Abstract
Transient global cerebral ischemia resulting from cardiac arrest is known to cause selective death in vulnerable neurons, including hippocampal CA1 pyramidal neurons. It is postulated that oxygen radicals, superoxide in particular, are involved in cell death processes. To test this hypothesis, we first used in situ imaging of superoxide radical distribution by hydroethidine oxidation in vulnerable neurons. We then generated SOD1 transgenic (Tg) rats with a five-fold increase in copper zinc superoxide dismutase activity. The Tg rats and their non-Tg wild-type littermates were subjected to 10 min of global ischemia followed by 1 and 3 d of reperfusion. Neuronal damage, as assessed by cresyl violet staining and DNA fragmentation analysis, was significantly reduced in the hippocampal CA1 region, cortex, striatum, and thalamus in SOD1 Tg rats at 3 d, as compared with the non-Tg littermates. There were no changes in the hippocampal CA3subregion and dentate gyrus, resistant areas in both SOD1 Tg and non-Tg rats. Quantitative analysis of the damaged CA1 subregion showed marked neuroprotection against transient global cerebral ischemia in SOD1 Tg rats. These results suggest that superoxide radicals play a role in the delayed ischemic death of hippocampal CA1 neurons. Our data also indicate that SOD1 Tg rats are useful tools for studying the role of oxygen radicals in the pathogenesis of neuronal death after transient global cerebral ischemia.
Keywords: superoxide dismutase, transgenic rat, superoxide radicals, transient global cerebral ischemia, delayed neuronal degeneration, DNA fragmentation
Free radicals with unpaired electrons are generally highly reactive molecules that initiate radical chain reactions and damage cellular macromolecules, including proteins, DNA, and lipids, ultimately leading to cell death. Superoxide radicals and other oxygen radicals have been implicated in neuronal cell death in acute CNS injury and in chronic neurodegenerative diseases (Kontos, 1985; Coyle and Puttfarcken, 1993; Chan, 1994). One particular role of oxygen free radicals in brain injury appears to involve reperfusion after cerebral ischemia (Chan, 1996). Reperfusion supplies oxygen to the ischemic region of the brain in which oxygen is being used in mitochondria to generate adenosine triphosphate, and superoxide radicals and H2O2 are produced as a by-product (Boveris and Chance, 1973). Mitochondria are known to be the site for the production of reactive oxygen species in cultured cortical neurons after exposure to NMDA (Dugan et al., 1995). Although several antioxidant enzymes (including superoxide dismutases [SODs], glutathione peroxidases, and catalase) process these oxygen radicals, when they are overproduced, they generally exceed the capacity of the endogenous antioxidant enzymes, causing oxidative stress or injury of brain cells during reperfusion after an ischemic insult. We have demonstrated that overexpression of cytosolic copper zinc (CuZn) SOD in transgenic (Tg) mice plays a protective role in several types of brain injury, including cold-injury-induced brain edema (Chan et al., 1991), transient focal cerebral ischemia (Kondo et al., 1997b), traumatic brain injury (Mikawa et al., 1996), and hypoxic and excitotoxic neuronal injury in cultures (Chan et al., 1990; Copin et al., 1996). Although reperfusion after transient global cerebral ischemia resulting from cardiac arrest is known to produce oxygen radicals that damage selective vulnerable neurons, it is not clear whether increased CuZn SOD activity will provide neuroprotection against this ischemic insult. Mice that overexpress SOD1 would be a perfect choice to address this issue. However, because of the plasticity of the arteries in most mouse strains, SOD1 Tg mice are not well suited for transient global cerebral ischemia, which requires the hypoplasticity of the posterior communicating arteries (Murakami et al., 1997, 1998a).
Despite recent success with the use of Tg and knock-out mutant mice in elucidating the oxidative mechanisms of brain injury after stroke, there are many obvious advantages to using the rat species for construction of Tg animals. The development of a Tg rat to be applied specifically toward gaining understanding of the underlying mechanisms of oxidative stress in stroke would provide many advantages over the use of Tg or knock-out mice. One of these advantages is the fact that rat global cerebral ischemia models are well established (Pulsinelli et al., 1982; Smith et al., 1984), whereas, application of these models is lacking or scarce in mice. In addition, the cerebrovascular structure, brain anatomy, various physiological parameters, blood hemodynamics, and many stroke risk factors have been characterized in rats. The larger size of the animal would permit additional studies, including but not limited to, electrophysiology, microdialysis, studies with systemic cardiovascular variables, regional cerebral blood flow and metabolism, multiorgan physiology, and interaction in both anesthetized and awake animals. For these reasons, as well as for ease of manipulation and performance of physiological measurements, Tg rats that overexpress SOD1 have been made. Using these SOD1 Tg rats, we now provide evidence that the death of vulnerable hippocampal CA1 neurons is significantly reduced after transient global cerebral ischemia, suggesting a role that superoxide radicals play in delayed ischemic neuronal death.
MATERIALS AND METHODS
Transgene purification and preparation. A 14 kbBamHI–EcoRI genomic fragment of the SOD1 gene that contains five exons, and its own promoter, was used for generating Tg rats. The SOD1 genomic DNA was purified from low-melting agarose gel after electrophoresis using EluTip (S & S). The eluted DNA was diluted to 2 ng/μl in 10 mm Tris, pH 7.4, 2 mm EDTA for injection.
Production of Tg rats. Female Sprague Dawley rats (60–90 gm; Charles River, Holister, CA) were superovulated with 15 U gonadotropin from pregnant mare serum (G4877; Sigma, St. Louis, MO) at 12 P.M. to 2 P.M. followed 48 hr later with 5–15 U human chorionic gonadotropin (CG-5; Sigma), mated to fertile Sprague Dawley males, and checked for “plugs” the following morning. Fertilized eggs were flushed from oviducts using M2 media (M7167; Sigma) at ∼2 P.M. the following day, incubated in hylauronidase (U/ml in M2 media) for 5 min at room temperature, washed through several changes of M2, placed in a 20 μl drop of M16 (M7292; Sigma) overlaid with mineral oil, and incubated at 37°C in 7% CO2.
Fertilized eggs with visible pronuclei were placed in an injection well prepared on a LabTek two-chamber slide with M2 media (50 μl M2 media spread to a diameter of ∼1 cm) and overlaid with mineral oil. The slide was secured to the stage of a Leica DMIRB inverted light microscope equipped with Nomarski DIC optics and two Narishige (Greenvale, NY) three-axis hanging joystick micromanipulators. The DNA was injected using an Eppendorf (Fremont, CA) transjector 5246 and needles fabricated from microcapillaries (TW100F-4; World Precision Instruments, Sarasota, FL) pulled using a Sutter Instruments (Novato, CA) model P-77 micropipette puller. DNA was injected at a concentration of 2 ng/μl.
