Abstract
The Drosophila mutants amnesiac,dunce (dnc), and rutabagawere isolated after associative conditioning tests, during which animals were trained to associate the presence of an odor with that of electric shocks (ES). In the absence of conditioning, the odor avoidance (OA) of these mutants was shown to be normal, indicating that their poor associative conditioning performance was attributable to specific learning or memory deficits. However, I show that the OA of the mutants is greatly decreased after their exposure to ES. This effect can last for hours. These results strongly suggest that part of the defect displayed by these mutants in associative conditioning tests does not correspond to a learning or memory deficit but might arise from abnormal sensitivity to stressful stimuli. I looked at the OA after ES of two previously characterized dncmutants. Df(1)N79f specifically decreases Dnc expression in the mushroom bodies, leading to a normal level of learning but decreased memory.Df(1)N79f mutants displayed a normal OA after ES. Df(1)N64j15 affects the entire brain expression of Dnc, leading to decreased learning and memory. Df(1)N64j15 animals showed a strong decrease of their OA after ES. Thus, the lack of Dnc “general” expression is most likely responsible for the OA defect, which would be responsible for the apparent learning defect after conditioning. In contrast, the Dnc phosphodiesterase accumulated in the mushroom bodies would be involved specifically in memory formation.
Keywords: Drosophila melanogaster, learning and memory mutants, cAMP, stress sensitivity, odor avoidance, conditioning controls
Several mutations affecting associative learning and memory have been characterized inDrosophila, including dunce (dnc),rutabaga (rut), amnesiac(amn), and linotte (lio) (Dudai et al., 1976, 1984; Quinn et al., 1979; Dura et al., 1993).dnc and rut encode, respectively, a phosphodiesterase and an adenylate cyclase (Chen et al., 1986; Levin et al., 1992), two enzymes involved with the cAMP pathway.amn encodes a putative neuropeptide that might regulate adenylate cyclase activity (Feany and Quinn, 1995), and lioencodes a putative tyrosine kinase (Dura et al., 1995) involved in adult brain development (Simon et al., 1998). Despite recent progress (Zhong and Wu, 1991; Qiu and Davis, 1993; Dauwalder and Davis, 1995), the precise physiological roles of the Drosophila proteins Amn, Dnc, and Rut are not fully understood. In particular, although Dnc and Rut accumulate in the mushroom bodies (Nighorn et al., 1991; Han et al., 1992), an insect structure involved in learning and memory (Erber et al., 1980; Davis, 1993; Hammer, 1993; de Belle and Heisenberg, 1994), they are also expressed in other parts of adult brain (Nighorn et al., 1991; Han et al., 1992) in which their possible roles are unknown.
The conditioning protocols originally used to isolate and characterize most of Drosophila learning and memory mutants associate an odor with electric shocks (ES) (Quinn et al., 1974; Tully and Quinn, 1985). Naturally, before the abnormal performance of the mutants could be linked to a learning or memory defect, it was shown that untrained mutants could react normally to the stimuli used for the conditioning and, in particular, that their odor avoidance (OA) was normal (Dudai et al., 1976, 1984; Quinn et al., 1979; Dura et al., 1993). However, the possibility that ES presentation during conditioning could itself affect odor perception was never explored. This issue is crucial when one needs to compare a putative learning or memory mutant with a reference wild-type stock, because the ES might differentially affect the two groups. Thus, to characterize a mutant deficient in associative learning or memory, it is essential to separate a bona fide learning or memory defect (related to the association of stimuli) from behavioral deficits merely related to abnormal perception of the stimuli after the conditioning treatment. I show here that the mutants amn, dnc, andrut display a very strong decrease of their OA after their exposure to ES. Two deficiencies affecting different sets ofdnc transcripts allowed the separation of memory defects from nonspecific deficits.
