Abstract
We investigated the influence of endogenous κ-opioids on the activity of supraoptic neurons in vivo. Administration of the κ-antagonist nor-binaltorphimine (200 μg/kg, i.v.), increased the activity of phasic (vasopressin), but not continuously active (oxytocin), supraoptic neurons by increasing burst duration (by 69 ± 24%) and decreasing the interburst interval (by 19 ± 11%). Similarly, retrodialysis ofnor-binaltorphimine onto the supraoptic nucleus increased the burst duration (119 ± 57% increase) of vasopressin cells but did not alter the firing rate of oxytocin cells (4 ± 8% decrease). Thus, an endogenous κ-agonist modulates vasopressin cell activity by an action within the supraoptic nucleus. To eliminate κ-agonist actions within the supraoptic nucleus, we infused the κ-agonist U50,488H (2.5 μg/hr at 0.5 μg/hr) into one supraoptic nucleus over 5 d to locally downregulate κ-receptor function. Such infusions reduced the spontaneous activity of vasopressin but not oxytocin cells and reduced the proportion of cells displaying spontaneous phasic activity from 26% in vehicle-infused nuclei to 3% in U50,488H-infused nuclei; this treatment also prevented acute inhibition of both vasopressin and oxytocin cells by U50,488H (1000 μg/kg, i.v.), confirming functional κ-receptor downregulation. In U50,488H-infused supraoptic nuclei, vasopressin cell firing rate was increased by nor-binaltorphimine (100 and 200 μg/kg, i.v.) but not to beyond that found in vehicle-treated nuclei, indicating that these cells were not U50,488H-dependent. Thus, normally functioning κ-opioid mechanisms on vasopressin cells are essential for the expression of phasic firing.
Keywords: electrophysiology; dendrites; oxytocin; vasopressin; opioid; dynorphin; U50,488H; nor-binaltorphimine; naloxone
Magnocellular neurosecretory cells in the hypothalamic supraoptic and paraventricular nuclei each send a single axon to the posterior pituitary (neurohypophysis) (Randle et al., 1986) that gives rise to 2000–10,000 neurosecretory varicosities (Tweedle et al., 1989; Jackson, 1993). These varicosities contain vasopressin or oxytocin in neurosecretory granules that are released into the circulation after Ca2+ entry through voltage-dependent channels, opened after depolarization by action potentials. Magnocellular neurosecretory cell terminals cannot sustain intrinsic repetitive firing (Bourque, 1990), thus, vasopressin and oxytocin secretion is primarily determined by action potentials initiated at the cell bodies.
Vasopressin cells display robust phasic activity (Wakerley et al., 1978); this patterning contrasts with that of continuously active oxytocin cells. Phasic bursts typically last for 20–40 sec at 5–10 spikes/sec and are separated by silent periods of ∼20 sec. As bursts are not synchronized between cells (Leng and Dyball, 1983), neurohypophysial vasopressin release is continuous rather than pulsatile. Nevertheless, phasic pattering is of physiological importance because it increases the efficiency of hormone release by making optimal use of the terminal membrane properties. The mechanisms which initiate and sustain bursts are well understood (Renaud, 1987); action potentials in vasopressin cells are followed by a depolarizing afterpotential (DAP) that sustains a plateau potential, increasing the probability of EPSPs triggering further action potentials (Bourque et al., 1986). However, the mechanisms that terminate bursts have yet to be fully elucidated.
Magnocellular neurosecretory cells possess between one and three dendrites that also contain neurosecretory granules, and both vasopressin and oxytocin are released by exocytosis from these dendrites (Pow and Morris, 1989). Vasopressin (Watson et al., 1982) and oxytocin (Levin and Sawchenko, 1993; Eriksson et al., 1996) cells also synthesize the κ-opioid peptide dynorphin; indeed the highest levels of expression seen in the CNS are in vasopressin cells (Molineaux et al., 1982). Dynorphin is present in the dendrites of vasopressin cells (Watson et al., 1982) and is copackaged with vasopressin in the same neurosecretory granules in the axon terminals (Whitnall et al., 1983), and is thus co-secreted with vasopressin. Exogenous dynorphin attenuates exocytosis from neurosecretory terminals (Rusin et al., 1997), but although co-released dynorphin clearly restrains oxytocin release, it does not influence vasopressin secretion from isolated neurohypophysial preparations (Bicknell et al., 1985; Bondy et al., 1988). Hence, the physiological significance of dynorphin expression in vasopressin cells is currently unknown. The supraoptic nucleus contains a high density of κ-receptors (Sumner et al., 1990) and systemic injection of a κ-agonist inhibits vasopressin and oxytocin cells (Pumford et al., 1993; Ludwig et al., 1997), hence, we hypothesized that dendritically released dynorphin may feedback to terminate bursts, modulating phasic activity in vasopressin cells.
Here, we determined in vivo whether endogenous κ-receptor activation modulates vasopressin and oxytocin cell activity. We addressed this question first by using a potent and selective κ-antagonist, nor-binaltorphimine (Portoghese et al., 1987), to acutely block the actions of endogenous dynorphin, and second by chronic local infusion of a potent and selective κ-agonist, U50,488H (Lahti et al., 1982), to induce functional supraoptic nucleus κ-receptor downregulation.
MATERIALS AND METHODS
Electrophysiology. Virgin, female Sprague Dawley rats (337 ± 11 gm; n = 8) were anesthetized by intraperitoneal injection of urethane (ethyl carbamate; 1.25 gm/kg) and a catheter inserted into the superior vena cava through the right jugular vein for administration of drugs. The pituitary stalk and right supraoptic nucleus were exposed by a transpharyngeal approach. An SNEX100 bipolar stimulating electrode (Clark Electromedical Instruments, Pangbourne, Reading, UK) was placed on the pituitary stalk to elicit antidromic action potentials in neurosecretory neurons, recorded using a glass microelectrode (15–40 MΩ) placed into the caudal supraoptic nucleus and conventional extracellular recording techniques. Antidromically identified neurons were confirmed as neurosecretory neurons by collision of antidromic action potentials by spontaneous orthodromic action potentials (Lincoln and Wakerley, 1974). The neurons were characterized as oxytocin or vasopressin cells by their activity pattern and, where necessary, their response to intravenous cholecystokinin-8-sulfate (CCK; 20 μg/kg; 0.5 ml/kg in 0.9% saline). Oxytocin cells transiently increase their activity after systemic CCK injection (Renaud et al., 1987), whereas vasopressin cells are unaffected or inhibited and also often show a distinct phasic pattern of spontaneous activity. At the end of all experiments, the rats were killed by intravenous anesthetic overdose (60 mg/kg pentobarbitone). The activity of five phasic vasopressin and four continuously active oxytocin neurons was recorded for >15 min before injection of the κ-antagonist, nor-binaltorphimine (100 and/or 200 μg/kg, i.v.; injections separated by 30 min).
