5 d, these rats were tested with a modified stimulus array, as previously described. As can be seen in Figure7, all rats continued to respond at a high rate of correct responses to the two concentrations of quinine and KCl but tended to press the quinine-associated lever whenever water was presented. Indeed, it may be that water is more qualitatively similar to quinine than KCl. Our main interest, however, was in how rats would respond to the remaining discriminable taste solutions, because the pattern of responses could reflect what would be seen if the effect of nerve transection were merely to reduce the perceived intensity of the taste stimuli. Compromised rats in the nerve transection groups frequently were disrupted at even the highest concentrations of taste stimuli and did not preferentially press the quinine-associated lever to low concentrations of both quinine and KCl. Thus, the CT and facial nerve transection most likely induced disruptions in taste quality, over and beyond any diminution of taste intensity.
Fig. 7.
Results of additional testing of control rats (see Materials and Methods). Percentages of correct responses ± SE as a function of stimulus with the normal stimulus array (i.e., the postsurgical test, top panels) and the altered array (lower panels) are shown. Note that the addition of a stimulus that could not be discriminated on the basis of taste (water) did not result in decreased performance to either quinine or KCl.
DISCUSSION
The use of the two-lever operant discrimination procedure provided a direct test of the hypothesis that the GL was necessary for the rat to distinguish quinine from KCl. Several lines of evidence led to the hypothesis that transection of this nerve would cause measurable impairments on this task: (1) of the major gustatory nerve branches, the GL appears to be the most responsive to quinine; (2) unlike the CT, this nerve contains fibers that respond to quinine with relatively narrow tuning and thus could differentially respond to the test stimuli; and (3) the GL innervates >60% of all rat taste buds. Unexpectedly, however, transection of this nerve had no effect on discrimination performance. In contrast, the CT was necessary for normal quinine–KCl discrimination to be maintained. Moreover, combined removal of the gustatory branches of the facial nerve (the CT and GSP) nearly eliminated the rat’s ability to discriminate quinine from KCl.
Given that sublingual and submaxillary salivary gland removal did not cause a similar degree of impairment, the consequence of CT transection cannot be explained simply as a secondary effect of partial denervation of these glands. Moreover, because the deficits seen in this group did not resemble the pattern of results seen in control rats during additional testing, it appears that CT transection produces deficits in taste quality coding rather than merely reducing perceived intensity. In addition, the fact that TRIPLE rats were unable to discriminate quinine from KCl at the concentrations tested raises our confidence that the rats were not using any nongustatory cues to perform appropriately in this task. Specifically, olfactory or trigeminal cues were not sufficient for the TRIPLE rats to perform at better than chance levels (Fig. 5). With these considerations in mind, it appears that the gustatory input of the seventh, but not the ninth, cranial nerve is critical in the normal discrimination of quinine from KCl. How can these unexpected results be reconciled with the known electrophysiological response properties of the gustatory nerves?
One possibility is based on human psychophysical reports of KCl as a complex, salty–bitter stimulus (van der Klaauw and Smith, 1995). Relative to the other gustatory nerves, the rat CT responds best to salts, and the GL responds best to alkaloids such as quinine. If GL transection were to remove the “bitterness” of both KCl and quinine, then the stimuli would remain discriminable because KCl would be rendered purely “salty,” and quinine would be rendered nonbitter. Furthermore, CT transection might remove the saltiness of the two compounds, rendering KCl purely bitter and thus more similar to quinine.
Although intuitively appealing, this hypothesis is oversimplified and receives little empirical support. The data most damaging to this interpretation are those showing the effect of nerve transection on NaCl versus KCl discrimination. By the same logic presented above, GL transection should disrupt such a discrimination by rendering KCl as purely salty, whereas CT transection should not, because it should actually make KCl less like NaCl. Contrary to this prediction, several experiments using a variety of behavioral techniques (including the two-lever operant conditioning task used in the current study) have demonstrated that CT transection, but not GL transection, impairs the ability of rats to discriminate NaCl from KCl (Spector and Grill, 1992;Markison et al., 1995; St. John et al., 1995, 1997b). Second, it is difficult to endorse the premise that GL transection removes the bitterness of taste stimuli given that it does not alter quinine preference–aversion functions as measured by two-bottle preference tests or brief-access licking tasks (Akaike et al., 1965; Yamamoto and Asai, 1986; Grill et al., 1992; St. John et al., 1994). Third, as mentioned earlier (see Results), CT transection did not preferentially disrupt responding to KCl in this study, as would be expected if the nerve section removed the saltiness of KCl (and did not affect the nonsalty quinine). These considerations help underscore the danger in interpreting rodent behavior in terms of human perceptual reports.