Injected zygotes were transferred the same day (8–10 P.M.) to the right oviduct of 24 hr postcoitus (with vasectomized Sprague Dawley male) “pseudopregnant” female Sprague Dawley rats. Pups were born 24 d from the day of transfer.
Fluorescence in situ hybridization. The site of transgene integration was determined by fluorescence in situ hybridization (FISH) on metaphase chromosome spreads prepared from blood lymphocytes, as described (Shi et al., 1994). The 12 kb SOD1 transgene fragment was labeled with digoxigenin-11-dUTP (Boehringer Mannheim, Indianapolis, IN) by nick translation (Enzo Biochem Inc., New York, NY) and detected using fluorescein isothiocyanate-conjugated sheep anti-digoxigenin Fab fragment (Boehringer Mannheim) (Shi et al., 1994). The slides were counterstained with propidium iodide and 4′6-diamidino-2-phenylindole in antifade solution (Oncor, Inc., Gaithersburg, MD) and photographed using ASA 400 Kodak Gold (Eastman Kodak, Rochester, NY) and a Zeiss Photoscope III.
Isoelectric focusing gel electrophoresis. Transgenic animals were identified by expression of the human SOD1 enzyme from red blood cell lysates using horizontal isoelectric focusing gel electrophoresis (pH 4.5–6.0; Multiphor; Pharmacia Biotech) and staining for SOD1 activity with nitroblue tetrazolium (Sigma) (Epstein et al., 1987). Five microliters of whole blood was lysed in 500 μl 2 mmEDTA and 0.5% NP-40. One microliter of lysate was mixed with 10 μl 1% glycine, loaded onto the gel, and electrophoresed for 2 hr at 2000 V and 12 A, limited with 0.1 m β-alanine cathode and 0.1m glutamic acid in 0.5 mH3PO4 anode. After electrophoresis, the gel was soaked with gentle mixing in the dark for 10 min in 100 ml of 0.036m phosphate buffer, pH 7.8, with added 16.1 mg nitroblue tetrazolium, 175 μlN,N,N′,N′-tetramethylethylenediamine (Sigma), and 20 μl 0.5 m EDTA, pH 8.0, and then removed from the solution, placed on a glass plate, and transferred to a light box to develop for 5–10 min. For analysis of transgene expression in tissues, 2–4 μg of tissue homogenates was electrophoresed as above.
Enzyme activity. The total activity of CuZn SOD was determined as previously described (Epstein et al., 1987).
Evaluation of anatomical background. To determine the anatomical background of the ischemia, we evaluated cerebral vasculature in both groups of animals using carbon black injection (Murakami et al., 1998a) with minor modifications. After anesthesia with ketamine (80 mg/kg) and xylazine (12 mg/kg), the rats were killed by transcardial perfusion with 200 ml of 10 U/ml heparin in saline and 300 ml of 3.7% formaldehyde in 0.1 m PBS. Carbon black, in an equal volume of 20% gelatin in H2O was injected from the ascending aorta. The brain was removed and fixed in 3.7% formaldehyde in PBS for 24 hr. The cerebral vasculature was observed with a dissecting microscope (Stemi 2000C, Zeiss).
Transient global cerebral ischemia. Transient global ischemia was induced by bilateral common carotid artery (CCA) occlusion and bleeding to lower the mean arterial blood pressure to 35–40 mmHg, using the previously described method of Smith et al. (1984) with some modifications. Male SOD1 Tg rats (325–540 gm) and control non-Tg littermates were anesthetized with 5% isoflurane in 70% N2O and 30% O2, and maintained during surgery at a level of 1.5–2% isoflurane in 70% N2O and 30% O2 under spontaneous breathing. The rectal temperature was controlled at 37.0 ± 0.5°C during surgery with a feedback-regulated heating pad. The femoral artery was exposed and catheterized with a PE-50 catheter to allow continuous recording of the arterial blood pressure and removal of blood samples for blood gas analysis. The right jugular vein was isolated and cannulated with a 23 gauge butterfly needle for intravenous injection of 500 U/kg heparin dissolved to 100 U/ml with 0.9% saline. After exposing the bilateral CCAs, blood was quickly withdrawn from the jugular vein cannula to achieve a reduction of the mean arterial blood pressure. When the blood pressure reached 40 mmHg, the bilateral CCAs were temporarily occluded with metal clips. The blood pressure was maintained at 35–40 mmHg by additional blood withdrawal and reinfusion. After 10 min, ischemia was terminated by removal of the CCA clips, and the blood was reinfused. Blood flow in the forebrain, measured by laser Doppler flowmetry, dropped to 10% of the basal level during ischemia and rapidly returned to 100% after reperfusion. After recovery of the arterial blood pressure, the arterial blood was collected for blood gas analysis. The animals regained consciousness and were maintained in an air-conditioned room at 20°C for 1 and 3 d after reperfusion.
At the end of the recovery period, the rats were deeply anesthetized with methoxyflurane, and the brains were removed and rapidly frozen. Coronal sections of the brains (20 μm) were cut with a cryostat and mounted onto slides. The sections were stained with cresyl violet for histological assessment of neuronal cell damage. The brain regions examined were the hippocampus, cortex, thalamus, and striatum. The histopathological damage was measured using a neuropathological scoring system of 0–6, in which 0 = no damage, 1 = 0–10%, 2 = 10–25%, 3 = 25–50%, 4 = 50–75%, 5 = 75–100%, and 6 = complete neuronal death (McBean et al., 1995). Scores for SOD1 Tg rats were compared with those for the non-Tg littermates by means of a nonparametric analysis using the Mann–Whitney U test. The lesion areas of the hippocampal CA1 subregion were quantified using the image analysis system of Swanson et al. (1990)with some modification. The present method was created to evaluate infarct size in focal ischemia by measuring areas of the stained sections that had optical densities exceeding a threshold value and was applied to measure the neuronal damage in the CA1 subregion after global ischemia without sampling error and observer bias. Fresh frozen brains were sectioned with a cryostat into a 20 μm thickness from the anterior side to the posterior side at 1 mm intervals, consecutively. The sections were mounted on slide glass and stained with cresyl violet using standard histological criteria. The stained images were scanned by a Color One scanner (Apple Computer, Cupertino, CA). In each hippocampal section the lengths of the unstained and total CA1 subregions were measured by the NIH image program. The area of the damaged and total CA1 pyramidal cell layer was calculated by integrating the length of the damaged and total CA1 pyramidal cell layers by the distance. The ratio of CA1 damage was calculated as (area of CA1damage/area of total CA1 subregion) × 100%. The hemisphere area at the posterior commissure level in the coronal sections was also measured using the same method to evaluate brain swelling and edema. Results were expressed as mean ± SE. The statistical significance of differences between the SOD1 Tg rat and non-Tg littermates was evaluated by Fisher’s protected least significant difference test followed by the nonparametric ttest. Significance between groups was assigned at a level of <5% probability.
