Abstract
In this study we investigated the role of protein kinase C (PKC) in associative learning of Apis mellifera. Changes in PKC activity induced by olfactory conditioning were measured in the antennal lobes, a brain structure involved in associative learning. Multiple conditioning trials inducing a memory different from that induced by a single conditioning trial specifically cause an increase in PKC activity. This increase begins 1 hr after conditioning, lasts up to 3 d, and is attributable to an increased level of constitutive PKC. The increased level of constitutive PKC consists of an early proteolysis-dependent phase and a late phase that requires RNA and protein synthesis. Inhibition of the pathways resulting in constitutive PKC selectively impairs distinct phases of multiple-trial induced memory. The inhibition of the proteolytic mechanism has an instant effect on an early phase of multiple-trial induced memory but does not affect acquisition and the late phase of memory. Blocking of the transient PKC activation during conditioning does not affect the induction of memory formation. Thus, the constitutive PKC in the antennal lobe seems to contribute to the early phase of memory that is induced by multiple-trial conditioning.
Keywords: antennal lobe, memory phases, olfactory learning, protein kinase C, thiol proteases, translation, transcription
The molecular analysis of learning and memory has confirmed the concept that memory consists of different phases, which are induced and supported by different mechanisms. The neural substrate of both short- and long-term memory is believed to reside in the synaptic connections between neurons. Recent work has documented convincingly the central role of second messenger-induced phosphorylation cascades in the modulation of neuronal activity and their involvement in learning and memory. One of these signaling cascades is the protein kinase C (PKC) pathway, which has been implicated in later stages of memory in vertebrate studies. A long-lasting activation and relocation of PKC consecutive to learning suggests a function of the PKC pathway in the expression of long-term memory (LTM) (Olds et al., 1989; Scharenberg et al., 1991; Van der Zee et al., 1992; Golski et al., 1995). Similar findings have been demonstrated for long-term potentiation (LTP) in the mammalian hippocampus, which is discussed as a cellular correlate of associative learning (Colley et al., 1989; Roberson et al., 1996). Mechanisms underlying the long-lasting PKC activation in LTP have been investigated, but the results are still controversial (Akers et al., 1986; Klann et al., 1993; Sacktor et al., 1993; Powell et al., 1994), and no conclusion can be drawn as to whether these mechanisms are also responsible for PKC modulation in associative learning.
The molecular mechanisms of learning and memory have been investigated extensively in Aplysia, Drosophila, and the honeybee, but very little is known about the role of PKC in associative learning in these invertebrates. However, PKC has been implicated in synaptic plasticity in Aplysia, Hermissenda, andDrosophila (Braha et al., 1990; Farley and Schuman, 1991;Sugita et al., 1992; McPhie et al., 1993; Sossin et al., 1994; Kane et al., 1997).
Associative learning in honeybees has several features similar to higher forms of learning observed in vertebrates (Menzel, 1990; Menzel and Müller, 1996). One learning paradigm is olfactory conditioning of the proboscis extension response (PER). This paradigm for olfactory learning consists of the pairing of an odor stimulus (CS) with a sucrose reward (US) (Menzel, 1985). Depending on the number of conditioning trials, different memories are induced. Whereas single-trial conditioning induces a memory (sLTM) that is insensitive to the inhibition of nitric oxide synthase (NOS) during training, multiple-trial conditioning additionally induces a distinct NO-dependent memory (mLTM) (Müller, 1996). Interestingly, in the honeybee brain, PKC is expressed strongly in two regions that are both involved in associative olfactory learning: the antennal lobe (AL) and the mushroom body (MB) (Hammer and Menzel, 1995; Menzel and Müller, 1996). In this study we investigated the role of PKC in the AL. We report here that multiple-trial conditioning selectively induces a long-lasting PKC activation after conditioning. This activation involves the formation of constitutively active PKC and is separable into two mechanistically different phases that contribute to distinct memory phases.
MATERIALS AND METHODS
Materials. [γ-32P]ATP (5000 Ci/mmol) was purchased from NEN Life Science Products (Brussels, Belgium), and E 64 was obtained from Biomol (Hamburg, Germany). Gö 7874 hydrochloride (Kleinschroth et al., 1995) and the protein kinase C inhibitor peptide (19–31) were obtained from Calbiochem–Novabiochem (Bad Soden, Germany). The ELISA plates were purchased from Falcon, Becton Dickinson (Heidelberg, Germany). All other chemicals were obtained from Sigma (Deisenhofen, Germany). All of the chemicals used were of analytical grade. Protein kinase C (PKC) was purified from bee brains, as has been described previously (Altfelder et al., 1991).
Animal treatment and injection procedure. Honeybees (Apis mellifera carnica) were caught at the hive entrance, immobilized by cooling, and mounted in metal harnesses by a strip of tape between the head and the thorax. Bees were fed with a sucrose solution (1.4 m) until satiation and kept in darkness in a container at 70% relative humidity and at 20–25°C until the training started the next day. Bees that were used in the long-term experiments also were kept in the containers between sessions and fed every evening with sucrose solution. Drugs were injected into the hemolymph of the thorax by using a microcapillary, as has been described previously (Müller and Hildebrandt, 1995).
Classical olfactory conditioning. Behavioral experiments were performed on different days and in different treatment combinations (drug injections). Each combination always included one PBS-injected control group. Olfactory conditioning of the proboscis extension response (PER) consisted of the temporal pairing of an odor (carnation) as the conditioned stimulus (CS) with sucrose as the unconditioned stimulus (US) (Müller, 1996). Three conditioning trials were presented, with an intertrial interval of 2 min. After successful conditioning, the presentation of the CS alone elicited PER. Animals that did not respond to the US before conditioning were excluded from the experiments (<5%). The PER was calculated for each treatment by dividing the sum of responding animals by the total number of animals tested after the respective treatment. Differences between the PBS-injected control and the drug-injected animals were evaluated by the χ2 test.
Preparation of tissues. Preparation was performed at different times after stimulation or conditioning. In experiments in which bees were killed at times longer than 1 hr after conditioning, the conditioned PER was tested 30 min before dissection. Only bees that showed conditioned PER in this test were dissected. The bees were cooled quickly in ice water, their heads were cut off, and the AL was isolated. Each AL was transferred into a separate capillary and homogenized on the surface of 10 μl of frozen extraction buffer [containing (in mm) 50 Tris-HCl, pH 7.5, 10 MgCl2, 1 EDTA, 1 EGTA, and 10 2-mercaptoethanol with 0.1 m NaCl). The whole procedure, from the cooling of the bee to the freezing of the AL, took ∼1 min. AL were kept in liquid nitrogen until they were used in the in vitrophosphorylation assay.
