Abstract
We have shown that N-acetylcysteine (NAC) promotes survival of sympathetic neurons and pheochromocytoma (PC12) cells in the absence of trophic factors. This action of NAC was not related to its antioxidant properties or ability to increase intracellular glutathione levels but was instead dependent on ongoing transcription and seemed attributable to the action of NAC as a reducing agent. Here, we investigate the mechanism by which NAC promotes neuronal survival. We show that NAC activates the Ras-extracellular signal-regulated kinase (ERK) pathway in PC12 cells. Ras activation by NAC seems necessary for survival in that it is unable to sustain serum-deprived PC12 MM17-26 cells constitutively expressing a dominant-negative form of Ras. Promotion of PC12 cell survival by NAC is totally blocked by PD98059, an inhibitor of the ERK-activating MAP kinase/ERK kinase, suggesting a required role for ERK activation in the NAC mechanism. In contrast, LY294002 and wortmannin, inhibitors of phosphatidylinositol 3-kinase (PI3K) that partially block NGF-promoted PC12 cell survival, have no effect on prevention of death by NAC. We hypothesized previously that the ability of NAC to promote survival correlates with its antiproliferative properties. However, although NAC does not protect PC12 MM17-26 cells from loss of trophic support, it does inhibit their capacity to synthesize DNA. Thus, the antiproliferative effect of NAC does not require Ras activation, and inhibition of DNA synthesis is insufficient to mediate NAC-promoted survival. These findings highlight the role of Ras-ERK activation in the mechanism by which NAC prevents neuronal death after loss of trophic support.
Keywords: apoptosis, neuronal survival, N-acetylcysteine, Ras, cell cycle, trophic factor withdrawal
Neuronal apoptotic death is an important aspect of nervous system development and disease and is regulated partly by trophic factors (Ellis et al., 1991). To dissect the mechanisms of trophic factor-mediated neuronal survival, we have used the rat PC12 pheochromocytoma cell line. PC12 cells die apoptotically after serum deprivation, and under serum-free conditions, NGF reversibly maintains the long-term survival of these cells (Greene, 1978; Batistatou and Greene, 1991; Rukenstein et al., 1991). This system has been used to uncover small molecules that mimic the survival-promoting action of NGF (Rukenstein et al., 1991; Farinelli and Greene, 1996; Park et al., 1996a). These molecules, in turn, provide insight into the pathways involved in neuronal survival and death.
The small molecule N-acetylcysteine (NAC) is highly effective in promoting long-term survival of trophic factor-deprived PC12 cells and sympathetic neurons (Ferrari et al., 1995). NAC also protects other cells from a variety of apoptotic stimuli including trophic factor deprivation (Shen et al., 1992; Mayer and Noble, 1994;Lin et al., 1995; Schultz et al., 1996) and enhances neuronal survival in animal models of forebrain ischemia and lower motor neuron degeneration (Knuckey et al., 1995; Henderson et al., 1996). NAC has been used to alleviate the symptoms of progressive myoclonus epilepsy (Hurd et al., 1996) and has been considered for treatment of a variety of disorders (Vyth et al., 1996; Herzenberg et al., 1997; Ponz de Leon and Roncucci, 1997).
NAC has a broad range of actions potentially relevant to its protective effects. These include antioxidant activity (Aruoma et al., 1989), enhancement of intracellular glutathione levels, inhibition of proliferation, and stimulation of transcription [for review, seeCotgreave (1997)]. Although the mechanism by which NAC promotes cell survival is unknown, it is widely assumed to be attributable to its antioxidant properties as well as to its elevation of glutathione.
In previous work, we provided evidence that questions these assumptions. For instance, NAC-promoted survival of sympathetic neurons and PC12 cells is not mimicked by other antioxidants, occurs at concentrations (20–60 mm) far higher than that required for antioxidant activity, is independent of glutathione (GSH) elevation, and requires ongoing transcription (Ferrari et al., 1995;Yan et al., 1995). Also, other thiol-containing reducing agents reproduce to some extent the ability of NAC to promote survival (Yan et al., 1995). Furthermore, NAC inhibits PC12 cell proliferation at the same concentrations required to prevent apoptosis. Other blockers of G1/S progression inhibit neuronal death caused by NGF deprivation (Farinelli and Greene, 1996; Park et al., 1996a), and this raised the possibility that the promotion of survival of NAC correlates with its arrest of proliferation (Ferrari et al., 1995). Taken together, these data suggested that NAC does not protect neuronal cells from loss of trophic support by working as an antioxidant but rather that it may act by reducing relevant thiol groups, thereby activating key pathways that lead to transcription of molecules that maintain neuronal survival.
With respect to regulation of transcription, reducing agents, NAC included, can affect the activity of transcription factors such as NF-κB and activator protein-1 (AP-1) (Sen and Packer, 1996) and can trigger induction of immediate early genes in several cell types (Meyer et al., 1993; Li et al., 1994). In addition, NAC activates the signal transduction molecule ERK in HeLa cells (Müller et al., 1997). The ERK pathway has been implicated in NGF-mediated PC12 cell survival (Xia et al., 1995) and seems required for NGF-mediated cell cycle arrest (Pumiglia and Decker, 1997) and for NGF-triggered AP-1 activity (Gille et al., 1992). Therefore the molecular components of the ERK pathway are potential candidates as targets and/or participants in the NAC mechanism.
Here, we explore the mechanisms by which NAC elicits survival of trophic factor-deprived PC12 cells. We find that NAC causes rapid activation of the Ras-ERK signaling pathway and that this is required for promotion of survival.
MATERIALS AND METHODS
Materials. Human recombinant NGF was a generous gift from Genentech (San Francisco, CA) and was used at a concentration of 100 ng/ml. N-Acetyl-l-cysteine (Sigma, St. Louis, MO) was made up in serum-free medium as an 800 mmstock solution, pH 7.4. LY294002 was purchased from Biomol (Plymouth Meeting, PA), and PD98059 was purchased from Alexis (San Diego, CA). Wortmannin was obtained from Sigma.
Cell culture. PC12, M7, and MM17-26 cells were maintained in RPMI 1640 supplemented with 5% fetal bovine serum and 10% heat-inactivated horse serum (JRH Biologicals, Lenexa, KS).
For survival experiments, PC12, M7, or MM17-26 cells were extensively washed with serum-free medium and plated in collagen-coated 24-well plates at a density of 20 × 104 cells per well (Rukenstein et al., 1991).
Cell counts. To determine the number of live cells in the survival experiments, we removed medium and replaced it with 0.1 ml of lysis buffer (Soto and Sonnenschein, 1985), which lyses the cell membrane and leaves the nuclei intact. The lysates are then loaded into a hemacytomer, and the intact nuclei were scored. All counts were done in triplicate and expressed relative to cells present on the day of plating ± SEM.
