Abstract
We tested the hypothesis that the neural networks for walking in the mudpuppy can be divided into a flexor and an extensor center, each of which contains collections of interneurons localized in the vicinity of their motoneuron pools. Combining a battery of techniques, we identified and localized the elbow flexor center and its motoneuron pool in the C2 segment and the elbow extensor center and its motoneuron pool in the C3 segment. Rhythmic flexion or extension of the limb in isolation could be induced by continuous trains of current pulses of the C2 or C3 segments, respectively. Independent activation could also occur after application of glutamate receptor agonist NMDA. Part of segment C2 in isolation generated rhythmic elbow flexor bursts, whereas part of segment C3 in isolation generated rhythmic elbow extensor bursts. An isolated region spanning the C3 roots generated both flexor and extensor bursts. The step cycle was modulated in a phase-dependent manner by stimulation of the dorsal roots, the ventral roots, or either of the two centers. The effects of ventral root stimulation were removed by deafferentation to block reafferent input attributable to muscle contraction induced by the stimulation. We conclude that the neural networks for walking contain at least a flexor and an extensor generator that are localized in close apposition to the motoneuron pools, that the two centers can work independently despite the fact that there are reciprocal inhibitory interconnections between them, and that sensory input interacts with the spinal neural networks to reset the ongoing walking rhythm in a phase-dependent manner.
Keywords: spinal cord, neural networks, locomotion, resetting, flexor center, extensor center, rhythmicity, deafferentation
The spinal cord in vertebrates is capable of generating rhythmic motor behaviors, such as locomotion, with or without sensory input (Székely et al., 1969; Delcomyn, 1980; Grillner, 1981, 1985; Pearson, 1993). The neural networks responsible for locomotion are composed of populations of interneurons and are often referred to as central pattern generators (CPGs). The organization of the CPGs for vertebrate locomotion is beginning to be understood at a cellular level. Grillner and his colleagues (Grillner et al., 1991, 1995; Grillner, 1996) identified classes of excitatory and inhibitory interneurons and elucidated for the first time the circuitry responsible for generating and coordinating the bending motion of the trunk required for swimming in the lamprey. The circuitry that produces swimming has also been examined in a tadpole (Roberts et al., 1986, 1995; Perrins, 1995). A pair of half centers that are mutually inhibitory is an essential feature of the characterized segmental circuits.
The circuitry for walking is much more difficult to determine experimentally because it involves coordinated limb movement about multiple joints in different limbs. Progress is being made to localize the networks for walking (Iwahara et al., 1991; Cazalets et al., 1995,1996; Bracci et al., 1996; Cowley and Schmidt, 1997; Kjaerulff and Kiehn 1996; Kremer and Lev-Tov, 1997) and to identify interneurons in the mammalian spinal cord that could be involved in generating walking (Edgley et al., 1988; Shefchyk et al., 1990; Viala et al., 1991;Hochman et al., 1994; Kjaerulff et al., 1994; MacLean et al., 1995;Kiehn et al., 1996; Raastad et al., 1996). However, the complexity of the mammalian spinal cord makes it extremely difficult to investigate the organization of the neuronal circuitry. The smallest functional unit was once assumed to be a center for each limb (Grillner, 1981). Based on pieces of indirect evidence, Grillner attempted to subdivide the CPGs into several subunits called “unit burst generators” (Grillner, 1981). He assumed that there is one network for each group of close synergists. This attractive hypothesis is technically difficult to test with the available experimental preparations. To circumvent the complexity of the mammalian nervous system, we developed an in vitro walking preparation from an amphibian, the mudpuppy (Necturus maculatus) (Wheatley and Stein, 1992;Wheatley et al., 1992, 1994; Jovanović et al., 1996a). We show in this study that the CPG for each limb can be divided into generators that produce flexor or extensor rhythms independently.
A second issue concerns the interplay between sensory input from the moving limb and the operation of CPGs. In the cat, stimulation of low-threshold extensor muscle afferents in the flexion phase initiates a new extensor burst but suppresses the flexor burst. Such stimulation in the extension phase prolongs the ongoing extensor burst and delays the onset of the flexor burst (Duysens and Pearson, 1980; Conway et al., 1987; Pearson, 1993; Guertin et al., 1995; Rossignol, 1996). Resetting effects of sensory input have also been shown in the neonatal rat (Kiehn et al., 1992; Iizuka et al., 1997). Here we show a phase-dependent resetting of the ongoing walking-like rhythm by stimulation of the dorsal roots, ventral roots, and the elbow flexor or extensor generators.
Part of this work has been reported in an abstract (Cheng et al., 1997).
MATERIALS AND METHODS
Twenty-nine adult mudpuppies (22–30 cm in length) were used for the experiments. The animals were anesthetized before surgery by application of 3-aminobenzoic acid ethyl ester (Sigma, St. Louis, MO) to the water bath (1–2 gm/l).
Retrograde labeling of motoneuron pools. We used two fluorescent tracers in complement to label the motoneuron pools of the elbow flexor (brachialis) and extensor (ulnae) muscles. The contours of these muscles were visible through the skin in these animals. Fluorogold (7% in DMSO–saline; Fluorochrome, Englewood, NJ) was injected into the belly of the elbow extensor in the left limb and the belly of the elbow flexor in the right limb in six animals (Richmond et al., 1994). Approximately 5 μl of the tracers was delivered using a 26 gauge needle over the course of 5 min in each muscle. A second tracer, fast blue (3% in DMSO–saline, 5 μl; Sigma), was used in two animals to avoid overestimation of the number of labeled motoneurons attributable to the diffusibility of fluorogold (Richmond et al., 1994). The animals were killed under anesthesia 3–22 d after injection. The target muscles were dissected and the tracks of injection within these muscles were verified. To examine the labeled cells, the spinal cord was isolated, fixed overnight in 4% paraformaldehyde/0.1 m sodium phosphate buffer, pH 7.2–7.4, at 4°C, transferred to 30% sucrose/H20 for 2 hr at 22°C (or overnight at 4°C) for cryoprotection, and sliced either coronally or horizontally in 40 μm cryostat sections (JUNG GM 3000; Leica Canada, Inc.). The sections were mounted sequentially onto slides (Fisherbrand; Fisher Scientific, Houston, TX), coverslipped (Cytoseal; Stephens Scientific), and inspected for the labeled motoneurons under a fluorescence microscope (Leitz, Wetzlar, Germany) equipped with type A filter (excitation bandpass 340–380, suppression long pass 430 nm) and dark-field–bright-field illumination.