In vitro phosphorylation assay. PKC activity was measured by the in vitro phosphorylation of exogenously added myristoylated alanine-rich C kinase substrate (MARCKS) protein from bovine brain, which is a specific substrate for PKC from honeybee brain (Müller, 1997a). Homogenates from single AL were phosphorylated in a final volume of 20 μl containing (in mm) 50 Tris-HCl, pH 7.5, 10 MgCl2, 1 EDTA, 1 EGTA, and 10 2-mercaptoethanol plus 0.1 m NaCl, 10 μm ATP, 1 μg of MARCKS protein, and 0.1 μCi [γ-32P]ATP (3000 Ci/mmol). PKC activators (1.5 mm CaCl2, 0.02 μg of diolein, and 0.8 μg of phosphatidylserine) were included in the mixture, unless indicated otherwise. To confirm the specific phosphorylation of MARCKS by PKC, we included the PKC inhibitor peptide (19–31) in the phosphorylation assay where indicated. The samples were phosphorylated for 20 sec at 15°C. The reaction was terminated by the addition of 3 μl of sample buffer (0.25 m Tris-HCl, pH 6.7, containing 20% glycerol, 5% SDS, and 5% 2-mercaptoethanol). SDS-PAGE and autoradiography were performed as described (Hildebrandt and Müller, 1995). Conditions of the film exposure were adjusted to keep the signals of labeled proteins in a linear range. For the calibration of the film exposure, a scintillation counter was used to determine 32P incorporation into MARCKS. Autoradiograms were scanned by a UMAX UC840 Scanner, and the 32P incorporation into the MARCKS protein was quantified by National Institutes of Health Image software. The statistical analysis was performed with Student’s t test.
PKC immunohistochemistry. Immunohistochemistry was performed as described in Müller (1997b). PKC immunoreactivity was visualized with polyclonal antiserum generated against calcium-dependent PKC purified from the honeybee brain and alkaline phosphatase coupled to the immunocomplex via the biotin streptavidin system.
RESULTS
Localization of PKC in the honeybee brain
The calcium-dependent PKC purified from honeybee brain shows remarkable similarities to the corresponding vertebrate PKC (Altfelder et al., 1991). Interestingly, the staining of honeybee brain with a polyclonal PKC antiserum reveals a strong, distinct labeling of the antennal lobes (AL) and the mushroom bodies (MB) (Fig.1). AL and MB are both involved in olfactory learning in the honeybee. Local cooling experiments and local injections of octopamine into the AL indicate that the AL may establish a memory trace independently of the MB (Erber et al., 1980; Hammer and Menzel, 1995). However, the AL primarily process chemosensory information, and PKC immunoreactivity is located predominantly in the AL interneurons. Sensory neurons projecting via the antennal nerve and innervating the rind areas of the glomeruli are stained very weakly. In contrast to the AL, the MB are involved in higher integrative functions and receive input from different sensory modalities (Menzel et al., 1994). The MB show high levels of PKC expression. Thus, because the AL predominantly process olfactory information, we concentrated on this structure of the honeybee brain to study the role of PKC in associative olfactory learning.
Fig. 1.

Comparison of the distribution of PKC in the honeybee brain with the neuropils critical to olfactory learning. The distribution of PKC in the adult honeybee brain, as analyzed by immunohistochemistry (A), is compared with the neuropils that are involved in olfactory learning (schematic).B, The antennal lobes (al), which receive mainly chemosensory input, show strong staining in the central core (asterisk), in the central regions of the glomeruli (g), and in the somata of the interneurons (arrow). Strong labeling is found throughout the mushroom bodies (mb), which process multimodal input and are innervated by AL interneurons. The lateral protocerebral lobes (lp) are stained also. In contrast, the optical lobes (ol) are stained very weakly. Scale bar, 100 μm.
Stimuli used in olfactory learning induce a transient PKC activation in the AL
To detect changes in PKC activity induced by in vivostimulation, we applied a fast phosphorylation assay similar to that described for the PKA (Hildebrandt and Müller, 1995), using MARCKS as a PKC-specific substrate. We recently demonstrated that MARCKS is phosphorylated specifically by PKC, but not by PKA or CaMII kinase from honeybee brain (Müller, 1997a). MARCKS also is phosphorylated by the calcium-independent PKC activity of PKM (see Table 2). In brain homogenate that was depleted of PKC, MARCKS phosphorylation was below 10% of the phosphorylation in the original homogenate containing PKC (see Table 2). To confirm further the specific phosphorylation of MARCKS by PKC, we included the PKC inhibitor peptide (19–31) in the phosphorylation assay. This PKC inhibitor peptide selectively inhibits MARCKS phosphorylation by PKC purified from honeybee brain (IC50 ≈ 10 μm) without affecting PKA and CaMII kinase activity. In brain homogenates it reduces MARCKS phosphorylation to below 10% of the phosphorylation in the absence of the inhibitor peptide (see Table 2). These data strongly confirm that MARCKS phosphorylation of honeybee brain homogenates is mediated exclusively by PKC.
Table 2.
Specificity of MARCKS phosphorylation and effect of the PKC inhibitor peptide (19–31)
| Kinase activity | MARCKS phosphorylation | |
|---|---|---|
| Control (%) | + PKC inhibitor peptide (%) | |
| PKC (+ Ca and DAG) | 100 ± 6 (8) | 8 ± 3 (8)* |
| PKM (− Ca and DAG) | 95 ± 6 (6) | 9 ± 4 (6)* |
| Brain homogenate depleted of PKC activity (+ Ca and DAG) | 9 ± 5 (4)* | 8 ± 4 (4)* |
| PKA (+ cAMP) | 6 ± 5 (4)* | 5 ± 4 (4)* |
| CaMII (+ Ca and calmodulin) | 10 ± 5 (4)* | 9 ± 5 (4)* |
32P incorporation into MARCKS induced by comparable activities of kinases purified from honeybee brain and homogenates depleted of PKC activity (Altfelder and Müller, 1991; Altfelder et al., 1991; Müller, 1997a). PKM was generated by partial proteolysis of purified PKC. Activities were measured in the absence and the presence of 100 μm PKC inhibitor peptide (19–31). Values were normalized with respect to the 32P incorporation into MARCKS induced by PKC (+ Ca and DAG). Means ± SEM are presented. The number of measurements is shown in parentheses. Values significantly different from the 32P incorporation into MARCKS induced by PKC are marked by an asterisk (p < 0.01; t test).