Thymidine incorporation. For thymidine incorporation experiments, M7 or MM17-26 cells were plated with media supplemented with 300 μm insulin into collagen-coated 24-well plates at a density of 20 × 104 cells per well. Thereafter, the cells were treated with different concentrations of NAC overnight. Thymidine incorporation were determined as described in Yan et al. (1995).
ERK-1 activity assay. Confluent 100 mm dishes of PC12 or MM17-26 cells treated with the indicated compounds in medium with serum were washed with PBS. Thereafter, ERK-1 was immunoprecipitated and assayed for activity as described previously (Loeb et al., 1992). In brief, ERK-1 was immunoprecipitated from cell extracts with anti-ERK-1 antibody (Santa Cruz, Santa Cruz, CA) at a dilution of 1:100 and protein A-Sepharose beads. The immunoprecipitates were washed and resuspended in a kinase buffer containing [γ-32P]ATP and myelin basic protein as substrate. The kinase reaction was incubated at 37°C for 30 min and terminated with the addition of Laemmli sample buffer. The samples were resolved on a 12% SDS-PAGE gel, blotted onto nitrocellulose, and stained with Ponceau S. The MBP bands were excised and submitted to scintillation counting.
Ras assay. A modified protocol of Qiu and Green (1992) was used. Confluent PC12 cells grown in 100 mm dishes were unfed for 3 d and then washed three times with Hank’s buffered saline with glucose (137 mm NaCl, 2.7 mm KCl, 1.2 mmCaCl2, 0.5 mm MgCl2, 25 mm HEPES, pH 7.4, and 1 mg/ml glucose) and labeled for 3 hr with 1–2 mCi of [32P]orthophosphate (New England Nuclear, Boston, MA). At the end of the labeling period, the cells were treated with the indicated compounds, washed with cold PBS, and lysed with 725 μl of cold lysis buffer (50 mm HEPES, pH 7.5, 1% Triton X-100, 100 mm NaCl, 5 mmMgCl2, 1 mg/ml BSA, 1% aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/μl trypsin inhibitor, and 1 mm PMSF). The samples were centrifuged at 15,000 ×g for 4 min at 4°C. The following reagents were added to the supernatant: 100 μl of 5 m NaCl, 50 μl of 10% deoxycholate salt, 25 μl of 2% SDS, and 100 μl of charcoal blocked with 10% BSA. The samples were vortexed, put on ice for 5 min, and centrifuged for 10 min at top speed in a tabletop microfuge to remove the charcoal. The supernatant of each sample was then split into two equal parts, one that was exposed to the anti-Ras antibody Y13–259 (Santa Cruz) and one that was not and constituted the background sample. Both samples were incubated with protein A-Sepharose preconjugated with rabbit anti-Ras antibody (Zymed, San Francisco, CA). After immunoprecipitation, the samples were washed eight times with cold wash buffer (50 mm HEPES, pH 7.4, 1% Triton X-100, 500 mm NaCl, 5 mm MgCl2, and 0.005% SDS) and resuspended in 20 μl of elution buffer (2 mm EDTA, 2 mm DTT, 0.2% SDS, 2 mmGTP, and 2 mm GDP). The samples were then incubated at 68°C for 20 min, and the guanidine species in the supernatant were resolved on polyethyleneimine-cellulose TLC plates in 0.9 mKH2PO4, pH 3.4, and were visualized by a phosphorimager. The radioactivity in GTP and GDP spots was quantified by densitometry, and the ratio of GTP bound to Ras was calculated as GTP/(GTP + 1.5 × GDP). The background was subtracted for each sample.
Northern blots. Cells were treated with 100 ng/ml NGF or 60 mm NAC in serum-containing medium for the indicated times. Total RNA was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Purified RNA was subjected to Northern blotting as described in Batistatou et al. (1992).
RESULTS
NAC activates the Ras-ERK pathway
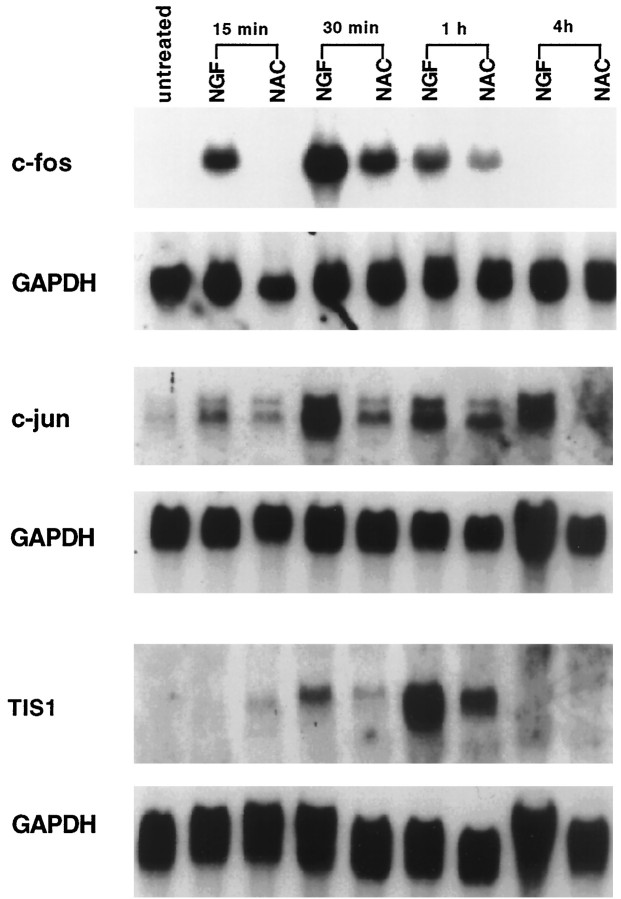
Our previous data demonstrated that NAC requires transcriptional activity to protect PC12 cells from trophic factor withdrawal. Consistent with this are reports that NAC induces c-fos and c-jun transcript levels in several cell systems (Li et al., 1994; Keogh et al., 1995). To test whether this was also true in our system and to elucidate the mechanism by which NAC maintains long-term survival, we treated PC12 cells with NAC for different times and measured the levels of c-fos or c-jun mRNA expression by Northern blotting. For these experiments, we chose NGF as a positive control because NGF also maintains long-term survival of PC12 cells and because its signaling pathways have been well studied and characterized (van der Geer et al., 1994). Figure 1 shows that both NAC and NGF transiently increase expression of the immediate early genes c-fos and c-jun as well as TIS1. However, induction of the immediate early genes by NAC was less intense in comparison with that by NGF. We also tested whether NAC, like NGF, elicits long-term gene regulation in PC12 cells by measuring mRNA levels for transin. This gene is greatly increased in expression after 2 d of NGF treatment (Machida et al., 1989). However, NAC did not increase transin mRNA levels after 2 d of exposure (data not shown). Thus, NAC does not reproduce all transcriptional activity of NGF.
Fig. 1.
NAC increases the expression of immediate early genes. PC12 cells in serum-containing medium were treated with 100 ng/ml NGF or 60 mm NAC. At the indicated times, the RNA was collected, blotted, probed with radiolabeled TIS1, c-fos, or c-jun, stripped, and reprobed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Similar findings were achieved in another independent experiment.