A more tenable basis for the discrepancy between the rodent electrophysiology and behavior is that the nerves are relatively functionally specialized (Frank, 1991), as is more clearly the case in the channel catfish and some other teleost fishes (Atema, 1971; Finger and Morita, 1985; Caprio et al., 1993; Finger, 1997). In catfish, the facial nerve innervates predominantly extraoral taste buds, whereas the GL and vagus nerves innervate intraoral taste buds. These nerves terminate in separate, highly specialized lobes in the brainstem. When the facial system is disrupted, catfish cannot locate food, although they can appropriately swallow or reject food placed in the oral cavity depending on its chemical nature. When the glossopharyngeal–vagal system is damaged, catfish can locate food but fail to initiate swallowing reflexes (Atema, 1971; Caprio et al., 1993).
In the rat, the 7th, 9th, and 10th cranial nerves terminate in partially overlapping but separate regions of the nucleus of the solitary tract (Hamilton and Norgren, 1984), and there is growing evidence that this relative segregation persists at more rostral levels of the central gustatory system (Halsell et al., 1996; Halsell and Travers, 1997). Such an anatomical organization might be expected to subserve functional differences between the peripheral nerves. We propose that sensory–discriminative taste function is based primarily on the gustatory input of the seventh cranial nerve. That is, input from the seventh nerve is channeled into neural circuits that serve to identify taste stimuli. Although the functional role of GL-derived taste input remains undetermined, we speculate, as have others, that it may be more involved with protective oromotor rejection reflexes (Travers et al., 1987; Frank, 1991; Grill et al., 1992; Grill and Schwartz, 1992). Affective (i.e., hedonic) responses to taste stimuli as well as nonspecific taste detection (presence or absence of any chemical cue regardless of quality), functions that do not require high resolution between chemical compounds, appear to be subserved by patterns of convergent input from the CT, GSP, and GL, depending on the taste stimulus (Pfaffmann, 1952; Yamamoto and Asai, 1986; Spector et al., 1990b, 1993, 1996b; Slotnick et al., 1991; Grill and Schwartz, 1992; Grill et al., 1992; Cauthon et al., 1994; St. John et al., 1994;St. John and Spector, 1996). As mentioned, the gustatory input from the 10th cranial nerve in the rat is thought to be involved with the protection of the airways. In support, behavioral evidence to date, including the present study, suggests that rats may be aguesic to sucrose and quinine (St. John et al., 1994; Spector et al., 1996b) when only the vagal taste receptors are left intact.
The supposition that the neural coding of the sensory features of a taste stimulus that subserves stimulus identification depends primarily on the input of the facial nerve is buttressed by the effects of gustatory nerve transection on performance in behavioral tasks that require the rat to compare sapid stimuli on the basis of quality. In such tasks, GL transection has never been found to interfere with performance (even in tasks involving quinine and other potent stimuli for this nerve), whereas considerable impairments consistently follow seventh nerve (or CT alone) transection (Table2). It is true that partial competence in taste discrimination tasks is sometimes demonstrated after combined neurotomy of the CT and GSP, which suggests that the input of the GL can support some sensory–discriminative taste function but only in a highly compromised form. Furthermore, given that TRIPLE rats in the current study did not perform at better than chance levels supports the view that the 10th nerve does not contribute much to coding the sensory features of taste stimuli that signal quality.
Table 2.
Effect of nerve transection on taste discrimination
| Task | GLX | CTX | 7THX | Reference2-a |
|---|---|---|---|---|
| Sucrose vs Maltose2-1002 | None | None | Large | Spector et al., 1997 |
| NaCl vs KClb,c | None1 | Large1,2 | Unknown2-1004 | 1Spector and Grill, 1992; 2St. John et al., 1995, 1997a |
| Expression of an NaCl taste aversion2-e | None1 | None2to Large1 | Large3 | 1Yamamoto et al., 1994b; 2St. John et al., 1997b; 3Spector et al., unpublished observations |
| Expression of Na-specific salt appetite2-1006 | None2 | Moderate1 | Unknown | 1Breslin et al., 1993, 1995; 2Markison et al., 1995 |
| Quinine vs citric acid2-1002 | None | Moderate | Moderate | St. John and Spector, 1997 |
| Quinine vs KCl2-c | None | Moderate | Large | this study |
When more than one reference is listed for a given task, the numbers indicate which reference refers to which result.
