Abstract
Ionotropic, nicotinic receptors have previously been shown to mediate both inhibitory (Cl-dependent) and excitatory (cationic) cholinergic responses in Aplysia neurons. We have used fast perfusion methods of agonist and antagonist application to reevaluate the effects on these receptors of a wide variety of cholinergic compounds, including a number of recently isolated and/or synthesized α toxins [α-conotoxin (αCTx)] fromConus snails. These toxins have been shown in previous studies to discriminate between the many types of nicotinic receptors now known to be expressed in vertebrate muscle, neuroendocrine, and neuronal cells. One of these toxins (αCTx ImI from the worm-eating snail Conus imperialis) revealed that two kinetically and pharmacologically distinct elements underlie the ACh-induced Cl-dependent response in Aplysia neurons: one element is a rapidly desensitizing current that is blocked by the toxin; the other is a slowly desensitizing current that is unaffected by the toxin. The two kinetically defined elements were also found to be differentially sensitive to different agonists. Finally, the proportion of the rapidly desensitizing element to the sustained element was found to be cell-specific. These observations led to the conclusion that two distinct nicotinic receptors mediate Cl currents inAplysia neurons. The receptor mediating the rapidly desensitizing Cl-dependent response shows a strong pharmacological resemblance to the vertebrate α-bungarotoxin-sensitive, α7-containing receptor, which is permeable to calcium and mediates a rapidly desensitizing excitatory response.
Keywords: nicotinic receptor, acetylcholine, α7, chloride, Aplysia, α-conotoxin ImI, suberyldicholine, methyllycaconitine, α-bungarotoxin, strychnine, dihydro-β-erythroidine
At least two nicotinic responses, differentiated by both their pharmacological and ionic properties, have been described in Aplysia neurons (Tauc and Gerschenfeld, 1962; Kehoe, 1972a,b). One is an excitatory response involving a nonspecific cationic channel (Ascher et al., 1978a); the other is an inhibitory response resulting from the gating of a Cl channel (Kehoe, 1972a).
The Cl-dependent inhibitory response was found to be blocked by α-bungarotoxin (αBTx) (Kehoe et al., 1976), tubocurarine (TC) (Tauc and Gerschenfeld, 1962; Kehoe, 1972b), dihydro-β-erythroidine (dβe), and strychnine (Kehoe, 1972b) and to be elicited by suberyldicholine (D6) (Ger and Zeimal, 1976; Kehoe, 1979). On the other hand, the cationic response was found to be blocked, among other compounds, by TC and hexamethonium (Tauc and Gerschenfeld, 1962), both of which were shown to block the open channel (Ascher et al., 1978b). From these pharmacological findings, parallels were drawn (Kehoe, 1979) (1) between the suberyldicholine-sensitive Aplysia receptor mediating the Cl-dependent response and the vertebrate nicotinic receptor found in muscle and (2) between the Aplysiareceptor mediating the cationic response and the vertebrate receptor mediating the fast EPSP in autonomic ganglion neurons.
Since that period, thanks to the use of single-channel recording, molecular biology techniques, and a more extensive battery of pharmacological tools, a vast number of vertebrate nicotinic receptor subtypes have been distinguished. The experiments described here used fast perfusion methods and an enlarged battery of cholinergic compounds to reevaluate the similarities between molluscan nicotinic receptors and the many newly defined vertebrate receptor subtypes. Among the antagonists used were α-toxins from predatory Conus snails [α-conotoxin (αCTx)]. These toxins have been extremely useful in discriminating between vertebrate nicotinic receptors. In particular, we tested the recently synthesized (McIntosh et al., 1994) αCTx ImI from the worm-eating snail, Conus imperialis. This toxin was shown to block αBTx-sensitive neuronal receptors from both mouse and rat (McIntosh et al., 1994; Pereira et al., 1996) and, when tested on nine recombinant nicotinic receptors expressed inXenopus oocytes (Johnson et al., 1995), to block, selectively, the two αBTx-sensitive homomeric receptors, α7 and α9. αCTx ImI was also shown to block the ACh response in frog muscle (McIntosh et al., 1994) but to be only weakly active or without effect on fish (McIntosh et al., 1994) or mammalian (Johnson et al., 1995) muscle. The only αBTx-resistant receptor that αCTx ImI has been shown to block is that mediating fast excitatory synaptic transmission in B neurons of the frog sympathetic ganglion (Tavazoie et al., 1997).
In the present study we have determined that the ACh-induced, αBTx-sensitive, Cl-dependent response in Aplysia neurons can be separated into two kinetically distinct currents mediated by two pharmacologically distinct receptors. One of those receptors shows a strong pharmacological resemblance to the αBTx-sensitive, α7-containing (Orr-Urtreger et al., 1997), calcium-permeable (Galzi et al., 1992; Seguela et al., 1993) receptor that mediates a rapidly desensitizing cationic response in vertebrate neurons.
MATERIALS AND METHODS
Experimental preparation. The experiments described in this paper were performed on cells from either the buccal or pleural ganglia of Aplysia californica. The ganglia were prepared as previously described (Kehoe, 1985). For experiments evaluating the cationic ACh response, identifiable, unpigmented small cells from the right pleural ganglion (Ascher et al., 1978a) were used. For experiments on the Cl-dependent cholinergic response, individually identifiable cells (B3, 6, 8, 9, or 10) of the buccal ganglia (Gardner and Kandel, 1977) were used, as were cells from two distinct cell groups (medial and posterior; Kehoe, 1972b) of the pleural ganglia.
Electrodes, voltage clamp, recording procedures, and treatment of data. All recordings were made in voltage clamp with hand-pulled microelectrodes made from a de Fonbrune microforge. For a description of the electrodes and the characteristics of the two-electrode voltage clamp, see Kehoe (1985). Continuous recordings were made on a Servogor 340 paper recorder and on a digital audio tape recorder. Records of currents elicited by agonist applications were digitized and sampled on-line on a Dell computer, via a Cambridge Electronic Design 1401 interface, using the Whole Cell Electrophysiology program from Strathclyde Electrophysiological Software.
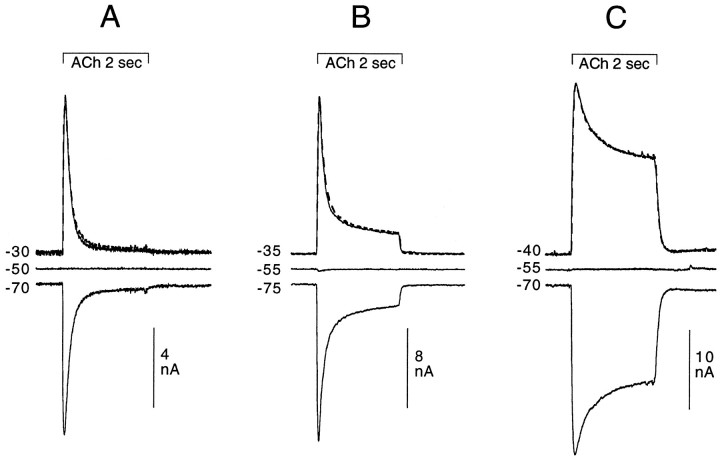
Most of the experiments presented here concern agonist-induced increases in Cl conductance. Illustrations of many of those experiments only show the outward current obtained at holding potentials less negative than ECl (e.g., between −25 and −40 mV). Although often not shown, records were made at higher holding potentials (at ECl as well as at potentials at which the response is inverted; for example, see Fig. 1) to establish that the drug-induced changes observed in the Cl-dependent response were neither voltage-dependent nor attributable to a change inECl.
Fig. 1.
Cl-dependent responses as a function of holding potential in three different cell types: 2 sec application of 200 μm ACh. Comparing A (posterior neuron and pleural ganglion), B (buccal cell 10), andC (buccal cell 3), it can be seen that for different cell types the Cl-dependent response shows markedly different desensitization kinetics over the 2 sec application of ACh. In all three cells, the response on one side of EClis the mirror image of the response on the other side ofECl; the dashed linesrepresent the responses measured in the three cells at −70, −75, and −70 mV, respectively, which have been inverted and superimposed on the responses taken at holding potentials less negative than but equidistant from ECl (solid lines).
Fast perfusion application of agonists and antagonists. The fast perfusion system used in these experiments is a modification of that described by Johnson and Ascher (1987). In the experiments reported here, three hand-pulled, thick-walled glass tubes, held together horizontally by heat shrink tubing, delivered either artificial seawater (ASW) or agonist- or antagonist-containing solutions to the soma of the cell under study. For these experiments, the tubes were pulled to obtain an opening of each tube that was usually on the order of 75–100 μm. The so-called “control tube,” which contained only ASW or antagonist-containing ASW, delivered a constant flow of solution over the cell under study except for the brief period during which one of the agonist-containing tubes was stepped (by a computer-controlled stepping motor) in front of that cell. Agonist then flowed over the cell for a brief (2 sec) period. The rest of the time, the control tube (from which ASW constantly flowed) was positioned in front of the cell, the agonist tubes were stepped aside, and the flow of agonist from those tubes was blocked by a solenoid-driven, computer-controlled valve. The usual procedure was to apply the agonist once per minute, and when two agonists were compared, the agonists were alternated; hence 2 min elapsed between the application of a given agonist. However, in a few experiments in which the control response appeared to diminish with repeated applications, the interapplication interval was set at 90 sec or 2 min.
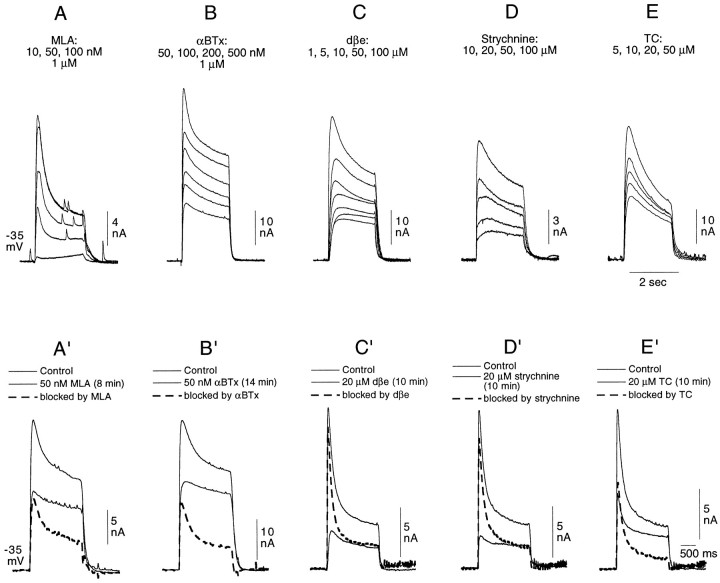
With only a few exceptions, antagonists were applied exclusively through the control tube (for exceptions see relevant text and figure legends). When concentration–response curves were established, antagonists were applied from the lowest to highest concentrations without intervening washes. A given concentration of antagonist was maintained until the blocking effect of the antagonist appeared to reach a plateau (usually 3–4 min). However, with certain compounds [in particular, methyllycaconitine (MLA), αBTx, and strychnine] this exposure time proved to be inadequate for obtaining a maximum blocking effect, so a few experiments were performed using a single concentration with longer exposure times (see Fig.4A′–E′ and Results).