To discriminate between two different forms of PKC activation, (1) transient calcium-dependent activation and (2) constitutive calcium-independent activation, we measured PKC activity in either the presence or the absence of activators for classical PKC [calcium and diacylglycerol (DAG)]. All of the stimuli used for olfactory conditioning induce comparable transient PKC activation in the AL regardless of the stimuli and the sequence of application (Fig.2). A similar, although prolonged, transient activation also is induced by three forward or backward pairings with intertrial intervals (ITI) of 2 min (Fig. 2). In the absence of PKC activators (calcium and DAG) in the in vitroassay, the PKC activity is reduced to <5% of the activity measured in presence of the activators. This suggests that in vivostimulation during conditioning causes a transient calcium-dependent activation of PKC in the AL.
Fig. 2.
PKC activation induced by stimuli used in olfactory conditioning. Animals received either a singleCS or US, a single forward (CS-US) or backward pairing (US-CS), or three forward or backward pairings delivered with an intertrial interval (ITI) of 2 min. Animals were dissected for the determination of PKC activity in the antennal lobes (AL) either immediately (0), 1.5, or 10 min after stimulation. In each experiment, values were normalized to PKC activity in unstimulated animals (−). Each column represents the mean ± SEM of n measurements as indicated by thenumbers on the bars. For all stimulations PKC activity at 0 is significantly different from PKC activity in unstimulated animals (p < 0.05;t test).
To confirm that PKC activation after stimulation takes place in vivo and is not produced during handling in vitro(i.e., during homogenization, phosphorylation, etc.), we analyzed thein vivo effect of PKC inhibitors on PKC activity. Of various available PKC inhibitors (chelerythrin, hypericin, calphostin C, GF 109203x, and sphingosin) only Gö 7874 (Kleinschroth et al., 1995) led to a specific and dose-dependent reduction of calcium-dependent activation of PKC when it was used in vitro(IC50 ≈ 0.5 μm). Interestingly, calcium-independent PKC activity of PKM was not blocked significantly by Gö 7874 (10 μm) in vitro (<10% reduction in MARCKS phosphorylation; p > 0.05;t test). Using appropriate substrates (Altfelder et al., 1991; Müller, 1997b), we showed that Gö 7874 at a concentration of 10 μm did not affect the activity of PKA and CaMII kinase purified from honeybee brain in vitro. In vivo application of Gö 7874 (10 μm final concentration) did not affect PKC activity measured in the AL of unstimulated animals in the presence of calcium and DAG. However, stimulus-induced PKC activation (Fig. 2) was fully suppressed in Gö 7874-treated animals (Table1). This, together with the finding that Gö 7874 does not inhibit calcium-independent PKC activity in vitro, suggests that Gö 7874 selectively interferes with calcium-dependent activation of PKC. Independent of the stimulation the animals received, the addition of PKC peptide inhibitor (100 μm) to the phosphorylation mixture strongly reduced MARCKS phosphorylation (Tables2, 3). This confirms that changes in MARCKS phosphorylation induced by in vivo stimulation reflect PKC activity.
Table 1.
In vivo effect of PKC inhibitor Gö 7874 on the transient, stimulus-induced PKC activation
| Stimulation | PBS | Gö 7874 |
|---|---|---|
| − | 1.00 ± 0.05 (20) | 1.12 ± 0.06 (21) |
| CS | 1.36 ± 0.05 (32)* | 0.95 ± 0.04 (21) |
| US | 1.32 ± 0.06 (16)* | 1.05 ± 0.07 (12) |
| 1× (CS-US) | 1.43 ± 0.06 (21)* | 1.06 ± 0.08 (7) |
| 1× (US-CS) | 1.36 ± 0.05 (38)* | 0.96 ± 0.06 (6) |
| 3× (CS-US) | 1.47 ± 0.05 (21)* | 1.00 ± 0.08 (8) |
| 3× (US-CS) | 1.42 ± 0.06 (15)* | n.d. |
Animals were injected with 1 μl Gö 7874 (1 mm) or PBS and received different stimulations 20 min later [(CS-US), forward pairing; (US-CS), backward pairing]. AL were prepared immediately after stimulation, and PKC activity was determined in the presence of calcium and DAG. In each experiment, values were normalized to PKC activity measured in PBS-injected, unstimulated animals. Mean values are presented as SEM. The number of measurements is shown in parentheses. Values significantly different from the corresponding PKC activity in unstimulated animals are marked by an asterisk (p < 0.05; t test).
Table 3.
Effect of the PKC inhibitor peptide (19–31) on stimulus-induced MARCKS phosphorylation in the AL
| Stimulation | Reduction of MARCKS phosphorylation by PKC inhibitor peptide | |
|---|---|---|
| − (Ca, DAG) (%) | + (Ca, DAG) (%) | |
| − | 92 ± 4 (8)* | 90 ± 3 (8)* |
| CS | 91 ± 4 (4)* | 91 ± 4 (4)* |
| US | 89 ± 5 (4)* | 90 ± 5 (4)* |
| 3 hr after one trial | 92 ± 5 (4)* | 95 ± 4 (4)* |
| 3 hr after three trials | 95 ± 5 (4)* | 91 ± 4 (4)* |
| 18 hr after one trial | 94 ± 5 (4)* | 94 ± 5 (4)* |
| 18 hr after three trials | 92 ± 4 (4)* | 93 ± 5 (4)* |
Animals were dissected unstimulated (−), directly after stimulation (CS or US), or at 3 hr, respectively, 24 hr after training (one or three trials). MARCKS phosphorylation was measured in the presence or absence of (100 μm) PKC inhibitor peptide (19–31). Mean values ± SEM indicate the reduction of kinase activity by PKC inhibitor peptide. The number of independent measurements is shown in parentheses. Values that are significantly different from the corresponding values in the absence of the PKC inhibitor peptide are marked by an asterisk (p < 0.01; t test).
Inhibition of transient PKC activation during conditioning does not interfere with associative olfactory learning
Forward and backward pairings both result in a comparable activation of PKC in the AL. This suggests that PKC might not be essential for the induction of memory during acquisition. To test this hypothesis, we investigated the effect of the PKC inhibitor Gö7874 on the acquisition and retention of memory induced by single- or multiple-trial conditioning. In neither case was the conditioned PER of Gö 7874-injected animals significantly different from that of PBS-injected control animals (Table 4). Thus, inhibition of the transient calcium-dependent activation of PKC during conditioning (Table 1) seems not to interfere with the mechanisms of associative learning.
Table 4.
