Abstract
Intravenous administration of interleukin-1 (IL-1) activates central autonomic neuronal circuitries originating in the nucleus of the solitary tract (NTS). The mechanism(s) by which blood-borne IL-1 regulates brain functions, whether by operating across the blood–brain barrier and/or by activating peripheral sensory afferents, remains to be characterized. It has been proposed that vagal afferents originating in the periphery may monitor circulating IL-1 levels, because neurons within the NTS are primary recipients of sensory information from the vagus nerve and also exhibit exquisite sensitivity to blood-borne IL-1. In this study, we present evidence that viscerosensory afferents of the vagus nerve respond to intravenously administered IL-1β. Specific labeling for mRNAs encoding the type 1 IL-1 receptor and the EP3 subtype of the prostaglandin E2 receptor was detected in situ over neuronal cell bodies in the rat nodose ganglion. Moreover, intravenously applied IL-1 increased the number of sensory neurons in the nodose ganglion that express the cellular activation marker c-Fos, which was matched by an increase in discharge activity of vagal afferents arising from gastric compartments. This response to IL-1 administration was attenuated in animals pretreated with the cyclooxygenase inhibitor indomethacin, suggesting partial mediation by prostaglandins. In conclusion, these results demonstrate that somata and/or fibers of sensory neurons of the vagus nerve express receptors to IL-1 and prostaglandin E2 and that circulating IL-1 stimulates vagal sensory activity via both prostaglandin-dependent and -independent mechanisms.
Keywords: nodose ganglion, viscerosensory, nucleus of the solitary tract, autonomic, cytokine, c-Fos, blood–brain barrier, neuroimmunomodulation, inflammation, acute-phase response
Inflammatory and infectious episodes trigger a host of systemic responses, including hyperthermia, increased pain sensitivity, altered metabolism, and increased secretion of liver acute phase proteins, adrenocorticotropin (ACTH), and glucocorticoids (Baumann and Gauldie, 1994). Details of how the immune system elicits these systemic responses have primarily been provided by studies using animal models in which acute inflammatory reactions were induced by the peripheral administration of bacterial cell wall components [e.g., lipopolysaccharides (LPS)] or cytokines (Dantzer, 1994; Tilders et al., 1994; Ericsson et al., 1996). Interleukin-1 (IL-1) is a potent mediator of systemic responses to infection (Besedovsky and del Rey, 1989) and inflammation (Rivier et al., 1989a). One systemic effect of IL-1, enhanced secretory activity of the hypothalamic–pituitary–adrenal (HPA) axis, is an important consequence of immune responses and is likely orchestrated via central autonomic neurocircuitries originating in the nucleus of the solitary tract (NTS) (Ericsson et al., 1994, 1996). Neurons in the NTS are highly responsive to intravenous administration of IL-1β, and their activation is concurrent with specific effector responses, such as activation of corticotropin-releasing factor neurons in the endocrine hypothalamus (Ericsson et al., 1994) and secretion of ACTH from the pituitary gland (Rivier et al., 1989b). Interestingly, functional lesions of ascending aminergic projections from the lower brainstem to the paraventricular nucleus of the hypothalamus attenuates IL-1-mediated activation of the HPA axis (Weidenfeld et al., 1989; Chuluyan et al., 1992; Ericsson et al., 1994).
These findings evoke fundamental questions regarding the mechanisms by which peripherally administered IL-1 regulates neuronal functions across the blood–brain barrier, and participation of vagal sensory pathways, circumventricular structures, and local signaling events across the blood–brain barrier have been considered (Watanabe et al., 1990; Dantzer, 1994; Cao et al., 1995; Ericsson et al., 1995; Watkins et al., 1995b; Elmquist et al., 1997; Ericsson et al., 1997). The neural responses are likely mediated by endogenous prostaglandins, because cyclooxygenase inhibitors attenuate important aspects of the IL-1- or LPS-induced systemic responses (Morimoto et al., 1989;Watanabe et al., 1990; Crestani et al., 1991; Dunn and Chuluyan, 1992;Kandasamy et al., 1995; Ericsson et al., 1997). The functional basis for these observations, however, remains to be determined. The NTS is a well characterized primary target of viscerosensory information transmitted via the vagal and glossopharyngeal nerves (Loewy, 1990). Subdiaphragmatic vagotomy effectively blocks hyperthermic, feeding, social exploratory, hyperalgesic, and adrenocortical responses after intraperitoneal administration of low-to-medium doses of IL-1 or LPS (Watkins et al., 1994a,b, 1995a; Bret-Dibat et al., 1995; Fleshner et al., 1995; Gaykema et al., 1995; Bluthe et al., 1996; Kapcala et al., 1996). In contrast, experiments that explored the potential involvement of vagal afferents in driving brain function after intravenous administration of inflammatory mediators have produced variable and inconclusive results (Katsuura et al., 1988; Wan et al., 1994; Sehic and Blatteis, 1996; Ericsson et al., 1997; Romanovsky et al., 1997).