In situ detection of superoxide anion (O2−) production. The spatial production of O2− during cerebral ischemia was investigated by the in situ detection of oxidized hydroethidine (HEt) method as previously described (Kondo et al., 1997a; Murakami et al., 1998b) with minor modifications. HEt (Molecular Probes, Eugene, OR) is taken up by living cells and oxidized to a red fluorescent dye, ethidium, specifically by O2−, but not by other reactive oxygen species in the cells (Bindokas et al., 1996). The rats were anesthetized with 2% isoflurane in 30% O2 and 70% N2O. The HEt solution (1 ml; 1 mg/ml in 1% dimethylsulfoxide with PBS) was administered intravenously 1 hr before killing. The rats were killed at 1 hr and 1 and 3 d after ischemia by transcardial perfusion with 10 U/ml heparin in saline and 3.7% formaldehyde. After post-fixation, the brains were cut into slices of 50 μm thickness at the level of the anterior commissure and the hippocampus using a vibratome and placed on glass slides. These sections were analyzed under fluorescent light (HBO 100 W/2, Zeiss), and fluorescence was assessed at excitation = 510–550 nm and emission > 580 nm for detection of ethidium. Photomicrographs of the hippocampus and the cortex were taken, and the intensity and expression patterns of the oxidized HEt were compared with control nonischemic brains and between each period after 10 min of global ischemia.
In situ detection of cells with fragmented DNA. DNA fragmentation after global ischemia was determined by the terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) method in the brains of Tg and control rats. Frozen hippocampal brain sections were stained as previously described (Murakami et al., 1998b) with minor modifications. In brief, frozen brain sections were fixed with 3.7% formaldehyde. After endogenous peroxidase was inactivated with 0.3% H2O2 for 30 min, the sections were immersed in terminal deoxynucleotidyl transferase (TdT) buffer (Life Technologies, Gaithersburg, MD) and incubated with TdT and biotin-16-uridine-5′-triphosphate (Boehringer Mannheim). After blocking with 2% bovine serum albumin in PBS, the sections were incubated with avidin-biotin-horseradish peroxidase (ABC kit; Vector Laboratories, Burlingame, CA) and visualized with 3 mm 3,3′-diaminobenzidine tetrahydrochloride and 18 mm hydrogen peroxide in PBS. The slides were counterstained with methyl green and mounted.
TUNEL-positive cells were quantified with a light microscope by a blinded investigator. The total number of cells and the number of TUNEL-positive cells within a grid were counted using high-powered magnification (400×). The ratio of the number of TUNEL-positive neurons to the total injured neurons was calculated and expressed as percent of the TUNEL-positive cells in each group.
RESULTS
Characterization of SOD1 Tg rats
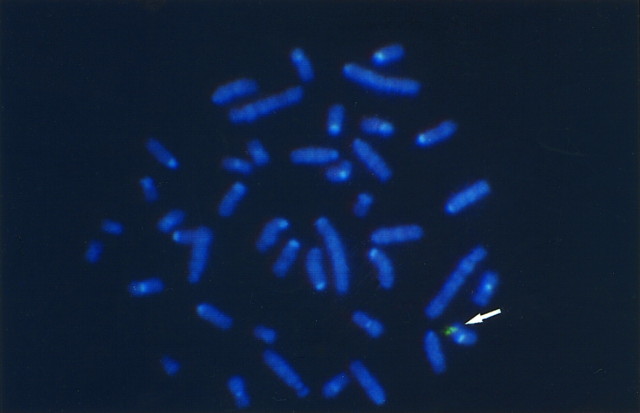
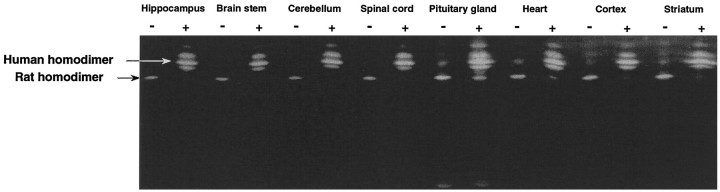
SOD1 Tg rats [number 66, University of California San Francisco (UCSF)] have been successfully produced. In this strain, the human SOD1 transgene was incorporated into a single chromosome as shown by FISH (Fig. 1). Because the human and rat SOD proteins comigrate in a nondenaturing gel system (Epstein et al., 1987), we developed a new nondenaturing isoelectric focusing gel electrophoresis (IFGE) method to separate these two proteins, thereby permitting semiquantitative assay of these two enzymes. The human homodimer and the heterodimer composed of human and rat segments were clearly demonstrated in various brain regions, including the hippocampus, brainstem, cerebellum, spinal cord, pituitary gland, cerebral cortex, and striatum, as well as in heart tissue in the IFGE gel (Fig. 2). Because of the high expression of the human transgene relative to the endogenous rat genes, the rat CuZn SOD homodimer was rarely visualized. Total CuZn SOD activity ranged from 8.2–27.2 U/mg in various brain regions as compared with the range of 2.5–5.3 U/mg in non-Tg rat counterparts (Table 1). The CuZn SOD activity, therefore, increased ∼5.2–6.2-fold in the Tg rats.
Fig. 1.
Incorporation of human CuZn SOD (SOD1) transgene into a single rat chromosome detected by fluorescence in situ hybridization. Note the spots of bright fluorescence located in one of the rat chromosomes.
Fig. 2.
Expression of human CuZn SOD in various brain regions and heart tissue in SOD1 Tg rat. The tissue homogenates from various brain regions and from heart tissue were subjected to isoelectric focusing gel electrophoresis. The short arrow indicates the homodimer of rat CuZn SOD. The long arrow indicates the homodimer of the human CuZn SOD. The band between the rat and human CuZn SOD homodimers is the rat/human heterodimer.
Table 1.
SOD activity in regions of the rat brain
| Region | Non-Tg | Tg | Fold Increase |
|---|---|---|---|
| U/mg ± SEM | U/mg ± SEM | ||
| Cortex | 2.5 ± 0.2 | 14.3 ± 1 | 5.8 |
| Striatum | 2.8 ± 0.3 | 18.2 ± 1.9 | 5.5 |
| Hippocampus | 4.5 ± 0.5 | 23.5 ± 3.7 | 5.2 |
| Brainstem | 5.2 ± 0.4 | 27 ± 2.6 | 5.3 |
| Cerebellum | 4.4 ± 0.6 | 27.2 ± 3.9 | 6.2 |
| Spinal cord | 4.5 ± 0.5 | 23.5 ± 3.7 | 5.2 |
The cerebral vasculature was analyzed to confirm that both anatomical backgrounds were the same in SOD1 Tg rats and non-Tg littermates. Cerebral vasculature was determined by carbon black injection (Fig.3). The method used herein allowed us to determine the structure of major blood vessels (Fig. 3A,B) in the brain and the posterior communicating artery (Fig.3C,D) that influence the ischemic status in global ischemia. There was no remarkable difference between SOD1 Tg rats and non-Tg littermates.