MATERIALS AND METHODS
All of the mutant stocks have a Canton-S background. This issue is important, because different wild-type backgrounds lead to different learning and memory aptitudes (Tully and Quinn, 1985). The three ethylmethane sulfonate mutations amn, dnc, andrut were originally induced in a Canton-S background. The background of the two P mutants rutP2080and lio1 was “cantogenized” by outcrossing w1118rutP2080/w1118females and w1118,lio1/+ females tow1118/Y (Canton-S) males for five generations. The lio2 mutant was cantogenized by outcrossing w1188,lio1/lio2 females to w1118/Y,lio1/lio1(Canton-S) males for five generations. ThewaDf(1)N79f/w+Y× CD(1), y w f andDf(1)N64j15/w+Y× CD(1), y w f (Canton-S) stocks were provided by Ronald Davis (Baylor College of Medicine, Houston, TX). A cantogenizedwaDf(1)N79f/w+Y× CD(1),y w f (Canton-S) stock was generated by outcrossing for nine generations waDf(1)N79f/w1118females to w1118/Y (Canton-S) males. Aw1118/w+Y× CD(1),y w f (Canton-S) stock was also generated.
For associative memory tests, flies were conditioned with the Pavlovian procedure developed by Tully and Quinn (1985), with a few adaptations. During training, groups of 50–100 flies were first exposed for 60 sec to a first odor (odor A) (either undiluted 3-octanol or 4-methylcyclohexanol), during which time they received ES (1.5 sec pulses of DC). After a 45 sec rest period, flies were exposed for 60 sec to the second odor (odor B), which was not paired with ES. Flies were then kept for 30 or 90 min in a vial with regular solid food. For memory testing, flies were transported to the choice point of a T maze, allowed to choose between the two odors for 120 sec, and counted. The performance index represents a normalized probability of correct choice. A score of 0 thus corresponds to a 50:50 distribution.
For OA tests, flies were treated in the upper chamber as for the associative conditioning, except that presentation of the second odor was omitted and replaced by exposure to air. For OA testing, treated animals were transported to the choice point of the T maze, allowed to choose between the new odor and air, and counted. A performance index was calculated as for associative conditioning. An index of 0 corresponds to a 50:50 distribution. An index of 100% corresponds to complete avoidance of the odor. Odors were used undiluted as by de Belle and Heisenberg (1994). Two groups of the same stock were run successively, and the side of the test tube with odor was alternated. To remove odor traces from the previous run before each new experiment and in the absence of flies, odor and fresh air, respectively, were aspirated through the relevant test tube for 1 min.
The correct perception of ES requires the presence of humid air (Tully and Quinn, 1985), and various voltage–humidity set-ups have been adopted as regular working conditions. In the present study, unless specified, 120 V–70% humidity was used (medium-humidity condition). A 60 V–90% humidity set-up was also used (high-humidity condition).
Statistical significance of the differences between two means, corresponding to mutant and control, were assessed with Student’st test. Comparisons among multiple means (see Fig. 1) were assessed with one-way ANOVA.
Fig. 1.
A, Half hour memory of normal [Canton-S (CS)] and mutant stocks. Flies were trained in the grid tube (position 1) and tested at the choice point (position 2). Bars represent mean ± SE performance index. These scores match previously published values (Tully and Quinn, 1985; Dura et al., 1993). **p < 0.01; ***p < 0.001; one-way ANOVA. n = 4 groups. B, OA of normal and mutant stocks after ES. OA to octanol (position 2) after presentation of ES combined with methylcyclohexanol (position 1) (see Materials and Methods). Left, CS,lio1, amn,rut1, anddnc1, 120 V were used together with medium humidity. Right, CS,lio2, andrutP2080, 60 V were used with high humidity. Note the similar scores displayed by Canton-S under both conditions. This experiment was performed blind as to genotype. *p < 0.05; **p < 0.01; ***p < 0.001; one-way ANOVA. n= 6 groups.
RESULTS
The olfactory associative conditioning protocol that produces the strongest learning scores consists of the following sequence (Fig.1A) (Tully and Quinn, 1985). A first odor is presented to a group of flies paired with pulses of ES. After a rest period, a second odor is presented in the absence of shock. To measure the association between the first odor and ES, flies are brought to a choice point from which they have free access to two compartments, each filled with one of the odors previously used during training. Animals, having learned and remembered the odor–ES association, will tend to avoid the corresponding odor.