The present study was designed to examine whether vagal viscerosensory afferents have the endogenous capacity to monitor and respond to elevated plasma levels of IL-1 and/or prostaglandins. The responsiveness of viscerosensory vagal afferents to intravenously administered IL-1β, as well as the relative involvement of endogenous prostaglandins, were examined using electrophysiological techniques and immediate-early gene technology (Morgan and Curran, 1991) on sections through the nodose ganglion of indomethacin- or vehicle-pretreated rats. In situ hybridization histochemical labeling was used to detect mRNAs encoding either IL-1 or prostaglandin E2 (PGE2) receptors within the nodose ganglion. The results demonstrate that blood-borne IL-1β activates vagal sensory functions in a partially prostaglandin-dependent manner and that sensory neurons in the nodose ganglia express receptors for IL-1 and PGE2.
MATERIALS AND METHODS
Animals. Adult male Sprague Dawley rats (260–340 gm; B & K Universal, Sollentuna, Sweden) were used, and all were certified to be free of rodent pathogens. The rats were housed individually in our animal facility for a minimum of 9 d before the onset of experiments at constant room temperature and a 12 hr light/dark cycle (lights on at 6:00 A.M.). Food and water were provided ad libitum up until the day before the injection. To normalize interindividual variation in gastrointestinal status, the animals were deprived of food but allowed access to water ad libitum for 24 hr before the time of injection. Rats were accustomed to experimental conditions by daily handling for a minimum of 7 d before the onset of experiments. These studies were approved by the Animal Welfare Committee at the Karolinska Institute (Stockholm, Sweden).
Recording IL-1-induced changes in afferent activity of the gastric vagal nerve. Rats (n = 19) were initially catheterized via the trachea under general anesthesia using sodium pentobarbital (60–70 mg/kg, i.p.; Apoteksbolaget, Umeå, Sweden), and the respiration of the animals was artificially maintained with a respirator (model 683; Harvard Apparatus, Helliston, MA). The femoral vein was simultaneously cannulated to allow drug administration. The jugular vein was cannulated for constant infusion of pentobarbital and a muscle relaxant, gallamine triethiodide (Sigma, St. Louis, MO). Blood pressure was monitored continuously from the femoral artery and maintained above 90 mmHg (systolic) by administering 4% Ficoll 70 (Pharmacia, Uppsala, Sweden) as needed. Rectal temperature was maintained at 37.5 ± 0.1°C using a heating pad and an infrared lamp (ATB-1100; Nihon-Kohden, Tokyo, Japan). All surgical procedures mentioned above were usually completed within 1 hr after the initial injection of anesthetic. A solution of pentobarbital and gallamine triethiodide was then administered intravenously at a rate of 10–20 mg/kg/hr by an infusion pump (STC-527; Terumo, Tokyo, Japan). During the experiment, the depth of anesthesia was determined by routinely monitoring the blood pressure. A midline incision was made in the abdomen through which one anterior subdiaphragmatic vagal nerve branch innervating the stomach was isolated under a binocular microscope and transected ∼1 cm proximal from the entrance of the stomach. Both the anterior and posterior subdiaphragmatic vagal trunks were cut to avoid involvement of vago-vagal reflexes. In three rats, the gastric sympathetic nerves were also crushed. The peripheral cut segment of the vagal nerve branch was placed in contact with a pair of bipolar platinum wire electrodes, and the afferent multiunit activity was amplified (time constant, 0.01 sec) (S-0476; Nihon-Kohden). The discharge activity was passed through a window discriminator (ME-1100; Nihon-Kohden), evaluated on a pulse counter, and recorded on a polygraph. The effect of IL-1β and/or indomethacin (10 mg/kg dissolved in 4% sodium bicarbonate buffer at 10 mg/ml; Sigma) on nerve activity was expressed as a percentage of the preadministration control activity. Initial attempts to monitor electrophysiological responsiveness of gastric vagal afferents to intravenous IL-1 in animals fed ad libitum (n = 4) demonstrated highly variable increases in discharge activity. After 24 hr food restriction, more consistent responses were obtained, and we therefore selected to conduct the entire study using food-restricted animals.
IL-1β protein (original specific biological activity in excess of 1 × 105 U/μg protein; A375 assay) (Nakano et al., 1988) was generated from a recombinant human IL-1β cDNA fragment encoding the 152 residue mature form of IL-1β. After its receipt, the material was thawed on ice, diluted 1:1 in 200 mm Tris-HCl buffer, pH 7.4, containing 0.2% BSA and then aliquoted in 1.5 μg batches, and refrozen at −70°C. Before its injection, an IL-1β aliquot was thawed and diluted to the appropriate concentration, depending on the size of the rats to be injected, in 40 mmsodium phosphate buffer containing 0.01% ascorbic acid. Injections of vehicle alone were composed of 0.01% BSA, 0.01% ascorbic acid, 10 mm Tris-HCl, and 36 mm sodium phosphate buffer, pH 7.4.
Intravenous administration of IL-1β to awake and freely moving rats. The procedures for administration of IL-1β to awake and freely moving rats has been described previously (Ericsson and Sawchenko, 1993). Briefly, indwelling catheters (PE-50; Becton Dickinson, Sparks, MD) containing sterile pyrogen-free heparin saline (500 U/ml) were surgically implanted in the right jugular vein of methoxyflurane-anesthetized rats. The catheter was positioned with the internal silastic tip in the atrium of the heart and then routed subcutaneously until its sealed end was exteriorized in the interscapular space. The present experiments were modified, however, in that the external part of the intrajugular catheter (50 cm in length) was protected from the animal by tunneling it through a fine steel spring and then anchoring it to a remote balancing device. This procedure completely avoided the need to approach the rat before and during the injections, reducing the potential risk of handling-related stress effects. After 2 d postsurgical recovery, the rats were preinjected intravenously with indomethacin (10 mg/kg) or with vehicle alone. One hour later, 2 μg/kg IL-1β or vehicle alone was injected in a total volume of 300 μl over 3 min. One hour after the last injection, rats were anesthetized and perfused. The injection procedure and the central effects of this cytokine preparation have been extensively characterized (Rivier et al., 1989b; Ericsson et al., 1994,1995, 1997; Ericsson and Sawchenko, 1993).