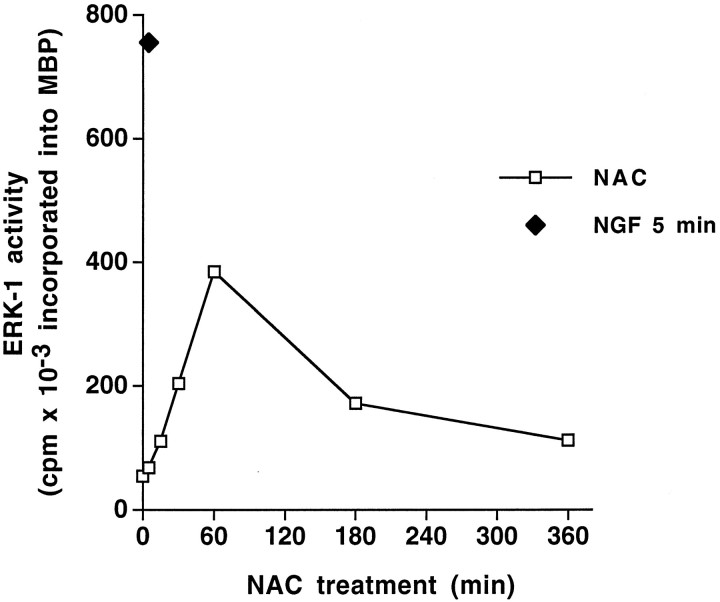
Because induction of immediate early genes can be mediated in part by activation of the ERK/MAPK pathway (Gille et al., 1992), we next evaluated the effect of NAC on ERK-1 activity. This was achieved by measuring kinase activity of immunoprecipitated ERK-1 using myelin basic protein as substrate. NAC increased ERK-1 activity in PC12 cells after 15 min of treatment (Fig. 2) and maximally stimulated activity by 60 min. We also observed that NAC brought about tyrosine phosphorylation of ERK-1 in cells treated with this compound for 30 min (data not shown). As in the case of immediate early gene induction, the kinetics as well as the intensity of ERK-1 activation by NAC differ from that reported for NGF. Maximal induction of ERK activity by NGF in PC12 cells occurs more rapidly and is more robust (Qiu and Green, 1992). For instance, we verified that at 5 min NGF-induced ERK activity is already twice that of the maximum activity induced by NAC (Fig. 2).
Fig. 2.
Time course of NAC-induced ERK-1 activity. PC12 cells grown in medium with serum were treated with 100 ng/ml NGF for 5 min or 60 mm NAC for the indicated times, after which the cells were collected and submitted to anti-ERK-1 immunoprecipitation (see Materials and Methods) and assessment of ERK-1 activity using myelin basic protein (MBP) as substrate. ERK-1 activity is expressed as the amount of 32P incorporated into MBP per 30 min.
Because of the high concentrations of NAC required to induce ERK-1 activity, it was conceivable that the increases observed were attributable to osmotic effects (Matsuda et al., 1995) rather than to a specific action of NAC alone. Therefore, we tested whether ERK-1 activity was also increased with 60 mm methionine. We observed no effect of such treatment on ERK-1 activity or on promotion of cell survival (data not shown).
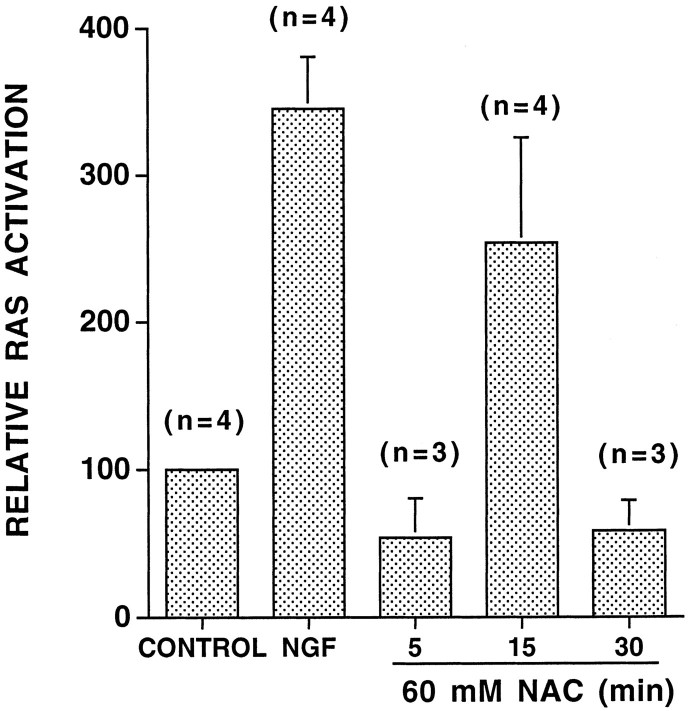
Many instances of ERK and immediate early gene activation are mediated via the GTP-binding Ras protein, which acts upstream from ERK (Waskiewicz and Cooper, 1995). We therefore measured Ras activity in NAC-treated PC12 cells by quantifying the relative proportion of Ras-GTP versus total Ras (see Materials and Methods). In four independent experiments, NAC treatment increased the level of Ras-GTP by an average of 2.5-fold (Fig. 3). This increase peaked after 15 min of exposure and returned to baseline levels after 30 min. Again, consistent with the differences between NGF and NAC in kinetics and intensity of ERK induction and immediate early gene regulation, NAC-stimulated Ras activity was both delayed and less robust in comparison with that by NGF (Qiu and Green, 1992) (Fig.3).
Fig. 3.
NAC activates Ras. PC12 cells loaded with [32P]orthophosphate were treated with no additives (control), with 100 ng/ml NGF for 5 min, or with 60 mm NAC for the indicated times. Ras activity is measured as the ratio of [32P]GTP/total nucleotides associated with Ras after immunoprecipitation (see Materials and Methods). The results are expressed relative to the activity present in untreated cells; n = number of independent experiments; data are expressed as the mean ± SEM.