Fig. 4.
A–E, Failure of MLA, αBTx, dβe, strychnine, and TC to discriminate between the two elements of the Cl-dependent response of buccal cells to 200 μm ACh. The data illustrated were obtained from five different cells. ACh (200 μm) was applied at 1 or 2 min intervals, and the antagonists were applied, at increasing concentrations, exclusively through the control tube. With the exception of 10 nm MLA, all concentrations of all five antagonists tested affected both elements of the Cl-dependent response.A′–B′, Comparison in the same cell of the block by 50 nm MLA and 50 nm αBTx of the rapidly desensitizing and sustained components of the two-component Cl-dependent response in buccal neurons elicited by a 2 sec application of 200 μm ACh. A complete recovery from the block by MLA was obtained before applying αBTx. C′–E′, Comparison in the same cell of the block by 20 μm dβe, 20 μm strychnine, and 20 μm TC of the rapidly desensitizing and sustained components of the Cl-dependent response elicited in a buccal neuron by a 2 sec application of 200 μm ACh. The three different antagonists were tested successively on the same cell. A thorough wash, resulting in the total recovery of the initial response amplitude, separated the three experiments. In A′–E′, the blocked responses, obtained by subtraction, are represented by dashed lines.
In addition to the ASW continuously flowing from the control tube onto the cell under study, a constant superfusion of the entire ganglion ensured the renewal of the bathing medium and the removal of the briefly applied agonists or antagonists from the chamber.
ASW and drug-containing solutions. The ASW contained 480 mm NaCl, 10 mm KCl, 10 mmCaCl2, 50 mm MgCl2, and 10 mm Na-HEPES, pH 7.8. All agonists and antagonists used were dissolved directly in ASW. αBTx and the α-conotoxins were prepared at 10 μm concentrations and were further diluted as needed. Unused 10 μm toxin solutions were frozen and kept for later experiments. At most a small diminution was noted in the efficacy of the toxins thus stored in solution.
Drugs. All α-conotoxins were supplied by JMM. αCTx ImI, αCTx MII, and αA-CTx EIVA were synthesized as previously described (McIntosh et al., 1994; Cartier et al., 1996;Jacobsen et al., 1997), and αCTx PnIA and αCTx PnIB were synthesized using methods described for αCTx MII (Cartier et al., 1996). MLA was obtained from Research Biochemicals (Natick, MA), and all other drugs were obtained from Sigma (St. Louis, MO).
Experimental n. Each conclusion concerning the effect of an agonist or an antagonist on a given receptor type was drawn from a minimum of three experiments and was usually corroborated by many more.
RESULTS
Although in some Aplysia cells, only one type of cholinergic receptor is expressed, and the response to ACh therefore reflects the increase in a single type of conductance (e.g., cationic), in other cells, more than one receptor type is present on the cell membrane, and ACh consequently elicits a multicomponent, multiconductance response; e.g., in the medial cells of the pleural ganglion ACh elicits both Cl- and K-dependent responses (Kehoe, 1972b), whereas both cationic and Cl-dependent responses are elicited in the B7 neurons of the buccal ganglia (Gardner and Kandel, 1977). From one animal to the next, ACh consistently gives the same type(s) of conductance change(s) in a given identifiable cell.
The results discussed in this paper concern cells in which ACh induces only an increase in Cl conductance or only an increase in cationic conductance. First, a description will be given of the kinetics of the Cl-dependent response as observed with fast perfusion techniques, followed by an analysis of its pharmacological properties, including the effects on the Cl-dependent response of αCTx ImI. A similar brief evaluation will then be made of the ACh-induced cationic response. The effects of a number of other α-conotoxins on both the Cl-dependent and cationic responses will then be described, before terminating with a summary, derived from the data presented here, of the pharmacological profiles of the Aplysia nicotinic receptors.
The ACh-induced increase in Cl conductance
Description of pure Cl-dependent responses to fast perfusion application of ACh in identified cells
Among the cells belonging to the poorly defined heterogeneous “posterior” groups of the left and right pleural ganglia are many cells that respond to ACh with a pure Cl-dependent response (J. Kehoe, unpublished data). ACh also activates a pure Cl-dependent response in a number of identifiable cells of the buccal ganglia (e.g., B3, 6, 8, 9, and 10; Gardner and Kandel, 1977). However, the kinetics of the Cl-dependent response to a fast perfusion application of ACh is different for these different cell types. Figure1A shows the response to a 2 sec ACh application that is typical of certain so-called posterior neurons: the Cl current declines rapidly to baseline before the end of the 2 sec ACh pulse. In contrast, the responses in the buccal cells identified above are characterized by a non-null current that is maintained throughout the ACh application. The relative level to which the ACh-induced current declines is, furthermore, cell-specific (Fig. 1B,C, respectively). This sustained current is of relatively small amplitude in B10 cells but is very prominent in B3 cells. It is technically difficult to give a meaningful quantitative evaluation of the proportion of rapidly desensitizing to sustained current in the different cell types. By its very nature the rapidly desensitizing element is probably never maximally expressed, and the dimensions of the perfusion system and its placement relative to the cell, which are forcibly not the same from experiment to experiment, are important determinants of the maximal amplitude of the desensitizing element and, hence, of its proportional contribution to the total response. Furthermore, the proportion of the two different elements varies as a function of ACh concentration—probably also because of the differential desensitization of the two elements. Despite these technical limitations, it is clear that consistent qualitative differences can be noted concerning the kinetic characteristics of the Cl-dependent response in different cell types. Under the conditions used here, the sustained current in cells B3 and B6 accounts for between 50 and 75% of the total Cl current activated by ACh at 100 or 200 μm, whereas in some neurons of the pleural ganglion no sustained current can be detected (Fig. 1A) at any ACh concentration. In other cell types (e.g., buccal cells B10 and B9 and the medial cells of the pleural ganglion), a sustained element exists but is less dominant than in the B3 and B6 cells.
No voltage dependence of the change in conductance can be detected in the responses illustrated in Figure 1, because the responses of a given cell taken at potentials equidistant from EClhave identical forms and amplitudes (see figure legend). Only cells with such voltage-independent conductance changes were used for experiments on the Cl-dependent response.
No experiments were specifically designed to determine whether the peak and plateau elements of the ACh-induced response showed differential selectivity for different anions. However, it can be stated that neither the rapidly desensitizing nor the sustained ACh-induced Cl-dependent response was blocked by intracellular sulfate ions, which have previously been shown (Kehoe and Vulfius, 1995) to block, selectively, the GABA-induced Cl-dependent response in the same and other cells.
Selective blockade by αCTx ImI of the rapidly desensitizing element of the Cl-dependent response
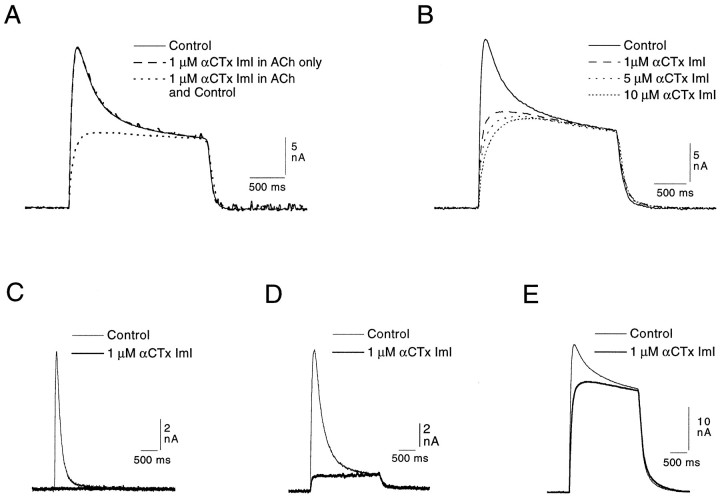
When the buccal cells used in the experiments illustrated above (Fig. 1) are exposed to αCTx ImI, the rapidly desensitizing element of the Cl-dependent response to ACh (200 or 250 μm) is completely eliminated (Fig.2A), and a toxin-resistant, seemingly nondesensitizing element is revealed. [This latter element will be referred to as the “sustained” response, because over longer ACh applications, and particularly in isolated cultured neurons (Kehoe, unpublished observations), it can be seen to desensitize, although still at a much slower rate than does the αCTx-sensitive element.] It can also be seen in Figure2A that adding 1 μm αCTx ImI exclusively to the ACh-containing solution had no effect on the response. It is only when the toxin is also added to the control tube solution, and hence bathes the cell before the ACh application, that the rapidly desensitizing element of the response is eliminated. Inversely, no further block is obtained by adding toxin to the ACh solution after the response has already been exposed to the toxin flowing through the control tube. Three experiments were made in which 1 or 5 μm toxin was added to the ACh solution, and all three confirmed that the presence of the toxin in the agonist solution does not alter the selectivity of the blockade; consequently, in all later experiments the toxin was added exclusively to the solution in the control tube.
Fig. 2.
Selective elimination by αCTx ImI of the rapidly desensitizing Cl-dependent response in identified neurons of the buccal and pleural ganglia (see Materials and Methods). A, αCTx ImI (1 μm) included in the ACh tube had no effect on the response (compare response presented as a solid line with that presented as an enhanced dashed line). The same concentration of αCTx ImI added to the control tube (hence bathing the cell before the ACh application), however, selectively blocked the desensitizing element of the response.B, In another cell of the same type, increasing concentrations of αCTx ImI failed to significantly alter the sustained element of the Cl-dependent response to 250 μmACh applied at 1 min intervals (records in toxin taken after a 4 min exposure to each concentration). C, In a neuron (posterior cell from the pleural ganglion; Kehoe, 1972a), which shows only the rapidly desensitizing element of the Cl-dependent response, 1 μm αCTx ImI completely blocks the response to 200 μm ACh. D, E, Selective block by 1 μm αCTx ImI of the rapidly desensitizing element of the response of two identified neurons of the buccal ganglion (B10 and B3, respectively) to 200 μm ACh. The two cells show different, characteristic proportions of the rapidly desensitizing and the sustained elements.