Tracer injections. Sensory neurons of the vagus nerve are located in the nodose and jugular ganglia. In the rat, however, these ganglia are fused with the petrosal ganglion to form a continuous ganglionic mass in which vagal and glossopharyngeal sensory neurons are partly interspersed (Altschuler et al., 1989). Therefore, tract-tracing studies were performed to specifically label vagal sensory neurons in the nodose ganglion. Three rats were anesthetized with methoxyflurane, and the right vagus nerve was exposed via a ventral incision and dissected free of surrounding connective tissue ∼1 cm distal to the caudal end of the nodose ganglion. A 2% (w/v) dispersion of the fluorochrome true blue, a fluorescent marker for retrograde axonal transport (Sigma), was freshly prepared in pyrogen-free saline, and 0.05–0.1 μl was injected with a 75N Hamilton syringe directly into the vagal nerve trunk. Tracer transport continued for 7 d to backfill the sensory neurons. The rats were then subjected to the catheterization procedure described above.
Perfusion and tissue preparation. The time of perfusion was between 10:00 A.M. and 1:00 P.M. to minimize diurnal variations in HPA axis activity. One hour after IL-1 or vehicle injection, the animals were rapidly killed with CO2 gas and immediately perfused via the ascending aorta with 0.9% NaCl containing 0.02% diethylpyrocarbonate for 10 min, followed by 300–400 ml of ice-cold fixative solution (4% paraformaldehyde in 0.1 m borate buffer, pH 9.5) for 20 min. Rats previously injected intravagally with the true blue tracer were perfused with 0.9% NaCl alone for 1 min before perfusion fixation. The nodose ganglion was cut centrally just external to the jugular foramen between the exit points of the pharyngeal ramus and the glossopharyngeal nerve. The ganglion was removed and post-fixed for 3 hr in the fixative solution containing 20% sucrose and subsequently cryoprotected in 0.02 mphosphate buffer containing 20% sucrose overnight at 4°C. Each ganglion was embedded in Tissue-Tek O.T.C. compound (Miles, Ekhart, IN) and frozen in a dry ice–acetone-chilled isopentane solution. The ganglia were cut in a cryostat chilled at −20°C into 12 μm thick sections, which were mounted onto Probe On Plus slides (Fischer Scientific, Houston, TX) in 14 parallel series and stored at −70°C until the in situ hybridization analysis.
Preparation of radioactively labeled cRNA probes. The preparation of radioactively labeled cRNA probes encoding the type 1 IL-1 receptor (IL-1Rt1), the PGE2 receptor, and c-Fos was performed as described previously (Simmons et al., 1989). In brief, sense and antisense cRNA probes were transcribed in vitrowith T3 or T7 RNA polymerase in the presence of [33P-UTP (New England Nuclear, DuMedical, Sollentuna, Sweden). After unincorporated nucleotides were removed using Quick Spin columns (Boehringer Mannheim, Indianapolis, IN), the specific activities of all the probes were 1–3 × 109 dpm/μg. IL-1Rt1 mRNA was transcribed from a 1.35 kb cDNA encoding part of the extracellular domain, as well as the entire transmembrane and cytoplasmic domains of the membrane-associated form of the IL-1Rt1 (Hart et al., 1993). In addition, an 885 bp cDNA fragment encoding part of the rat PGE2 receptor isoform, EP3α, was initially generated in our laboratory by reverse transcriptase-PCR using sequence-specific (Takeuchi et al., 1993) oligonucleotide primers. The EP3α cDNA clone encodes a large sequence (837 bp) common to all isoforms of the EP3 receptor and a short (46 bp) sequence unique to the EP3α isoform (Takeuchi et al., 1993). Antisense probes transcribed from this sequence should hybridize to mRNAs encoding all EP3 isoforms. Radiolabeled antisense and sensec-Fos probes were generated from a cDNA clone (Curran et al., 1987) encoding the rat Fos protein. All restriction enzymes and RNA polymerases were obtained from Promega (Madison, WI).