Ras activity is necessary for NAC-mediated survival
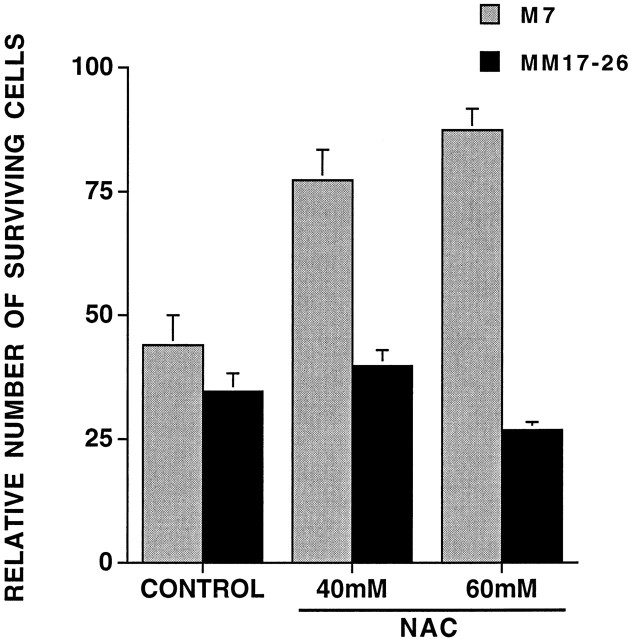
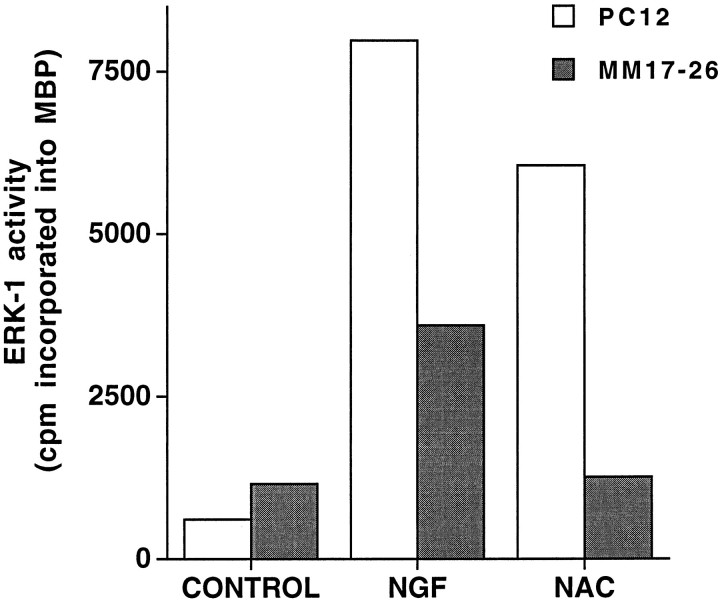
We next determined whether Ras activity is necessary for NAC-mediated neuronal survival. For this purpose, we used the PC12 MM17-26 cell line that constitutively expresses high levels of Ha-Ras(Asn17), a dominant-negative form of Ras, and the PC12 M7 cell line that was transfected with the control construct (Szeberényi et al., 1990). MM17-26 and M7 cells proliferate in medium with serum, die in response to serum withdrawal, and can be rescued under such conditions by addition of NGF or insulin (Yao and Cooper, 1995) (data not shown). However, in contrast to its effects on wild-type PC12 (Ferrari et al., 1995) and on M7 cells, NAC did not maintain MM17-26 cells under serum-free conditions (Fig.4). We also observed that NAC does not increase ERK-1 activity in the MM17-26 cells (Fig.5) or elevate c-fos transcript levels (data not shown), thus confirming that NAC does not activate the Ras pathway in this line. These data thus suggest that NAC requires Ras activity to maintain survival as well as to activate the ERK pathway.
Fig. 4.
NAC does not protect MM17-26 cells from apoptosis caused by withdrawal of trophic support. Replicate cultures of PC12 cells transfected with control vector (M7) or dominant-negative Ras (MM17-26) were cultured in serum-free medium treated with no additives (control) or 40 or 60 mm NAC for 2 d. Cells were counted, and results are expressed relative to initial plating number (n = 3).
Fig. 5.
NAC induces ERK-1 activity in PC12 but not in MM17-26 cells. Cells grown in medium supplemented with serum were treated with no additives (control), 100 ng/ml NGF, or 60 mm NAC for 30 min, after which ERK-1 was immunoprecipitated and assessed for activity as described in Materials and Methods. Activity is expressed as the amount of 32P incorporated into myelin basic protein in 30 min. This experiment is representative of three others.
ERK activation plays a role in NAC-mediated survival
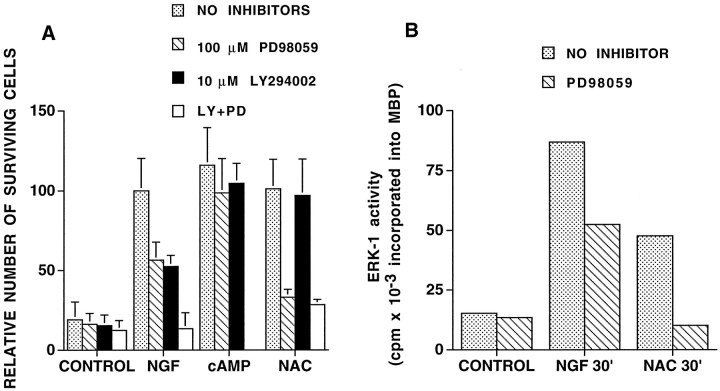
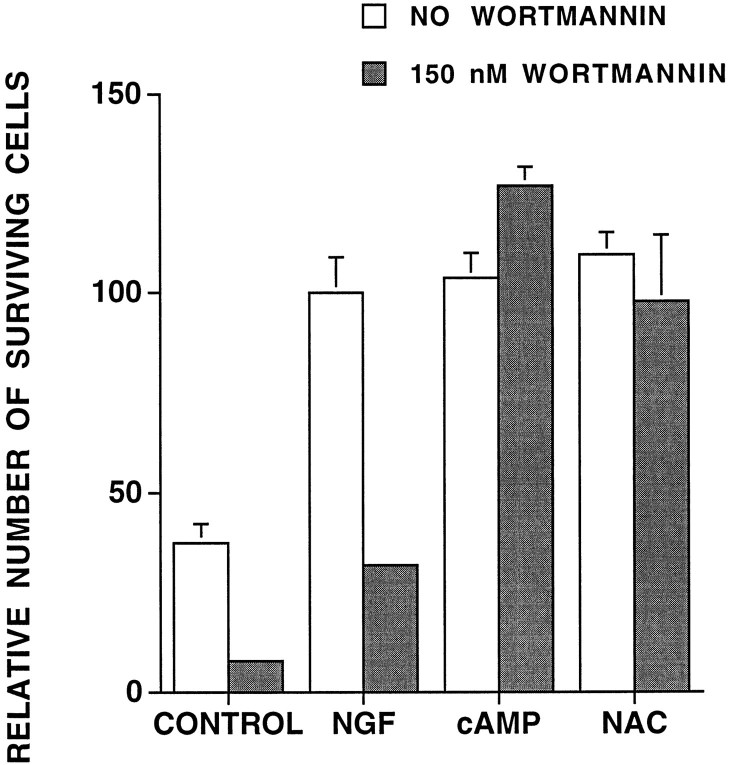
We next sought to distinguish which of the signaling pathways downstream from Ras is required for NAC-induced cell survival. Recent findings implicate phosphatidylinositol 3-kinase (PI3 kinase) activity as a signaling component in trophic factor-maintained cell survival (Rodriguez-Viciana et al., 1994; Dudek et al., 1997; Kennedy et al., 1997) and indicate that PI3 kinase regulation may be downstream as well as independent of Ras (Kodaki et al., 1994; Rodriguez-Viciana et al., 1994; Yao and Cooper, 1995). We thus investigated whether PI3 kinase activity is necessary for NAC-induced survival by using the PI3 kinase inhibitor LY294002 (Vlahos et al., 1994); 10 μm LY294002 decreased by ∼50% NGF-promoted cell survival of PC12 cells in serum-free medium (Fig.6A). However, it had no significant effect on survival of NAC-treated cells. We also used wortmannin, another PI3 kinase inhibitor (Kimura et al., 1994) (Fig. 7). Although wortmannin substantially inhibited NGF-dependent survival, it had no effect on survival of NAC-treated cells. Neither drug affected survival promoted by CPT-cAMP, which was included as a control for toxic activities of the drugs.