In vitro walking preparation. The preparation contained the first five segments of the spinal cord, the brachial nerves, and the forelimb(s). A segment border is defined as midway between two adjacent spinal dorsal roots. The procedures for surgical dissection, electromyography (EMG), and induction of walking-like limb movement were described in detail elsewhere (Wheatley et al., 1992). Briefly, after a dorsal laminectomy under anesthesia, the first five segments of the spinal cord were isolated from the rest of the body with one or both forelimbs attached by the brachial nerves. The paraspinal muscles were removed. Pairs of fine teflon-insulated silver wires (75 μm in diameter) were inserted into the elbow flexor and extensor muscles for EMG recording. The preparation was placed dorsal side up in a recording chamber superfused with continuously oxygenated Ringer’s solution at a rate of 2–5 ml/min (NaCl 115 mm, KCl 2 mm, CaCl2 2 mm, MgCl2 1.8 mm, HEPES 5 mm, and glucose 1 gm/l, pH 7.35). The cord and forelimb(s) were stabilized by pinning the vertebrate column and the procoracoid cartilage to the base of the chamber coated with Sylgard resin (Dow Corning). Deafferentation was performed in some animals by cutting the dorsal roots within the spinal canal. Walking-like motion of the leg(s) was induced by bath application of 20–80 μm NMDA (Sigma) with 5–20 μmd-serine, which potentiates the effect of NMDA (Wheatley et al., 1992).
Microstimulation. Motor responses were elicited by stimulation of regions of the spinal cord with constant cathodic currents (Master 8; A.M.P.I., Jerusalem, Israel) through a stimulus isolator (NL800, Digitimer, Ltd.). The surface of the cord dorsum was first stimulated systematically with concentric tungsten electrodes (negative pole, ∼100 μm in diameter) at a spatial resolution of 1.0 mm. At spots where extension or flexion was induced by trains of stimulation with the lowest threshold current, a tungsten microelectrode (∼10-μm-diameter tips, 1–2 MΩ; Micro Probe, Gaithersburg, MD) was then inserted into the predicted areas of the gray matter (200–500 μm deep) where interneurons were densely populated (see Fig. 1B). Continuous trains of current pulses (40 Hz, 0.2 msec duration) were used to find regions where rhythmic flexion or extension of the limb could be induced by unpatterned stimulation. Peak current amplitudes ranged between 2 and 8 μA, which corresponded to an estimated stimulation volume of 100 μm radius (Ranck, 1981; Yeomans, 1990). The tracks of electrodes were examined in 40 μm frozen sections stained with cresyl violet and were confirmed to be in the intermediate areas of the gray matter.
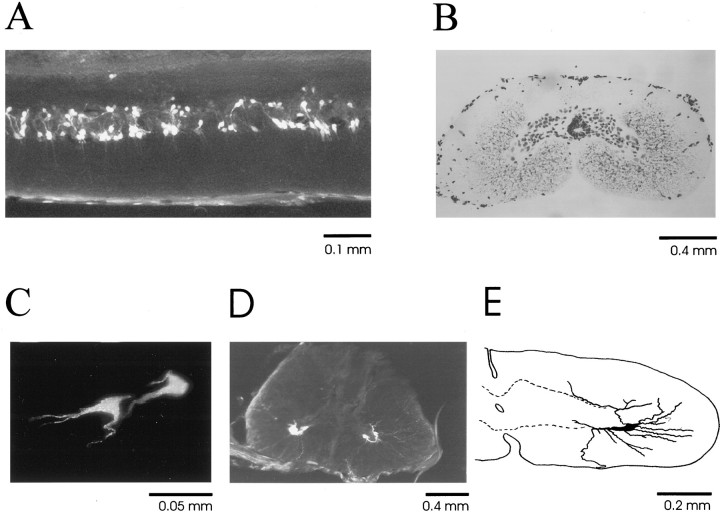
Fig. 1.
Microphotographs of the spinal cord.A, Motoneurons labeled retrogradely with fluorogold in a 40-μm-thick horizontal section of the extensor motoneuron pool taken from the C3 segment (midline top and rostral right). B, Histology of the mudpuppy spinal cord stained with cresyl violet in a 40-μm-thick coronal section from the C2 segment. There are ∼80 cells in the gray matter on each side. One to three motoneurons are typically labeled retrogradely on each side, as shown inC and D. C, Two extensor motoneurons are labeled retrogradely in a 40-μm-thick coronal section from the C3 segment. D, At the C3 ventral root entry level, both extensor motoneurons (left) and flexor motoneurons (right) are labeled. The C3 ventral root can be seen in this section. E, Partial reconstruction of a flexor motoneuron showing the morphological details of its processes and orientation in the cord. The dotted line indicates the border of the gray matter.
Surgical isolation of regions of the cervical cord. With the aid of a surgical dissection microscope (Leica), fine insect pins and iridectomy scissors were used to isolate regions of the cord. The unwanted regions were removed by vacuum suction to ensure complete isolation. In cases in which two regions of the cord were necessary, a gap of at least 1 mm was produced between the separated regions attributable to the tension within the spinal cord.
Resetting “walking” rhythm by stimulation of regions of the cord, dorsal roots, and ventral roots. Trains of constant current pulses (40 Hz, 0.2 msec pulse duration, six pulses) were delivered to specific regions of the cord dorsum (C2 or C3), dorsal roots, or ventral roots every four to six step cycles. The stimulus intensity was 1.2× motor thresholds (6–12 μA). The mean durations of the step cycles before and after each stimulated step were measured and compared (Student’s t test). The phasic effects of the perturbation were examined by plotting the cycle duration of the stimulated steps against the timing of stimulation in each cycle. The slope of the best fitting straight line gave a measure of the degree of resetting (1 represents complete resetting; Stein and Lee, 1981).
Correlation analyses. The phase relationship between the flexor and extensor EMG bursts was quantified by cross-correlation analysis, and the rhythmicity of the flexor and extensor bursts was tested by auto-correlation analysis whenever necessary. At least 50 step cycles were included for the analyses. A positive value for the peak near zero in a cross-correlation indicates that the two signals (flexor and extensor EMGs) are relatively in phase, whereas a negative value indicates that they are relatively out of phase. A value of 1 represents a perfect correlation. The exact phase relationship can be determined by measuring the time delay (see Fig. 3) as a fraction of the complete step cycle.
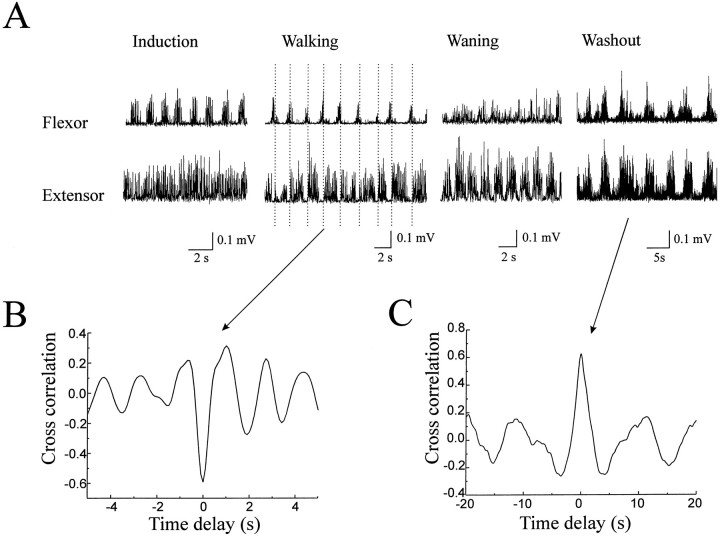
Fig. 3.