Microdialysis. Rats (290 ± 18 gm; n = 8) were prepared for electrophysiological recording of vasopressin and oxytocin neurons from the supraoptic nucleus and microdialysis application (retrodialysis) of drugs as previously described (Ludwig and Leng, 1997). Briefly, the supraoptic nucleus and pituitary stalk were exposed as described above. After removal of the meninges, an in-house-designed U-shaped microdialysis probe (total membrane length 2.0 mm; Spectra/Por RC Hollow Fibers, Spectrum Med. Inc., Houston, TX) was bent to position the loop of the membrane flat onto the exposed ventral surface of the brain over the ventral glial lamina of the supraoptic nucleus. The recording electrode was placed in the supraoptic nucleus through the center of the dialysis loop. The supraoptic nucleus was dialyzed with artificial CSF (aCSF; pH 7.2, composition in mm: NaCl 138, KCl 3.36, NaHCO39.52, Na2HPO4 0.49, urea 2.16, CaCl2 1.26, and MgCl2 1.18) at a flow rate of 2 μl/min for between 15 and 45 min before the inclusion ofnor-binaltorphimine (200 μg/ml) in the dialysate (n = 10).
Chronic intrasupraoptic nucleus U50,488H infusion. Rats were anesthetized with 5% halothane in a mixture of O2 and N2O (both flow rates at ∼500 ml/min) and a 28 gauge stainless steel cannula containing U50,488H or Ringer’s solution (in mm: 147 NaCl, 4 KCl, and 2.5 CaCl2) was inserted immediately dorsal to the right supraoptic nucleus (0.9 mm caudal and 1.7 mm lateral to bregma and 9.1 mm below the surface of the skull). The cannula was attached via silicone tubing (0.25 mm internal diameter; 0.91 mm wall thickness) to a subcutaneous Alzet model 2002 miniosmotic pump (Charles River UK Ltd., Margate, Kent, UK) set to deliver U50,488H at 2.5 μg/hr or Ringer’s solution at 0.5 μl/hr. The cannula was secured using dental acrylic bonded to stainless steel screws inserted in the skull. After surgery, the rats were housed individually with ad libitum access to food and water.
On the sixth day after implantation of the minipump, rats were prepared for electrophysiological recording from the right supraoptic nucleus as above. The spontaneous activity of 64 supraoptic neurons was recorded from U50,488H-infused rats (289 ± 6 g; n = 48) and 38 neurons from Ringer’s-infused rats (284 ± 6 g;n = 15). After completion of the experiments, the tip of the infusion cannula was located by section through the supraoptic nucleus. Only recordings from animals in which the cannula containing U50,488H was found to be located immediately adjacent to the supraoptic nucleus were included in the drug-treated group. In a small number of cases (n = 3) in which the cannula containing U50,488H missed the supraoptic nucleus, recordings were included in the control group.
In addition to recording spontaneous activity, the activity of supraoptic nucleus neurons (five vasopressin neurons each from U50,488H-infused and control rats, four oxytocin neurons from U50,488H-infused rats, and two from control rats) were recorded from rats that were injected intravenously at 15 min intervals with increasing doses of U50,488H (10, 100, and 1000 μg/kg) followed bynor-binaltorphimine (100 and 200 μg/kg) and then the general opioid antagonist, naloxone (5000 μg/kg).
Vasopressin cells recorded from U50,488H-infused supraoptic nuclei were relatively inactive, so we used hypertonic saline infusion to increase the activity of some of these cells. To this end, rats were prepared for U50,488H infusion (n = 20) or for Ringer’s infusion (n = 5) into the right supraoptic nucleus and for electrophysiological recording of the activity of supraoptic neurons 5 d later, as described above, but with the addition of a femoral intravenous catheter for the infusion of hypertonic saline. The activity of four vasopressin neurons was recorded from the supraoptic nuclei of four U50,488H-infused rats throughout a 2 hr infusion of 2 m NaCl (0.026 ml/min, i.v). Further recordings of both vasopressin and oxytocin neurons were made from both U50,488H- and Ringer’s-infused supraoptic nuclei during infusion of hypertonic saline over shorter periods.
Firing rate analysis. Neuronal activity was recorded onto computer and analyzed off-line using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Neurons that fired less than one spontaneous action potential every 10 sec were categorized as silent. Phasic activity was characterized using the “bursts” script in Spike2; a burst being defined as activity lasting at least 5 sec containing at least 20 action potentials, and with at least 5 sec interval between bursts during which there was less than one action potential every 5 sec. Active neurons that did not display periods of silence of sufficient duration to be recognized as bursts were categorized as continuously active. The mean firing rate and, when appropriate, the mean intraburst firing rate, burst duration, interburst interval, and activity quotient (proportion of time active relative to total time) of each neuron was calculated before and during drug administration.
Drugs. Naloxone hydrochloride was purchased from Sigma (Poole, Dorset, UK), CCK from Bachem (Saffron Walden, Essex, UK) and (±)-trans-U50,488 methane sulfonate andnor-binaltorphimine dihydrochloride from Research Biochemicals International, Semat Technical Ltd. (St. Albans, Herts, UK).
Statistics. All data are given as the mean ± SEM, and all statistical tests were completed on the SigmaStat software package (Jandel Scientific GmbH, Erkrath, Germany). Within groups, data were analyzed using the Wilcoxon signed rank test or, when appropriate, using Friedman one-way repeated measures (RM) ANOVA on ranks, and when χ2 was significant, post hoc analyses were performed using Student Newman–Keuls tests. Between groups, the data were analyzed by Student’s t test or two-way RM ANOVA. When the F-ratio was significant, post hocanalyses were again performed using Student Newman–Keuls tests. The χ2 test was applied to the proportion data.
RESULTS
Effects of intravenous nor-binaltorphimine on phasically firing supraoptic neurons
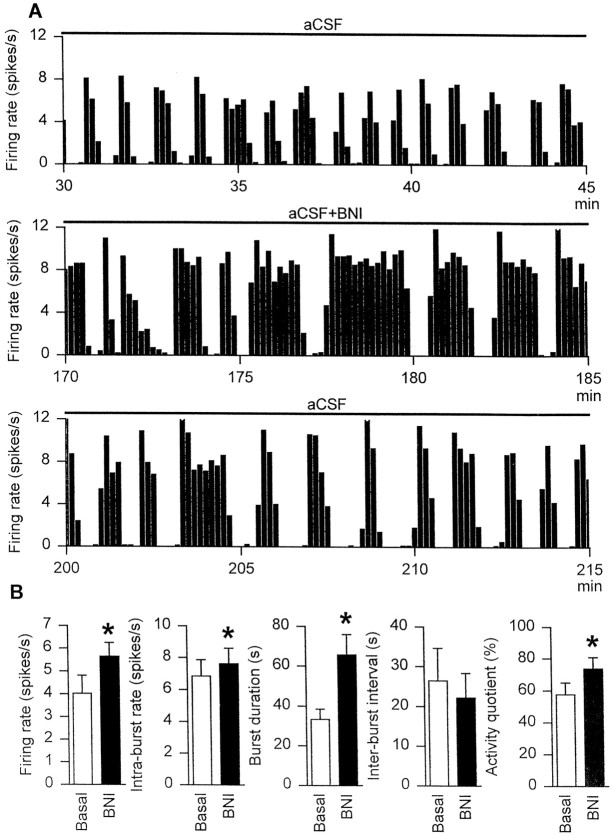
In each of five experiments, a supraoptic neuron displaying typical phasic firing activity was selected for testing. The spontaneous firing rate of each of the five phasic cells recorded (mean 4.7 ± 0.5 spikes/sec) was increased after 100 and 200 μg/kgnor-binaltorphimine [mean change 0.9 ± 0.3 and 1.6 ± 0.5 spikes/sec (intravenous), respectively; bothp < 0.05, Friedman RM ANOVA followed by Student Newman–Keuls test]. The spontaneous intraburst firing rate (6.6 ± 0.6 spikes/sec) was not affected by intravenousnor-binaltorphimine, but the burst duration was increased by 63.8 ± 35.9 sec and 853.7 ± 548.0 sec from 80.2 ± 21.3 sec after 100 and 200 μg/kg nor-binaltorphimine (bothp < 0.05), whereas the intraburst interval of 27.8 ± 5.0 sec was decreased by 6.1 ± 3.4 and 14.6 ± 6.6 sec after the two injections (both p < 0.05). Indeed, two of the five cells were continuously active after the higher dose of nor-binaltorphimine.