Fig. 3.
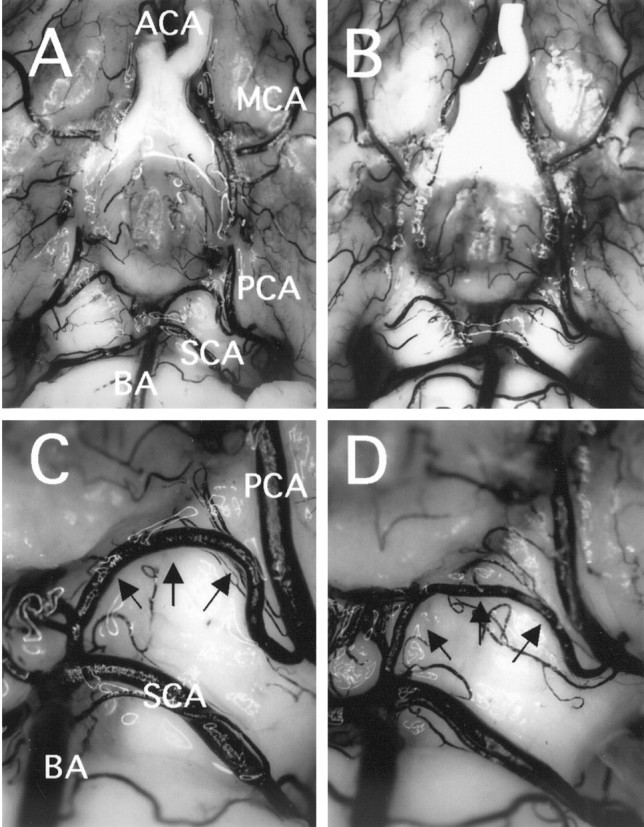
Photographs showing similarity in cerebral vasculature in non-Tg littermates (left) and SOD1 Tg rats (right). The major blood vessels involved in the circle of Willis were almost the same in both animals (A, B). The plasticity of the posterior communicating artery (arrows) that influences the outcome of hippocampal injury after global ischemia was similar between the two animals (C, D). ACA, Anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; SCA, superior cerebellar artery; BA, basilar artery.
Production of O2− during global cerebral ischemia
Before the use of SOD1 Tg rats for transient global cerebral ischemia studies, we first wanted to verify the increased production of O2− in the brains of animals after transient cerebral ischemia, especially in the areas with vulnerable neurons (i.e., hippocampal CA1 subregion). Therefore, production of O2− was determined using HEt in the cortex and hippocampal CA1 and CA3 subregions at 1 hr and 1 and 3 d after 10 min global ischemia. As previously observed byKondo et al. (1997a), O2− production was shown by oxidized HEt signals as small particles in the cytosol, suggesting possible mitochondrial production of O2− under normal physiological conditions (Fig.4A–C).
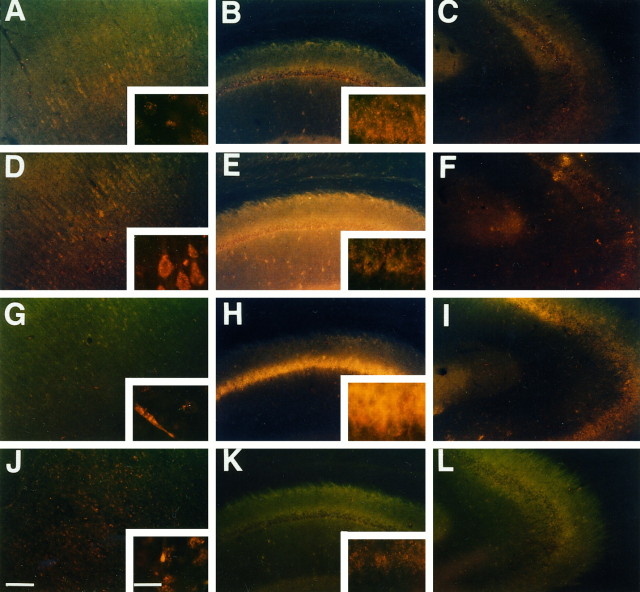
Fig. 4.
Superoxide radical imaging in ischemic brain. Representative photographs showing the production of O2− by the presence of oxidized HEt in cortex (left), hippocampal CA1(middle), and CA3 (right) subregions. As previously observed by Kondo et al. (1997a), O2− production was demonstrated under normal physiological conditions by oxidized HEt signals appearing as small particles in the cytosol, suggesting possible mitochondrial production of O2− (A–C). At 1 hr after ischemia and reperfusion, the diffuse cytosolic oxidized HEt signal was observed in cortical cells (D). Although a high background could be observed, the cytosolic signals were not increased in the hippocampal CA1(E) and CA3 (F) subregions. At 1 d after ischemia, the diffuse cytosolic signals attenuated to preischemic levels in cortical cells except for endothelial cells (G). However, the marked diffuse cytosolic signal remained in the hippocampal CA1subregion (H) but not in the CA3 subregion (I). The diffuse signal was not found at 3 d after ischemia (J–L). Scale bar, 200 μm (lower magnification); 20 μm (higher magnification).
At 1 hr after ischemia and reperfusion, the increase in the diffuse cytosolic signal of oxidized HEt was markedly observed in cortical cells (Fig. 4D). In the hippocampus the diffuse cytosolic signal of oxidized HEt was also observed, but the intensity was similar to that of the preischemic level (Fig.4E,F). At 1 d after ischemia, the diffuse cytosolic signals in cortical cells returned to the preischemic level, and, except for the endothelial cells (Fig. 4G), the marked expression of the signal was present in the hippocampal CA1subregion (Fig. 4H). The cytosol was filled with diffuse oxidized HEt fluorescence in these cells. However, signals were not observed in the CA3 subregion (Fig.4I). The oxidized HEt signals decreased at 3 d after ischemia (Fig. 4J–L). The observation of increased production of O2− in vulnerable brain regions prompted us to proceed to the use of SOD1 Tg rats for the transient global cerebral ischemia studies.