Chemically induced independent activation of the elbow flexor and extensor centers. A, Rhythmic flexor EMG bursts developed first after bath application of NMDA (60 μm) while the extensor was still tonically active (Induction). Alternation of flexor and extensor bursts then emerged with well coordinated rhythmic limb movement (Walking). The vertical dotted linesindicate the transition of activation from the flexor to the extensor muscles. The flexor turned to tonic activation while rhythmic extensor bursts were still evident when the walking-like rhythm started to deteriorate after several hours of walking-like movement (Waning). The flexor and extensor bursts became synchronized for a short period during washout of NMDA (Washout). The EMG signals have been low-pass filtered at 20 Hz and rectified. B, Cross-correlation analysis shows the antiphase relationship (negative peak value at 0) between the flexor and extensor bursts during walking. C, During washout, the flexor and extensor bursts were synchronized, as shown by the in-phase relationship in cross-correlation (positive peak value at 0).
RESULTS
Motoneuron pools innervating the elbow flexor and extensor muscles
Retrograde labeling by fluorescent tracers (fluorogold and fast blue) revealed the patterns of distribution of motoneurons innervating brachialis and extensor ulnae of the limb. The labeling was consistent between animals using both tracers. Figure1 shows the distribution and morphology of the labeled cells. The flexor pool was localized mainly in the caudal part of the C2 segment (∼3.0 mm long), and the extensor pool was localized in the caudal part of the C3 segment (∼4.0 mm long). There was an overlap region at the rostral C3 segment (∼0.2–0.4 mm) where motoneurons of both the flexor and extensor muscles were labeled. Part of the extensor motoneuron pool from the C3 segment is shown in Figure 1A (horizontal section, midline top, rostral right). The motoneurons were organized approximately into two columns of cells. In the coronal plane, ∼80 cells in the gray matter of each side were typically stained with cresyl violet within each 40 μm section (Fig. 1B). The cells around the central canal were excluded from the counting because they appeared to be ependymal cells (not labeled in silver staining). One to three motoneurons were labeled retrogradely in each 40 μm coronal section, and the majority of the neurons in the gray matter appeared to be interneurons. The labeled motoneurons are exemplified in coronal sections (Fig.1C,D) from the extensor pool and the overlapping region. Two extensor motoneurons were labeled retrogradely on the left side of a 40-μm-thick coronal section from the caudal C3 segment (Fig. 1C). Both extensor (Fig. 1D,left) and flexor (Fig. 1D,right) motoneurons were labeled at the entry level of the C3 ventral root, which can be seen in this section. Partial reconstruction of a flexor motoneuron in the C2 segment shows the morphological details of its processes and orientation in the cord (Fig.1E). The cell bodies of these motoneurons were ∼35–45 μm in diameter. The dendritic trees extended into the white matter of the ipsilateral side toward the edge of the cord. The axons were found to join the ventral roots either at the level of ventral root formation (Fig. 1D,E) or to ascend or descend up to ∼3 mm within the ventral column of the white matter before entering the ventral roots (data not shown). Note that the sizes of the cells are not corrected for the shrinkage of the cord because of the dehydration process for fixation.
Identification of the elbow flexor and extensor centers
The flexor and extensor centers for the elbow joint were first identified by the use of microstimulation in six preparations. The cord dorsum was stimulated systematically with 1 mm resolution by continuous trains of constant current pulses, and the threshold for flexor and extensor responses was mapped (see Materials and Methods). Stimulation of the C2 segment induced flexor responses. The lowest threshold for the elbow flexor responses was localized in the middle of this region, ∼0.5 mm lateral to the midline of the cord but medial to the flexor motoneuron column. At this spot, continuous trains of pulses induced rhythmic flexion of the forelimb about the elbow joint and rhythmic elbow flexor bursts (Fig. 2). Note that these rhythmic responses occurred without activation of the elbow extensor. Their counterparts in the contralateral limb were also silent.
Fig. 2.
Electrically induced independent activation of the elbow flexor and extensor centers. Stimulation by continuous trains of constant current pulses at 40 Hz of the C2 segment medial to the flexor motoneuron pool induced rhythmic bursts of the elbow flexor muscle, whereas stimulation of the caudal part of the C3 segment produced rhythmic extensor bursts, all on the ipsilateral side of stimulation (I-). The flexor and extensor muscles on the contralateral limb (C-) did not respond to the stimulation. The EMG has been rectified and low-pass-filtered at 30 Hz for clarity.
Stimulation of the C3–C5 segments evoked extensor responses. The lowest threshold was localized in the caudal C3 segment, medial to the extensor motoneuron column. At this location, continuous trains of pulses induced rhythmic extension of the limb and elbow extensor bursts (Fig. 2). Again, the extensor bursts were independent of its antagonist and its counterpart of the contralateral limb.
Stimulation of a region (∼2 mm long) immediately rostral to the C3 dorsal root induced rhythmic flexion and extension of the wrist without noticeable EMG activities of the elbow flexor and extensor muscles. At higher intensity, stimulation of this region induced rhythmic alternation or co-contraction of flexor and extensor bursts of the elbow (data not shown). Stimulation of the rostral part of C2 segment caused protraction of the limb about the shoulder joint. Retraction of the limb about the shoulder was induced by stimulation of caudal C4 and rostral C5.
Independent rhythmic activation of the elbow flexor and extensor muscles was also observed during bath application of the glutamate receptor agonist NMDA in some preparations. Figure3 shows an example of such chemically induced independent activation. Figure 3A shows that rhythmic flexor bursts developed first during induction of the walking-like motion while excitation of the extensor was still tonic (Induction). Then a regular walking-like rhythm became well established, and rhythmic alternation of flexor and extensor bursts emerged (Fig. 3A, Walking). The flexor turned to more tonic activation, whereas rhythmic extensor bursts were still evident when the walking-like rhythm started to wane after several hours of walking (Fig. 3A, Waning). The flexor and extensor bursts became synchronized for a short period during washout of NMDA (Fig. 3A, Washout). It was noticed that the limb movement became jerky, and the range of motion was reduced in conditions in which one of the muscles was tonically active or the bursts of the antagonist muscles were synchronized. The cycle duration increased from 1.8 sec (Fig. 3A,Walking) to 8.2 sec (Fig. 3A,Washout). Cross-correlation analysis demonstrated an antiphase relationship (negative peak value near 0) between the elbow flexor and extensor bursts during walking and an in-phase relationship (positive value near 0) during washout, as shown in Figure 3,B and C, respectively.
Surgical isolation of the elbow flexor and extensor centers
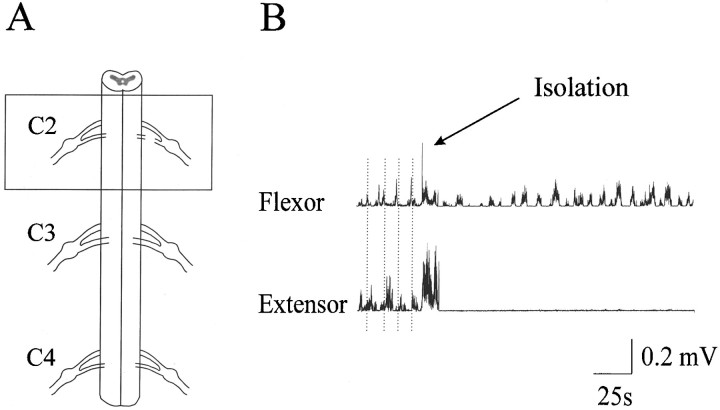
This part of the study was performed in nine preparations. An isolated region spanning the C2 spinal roots (∼4 mm long) generated rhythmic elbow flexor EMG bursts in the presence of NMDA (60 μm) and d-serine (15 μm). A schematic representation of the isolated region is shown in Figure4A. This region of the cord was connected to the forelimb by the C2 ventral root. The C2 dorsal root was cut, and the rest of the cord was removed. An example of rhythmic flexor bursts before and after the surgical isolation is shown in Figure 4B. The extensor bursts were totally abolished by the isolation whereas the flexor bursts remained rhythmic, albeit at a slower pace.