Effects of retrodialysis of nor-binaltorphimine onto the supraoptic nucleus on phasically firing cells
Six phasic cells, recorded from five rats, were tested during local application of nor-binaltorphimine by retrodialysis onto the supraoptic nucleus. The mean spontaneous firing rate of the six cells was 4.0 ± 0.8 spikes/sec, and this was not affected by dialysis of aCSF for between 15 and 45 min before the inclusion ofnor-binaltorphimine in the dialysate. Each cell exhibited a progressive increase in activity over the course ofnor-binaltorphimine retrodialysis (200 μg/ml at 2 μl/min over 47–145 min) such that the mean firing rate was increased to 5.6 ± 0.6 spikes/sec (p < 0.05; Wilcoxon signed rank test) during the last 15 min of retrodialysis (Fig.1). The latency to a first detectable clear response to retrodialysis of nor-binaltorphimine (>25% increase in firing rate from basal, averaged in 1 min bins and sustained over at least 3 min) of four of the six cells recorded was 14 ± 4 min; a fifth cell was clearly excited only after 109 min of nor-binaltorphimine retrodialysis, and the sixth cell showed only a small excitation (4.4%) by the final 15 min of recording before the cell was lost (50 min after the onset of dialysis). The intraburst firing rate, burst duration, and activity quotient, but not the interburst interval, also increased significantly (allp < 0.05) during intrasupraoptic nucleusnor-binaltorphimine administration.
Fig. 1.
A, The firing rate (averaged in 10 sec bins) of a phasic supraoptic neuron immediately before (top panel), during the final 15 min of (middle panel), and after (bottom panel) inclusion of nor-binaltorphimine (200 μg/ml) in the dialysate (aCSF at 2 μl/min) retrodialyzed directly onto the nucleus.B, The mean firing rate, intraburst firing rate, burst duration, interburst interval, and activity quotient of six phasic supraoptic nucleus neurons recorded from five rats treated as inA. *p < 0.05 versus basal; Wilcoxon signed rank test.
Effects of intravenous nor-binaltorphimine on oxytocin cells
In four experiments, a supraoptic neuron displaying typical continuous firing activity was selected for testing, after confirmation in each case that the cell selected was an oxytocin cell by observing its response to injection of CCK (excited by 1.0 ± 0.5 spikes/sec after 20 μg/kg CCK, i.v.). The mean spontaneous firing rate of the four cells was 3.7 ± 1.3 spikes/sec. The activity of none of these cells was affected by nor-binaltorphimine (200 μg/kg, i.v.); 0.3 ± 0.4 spikes/sec decrease in firing rate over 30 min (p = 0.75; Wilcoxon signed rank test).
Effects of nor-binaltorphimine retrodialysis onto the supraoptic nucleus on oxytocin cells
Four continuously active oxytocin cells (0.6 ± 0.3 spikes/sec increase in firing rate over 5 min after 20 μg/kg CCK, i.v.) were recorded from three rats during retrodialysis application ofnor-binaltorphimine onto the supraoptic nucleus for between 55 and 90 min. The firing rate of the oxytocin cells was 3.5 ± 0.9 spikes/sec over the 15 min immediately beforenor-binaltorphimine administration, and this was unchanged throughout the infusion for each of the cells (3.1 ± 0.7 spikes/sec mean firing rate during the final 15 min ofnor-binaltorphimine administration).
Effects of chronic U50,488H infusion into the supraoptic nucleus on the spontaneous activity of supraoptic neurons
The spontaneous firing rates of 64 neurons (mean latency from antidromic stimulation, 11.2 ± 0.6 msec) were recorded from U50,488H-infused and 38 neurons (mean latency, 10.9 ± 0.7 msec) from Ringer’s-infused supraoptic nuclei. In Ringer’s-infused rats, 10 of 38 cells were recognized as firing phasically. These cells had mean burst durations between 19 and 46 sec, mean intraburst firing rates between 3.8 and 7.1 Hz, mean interburst intervals between 11 and 88 sec, and >95% (mean, 96.6 ± 0.0%) of spikes occurred within recognized bursts. Five cells were categorized as irregular, displaying some activity recognized as bursts by the analysis algorithm, but typically <50% (28.2 ± 7.5%) of all spikes occurred within bursts. Ten cells showed little or no spontaneous activity and 13 were continuously active without bursts. The proportions of cells exhibiting these activity patterns were different in U50,488H-infused supraoptic nuclei (p = 0.001; χ2 test); in the U50,488H-infused supraoptic nuclei only 2 of 64 cells exhibited phasic activity (Table 1), 23 were irregular, 22 silent, and 17 continuous. The mean spontaneous firing rates of the cells recorded from Ringer’s- and U50,488H-infused supraoptic nuclei were 2.4 ± 0.4 (n = 38) and 1.5 ± 0.3 (n = 64) spikes/sec, respectively, and these were not significantly different (p = 0.07; Student’s t test).
Table 1.
The proportions of supraoptic neurons displaying different spontaneous activity patterns after 5 d of U50,488H (2.5 μg/hr at 0.5 μl/hr) or Ringer’s infusion into the supraoptic nucleus. The proportions of cells exhibiting each of the firing patterns were significantly different (p = 0.001, χ2test).
| Treatment | Spontaneous activity pattern | |||
|---|---|---|---|---|
| Continuous | Phasic | Irregular | Silent | |
| Ringer’s solution | 13/38 (34%) | 10/38 (26%) | 5/38 (13%) | 10/38 (26%) |
| U50,488H | 17/64 (27%) | 2/64 (3%) | 23/64 (36%) | 22/64 (34%) |
In Ringer’s-infused supraoptic nuclei, 45% of neurons were identified as vasopressin cells by their phasic activity pattern (n = 10) or by inhibition (0.7 ± 0.4 spikes/sec decrease in firing rate averaged over 5 min; n = 7) after intravenous CCK (20 μg/kg, i.v.); 18% were identified as oxytocin cells by their transient excitatory response to intravenous CCK (0.7 ± 0.3 spikes/sec increase). The remaining 37% could not be identified because they were silent (n = 10) or were lost before injection of CCK (n = 4).