A major drawback of this procedure is that flies make their olfactory choice after having received strong ES, which might itself induce behavioral changes unrelated to learning and memory, only indirectly affecting learning and memory performances. In particular, because unimpaired olfaction is a prerequisite for the correct interpretation of results in the olfactory associative conditioning protocol, I tested the OA of wild-type and mutant flies after presentation of ES (Fig.1B). In this test, a first repellent odor is presented to the flies in association with ES. The flies are then brought to the choice point, where their reactivity to a second repellent odor is measured. This protocol is thus similar to the associative conditioning procedure, but the second odor is not presented during the first phase, whereas the first odor, which has been associated with the shocks, is not presented during the test. Such control of the OA of the mutants is more relevant to the associative conditioning protocol than the direct test of naive animals. The fact that the second odor, used to test OA, is novel to the flies at the time of testing precludes interference from phenomena related to multiple presentations, such as habituation.
After presentation of ES combined with the first odor, the mutantsamn, dnc1,rut1, andrutP2080 displayed strongly reduced avoidance of a second odor compared with normal flies (Fig.1B). On the contrary,lio1 andlio2 behaved in the same way as wild-type flies. Thus, although naive amn, dnc, andrut mutants have been shown in previous studies to react normally to odors (Dudai et al., 1976, 1984; Quinn et al., 1979), their OA is dramatically reduced after presentation of ES, the stimulus normally used for aversive conditioning.
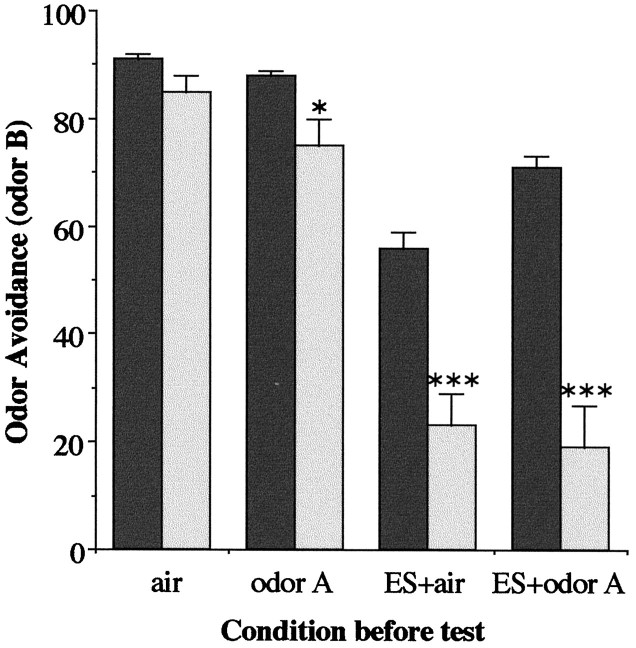
The amn mutant was chosen, together with the reference wild-type stock Canton-S, to analyze in more detail the effect of ES on OA. amn displays an abnormal OA to odor B after presentation of ES, whether ESs are delivered in combination with fresh air or with odor A (Fig. 2). This result indicates that ES presentation is the main cause of the abnormal OA and not preexposure to a first repellent odor. In the case of Canton-S, avoidance of odor B is less efficient when ES is associated with air rather than with odor A (Fig. 2). A plausible explanation for this is that Canton-S flies, having learned the association between the air flow and ES, tend to avoid air during the test. Consequently, to measure any learning-independent effects of ES on OA, it is imperative to present ES associated with odor A, a condition which will not be one of the options in the test.
Fig. 2.
OA of Canton-S (black) andamn (light gray) strains after various experimental conditions. Odor A is benzaldehyde, and the OA is measured to methylcyclohexanol. No difference is detected between Canton-S andamn after air presentation (t test;p = 0.054; t = 2.2). After presentation of odor A alone, amn avoidance is marginally reduced (t test; *p = 0.041; t = 2.3), whereas it is strongly reduced after presentation of ES (ES+air) (ttest; ***p = 0.0006; t = 4.9) or ES combined with odor A (ES+odor A) (ttest; ***p = 0.0007; t = 4.8).n = 6 groups.