Delayed neuronal injury after transient global cerebral ischemia
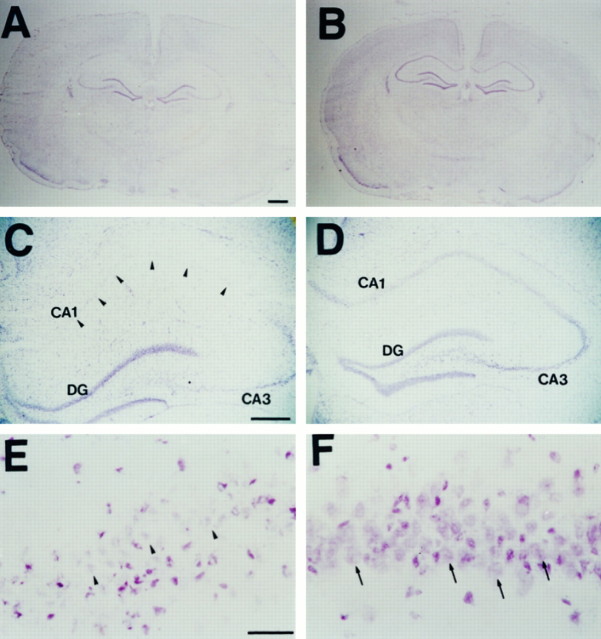
There were no marked differences in mortality between SOD1 Tg rats (10%) and non-Tg littermates (7.7%) within 72 hr after global ischemia and reperfusion. No significant difference was observed in any physiological parameters between the two groups (Table2). As shown in Figure5, the ischemic damage can be defined by cresyl violet staining. Ischemic change and severe brain swelling at 3 d after global ischemia were seen in the non-Tg littermates (Fig. 5A). In contrast, this damage was reduced in the SOD1 Tg rats. Additionally, the quantitative analysis of the area of hemisphere at the level of the posterior commissure clearly showed that the swelling was markedly reduced in SOD1 Tg rats (57.3 ± 0.8 mm2) compared with non-Tg littermates (62.2 ± 1.2; p < 0.01). The pyramidal neurons were not clearly stained in the non-Tg littermates, especially in the hippocampal CA1 subregion (Fig. 5C,E). However, many CA1 neurons in the SOD1 Tg rats were clearly observed as being without degeneration (Fig.5D,F). The grading data of the neuronal damage were significantly reduced in the cortex of SOD1 Tg rats compared with non-Tg littermates, striatum, thalamus, and in the hippocampal CA1 subregion, whereas no significant changes were seen after ischemia and reperfusion in the dentate gyrus (Fig.6A). There was no significant reduction of injuries in the CA3 subregion, although a trend toward significance was observed. This was because damage to the CA3 subregion did occur in the SOD1 Tg rats. The quantitative analysis of the damaged area of the CA1subregion showed a markedly protective effect against global ischemia in SOD1 Tg rats compared with non-Tg littermates 3 d after ischemia (Fig. 6B). However, no significant differences were detected 1 d after reperfusion.
Table 2.
Physiological parameters
| Genotype | Arterial blood gas analysis | Mean arterial blood pressure (mmHg) | ||||
|---|---|---|---|---|---|---|
| pH | pO2 (torr) | pCO2(torr) | Preischemia | Ischemia | Postischemia | |
| SOD1 Tg | 7.39 ± 0.02 | 118.8 ± 11.9 | 44.9 ± 1.6 | 104.0 ± 3.1 | 36.4 ± 0.4 | 125.0 ± 10.0 |
| Non-Tg | 7.34 ± 0.03 | 116.4 ± 10.4 | 50.1 ± 3.7 | 102.6 ± 2.2 | 36.8 ± 0.5 | 128.8 ± 4.1 |
All data are expressed as mean ± SE. The arterial samples were collected just after reperfusion. No significant difference was observed between SOD1 Tg rats and non-Tg littermates (n= 5, p > 0.05).
Fig. 5.
Microscopic photographs showing neuronal damage stained with cresyl violet in SOD1 Tg rats and non-Tg littermates 3 d after transient global ischemia. More severe edema was observed in non-Tg littermates (A) than in Tg rats (B). The CA1 pyramidal cell layer in non-Tg littermates was weakly stained by cresyl violet (C, arrowheads), and the nuclei of CA1 neurons were shrunken (E,arrowheads) compared with those of SOD1 Tg rats (D, F, arrows). Scale bars: A, B, 1 mm; C,D, 500 μm; E, F, 10 μm.
Fig. 6.
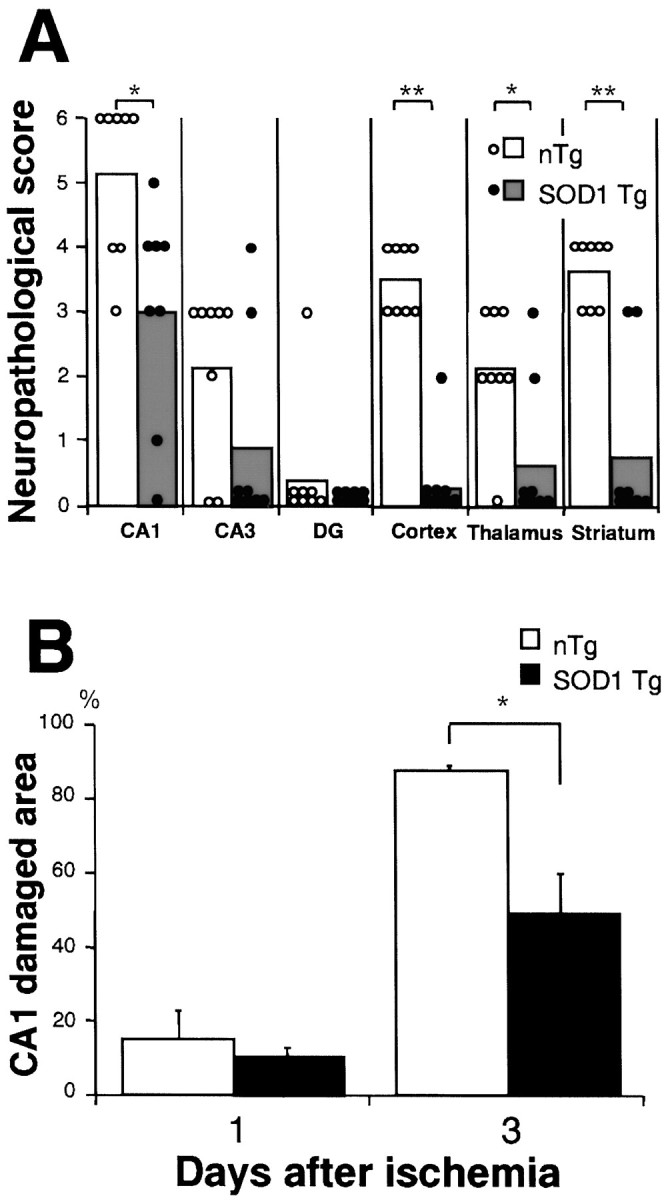
Neuronal injury after transient global cerebral ischemia in rats. A, Data showing the scatterplot of the neuropathological scores for a variety of brain areas for SOD1 Tg rats (closed circles, n = 8) and non-Tg littermates (open circles, n = 8) after global ischemia of 10 min and reperfusion of 3 d. Each column shows the mean score of injury of each group. *p < 0.03, **p < 0.01, significantly different from non-Tg littermates (Mann–WhitneyU test). B, The quantitative analysis of the damaged area of the CA1 subregion at 1 and 3 d after 10 min of global ischemia, and reperfusion showed a marked increase in the size of the damaged area between 1 and 3 d in both groups. The protective effect of CuZn SOD against global ischemia was significant in SOD1 Tg rats (48.4 ± 11.7%; closed bar) compared with non-Tg littermates (87.3 ± 1.7%;open bar; n = 6,p < 0.01) at 3 d after ischemia. However, no significant differences were observed 1 d (n = 4) after ischemia. Values are mean ± SE (Fisher’s protected least significant difference test followed by nonparametrict test).