Fig. 4.
Isolation of the flexor center. A, Schematic diagram of the surgery. The box highlights the isolated part of the C2 segment (∼4 mm long). This region alone generated rhythmic elbow flexor EMG bursts through the C2 ventral root. The C2 dorsal root was cut, and the rest of the cord was removed.B, Rhythmic alternating flexor and extensor bursts were evident in the presence of NMDA (60 μm) before the isolation. The vertical dotted lines indicate the transition from flexor bursts to extensor bursts. The extensor bursts were totally abolished by the surgical isolation as indicated, whereas rhythmic flexor bursts remained.
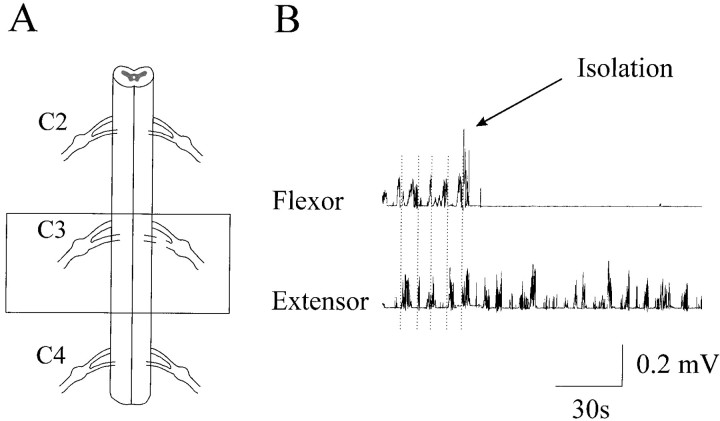
Similarly, an isolated part (∼4 mm long) of the C3 segment generated rhythmic elbow extensor EMG bursts in the presence of NMDA (80 μm) and d-serine (20 μm) through the C3 ventral root that connected this part of the cord with the forelimb. The C3 dorsal root was cut, and the rest of the cord was removed. Figure 5 shows the rhythmic elbow extensor bursts before and after the isolation, together with a schematic representation of the isolated region. The flexor bursts were completely abolished by the isolation, whereas the extensor bursts were still evident, although the rhythmicity was less regular, and the cycle duration was prolonged.
Fig. 5.
Isolation of the extensor center.A, Schematic illustration of the surgery. Thebox highlights the isolated part of the C3 segment (∼5 mm long) that generated rhythmic elbow extensor EMG bursts through the C3 ventral root. The C3 dorsal root was cut and the rest of the cord was removed. B, Rhythmic alternating flexor and extensor bursts were evident in the presence of NMDA (80 μm) before the isolation. The vertical dotted lines indicate the flexor and extensor transition of activation. The flexor bursts were totally abolished by the surgical isolation, whereas rhythmic extensor bursts remained.
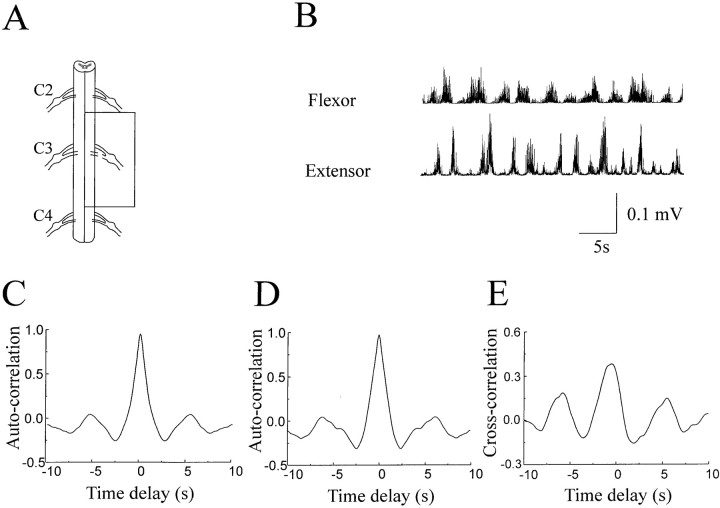
An isolated region spanning the C3 roots (∼8 mm long) generated rhythmic flexion and extension alternation of the limb and elbow flexor and extensor bursts through the C3 ventral root. This isolated part of the cord is schematically shown in Figure6A. Note that the contralateral half of the cord was also removed through the midsagittal line. The movement of the leg generated by this isolated segment was noted to be less smooth, and the range of motion was also reduced. The flexor and extensor EMG bursts, however, remained rhythmic, as exemplified in Figure 6B. Auto-correlation analysis confirmed the rhythmicity of the flexor and extensor activation with a cycle duration of ∼5 sec (Fig. 6C,D). The phase relationship between the flexor and extensor bursts was quantified by cross-correlation analysis. Although the flexor and extensor bursts were less regular when compared with the less reduced preparation (Fig. 3), they were clearly coupled with a time delay of 1.2 sec (Fig. 6E).
Fig. 6.
Rhythmic flexor and extensor EMG bursts were generated by a region spanning the C3 ventral root (∼8 mm long). Thebox in the schematic illustration of the surgery (A) highlights the isolated region. C3 dorsal root was cut. The rest of the cord, including the contralateral side, was removed. This isolated region produced rhythmic flexor and extensor bursts through the C3 ventral root in the presence of 80 μm NMDA (B). Auto-correlation analysis confirmed the rhythmicity of the flexor (C) and extensor (D) bursts. Cross-correlation analysis revealed the coupling of the flexor and extensor activation with a time delay of ∼1.2 sec (E).
Separation of the two centers by a transverse section just rostral to the C3 dorsal root revealed different rhythms of flexor and extensor bursts. Figure 7 shows an example of such experiments. The histogram in Figure 7 depicts the onset delay between the flexor and extensor bursts before and after the separation, together with the schematic position of the section (inset). The flexor–extensor onset delay was relatively fixed at 0.8 sec (Fig.7, filled bars) before the separation. The rhythms generated by the two centers were decoupled by the separation (Fig. 7, open bars), leading to a randomized flexor–extensor onset relationship. The cycle durations were also prolonged, particularly for the extensor bursts.
Fig. 7.
Decoupling of the two centers by surgical separation of the cord. The onset delay between the flexor and extensor EMG bursts was relatively fixed (filled bars) before the separation at the position shown in theinset. After the separation, the rhythms generated by the two centers were decoupled as indicated by the wide spread of the onset delay between the flexor and extensor bursts (open bars).