Inhibition of transient PKC activation during conditioning does not affect associative olfactory memory
| Conditioning | 1× (CS-US) | 3× (CS-US) | ||
|---|---|---|---|---|
| Injection | PBS | Gö7874 | PBS | Gö 7874 |
| 1 trial | 0.03 (79) | 0.00 (49) | 0.05 (76) | 0.02 (67) |
| 2 trial | — | — | 0.49 (77) | 0.47 (69) |
| 3 trial | — | — | 0.61 (78) | 0.69 (70) |
| 3 hr | 0.46 (38) | 0.51 (40) | 0.74 (43) | 0.83 (40) |
| 18 hr | 0.35 (60) | 0.38 (30) | 0.63 (46) | 0.62 (43) |
| 2 d | 0.52 (52) | 0.52 (35) | 0.75 (38) | 0.65 (38) |
| 3–4 d | 0.52 (44) | 0.45 (29) | 0.79 (24) | 0.63 (32) |
Animals were injected with 1 μl Gö 7874 (1 mm) or PBS and received one [1× (CS-US)] or three forward pairings [3× (CS-US)] 20 min later. The data show the probability of the conditioned PER during the acquisition and at different times after conditioning (the number of animals is shown in parentheses). The values of Gö 7874-injected animals are in no case significantly different from the corresponding values from control animals (PBS) (p > 0.05; χ2test).
Learning-induced long-lasting changes in PKC activation
Training-induced long-lasting changes in PKC activation as well as in PKC distribution have been observed in various species (Bank et al., 1988; Olds et al., 1989; Burchuladze et al., 1990; Van der Zee et al., 1992; Golski et al., 1995). In these studies it has been shown that learning induces changes of membrane-bound PKC activity or of PKC activity in subcellular fractions in the range of 10–40% of the corresponding values from control animals. Therefore, we have analyzed changes in total PKC activity in the AL of honeybees at different times after single- and three-trial conditioning. Only bees that showed conditioned PER after conditioning were used. This enabled us to compensate for the distinct levels in conditioned PER, depending on the number of conditioning trials, and to detect potential differences in the mechanisms underlying single- and multiple-trial conditioning. Untreated animals and animals that had received the corresponding backward pairings (US-CS), which cause no excitatory learning, were used as controls.
No change in PKC activity was detectable in animals of the control groups at different times after stimulation (data not shown). The same holds for animals that showed conditioned PER after single-trial conditioning, which induces sLTM (Fig.3). In contrast, after three-trial conditioning, which induces mLTM, a characteristic change of PKC activity was detected. This learning-induced MARCKS phosphorylation is PKC-specific, because it is strongly inhibited by the addition of the PKC inhibitor peptide to the phosphorylation assay (Table 3). The decrease in PKC activity observed ∼30 min after training was highly variable and not significantly different from controls. Increased PKC activity was detected first at 1 hr and reached its maximum ∼3 hr after training. It remained at this elevated level until 3 d after conditioning (Fig. 3). However, at 4 and 5 d after conditioning, PKC activity was not distinguishable from the activity measured in untrained animals. The observed 20–30% increase of total PKC activity is in agreement with changes within the same range observed for membrane-associated PKC in other systems (Bank et al., 1988; Olds et al., 1989; Burchuladze et al., 1990; Golski et al., 1995). The specific change in PKC activity, only detected in animals that show a conditioned PER after three-trial conditioning, indicates a role of the long-lasting elevation of PKC activity in the formation of this distinct memory.
Fig. 3.
Time course of PKC activity after one and three conditioning trials. PKC activity in the AL was determined at different times after one or three conditioning trials (ITI, 2 min). In each experiment, values were normalized to PKC activity in unstimulated naive animals (dashed line). Each pointrepresents the mean ± SEM of n measurements. Values that are significantly different from PKC activity in unstimulated naive animals are marked by an asterisk(p < 0.05; t test).
Long-lasting PKC activation is attributable to constitutively active PKC
Evidence from other studies suggests that PKC activation after learning involves the formation of a constitutively active form of PKC that is independent of calcium and DAG (Klann et al., 1991, 1993). To distinguish between transient and constitutive activity, we measured PKC activity in the presence and absence of calcium and DAG. Again, the MARCKS phosphorylation measured either in the presence or in the absence of calcium and DAG is PKC-specific, because it is strongly reduced by the addition of the PKC inhibitor peptide to the phosphorylation assay (Table 3).
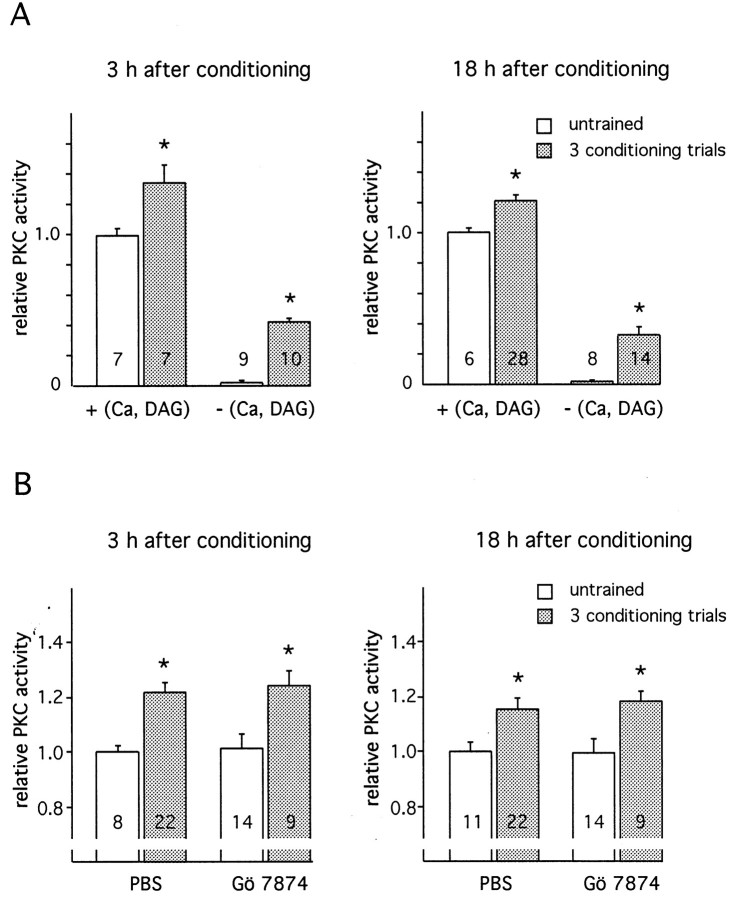
Whereas constitutive activity is marginal in untrained animals (<5% of basal calcium-dependent PKC activity), three-trial conditioning produces an at least 600% increase in constitutive activity, as compared with that in untrained animals (Fig.4A). This increase is similar at 3 and 18 hr after conditioning and corresponds to the learning-induced elevation of PKC activity measured in the presence of calcium and DAG. Hence, the constitutive PKC activity may be fully responsible for the long-lasting PKC activation induced by conditioning. Whereas the transient activation of PKC induced by single or paired stimuli is blocked in the presence of the PKC inhibitor Gö 7874 (Table 1), long-lasting activation 3 and 18 hr after conditioning is not susceptible to this inhibition (Fig.4B). Thus, the calcium independence and the lack of inhibition by Gö 7874 support the hypothesis that long-lasting and transient PKC activation are mediated by different mechanisms.