DNA fragmentation
To determine the role of CuZn SOD in DNA fragmentation in delayed neuronal damage, we used in situ TUNEL staining to evaluate the contribution of DNA fragmentation to neuronal damage after global ischemia. We did not observe DNA-fragmented nuclei labeled by TUNEL staining at 1 d after global ischemia (Fig.7A,B). At 3 d after global ischemia, the TUNEL-positive cells were restricted to the hippocampal CA1 subregion (Fig. 7C,D), and the number of TUNEL-positive cells observed in the CA1subregion of the non-Tg littermates (Fig. 7C) was much greater than in the SOD1 Tg rats (Fig. 7D).
Fig. 7.
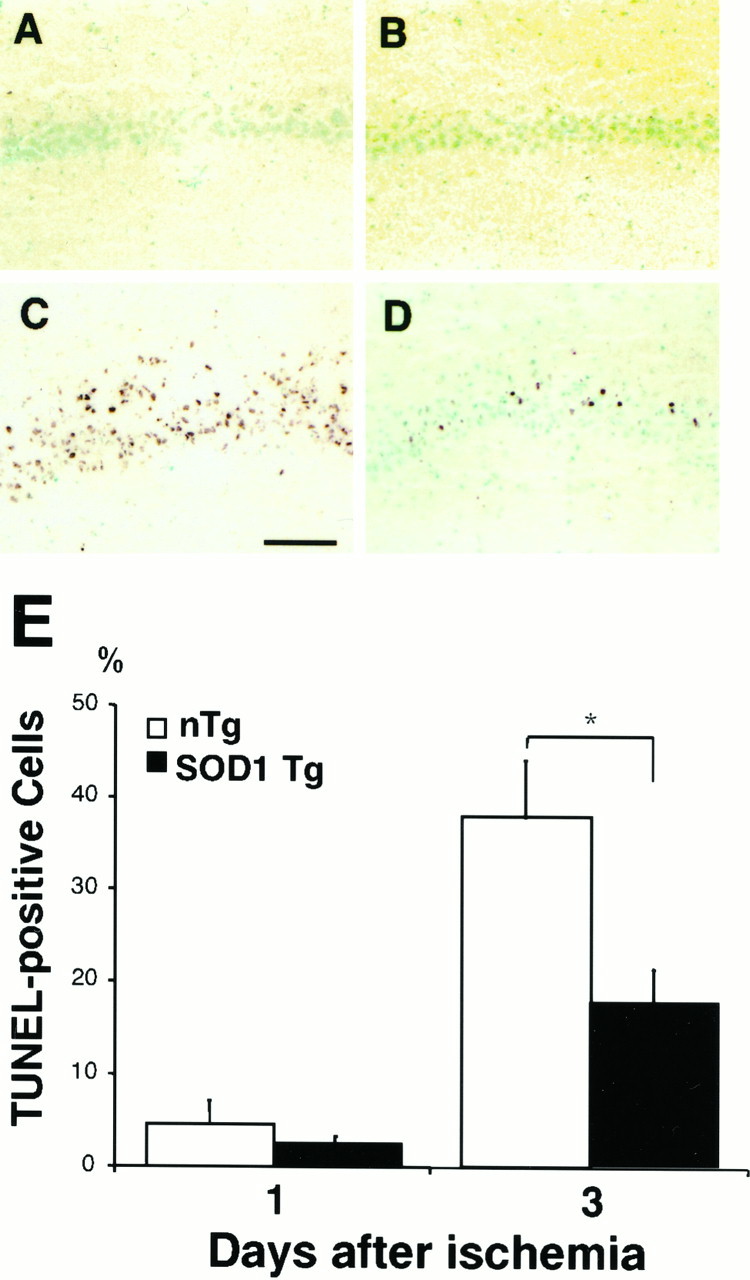
DNA fragmentation. Representative photographs showing DNA fragmentation by TUNEL staining at 1 d (A, B) and 3 d (C, D) after 10 min of global ischemia and reperfusion. The DNA-fragmented cells labeled by TUNEL staining were restricted to the hippocampal CA1 subregion at 3 d after global ischemia, but not at 1 d after global ischemia. A marked number of TUNEL-positive cells was observed in the CA1 subregion in non-Tg littermates (C), compared with SOD1 Tg rats (D). Scale bar, 500 μm. Quantitative analysis showed the ratio of TUNEL-positive neurons in the hippocampal CA1 subregion after global ischemia and reperfusion. The ratio was significantly ameliorated in Tg rats (closed bar) compared with non-Tg littermates (open bar) at 3 d (n = 6; p < 0.03). However, the ratio was the same between Tg and non-Tg rats at 1 d (n = 4). Values are mean ± SE (Fisher’s protected least significant difference test followed by nonparametrict test). E, Quantitative analysis of TUNEL-positive cells in the hippocampal CA1 subregion in SOD1 Tg rats and non-Tg littermates after transient global cerebral ischemia. TUNEL-positive cells in the hippocampal CA1subregion were counted by imaging analysis. There was a significant reduction in these DNA-fragmented cells in SOD1 Tg rats as compared with non-Tg littermates at 3 d after 10 min of ischemia. *p < 0.03, Fisher’s protected least significant difference test followed by nonparametric t test.