In situ hybridization histochemistry. In situhybridization histochemical analysis was used to detect cells expressing mRNAs encoding IL-1Rt1, the EP3 receptor, andc-Fos in the nodose ganglia from treated rats as described previously (Simmons et al., 1989). Briefly, slides with nodose ganglion sections were dried overnight in vacuo at room temperature. The sections were additionally post-fixed with 4% paraformaldehyde, pH 7.4, for 10 min, washed with PBS, and digested in preheated proteinase K solution (10 mg/ml proteinase K, 0.1 mTris-HCl, pH 8.0, and 0.05 m EDTA, pH 8.0) for 2 min at 37°C. The sections were then rinsed in TE buffer (0.1m Tris-HCl, pH 8.0, and 0.05 m EDTA, pH 8.0), dehydrated with ethanol, and dried in vacuo at room temperature overnight. Radioactively labeled cRNA probes (1–3 × 109 cpm/μg) were diluted in hybridization buffer [final concentrations of 41% (v/v) formamide, 247 mmNaCl, 8.2 mm Tris-HCl, pH 8.0, 0.82 mm EDTA, 0.82× Denhardt’s solution, 8.2% (w/v) dextran sulfate, 411 mg/ml yeast tRNA, and 8.2 mm DTT]. The final hybridization solution, 100–120 μl containing 106 cpm of either Fos, IL-1Rt1, or EP3 receptor antisense or sense cRNA probe, was applied to each slide and covered with a coverslip. All probes were hybridized at 60°C for 16–18 hr. Slides were subsequently rinsed in 4× SSC (1× SSC: 150 mm NaCl and 15 mm sodium citrate, pH 7.0), and the tissue sections were digested with 20 μg/ml RNase A in 0.5 m NaCl, 0.01 m Tris-HCl, and 0.001 m EDTA, pH 8.0, at 37°C for 30 min. The slides were washed in decreasing concentrations of SSC, ending in a final stringency wash of 0.1× SSC for 30 min at 76–78°C. Sections were then dehydrated with ethanol, dried in vacuo, subsequently defatted in increasing concentrations of ethanol and xylenes, air-dried, and dipped in Kodak NTB-2 (Eastman Kodak, Rochester, NY) nuclear track emulsion. Slides were exposed for 14–30 d at 4°C and then developed with Kodak D-19 developer for 4 min at 14–15°C. Sections were counterstained with 0.1% cresyl violet, dehydrated with graded ethanols, and coverslipped. Tissue sections from rats that were preinjected with true blue into the vagal nerve trunk were subjected to the in situ hybridization histochemical procedure as described above, except that light exposure was minimized and sections were not counterstained. To protect the fluorescent dye from the fading effects of xylenes and photochemicals, the sections were also precoated in isoamylacetate containing 2% Collodion (Electron Microscopy Sciences, Inc.) before defatting and coating the slides with the nuclear track emulsion.
Quantitating IL-1-induced changes in c-Fos expression.Resting cells normally express c-Fos mRNA and protein only at nondetectable-to-low levels, whereas physiological activation rapidly elevates intracellular levels of the transcription factor (Morgan and Curran, 1991). In the present study, we tested whether sensory neurons within the nodose ganglion respond to intravenously injected IL-1β by measuring the cellular expression ofc-Fos mRNA using in situ hybridization histochemistry. A minimum of 140 neurons were plotted from each nodose ganglion of vehicle/IL-1-, indomethacin/IL-1-, indomethacin/vehicle-, or vehicle/vehicle-injected rats, using camera lucida. Only neurons with clearly visible nuclei were plotted. The area of each neuron was determined at high magnification under Nomarski-enhanced phase contrast optics. Plots and grain counts were done by an observer who was blind to the treatment conditions of each section. A neuron was considered to be specifically labeled for c-Fos mRNA if the silver grains overlaying it exceeded the mean background level +2 SD (as measured over 20 similarly sized areas immediately surrounding the tissue section). This criterion differs from that used by others who define specific labeling to be more than five times the background levels determined from tissue sections hybridized in parallel with a sense probe. Our procedure more accurately estimates background levels by eliminating variations between slides and probes, although there is still the risk of nonspecific hybridization of the probe to the tissue. Nevertheless, a pilot experiment revealed no significant differences between background levels determined by these two criteria. Specific labeling according to the conventional or our present technique was defined as more than 5 ± 0.6 or 5 ± 0.4 grains per neuron, respectively (20 circular areas with a diameter of 50 μm were analyzed per slide; n = 5).
Statistical analysis. Data are expressed as the mean ± SEM. Comparisons were initially made by ANOVA, followed by Scheffé’s (c-Fos induction) or Dunnett’s multiple range (vagal discharge activity) post hoc tests.
RESULTS
Vagal sensory neurons express receptors for IL-1 and PGE2
We measured the local expression of mRNAs encoding IL-1Rt1 and the EP3 subtype of the PGE2 receptor (EP3R) within the nodose ganglion byin situ hybridization histochemical analysis using33P-labeled antisense cRNA probes. The IL-1Rt1-specific probe revealed labeling of weak-to-medium intensity exclusively over neuron-like cells in the rat nodose ganglion (Fig.1A). These neurons were evenly distributed throughout the ganglion, and quantitative evaluation revealed that 26.5 ± 3.4% of the neurons in the nodose ganglion (n = 3) expressed mRNA for IL-1Rt1. Analysis for expression of the rat EP3R in nodose ganglion sections showed high levels of EP3R mRNA exclusively within neuron-like cells throughout the ganglionic mass (Fig. 1C,E). Ganglion sections, hybridized in parallel with 33P-labeled sense control probes for each of the receptors, did not show specific labeling above background levels (Fig.1B,D). In addition, the expression of IL-1Rt1 and EP3R mRNAs in the nodose ganglia, both in terms of the number of receptor-expressing cells and in the relative intensity of labeling for each mRNA, did not visually differ between rats that had received injections of either human recombinant IL-1β (2 μg/kg) or vehicle alone 60 min before perfusion fixation (data not shown).
Fig. 1.