The sustained element of the response remained essentially unaffected, even when the toxin concentration was increased by 1 order of magnitude to 10 μm (Fig. 2B). With more prolonged applications of toxin (e.g., 10 min), a slight diminution (∼10–15%) in the sustained element could sometimes be noted.
In Figure 2C–E, 1 μm αCTx ImI produces a similar selective and total block of the rapidly desensitizing element of the Cl-dependent response in the three cell types previously described in Figure 1. The net response in the buccal cells thus appears to be composed of a toxin-sensitive, rapidly desensitizing element and a toxin-resistant, sustained element, each of greater or lesser relative amplitude, depending on the cell studied.
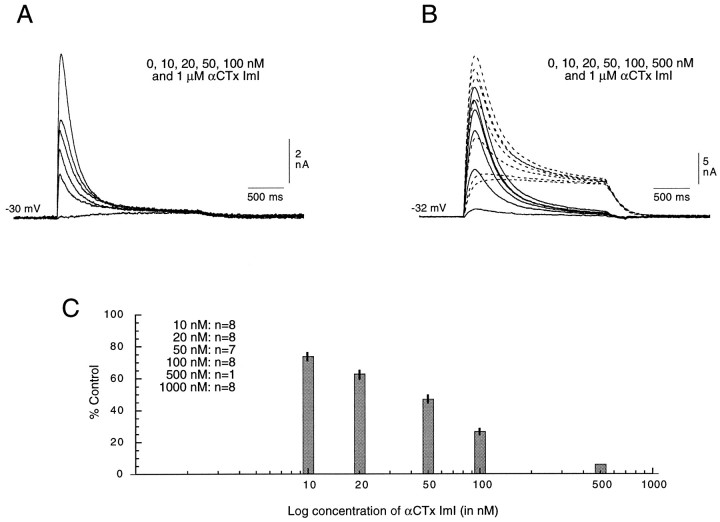
The progressive decline of the rapidly desensitizing element of the Cl-dependent response with increasing concentrations of αCTx ImI can be seen in Figure 3A in an unidentified buccal neuron that has almost no sustained element. Given that in cells having only the rapidly desensitizing element, the total response was eliminated by 1 μm toxin (Fig.3A), that concentration of αCTx ImI was assumed to completely eliminate the rapidly desensitizing response even in cells in which it was accompanied by the sustained response—a hypothesis supported by the form of the response in toxin-treated cells. Data from a cell with both response elements are illustrated in Figure3B. To better evaluate the effect of the toxin on the rapidly desensitizing element in such cells, the presumably pure sustained element seen in 1 μm toxin was subtracted from the total response obtained in increasing toxin concentrations (from 0 to 500 nm). The resulting traces (Fig. 3B, solid lines) represent the progressive toxin-induced decline of the rapidly desensitizing element of that response.
Fig. 3.
Progressive diminution in the rapidly desensitizing element of the Cl-dependent response of identified buccal cells with increasing concentrations of αCTx ImI. In all experiments, 200 μm ACh was applied at 1 min intervals, and increasing concentrations of αCTx ImI were introduced successively in the control tube of the fast perfusion system. The cells were exposed to a continual flow of each concentration for ∼3–4 min. A, Records from a buccal cell having only a very weak sustained element.B, Records from a buccal cell with a prominent sustained element. The response in 1 μm αCTx ImI was subtracted from the response at each of the other concentrations (dashed lines) to obtain records of only the rapidly desensitizing, toxin-sensitive responses (solid lines).C, Data from eight experiments were used to calculate the average reduction of the desesensitizing element of the control response with increasing concentrations of αCTx ImI (10, 20, 50, 100, and 500 nm and 1 μm). For cells having both rapidly desensitizing and sustained responses, the amplitude of the rapidly desensitizing element was obtained by subtracting the response obtained in 1 μm toxin from that obtained at each of the other concentrations. Because identical concentrations of toxin were not used in each experiment, the n values have been indicated in the inset. The SEs have been indicated for each concentration, except for 500 nm, for which only one point was obtained, and for 1 μm, which yielded a total block (either by definition, see above, or by direct reading of the data) of the desensitizing response in all cases. The average IC50 for the eight experiments illustrated here was estimated by interpolation to be 47 nm.
Data from eight different experiments were used to calculate the average percent reduction of the rapidly desensitizing response with increasing concentrations of αCTx ImI (10, 20, 50, 100, and 500 nm and 1 μm). Under the conditions used here, the response to 200 μm ACh was reduced by half with 47 nm αCTx ImI (determined by interpolation using a semilog plot of the data included in Fig. 3C) and was reduced (as defined above) to 0% by 1 μm toxin. No clear distinction could be made between the concentration–response curves obtained from cells showing only the rapidly desensitizing element and those obtained from cells having both elements.
In contrast, even 20 μm αCTx ImI (the highest concentration tested) failed to reduce by half the sustained Cl-dependent response to 200 μm ACh. Consequently, the sensitivity of the two Cl-dependent responses to the toxin differs by at least 400-fold.
Failure of other antagonists to distinguish between the rapidly desensitizing and sustained elements of the Cl-dependent response
In most experiments performed with αBTx, MLA, dβe, strychnine, and TC, the antagonist was applied to the cell exclusively through the control tube. As can be seen in Figure4A–E, both elements of the Cl-dependent response were diminished by these five antagonists at all concentrations used in these experiments, with the exception of 10 nm MLA, which produced a very small, but selective, reduction in the rapidly desensitizing element. With none of these antagonists was it possible to eliminate selectively one of the two components of the Cl-dependent response.
Differential effectiveness of nicotinic antagonists in blocking the rapidly desensitizing Cl-dependent response
At first approximation it is possible to determine the relative effectiveness of the different antagonists in blocking the rapidly desensitizing Cl-dependent response from experiments using cells having both the rapidly desensitizing and sustained elements (Fig.4A–E). However, to better evaluate the concentrations necessary to reduce the rapidly desensitizing component by half, a series of experiments (not illustrated) was performed on posterior pleural neurons or on buccal neurons showing at most a very small sustained component (see an example of such an experiment using αCTx ImI in Fig. 3A). At least two or three concentration–response curves were obtained in experiments of this type for αCTx ImI as well as for the five other classical antagonists. In all experiments, 200 μm ACh was applied for 2 sec at 1 min intervals, and the antagonist was included exclusively in the control tube solution. A relatively clear separation of the antagonists into three groups was revealed by such experiments: with the short applications typical of the protocol used here, αCTx ImI, MLA, and αBTx were approximately equally effective in blocking the rapidly desensitizing response, with 40–50 nm being the concentration required to block by half the response to 200 μm ACh. For dβe, strychnine, and TC, the required concentration for such a block was in the micromolar range, with TC being the least effective.
To further define the relative efficacy of the different antagonists, an antagonist concentration approximately equal to that necessary for blocking the rapidly desensitizing component by half was tested independently, i.e., not as part of a series of concentrations used for establishing concentration–response curves, and was applied for up to at least 8–10 min to make certain that the relatively short exposure to the antagonist given in the concentration–response experiments did not yield an underestimation of its blocking capacity. Thus, the effect of 50 nm MLA on the two-component Cl-dependent response was compared with that of the same concentration of αBTx (Fig.4A′,B′), whereas the effects of dβe, strychnine, and TC, at 20 μm each, were similarly compared (Fig.4C′–E′). In these figures, the control response and the response in the presence of the antagonist are both represented by solid lines, whereas the difference between the control response and that taken after exposure to the antagonist (the so-called “blocked response”) is represented by dashed lines.
Findings obtained with the experiments of the type shown in Figure4A′–E′ combined with those from experiments designed to establish concentration–response curves confirmed that for blocking the rapidly desensitizing element of the response, αCTx ImI, MLA, and αBTx are similarly effective and that both dβe and strychnine are more effective than TC, with the IC50 values obtained for the latter three antagonists being ∼2, 5, and 20 μm, respectively. It can furthermore be seen by comparing Figure 4,A and A′ and B and B′, that a longer exposure to a given concentration of either MLA (50 nm) or αBTx (50 nm) leads to a significantly more complete block of the rapidly desensitizing element. Additional experiments (data not shown) suggested that, under “equilibrium” conditions (defined as a complete stabilization of the response amplitude), 10 nm αBTx was sufficient for blocking the rapidly desensitizing element by half.
Differential effectiveness of nicotinic antagonists in blocking the sustained Cl-dependent response
An indication concerning the effectiveness of most antagonists to block the sustained Cl-dependent response is already present in the data illustrated in Figure 4, A–E and A′–E′. As was seen above, αCTx ImI fails to block the sustained element. For the other antagonists, however, the rank order of effectiveness is quite similar to that seen for the blockade of the rapidly desensitizing element; i.e., MLA and αBTx are more effective than dβe, strychnine, or TC, and both dβe and strychnine are more effective than TC (Fig. 4, compare C′–E′).
In experiments such as those illustrated in Figure 4, the block of the sustained element was estimated from the response amplitude at the end of the 2 sec ACh application, so any “washing off” of the antagonist that might occur during the application of the agonists would yield an inaccurate estimate of its blocking capacity. Consequently, a series of experiments comparing the efficacy of various antagonists was performed in which the antagonist was present first only in the control tube solution and then in both the control and ACh-containing solution. Only one agonist concentration was used for a given comparison. Adding the antagonist to the ACh-containing solution failed to enhance the block by αBTx and only enhanced the block obtained with the other antagonists by at most 20%, so the general conclusions were not significantly affected by that aspect of the methodology.
Differential effectiveness of nicotinic agonists in eliciting the two Cl-dependent responses
To evaluate the relative capacity of different agonists to activate the rapidly desensitizing and sustained Cl-dependent responses, experiments were done in which the response to a fixed concentration of ACh (100 μm) was compared, in the same experiment on the same cell, to those elicited by 10, 50, 100, and often 200 μm concentrations of another cholinomimetic. The concentration of ACh used in these experiments was far from maximal, because the amplitudes of both elements of the Cl-dependent response to ACh continue to increase with increasing concentrations well beyond the 100 μm concentration used here (data not shown).