Resetting of the walking-like rhythm
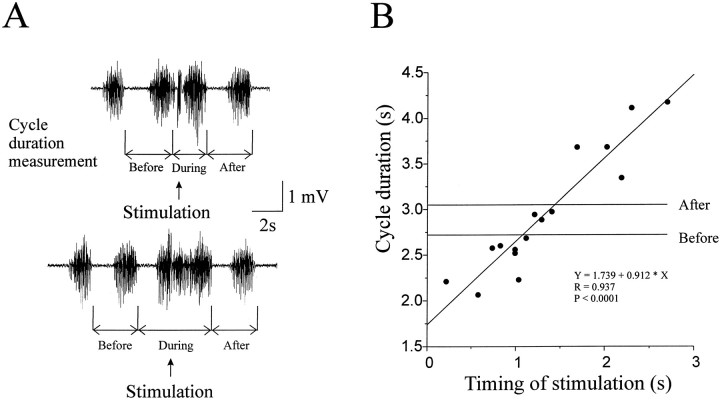
Low-intensity stimulation (a train of six pulses of 40 Hz at 1.2× motor threshold) of each dorsal root (C2–C4) evoked motor responses in both the flexor and extensor muscles and reset the ongoing walking-like rhythm in a phase-dependent manner (n = 6). Figure8 illustrates the resetting effects of C3 dorsal root stimulation. The cycle duration was measured as offset-to-offset of adjacent flexor bursts, as shown in Figure8A. The stimulated step (Fig. 8A,During) was either shortened (top trace) or prolonged (bottom trace) depending on the timing of stimulation. Plotting the cycle duration against the timing of the stimulation (0 represents offset of flexion) revealed that the cycle duration varied as a function of the timing of stimulation. The slope of the best straight line fit, 0.912, indicates the effectiveness of stimulation in resetting the rhythm (a slope of 1 represents perfect resetting). Stimulation of the C3 dorsal root showed the strongest effects among the three dorsal roots. Also shown in Figure 8 are the mean cycle durations immediately before and after the stimulated step, which were not significantly different from each other in this case. A small but statistically significant slope also occurred in the step cycle immediately after the stimulated step in two of the six preparations. The resetting effects of stimulating different roots (C2-C4) were compared. Preferential effects on flexor or extensor rhythms from a particular dorsal root were not found.
Fig. 8.
Resetting the walking-like rhythm by dorsal root stimulation. A, Examples of raw recordings of the flexor EMG showing the measurement of cycle duration and the effects of stimulation on the cycle duration. A train of 6 pulses of 40 Hz at 1.2× the motor threshold intensity was delivered to the C3 dorsal root at times indicated by arrows. The cycle duration of the disturbed step (During) was either shortened (top trace) or prolonged (bottom trace) depending on the timing of stimulation. The cycle durations of the steps immediately before and after the disturbed step were not substantially affected.B, The cycle duration of the disturbed steps was plotted against the timing of stimulation. The effect was phase-dependent. The slope (0.912) of the best straight line fit indicates the resetting effectiveness, with 1 indicating a perfect resetting. The mean cycle durations of steps before and after the stimulated steps are also shown and are not significantly different.
Resetting of the walking-like rhythm was also induced by stimulation of the ventral roots (C2–C4) in a phase-dependent manner (n = 4). Deafferentation by dorsal rhizotomy abolished the resetting effect of ventral root stimulation. Figure9 shows an example of such observations. Stimulating the C3 ventral root partially reset the cycle duration (Fig. 9A). This effect was removed by rhizotomy of all the dorsal roots (Fig. 9B). The mean cycle durations of the steps immediately before and after the stimulated steps were not significantly different. The average of these means is shown in Figure9, A and B, horizontal line.
Fig. 9.
Resetting the walking-like rhythm by reafferent input consequent to limb movement. A, Stimulation of the ventral root C3 by a train of 6 pulses of 40 Hz at 1.2× the motor threshold intensity modulated the cycle duration in a phase-dependent manner and partially reset the walking-like rhythm. B, Deafferentation by dorsal rhizotomy abolished the resetting effect of ventral root stimulation. The average of the mean cycle durations of the steps immediately before and after the stimulated cycle is shown as a horizontal line in A andB. C, Dorsal root cutting, per se, significantly affected the walking cadence. Cutting the C3 dorsal root shortened the cycle duration compared with the precutting control (Ctl), whereas cutting the C2 dorsal root prolonged the cycle duration. Cutting the C4 and C5 dorsal roots also shortened the cycle duration.
The effects of naturally occurring sensory input on the walking-like rhythm were investigated by comparing the average cycle durations before and after cutting each of the C2–C4 dorsal roots. An observation consistent between preparations (n = 4) was that the cycle duration decreased after the C3 dorsal root was cut, regardless of the order of root cutting. Conversely, cutting the C2 dorsal root resulted in a longer cycle duration in all of the preparations. After all the dorsal roots of the preparation were cut, the walking-like rhythm became faster in three of the four preparations. Figure 9C illustrates the results from a representative experiment. Note that cutting the C4 and C5 dorsal roots also shortened the cycle duration in this preparation.
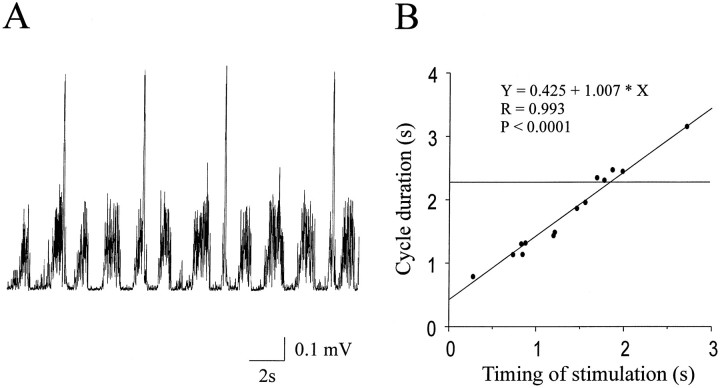
Resetting of the walking-like rhythm was also induced by stimulating regions of the cord (n = 4). Figure10 shows an example of such effects. The extensor bursts were terminated prematurely whenever stimulation was delivered to the flexor center in the C2 segment (Fig.10A). The walking-like rhythm was completely reset in a phase-dependent manner, as indicated by the slope (1.007) of the best straight line fit (Fig. 10B). The mean cycle durations of the steps immediately before and after the stimulated steps were not significantly different. The average of these mean durations is shown in Figure 10B (horizontal line). Conversely, stimulating the extensor center in the C3 segment initiated or prolonged the extensor bursts and terminated or delayed the onset of the flexor bursts (data not shown). This reciprocal inhibition between the two centers was observed in all the preparations tested.
Fig. 10.
Resetting the walking-like rhythm by stimulation of the flexor center. A, The extensor bursts were terminated prematurely whenever stimulation was delivered to the flexor center in the C2 segment as indicated by the stimulus artifacts (four large brief peaks). B, The cycle duration was modulated, and the walking-like rhythm was completely reset in a phase-dependent manner. The mean cycle duration of the steps immediately before and after the stimulated steps is indicated by the horizontal line.