Fig. 6.
Effect of MEK and PI3 kinase inhibitors on NAC-mediated PC12 cell survival (A) and of MEK inhibition on NAC-induced ERK-1 activity (B).A, For the survival experiments, cells in serum-free medium were pretreated with no inhibitors, with 100 μmPD98059, and/or with 10 μm LY294002 for 1 hr before addition of no additives (CONTROL), 100 ng/ml NGF, 100 μm 8-(4-chlorophenylthio)-cAMP (CPT-cAMP), or 60 mm NAC. Surviving cells were counted after 24 hr, and results are expressed relative to NGF-treated cells in the absence of inhibitors (n = 3). B, For the assessment of ERK-1 activity, cells in complete medium were pretreated with no inhibitor or with PD98059 for 1 hr before addition of no additives (CONTROL), 100 ng/ml NGF or 60 mm NAC for 30 min, after which ERK-1 was immunoprecipitated and assessed for kinase activity (see Materials and Methods). Activity is expressed as the amount of 32P incorporated into myelin basic protein in 30 min.
Fig. 7.
The PI3 kinase inhibitor wortmannin does not affect NAC-mediated survival. PC12 cells in serum-free medium were pretreated with 150 nm wortmannin for 30 min and then 60 mm NAC for 9 hr. Wortmannin was added every 2 hr to compensate for breakdown. Results are expressed relative to survival obtained in NGF-treated cells in the absence of wortmannin (n = 3).
In contrast, PD98059, an inhibitor of a kinase downstream of Ras and upstream of ERKs (MAPKK/MEK) (Alessi et al., 1995), reduced NAC-induced survival to near control levels (Fig. 6A). In contrast, NGF-promoted survival was decreased by approximately half. Consistent with these observations, PD98059 inhibited NAC-induced ERK-1 activity to basal levels and only partially blocked NGF-induced ERK-1 activity (Fig. 6B). When the cells were treated with both LY294002 and PD98059, all survival-promoting activity by NGF was abolished. In contrast, the combination of drugs had little if any further inhibitory effect on NAC-mediated survival in comparison with that with PD98059 alone. PD98059 had no effect on cAMP-induced survival. These results indicate, firstly, that the ERK pathway is necessary for NAC-mediated survival. Secondly, they show that the ERK and PI3 kinase pathways contribute differently to NAC- and NGF-mediated survival. Although NAC seems to rely primarily on the ERK pathway to exert its survival-promoting effect, NGF-promoted survival seems to use both the ERK and PI3 kinase pathways. An additional point is that consistent with past findings (Alessi et al., 1995), PD98059 does not completely abolish activation of ERK-1 by NGF (Fig.6B), and thus there may be an underestimation of the role of MAPKK in NGF-mediated survival.
NAC-mediated cell cycle inhibition is not sufficient for survival
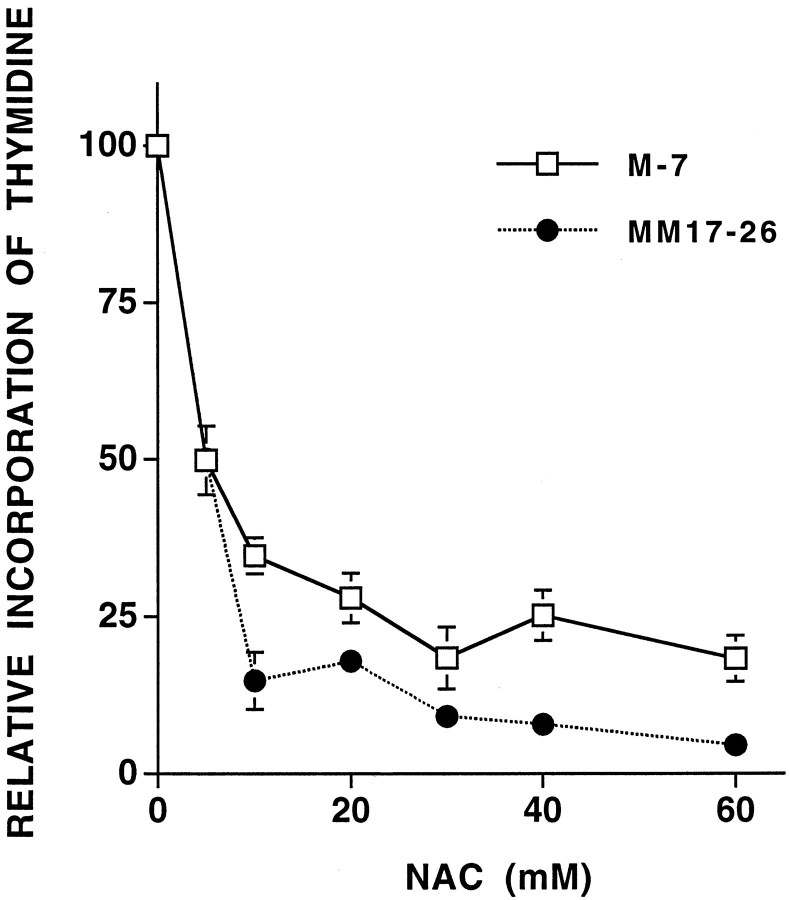
In a previous report, we demonstrated that NAC inhibits PC12 cell proliferation and hypothesized that this is causally related to promotion of survival (Ferrari et al., 1995). We therefore examined the relationship between Ras activity and NAC-mediated cell cycle arrest. Thymidine incorporation was used as a measure of cell cycle progression. Despite the inability of NAC to promote survival of MM17-26 cells, it inhibited thymidine incorporation in this line (Fig.8). Maximal inhibition of thymidine incorporation with NAC occurred at 30–60 mm, concentrations that provided significant protection against trophic factor withdrawal for PC12 cells transfected with control vector (M7) but not for MM17-26 cells (Fig. 4). These findings suggest that the effect of NAC on cell cycle arrest is independent of Ras activation.
Fig. 8.
NAC inhibits thymidine incorporation in both M7 and MM17-26 cells. The cells were cultured in serum-free medium supplemented with 100 μm insulin and were treated with increasing concentrations of NAC overnight. Results are expressed relative to cells that were not treated with NAC (n= 3).