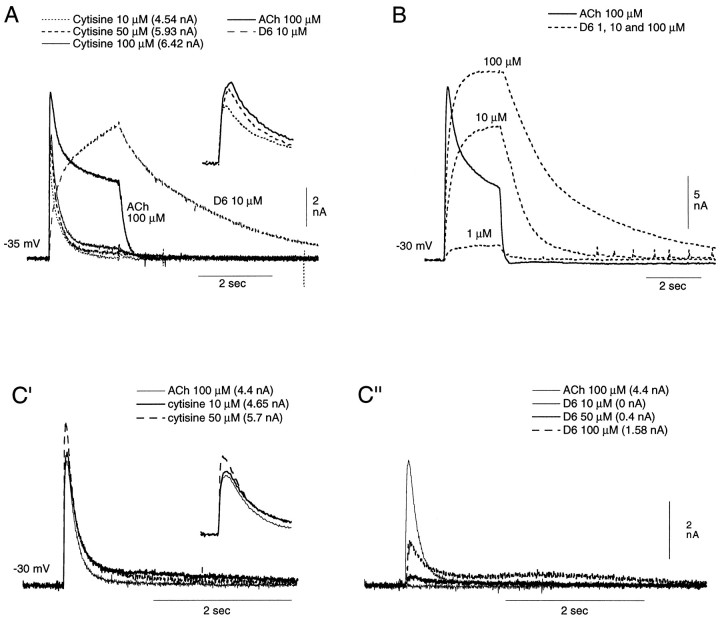
It was found that whereas nicotine and cytisine both preferentially activate the rapidly desensitizing element of the Cl-dependent response, suberyldicholine preferentially activates the sustained element. In Figure 5, the responses to increasing concentrations of cytisine and of suberyldicholine are compared in buccal cells, which show a prominent sustained Cl-dependent component (Fig. 5A,B), with the responses to the same agonists in a posterior neuron (pleural ganglion), which shows only the rapidly desensitizing response (Fig. 5C, left, right). It can be seen that the response to cytisine is essentially identical in the two cell types; at the concentrations used here, it consists almost exclusively of a rapidly desensitizing element, even in cells having a marked sustained response to ACh (Fig. 5A). At concentrations greater than 100 μm (data not shown), cytisine becomes less specific and, even during the 2 sec cytisine pulse, elicits a weak sustained Cl current in cells in which ACh elicits such a current. It should be noted that the response to 10 μm cytisine is approximately equal in amplitude to that of the rapidly desensitizing element of the response to 100 μm ACh (estimated by subtraction of the sustained element from the total ACh response in Fig. 5A to be 4.3 nm). The peak currents elicited by the three cytisine concentrations are indicated in Figure 5.
Fig. 5.
Preferential activation, by cytisine and D6, of the rapidly desensitizing and sustained Cl-dependent responses, respectively. A, Comparison, in a buccal cell showing a marked sustained element in the ACh Cl-dependent response, to 100 μm ACh, 10 μm D6, and increasing concentrations of cytisine (10, 50, and 100 μm). Note that at the concentrations applied here, there is practically no evidence of a sustained Cl-dependent response to cytisine, and that the response to 10 μm cytisine (4.54 nA) is equal in amplitude to the rapidly desensitizing response to 100 μmACh (4.53 nA, obtained by subtraction of the sustained element from the total ACh response). For easier evaluation of the cytisine-induced currents, they have been reproduced in the inset with an expanded x-axis and a reduced y-axis.B, Responses of a similar buccal cell to 100 μm ACh and to increasing concentrations of suberyldicholine (1, 10, and 100 μm). Note that the sustained response to 10 μm suberyldicholine is larger than that to 100 μm ACh. C, Responses to increasing concentrations of both cytisine (C′; cytisine and ACh records presented in inset with expandedx-axis and reduced y-axis) and suberyldicholine (C") of a buccal cell showing a predominantly rapidly desensitizing response to 100 μmACh (C′ and C"). Note that the responses to all three agonists desensitize during the 2 sec application, and that the response to 10 μm cytisine (C′) is larger than that to 100 μm ACh, whereas no response can be detected to 10 μm suberyldicholine (C"). The desensitizing response to 100 μmsuberyldicholine (C") is only approximately one-third that of the response to the same concentration of ACh.
Even at concentrations at which a 2 sec agonist pulse fails to elicit a sustained response to the above-mentioned cholinomimetics (nicotine and cytisine), it was possible to observe a slowly developing, low-amplitude activation of that response when either nicotine (>20 μm) or cytisine (>10 μm) was applied continually through the control tube. This more slowly developing background activation of the receptor mediating the sustained response resulted in a weak diminution in the corresponding sustained element of the ACh response elicited during the period that one of the two above cholinomimetics flowed through the control tube.
Nicotine and cytisine both induce a marked desensitizing block of the receptor they preferentially activate (see below), with nicotine inducing the most persistent blockade. For example, a 10 min wash was required for the rapidly desensitizing element of the Cl-dependent ACh response to recover its initial amplitude after a 2 sec application of 200 μm nicotine. Such a “desensitizing blockade” explains, for example, the failure of the amplitude of the response to cytisine to increase significantly with increasing cytisine concentrations (Fig. 5A,C′, insets).
A selective activation of the sustained element of the Cl-dependent response, in contrast, is obtained with D6. A comparison of the records in Figure 5B and those illustrated in Figure 5C"reveals the distinct preferential activation by suberyldicholine of the sustained element. In Figure 5B, it can be seen that even with 1 μm suberyldicholine, a clear sustained element is elicited, and at 10 μm the sustained response to that agonist is considerably larger than that elicited by 100 μm ACh (compare the response at the end of the 2 sec agonist application to 100 μm ACh with that to 10 μm suberyldicholine). In Figure 5C", it can, in contrast, be seen that in a cell showing at most a very weak sustained element in the ACh response, suberyldicholine elicits no response at 10 μm, only a very weak rapidly desensitizing element at 50 μm, and, at 100 μm, a rapidly desensitizing response that is only approximately one-third that elicited by the same concentration of ACh. These data were obtained during the same experiment and from the same cell as those illustrated in Figure 5C′ comparing the responses to cytisine and ACh.
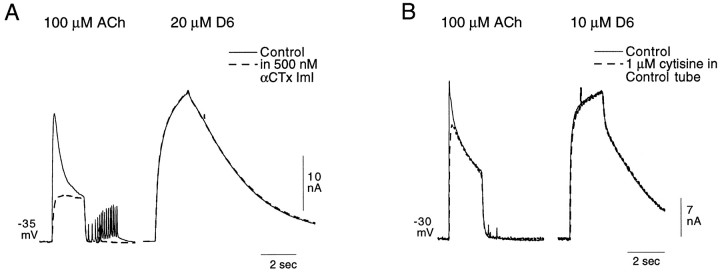
A study of the effects of αCTx ImI on the responses of buccal cells to ACh and suberyldicholine confirms the selective action of suberyldicholine as described above. In Figure6A, the responses of a buccal neuron to 100 μm ACh and to 20 μmsuberyldicholine were evaluated before and after inclusion of 500 nm αCTx ImI in the control solution. In the experiment illustrated in Figure 6A, ACh elicited both the rapidly desensitizing and sustained elements of the Cl-dependent response. The response to 20 μm suberyldicholine, on the other hand, is exclusively of the sustained type and is considerably larger than the sustained response elicited by 100 μmACh. αCTx ImI had no effect on the suberyldicholine response, which is consistent with the failure of low concentrations of suberyldicholine to elicit a response in cells having only the rapidly desensitizing Cl current (Fig. 5C"). The rapidly desensitizing element of the ACh response, on the other hand, was completely eliminated.
Fig. 6.
Further evidence for the differentiation, by suberyldicholine and cytisine, of two distinct Cl-dependent responses.A, Effect of 500 nm αCTx ImI on the responses to 100 μm ACh and 20 μmsuberyldicholine of a buccal cell showing a prominent sustained element in the two-component Cl-dependent response. Note the selective elimination in the ACh response of the rapidly desensitizing element and a failure of the toxin to affect the response to suberyldicholine. The periodic rapid deflections seen in the ACh records inA represent spontaneous synaptic activity.B, Effect of 1 μm cytisine (applied in the control tube only) on the ACh response of a buccal cell to 100 μm ACh and 10 μm suberyldicholine. Cytisine can be seen to block only the rapidly desensitizing element of the ACh response, whereas it has no effect on the response to suberyldicholine.
As mentioned above, both nicotine and cytisine cause a desensitizing block of the receptor they preferentially activate (that mediating the desensitizing Cl-dependent response). A selective but sometimes partial block of the rapidly desensitizing element of the ACh response can thereby be obtained by applying low concentrations of nicotine or cytisine through the control tube. The block by 1 μmcytisine of the rapidly desensitizing response to 100 μmACh is shown in Figure 6B. It can be seen in the same figure that the response to suberyldicholine, which, at 10 μm selectively activates the sustained Cl-dependent response (Fig. 5), is unaffected by the cytisine application, as is the sustained element of the ACh response.
The ACh-induced nonspecific cationic response
The cholinergic receptor gating the cationic response is the only cholinergic receptor found on a small group of cells in the right pleural ganglion (Ascher et al., 1978a). Cells from this group were chosen for evaluating the pharmacological characteristics of that receptor.
Effects of αCTx ImI and other antagonists on the nicotinic receptor mediating the cationic cholinergic response
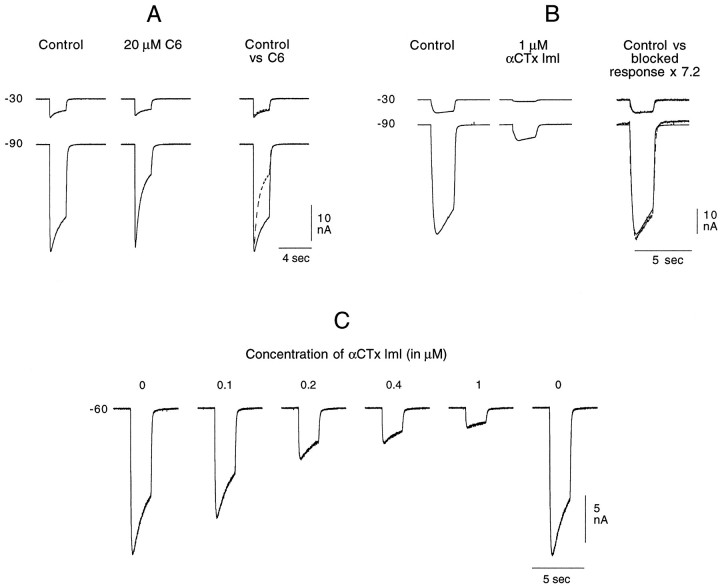
The blocking effect of many antagonists (e.g., TC and hexamethonium) acting on the receptor mediating the cationic response is highly sensitive to membrane voltage, presumably because such antagonists act only on the open channel (Ascher et al., 1978b; Slater and Carpenter, 1982). Such a voltage-dependent blockade of the ACh cationic response is shown in Figure7A. As described in Materials and Methods, in most experiments the antagonist was not included in the agonist-containing solution. However, because of the type of blockade obtained with many compounds on the cationic response (i.e., an open channel block), the antagonist was introduced in both the control and ACh solutions in these experiments. When using a sufficiently fast perfusion, 20 μm hexamethonium (C6) flowing continually through the control tube has no effect on the cationic response (data not shown). It is only when hexamethonium is present after the channel has been opened by the ligand (i.e., added to the ACh-containing solution) that a blockade occurs. This can be seen in Figure7A by comparing the initial amplitudes of the responses obtained at −90 mV before and after application of ACh and C6. During the 2 sec application of the C6-containing ACh solution, there is a rapidly developing block of the response at −90 mV. At −30 mV, this blockade is minimal. The blockade of the same receptor by TC was shown to have a similar voltage sensitivity (Ascher et al., 1978b).