DISCUSSION
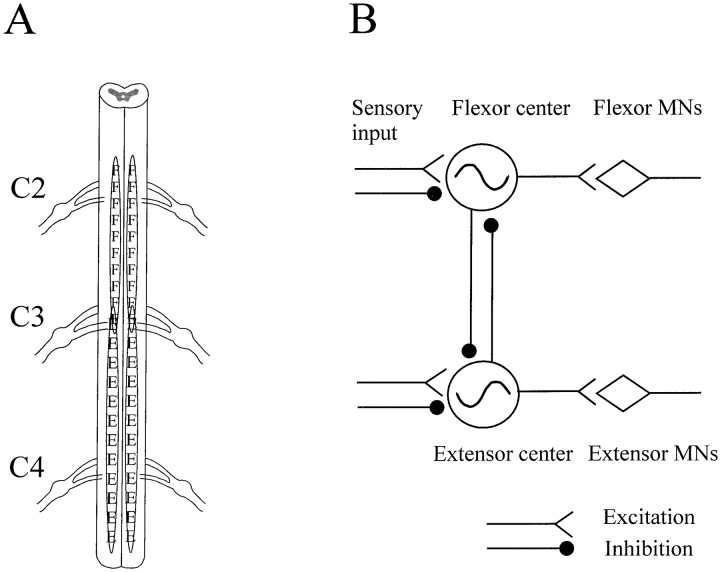
The results demonstrate that the neuronal networks for rhythmic flexor and extensor activation required for walking are distinctly localized in regions of the spinal cord. The elbow flexor center was identified and localized in the C2 segment. The elbow extensor center was localized in the C3 and C4 segments (Fig.11A). The motoneuron pool for the flexor or extensor muscle was localized in close apposition to its pattern generator as revealed by the retrograde-labeling experiments. The flexor and extensor centers can oscillate independently. These findings represent the first direct evidence that the so-called central pattern generators for walking can be divided into smaller functional subunits (Grillner, 1981). The results further show that the elbow flexor and extensor centers are interconnected by inhibitory connections and that sensory input plays an important role in determining the rhythmicity of these centers. Together, these data constitute the basis for an updated model of the neural network for walking (Fig. 11B). This model only incorporates data for elbow movements and may need to be extended, for example, to include protraction and retraction movements at the shoulder.
Fig. 11.
Schematic localization of the elbow flexor and extensor centers and modeling of the neural networks required for walking. A, The localization of the flexor center in the C2 and part of C3 segments (F) and the extensor center in the C3 and C4 segment (E) are based on the integration of data from electrical stimulation and surgical isolation experiments. A small overlap region at C3 spinal root level is also shown. B, A model of neural networks required for walking is drawn based on the data presented in this work. This model contains independent flexor and extensor generators that drive the flexor motoneuron pool and extensor motoneuron pool, respectively. There are inhibitory interconnections between the two centers. Somatosensory input makes both excitatory and inhibitory connections with each of the two centers, modulates the activity of the neural network, and resets the ongoing walking-like rhythm in a phase-dependent manner.
Flexor and extensor centers
The conceptual framework for the organization of the central pattern generator for walking has been strongly influenced by the half-center model of Brown (1911) that was developed to account for the alternating activation of flexor and extensor muscles in the cat during walking. Brown envisaged that each pool of motoneurons for flexor or extensor muscles is driven by a corresponding half center of interneurons. Between the two half centers are inhibitory connections that ensure alternating activation of flexor and extensor muscles. This model assumes that rhythmicity in flexor and extensor half centers depends on the connectivity between the two half centers. Instead, our results showed that each of the two centers can generate rhythmic motor output for walking, independent of the other. This is indicated in Figure 11B by the presence of separate rhythm generators for the flexor and extensor. Evidence for the separation came from the following several distinct experiments: (1) continuous trains of electrical stimulation to the flexor center (or the extensor center) induced rhythmic flexion (or extension) of the limb, independent of its antagonist and its counterpart in the contralateral limb (Fig. 2); (2) independent activation of one center was also sometimes induced by the glutamate receptor agonist NMDA (Fig. 3); and (3) surgical isolation and separation of the regions of the cord provided unequivocal evidence for the existence of two distinct rhythmogenic centers. An isolated region of the C2 segment generated rhythmic flexion (Fig. 4). Similarly, part of the C3 segment in isolation generated rhythmic extensor bursts (Fig. 5). Separation of the two regions produced independent rhythms that had no phase relationship to one another (Fig. 7).
Thus, electrical, chemical, and anatomical evidence all argue strongly for the presence of two separate rhythm generators. A similar situation may apply to mammals as well, as suggested by recent work showing some independence of flexor and extensor activity in the neonatal rat (Hochman and Schmidt, 1998) and in the spinal cat (R. B. Stein, K. G. Pearson, D. J. Bennett, and S. J. DeSerres, unpublished observations). Generation of the rhythm may arise from interneurons with pacemaker properties or from neural networks within a center.Hochman et al. (1994) recorded NMDA-mediated membrane oscillations in interneurons in rat spinal cord slices in the absence of synaptic interaction. On the other hand, Wheatley et al. (1994) found that the majority of the interneurons in the mudpuppy spinal cord had their peak firing at the transitions between flexion and extension phases and so may be responsible for switching the rhythm from one phase to another.
The neural network for walking may be divisible into even smaller units (for review, see Rossignol, 1996). We have only recorded EMGs of the flexor and extensor muscles at the elbow. However, we did notice that stimulation of a region caudal to C3 dorsal root induced rhythmic extension of the wrist without noticeable EMG activities of the elbow flexor and extensor muscles. Further stimulation of the rostral part of the C2 segment activated the shoulder flexors and produced a protraction of the limb. Therefore, there appears to be a rostro–caudal gradient generating activity in shoulder, elbow, and wrist muscles that could be normally coupled with some phase shift like the segmental oscillators in the lamprey (for review, see Grillner et al., 1991).
The surgical isolation experiments alone do not provide enough information to determine the precise boundary of the flexor and extensor centers (Fig. 11A). Part of the flexor generator may extend to overlap the region of the extensor generator (or vise versa), and its activity may be masked after the removal of its motoneuron pool by the surgery. However, evidence from microstimulation experiments argues against the possibility of such overall overlap. Isolated rhythmic flexor activity was induced by stimulating the C2 segment, and likewise, isolated extensor activity was generated by stimulating the C3 segment (Fig. 2), indicating distinct localization of the flexor and extensor centers. One could argue that the stimulation excited the motoneuron pool directly, as well as the pattern generator, and thus activity may be seen only near the stimulation site. However, this possibility is unlikely, because the stimulation volume with the present parameters was estimated to be small (within 0.2 mm radius) relative to the length of the motoneuron column, which could be as long as 4 mm, and thus the motor output was likely produced by the pattern generator.