F2-1002: Conditioned shock avoidance taste discrimination task.
Two-lever operant taste discrimination task.
F2-1004: Amiloride, which inhibits a portion of the NaCl-evoked taste activity in the facial nerve (Heck et al., 1984;Brand et al., 1985; Sollars et al., 1997), completely impairs NaCl versus KCl discrimination performance (Spector et al., 1996a).
Taste aversion generalization task. AlthoughSt. John et al. (1997) failed to replicate the severity of CTX-induced deficits in the acquisition of a NaCl CTA seen by Yamamoto et al. (1994b), seventh nerve neurotomy causes profound deficits (Spector et al., unpublished observations).
F2-1006: Response of furosemide-injected rats to an array of chloride salts.
The peripheral distribution of taste buds is consistent with the hypothesized functional roles of the gustatory nerves. The position of the taste buds innervated by the 10th nerve appears to be less than optimal for taste stimulus sampling. In contrast, their location in the laryngeal epithelium would seem well suited to protect the airways. Likewise, taste buds specialized for the identification and discrimination of taste solutions might be expected to lie on the surfaces that first contact taste stimuli (e.g., the anterior tongue). Although this anatomical reality is consistent with the hypothesized functional role of the facial nerve, it raises the possibility that rats in this experiment may have learned to discriminate quinine from KCl on the basis of that information alone. If so, it is not surprising that discriminative behavior was lost after complete or partial facial nerve transection. What remains a question is whether rats, given enough time, might eventually be able to “relearn” the discrimination on the basis of posterior tongue input alone. Rats in the CT and GLX + CTX group did show an improvement in discriminative behavior over the 5 d postsurgical test, but rats in the 7TH group did not (data not shown). Perhaps >5 d would be required, or perhaps posterior tongue input cannot serve as the sole discriminative signal. A more straightforward test of these possibilities than allowing a longer postsurgical test interval would be to perform the facial nerve transection first and to examine whether such rats could ever be trained on a quinine versus KCl task.
Over and beyond the possibility that under some circumstances the GL alone might provide a signal that qualitatively differentiates quinine from KCl, there remains the paradox (based on the electrophysiology) that rats in this experiment did form a discrimination that was dependent on the facial nerve. Why does disruption of facial nerve input have such severe effects on the behavioral discrimination of quinine from KCl, given that this nerve does not ostensibly contain units that are differentially responsive to these compounds? Quinine-responsive fibers of the CT (H-units) also respond to nonsodium salts such as KCl (Frank et al., 1983; Dahl et al., 1997). Because H-units do not differentially respond to these stimuli (at concentrations examined), it is surprising that this nerve provides valuable information for such a behavioral discrimination. Nonetheless, the behavioral results make it clear that the CT must contain neural elements that are differentially responsive to the stimuli tested, whether such differential responsiveness takes the form of a spatial or temporal pattern (Nagai and Ueda, 1981; DiLorenzo, 1989; Erickson et al., 1994).
From a spatial coding perspective, it is possible that more specific quinine-responsive units exist in the CT but have not been located because of an unknown sampling bias. Other researchers have been puzzled by the difficulty in locating neurons responsive to moderate concentrations of quinine at several levels of the gustatory system (Ogawa et al., 1968, 1984; Frank et al., 1983; Ogawa and Hayama, 1984;Nakamura and Norgren, 1991; Nishijo and Norgren, 1991; Halsell et al., 1993; DiLorenzo and Monroe, 1995), in light of the strong activation of c-fos protein (thought to be a marker of neuronal activity) and the robust behavioral responses to far lower concentrations (Koh and Teitelbaum, 1961; St. John et al., 1994; Yamamoto et al., 1994a; Harrer and Travers, 1996; St. John and Spector, 1996; Thaw, 1996). Similarly, perhaps there are facial nerve units that respond to KCl but not to quinine regardless of the bandwidth of their tuning. For example, only 53% of H-units in the Frank et al. (1983) study had a response to quinine above the chosen criterion for activation, and this percentage was based on the response to 0.02 m quinine, which is 1.3 log10 units higher than the highest concentration used in the current behavioral study. Given that rats can detect concentrations 1000 times lower than 0.02 m, the peripheral gustatory system must be capable of responding to concentrations far weaker than those typically used in electrophysiological studies (Koh and Teitelbaum, 1961; St. John and Spector, 1996; Thaw, 1996). The use of lower concentrations might reveal greater fiber specificity than previously suggested. Moreover, although the response properties of single fibers in the CT have been extensively studied, those of the GSP have yet to be comprehensively characterized.