Fig. 7.
Effect of C6 and αCTx ImI on the ACh-induced cationic response. ACh was applied on the cell soma for 2 sec once every minute (A, B) or once every 90 sec (C). Between each ACh application, the holding potential was alternately set at −30 or −90 mV. A, C6 (20 μm) present in both the control and agonist tubes blocks the cationic response to 200 μm ACh only when the ligand-gated channel is open, and it does so in a voltage-dependent manner. The records taken in the presence of C6 are superimposed on the control records in the right column (control vs C6). Whereas at −30 mV the responses are essentially identical in the absence (control, solid line) and presence (C6, dashed line) of the antagonist, at −90 mV, a reduction in amplitude rapidly develops during the 2 sec application. Note, however, that even at −90 mV the response amplitude at the beginning of the 2 sec application is unaffected by the presence of C6. These records were taken after 5 min exposure to C6 through the control tube. B. αCTx ImI (1 μm) blocks the cationic response to 250 μm ACh in a non-voltage-dependent manner. The toxin, flowing continuously and only from the control tube, reached its maximum blocking effect within 3 min. The records shown in the middle column were taken after ∼7 min exposure to αCTx ImI. In the right column, the control records have been superimposed on the records taken in the presence of toxin, which have been multiplied by 7.2. At both potentials, the amplified toxin records superimpose directly on the control responses, showing that there was no voltage-dependent element in the toxin block of the cationic ACh response. C, Progressive and reversible block of the cationic response to 250 μm ACh with increasing concentrations of αCTx ImI, with each increasing toxin concentration being placed in the control tube after the stabilization of the response amplitude at the previous toxin concentration. Ten minutes after returning toxin-free ASW to the control tube, the response returned to its control amplitude.
Unlike the case with hexamethonium, the channel does not need to be open for αCTx ImI (1 μm) to block the cationic response (Fig. 7B). With αCTx ImI it was unnecessary to add the antagonist to the ACh-containing solution. Including the toxin only in ASW flowing through the control tube was sufficient to induce a rapid, marked, and reversible block of the response that is apparent immediately on application of ACh. Again, in contrast to the block by hexamethonium, that with αCTx ImI shows no voltage dependence. This is seen in the last set of records of Figure 7B, in which the two records taken in the presence of the toxin (at −30 and −90 mV) are both seen to superimpose on the control records when they are both multiplied by the same factor (7.2).
To establish the concentration dependence of the toxin-induced block, increasing concentrations of toxin were applied successively through the control tube of the fast perfusion system. One of four such experiments is shown in Figure 7C, in which 250 μm ACh was used. The response rapidly stabilized at each toxin concentration, and in all experiments the effect was completely reversible after a 10–15 min wash. The IC50 value for the experiment illustrated in Figure 7C, which was obtained by interpolation using a semilog plot, was 160 nm. In the three other experiments, performed using 200 μm ACh, only three toxin concentrations were used (50, 100, and 200 nm). The IC50 values obtained in those experiments were estimated by the same method to be ∼120, 150, and 170 nm, respectively, giving an average IC50 of 150 nm. The concentration required for an equivalent, non-voltage-dependent blockade by either dβe or strychnine (Slater and Carpenter, 1982) was ∼2 orders of magnitude higher.
Failure of most cholinomimetics to activate the cationic response
None of the selective agonists discussed above (cytisine, nicotine, and suberyldicholine) elicited a response in the cells showing only the cationic response to ACh. This was true whether a low concentration (1 or 10 μm) or a high concentration (200 μm) of agonist was used.
It was hypothesized that perhaps the responses were not being detected because of an extremely rapid desensitization of the underlying receptor by the agonist. However, no rapidly desensitizing cationic response was revealed when the speed of the concentration jump was increased by reducing the diameter of the fast perfusion tubes. Second, it would be expected that if such a desensitizing response existed but was somehow undetectable with the fast perfusion system used, it should be possible to detect a strong “blocking” effect by the seemingly inactive agonist, similar to that seen on the rapidly desensitizing Cl-dependent response when 1 μm nicotine or cytisine was applied by the control tube (see Fig. 6B). This was not found to be the case; a 100 μm concentration of either nicotine or cytisine was required to reduce the cationic response by half. Suberyldicholine failed to have a significant blocking effect on the cationic response, even at 100 μmconcentrations.
The only agonist tested (other than ACh and carbachol) that was capable of eliciting the cationic response was dimethyl-4-phenyl-piperazinium (DMPP), and the response to 100 μm DMPP was only ∼10–20% of that to an equivalent concentration of ACh. Furthermore, DMPP was not a selective agonist, because it activated, albeit weakly, both of the Cl-dependent responses as well.
Effects of other toxins from Conus snails (αCTx MII, αA-CTx EIVA, αCTx PnIA, and αCTx PnIB) on the nicotinic ACh responses
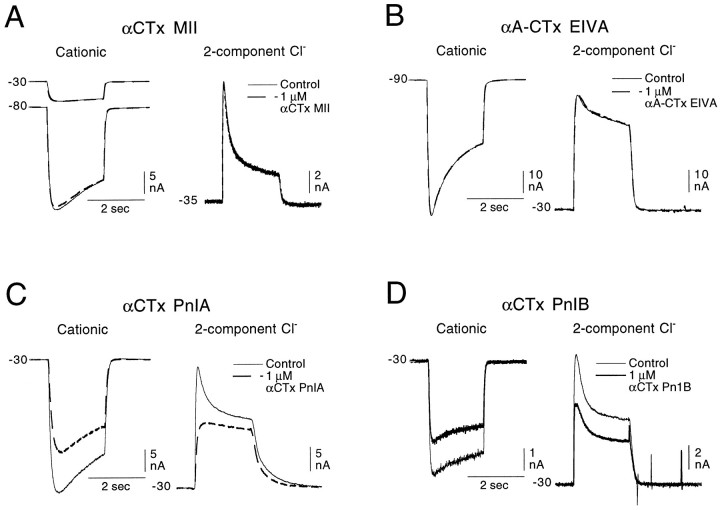
αCTx MII is a toxin isolated from the venom of a fish eatingConus snail, Conus magus. It has been found to be a highly effective antagonist on recombinant α3β2 receptors (Cartier et al., 1996). α3 and β2 subunits are found in mammalian neurons, including those of autonomic ganglia, and the antagonist profile of the receptor mediating the fast EPSP in autonomic ganglion neurons is similar to that of the Aplysia receptor mediating the cationic response; therefore, αCTx MII was tested on the cholinergic Aplysia receptors to see whether it would act selectively on the receptor mediating the cationic response. As can be seen in Figure 8A, at 1 μm (3 orders of magnitude higher than the IC50 value for the block of recombinant α3β2 vertebrate receptors), it had no effect on the cationic response and no effect on either element (rapidly desensitizing or sustained) of the two-component Cl-dependent response.
Fig. 8.
Effect of αCTx MII, αA-CTx EIVA, αCTx PnIA, and αCTx PnIB on the cationic and Cl-dependent responses to 100 or 200 μm ACh. A, B, Neither αCTx MII (1 μm) nor αA-CTx EIVA (1 μm) has an effect on either the cationic response of the unpigmented pleural ganglion cells (A, B, left records) or on either component of the Cl-dependent responses in buccal neurons (A, B, right records). C, αCTx PnIA (1 μm) weakly blocks the cationic response as well as the sustained Cl-dependent response (left and right columns, respectively) and strongly blocks the rapidly desensitizing Cl-dependent response in the same cell (right column). D, αCTx PnIB (1 μm) causes a small reduction in all three nicotinic responses (cationic, rapidly desensitizing, and sustained Cl-dependent responses). No agonist effect of any of the four toxins could be detected.
We also tested on the Aplysia receptors a toxin belonging to the newly defined αA-conotoxin family. This toxin, αA-CTx EIVA, which was isolated from the fish-eating snail Conus ermineus, was shown by Jacobsen et al. (1997) to block, at nanomolar concentrations, both Torpedo and mouse α1-containing muscle receptors expressed in Xenopusoocytes but to be without effect on neuronal αBTx-sensitive receptors. Because the Aplysia neuronal receptor mediating the sustained Cl-dependent response shows some pharmacological similarities to the mammalian skeletal muscle receptor (e.g., blocked by αBTx and activated by suberyldicholine), this toxin looked like a possible candidate for a selective antagonist of that receptor. As can be seen in Figure 8B, αA-CTx EIVA also fails to affect any of the Aplysia ACh receptors studied.
The effects of two other α-conotoxins isolated from the venom of the molluscivorous snail Conus pennaceus were previously studied on Aplysia neurons bearing the nicotinic receptor mediating the cationic response. Fainzilber et al. (1994) found that bath application of 0.5–1 μm concentrations of either αCTx PnIA or αCTx PnIB elicited, like ACh, a depolarization. Furthermore, they observed a desensitizing block of the depolarizing response to ACh as long as the toxin bathed the cell. Using synthesized αCTx PnIA and PnIB, we were unable to duplicate the agonist effect observed byFainzilber et al. (1994) on the cells responding to ACh with a cationic response or to observe any agonist effect on the cells responding to ACh with a two-component Cl-dependent response. Both toxins did, however, act as weak antagonists of these responses, as can be seen in Figure 8, C and D. Both toxins (at 1 μm) caused a small, reversible reduction in the cationic response (Fig. 8C,D, left records). In Figure 8C, right records, it can be seen that 1 μm αCTx PnIA almost completely blocked the rapidly desensitizing Cl-dependent response but also partially blocked the sustained element; αCTx PnIB (Fig. 8D, right records) slightly blocked both of the Cl-dependent elements (Fig. 8D, right column), showing a somewhat more marked diminution of the sustained response.
Pharmacological profiles of the receptors mediating the ionotropic responses in Aplysia neurons
From the findings reported above, three distinct “nicotinic” receptors can be distinguished, and Figures9 and 10summarize the pharmacological characteristics of the three responses they mediate: cationic, rapidly desensitizing Cl, and sustained Cl.
Fig. 9.