In U50,488H-infused supraoptic nuclei, 36% of neurons were identified as vasopressin cells (phasic, n = 2) or inhibited (0.3 ± 0.1 spikes/sec decrease in firing rate averaged over 5 min; n = 21) by intravenous CCK (20 μg/kg, i.v.), and 12% were identified as oxytocin cells (1.4 ± 0.5 spikes/sec increase in firing rate after intravenous CCK). The remainder were silent (n = 22) or were lost before injection of CCK (n = 11). The proportion of cells categorized as oxytocin or vasopressin cells were similar in the Ringer’s- and U50,488H-infused supraoptic nuclei (p = 0.40, χ2 test; Table 2).
Table 2.
The proportions of supraoptic neurons identified as vasopressin or oxytocin cells after 5 d of U50,488H (2.5 μg/hr at 0.5 μl/hr) or Ringer’s infusion into the supraoptic nucleus. The proportions of cells in each of the categories were similar in the U50,488H- and Ringer’s-infused supraoptic nuclei (p = 0.40, χ2 test).
| Treatment | Supraoptic nucleus cell type | ||
|---|---|---|---|
| Vasopressin | Oxytocin | Unclassified | |
| Ringer’s solution | 17/38 (45%) | 7/38 (18%) | 14/38 (37%) |
| U50,488H | 23/64 (36%) | 8/64 (12%) | 33/64 (52%) |
The mean spontaneous firing rate of the vasopressin cells recorded from U50,488H-infused supraoptic nuclei was 1.8 ± 0.5 spikes/sec (n = 23), significantly lower (p= 0.04, Student’s t test) than that of vasopressin cells from Ringer’s-infused supraoptic nuclei (3.2 ± 0.5 spikes/sec;n = 17). By contrast, the mean spontaneous firing rates of the oxytocin cells recorded from the Ringer’s- and U50,488H-infused supraoptic nuclei were similar (p = 0.71, Student’s t test), at 2.8 ± 0.9 (n = 7) and 3.2 ± 0.8 spikes/sec (n = 8), respectively.
Effects of κ-opioid drugs on the activity of vasopressin cells after chronic intrasupraoptic nucleus infusion of U50,488H
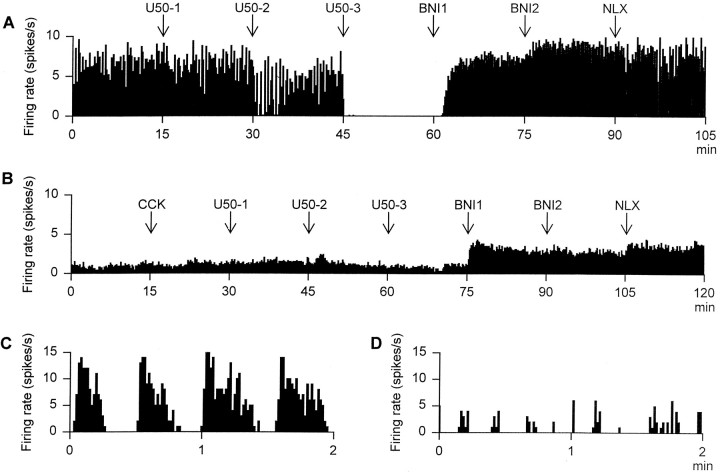
After intravenous Ringer’s infusion, U50,488H inhibited all five phasic cells tested in a dose-dependent,nor-binaltorphimine-reversible manner (Figs.2A, 3A); this inhibition was manifested as a decrease in the intraburst firing rate and in the proportion of time active (both p < 0.05, two-way RM ANOVA followed by Student Newman–Keuls test) and was maximal after 1000 μg/kg U50,488H, which silenced all five cells for at least 15 min. This inhibition was promptly reversed after intravenous administration of 100 μg/kgnor-binaltorphimine. After nor-binaltorphimine administration, the mean firing rate of these cells was similar to that of the phasic cells recorded from untreated supraoptic nuclei after administration of nor-binaltorphimine. Administration of an excess of the wide-spectrum opioid antagonist naloxone (5000 μg/kg, i.v.) did not further alter the activity of the cells.
Fig. 2.
A, The firing rate (averaged in 10 sec bins) of a phasic cell recorded from a rat in which Ringer’s solution (0.5 μl/hr) had been infused into the supraoptic nucleus for 5 d before recording. The rat was administered the following drugs at 15 min intervals: 10 (U50-1), 100 (U50-2), and 1000 (U50-3) μg/kg intravenous U50,488H followed by 100 (BNI1) and 200 (BNI2) μg/kg intravenous nor-binaltorphimine and then 5000 μg/kg intravenous naloxone (NLX).B, The firing rate (averaged in 10 sec bins) of a continuously active vasopressin cell recorded from a rat in which U50,488H (2.5 μg/hr) had been infused into the supraoptic nucleus for 5 d before recording. The rat was administered drugs as inA, except that CCK (20 μg/kg, i.v.) was administered before the first dose of U50,488H. C, The spontaneous firing rate (in 1 sec bins) of the cell recorded inA, showing clear phasic activity. D, The spontaneous firing rate (in 1 sec bins) of an irregularly firing cell recorded from a supraoptic nucleus that had been infused with U50,488H (2.5 μg/hr) over 5 d. The cell shows “clusters” of action potentials that were typical of irregularly firing cells in U50,488H-infused rats.
Fig. 3.