To determine the temporal pattern of DNA fragmentation after global ischemia, we counted TUNEL-positive cells in the hippocampal CA1 subregion at 1 and 3 d (Fig. 7E). Quantitative analysis showed that the TUNEL-positive neurons in the hippocampal CA1 subregion did not appear 1 d after global ischemia and reperfusion. Although DNA-fragmented cells were observed 3 d after global ischemia in both groups, DNA fragmentation damage was significantly ameliorated in the Tg rats compared with the non-Tg littermates (n = 6,p < 0.03, Fisher’s protected least significant difference test, followed by the nonparametric t test). However, the same level of TUNEL-stained cells was observed between the Tg and non-Tg rats 1 d after reperfusion (n = 4).
DISCUSSION
Early superoxide radical formation in hippocampal CA1subregion after transient global cerebral ischemia in rats
It is well established that transient global cerebral ischemia manifested by cardiac arrest causes selective neuronal death in vulnerable regions, such as hippocampal CA1 pyramidal cells, Purkinje cells of the cerebellum, and neurons in the third to fifth layers of the cerebral cortex (Kirino, 1982; Pulsinelli et al., 1982). The cell death mechanisms of these vulnerable neurons after transient cerebral ischemia have been extensively studied and are attributed to many factors, including glutamate neurotoxicity, calcium, expression of various genes, mitochondrial dysfunction, and oxygen free radicals (Abe et al., 1995; Ito et al., 1997). The oxygen free radical hypothesis is especially attractive because of the phenomenon of oxygen radical production, superoxide radicals in particular, that is associated with reperfusion injury (McCord, 1985; Chan, 1996). However, it is technically difficult to prove or disprove this hypothesis because of the lack of a quantitative method for oxygen radical measurement in the ischemic brain and the lack of a direct correlation between the increased antioxidant levels and their neuroprotection in experimental animal models of transient global cerebral ischemia (Chan et al., 1996).
To address the first issue, we have developed an in situimaging method for superoxide radical measurement (Kondo et al., 1997a;Murakami et al., 1998b). This method is based on the selective oxidation of HEt by superoxide radicals (Bindokas et al., 1996). At 1 hr after 10 min of ischemia and reperfusion, we observed diffuse cytosolic expression of oxidized HEt signals in cortical cells (Fig. 4D). These oxidized HEt signals were significantly increased in vulnerable hippocampal CA1neurons, whereas they were not observed in the ischemia-resistant CA3 neurons. The increased level of superoxide radicals precedes the occurrence of the majority of neuronal cell deaths and DNA fragmentation detected 3 d after reperfusion in rats (Figs. 5, 7). These data prompted our further investigation of the role of SOD1 in CA1 neuroprotection after transient global cerebral ischemia.
The making of SOD1 Tg rats for transient global cerebral ischemia
CuZn SOD has been extensively used in attempts to reduce brain injury induced by ischemia and reperfusion. Various degrees of success and failure were obtained in neuroprotection when exogenous CuZn SOD was used (Chan et al., 1993). However, the neuroprotective role of CuZn SOD in transient focal cerebral ischemia was found in Tg mice overexpressing SOD1 activity (Kinouchi et al., 1991; Yang et al., 1994). Conversely, increased neuronal injury was observed in knock-out mutant mice deficient in SOD1 activity (Kondo et al., 1997b). However, the extremely high mortality of mice that were subjected to global cerebral ischemia and the variability in the anatomy of the cerebrovasculature and in the genetic background of the Tg and knock-out mutant mice prompted us to produce SOD1 Tg rats. Using standard pronuclear DNA injection in rat embryos, we have succeeded in making several strains of transgenic rats that overexpress human CuZn SOD activity in the brain and in other systemic organs (E. Carlson, C. Epstein, and P. H. Chan, unpublished data). A heterozygous Tg strain (number 66 UCSF) that overexpresses CuZn SOD has been fully characterized by FISH (Fig. 1), IFGE (Fig. 2), and by direct enzymatic assay (Table 1). Additional Southern blots and reverse transcriptase PCRs have been used during the breeding to confirm both the SOD1 gene and the mRNA, respectively (data not shown). There were no observable phenotypic differences between the SOD1 Tg rats and the littermates.
Reduction of neuronal death and DNA fragmentation in vulnerable hippocampal CA1 neurons after transient global ischemia
When non-Tg rats were subjected to 10 min ischemia they developed selective neuronal damage at 3 d in vulnerable regions, including the hippocampal CA1 subregion, cerebral cortex, thalamus, and striatum, whereas the neurons in the dentate gyrus were not affected (Fig. 6A). The hippocampal CA1subregion was the most vulnerable region, and 90% of the CA1 neurons were lost (Fig. 6A,B). In contrast, neuronal damage in various brain regions was significantly reduced in SOD1 Tg rats (Fig. 7A,B) at 3 d after reperfusion. The reduction of cell damage in the CA1 region was ∼50% (Fig. 6B) in Tg rats. Although there was no significant cell damage in the CA1 subregion at 1 d after reperfusion in either SOD1 Tg rats or their littermates (Fig. 6B), the significant increase at 3 d in the non-Tg animals suggests that a delayed neurodegeneration occurs in the vulnerable CA1neurons and that increased endogenous SOD1 activity can significantly prevent cell death. Although a neuroprotective effect has been observed in vulnerable neurons of SOD1 Tg rats after transient global cerebral ischemia, it is not clear whether this neuroprotection is permanent. Such issues can be addressed by future studies of long-delayed cell injury and recovery after ischemia (i.e., 6 months).
The mechanisms of oxidative stress-induced delayed death of hippocampal CA1 pyramidal cells after transient global cerebral ischemia are unclear. Recent studies, although still somewhat controversial, have identified some apoptotic features by biochemical and morphological evidence such as TUNEL (MacManus et al., 1993;Sei et al., 1994; Petito et al., 1997) and internucleosomal DNA fragmentation as indicated by the DNA laddering pattern (Héron et al., 1993). Because TUNEL staining indicates DNA damage, and its specificity for apoptosis is questionable, we have used TUNEL staining only as an indication of DNA damage in cells. Whereas only a small fraction of cells (<5%) are TUNEL-positive 1 d after reperfusion, we have demonstrated a tremendously delayed increase in TUNEL-positive cells in the hippocampal CA1 subregion in wild-type animals 3 d after reperfusion (Fig. 7E) with up to 40% of the total cells being TUNEL-positive. The TUNEL-positive cells are likely caused by the increased level of superoxide radicals during reperfusion because <20% of the hippocampal CA1cells are TUNEL-positive in SOD1 Tg rats at 3 d after reperfusion. Our data also suggest that if TUNEL-positive cells are mainly apoptotic in nature, the damage to hippocampal CA1 neurons would involve both necrosis (60%) and apoptosis. However, much more stringent criteria for apoptosis (i.e., DNA laddering, caspases induction, cytochrome c release, and ultrastructural features) will be needed in future studies so that the apoptosis process can be confirmed. Whatever cell death processes are involved in the delayed death of hippocampal CA1 neurons, it is mediated by superoxide radicals. Because only a 50% reduction in cell death is achieved in SOD1 Tg rats after transient global cerebral ischemia, mechanisms or factors other than superoxide radicals are likely to be involved. It is also noteworthy that superoxide production in the hippocampal CA1 subregion is well developed 24 hr after ischemia, at a time when neuronal damage is not yet observed. This might suggest that superoxide radicals do not immediately damage these neurons but that an interval of time is required for the full expression of the injury. This delayed cell injury might provide a window of opportunity for therapeutic interventions using antioxidants. Additional therapeutic or pharmacological regimens in SOD1 Tg rats will be useful to further dissect the mechanisms involved in delayed vulnerable cell death after transient global cerebral ischemia.