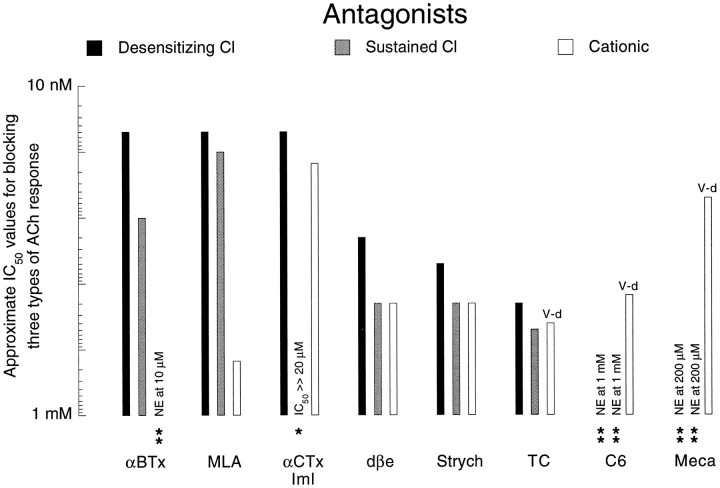
Relative ability of eight antagonists to block the three types of nicotinic response to 200 μm ACh expressed in approximate IC50 values. Estimates of IC50values were made by visual inspection of the data and have been established with the notion of obtaining a weighted rank ordering rather than of obtaining rigorously defined IC50 values (see Results). A perusal of a given tone of column (black, gray, or white) permits an evaluation of the differential facility with which the different antagonists block a given response type. A comparison of the three column tones for each antagonist reveals the ability of a given antagonist to discriminate between the different response types. Empty columns with two asterisks are cases in which no effect (NE) on the ACh response was seen with the maximum concentration tested, the value of which is indicated in lieu of the data column. One asterisk indicates that the antagonist can induce a slight reduction in the tagged response, but that the highest concentration used failed to block the response by half (only case being that of 20 μm αCTx ImI on the sustained Cl response). V-d, Instances in which the block of the cationic response was voltage-dependent.
Fig. 10.
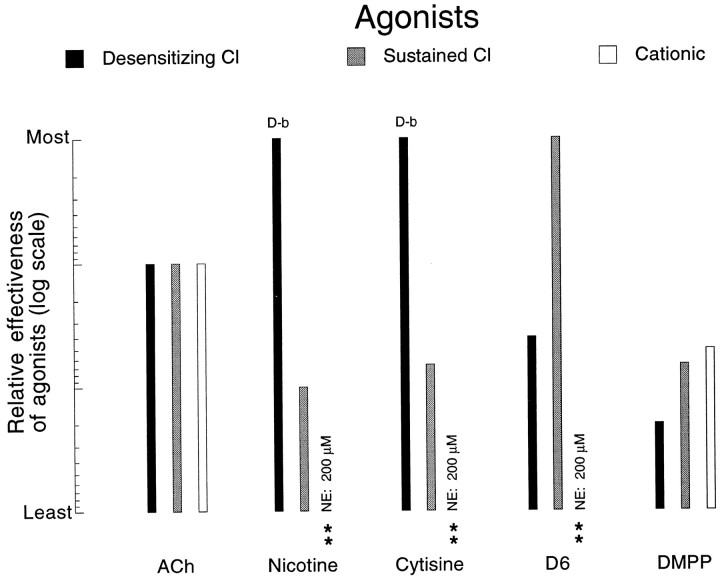
Relative effectiveness of different cholinomimetics in eliciting the three different types of nicotinic response. The log scale on which the data have been plotted shows, for example, that under the criteria used here (see Results), nicotine and cytisine are at least 10 times more effective than ACh in eliciting the rapidly desensitizing Cl-dependent response, whereas DMPP is 20 times less effective. Because of the desensitizing block (D-b) that nicotine and cytisine induce in the desensitizing Cl response, only low concentrations (1–20 μm) of those agonists were used in determining their relative efficacy on that receptor. The double asteriskindicates cases in which no response at all could be elicited (NE, no effect) with the highest concentration tested (200 μm).
Antagonists
Figure 9 summarizes the results obtained using the cholinergic anatagonists on the three nicotinic response types. The efficacy of the antagonists, presented on a log scale, has been indicated as the approximate antagonist concentration necessary to reduce the response to 200 μm ACh by half (IC50). The values included in the figure are those estimated in experiments in which the standard protocol was used. Lower IC50 values were obtained when longer exposures were used for evaluating the blockade by αBTx and MLA of the desensitizing Cl response (see above). The IC50 values presented in Figure 9 are very approximate, “rounded off” estimates obtained by visual inspection of many experiments performed on a variety of cell types. This approach was used in preference to a simple “greater or less than” analysis, because it permits an easier comparison across and within response types and offers a calibrated, weighted rank ordering. However, these values cannot be taken as rigorously quantitative estimates of IC50 values, for which it would have been necessary to work always on exactly the same cell type and to use a less-variable fast perfusion system. In some cases, the efficacy of an antagonist on a given response was so low that no IC50 could be obtained with the antagonist concentrations used (Fig. 9, see legend for details).
The pharmacological profiles revealed in Figure 9 permit a very clear separation between the cationic response and the Cl-dependent responses and show that the separation between the two Cl-dependent responses, in contrast, depends almost exclusively on αCTx ImI.
Agonists
The agonists offer a more detailed differentiation between the three responses, as can be seen in Figure 10. To obtain approximate estimates of relative efficacy of different agonists, the responses to varying concentrations of a given cholinomimetic were compared with the response obtained with 100 μm ACh. With agonists capable of inducing a desensitizing block of the activated receptor(s) (e.g., nicotine and cytisine on the receptor mediating the desensitizing Cl-dependent response), only low concentrations were used for such an estimate of efficacy.
Figure 10 clearly displays the preferential activation by nicotine and cytisine on the receptor mediating the desensitizing Cl response and the preferential activation by suberyldicholine of the receptor mediating the sustained Cl response. Even more striking is the relative ineffectiveness of all agonists tested here in activating the receptor mediating the cationic response.
DISCUSSION
Two pharmacologically distinct receptors mediate Cl-dependent responses in Aplysia neurons
The dissection by αCTx ImI of the ACh-induced Cl current in Aplysia neurons into a toxin-sensitive, rapidly desensitizing element and a toxin-resistant, sustained element and the differential activation of these two elements by different agonists strongly suggest that two different cholinergic receptors mediate the two kinetically defined increases in Cl conductance. This conclusion is reinforced by the finding that the relative proportion of the two elements is cell-specific.
While studying cholinergic synapses in the buccal ganglion, Gardner and Kandel (1977) noted that, in some cells, the synaptically activated or ACh-induced Cl-dependent response diminished with repetition, whereas in others it did not. The authors’ conclusion that “rate of desensitization is … an additional criterion for characterizing otherwise similar receptors for neurotransmitters” appears to receive support from the pharmacological findings reported here.
That more than one receptor subtype for a given transmitter can mediate a Cl conductance increase was recently demonstrated in the vertebrate GABAergic system, in which pharmacologically and molecularly distinct GABAA and GABAC receptors were shown to mediate rapidly desensitizing and slowly desensitizing Cl currents, respectively (for review, see Polenzani et al., 1991; Feigenspan and Bormann, 1994; Bormann and Feigenspan, 1995; Johnston, 1996;Lukasiewicz, 1996), and to yield two-component Cl-dependent responses in cells in which the two receptor types co-exist (Qian and Dowling, 1993). A similar two-component, glutamate-induced, Cl-dependent current has been seen in lobster neurons of the stomatogastric ganglion (Cleland and Selverston, 1995), but no pharmacological separation of the two elements has as yet been established.
A pharmacological comparison of Aplysia and vertebrate nicotinic receptors
αBTx-sensitive receptors in vertebrates and in Aplysia
Four distinct αBTx-sensitive vertebrate receptors have been pharmacologically and molecularly described; three of them, containing either α7, α8, or α9 gene product, respectively, have been shown to form functional homomeric receptors when the appropriate α subunit is expressed in oocytes (Couturier et al., 1990; Elgoyhen et al., 1994;Gerzanich et al., 1994). The fourth αBTx-sensitive receptor, found in skeletal muscle, is heteromeric, being composed of either α1β1γδ or α1β1εδ subunits. Because the α8-containing receptor, found as yet only in chick, has many pharmacological characteristics that are unlike those of any of the Aplysiareceptors (see Gerzanich et al., 1994), that receptor will not be discussed further here.
Is the receptor mediating the rapidly desensitizing Cl-dependent response in Aplysia a relative of α7?
Receptors containing the α7 gene product appear to account for most (for exception, see Pugh et al., 1995) if not all (seeOrr-Urtreger et al., 1997) neuronal αBTx-sensitive receptors in all species other than chick, where receptors containing the α8 subunit are also present. In vertebrate preparations in which they have been studied, receptors formed of α7 subunits have been shown to be permeable to calcium and to mediate a cationic, rapidly desensitizing, response (for review, see McGehee and Role, 1995; Colquhoun and Patrick, 1997a).
The Aplysia receptor mediating the rapidly desensitizing Cl-dependent response shows a striking pharmacological resemblance to the native, αBTx-sensitive receptor in chick ciliary ganglion and differs only slightly from the chick α7 recombinant receptors expressed in oocytes (Table 1). For both the Aplysia receptor and the chick α7 receptor, nicotine and cytisine (at low concentrations) are highly effective agonists, and on both the Aplysia receptor and the in vivo α7 chick receptor, these same agonists induce a desensitizing block (Table1, Agonists; Zhang et al., 1994), which is not, however, seen for the chick α7 recombinant receptors (Gerzanich et al., 1994). DMPP is a very ineffective agonist in both preparations (Table 1, Agonists).
Table 1.