The potential for differences in the functional roles of the gustatory nerves has important implications for theories of taste quality coding. These theories often assume that all taste-responsive units play an equal role is quality coding, but in fact, this might not be the case at some levels of the nervous system. For example, if GL input cannot be shown to make a significant contribution to sensory–discriminative function, then there would be little justification for incorporating the taste response properties of its units into a model of quality coding. That is not to say, of course, that GL input might not make a significant (or even substantial) contribution to other aspects of gustatory processing, which may be manifest in affective behavior or cephalic phase responses to tastants. It should also be stressed that whatever the functional roles of the gustatory nerves, they are not necessarily conserved across all species of vertebrates or even mammals. Accordingly, electrophysiological data from gustatory nerves should be evaluated in the context of a variety of functions with respect to the given species under examination.
Footnotes
This work was supported by Grant R01-DC01628 from the National Institute on Deafness and Other Communication Disorders. A.C.S. is the recipient of a Research Career Development Award (K04-DC00104) from the National Institute on Deafness and Other Communication Disorders, and S.J.S. is the recipient of a Graduate Research Fellowship from the National Science Foundation. We thank Mircea Garcea, Nick Guagliardo, Kim Robertson, Jessica Couch, and Brian Sauer for technical assistance.
Parts of this paper were presented in a dissertation in partial fulfillment of a Doctor of Philosophy degree from the University of Florida and were also presented at the 12th International Symposium on Olfaction and Taste, San Diego, CA, July 1997.
Correspondence should be addressed to Dr. Alan C. Spector, Department of Psychology, University of Florida, Gainesville, FL 32611-2250.
Dr. St. John’s present address: Department of Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore, MD 21201.
REFERENCES
- 1.Akaike N, Hiji Y, Yamada K. Taste preference and aversion in: rats following denervation of the chorda tympani and the IXth nerve. Kumamoto Med J. 1965;18:108–109. [PubMed] [Google Scholar]
- 2.Andrew BL. A functional analysis of the myelinated fibres of the superior laryngeal nerve of the rat. J Physiol (Lond) 1956;133:420–432. doi: 10.1113/jphysiol.1956.sp005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atema J. Structures and functions of the sense of taste in the catfish (Ictalurus natalis). Brain Behav Evol. 1971;4:273–294. doi: 10.1159/000125438. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau JC, Hoang NK, Oravec J, Do LT. Rat neurophysiological taste responses to salt solutions. Chem Senses. 1983;8:131–150. [Google Scholar]
- 5.Boudreau JC, Sivakumar L, Do LT, White TD, Oravec J, Hoang NK. Neurophysiology of geniculate ganglion (facial nerve) taste systems: species comparisons. Chem Senses. 1985;10:89–127. [Google Scholar]
- 6.Boudreau JC, Do LT, Sivakumar L, Oravec J, Rodriguez CA. Taste systems of the petrosal ganglion. Chem Senses. 1987;12:437–458. [Google Scholar]
- 7.Brand JG, Teeter JH, Silver WL. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 1985;334:207–214. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- 8.Breslin PAS, Spector AC, Grill HJ. Chorda tympani section decreases the cation specificity of depletion-induced sodium appetite in rats. Am J Physiol. 1993;264:R319–R323. doi: 10.1152/ajpregu.1993.264.2.R319. [DOI] [PubMed] [Google Scholar]
- 9.Breslin PAS, Spector AC, Grill HJ. Sodium specificity of salt appetite in Fischer 344 and Wistar rats is impaired by chorda tympani nerve transection. Am J Physiol. 1995;269:R250–R356. doi: 10.1152/ajpregu.1995.269.2.R350. [DOI] [PubMed] [Google Scholar]
- 10.Brown BW, Hollander M. Statistics: a biomedical introduction. Wiley; New York: 1977. [Google Scholar]