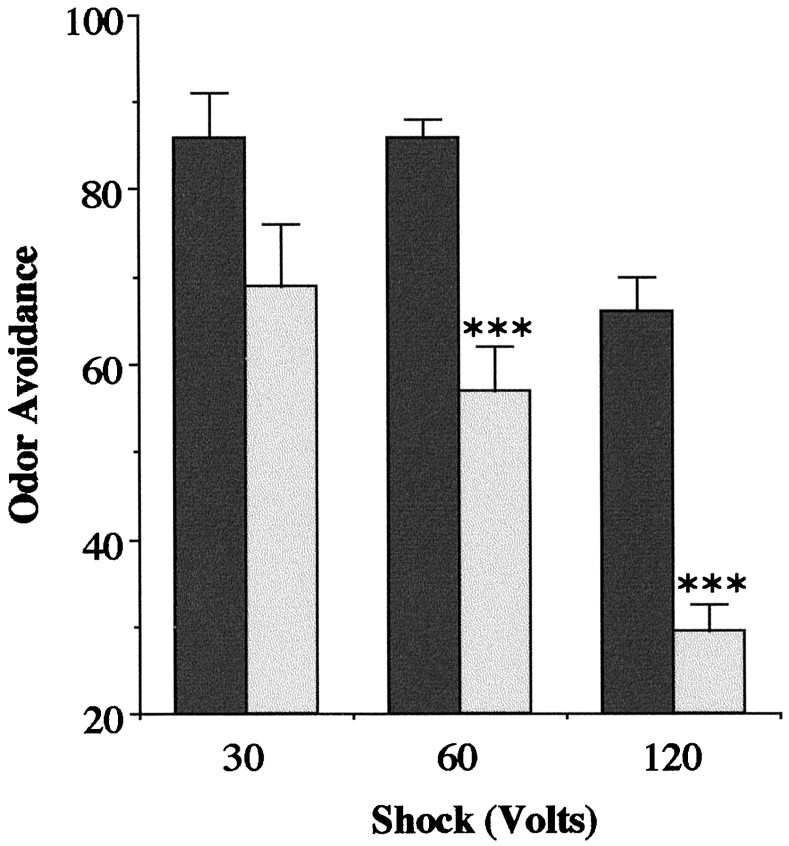
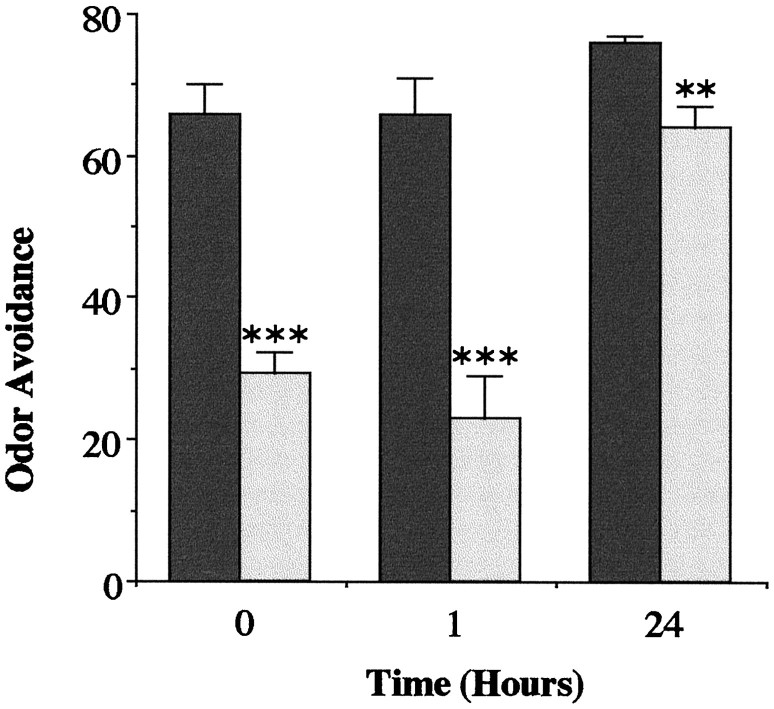
If amn displays a strong OA decrease after a 120 V ES presentation, Canton-S is also affected at this voltage (Fig.3). This is apparently attributable to the degree of sensitivity, because Canton-S is unaffected at 60 V (with medium humidity; see Materials and Methods), whereas amnflies are abnormal. Thus, it seems that the threshold for ES perturbation is lower in amn mutants than in normal flies. At this stage, the nature of the physiological changes induced by ES remains to be determined; ES could affect olfaction per se, or it could produce a more general effect on brain activity. Whatever the case, the concern is to understand to what degree the decreased OA affects the performance of the mutants after associative conditioning. In particular, could it account for part of the lasting deficit ofamn, which has been interpreted up to now as a memory problem? Indeed, testing OA 1 hr after ES presentation shows no amelioration of the amn defect (Fig.4), which has only partially recovered at 24 hr.