Fig. 4.
Characterization of the long-lasting PKC activation induced by three-trial conditioning. A, PKC activity in the AL was measured at 3 and 18 hr after three-trial conditioning (ITI, 2 min) in the presence or absence of calcium and diacylglycerol (DAG). In a control group of untrained animals the PKC activity was measured by following the same procedure as in trained animals. In each experiment, values were normalized with respect to PKC activity in untrained animals, measured in the presence of calcium and DAG. B, Animals were dissected at 3 and 18 hr after three-trial conditioning. Conditioned animals were each injected with 1 μl of either Gö 7874 (1 mm) or PBS 20 min before dissection. Untrained animals were handled in parallel in the same way as trained animals. In all experimental groups the PKC activity was measured in the presence of calcium and DAG. In each experiment, values were normalized with respect to PKC activity in PBS-injected untrained animals. Each column represents the mean ± SEM of n measurements as indicated by the numbers on the bars(*p < 0.05; t test).
PKC activation after three-trial conditioning is separable into two mechanistically different phases
Because PKC activation is induced as early as 1 hr after three-trial conditioning but remains stable until 3 d later, different mechanisms may be responsible for the elevation at different times after conditioning. Permanent membrane translocation and proteolytic formation of the catalytic fragment PKM have been proposed in this regard (Akers et al., 1986; Klann et al., 1993; Sacktor et al., 1993). However, the observed long-lasting increase in PKC activity (see Fig. 3) should, finally, require a change of gene expression. We investigated the contribution of these different mechanisms to long-lasting PKC activation for two time intervals: 2–4 hr (3 hr) and 14–24 hr (18 hr) after conditioning.
To test whether long-lasting PKC activation involves membrane translocation, we quantified PKC with antibodies in soluble and particulate fractions of AL homogenate at different times after conditioning. Using the ELISA technique (Müller, 1997b), we detected no change in total PKC expression or PKC distribution (data not shown). Nevertheless, subtle changes that can cause drastic alterations of PKC activity, which are not detected by this assay, may occur. It is also possible that the learning-induced changes of PKC activity described above are attributable to PKC isoforms that are not detected with the antibodies that were used. To detect a possible formation of the constitutively active fragment PKM directly, we performed immunoblot analysis. Unfortunately, none of the presently available antibodies against honeybee PKC did detect PKM.
Because the direct detection of PKM was not possible, the in vivo effect of protease inhibitors on conditioning-induced PKC activation was investigated. The formation of PKM probably is mediated by the thiol protease calpain (Suzuki et al., 1992). Calpain homologs have been purified from honeybee brains and can be inhibited by the thiol protease inhibitor E 64 (Müller and Altfelder, 1991) (our unpublished results). E 64 was injected before conditioning, because the induction of a proteolytic mechanism in LTP was described as taking place during or shortly after training (Sacktor et al., 1993). Elevation of PKC activity 3 hr after conditioning was blocked in E 64-treated bees, as compared with that in PBS-injected control animals (Fig. 5A). However, 18 hr after conditioning, PKC activation in E 64-treated bees was not distinguishable from that in PBS-injected controls. PKC activity in untrained animals was not changed by E 64. Hence, a proteolytic mechanism is required for the induction of PKC activation during the first hour after conditioning.
Fig. 5.
Effect of E 64, actinomycin D, and anisomycin/cycloheximide on conditioning-induced PKC activation.A, Animals were injected with 1 μl of the thiol protease inhibitor E 64 (1 mm) or PBS 20 min before three-trial conditioning (ITI, 2 min). PKC activity was measured at 3 and 18 hr after conditioning. B, Animals were injected with 1 μl of actinomycin D (2 mg/ml), anisomycin/cycloheximide (CHX; 2.5 respectively, 5 mg/ml), or PBS 1 hr after three-trial conditioning. PKC activity was measured at 3 and 18 hr after conditioning. PKC activity was determined in parallel in groups of untrained animals, injected according to the protocol for trained animals. In each experiment, values were normalized to PKC activity in PBS-injected untrained animals. Each column represents the mean ± SEM of n measurements as indicated by the numbers on the bars(*p < 0.05; t test).
To investigate the contribution of gene expression to PKC activation after conditioning, we tested transcription and translation inhibitors for their ability to interfere with PKC activation in vivo. We assumed that RNA and protein synthesis are not required during, but after, training. Therefore, inhibitors of RNA (actinomycin D) and protein synthesis (a mixture of cycloheximide and anisomycin) were injected 1 hr after training. Actinomycin D did not interfere with PKC activation 3 hr after conditioning. However, 18 hr after conditioning, no activation of PKC was measured in bees treated with transcription or translation inhibitors. Taken together, these data suggest that the early phase of conditioning-induced PKC activation is dependent on proteolysis and is not required for the induction of the late phase of conditioning-induced PKC activation, which requires the synthesis of RNA and proteins.
Memory formation after three-trial conditioning also can be dissected into different phases
To test whether the long-lasting PKC activation is related functionally to olfactory learning, we analyzed E 64 and actinomycin D, which suppress PKC activation at different times after multiple-trial conditioning, with regard to their effect on memory formation.