Comparison of the relative effectiveness of agonists and antagonists on the Aplysia receptor mediating the rapidly desensitizing Cl-dependent response and on the avian α7-containing receptor
| Receptor type | Agonists | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Aplysia Cl rapidly desensitizing | Nicotine | ≥ | Cytisine | > | ACh | >> | DMPP | This paper |
| α7 chick in vivo | Nicotine | ≥ | Cytisine | >> | ACh | > | DMPP | Zhang et al., 1994 |
| α7 chick immunoisolated inhibition αBTx binding | Nicotine | ≥ | Cytisine | > | ACh | Anand et al., 1993a,b | ||
| α7 chick homomeric | Nicotine | ≥ | Cytisine | > | ACh | >> | DMPP | Couturier et al., 1990; Bertrand et al., 1992; Gerzanich et al., 1994; however, see Amar et al., 1993 |
| α7 chick homomeric inhibition αBTx binding | Cytisine | > | Nicotine | > | ACh | Anand et al., 1993a | ||
| Receptor type | Antagonists | References | ||||||
|---|---|---|---|---|---|---|---|---|
| AplysiaCl rapidly desensitizing | MLA | ≈ | αBTx | >> | Strychnine | > | TC | This paper |
| α7 chick in vivo | αBTx | > | αBTx MLA | >> >> | Strychnine | > | TC TC | Zhang et al., 1994; 1996,Vijayaraghavan et al., 1992 |
| α7 chick immunoisolated inhibition αBTx binding | MLA | ≥ | αBTx αBTx | > >> | Strychnine TC | ≥ ≥ | TC Strychnine | Pugh et al., 1995,Anand et al., 1993a,b |
| α7 chick homomeric | αBTx | >> | TC | > | Strychnine | Gerzanich et al., 1994 | ||
| α7 chick homomeric inhibition αBTx binding | αBTx | >> | TC | ≥ | Strychnine | Anand et al., 1993a,b | ||
Italics have been used to indicate the cases in which an agonist induces a desensitizing block on the receptor being activated.
The effects of cholinergic antagonists also suggest a striking similarity between the same two receptors (Table 1, Antagonists). They are both blocked by low concentrations of MLA and αBTx (Zhang et al., 1994). Whereas the Ki determined by competitive binding of avian brain αBTx binding sites (presumably α7-containing receptors) was found to be on the order of 1 nm(Vijayaraghavan et al., 1992; Anand et al., 1993b), a half-amplitude steady-state block of the rapidly desensitizing Cl-dependent response in Aplysia neurons (after 15 min exposure) was obtained with 10 nm αBTx. A higher sensitivity of both theAplysia and chick receptors to MLA was likewise revealed by experiments with longer exposures to that antagonist. The native avian receptor and the Aplysia receptor are also both blocked by strychnine and TC at concentrations ∼3 orders of magnitude higher than those required for MLA and αBTx (Vijayaraghavan et al., 1992;Anand et al., 1993a; Pugh et al., 1995; Zhang et al., 1994, 1996). Similar profiles emerge from binding studies on immunoisolated chick brain α7-containing receptors or from studies on recombinant chick α7 receptors, except that in those preparations, TC has been found to be slightly more rather than less effective than strychnine (Table 1, Antagonists).
αCTx ImI, which is the only antagonist to differentiate the rapidly desensitizing element of the Cl-dependent response inAplysia from the sustained element, has not yet been tested on the chick α7-containing receptor. However, this toxin was shown to block the rat α7 recombinant receptor (IC50, 220 nm; Johnson et al., 1995), the αBTx-sensitive, α7-containing (Orr-Urtreger et al., 1997) receptor in rat hippocampus (IC50, 85 nm; Pereira et al., 1996), as well as the presumably α7-containing receptor in human neuroendocrine carcinoma cells (Codignola et al., 1996).
Rat, human, and bovine α7-containing receptors show pharmacological properties similar to those of the in vivo chick α7-containing receptors, except for the finding that DMPP is a potent and efficacious agonist on the mammalian receptors (Alkondon and Albuquerque, 1993; Seguela et al., 1993; Garcia-Guzman et al., 1995;Albuquerque et al., 1997).
The parallel drawn between the Aplysia receptor mediating the rapidly desensitizing Cl-dependent response and the avian α7-containing receptor is reinforced by the finding of Galzi et al. (1992) that a substitution and/or addition of three amino acids in the channel domain of the avian α7 subunit was sufficient to transform the homomeric recombinant α7 receptor from an ACh-gated cationic channel to an ACh-gated anionic channel.
Given the pharmacological resemblance of the Aplysiareceptor mediating the rapidly desensitizing Cl-dependent response to the calcium-permeable α7 vertebrate receptor, it is perhaps necessary to clarify that the ACh-induced Cl-dependent currents observed inAplysia neurons persist in Ca-free external solutions as well as in cells perfused, in the whole-cell patch-clamp configuration, with 20 mm BAPTA in the internal solution (Kehoe, unpublished findings).
Does the Aplysia receptor mediating the sustained Cl-dependent response resemble either α9 or the skeletal muscle receptor?
α9. The α9 subunit has been cloned only from rat (Elgoyhen et al., 1994), and only a few and often incomplete studies have evaluated the pharmacological characteristics of the receptor, whether it be in its native habitat of cochlear hair cells (rat,Bartolami et al., 1993; Chen et al., 1996; guinea pig, Kakehata et al., 1993; Erostegui et al., 1994; Kujawa et al., 1994, Chen et al., 1996; chick, Fuchs and Murrow, 1992, McNiven et al., 1996; frog, Guth et al., 1994) (for review, see Fuchs, 1996; Guth and Norris, 1996) or in its recombinant form expressed in oocytes (Elgoyhen et al., 1994; Johnson et al., 1995).
For all species, ACh is a more potent agonist on the α9 receptor than is suberyldicholine, nicotine is ineffective even at 100 μm concentrations, and, in guinea pig, DMPP was found to be as potent as ACh (see references above). These agonist properties do not coincide with those of the Aplysia receptor mediating the sustained Cl-dependent response. A greater similarity is seen when comparing the effects of antagonists on the same two receptor types, because both are blocked by αBTx, strychnine, and TC (see references cited above for α9). However, whereas the homomeric α9 receptor was found to be blocked by αCTx ImI with an IC50 of 1.8 μm (Johnson et al., 1995), even a 10 times higher concentration clearly failed to reduce by half the sustained Cl-dependent response in Aplysia.
Skeletal muscle receptor. The only striking similarity between the mammalian muscle receptor and the Aplysiareceptor mediating the sustained Cl-dependent response is offered by their common marked sensitivity to suberyldicholine (see Figs. 5, 10 for the Aplysia response; Luetje and Sine and Steinbach, 1986, 1987; Patrick, 1991; Cooper et al., 1996 for muscle cells). The clearest discrepancy in the two agonist profiles concerns the relatively high sensitivity of muscle to DMPP (Luetje and Patrick, 1991; Cooper et al., 1996; Yost and Winegar, 1997), which is a very poor agonist for either of the receptors underlying Cl-dependent responses in Aplysia. Although many of the same antagonists are effective on the skeletal muscle receptor and on theAplysia receptor mediating the sustained Cl-dependent response, neither the relative potencies nor the threshold concentrations of the antagonists appear to be the same for the two receptor types (Ward et al., 1990; Walther, 1968; McIntosh et al., 1994; Johnson et al., 1995; Martin et al., 1996; Jacobsen et al., 1997).
The more thorough pharmacological evaluation provided here of the suberyldicholine-sensitive Aplysia receptor puts in doubt the parallel previously drawn between it and the vertebrate skeletal muscle receptor (Kehoe, 1979) and fails to suggest another vertebrate counterpart of that molluscan receptor.
Does the αBTx-resistant receptor mediating the cationic response in Aplysia have a vertebrate relative?
The receptor mediating the cationic, αBTx-resistant response inAplysia neurons distinguishes itself from all of the vertebrate nicotinic receptors by its failure to be activated by most of the agonists studied here (nicotine, cytisine, and suberyldicholine) as well as by decamethonium and succinylcholine (Kehoe, unpublished data). This newly obtained agonist profile renders obsolete the parallel, based excluisvely on antagonist data, drawn between theAplysia receptor mediating the cationic response and known ganglionic receptors in vertebrates (Kehoe, 1979).
The cationic response in Aplysia is unaffected by the highly selective α3β2 antagonist αCTx MII, is blocked in a primarily voltage-dependent way by mecamylamine, hexamethonium, and TC, and is blocked in a presumably competitive manner by αCTx ImI, strychnine, and dβe. Many, but never all, of these characteristics are shared by a number of the vertebrate αBTx-resistant recombinant neuronal receptors (Johnson et al., 1995; McGehee and Role, 1995; Cartier et al., 1996; Harvey and Luetje, 1996; Harvey et al., 1996; Tavazoie et al., 1997).
The failure to find a recombinant vertebrate receptor showing a profile similar to that of the Aplysia cationic response is hardly surprising, given the difficulty that such comparisons have encountered in preparations for which the possible contributing gene products in the native receptors are known (e.g., the rat superior cervical ganglion; Covernton et al., 1994; Lewis et al., 1997; Sivilotti et al., 1997), and given recent indications that most neuronal αBTx-resistant receptors are composed of more than two types of subunit, all of which can affect the agonist and antagonist profiles (McGehee and Role, 1995;Ramirez-Latorre et al., 1996; Colquhoun and Patrick, 1997b).
α-Conotoxin specificity
The findings reported here once more confirm the specificity of α-conotoxins. First, whereas all classical cholinergic antagonists failed to discriminate between the two Cl-dependent responses inAplysia, αCTx ImI from the worm-eating C. imperialis selectively eliminated one of them without affecting the other.
It is interesting to note that neither αCTx EVIA nor αCTx MII, both from fish-eating snails, affected any of the Aplysiareceptors. Other than αCTx ImI, which comes from a worm-eating snail, the only conotoxins or toxic elements (Elliott and Raftery, 1979) thus far shown to affect Aplysia receptors are from molluscivorous Conus snails (Fig. 8; Elliott and Kehoe, 1978).
Footnotes
This research was supported by the Centre National de la Recherche Scientifique (URA 1857) (to J.K.) and by National Institutes of Health Grants MH 53631 and GM 48677 (to J.M.M.). We thank Micha Spira for suggesting the use of αCTx ImI and for participation in some of the early experiments in which the toxin was tested.
Correspondence should be addressed to JacSue Kehoe, Laboratoire de Neurobiologie, Ecole Normale Supérieure, 46 rue d’Ulm, Paris 75005, France.