The finding of distinctly localized rhythmogenic centers may reflect a principle of neural network organization that is common across species. Earlier studies have demonstrated that rhythmic output could be generated from isolated spinal cord segments. The minimal number of segments required was found to be eight in the dogfish (Grillner, 1974), four in the lamprey (Cohen and Wallén, 1980; Grillner et al., 1982), two in the cat (Deliagina et al., 1983), and one in the turtle (Mortin and Stein, 1989; Stein et al., 1995). In the chick embryo, one segment was also able to generate rhythmic locomotor-like activities (Ho and O’Donovan, 1993). Furthermore, a single hemisegment was observed to produce locomotor-like output in the neonatal rat (Cowley and Schmidt, 1997). These observations together suggest that more complex neural networks with greater adaptability are probably built on the small modular pattern generators.
Inhibitory coupling
Our observations do not contradict Brown’s (1911, 1914) suggestion that reciprocal inhibition contributes to the normal rhythmic pattern. Stimulation of one center could terminate its antagonist and completely reset the walking-like rhythm (Fig. 10). Mutual inhibitory connections between the flexor and extensor centers are therefore included in Figure 11B. They are presumably important for coordinating rhythmic limb movements by alternating activation of the flexors and extensors. The limb movements became jerky, and the range of motion was reduced in conditions in which one of the muscles became tonically active or the bursts of both muscles became synchronized (Fig. 3). Synchronized activation of the flexors and extensors has also been seen after blocking inhibitory transmission in the mudpuppy (Jovanović et al., 1996b) and in the neonatal rat (Cowley and Schmidt, 1994). This represents further evidence that the inhibitory connections are not essential for rhythm generation but are important for coordinated gait. It also suggests that there are weaker excitatory connections between the flexor and extensor centers, but these have not been included in Figure11B.
Sensory modulation
Afferent input resets and profoundly modulates the centrally generated rhythm. Our results show that the step cycle could be lengthened or shortened in a phase-dependent manner by low-intensity stimulation of the dorsal roots (Fig. 8). This is indicated by the presence of both excitatory and inhibitory connections onto the flexor and extensor centers in Figure 11B. Ventral root stimulation produced a weaker degree of resetting (Fig. 9), but this is probably mediated by reafferent sensory input induced by muscle contraction and limb movement because dorsal rhizotomy completely abolished the effects. The sensory input appears to be representative of naturally occurring sensory information resulting from limb movement during locomotion. Indeed, dorsal rhizotomy alone induced consistent changes in step cycle duration, indicating that sensory input as a consequence of the walking motion interacts with the rhythmogenic networks and modulates the walking cadence. It appears that dorsal roots C2 and C3 carry the major portion of the information that affects the rhythm. The input through the C3 dorsal root on balance prolongs the step cycle duration, and that through the C2 dorsal root on balance shortens the cycle duration. The overall effects of dorsal rhizotomy appear to be in favor of a faster rhythm, in agreement with findings in the rat (Iakhnitsa et al., 1987; Atsuta et al., 1991; Iwahara et al., 1991). The cellular basis for this interaction remains to be resolved, but the transitional interneurons described previously (Wheatley et al., 1994) may be involved. Functionally, sensory input in the extension phase may serve as a positive feedback to enhance the striding force and increase the range of the limb movement, thus increasing the step cycle durations (Pearson, 1995; Whelan et al., 1995). Sensory input in the flexion phase, on the other hand, may be related to landing of the foot and used to trigger the onset of extensor activation, leading to shortened step cycles.
As well as afferent effects on the CPG, the CPG affects afferent transmission. The same stimulus could lengthen or shorten the step cycle depending on its time in the cycle. The gain of many sensorimotor pathways is modulated in a phase-dependent manner during a variety of locomotor behaviors in humans and other mammals (Stein and Capaday 1988; Brooke et al., 1997). Presynaptic inhibition appears to be involved in gating the sensory input (Stein, 1995; Brooke et al., 1997). How the activities of the networks for walking affect sensorimotor transmission in the mudpuppy requires further investigation.
The length of the hemisected spinal cord necessary for generation of the elbow flexor and extensor rhythm is relatively small (8 mm) (Fig.6) and the cord contains a small number of relatively large interneurons (Fig. 1B). The organization of the flexor and extensor centers can be studied separately (Fig.11B), and the connectivity between the two centers and between the CPGs and the sensory input is becoming clear. Thus, the mudpuppy seems a very promising preparation for finally elucidating the circuitry for vertebrate walking and its modulation.
Footnotes
This work was supported by grants to R.B.S. from the Medical Research Council of Canada and the NeuroScience Network of Centers of Excellence (NCE). J.C. is supported by postdoctoral fellowships from NCE and the Alberta Heritage Foundation for Medical Research. We thank Drs. K. G. Pearson and M. Gorassini for helpful comments on an earlier version of this manuscript. Special thanks to Y. Tharani for excellent technical assistance.
Correspondence should be addressed to Dr. Richard B. Stein, Division of Neuroscience, University of Alberta, 513 HMRC, Edmonton, AB, Canada, T6G 2S2.
REFERENCES
- 1.Atsuta Y, Garcia-Rill E, Skinner RD. Control of Locomotion in vitro. I. Deafferentation. Somatosens Mot Res. 1991;8:45–53. doi: 10.3109/08990229109144728. [DOI] [PubMed] [Google Scholar]
- 2.Bracci E, Ballerini L, Nistri A. Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J Neurosci. 1996;16:7063–7076. doi: 10.1523/JNEUROSCI.16-21-07063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci. 1911;84:308–319. [Google Scholar]
- 5.Brown TG. On the nature of the fundamental activity of the nervous centres: together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol (Lond) 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazalets JR, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci. 1996;16:298–306. doi: 10.1523/JNEUROSCI.16-01-00298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Stein RB, Jovanović K, Yoshida K. Localization, activation, and modulation of networks for walking in mudpuppy spinal cord. Soc Neurosci Abstr. 1997;23:207. doi: 10.1523/JNEUROSCI.18-11-04295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AH, Wallén P. The neuronal correlate of locomotion in fish. “Fictive swimming” induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- 10.Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- 11.Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-d-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 12.Cowley KC, Schmidt BJ. Regional distribution of locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- 13.Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- 14.Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillation is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res. 1983;53:81–90. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- 15.Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- 16.Edgley SA, Jankowska E, Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. J Physiol (Lond) 1988;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillner S. On the generation of locomotion in the spinal dogfish. Exp Brain Res. 1974;20:459–470. doi: 10.1007/BF00238013. [DOI] [PubMed] [Google Scholar]