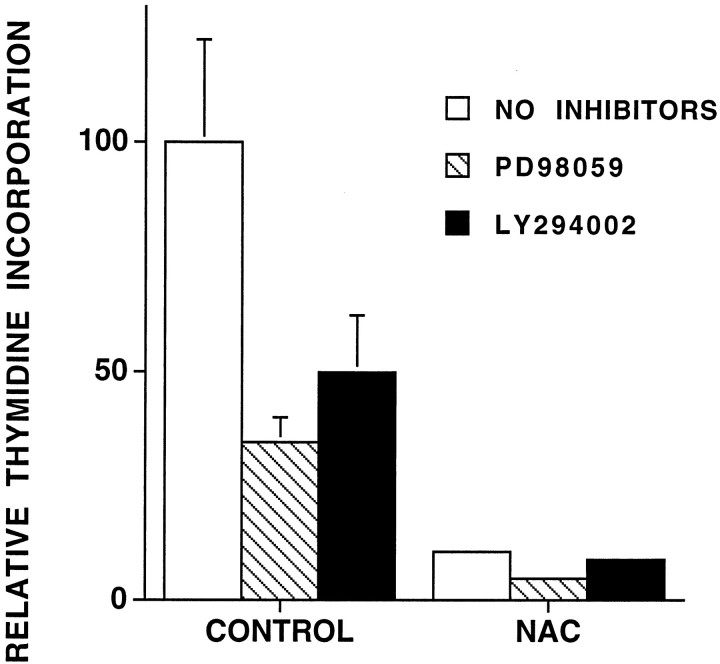
Additional thymidine incorporation experiments were performed with PC12 cells exposed to PD98059 or LY294002. Although these pharmacological inhibitors were not toxic (Fig. 6), they inhibited thymidine incorporation at least by half in control cells cultured in the absence of NAC (Fig. 9). This was most likely because the trophic factors present in serum may in part depend on MEK and PI3 kinase activity to promote cell proliferation. Thymidine incorporation in the presence of NAC was unaffected by either of the inhibitors and remained far below that in NAC-untreated controls. This indicates that NAC does not require MEK or PI3 kinase (PI3K) activity to arrest proliferation.
Fig. 9.
MEK and PI3 kinase inhibitors do not reverse NAC-induced cell cycle arrest. PC12 cells in serum-containing medium were preincubated with 100 μm PD98059 or 10 μm LY294002 for 1 hr and then were incubated with or without 60 mm NAC overnight. Measurements of thymidine incorporation were performed as described in Materials and Methods. Results are expressed relative to the untreated control (n = 3).
DISCUSSION
Mechanism by which NAC protects neurons from loss of trophic deprivation
At concentrations of 20–60 mm, NAC protects neuronal cells from death evoked by withdrawal of trophic support (Ferrari et al., 1995). Our aim here was to determine the mechanism by which this occurs. Because NAC is an antioxidant and elevates another antioxidant, glutathione, it is often assumed that such activities account for all of the neuroprotective actions of NAC. Conversely, protection by NAC is frequently assumed to indicate that oxidative stress is a cause of death. However, as reviewed above, there were multiple reasons to discount this mechanism in the case of protection from trophic deprivation.
Starting with the observation that NAC requires transcription to protect PC12 cells and that it induces immediate early genes in several cell systems, we showed that NAC induces c-fos, c-jun, and TIS1 in PC12 cells. This observation led us to follow the signaling pathway upstream and to determine that NAC stimulates activation of ERK-1 and of p21Ras. Furthermore, NAC was unable to protect cells overexpressing a dominant-negative form of Ras from loss of trophic support, thus indicating that Ras plays a required role in the survival mechanism of NAC. Abolition of NAC-promoted survival with PD98059 further indicates a necessary role for MEK activity and is consistent with a role for ERK activation as well (Xia et al., 1995). The implication of Ras activation in NAC-promoted neuronal survival is consistent with reports that a constitutively active form of Ras rescues NGF-deprived sympathetic neurons and PC12 cells (Rukenstein et al., 1991; Nobes et al., 1996).
Activation of the Ras-ERK pathway leads to induction of immediate early genes including c-fos and c-jun. It has been observed that reducing agents, including NAC, can directly activate transcription factors, apparently by reduction of critical thiol groups (Abate et al., 1990;Meyer et al., 1993). However, our findings with PC12 MM17-26 cells indicate that induction by NAC of at least c-fos occurs via Ras rather than by direct action on transcription factors. Nevertheless, we cannot exclude that such direct activation of transcription contributes to NAC-promoted neuronal survival.
The mechanism by which NAC activates Ras is presently unclear. One possibility was via tyrosine phosphorylation and activation of the SH2-containing protein (SHC) protein that mediates stimulation of Ras by growth factors such as NGF (Basu et al., 1994). However, we measured tyrosine phosphorylation of SHC immunoprecipitated from cells treated with NAC for various times and found no significant effect (data not shown). An alternative possibility was NAC-mediated nitrosylation of Ras. Recent findings indicate that nitrosylation of a critical cysteine residue enhances Ras activity (Lander et al., 1997). Moreover, NAC increases nitric oxide (NO) release from protein-bound stores in vascular tissue (Muller et al., 1996). We therefore pretreated PC12 cells with the NO synthase inhibitor N-nitro arginine methyl ester (l-NAME), but this did not block NAC-mediated survival (data not shown). A third prospect is that NAC directly activates Ras by virtue of its reducing potential. The affinity of G-proteins for GTP measured in vitro is significantly increased in the presence of reducing agents (Carty and Iyengar, 1994). Moreover, a critical redox-sensitive cysteine residue has been identified in Ras (Lander et al., 1997). This mechanism is supported by our observations that reducing agents in addition to NAC have survival-promoting activity (Yan et al., 1995) and is compatible with the high concentrations of NAC that are required to prevent death in our systems.
Comparing the actions of NGF and NAC
NAC shares with NGF and other growth factors the capacity to maintain neuronal survival. Like NGF, NAC activates the Ras-ERK pathway and induces immediate early genes. However, NAC does not completely mimic the trophic actions of NGF. For instance, unlike NGF, NAC does not elicit or maintain neurites or induce the late gene transin. This reinforces past observations that promotion of survival alone is not necessarily sufficient for maintenance or promotion of differentiation (Batistatou and Greene, 1991; Ferrari et al., 1995).
Constitutive activation of Ras mimics at least some of the differentiation-promoting actions of NGF including stimulation of neurite outgrowth (Bar-Sagi and Feramisco, 1985), and activation of the Ras pathway plays a required role in NGF-stimulated neuronal differentiation (Szeberényi et al., 1990). Why then does Ras activation by NAC not drive differentiation? Stimulation of Ras by NAC is of slower onset, reaches lower levels, and is far less sustained than that with NGF. The stronger, sustained activation of the Ras-ERK pathway by NGF has been suggested to be required for the induction of neurite outgrowth (Qiu and Green, 1992). Thus, one reason that NAC mimics only the survival-promoting actions of NGF might be its relatively weak and nonsustained degree of Ras stimulation.