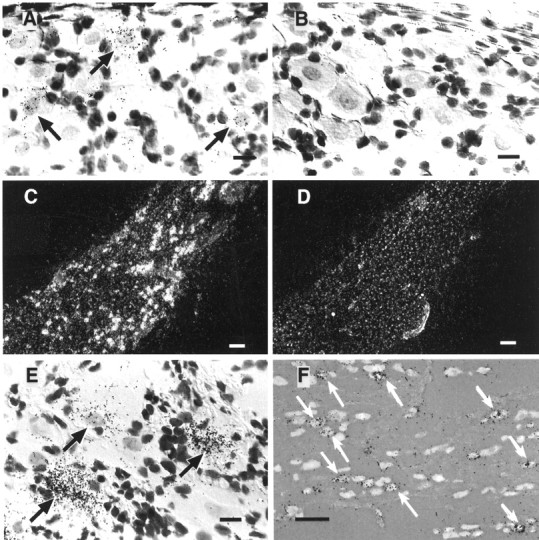
Sensory neurons in the nodose ganglion express receptors for IL-1 and PGE2. An antisense (A) or sense (B) cRNA probe encoding the IL-1Rt1 was hybridized in situ to nodose ganglion sections from a rat that had previously received an injection with vehicle only 60 min before perfusion fixation. Specific hybridization with the antisense IL-1 receptor probe resulted in the accumulation of silver grains over cells throughout the nodose ganglion (A), whereas no specific labeling was obtained in parallel sections after hybridization with the sense control probe (B). The arrows in the bright-field micrograph inA indicate cells typical of those expressing the IL-1 receptor in the nodose ganglion. Cells expressing mRNA encoding the EP3 receptor were identified in sections throughout the nodose ganglion after hybridization with a specific antisense cRNA probe (C; dark-field micrograph), whereas parallel tissue sections hybridized with a sense probe showed no labeling above background levels (D). Arrows inE identify specific labeling for EP3 receptor mRNA over neuron-like cells (bright-field micrograph). These receptor-expressing cells in the nodose ganglion send projections to the vagus nerve, as was revealed by their labeling with the retrograde tracer true blue (F), which was injected into the vagal fiber trunk ∼1 cm distal to the caudal pole of the nodose ganglion. These rats showed extensive and specific in situ labeling with the EP3R antisense probe in retrogradely filled neurons (F). Arrows depict double-labeled cells. Scale bars: A, B,E, 30 μm; C, D, 300 μm; F, 120 μm.
In some rats (n = 3), the retrograde tracer true blue was injected into the vagus nerve to characterize the nodose ganglion neurons that emanate vagal sensory afferents. In situhybridization analysis of ganglia from these rats showed specific labeling of EP3R mRNA exclusively localized (Fig. 1F) over neuronal cells in the nodose ganglion that were retrogradely filled with true blue (24.9 ± 5.2% of all the retrogradely labeled cells expressed EP3R mRNA; n = 3). These results demonstrate that EP3R-expressing cells in the nodose ganglion are vagal sensory neurons, which likely receive and relay information from the thoracic and/or abdominal compartments. It was difficult to obtain a reasonable signal using the IL-1Rt1 antisense probe in combination with true blue fluorescence because of a loss of sensitivity in the hybridization procedure, but the overall similarity in morphology and distribution between neurons expressing these two receptors strongly suggests that IL-1Rt1 mRNA is also expressed in vagal sensory neurons. Finally, attempts to analyze adjacent tissue sections that were independently labeled for IL-1Rt1 or EP3R mRNA did not reliably detect neuronal colocalization of these mRNA species.
Effects of blood-borne IL-1β on sensory neurons in the nodose ganglion
Although in situ hybridization analysis showed only a few widely scattered neuron-like cells expressing c-Fos mRNA in the nodose ganglion of vehicle/vehicle-injected rats (11.10 ± 0.79%; n = 15) (Fig. 2), a substantial increase in their number was seen after IL-1β administration (2 μg/kg, i.v.; 28.40 ± 1.66%;n = 8; p < 0.0001) (Fig. 2). These IL-1-responsive neurons were generally distributed throughout the nodose ganglionic mass but had a tendency to aggregate caudally (Fig.2). Interestingly, IL-1 injections resulted in the widespread activation of a population of smaller cells with densely counterstained nuclei, presumably of glial or possibly vascular origin (Fig. 2). Rats pretreated with indomethacin (10 mg/kg, i.v.), a general inhibitor of prostaglandin synthesis, before the IL-1 challenge, revealed a significant reduction in the number of c-Fos-expressing neurons (21.70 ± 1.50%; n = 9; p= 0.0093, compared with vehicle/IL-1-injected rats) (Fig. 2). The indomethacin/IL-1-treated rats did, however, still show an elevated number of c-Fos-expressing neurons in the nodose ganglion compared with vehicle/vehicle-injected control rats (p < 0.0001) (Fig. 2). Rats injected with indomethacin/vehicle also exhibited a slight but nonsignificant increase in the number of c-Fos cells in the ganglion compared with those in the vehicle/vehicle control group (14.73 ± 1.04%; n = 6; p = 0.28).
Fig. 2.