- 11.Cano J, Rodriguez-Echandia EL. Degenerating taste buds in sialectomized rats. Acta Anat. 1980;106:487–492. doi: 10.1159/000145218. [DOI] [PubMed] [Google Scholar]
- 12.Caprio J, Brand JG, Teeter JH, Valentincic T, Kalinoski DL, Kohbara J, Kumazawa T, Wegert S. The taste system of channel catfish: from biophysics to behavior. Trends Neurosci. 1993;16:192–197. doi: 10.1016/0166-2236(93)90152-c. [DOI] [PubMed] [Google Scholar]
- 13.Catalanotto FA, Sweeney EA. Long-term effects of selective desalivation on taste acuity in rats. Arch Oral Biol. 1973;18:941–952. doi: 10.1016/0003-9969(73)90174-x. [DOI] [PubMed] [Google Scholar]
- 14.Cauthon R, Garcea M, Spector AC. Taste-guided unconditioned licking to suprathreshold sodium chloride concentrations is unaffected by selective lingual denervation. Chem Senses. 1994;19:452. [Google Scholar]
- 15.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 16.Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450:25–38. doi: 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- 17.DiLorenzo PM. Across unit patterns in the neural response to taste: vector space analysis. J Neurophysiol. 1989;62:823–833. doi: 10.1152/jn.1989.62.4.823. [DOI] [PubMed] [Google Scholar]
- 18.DiLorenzo PM, Monroe S. Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. J Neurophysiol. 1995;74:258–272. doi: 10.1152/jn.1995.74.1.258. [DOI] [PubMed] [Google Scholar]
- 19.Erickson RP, DiLorenzo PM, Woodbury MA. Classification of taste responses in brain stem: membership in fuzzy sets. J Neurophysiol. 1994;71:2139–2150. doi: 10.1152/jn.1994.71.6.2139. [DOI] [PubMed] [Google Scholar]
- 20.Finger TE. Feeding patterns and brain evolution in ostariophysean fishes. Acta Physiol Scand. 1997;161:59–66. [PubMed] [Google Scholar]
- 21.Finger TE, Morita Y. Two gustatory systems: facial and vagal gustatory nuclei have different brainstem connections. Science. 1985;227:776–778. doi: 10.1126/science.3969566. [DOI] [PubMed] [Google Scholar]
- 22.Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- 23.Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in the chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- 24.Ganchrow JR, Ganchrow D. Long-term effects of gustatory neurectomy on fungiform papillae in the young rat. Anat Rec. 1989;225:224–231. doi: 10.1002/ar.1092250308. [DOI] [PubMed] [Google Scholar]
- 25.Geran LC, Guagliardo NA, Spector AC. Chorda tympani transection increases KCl detection threshold in rats as measured by a two lever operant conditioning paradigm. Chem Senses. 1997;122:685. [Google Scholar]
- 26.Grill HJ, Schwartz GJ. The contribution of gustatory nerve input to oral motor behavior and intake-based preference. II. Effects of combined chorda tympani and glossopharyngeal nerve section in the rat. Brain Res. 1992;573:105–113. doi: 10.1016/0006-8993(92)90118-s. [DOI] [PubMed] [Google Scholar]
- 27.Grill HJ, Schwartz GJ, Travers JB. The contribution of gustatory nerve input to oral motor behavior and intake-based preference. I. Effects of chorda tympani or glossopharyngeal nerve section in the rat. Brain Res. 1992;573:95–104. doi: 10.1016/0006-8993(92)90117-r. [DOI] [PubMed] [Google Scholar]
- 28.Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol. 1997;78:920–938. doi: 10.1152/jn.1997.78.2.920. [DOI] [PubMed] [Google Scholar]
- 29.Halsell CB, Travers JB, Travers SP. Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Res. 1993;632:161–173. doi: 10.1016/0006-8993(93)91151-h. [DOI] [PubMed] [Google Scholar]
- 30.Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience. 1996;72:185–197. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- 32.Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 1997;22:133–140. doi: 10.1093/chemse/22.2.133. [DOI] [PubMed] [Google Scholar]
- 33.Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996;711:125–137. doi: 10.1016/0006-8993(95)01410-1. [DOI] [PubMed] [Google Scholar]