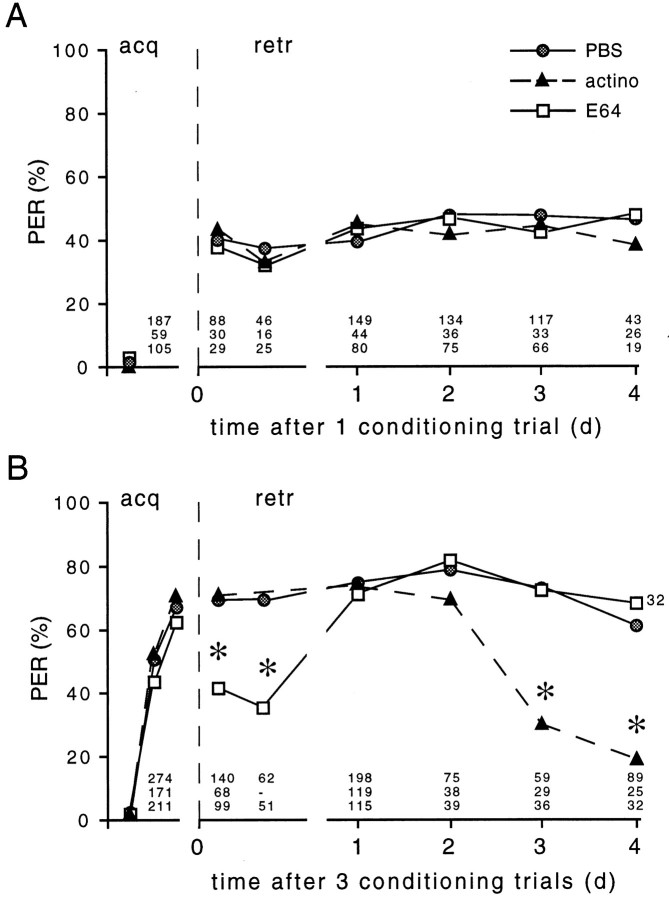
Whereas E 64 does not affect the conditioned PER at any time after single-trial conditioning, it significantly reduces the conditioned PER during the first hour after three-trial conditioning (Fig.6). The acquisition, however, does not differ from that of PBS-injected animals. Interestingly, the effect of E 64 on the conditioned PER, similar to the suppression of PKC activation by E 64, is transient, and the conditioned PER tested at times longer than 1 d after three-trial conditioning is not compromised by E 64. The coincidence of these time windows suggests that the proteolysis-dependent PKC activation might contribute selectively to an early phase of multiple-trial induced memory.
Fig. 6.
Dissection of memory phases by inhibition of thiol proteases or transcription. Animals were injected with 1 μl of PBS or 1 μl of the thiol protease inhibitor E 64 (1 mm) 20 min before conditioning, or they were injected with 1 μl of PBS or actinomycin D (2 mg/ml) 1 hr after conditioning. Animals received either one conditioning trial (A) or three conditioning trials (B). The probability of the CS-elicited proboscis extension response (PER) was tested during acquisition (acq) and at different times after conditioning (retr). The PER of animals that received PBS injection before or after conditioning did not differ between the groups. Thus the values were combined in the PBS control group. The data show the PER of n animals, as indicated. Sequence from top to bottom: PBS, actino, and E 64 (*p < 0.001; χ2test).
Actinomycin D, like E 64, does not change the conditioned PER after single-trial conditioning (Fig. 6). Although actinomycin D suppresses the increase in PKC activity already at 1 d after conditioning, the conditioned PER of actinomycin-treated animals is not affected until 3 d after conditioning. Thus, the late phase of PKC activation in the AL, requiring RNA and protein synthesis, does not coincide with a defect in memory formation.
DISCUSSION
In this study we have investigated the role of PKC in associative learning and memory in the honeybee. The method of shock freezing the bees in liquid nitrogen allowed us to preserve the in vivostate of activation of PKC and to measure directly the PKC activity in homogenates of the frozen AL by specific substrate phosphorylation. The MARCKS protein serves as a highly specific substrate for PKC from honeybee brain (Müller, 1997a). Using the PKC inhibitor peptide (19–31), we demonstrated that MARCKS is phosphorylated by calcium-dependent as well as calcium-independent PKC (Table 2). Thus, we were able to monitor changes in both forms of PKC activity. Interestingly, the PKC inhibitor Gö 7874 inhibits only calcium-dependent PKC activity and is not effective on calcium-independent PKC in vitro. Thus, Gö 7874 was used to distinguish between these different forms of PKC activation. However, a detailed analysis of the calcium-dependent and calcium-independent PKC isoforms in the honeybee is still missing.
Although this method allowed us to determine the time course of PKC activation with a resolution of ∼1 min, we did not detect a learning-specific modulation of PKC activity at the stage of acquisition. Single and combined stimuli, used in olfactory conditioning, evoked a comparable, transient, and second messenger-dependent activation of PKC, irrespective of whether the stimulations resulted in the induction of memory or not. Interestingly,in vivo inhibition of PKC during conditioning did not affect acquisition and memory at different times after training (Table 4). These findings suggest that the activation of PKC immediately after conditioning is not essential for the induction of memory but, rather, may be attributed to a role for PKC in the processing of chemosensory information in the AL in general. In contrast, three-trial conditioning that promotes the formation of mLTM specifically leads to a long-lasting but reversible activation of PKC from 1 hr up to 3 d after conditioning.
This is in agreement with behavioral studies in vertebrates, in which PKC inhibitors interfered with memory formation, whereas the acquisition of memory was not compromised (Burchuladze et al., 1990;Zhao et al., 1994). A membrane translocation and regional redistribution of PKC in the hippocampus were detected within 1 hr, but also 1 d or more after learning, suggesting that learning might lead to a long-lasting activation of PKC (Bank et al., 1988; Olds et al., 1989, 1990; Scharenberg et al., 1991; Van der Zee et al., 1992;Golski et al., 1995). However, it was not shown whether these PKC-related changes are reversible.
The role and the time course of PKC activity were investigated in more detail in the vertebrate model system for synaptic plasticity, LTP. Although PKC is not involved in the initial induction, it contributes to the expression of LTP at later times (Colley et al., 1989; Roberson et al., 1996). A long-lasting activation of PKC in LTP was demonstrated by using assays for kinase activity, and PKC inhibitor studies confirmed the requirement for ongoing PKC activity during the first hour after LTP induction (Lovinger et al., 1987; Colley et al., 1990;Klann et al., 1991, 1993; Wang and Feng, 1992). Interestingly, injection of the PKC inhibitor polymyxin B (PMXB) before the induction of LTP did not affect initial potentiation but led to a decay of LTP within 2 hr after induction. This decay also was observed when PMXB was injected 15 or 30 min after LTP induction. Another inhibitor, H7, which interacts with the catalytic domain of PKC, was effective even when it was injected up to 4 hr after LTP induction (Colley et al., 1990). These data suggest the existence of two postinitiation components of PKC activation in LTP.
Permanent membrane translocation, proteolytic removal of the regulatory region leading to generation of constitutively active PKM, and phosphorylation of PKC may be responsible for persistent activation of PKC (Akers et al., 1986; Suzuki et al., 1992; Klann et al., 1993;Sacktor et al., 1993; Powell et al., 1994; Osten et al., 1996). However, it remains unclear how these mechanisms are related to the function of PKC in learning in vivo.