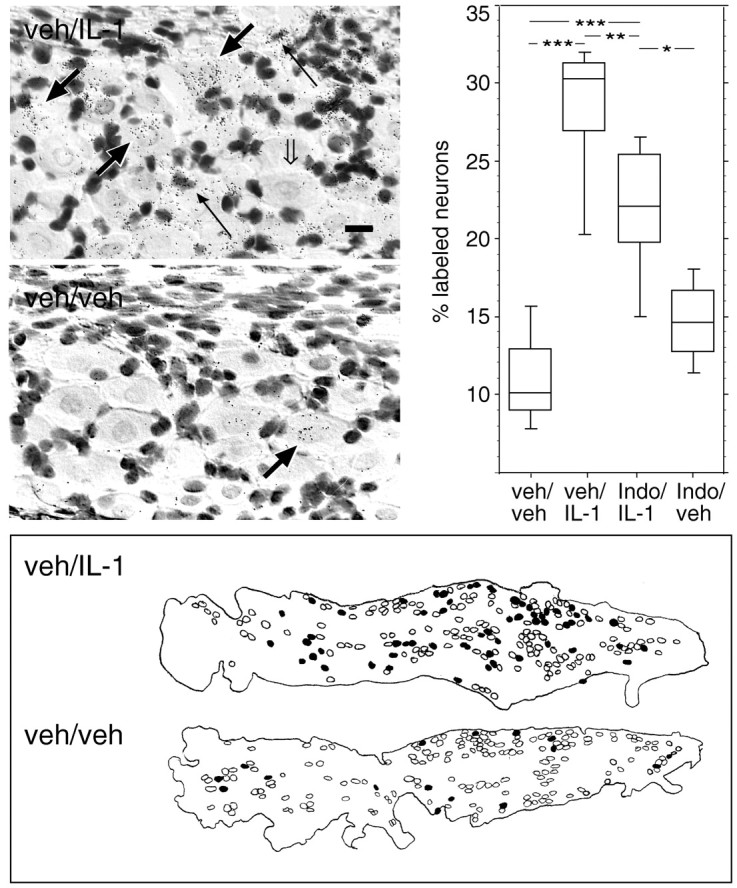
Sensory neurons in the nodose ganglion are responsive to blood-borne IL-1. Photomicrographs in the top left panels show in situ hybridization analysis with a 33P-labeled cRNA antisense probe encoding the cellular activation marker c-Fos to 12 μm sections through the nodose ganglia of rats injected with 2 μg/kg human recombinant IL-1β (top photomicrograph) or vehicle alone (bottom photomicrograph) 60 min before perfusion fixation. Note the accumulation of black silver grains, corresponding to specific labeling for c-Fos mRNA, over neuron-like cells in IL-1- but not in vehicle-injected rats. Parallel sections hybridized with a c-Fos cRNA sense probe displayed background levels of silver grains throughout the ganglion (data not shown). Thick black or open arrows, respectively, indicate neurons that either do or do not expressc-Fos mRNA. Thin black arrowsindicate specific labeling for c-Fos mRNA over non-neuronal cells. Scale bar (in top photomicrograph), 25 μm. Top right panel shows results from the quantitative evaluation of neurons specifically expressingc-Fos mRNA in the nodose ganglia of vehicle/vehicle-, vehicle/IL-1-, indomethacin/IL-1-, or indomethacin/vehicle-injected rats. Silver grains were visually counted over neurons plotted with a camera lucida. The data are displayed in box plots in which the horizontal lines in each boxcorrespond to the 25th percentile, the median, and the 75th percentile, and the range for each group is indicated by the extent of thevertical lines. *p = 0.0134; **p = 0.0093; *** p < 0.0001. The bottom panel is a schematic drawing showing the distribution of c-Fos-expressing cells within the nodose ganglion of IL-1- or vehicle-injected rats. Cells having a neuronal morphology and a clearly delineated nucleus were plotted throughout the ganglion using a camera lucida. Sensory neurons were defined as being specifically labeled or unlabeled with the c-Fosantisense mRNA probe and are represented by filled oropen circles, respectively. The outline of each ganglion is shown with the caudal pole of the ganglion positioned to theright.
Intravenously administered IL-1β triggers increased discharge activity of gastric vagal afferents via a prostaglandin-dependent mechanism
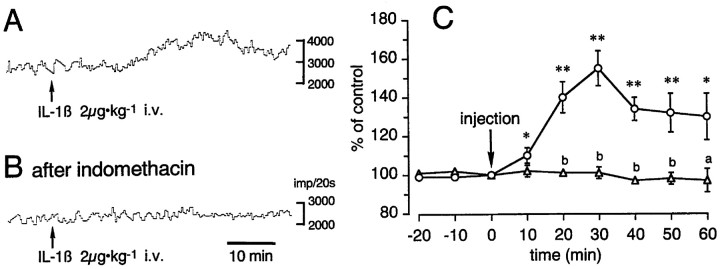
Elevated cellular activity, as indicated by an increase inc-Fos expression, in vagal sensory neurons of IL-1-injected rats was matched by a sharp rise in discharge activity of vagal sensory afferent fibers. Electrophysiological analysis showed that the administration of 2 μg/kg human recombinant IL-1β caused a pronounced increase in the discharge activity of gastric vagal afferents (Fig.3A,C). The response was noticeable within minutes after drug administration, manifested peak values (155 ± 9% of preinjection discharge levels) by 30 min (n = 5), and was still elevated (130 ± 12% of preinjection discharge levels) after 60 min. Vehicle-injected control rats (n = 6) did not display significant changes in afferent discharge activity compared with preinjection levels. Furthermore, administration of indomethacin before IL-1β injection completely blocked the IL-1-mediated rise in discharge activity (n = 5) (Fig. 3), indicating that these IL-1 effects on gastric afferents are dependent on endogenous prostaglandins. The initial injection of indomethacin alone did not alter the pattern of afferent discharge activity during the 60 min preceding the IL-1 injection (data not shown).