Fig. 3.
OA after ES of Canton-S (black) andamn (light gray) as a function of ES intensity. No difference is detected at 30 V (t test;p = 0.072; t = 2.0). A significant difference is detected at 60 V (t test; ***p = 0.0005; t = 5.1) and at 120 V (t test; ***p = 0.0001;t = 7.0). Odors as in Figure 2.n = 6 groups.
Fig. 4.
OA of Canton-S (black) andamn (light gray) as a function of the delay between ES delivery and testing. A significant difference is detected at all points: t = 0 hr, ttest; ***p = 0.0001; t = 7.0;t = 1 hr, t test; ***p = 0.0003; t = 5.3;t = 24 hr, t test; **p= 0.010; t = 3.7. Odors as in Figure 2.n = 6 groups, except n = 4 groups at 24 hr.
A fundamental issue now is to differentiate learning and memory defects from unrelated deficits. The Dnc phosphodiesterase and the Rut adenylyl cyclase are preferentially expressed in the mushroom bodies, but these enzymes are also found in other parts of the brain (Nighorn et al., 1991; Han et al., 1992). The mushroom body expression might indeed be related to learning and/or memory, whereas the “general” expression might be involved in the nonassociative response described here. To test this hypothesis, I looked at the OA after ES of two dncdeficiencies, which remove different sets of dnc transcripts (Qiu and Davis, 1993). The first deficiency,Df(1)N79f, dramatically decreased Dnc expression in the mushroom bodies. Individuals carrying this deficiency displayed a normal OA after ES (Fig. 5). On the contrary, Df(1)N64j15 affected the general Dnc expression, as well as the mushroom body expression. These animals showed a strong decrease of their OA after ES (Fig. 5).
Fig. 5.
OA after ES ofDf(1)N/w+Y males (light gray) and control CD(1), y w fsisters (black). OnlyDf(1)N64j15/w+Ymales are affected (t test; **p = 0.005; t = 2.77). Odors as in Figure1B. n = 12 groups forN64j15; n = 16 groups for N79f. The standard condition of 60 V–high humidity was used for this experiment.waDf(1)N79f/w+Y(Canton-S) males displayed a 90 min memory score of 30.6 ± 4.3 compared with 65.1 ± 5.4 forw1118/w+Y(Canton-S) control males (n = 6 groups;t test; ***p = 0.0005;t = 5).
DISCUSSION
The present results strongly suggest that part of the learning and memory deficits displayed by the mutants amn,dnc, and rut in an associative conditioning protocol could be attributable to effects of the ES on some aspect of perception in the animals. It is possible that ES alters faculties in the mutants required not only for the test but also for the training phase of associative conditioning, and it is likely that these faculties are progressively deteriorated as the shock pulses are delivered. In summary, an altered physiology in the mutants after ES presentation could lead to an apparent defect in associative conditioning because of weaker acquisition during training and/or because of poorer performance during the test.
This work highlights the need for two-step controls in associative learning and memory experiments, especially in which a strong stimulus is used to condition the animals. In particular, the effect of the aversive unconditioned stimulus on the ability of the animals to perceive the conditioned stimulus should be investigated. Thus, although the mutants studied here have been shown to perform poorly under many different associative conditioning protocols (Aceves-Pina and Quinn, 1979; Folkers, 1982; Mariath, 1985), the unconditioned stimulus was generally stressful.
Two strategies can now be adopted to differentiate learning and memory defects from unspecific deficits. First, genetic dissection might reveal that a specific protein isoform and/or expression in a particular subdomain is involved in only one type of behavior. Such an approach was performed successfully with dnc (Table1) by testing two previously characterized deficiencies (Qiu and Davis, 1993). Thus, a dramatic and specific decrease in Dnc mushroom body product correlates with normal OA after ES. These animals showed a specific memory defect. On the contrary, elimination of the entire brain expression of Dnc leads to a strongly reduced OA after ES. The fact that only the latter animals showed an apparent learning deficit suggests that this might be a secondary consequence of the nonassociative deficit induced by ES. In this hypothesis, the Dnc phosphodiesterase accumulated in the mushroom bodies would be involved specifically in memory formation.
Table 1.