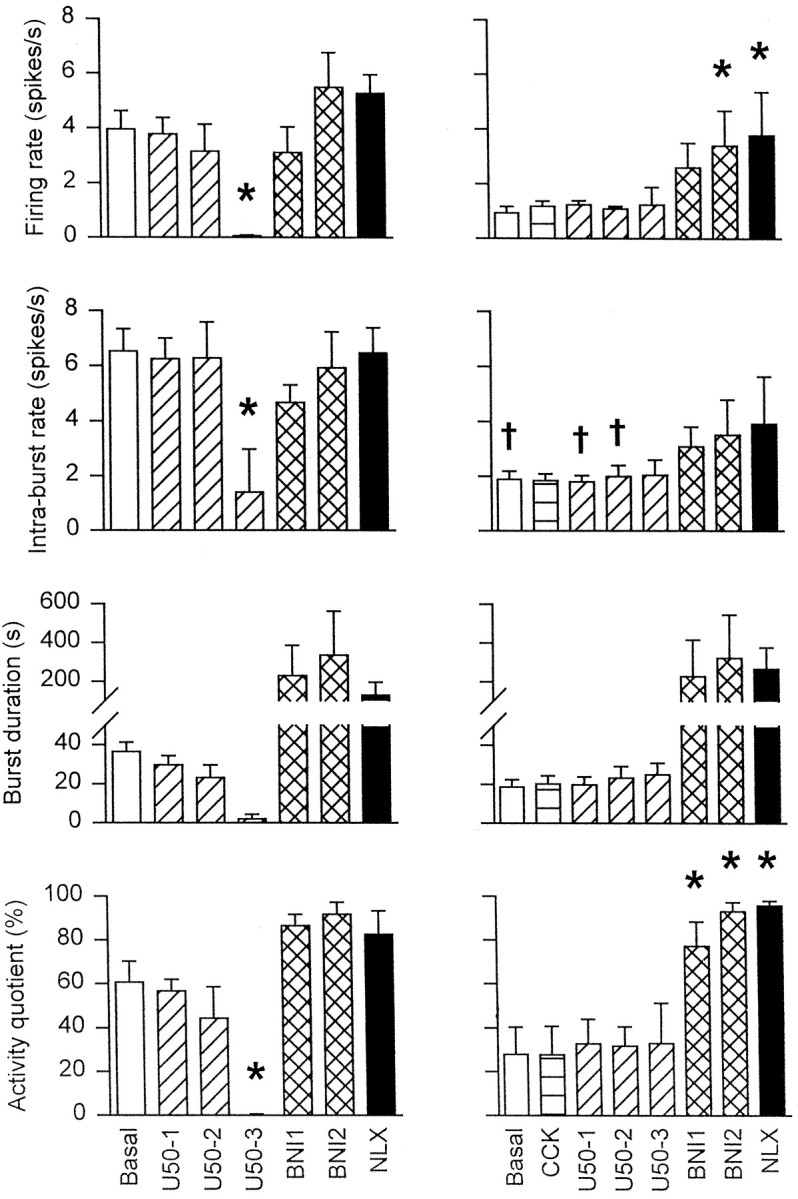
The mean firing rate, intraburst firing rate, burst duration, and activity quotient of five phasic cells in Ringer’s-infused supraoptic nuclei (left panels), and five cells in U50,488H-infused supraoptic nuclei (right panels) over 15 min periods before and after administration of CCK (20 μg/kg, i.v.), U50,488H (10, 100, and 1000 μg/kg, i.v.;U50-1,U50-2, andU50-3, respectively),nor-binaltorphimine (100 and 200 μg/kg, i.v.;BNI1 and BNI2, respectively), and naloxone (5000 μg/kg, i.v.; NLX) as shown in Figure 2. Two-way RM ANOVA followed by Student Newman–Keuls test: *p < 0.05 versus basal,†p < 0.05 versus matched treatment group in Ringer’s-infused rats.
The mean intraburst firing rates, burst durations, interburst intervals, and activity quotients of the two phasic cells recorded from U50,488H-infused supraoptic nuclei were 5.1 and 4.2 spikes/sec, 48.1 and 35.1 sec, 17.2 and 14.7 sec, and 73.7 and 70.5%, respectively. After 10 and 100 μg/kg intravenous U50,488H, the intraburst firing rates, burst durations, interburst intervals, and activity quotients of the former cell were not reduced at 5.2 and 5.1 spikes/sec, 141.3 and 73.9 sec, 8.6 and 6.5 sec, and 94.2 and 91.9%, respectively. The recording from this cell was lost 10 min after injection of the 100 μg/kg intravenous dose of U50,488H. The recording from the latter cell was lost before acute administration of intravenous U50,488H.
Five vasopressin cells, each from a different rat, were recorded during administration of increasing doses of U50,488H followed bynor-binaltorphimine (Fig. 2). The activity of these cells was not significantly altered by administration of CCK (0.2 ± 0.2 spikes/sec increase). Like the phasic cell recorded from a U50,488H-infused supraoptic nucleus, these cells were not inhibited by intravenous U50,488H, even at the 1000 μg/kg dose (Fig.3). After nor-binaltorphimine, the activity of each of these cells was increased, but not to levels above that of phasic cells in Ringer’s-infused supraoptic nuclei after administration of nor-binaltorphimine. Again, naloxone (5000 μg/kg, i.v.) did not further alter the activity of the cells.
Effects of κ-opioid drugs on the activity of oxytocin cells after chronic intrasupraoptic nucleus infusion of U50,488H
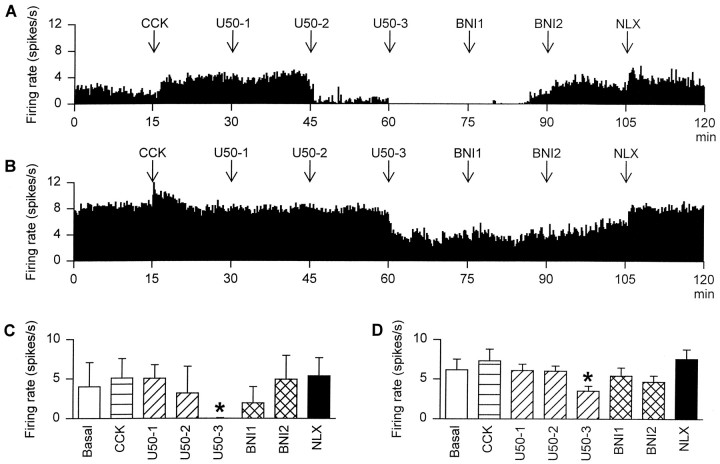
After both Ringer’s infusion (n = 2) and U50,488H infusion (n = 4), intravenous U50,488H inhibited identified oxytocin cells in a dose-dependent,nor-binaltorphimine-reversible manner (Fig.4). However, after the 1000 μg/kg dose of U50,488H the spontaneous activity of both of the cells in Ringer’s-infused rats was virtually abolished, as previously observed in noninfused rats at this dose (Pumford et al., 1993; Ludwig et al., 1997). By contrast the four cells in U50,488H-infused rats remained active after this dose, although at a rate significantly lower than the initial spontaneous activity (p < 0.05; Student Newman–Keuls test). This inhibition was reversed bynor-binaltorphimine (200 μg/kg, i.v.) in Ringer’s-treated rats, but in U50,488H-treated rats, full recovery from inhibition by intravenous U50,488H was not evident until after injection of naloxone (5000 μg/kg, i.v.).
Fig. 4.
The firing rate (averaged in 10 sec bins) of oxytocin cells recorded from rats in which (A) Ringer’s solution (0.5 μl/hr) or (B) U50,488H (2.5 μg/hr) had been infused into the supraoptic nucleus for 5 d before recording. The rats were intravenously administered the following drugs at 15 min intervals: 20 μg/kg CCK, 10 (U50-1), 100 (U50-2), and 1000 (U50-3) μg/kg U50,488H followed by 100 (BNI1) and 200 (BNI2) μg/kgnor-binaltorphimine, and finally 5000 μg/kg naloxone (NLX). C, The mean firing rate of oxytocin cells recorded from two control rats. D, The mean firing rate of four oxytocin cells recorded from U50,488H-infused rats. *p < 0.05 versus basal; two-way RM ANOVA followed by Student Newman–Keuls test.