Fig. 3.
Afferent activity of the gastric vagal nerve responds to intravenous administration of IL-1β (2 μg/kg).A and B show sample recordings from individual rats injected with vehicle/IL-1 or indomethacin/IL-1, respectively. C summarizes the responses from 19 rats. The ordinate indicates the magnitude of the response (>1 min), which is expressed as the percentage of the preadministration control value (taken 1 min immediately before injection of IL-1β or vehicle). The abscissa shows time in minutes in which 0 indicates the onset of injection. Circles show the data from vehicle/IL-1β-injected rats (n = 5), andtriangles show the data from indomethacin/IL-1β-injected rats (n = 5). Eachpoint and vertical bar indicate the mean ± SEM. *p < 0.05; **p < 0.01, comparing the vehicle/IL-1β-injected with the vehicle/vehicle-injected groups. a,p < 0.05; b, p < 0.01, comparing the indomethacin/IL-1β with the vehicle/IL-1β groups.
DISCUSSION
The activation of central autonomic neurocircuitries during peripheral inflammatory processes raises fundamental questions about the mechanism(s) by which immune–neural communication occurs, whether via humoral effects across the blood–brain barrier or by activating peripheral sensory afferents. The present investigation explored the responsiveness of vagal sensory neurons in the nodose ganglion to intravenous IL-1β. Our data demonstrate that nodose ganglion neurons express IL-1Rt1 and EP3 receptors under basal conditions and that administration of IL-1β potently stimulates vagal sensory neurons in a partially prostaglandin-dependent manner, as measured by the expression of the cellular activation marker c-Fos and electrophysiological recordings.
Vagal sensory afferents are responsive to blood-borne IL-1
Central responses to immune challenges within the peritoneum depend on intact abdominal vagal afferents (Watkins et al., 1995b). Although this clearly suggests that vagal mechanisms mediate aspects of the systemic responses to immunological challenges within the abdominal cavity, the involvement of vagal viscerosensory afferents in mediating central responses to intravenous IL-1 or LPS remain controversial (Katsuura et al., 1988; Wan et al., 1994; Sehic and Blatteis, 1996;Ericsson et al., 1997; Romanovsky et al., 1997). Here, we demonstrate that intravenous infusion of IL-1β (2 μg/kg) causes nuclear induction of the transcription factor c-Fos within sensory neurons of the nodose ganglion. This marker of cellular activation has been used to identify activated central neural circuitries after an IL-1 challenge (Ericsson et al., 1994). The induction ofc-Fos in nodose ganglion neurons in response to IL-1 strongly suggests that vagal sensory functions are triggered by this stimulus, which is further supported by our findings that gastric vagal afferents display rapid and lasting increases in discharge activity after an IL-1 challenge. This effect was also shown in animals whose gastric sympathetic nerves were crushed (data not shown), demonstrating that the IL-1-induced increase in gastric vagal afferent activity is not secondary to changes in the efferent activity of the vagus and/or sympathetic nerves. However, it remains to be analyzed whether the vagal responses to IL-1 were produced by recruitment of silent afferent fibers and/or by increases in spontaneous afferent firing rates.
These findings reveal that the activation of vagal viscerosensory afferents induced by IL-1β occurs not only when the cytokine is delivered to the abdominal cavity, but also when it is introduced into the general circulation. This is in agreement with findings that intravenous IL-1β stimulate hepatic afferents (Niijima, 1996) and that selective hepatic vagotomy block fever responses to low levels of circulating LPS (Sehic and Blatteis, 1996; Simons et al., 1998). In contrast, abdominal vagotomies do not interfere with the activation of central neurocircuitries, fever, or endocrine responses after intravenous administration of moderate-to-high doses of IL-1 or LPS (Katsuura et al., 1988; Wan et al., 1994; Ericsson et al., 1997;Romanovsky et al., 1997). Collectively, these findings suggest that abdominal vagal afferents mediate central responses to low levels of circulating IL-1 or LPS and that other mechanisms, including local signaling events across the blood–brain barrier and/or thoracic vagal and nonvagal afferents, are involved in mediating central responses to moderate-to-high plasma levels of IL-1 or LPS.
Neurons in the nodose ganglion receive viscerosensory information from pharynx, larynx, and the abdominal and thoracic compartments. Although these neurons do not display strict somatotopic organization within the ganglion, the sensory neurons receiving input from the abdominal organs are generally distributed in the caudal and middle portions of the ganglia (Sharkey et al., 1984; Gwyn et al., 1985; Green and Dockray, 1987), whereas those with afferent input from the larynx, esophagus, and the aortic depressor nerve tend to aggregate within the rostral pole of the ganglion (Altschuler et al., 1989; Hopkins and Armour, 1989; Uno et al., 1996). Our findings of c-Fos-labeled sensory neurons within the caudal, middle, and rostral divisions of the nodose ganglion are consistent with the simultaneous activation of gastric (present results; Kurosawa et al., 1997), hepatic (Niijima, 1996), and possibly thoracic vagal afferents in response to circulating IL-1. Extended studies are required to determine to what extent each vagal branch is involved in responding to an intravenous IL-1 challenge.