- 34.Heck GI, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 35.Jacquin MF. Gustation and ingestive behavior in the rat. Behav Neurosci. 1983;97:98–109. doi: 10.1037//0735-7044.97.1.98. [DOI] [PubMed] [Google Scholar]
- 36.Koh SD, Teitelbaum P. Absolute behavioral taste thresholds in the rat. J Comp Physiol Psych. 1961;54:223–229. doi: 10.1037/h0048474. [DOI] [PubMed] [Google Scholar]
- 37.Markison S, St. John SJ, Spector AC. Glossopharyngeal nerve transection does not compromise the specificity of taste-guided sodium appetite in rats. Am J Physiol. 1995;269:R215–R221. doi: 10.1152/ajpregu.1995.269.1.R215. [DOI] [PubMed] [Google Scholar]
- 38.Miller IJ. Gustatory receptors of the palate. In: Katsuki Y, Sato M, Takagi S, Oomura Y, editors. Food intake and chemical senses. University of Tokyo; Tokyo: 1977. pp. 173–186. [Google Scholar]
- 39.Morrison GR. Behavioural response patterns to salt stimuli in the rat. Can J Psychol. 1967;21:141–152. [Google Scholar]
- 40.Nagai T, Ueda K. Stoichastic properties of gustatory impulse discharges in rat chorda tympani fibers. J Neurophysiol. 1981;45:574–592. doi: 10.1152/jn.1981.45.3.574. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Norgren R. Gustatory responses of neurons in the nucleus of the solitary tract of behaving rats. J Neurophysiol. 1991;66:1232–1248. doi: 10.1152/jn.1991.66.4.1232. [DOI] [PubMed] [Google Scholar]
- 42.Nanda R, Catalanotto FA. Long-term effects of surgical desalivation upon taste acuity, fluid intake, and taste buds in the rat. J Dent Res. 1981;60:69–76. doi: 10.1177/00220345810600011401. [DOI] [PubMed] [Google Scholar]
- 43.Nejad MS. The neural activities of the greater superficial petrosal nerve of the rat in response to chemical stimulation of the palate. Chem Senses. 1986;11:283–293. [Google Scholar]
- 44.Nejad MS, Beidler LM (1987) Taste responses of the cross-regenerated greater superficial petrosal and chorda tympani nerves of the rat. Ann NY Acad Sci 523–526.
- 45.Nishijo H, Norgren R. Parabrachial gustatory neural activity during licking by rats. J Neurophysiol. 1991;66:974–985. doi: 10.1152/jn.1991.66.3.974. [DOI] [PubMed] [Google Scholar]
- 46.Oakley B. Altered temperature and taste responses from cross-regenerated sensory nerves in the rat’s tongue. J Physiol (Lond) 1967;188:353–371. doi: 10.1113/jphysiol.1967.sp008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa H, Hayama T. Receptive fields of solitario-parabrachial relay neurons responsive to natural stimulation of the oral cavity in rats. Exp Brain Res. 1984;54:359–366. doi: 10.1007/BF00236237. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol (Lond) 1968;199:223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa H, Imoto T, Hayama T. Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Exp Brain Res. 1984;54:349–358. doi: 10.1007/BF00236236. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffmann C. Taste preference and aversion following lingual deafferentation. J Comp Physiol Psych. 1952;45:393–400. doi: 10.1037/h0057377. [DOI] [PubMed] [Google Scholar]
- 51.Pfaffmann C. Gustatory nerve impulses in rat, cat, and rabbit. J Neurophysiol. 1955;18:429–440. doi: 10.1152/jn.1955.18.5.429. [DOI] [PubMed] [Google Scholar]
- 52.Shingai T. Water fibers in the superior laryngeal nerve of the rat. Jpn J Physiol. 1980;30:305–307. doi: 10.2170/jjphysiol.30.305. [DOI] [PubMed] [Google Scholar]
- 53.Slotnick BM, Sheelar S, Rentmeister-Bryant H. Transection of the chorda tympani and insertion of ear pins for stereotaxic surgery: equivalent effects on taste sensitivity. Physiol Behav. 1991;50:1123–1127. doi: 10.1016/0031-9384(91)90571-5. [DOI] [PubMed] [Google Scholar]
- 54.Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol. 1991;65:1098–1113. doi: 10.1152/jn.1991.65.5.1098. [DOI] [PubMed] [Google Scholar]
- 55.Sollars SI, Hill DL. Amiloride sensitivity of the greater superficial petrosal nerve in Sprague-Dawley and Fischer 344 rats. Soc Neurosci Abstr. 1997;23:1037. [Google Scholar]