In Drosophila, the role of PKC was investigated only for the associative conditioning of courtship: transgenic flies with reduced PKC activity do not express initial learning in their behavior, although the expression of memory after conditioning is not compromised (Kane et al., 1997). These data suggest that the immediate performance of the task during acquisition and the induction of memory formation may be mediated by different mechanisms acting in parallel. However, in these transgenic flies PKC is inhibited permanently, and the effect of a precisely timed inhibition of PKC during or at different times after conditioning cannot be determined.
In the AL of the honeybee, we have dissected different mechanisms contributing to long-lasting PKC activation at distinct times after conditioning. A proteolytic mechanism, probably involving the thiol protease calpain, is needed during training for the induction of PKC activation in the range of just a few hours after conditioning, whereas PKC activation ∼18 hr after conditioning is dependent on RNA and protein synthesis. Both mechanisms are independent of each other (see Fig. 5).
The dissection of memory phases in Drosophila has led to the concept that memory, at a distinct time after learning, may consist of mechanistically different components (Tully et al., 1990, 1994). In the honeybee it has been demonstrated that, depending on the numbers of conditioning trials, at least two distinguishable memories are induced: mLTM induced by multiple-trial conditioning is strongly reduced by NOS inhibition, whereas sLTM, induced by single-trial conditioning, is not affected by this treatment (Müller, 1996). Our data now present additional evidence that PKC also contributes specifically to mLTM: (1) only multiple-trial conditioning induces a long-lasting, but reversible, activation of PKC, which depends on an early proteolytic mechanism and requires RNA and protein synthesis in the late phase; (2) inhibition of these mechanisms selectively interferes with distinct phases of mLTM.
However, only the protease inhibitor E 64 has a coinciding effect on memory and on suppression of PKC activation, whereas the effect of actinomycin D affects memory and PKC activation in different time windows (compare Figs. 5 and 6). Because PKC activity was determined exclusively for the AL whereas drug injection was performed systemically, these results may reflect directly the contribution of the AL to distinct phases of associative learning.
Because E 64 exerts its action on PKC and PER within coinciding time windows, the activation of the PKC in the AL may contribute to an early phase of mLTM. Interestingly, application of E 64 selectively suppresses the conditioned PER in the range of hours, but neither interferes with acquisition nor with the late phase of mLTM (Fig. 6). Thus, the rapidly induced proteolysis-dependent activation of PKC in the AL might serve to bridge the time gap until other RNA- and protein synthesis-dependent mechanisms take effect.
The RNA and protein synthesis-dependent PKC activation in the AL, however, seems to have no immediate effect on memory formation. This suggests that, in the range of days after conditioning, other brain areas than the AL are involved predominantly in memory formation. This is in agreement with findings that the AL and the MB seem to contribute to different features of memory formation (Erber et al., 1980; Hammer and Menzel, 1995). Therefore, the late phase of PKC activation in the AL may be only one of several parallel mechanisms that contribute to the formation of mLTM with different time courses and occur in different brain compartments. Although we have evidence that the proteolytic activation of PKC in the AL contributes to an early phase of mLTM, future analysis will reveal the role of PKC in brain areas like the MB and its interaction with the RNA and protein synthesis-dependent PKC activation in the AL.
An interesting speculation is that long-time activation of PKC may depend on a preceding activation of PKA. PKA and its nuclear substrate, cAMP response element-binding proteins (CREB), have been shown to mediate changes in gene expression in long-term facilitation, late LTP, and LTM (Frey et al., 1993; Kaang et al., 1993; Bourtchouladze et al., 1994; Yin et al., 1994; Abel et al., 1997). Future analysis will have to show whether PKC is a direct target of conditioning-induced gene expression or whether the expression of another protein could be responsible for constitutive PKC activation. Moreover, it is of special interest to learn how these changes in PKC activity affect the phosphorylation and function of substrates and thus finally contribute to structural changes in LTM.
Footnotes
This investigation was supported by the Deutsche Forschungsgemeinschaft (SFB 515/C3). We would like to thank R. Menzel for helpful suggestions on an earlier version of this manuscript and D. Alexander and S. Meuser for their help with the preparation of this manuscript.
Correspondence should be addressed to Dr. Uli Müller, Institut für Neurobiologie der Freien Universität Berlin, Königin-Luise-Strasse 28/30, D-14195 Berlin, Germany.
REFERENCES
- 1.Abel T, Nguyen PV, Barad M, Deuel AS, Kandel E, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 2.Akers RF, Lovinger DM, Colley PA, Linden DJ, Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986;231:587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- 3.Altfelder K, Müller U, Menzel R. Ca2+/calmodulin and Ca2+/phospholipid-dependent protein kinases in the neuronal tissue of the honeybee Apis Mellifera. Insect Biochem. 1991;21:479–486. [Google Scholar]
- 4.Bank B, DeWeer A, Kuzirian AM, Rasmussen H, Alkon DL. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci USA. 1988;85:1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of facilitation in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 7.Burchuladze R, Potter J, Rose SPR. Memory formation in the chick depends on membrane-bound protein kinase C. Brain Res. 1990;535:131–138. doi: 10.1016/0006-8993(90)91831-z. [DOI] [PubMed] [Google Scholar]
- 8.Colley PA, Sheu F-S, Routtenberg A. Dose-dependent phorbol ester facilitation or blockade of hippocampal long-term potentiation: relation to membrane/cytosol distribution of protein kinase C activity. Brain Res. 1989;495:205–216. doi: 10.1016/0006-8993(89)90214-x. [DOI] [PubMed] [Google Scholar]
- 9.Colley PA, Sheu F-S, Routtenberg A. Inhibition of protein kinase C blocks two components of LTP persistence, leaving initial potentiation intact. J Neurosci. 1990;10:3353–3360. doi: 10.1523/JNEUROSCI.10-10-03353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erber J, Masuhr T, Menzel R. Localization of short-term memory in the brain of the honeybee Apis mellifera. Physiol Entomol. 1980;5:343–358. [Google Scholar]