Effects of hyperosmotic stimulation on the activity of supraoptic neurons after chronic intrasupraoptic nucleus infusion of U50,488H
Because vasopressin cells in U50,488H-infused supraoptic nuclei were relatively inactive, we went on to test whether phasic activity could be induced by increasing the activity of these cells by infusion of hypertonic saline. Four irregularly firing supraoptic cells, each from a different rat, were identified as putative vasopressin cells by their inhibitory response to CCK (firing rate reduced by 0.5 ± 0.3 spikes/sec after CCK, 20 μg/kg, i.v.). The mean basal firing rate of these cells was 1.3 ± 0.4 spikes/sec. Each showed a progressive increase in firing rate during infusion of 2 mhypertonic saline reaching a mean maximum firing rate (achieved between 30 and 120 min after the onset of the infusion) of 3.7 ± 0.9 spikes/sec. The mean firing rate reached by putative vasopressin cells in time-matched recordings from Ringer’s-infused supraoptic nuclei (n = 5) was significantly greater at 8.6 ± 1.5 spikes/sec (p = 0.02, Student’s ttest; three phasic cells and two continuous cells inhibited by CCK).
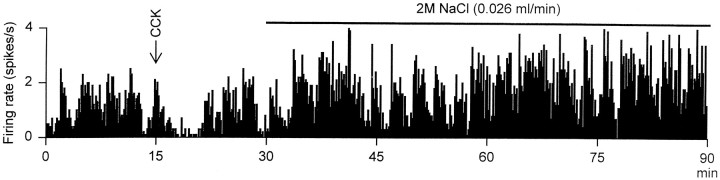
Over the course of the 2 hr intravenous hypertonic saline infusion, the firing patterns of two of the four cells recorded from infused rats satisfied the criteria for classification as phasic, as identified by the algorithm that consistently and appropriately identified phasic bursts in Ringer’s-infused nuclei. However, this activity was not typical of that seen in phasic vasopressin cells in untreated rats (Fig. 5). In untreated rats, phasic bursts are generally clearly defined, comprising an initial high-frequency discharge, a plateau of activity at a relatively constant mean rate but of variable duration, followed by an abrupt termination and subsequent quiescence. The program we used here to quantify characteristics of phasic bursts, thus, typically recognizes >95% of all action potentials as occurring within bursts. This was not true of the “phasic” cells in U50–588H-treated nuclei (Fig. 5) in which activity, although episodic, was highly irregular, and episodes of activity were not clearly separated by silent intervals. Such activity is not normally seen in either oxytocin cells or vasopressin cells in untreated rats.
Fig. 5.
The firing rate (averaged in 10 sec bins) of an irregularly active cell recorded from a rat in which U50,488H (2.5 μg/hr) had been infused into the supraoptic nucleus for 5 d before recording. The cell was inhibited by CCK (20 μg/kg, i.v.), identifying it as a vasopressin cell, and excited by intravenous infusion of 2 m NaCl (0.026 ml/min), clearly showing that this cell was not induced to fire in the robust phasic pattern typical of vasopressin cells during stimulation with hypertonic saline.
DISCUSSION
Here, we have shown that termination of phasic bursts by vasopressin cells involves an endogenous κ-opioid agonist because intrasupraoptic nucleus κ-antagonist administration prolongs burst duration. Furthermore, chronic supraoptic nucleus κ-receptor activation virtually eliminates phasic activity in vasopressin cells, indicating that normally functioning κ-receptors are essential for the expression of phasic activity. Finally, although both oxytocin and vasopressin cells develop tolerance to U50,488H, these cells do not develop κ-opioid dependence because κ-antagonist administration does not induce hyperexcitation of U50,488H-tolerant magnocellular neurosecretory cells.
Acute effects of κ-receptor antagonism on vasopressin cells
To determine whether the effects of systemicnor-binaltorphimine were exerted within the supraoptic nucleus, we retrodialyzed nor-binaltorphimine onto the supraoptic nucleus; this causes little morphological disruption and allows simultaneous recording of magnocellular cell activity (Ludwig and Leng, 1997). When applied using this technique, Fluorogold penetrates only ∼200 μm from the brain surface (Ludwig and Leng, 1997), a depth that corresponds to the ventral glial lamina of the supraoptic nucleus that contains the dendrites and not the cell bodies of supraoptic neurons (Armstrong et al., 1982). Furthermore, 10−6m tetrodotoxin (TTX) blocks action potentials in magnocellular cells, but retrodialysis of 10−4m TTX does not generally block antidromically evoked action potentials, implying that the concentration achieved at the cell bodies remains at least two orders of magnitude below the dialysate concentration (Ludwig and Leng, 1997). Thus, diffusion of the κ-antagonist away from the dialysis probe is probably highly restricted, and the effective intranuclear concentration achieved during retrodialysis may be estimated as ∼1 μg/ml. Because there is a high density of κ-receptors in, but not adjacent to, the supraoptic nucleus (Sumner et al., 1990), the effects of retrodialyzed nor-binaltorphimine are probably exerted within the nucleus. Nor-binaltorphimine excited most cells ∼15 min into retrodialysis. The reduced/delayed responses of two cells may reflect a greater diffusion distance between the recorded cell and the dialysis membrane.
Although both systemic and retrodialyzed nor-binaltorphimine increased the burst duration of phasic vasopressin cells, only systemicnor-binaltorphimine decreased the intraburst interval, and only retrodialyzed nor-binaltorphimine increased the intraburst firing frequency. The simplest explanation of these observations is that the reduced interburst interval results fromnor-binaltorphimine actions outside the supraoptic nucleus, probably by increasing excitatory input activity while the increased burst duration and intraburst firing rate result, at least in part, from intranuclear actions.
The present results cannot differentiate between presynaptic and postsynaptic actions of nor-binaltorphimine on vasopressin cells. κ-agonists hyperpolarize presynaptic terminals of the chick ciliary ganglion by inhibiting the K+-inward rectifier current (Fletcher and Chiappinelli, 1993), and blocking presynaptic K+ channels prevents κ-agonist inhibition of EPSCs in CA3 pyramidal cells (Simmons and Chavkin, 1996). Dendritically released dynorphin might also modulate vasopressin cell activity by a presynaptic action, as observed for the actions of dendritically released oxytocin on oxytocin cells (Kombian et al., 1997). Dynorphin might also act postsynaptically because U50,488H reduces voltage-activated Ca2+ currents in cultured cells dissociated from the supraoptic nuclei of neonatal rats (Mason et al., 1988). Dynorphin also decreases postsynaptic potential amplitude and suppresses the Ca2+ component of action potentials in supraoptic neurons (Inenaga et al., 1994), and U50,488H reduces the magnitude of DAPs (C. H. Brown, M. Ghamari-Langroudi, G. Leng, and C. W. Bourque, unpublished observations). Dendritic vasopressin co-released with dynorphin is also autoinhibitory (Ludwig and Leng, 1997). Thus, vasopressin and dynorphin may act together to terminate bursts, generating phasic activity necessary for efficient systemic vasopressin release. Whether these effects of dynorphin and vasopressin result from presynaptic and/or postsynaptic actions remains to be established.