Genetic dissection of dnc behavioral functionsa
| Genotype | Dnc mushroom body expression | Dnc general brain expression | OA after ES | Learning | Memory (90 min) |
|---|---|---|---|---|---|
| Canton-S | + | + | + | + | + |
| dnc1 | − | − | − | − | − |
| Df(1)N79f | −1-b | + | + | + | − |
| Df(1)N64j15 | − | − | − | − | − |
Data reprinted from Qiu and Davis (1993), except for OA.
It should be noted thatDf(1)N79f very strongly decreases Dnc expression in the mushroom bodies but does not eliminate this expression completely (Qiu and Davis, 1993).
Second, the mutants could be studied under conditions designed to reduce stress to prevent the occurrence of nonspecific defects which might interfere with the conditioning procedure. Thus, it has been seen that dnc, which apparently displays a learning defect when conditioned with a negative stimulus (ES), learns normally when a positive stimulus (sugar) is used in association with odors (Tempel et al., 1983). This observation supports the idea that Dnc is specifically required for associative memory but not for associative learning.
Footnotes
This work was supported by the Human Frontier Science Organization, the Fondation pour la Recherche Médicale, the Association pour la Recherche contre le Cancer, and the Centre National de la Recherche Scientifique (ATIPE 7). Part of this work has been performed at the Universität Würzburg (Lehrstuhl für Genetik, Am Hubland, D-97074 Würzburg, Germany), and I thank researchers from that laboratory for their valuable criticisms of this work. I thank Ronald Davis for providing fly stocks and Jean-Maurice Dura, Yves Fregnac, Martin Heisenberg, Raphael Hitier, Lucy Vincent, and Jean-Didier Vincent for their helpful comments on a previous version of this manuscript.
Correspondence should be addressed to Dr. Thomas Préat, Institut Alfred Fessard, Centre National de la Recherche Scientifique, 91190 Gif-sur-Yvette, France.
REFERENCES
- 1.Aceves-Pina EO, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;15:93–95. doi: 10.1126/science.206.4414.93. [DOI] [PubMed] [Google Scholar]
- 2.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Nat Acad Sci USA. 1986;83:9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dauwalder B, Davis RL. Conditional rescue of the dunce learning/memory and female fertility defects with Drosophila or rat transgenes. J Neurosci. 1995;15:3490–3499. doi: 10.1523/JNEUROSCI.15-05-03490.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- 5.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 6.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. Dunce, a mutant of Drosophila, deficient in learning. Proc Natl Acad Sci USA. 1976;5:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudai Y, Zvi S, Segel S. A defective conditioned behavior and a defective adenylate cyclase in the Drosophila mutant rutabaga. J Comp Physiol [A] 1984;155:569–576. [Google Scholar]
- 8.Dura JM, Préat T, Tully T. Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster. J Neurogenet. 1993;9:1–14. doi: 10.3109/01677069309167272. [DOI] [PubMed] [Google Scholar]
- 9.Dura JM, Taillebourg E, Préat T. The Drosophila learning and memory gene linotte encodes a putative receptor tyrosine kinase homologous to the human RYK gene product. FEBS Lett. 1995;370:250–254. doi: 10.1016/0014-5793(95)00847-3. [DOI] [PubMed] [Google Scholar]
- 10.Erber J, Masuhr T, Menzel R. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiol Entomol. 1980;5:343–358. [Google Scholar]
- 11.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 12.Folkers E. Visual learning and memory of Drosophila melanogaster wild type c-s and the mutants dunce, amnesiac, turnip and rutabaga. J Insect Physiol. 1982;6:535–539. [Google Scholar]
- 13.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 14.Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 15.Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 16.Mariath HA. Operant conditioning in Drosophila melanogaster wild-type and learning mutants with defects in the cyclic AMP metabolism. J Insect Physiol. 1985;10:779–787. [Google Scholar]
- 17.Nighorn AM, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y, Davis RL. Genetic dissection of the learning/memory gene dunce of Drosophila melanogaster. Genes Dev. 1993;7:1447–1458. doi: 10.1101/gad.7.7b.1447. [DOI] [PubMed] [Google Scholar]
- 19.Quinn WG, Harris W, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- 21.Simon AF, Boquet I, Synguélakis M, Préat T (1998) The Drosophila putative kinase Linotte (Derailed) prevents central brain axons from converging on a newly described interhemispheric ring. Mech Dev, in press. [DOI] [PubMed]
- 22.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci USA. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–200. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]