- 11.Farley J, Schuman E. Protein kinase C inhibitors prevent induction and continued expression of cell memory in Hermissenda type B photoreceptors. Proc Natl Acad Sci USA. 1991;88:2016–2020. doi: 10.1073/pnas.88.5.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey U, Huang Y-Y, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 13.Golski S, Olds JL, Mishkin M, Olton DS, Alkon DL. Protein kinase C in the hippocampus is altered by spatial but not cued discriminations: a component task analysis. Brain Res. 1995;676:52–62. doi: 10.1016/0006-8993(95)00080-a. [DOI] [PubMed] [Google Scholar]
- 14.Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt H, Müller U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J Neurobiol. 1995;27:44–50. doi: 10.1002/neu.480270105. [DOI] [PubMed] [Google Scholar]
- 16.Kaang BK, Kandel ER, Grant SG. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- 17.Kane NS, Robichon A, Dickinson JA, Greenspan RJ. Learning without performance in PKC-deficient Drosophila. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- 18.Klann E, Chen S, Sweatt JD. Persistent protein kinase activation in the maintenance phase of long-term potentiation. J Biol Chem. 1991;266:24253–24256. [PubMed] [Google Scholar]
- 19.Klann E, Chen S-J, Sweatt JD. Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci USA. 1993;90:8337–8341. doi: 10.1073/pnas.90.18.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinschroth J, Hartenstein J, Rudolph C, Schächtele C. Novel indolocarbazole protein kinase C inhibitors with improved biochemical and physicochemical properties. Bioorg Med Chem Lett. 1995;5:55–60. [Google Scholar]
- 21.Lovinger DM, Wong KL, Murakami K, Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987;436:177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- 22.McPhie DL, Matzel LD, Olds JL, Lester DS, Kuzirian AM, Alkon DL. Cell specificity of molecular changes during memory storage. J Neurochem. 1993;60:646–651. doi: 10.1111/j.1471-4159.1993.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 23.Menzel R. Learning in honeybees in an ecological and behavioral context. In: Hölldobler B, Lindauer M, editors. Experimental behavioral ecology. Fischer; Stuttgart, Germany: 1985. pp. 55–74. [Google Scholar]
- 24.Menzel R. Learning, memory and “cognition” in honeybees. In: Kesner RP, Olten DS, editors. Neurobiology of comparative cognition. Erlbaum; Hillsdale, NY: 1990. pp. 237–292. [Google Scholar]
- 25.Menzel R, Müller U. Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- 26.Menzel R, Durst C, Erber J, Eichmüller S, Hammer M, Hildebrandt H, Mauelshagen J, Müller U, Rosenboom H, Rybak J, Schäfer S, Scheidler A. The mushroom bodies in the honeybee: from molecules to behaviour. In: Schildberger K, Elsner N, editors. Neural basis of behavioural adaptations. Fischer; Stuttgart, Germany: 1994. pp. 81–102. [Google Scholar]
- 27.Müller U. Inhibition of nitric oxide synthase impairs a distinct form of long-term memory in the honeybee, Apis mellifera. Neuron. 1996;16:541–549. doi: 10.1016/s0896-6273(00)80073-2. [DOI] [PubMed] [Google Scholar]
- 28.Müller U. Insect 86 kDa protein kinase C substrate is a filament-interacting protein regulated by Ca2+/calmodulin and phosphorylation. Brain Res. 1997a;757:24–30. doi: 10.1016/s0006-8993(96)01435-7. [DOI] [PubMed] [Google Scholar]
- 29.Müller U. Neuronal cAMP-dependent protein kinase type II is concentrated in mushroom bodies of Drosophila melanogaster and the honeybee Apis mellifera. J Neurobiol. 1997b;33:33–44. doi: 10.1002/(sici)1097-4695(199707)33:1<33::aid-neu4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Müller U, Altfelder K. The Ca2+-dependent proteolytic system—calpain-calpastatin—in the neural tissue of the honeybee Apis mellifera. Insect Biochem. 1991;21:473–477. [Google Scholar]
- 31.Müller U, Hildebrandt H. The nitric oxide/cGMP system in the antennal lobe of Apis mellifera is implicated in integrative processing of chemosensory stimuli. Eur J Neurosci. 1995;7:2240–2248. doi: 10.1111/j.1460-9568.1995.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 32.Olds JL, Anderson ML, McPhie DL, Staten LD, Alkon DL. Imaging of memory-specific changes in the distribution of PKC in the hippocampus. Science. 1989;245:866–869. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- 33.Olds JL, Golski S, McPhie DL, Olton D, Mishkin M, Alkon DL. Discrimination learning alters the distribution of protein kinase C in the hippocampus of rats. J Neurosci. 1990;10:3707–3713. doi: 10.1523/JNEUROSCI.10-11-03707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mζ in long-term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell CM, Johnston D, Sweatt JD. Autonomously active protein kinase C in the maintenance phase of N-methyl-d-aspartate receptor-independent long-term potentiation. J Biol Chem. 1994;269:27958–27963. [PMC free article] [PubMed] [Google Scholar]
- 36.Roberson ED, English JD, Sweatt JD. A biochemist’s view of long-term potentiation. Learn Memory. 1996;3:1–24. doi: 10.1101/lm.3.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharenberg AM, Olds JL, Schreurs BG, Craig AM, Alkon DL. PKC redistribution within CA3 stratum oriens occurring during acquisition of NM conditioning in the rabbit. Proc Natl Acad Sci USA. 1991;88:6637–6641. doi: 10.1073/pnas.88.15.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sossin WS, Sacktor TC, Schwartz JH. Persistent activation of protein kinase C during the development of long-term facilitation in Aplysia. Learn Memory. 1994;1:189–202. [PubMed] [Google Scholar]
- 40.Sugita S, Goldsmith JR, Baxter DA, Byrne JD. Involvement of protein kinase C in serotonin-induced spike broadening and synaptic facilitation in sensorimotor connections of Aplysia. J Neurophysiol. 1992;68:643–651. doi: 10.1152/jn.1992.68.2.643. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Okumura-Noji K, Ogura A, Tanaka R, Nakamura K, Kudo Y. Calpain may produce a Ca2+-independent form of kinase C in long-term potentiation. Biochem Biophys Res Commun. 1992;189:1514–1520. doi: 10.1016/0006-291x(92)90247-i. [DOI] [PubMed] [Google Scholar]
- 42.Tully T, Boynton S, Brandes C, Dura JM, Mihalek R, Preat T, Villella A. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harbor Symp Quant Biol. 1990;55:203–211. doi: 10.1101/sqb.1990.055.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila melanogaster. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 44.Van der Zee EA, Compaan JC, de Boer M, Luiten PGM. Changes in PKCγ immunoreactivity in mouse hippocampus induced by spatial discrimination learning. J Neurosci. 1992;12:4808–4815. doi: 10.1523/JNEUROSCI.12-12-04808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JH, Feng DP. Postsynaptic protein kinase-C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci USA. 1992;89:2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant-negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 47.Zhao WQ, Gibbs ME, Sedman GL, Ng KT. Effect of PKC inhibitors and activators on memory. Behav Brain Res. 1994;60:151–160. doi: 10.1016/0166-4328(94)90142-2. [DOI] [PubMed] [Google Scholar]