In addition, unlike NGF, NAC does not stimulate Trk and is therefore unlikely to reproduce the full repertoire of signaling events evoked by NGF. An important pathway stimulated by NGF is activation of PI3K (Stephens et al., 1994). As suggested previously (Yao and Cooper, 1995) and confirmed here, this pathway seems to account at least in part for NGF-promoted survival of PC12 cells. In contrast, inhibitors of this pathway had no effect on survival stimulated by NAC. This suggests that NAC does not activate PI3K. This is also consistent with the inability of NAC, in contrast to NGF, to maintain survival of MM17-26 cells.
Our observations are consistent with the presence of at least two alternative signaling pathways that contribute to NGF-promoted PC12 cell survival: the Ras-ERK pathway and the PI3K pathway. Depending on cellular context, the relative contributions of these pathways may vary. For instance, the Ras-ERK pathway seems to be required for NGF-mediated survival of chick sensory but not sympathetic neurons (Borasio et al., 1993), and it has been reported that PD98059 does not affect survival of cultured rat sympathetic neurons (Virdee and Tolkovsky, 1995, 1996). Our findings indicate that NAC differs from NGF in using only the Ras-ERK pathway to maintain survival.
In response to loss of trophic support, PC12 and other cell types show an increased JUN kinase (JNK)/p38 kinase activity (Xia et al., 1995). Evidence has been provided with PC12 cells that this increase is required for death, and a model has been proposed in which survival occurs when the elevation of JNK/p38 activity is suppressed and ERK kinase activity is stimulated (Xia et al., 1995). We showed previously that NAC inhibits the induction of JNK activity caused by serum withdrawal (Park et al., 1996b). Thus NAC, like NGF, fulfills both proposed criteria for promotion of survival: suppression of JNK and activation of ERK.
An additional point is that survival mediated by CPT-cAMP was insensitive to both PI3K inhibitors and to PD90859. CPT-cAMP requires PKA to promote survival (Rukenstein et al., 1991), and our findings suggest that unlike NAC and NGF, it acts independently of PI3K or Ras-ERK stimulation. However, it should be noted that a Ras antibody was reported to interfere with cAMP-mediated survival of rat sympathetic neurons (Nobes and Tolkovsky, 1995).
Recently, it was reported that NAC pretreatment of PC12 cells interferes with NGF-dependent signaling and neurite outgrowth, and it was suggested that NAC interferes with redox-sensitive steps in the NGF mechanism (Kamata et al., 1996). An alternative interpretation raised by our findings is that transient activation of Ras-ERK signaling by NAC during pretreatment causes a compensatory refractoriness of this pathway during subsequent exposure to NGF.
NAC and the cell cycle
We showed previously that NAC inhibits DNA synthesis and suggested that the capacity of NAC to prevent neuronal cell death was because of its antiproliferative activity (Ferrari et al., 1995). This was consistent with other evidence that death evoked by trophic factor withdrawal involves inappropriate activation of cell cycle components (Ferrari and Greene, 1994; Farinelli and Greene, 1996; Park et al., 1996a). The present observation that NAC inhibits cell cycle progression by PC12 MM17-26 cells but does not support them suggests that suppression of proliferation is insufficient to account for the neuroprotective action of NAC against loss of trophic support. Our findings do not distinguish between the possibilities that NAC-promoted cell cycle arrest is irrelevant for support of survival or is required in addition to stimulation of the Ras-ERK pathway.
The mechanism by which NAC blocks DNA synthesis is unknown. The antiproliferative action of NAC on MM17-26 cells indicates that activation of the Ras-ERK pathway is not required for this effect. This is supported by the inability of PD98059 to interfere with NAC-induced cell cycle arrest. Thus the stimulation of Ras-ERK and the inhibition of DNA synthesis by NAC seem to be mediated by different mechanisms.
In summary, we show here that the mechanism by which NAC rescues neuronal cells from loss of trophic support requires activation of the Ras-ERK pathway. This is most likely because of the reducing activity of this compound rather than its actions as an antioxidant or regulator of GSH levels. NAC is presently being considered for treatment of a variety of human diseases including cancer, acquired immunodeficiency syndrome, and neurodegenerative disorders (Vyth et al., 1996;Herzenberg et al., 1997; Ponz de Leon and Roncucci, 1997). In light of this, it is important to understand more clearly the mechanisms of action of NAC, both for predicting possible side-effects and for designing additional agents with comparable or superior activity.
Footnotes
This work was supported by grants from the National Institute of Neurological Diseases and Stroke, the Amyotrophic Lateral Sclerosis Association, and the Blanchette Rockefeller Foundation. We thank Dr. A. Rukenstein for aid with cell culture, Drs. S. H. Green and H. Q. Qi for sharing advice on the assay for Ras activity, Dr. Geoffrey Cooper for providing the M7 and MM17-26 cell lines, and C. Albuquerque for critically reading this manuscript.
Correspondence should be addressed to Dr. Chao Yun Irene Yan, Department of Pathology, College of Physicians and Surgeons, 15-401, Columbia University, 630 West 168th Street, New York, NY 10032.