- 18.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brookhardt JM, Mountcastle VB, editors. Handbook of physiology, Sec 2, The nervous system. American Physiological Society; Bethesda, MD: 1981. pp. 1179–1236. [Google Scholar]
- 19.Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985;228:143–149. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- 20.Grillner S. Neural networks for vertebrate locomotion. Sci Am. 1996;274:64–69. doi: 10.1038/scientificamerican0196-64. [DOI] [PubMed] [Google Scholar]
- 21.Grillner S, McClellan A, Sigvardt K, Wallén P, William T. On the neural generation of “fictive locomotion” in a lower vertebrate nervous system in vitro. In: Sjölund B, Björklund A, editors. Brain stem control of spinal mechanisms. Elsevier; Amsterdam: 1982. pp. 273–295. [Google Scholar]
- 22.Grillner S, Wallén P, Brodin L. Neural network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties and simulation. Annu Rev Neurosci. 1991;14:169–199. doi: 10.1146/annurev.ne.14.030191.001125. [DOI] [PubMed] [Google Scholar]
- 23.Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Orlovsky GN, Wallen P. Neural network that coordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 24.Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol (Lond) 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho S, O’Donovan MJ. Regionalization and intersegmental coordination of rhythm-generating networks in the spinal cord of the chick embryo. J Neurosci. 1993;13:1354–1371. doi: 10.1523/JNEUROSCI.13-04-01354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochman S, Schmidt BJ (1998) Whole-cell recordings of lumbar motoneurons during locomotor-like activity in the in vitroneonatal rat spinal cord. J Neurophysiol, in press. [DOI] [PubMed]
- 27.Hochman S, Jordan LM, MacDonald JF. N-methyl-d-aspartate receptor-mediated voltage oscillations in neurons surrounding the central canal in slices of rat spinal cord. J Neurophysiol. 1994;72:565–577. doi: 10.1152/jn.1994.72.2.565. [DOI] [PubMed] [Google Scholar]
- 28.Iakhnitsa IA, Bulgakova NV, Piliavskii AL. Kinematic analysis of the different types of locomotor movement in rats after deafferentation. Neirofiziologiya. 1987;19:520–525. [PubMed] [Google Scholar]
- 29.Iizuka M, Kiehn O, Kudo N. Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Exp Brain Res. 1997;114:193–204. doi: 10.1007/pl00005628. [DOI] [PubMed] [Google Scholar]
- 30.Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Locomotion induced by spinal cord stimulation in the neonate rat in vitro. Somatosens Mot Res. 1991;8:281–287. doi: 10.3109/08990229109144751. [DOI] [PubMed] [Google Scholar]
- 31.Jovanović K, Petrov T, Greer JJ, Stein RB. Serotonergic modulation of the mudpuppy (Necturus maculatus) locomotor pattern in vitro. Exp Brain Res. 1996a;111:57–67. doi: 10.1007/BF00229556. [DOI] [PubMed] [Google Scholar]
- 32.Jovanović K, Petrov T, Stein RB. Modulatory action of inhibitory neurotransmitters on the mudpuppy (Necturus maculatus). Soc Neurosci Abstr. 1996b;22:1376. [Google Scholar]
- 33.Kiehn O, Iizuka M, Kudo N. Resetting from low threshold afferents of N-methyl-d-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci Lett. 1992;184:43–46. doi: 10.1016/0304-3940(92)90800-m. [DOI] [PubMed] [Google Scholar]
- 34.Kiehn O, Johnson BR, Raastad M. Plateau potentials in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience. 1996;75:263–273. doi: 10.1016/0306-4522(96)00250-3. [DOI] [PubMed] [Google Scholar]
- 35.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjaerulff O, Barajon I, Kiehn O. Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. J Physiol (Lond) 1994;478:265–273. doi: 10.1113/jphysiol.1994.sp020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremer E, Lev-Tov A. Localization of spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- 38.MacLean JN, Hochman S, Magnuson DSK. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neurosci Lett. 1995;197:9–12. doi: 10.1016/0304-3940(95)11882-w. [DOI] [PubMed] [Google Scholar]
- 39.Mortin LI, Stein PSG. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J Neurosci. 1989;9:2285–2296. doi: 10.1523/JNEUROSCI.09-07-02285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- 41.Pearson KG. Proprioceptive regulation of locomotion. Curr Opin Neurobiol. 1995;5:786–791. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 42.Perrins R. The roles of central cholinergic and electrical synapses made by spinal motoneurones in Xenopus embryos. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and gamma motor systems. Academic; New York: 1995. pp. 48–50. [Google Scholar]
- 43.Raastad M, Johnson BR, Kiehn O. The number of postsynaptic currents necessary to produce locomotor-related cyclic information in neurons in the neonatal rat spinal cord. Neuron. 1996;17:729–738. doi: 10.1016/s0896-6273(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 44.Ranck JB. Extracellular stimulation. In: Patterson MM, Keener RP, editors. Electrical stimulation research techniques. Academic; New York: 1981. pp. 2–34. [Google Scholar]
- 45.Richmond FJR, Gladdy R, Creasy JL, Kitamura S, Smits E, Thomson DB. Efficacy of seven retrograde tracers, compared in multiple-labelling studies of feline motoneurones. J Neurosci Methods. 1994;53:35–46. doi: 10.1016/0165-0270(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 46.Roberts A, Soffe SR, Dale N. Spinal interneurons and swimming in frog embryos. In: Grillner S, Stein PSG, Stuart D, Forssberg H, Herman RM, editors. Neurobiology of vertebrate locomotion. Macmillan; London: 1986. pp. 279–306. [Google Scholar]
- 47.Roberts A, Tunstall MJ, Wolf E. Properties of networks controlling locomotion and significance of voltage dependency of NMDA channels: simulation study of rhythmic generations sustained by positive feedback. J Neurophysiol. 1995;73:485–495. doi: 10.1152/jn.1995.73.2.485. [DOI] [PubMed] [Google Scholar]
- 48.Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT, editors. Handbook of physiology, Sec 12, Exercise: regulation and integration of multiple systems. Oxford UP; New York: 1996. pp. 173–216. [Google Scholar]
- 49.Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- 50.Stein PSG, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein RB. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–544. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 52.Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci. 1988;11:328–332. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- 53.Stein RB, Lee RG. Tremor and clonus. In: Brooks VB, editor. Handbook of physiology, Sec 1, The nervous system, Vol II, Motor control. American Physiological Society; Bethesda, MD: 1981. pp. 235–243. [Google Scholar]
- 54.Székely G, Czéh G, Vörös G. The activity pattern of limb muscles in freely moving normal and deafferented newts. Exp Brain Res. 1969;9:53–62. doi: 10.1007/BF00235451. [DOI] [PubMed] [Google Scholar]
- 55.Viala D, Viala G, Jordan L. Interneurones of the lumbar cord related to spontaneous locomotor activity in the rabbit. I. Rhythmically active interneurones. Exp Brain Res. 1991;84:177–186. doi: 10.1007/BF00231773. [DOI] [PubMed] [Google Scholar]
- 56.Wheatley M, Stein RB. An in vitro preparation of the mudpuppy for simultaneous intracellular and electromyographic recording during locomotion. J Neurosci Methods. 1992;42:129–137. doi: 10.1016/0165-0270(92)90143-2. [DOI] [PubMed] [Google Scholar]
- 57.Wheatley M, Edamura M, Stein RB. A comparison of intact and in vitro locomotion in an adult amphibian. Exp Brain Res. 1992;88:609–614. doi: 10.1007/BF00228189. [DOI] [PubMed] [Google Scholar]
- 58.Wheatley M, Jovanović K, Stein RB, Lawson V. The activity of interneurons during locomotion in the in vitro Necturus spinal cord. J Neurophysiol. 1994;71:2025–2032. doi: 10.1152/jn.1994.71.6.2025. [DOI] [PubMed] [Google Scholar]
- 59.Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res. 1995;103:20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]
- 60.Yeomans JS. Principles of brain stimulation. Oxford UP; Oxford: 1990. [Google Scholar]