Acute effects of κ-receptor antagonism on oxytocin cells
Neither systemic nor retrodialyzed nor-binaltorphimine altered the activity of oxytocin cells. Because vasopressin cells were strongly excited by the κ-antagonist, and oxytocin cells are as sensitive as vasopressin cells to κ-agonists (Pumford et al., 1993), the failure of nor-binaltorphimine to affect oxytocin cells probably does not reflect a failure to antagonize endogenous κ-agonists. In lactating rats, oxytocin and dynorphin are upregulated in paraventricular nucleus magnocellular cells (Eriksson et al., 1996) and, in the neurohypophysis, endogenous dynorphin, probably derived from oxytocin cells, attenuates stimulated release from oxytocin terminals (Bondy et al., 1988; Summy-Long et al., 1990). However, μ- and κ-receptor antagonism does not alter oxytocin cell activity, even after stimulation with hypertonic saline (Shibuki et al., 1988). Thus, dynorphin modulates systemic oxytocin secretion solely by an action within the neurohypophysis.
Effects of chronic κ-receptor activation on vasopressin cells
Assuming that dendritic vasopressin release is coupled to the electrical activity of vasopressin cells or to events associated with this activity, vasopressin cells are likely to be challenged intermittently with κ-agonists during phasic activity. However, although acute κ-receptor antagonism increased burst duration, phasic activity persisted. The natural explanation would be that there are other factors that contribute to burst termination. However, because κ-receptors expressed by vasopressin cells may lie in close proximity to the site of dendritic dynorphin release, these receptors may be exposed to high dynorphin concentrations. If so, even local administration of antagonists may poorly compete with endogenous agonists for receptor binding. We therefore tested our hypothesis of κ-receptor modulation of phasic firing by attempting to induce local knock-out of functional κ-receptors in the supraoptic nucleus. The method we chose was to chronically infuse, into one supraoptic nucleus, high concentrations of a potent and selective κ-agonist to induce local receptor downregulation. Again, such infusions cannot differentiate between direct effects on the vasopressin neurons themselves or presynaptic effects on their afferent inputs. Nevertheless, after chronic U50,488H infusion, supraoptic neurons were not affected by acute intravenous U50,488H injection, even at doses that consistently silenced phasic cells in Ringer’s-infused nuclei. Thus, the remaining activity in U50,488H-infused nuclei was tolerant to inhibition by U50,488H. As predicted, classical phasic firing patterns were virtually absent from the supraoptic nucleus. This effect is specific to chronic κ-receptor activation because phasically active cells are still present after chronic intracerebroventricular infusion of the μ-agonist, morphine, at doses that induce tolerance and dependence in supraoptic nucleus oxytocin cells (Bicknell et al., 1988). U50,488H infusion did not markedly affect the spontaneous behavior of oxytocin cells and did not prevent the responsiveness of either cell type to CCK or to osmotic challenge, although the magnitude of the response of vasopressin cells to osmotic challenge was impaired.
Subsequent κ-antagonist administration increased the activity of vasopressin neurons recorded from U50,488H-infused nuclei. However, this excitation was by an elevated firing rate in a continuous pattern rather than in the initiation of phasic activity. Because the κ-antagonist-induced excitation did not “overshoot” the activity seen in vasopressin cells without any experimental manipulations, it is unlikely that this excitation represents withdrawal excitation analogous to that seen for oxytocin cells challenged with chronic morphine infusion (Bicknell et al., 1988). Thus, vasopressin cells develop tolerance to, but not dependence on, U50,488H.
In the rat, osmotic stimuli and hemorrhage release both oxytocin and vasopressin, but after these stimuli oxytocin cells are continuously, rather than phasically, active (Wakerley et al., 1978). After hemorrhage (Wakerley et al., 1975) or systemic osmotic stimuli (Brimble and Dyball, 1977), vasopressin cells are phasically active, and when plasma Na+ rises, the cells first respond by increasing the proportion of time in which they are active (Brimble and Dyball, 1977). During chronically maintained stimulation, vasopressin secretion remains high, but vasopressin cells fire in shorter, more intense bursts (Wakerley et al., 1978). After chronic U50,488H infusion, only 3% of cells displayed spontaneous phasic activity, and half of the cells tested fired in an irregular pattern during hypertonic saline infusion. However, even after prolonged infusion of >3 ml of 2 m NaCl, this “episodic” activity was not typical of that normally associated with vasopressin cells (Fig. 5). Thus, it appears that chronic intrasupraoptic U50,488H infusion induces changes in the membrane properties of vasopressin cells that prevent the expression of “classical” phasic activity.
Effects of chronic κ-receptor activation on oxytocin cells
During chronic central administration of the μ-agonist, morphine, oxytocin cells develop both tolerance and dependence. Tolerance is manifested as an increase in the dose of morphine required to inhibit the oxytocin cells (Pumford et al., 1991). Dependence is seen as a rebound hyperexcitation after morphine withdrawal that results in a marked increase in oxytocin cell firing rate (Leng et al., 1989) and a consequent systemic oxytocin hypersecretion (Bicknell et al., 1988). Here, U50,488H was less effective at inhibiting oxytocin cells after chronic U50,488H administration, indicating that this treatment induces κ-opioid tolerance in oxytocin cells. Morphine-tolerant oxytocin cells do not exhibit cross-tolerance to U50,488H, indicating that μ- and κ-opioid tolerance are separate processes (Pumford et al., 1993). Unlike morphine withdrawal, U50,488H withdrawal did not induce a rebound hyperexcitation. Clearly, oxytocin cells do not develop dependence on opioids in general but rather on μ-opioids in particular.
Physiological significance
The generation of phasic activity of vasopressin cells in vivo appears to involve an autoinhibitory effect of dynorphin at the level of the somata/dendrites of vasopressin cells or presynaptically on their afferent inputs. Furthermore, chronic activation of κ-receptors in the supraoptic nucleus reduces the ability of vasopressin cells to fire phasically, but does not induce κ-opioid dependence in these cells. Thus, normally functioning supraoptic nucleus κ-opioid receptor mechanisms are essential for the expression of phasic activity by vasopressin cells.
Footnotes
Supported by the Medical Research Council (C.H.B.) and a Deutsche Forschungsgemeinschaft Research Fellowship (M.L.). We are grateful to Mr. Martyn Link for technical assistance.
Correspondence should be addressed to Dr. Colin Brown, Department of Physiology, University Medical School, Edinburgh EH8 9AG, UK.