REFERENCES
- 1.Abate C, Patel L, Rauscher FJ, III, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Cuenda A, Cohen O, Dudley DT, Saltiel AR. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinases kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Aruoma OJ, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 5.Basu T, Warne PH, Downward J. Role of Shc in the activation of Ras in response to epidermal growth factor and nerve growth factor. Oncogene. 1994;9:3483–3491. [PubMed] [Google Scholar]
- 6.Batistatou A, Greene LA. Aurintricarboxilic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991;115:461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batistatou A, Volonté C, Greene LA. Nerve growth factor employs multiple pathways to induce primary response genes in PC12 cells. Mol Biol Cell. 1992;3:363–371. doi: 10.1091/mbc.3.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borasio GD, Markus A, Wittinghofer A, Barde YA, Heumann R. Involvement of ras p21 in neurotrophin-induced response of sensory, but not sympathetic neurons. J Cell Biol. 1993;121:665–672. doi: 10.1083/jcb.121.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carty DJ, Iyengar R. Guanosine 5′-o-(γ-thio-triphosphate binding assay for solubilized G proteins. Methods Enzymol. 1994;237:38–44. doi: 10.1016/s0076-6879(94)37051-6. [DOI] [PubMed] [Google Scholar]
- 10.Cotgreave IA. N-Acetylcysteine: pharmacological consideration and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 11.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine–threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 12.Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 13.Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996;16:1150–1162. doi: 10.1523/JNEUROSCI.16-03-01150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari G, Greene LA. Proliferative inhibition by dominant-negative Ras rescues naive and neuronally differentiated PC12 cells from apoptotic death. EMBO J. 1994;13:5922–5928. doi: 10.1002/j.1460-2075.1994.tb06937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari G, Yan CYI, Greene LA. N-Acetylcysteine (d- and l-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995;15:2857–2866. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 17.Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson JT, Jahaveri M, Kopko S, Roder JC. Reduction of lower motor neuron degeneration in wobbler mice by N-acetyl-l-cysteine. J Neurosci. 1996;16:7574–7582. doi: 10.1523/JNEUROSCI.16-23-07574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurd RW, Wilder BJ, Helveston WR, Uthman BM. Treatment of four siblings with progressive myoclonus epilepsy of the Unverricht-Lundborg type with N-acetylcysteine. Neurology. 1996;47:1264–1268. doi: 10.1212/wnl.47.5.1264. [DOI] [PubMed] [Google Scholar]
- 21.Kamata H, Tanaka C, Yagisawa H, Matsuda S, Gotoh Y, Nishida E, Hirata H. Suppression of nerve growth factor-induced neuronal differentiation of PC12 cells. N-Acetylcysteine uncouples the signal transduction from Ras to the mitogen-activated protein kinase cascade. J Biol Chem. 1996;271:33018–33025. doi: 10.1074/jbc.271.51.33018. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, Hay H. The PI3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 23.Keogh BP, Tresini M, Cristofalo VJ, Allen RG. Effects of cellular aging on the induction of c-fos by antioxidant treatments. Mech Ageing Dev. 1996;86:151–160. doi: 10.1016/0047-6374(95)01689-9. [DOI] [PubMed] [Google Scholar]
- 24.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toke S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;29:18961–18967. [PubMed] [Google Scholar]
- 25.Knuckey NW, Palm D, Primiano M, Epstein MH, Johanson CE. N-Acetylcysteine enhances hippocampal neuronal survival after transient forebrain ischemia in rats. Stroke. 1995;26:305–311. doi: 10.1161/01.str.26.2.305. [DOI] [PubMed] [Google Scholar]
- 26.Kodaki T, Wolscholski R, Hallberg B, Rodriguez-Vinciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 27.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21ras. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Wang G, Wang R, Spector A. The redox active components H2O2 and N-acetyl-l-cysteine regulate expression of c-jun and c-fos in lens systems. Exp Eye Res. 1994;59:179–190. doi: 10.1006/exer.1994.1096. [DOI] [PubMed] [Google Scholar]
- 29.Lin KI, Lee SH, Narayanan R, Baraban JM, Hardwick JM, Ratan RR. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loeb DM, Tsao H, Cobb MH, Greene LA. NGF and other growth factors induce an association between ERK1 and the NGF receptor, gp140prototrk. Neuron. 1992;9:1053–1065. doi: 10.1016/0896-6273(92)90065-l. [DOI] [PubMed] [Google Scholar]
- 31.Machida CM, Rodland KD, Matrisian L, Magun BE, Ciment G. NGF induction of the gene encoding the protease transin accompanies neuronal differentiation in PC12 cells. Neuron. 1989;2:1587–1596. doi: 10.1016/0896-6273(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda S, Kawasaki H, Morigushi T, Gotoh Y, Nishida E. Activation of protein kinase cascades by osmotic shock. J Biol Chem. 1995;270:12781–12786. doi: 10.1074/jbc.270.21.12781. [DOI] [PubMed] [Google Scholar]
- 33.Mayer M, Noble M. N-Acetyl-l-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller B, Kleshyov AL, Stoclet J. Evidence for N-acetylcysteine-sensitive nitric oxide storage as dinitrosyl-iron complexes in lipopolysaccharide-treated rat aorta. Br J Pharmacol. 1996;119:1281–1285. doi: 10.1111/j.1476-5381.1996.tb16034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller JM, Cahill MA, Rupec RA, Baeuerle PA, Nordheim A. Antioxidants as well as oxidants activate c-fos via Ras-dependent activation of extracellular-signal-regulated kinase 2 and Elk-1. Eur J Biochem. 1997;244:45–52. doi: 10.1111/j.1432-1033.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 37.Nobes CD, Tolkovsky AM. Neutralizing anti-p21ras Fabs suppress rat sympathetic neuron survival induced by NGF, LIF, CNTF and cAMP. Eur J Neurosci. 1995;7:344–350. doi: 10.1111/j.1460-9568.1995.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 38.Nobes CD, Reppas JB, Markus A, Tolkovsky AM. Active p21ras is sufficient for rescue of NGF-dependent rat sympathetic neurons. Neuroscience. 1996;70:1067–1079. doi: 10.1016/0306-4522(95)00420-3. [DOI] [PubMed] [Google Scholar]
- 39.Park DS, Farinelli SE, Greene LA. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J Biol Chem. 1996a;271:8161–8169. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- 40.Park DS, Stefanis L, Yan CYI, Farinelli SE, Greene LA. Ordering the cell death pathway. Differential effects of BCL2: an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J Biol Chem. 1996b;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 41.Ponz de Leon M, Roncucci L. Chemoprevention of colorectal tumors: role of lactulose and of other agents. Scand J Gastroenterol [Suppl] 1997;222:72–75. doi: 10.1080/00365521.1997.11720724. [DOI] [PubMed] [Google Scholar]
- 42.Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu M, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesenbroek B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 45.Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 48.Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca2+-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol Appl Pharmacol. 1992;112:32–40. doi: 10.1016/0041-008x(92)90276-x. [DOI] [PubMed] [Google Scholar]
- 49.Soto AM, Sonnenschein C. The role of estrogens on the proliferation of human breast tumor cells. J Steroid Biochem. 1985;23:87–94. doi: 10.1016/0022-4731(85)90265-1. [DOI] [PubMed] [Google Scholar]
- 50.Stephens RM, Loeb DM, Copelan TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 51.Szeberényi J, Cai H, Cooper GM. Effect of a dominant inhibitor Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 53.Virdee K, Tolkovsky AM. Activation of p44 and p42 MAP kinases is not essential for the survival of rat sympathetic neurones. Eur J Neurosci. 1995;7:2159–2169. doi: 10.1111/j.1460-9568.1995.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 54.Virdee K, Tolkovsky A. Inhibition of p42 and p44 mitogen-activated protein kinase activity by PD98059 does not suppress nerve growth factor-induced survival of sympathetic neurons. J Neurochem. 1996;67:1801–1805. doi: 10.1046/j.1471-4159.1996.67051801.x. [DOI] [PubMed] [Google Scholar]
- 55.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 1-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 56.Vyth A, Timmer A, Bossuyt PMM, Louwerse ES, Vianney de Jong JMB. Survival in patients with amyotrophic lateral sclerosis, treated with an array of antioxidants. J Neurol Sci [Suppl] 1996;139:99–103. doi: 10.1016/0022-510x(96)00071-8. [DOI] [PubMed] [Google Scholar]
- 57.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 58.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 59.Yan CYI, Ferrari G, Greene LA. N-Acetylcysteine-promoted survival of PC12 cells is glutathione-independent but transcription-dependent. J Biol Chem. 1995;270:26827–26832. doi: 10.1074/jbc.270.45.26827. [DOI] [PubMed] [Google Scholar]
- 60.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]