Our success in making SOD1 Tg rats also provides an impetus for stroke researchers and neuroscientists to develop and to employ these animals for studying the oxidative mechanisms in acute brain injuries and chronic neurodegenerative diseases. Some of these studies are currently being undertaken in our laboratory.
Footnotes
This work was supported by National Institutes of Health contract, “Transgenic Rat for Stroke Research”, NO1-NS-5–2334 and NO1-NS-8–2386 (P.H.C.), and National Institutes of Health Grants NS 14543 (P.H.C.), NS 25372 (P.H.C.), NS 36147 (P.H.C.), and AG 08938 (C.J.E., P.H.C.). P.H.C. is a recipient of the Jacob Javits Neuroscience Investigator Award. We thank Cheryl Christensen for editorial assistance.
Correspondence should be addressed to Dr. Pak H. Chan, Neurosurgical Laboratories, Stanford University, 701B Welch Road, #148, Palo Alto, CA 94304.
REFERENCES
- 1.Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- 2.Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994;4:59–65. doi: 10.1111/j.1750-3639.1994.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 5.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH, Chu L, Chen SF, Carlson EJ, Epstein CJ. Reduced neurotoxicity in transgenic mice overexpressing human copper-zinc superoxide dismutase. Stroke. 1990;21:III80–III82. [PubMed] [Google Scholar]
- 7.Chan PH, Yang GY, Chen SF, Carlson E, Epstein CJ. Post-traumatic brain injury and edema are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991;21:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- 8.Chan PH, Kinouchi H, Epstein CJ, Carlson E, Chen SF, Imaizumi S, Yang GY. Role of superoxide dismutase in ischemic brain injury: reduction of edema and infarction in transgenic mice following focal cerebral ischemia. Prog Brain Res. 1993;96:97–104. doi: 10.1016/s0079-6123(08)63260-4. [DOI] [PubMed] [Google Scholar]
- 9.Chan PH, Epstein CJ, Kinouchi H, Kamii H, Chen SF, Carlson E, Gafni J, Yang G, Reola L. Neuroprotective role of CuZn-superoxide dismutase in ischemic brain damage. Adv Neurol. 1996;71:271–280. [PubMed] [Google Scholar]
- 10.Copin J-C, Reola LF, Chan TYY, Li Y, Epstein CJ, Chan PH. Oxygen deprivation but not a combination of oxygen, glucose, and serum deprivation induces DNA degradation in mouse cortical neurons in vitro: attenuation by transgenic overexpression of CuZn-superoxide dismutase. J Neurotrauma. 1996;13:233–243. doi: 10.1089/neu.1996.13.233. [DOI] [PubMed] [Google Scholar]
- 11.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 12.Dugan LL, Sensi SL, Canzoniero LMT, Handran SD, Rothman SM, Lin T-S, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci USA. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Héron A, Pollard H, Dessi F, Moreau J, Lasbennes F, Ben-Ari Y, Charriaut-Marlangue C. Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem. 1993;61:1973–1976. doi: 10.1111/j.1471-4159.1993.tb09843.x. [DOI] [PubMed] [Google Scholar]
- 15.Ito U, Kirino T, Kuroiwa T, Klatzo I. Maturation phenomenon in cerebral ischemia II. Neuronal recovery and plasticity. Springer; Berlin: 1997. [Google Scholar]
- 16.Kinouchi H, Epstein CJ, Mizui T, Carlson EJ, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Li Y, Sato S, Murakami K, Copin J-C, Huang T-T, Epstein CJ, Chan PH (1997a) Subcellular localization of superoxide anions following focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab [Suppl 1] 17:S102.
- 19.Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997b;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kontos HA. George E. Brown memorial lecture: oxygen radicals in cerebral vascular injury. Circ Res. 1985;57:508–516. doi: 10.1161/01.res.57.4.508. [DOI] [PubMed] [Google Scholar]
- 21.MacManus JP, Buchan AM, Hill IE, Rasquinha I, Preston E. Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett. 1993;164:89–92. doi: 10.1016/0304-3940(93)90864-h. [DOI] [PubMed] [Google Scholar]
- 22.McBean DE, Winters V, Wilson AD, Oswald CB, Alps BJ, Armstrong JM. Neuroprotective efficacy of lifarizine (RS-87476) in a simplified rat survival model of 2 vessel occlusion. Br J Pharmacol. 1995;116:3093–3098. doi: 10.1111/j.1476-5381.1995.tb15110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 24.Mikawa S, Kinouchi H, Kamii H, Gobbel GT, Chen SF, Carlson E, Epstein CJ, Chan PH. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Kondo T, Epstein CJ, Chan PH. Overexpression of CuZn-superoxide dismutase reduces hippocampal injury after global ischemia in transgenic mice. Stroke. 1997;28:1797–1804. doi: 10.1161/01.str.28.9.1797. [DOI] [PubMed] [Google Scholar]
- 26.Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998a;780:304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- 27.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998b;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petito CK, Torres-Munoz J, Roberts B, Olarte J-P, Nowak TS, Jr, Pulsinelli WA. DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:967–976. doi: 10.1097/00004647-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 30.Sei Y, Von Lubitz KJ, Basile AS, Borner MM, Lin RC, Skolnick P, Fossom LH. Internucleosomal DNA fragmentation in gerbil hippocampus following forebrain ischemia. Neurosci Lett. 1994;171:179–182. doi: 10.1016/0304-3940(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 31.Shi YP, Huang TT, Carlson EJ, Epstein CJ. The mapping of transgenes by fluorescence in situ hybridization on G-banded mouse chromosomes. Mamm Genome. 1994;5:337–341. doi: 10.1007/BF00356551. [DOI] [PubMed] [Google Scholar]
- 32.Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjö BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 33.Swanson RA, Morton MT, Wu GT, Savalos R, Davidson C, Sharp FR. A semi-automated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]