REFERENCES
- 1.Armstrong WE, Scholer J, McNeill TH. Immunocytochemical, Golgi and electron microscopic characterization of putative dendrites in the ventral glial lamina of the rat supraoptic nucleus. Neuroscience. 1982;7:679–694. doi: 10.1016/0306-4522(82)90074-4. [DOI] [PubMed] [Google Scholar]
- 2.Bicknell RJ, Chapman C, Leng G. Effects of opioid agonists and antagonists on oxytocin and vasopressin release in vitro. Neuroendocrinology. 1985;41:142–148. doi: 10.1159/000124168. [DOI] [PubMed] [Google Scholar]
- 3.Bicknell RJ, Leng G, Lincoln DW, Russell JA. Naloxone excites oxytocin neurones in the supraoptic nucleus of lactating rats after chronic morphine treatment. J Physiol (Lond) 1988;396:297–317. doi: 10.1113/jphysiol.1988.sp016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondy CA, Gainer H, Russell JT. Dynorphin A inhibits and naloxone increases the electrically stimulated release of oxytocin but not vasopressin from the terminals of the neural lobe. Endocrinology. 1988;122:1321–1327. doi: 10.1210/endo-122-4-1321. [DOI] [PubMed] [Google Scholar]
- 5.Bourque CW. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol (Lond) 1990;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourque CW, Randle JC, Renaud LP. Non-synaptic depolarizing potentials in rat supraoptic neurones recorded in vitro. J Physiol (Lond) 1986;376:493–505. doi: 10.1113/jphysiol.1986.sp016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brimble MJ, Dyball RE. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol (Lond) 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson M, Ceccatelli S, Uvnas-Moberg K, Iadarola M, Hokfelt T. Expression of Fos-related antigens, oxytocin, dynorphin and galanin in the paraventricular and supraoptic nuclei of lactating rats. Neuroendocrinology. 1996;63:356–367. doi: 10.1159/000126976. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher GH, Chiappinelli VA. The actions of the kappa 1 opioid agonist U-50,488 on presynaptic nerve terminals of the chick ciliary ganglion. Neuroscience. 1993;53:239–250. doi: 10.1016/0306-4522(93)90302-v. [DOI] [PubMed] [Google Scholar]
- 10.Inenaga K, Nagatomo T, Nakao K, Yanaihara N, Yamashita H. Kappa-selective agonists decrease postsynaptic potentials and calcium components of action potentials in the supraoptic nucleus of rat hypothalamus in vitro. Neuroscience. 1994;58:331–340. doi: 10.1016/0306-4522(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 11.Jackson MB. Passive current flow and morphology in the terminal arborizations of the posterior pituitary. J Neurophysiol. 1993;69:692–702. doi: 10.1152/jn.1993.69.3.692. [DOI] [PubMed] [Google Scholar]
- 12.Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 13.Lahti RA, VonVoigtlander PF, Barsuhn C. Properties of a selective kappa agonist, U-50,488H. Life Sciences. 1982;31:2257–2260. doi: 10.1016/0024-3205(82)90132-1. [DOI] [PubMed] [Google Scholar]
- 14.Leng G, Dyball RE. Intercommunication in the rat supraoptic nucleus. Q J Exp Physiol. 1983;68:493–504. doi: 10.1113/expphysiol.1983.sp002742. [DOI] [PubMed] [Google Scholar]
- 15.Leng G, Russell JA, Grossmann R. Sensitivity of magnocellular oxytocin neurones to opioid antagonists in rats treated chronically with intracerebroventricular (i.c.v.) morphine. Brain Res. 1989;484:290–296. doi: 10.1016/0006-8993(89)90372-7. [DOI] [PubMed] [Google Scholar]
- 16.Levin MC, Sawchenko PE. Neuropeptide co-expression in the magnocellular neurosecretory system of the female rat: evidence for differential modulation by estrogen. Neuroscience. 1993;54:1001–1018. doi: 10.1016/0306-4522(93)90591-3. [DOI] [PubMed] [Google Scholar]
- 17.Lincoln DW, Wakerley JB. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol (Lond) 1974;242:533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurones in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig M, Brown CH, Russell JA, Leng G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol (Lond) 1997;505:145–152. doi: 10.1111/j.1469-7793.1997.145bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason WT, Cobbett P, Inenaga K, Legendre P. Ionic currents in cultured supraoptic neurons: actions of peptides and transmitters. Brain Res Bull. 1988;20:757–764. doi: 10.1016/0361-9230(88)90088-3. [DOI] [PubMed] [Google Scholar]
- 21.Molineaux CJ, Feuerstein G, Faden AL, Cox BM. Distribution of immunoreactive dynorphin in discrete brain nuclei: comparison with vasopressin. Neurosci Lett. 1982;33:179–184. doi: 10.1016/0304-3940(82)90248-8. [DOI] [PubMed] [Google Scholar]
- 22.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 23.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 24.Pumford KM, Leng G, Russell JA. Morphine actions on supraoptic oxytocin neurones in anaesthetized rats: tolerance after i.c.v. morphine infusion. J Physiol (Lond) 1991;440:437–454. doi: 10.1113/jphysiol.1991.sp018717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pumford KM, Russell JA, Leng G. Effects of the selective kappa-opioid agonist U50,488 upon the electrical activity of supraoptic neurones in morphine-tolerant and morphine-naive rats. Exp Brain Res. 1993;94:237–246. doi: 10.1007/BF00230291. [DOI] [PubMed] [Google Scholar]
- 26.Randle JC, Bourque CW, Renaud LP. Serial reconstruction of Lucifer yellow-labelled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986;17:453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]
- 27.Renaud LP. Magnocellular neuroendocrine neurons: update on intrinsic properties, synaptic inputs and neuropharmacology. Trends Neurosci. 1987;10:498–501. [Google Scholar]
- 28.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- 29.Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. κ-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci. 1997;17:6565–6574. doi: 10.1523/JNEUROSCI.17-17-06565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuki K, Leng G, Way S. Effects of naloxone and of intraperitoneal hypertonic saline upon oxytocin release and upon supraoptic neuronal activity. Neurosci Lett. 1988;88:75–80. doi: 10.1016/0304-3940(88)90318-7. [DOI] [PubMed] [Google Scholar]
- 31.Simmons ML, Chavkin C. κ-opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol Pharmacol. 1996;50:80–85. [PubMed] [Google Scholar]
- 32.Summy-Long JY, Rosella-Dampman LM, McLemore GL, Koehler E. Kappa opiate receptors inhibit release of oxytocin from the magnocellular system during dehydration. Neuroendocrinology. 1990;51:376–384. doi: 10.1159/000125364. [DOI] [PubMed] [Google Scholar]
- 33.Sumner BE, Coombes JE, Pumford KM, Russell JA. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–645. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- 34.Tweedle CD, Smithson KG, Hatton GI. Neurosecretory endings in the rat neurohypophysis are en passant. Exp Neurol. 1989;106:20–26. doi: 10.1016/0014-4886(89)90140-4. [DOI] [PubMed] [Google Scholar]
- 35.Wakerley JB, Poulain DA, Dyball RE, Cross BA. Activity of phasic neurosecretory cells during haemorrhage. Nature. 1975;258:82–84. doi: 10.1038/258082a0. [DOI] [PubMed] [Google Scholar]
- 36.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 37.Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, Van Wimersma Greidanus TB. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- 38.Whitnall MH, Gainer H, Cox BM, Molineaux CJ. Dynorphin-A-(1–8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983;222:1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]