- 56.Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol. 1992;263:R169–R176. doi: 10.1152/ajpregu.1992.263.1.R169. [DOI] [PubMed] [Google Scholar]
- 57.Spector AC, Andrews-Labenski J, Letterio FC. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 1990a;47:795–803. doi: 10.1016/0031-9384(90)90099-p. [DOI] [PubMed] [Google Scholar]
- 58.Spector AC, Schwartz GJ, Grill HJ. Chemospecific deficits in taste detection after selective gustatory deafferentation in rats. Am J Physiol. 1990b;258:R820–R826. doi: 10.1152/ajpregu.1990.258.3.R820. [DOI] [PubMed] [Google Scholar]
- 59.Spector AC, Travers SP, Norgren R. Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behav Neurosci. 1993;107:694–702. doi: 10.1037//0735-7044.107.4.694. [DOI] [PubMed] [Google Scholar]
- 60.Spector AC, Guagliardo NA, St. John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996a;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci. 1996b;110:1096–1109. [PubMed] [Google Scholar]
- 62.Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;41:R1210–R1228. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- 63.St. John SJ, Spector AC. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus). Behav Neurosci. 1996;110:1456–1468. doi: 10.1037//0735-7044.110.6.1456. [DOI] [PubMed] [Google Scholar]
- 64.St. John SJ, Spector AC. The effect of gustatory nerve transections on taste discriminations involving quinine in rats. Chem Senses. 1997;22:800. [Google Scholar]
- 65.St. John SJ, Garcea M, Spector AC. Combined, but not single gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci. 1994;108:131–140. doi: 10.1037//0735-7044.108.1.131. [DOI] [PubMed] [Google Scholar]
- 66.St. John SJ, Markison S, Spector AC. Salt discriminability is related to number of regenerated taste buds after chorda tympani nerve transection in rats. Am J Physiol. 1995;269:R141–R153. doi: 10.1152/ajpregu.1995.269.1.R141. [DOI] [PubMed] [Google Scholar]
- 67.St. John SJ, Markison S, Spector AC. Chorda tympani transection disrupts taste aversion learning to potassium chloride, but not sodium chloride. Behav Neurosci. 1997a;111:188–194. doi: 10.1037//0735-7044.111.1.188. [DOI] [PubMed] [Google Scholar]
- 68.St. John SJ, Markison S, Guagliardo NA, Hackenberg TD, Spector AC. Chorda tympani nerve transection and selective desalivation differentially disrupt two-lever salt discrimination performance in rats. Behav Neurosci. 1997b;111:450–459. [PubMed] [Google Scholar]
- 69.Thaw AK. Changes in taste threshold over the life span of the Sprague-Dawley rat. Chem Senses. 1996;21:189–193. doi: 10.1093/chemse/21.2.189. [DOI] [PubMed] [Google Scholar]
- 70.Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec. 1990;227:373–379. doi: 10.1002/ar.1092270313. [DOI] [PubMed] [Google Scholar]
- 71.Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis int the rat. Behav Brain Res. 1987;25:233–246. doi: 10.1016/0166-4328(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 72.van der Klaauw NJ, Smith DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol Behav. 1995;58:295–306. doi: 10.1016/0031-9384(95)00056-o. [DOI] [PubMed] [Google Scholar]
- 73.Whitehead MC, Frank ME, Hettinger TP, Hou L-T, Nah H-D. Persistence of taste buds in denervated fungiform papillae. Brain Res. 1987;405:192–195. doi: 10.1016/0006-8993(87)91008-0. [DOI] [PubMed] [Google Scholar]
- 74.Yamada K. Gustatory and thermal responses in the glossopharyngeal nerve of the rat. Jpn J Physiol. 1966;16:599–611. doi: 10.2170/jjphysiol.16.599. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto T, Asai K. Effects of gustatory deafferentation on ingestion of taste solutions as seen by licking behavior in rats. Physiol Behav. 1986;37:299–305. doi: 10.1016/0031-9384(86)90237-4. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav. 1994a;56:1197–1202. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Some critical factors involved in formation of conditioned taste aversion to sodium chloride in rats. Chem Senses. 1994b;19:209–217. doi: 10.1093/chemse/19.3.209. [DOI] [PubMed] [Google Scholar]