REFERENCES
- 1.Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CTF, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;80:1117–1136. [PubMed] [Google Scholar]
- 2.Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons: I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 3.Amar M, Thomas P, Johnson C, Lunt GG, Wonnacott S. Agonist pharmacology of the neuronal α7 nicotinic receptor expressed in Xenopus oocytes. FEBS Lett. 1993;327:284–288. doi: 10.1016/0014-5793(93)81005-k. [DOI] [PubMed] [Google Scholar]
- 4.Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993a;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- 5.Anand R, Peng X, Ballesta JJ, Lindstrom J. Pharmacological characterization of α-bungarotoxin sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of α7 and α8 subunit containing subtypes. Mol Pharmacol. 1993b;44:1046–1050. [PubMed] [Google Scholar]
- 6.Ascher P, Marty A, Neild TO. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol (Lond) 1978a;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascher P, Marty A, Neild TO. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol (Lond) 1978b;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartolami S, Ripoll C, Eybalin M. Anticholinergic effects of strychnine in the cochlea do not involve muscarinic receptors. NeuroReport. 1993;4:1003–1006. doi: 10.1097/00001756-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric α7 receptor. Neurosci Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- 10.Bormann J, Feigenspan A. GABA C receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 11.Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-Conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Lebland C, Bobbin RP. Differences in cholinergic responses from outer hair cells of rat and guinea pig. Hear Res. 1996;98:9–17. doi: 10.1016/0378-5955(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 13.Cleland TA, Selverston AI. Glutamate-gated inhibitory currents of central pattern generator neurons in the lobster stomatogastric ganglion. J Neurosci. 1995;15:6631–6639. doi: 10.1523/JNEUROSCI.15-10-06631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codignola A, McIntosh JM, Cattaneo MG, Vicentini LM, Clementi F, Sher E. α-Conotoxin imperialis I inhibits nicotine-evoked hormone release and cell proliferation in human neuroendocrine carcinoma cells. Neurosci Lett. 1996;206:53–56. doi: 10.1016/0304-3940(96)12423-x. [DOI] [PubMed] [Google Scholar]
- 15.Colquhoun LM, Patrick JW. Pharmacology of neuronal nicotinic acteylcholine receptor subtypes. Adv Pharmacol. 1997a;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- 16.Colquhoun LM, Patrick JW. α3, β2, and β4 form heterotrimeric neuronal nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 1997b;69:2355–2362. [PubMed] [Google Scholar]
- 17.Cooper JC, Gutbrod O, Witzemann V, Methfessel C. Pharmacology of the nicotinic acetylcholine receptor from fetal rat muscle expressed in Xenopus occytes. Eur J Pharmacol. 1996;309:287–298. doi: 10.1016/0014-2999(96)00294-4. [DOI] [PubMed] [Google Scholar]
- 18.Couturier S, Bertrand D, Matter J-M, Hernandez M-C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by αBTx. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 19.Covernton PJO, Kojima H, Sivilotti LG, Gibb AJ, Colquhoun D. Comparison of neuronal nicotinic receptors in rat sympathetic neurones with subunit pairs expressed in Xenopus oocytes. J Physiol (Lond) 1994;481:27–34. doi: 10.1113/jphysiol.1994.sp020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 21.Elliott EJ, Kehoe JS. Cholinergic receptor in Aplysia neurons: activation by a venom component from the marine snail Conus californicus. Brain Res. 1978;156:387–390. doi: 10.1016/0006-8993(78)90525-5. [DOI] [PubMed] [Google Scholar]
- 22.Elliott EJ, Raftery MA. Venom of marine snail Conus californicus: biochemical studies of a cholinomimetic component. Toxicon. 1979;17:259–268. doi: 10.1016/0041-0101(79)90216-2. [DOI] [PubMed] [Google Scholar]
- 23.Erostegui C, Norris CH, Bobbin RP. In vitro pharmacologic characterization of a cholinergic receptor on outer hair cells. Hear Res. 1994;74:135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 24.Fainzilber M, Hasson A, Oren R, Burlingame AI, Gordon D, Spira ME, Zlotkin E. New mollusc-specific α-conotoxins block Aplysia neuronal acetylcholine receptors. Biochemistry. 1994;33:9523–9529. doi: 10.1021/bi00198a018. [DOI] [PubMed] [Google Scholar]
- 25.Feigenspan A, Bormann J. Differential pharmacology of GABA A and GABA C receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs PA. Synaptic transmission at vertebrate hair cells. Curr Opin Neurobiol. 1996;6:514–519. doi: 10.1016/s0959-4388(96)80058-4. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci. 1992;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galzi JL, Devillers-Thiéry A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–504. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Guzman M, Sala F, Sala S, Campos-Caro A, Stühmer W. α-Bungarotoxin-sensitive nicotinic receptors on bovine chromaffin cells: molecular cloning, functional expression and alternative splicing of the α7 subunit. Eur J Neurosci. 1995;7:647–655. doi: 10.1111/j.1460-9568.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 30.Gardner D, Kandel ER. Physiological and kinetic properties of cholinergic receptors activated by multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977;40:333–348. doi: 10.1152/jn.1977.40.2.333. [DOI] [PubMed] [Google Scholar]
- 31.Ger BA, Zeimal EV. Pharmacological study of two kinds of cholinoreceptors on the membrane of identified completely isolated neurones of Planorbarius corneus. Brain Res. 1976;107:527–540. doi: 10.1016/0006-8993(77)90443-7. [DOI] [PubMed] [Google Scholar]
- 32.Gerzanich V, Anand R, Lindstrom J. Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- 33.Guth PS, Norris CH. The hair cell acetylcholine receptors: a synthesis. Hear Res. 1996;98:1–8. doi: 10.1016/0378-5955(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 34.Guth PS, Dunn A, Kronomer K, Norris CH. The cholinergic pharmacology of the frog saccule. Hear Res. 1994;75:225–232. doi: 10.1016/0378-5955(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 35.Harvey SG, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor β subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen R, Yoshikami D, Ellison M, Martinez J, Gray WR, Cartier GE, Shon KJ, Groebe DR, Abramson SN, Olivera BM, McIntosh JM. Differential targeting of nicotinic acetylcholine receptors by novel αA-conotoxins. J Biol Chem. 1997;272:22531–22537. doi: 10.1074/jbc.272.36.22531. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DS, Martinez J, Elgoyhen AB, Heinemann SF, McIntosh JM. α-Conotoxin ImI exhibits subtype-specific nicotinic acetylcholine receptor blockade: preferential inhibition of homomeric α7 and α9 receptors. Mol Pharmacol. 1995;18:194–199. [PubMed] [Google Scholar]
- 39.Johnston GAR. GABA C receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- 40.Johnson J, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 41.Kakehata S, Nakagawa T, Takasaka T, Akaike N. Cellular mechanism of acetylcholine-induced response in dissociated outer hair cells of guinea pig cochlea. J Physiol (Lond) 1993;463:227–244. doi: 10.1113/jphysiol.1993.sp019592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kehoe JS. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol (Lond) 1972a;225:85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehoe JS. Three acetylcholine receptors in Aplysia neurones. J Physiol (Lond) 1972b;225:115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kehoe JS. Acetylcholine receptors on molluscan neurones. In: Olive G, editor. Advances in Pharmacology and Therapeutics, Vol 8. Pergamon; Oxford: 1979. pp. 285–298. [Google Scholar]
- 45.Kehoe JS. Synaptic block of a transmitter-induced potassium conductance in Aplysia neurones. J Physiol (Lond) 1985;369:399–437. doi: 10.1113/jphysiol.1985.sp015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kehoe JS, Vulfius CA (1995) Comparisons and interactions between three transmitter-induced Cl-dependent responses inAplysia neurons. Soc Neurosci Abstr 21.
- 47.Kehoe JS, Sealock R, Bon C. Effects of α-toxins from Bungarus multicinctus and Bungarus caeruleus on cholinergic responses in Aplysia neurones. Brain Res. 1976;107:527–540. doi: 10.1016/0006-8993(76)90142-6. [DOI] [PubMed] [Google Scholar]
- 48.Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear Res. 1994;74:122–134. doi: 10.1016/0378-5955(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 49.Lewis TM, Harkness PC, Sivilotti LG, Colquhoun D, Millar NS. The ion channel properties of a rat recombinant neuronal nicotinic receptor are dependent on the host cell type. J Physiol (Lond) 1997;505:299–306. doi: 10.1111/j.1469-7793.1997.299bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luetje CW, Patrick J. Both α and β subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukasiewicz PD. GABAC receptors in the vertebrate retina. Mol Neurobiol. 1996;12:181–194. doi: 10.1007/BF02755587. [DOI] [PubMed] [Google Scholar]
- 52.Martin M, Czajkowski C, Karlin A. The contributions of aspartyl residues in the acetylcholine receptor γ and δ subunits to the binding of agonists and competitive antagonists. J Biol Chem. 1996;271:13497–13503. doi: 10.1074/jbc.271.23.13497. [DOI] [PubMed] [Google Scholar]
- 53.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 54.McIntosh JM, Yoshikami D, Mahe E, Nielsen DB, Rivier JE, Gray WR, Olivera BM. A nicotinic acetylcholine receptor ligand of unique specificity, α-conotoxin ImI. J Biol Chem. 1994;269:16733–16739. [PubMed] [Google Scholar]
- 55.McNiven AI, Yuhas WA, Fuchs PA. Ionic dependence and agonist preference of an acetylcholine receptor in hair cells. Aud Neurosci. 1996;2:63–77. [Google Scholar]
- 56.Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, de Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira EF, Alkondon M, McIntosh JM, Albuquerque EX. α-Conotoxin ImI: a competitive antagonist at α-bungarotoxin-sensitive neuronal nicotinic receptors in hippocampal neurons. J Pharmacol Exp Ther. 1996;278:1472–1483. [PubMed] [Google Scholar]
- 58.Polenzani L, Woodward RM, Miledi R. Expression of mammalian γ-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci USA. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pugh PC, Corriveau RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding α-bungarotoxin. J Pharmacol Exp Ther. 1995;47:717–725. [PubMed] [Google Scholar]
- 60.Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 62.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sine SM, Steinbach JH. Activation of acetylcholine receptors on clonal mammalian BC2H-1 cells by low concentrations of agonist. J Physiol (Lond) 1986;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sine SM, Steinbach JH. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol (Lond) 1987;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J Physiol (Lond) 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slater NT, Carpenter DO. Blockade of acetylcholine-induced inward currents in Aplysia neurons by strychnine and desipramine: effect of membrane potential. Cell Mol Neurobiol. 1982;2:53–58. [Google Scholar]
- 67.Tauc L, Gerschenfeld HM. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- 68.Tavazoie SF, Tavazoie MF, McIntosh JM, Olivera BM, Yoshikami D. Differential block of nicotinic synapses on B versus C neurones in sympathetic ganglia of frog by alpha-conotoxins MII and IMI. Br J Pharmacol. 1997;120:995–1000. doi: 10.1038/sj.bjp.0700993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijayaraghavan S, Pugh PC, Zhang Z-w, Rathouz MM, Berg DK. Nicotinic receptors that bind α-bungarotoxin on neurons raise intracellular free Ca2+. Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 70.Walther H. On receptor mechanism of strychnine at the motor endplate. Acta Biol Med Ger. 1968;21:367–376. [PubMed] [Google Scholar]
- 71.Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- 72.Yost CS, Winegar BD. Potency of agonists and competitive antagonists on adult- and fetal-type nicotinic acetylcholine receptors. Cell Mol Neurobiol. 1997;17:35–50. doi: 10.1023/A:1026325020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang ZW, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 74.Zhang AW, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to α-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]