Prostaglandins mediate aspects of the vagal responses to peripheral inflammatory stimuli
Prostaglandins mediate central responses after peripheral cytokine administration. Pharmacological blockade of endogenous cyclooxygenase activity inhibit hyperthermia (Morimoto et al., 1989) and ACTH responses to intravenously administered IL-1β (Watanabe et al., 1990). Indomethacin pretreatment also reduces or prevents IL-1-mediated corticosterone secretion (Dunn and Chuluyan, 1992; Kandasamy et al., 1995), central c-Fos and hypothalamic CRF mRNA induction (Ericsson et al., 1997), behavioral responses (Crestani et al., 1991), and hypothalamic noradrenaline depletion (Dunn and Chuluyan, 1992). Moreover, infusion of IL-1 results in elevated plasma levels of PGE2 (Rotondo et al., 1988; Watanobe et al., 1995), and intravenous administration of PGE2, like IL-1, induces ACTH secretion (Hollingsworth et al., 1995; Watanobe et al., 1995; Young et al., 1996). Although collectively these data indicate that prostaglandins participate in various aspects of the host systemic response to IL-1, limited evidence is available on how different prostaglandin subtypes influence central functions. Interestingly, several studies have demonstrated the contribution of prostaglandins in vagal afferent transmission. For example, PGE1, PGE2, and PGF2α exhibit multifaceted regulatory actions on vagal afferents transmitting cardiac, baroreceptor, and pulmonary sensory information (Kalix, 1979; Bergren et al., 1984; Panzenbeck et al., 1988; Taguchi et al., 1992; Lee and Morton, 1995). In addition, prostacyclin attenuates baroreflex control of renal nerve activity via vagal afferents (Zucker et al., 1988), and tromboxane induces rapid, shallow breathing via vagal pulmonary receptors (Karla et al., 1992; Carrithers et al., 1994). Here, we show that prostaglandins are likely to contribute to vagal responses after administration of IL-1, because these responses were attenuated by indomethacin pretreatment. Similarly, increases in vagal hepatic afferent activity after intravenous administration of IL-1 are partly mediated via prostaglandins (Niijima, 1996). It remains to be determined whether prostaglandins exert their own independent actions on vagal sensory neurons or whether they augment the actions of IL-1. For instance, prostaglandins may influence the expression and/or function of IL-1 receptors on vagal afferents (see below), similar to the effects of PGE2 on sensitizing vagal responses to bradykinin (Staszewska-Barczak, 1983) and capsaicin (Lee and Morton, 1995). The effective blockade of IL-1-mediated increase in gastric vagal discharge activity in indomethacin-pretreated rats contrasts to the findings of a population of prostaglandin-independent IL-1-responsive neurons in the nodose ganglion. This suggests that distinct vagal viscerosensory afferents are differentially dependent on prostaglandins in their response to blood-borne IL-1. Collectively, these data show that prostaglandins regulate a variety of vagus-dependent processes and that they are likely to contribute in part to aspects of IL-1-mediated activation of vagal sensory functions.
Sensory neurons in the nodose ganglion express IL-1 and prostaglandin receptors
The partial involvement of prostaglandins in IL-1-induced vagal sensory activation suggests that cells in the nodose ganglion express both IL-1 and prostaglandin receptors. Evidence for this has been based on receptor-binding studies examining prostacyclin binding on vagal afferents (Matsumura et al., 1995), as well as IL-1 receptor antagonist binding on glomus cells located within vagus nerve-associated paraganglia (Goehler et al., 1997). Paraganglia are distributed along the vagus nerve trunks in the abdomen and thorax, and they receive rich innervation from vagal afferents (Berthoud et al., 1995). It remains, however, to be ascertained whether these glomus cells actually express the transmembrane-signaling IL-1Rt1 and whether they interact with vagal sensory afferents. Here, we provide the first experimental data to show that vagal sensory neurons themselves synthesize mRNA encoding receptors for both IL-1 and PGE2. These findings demonstrate an alternative and more direct means by which IL-1 and PGE2 may regulate sensory functions of the vagus nerve.
Functional considerations
The hepatic vagal branch is implicated in driving centrally regulated systemic responses to peripheral immune stimuli, such as hyperalgesia (Watkins et al., 1994a) and hyperthermia (Simons et al., 1998). On the other hand, adrenocortical responses to intraperitoneal IL-1 are attenuated after subdiaphragmatic, but not hepatic, vagotomy (Fleshner et al., 1995), suggesting that gastrointestinal vagal afferents activate central neurocircuitries involved in stress-related endocrine responses. In accordance, the kinetics of the electrophysiological responses in our study (i.e., discharge activity increases within 10 min and peaks 30 min after IL-1 administration) mimic the kinetics of cytokine-induced ACTH secretion (Rivier et al., 1989b) and hyperthermia (Simrose and Fewell, 1995). The IL-1-responsive viscerosensory neurons in the nodose ganglion identified in the present study are thus likely to participate in the central component of cytokine-responsive endocrine, nociceptive, and fever responses. Our current results, revealing that increased discharge activity of the gastric vagal branches to intravenous IL-1 is dependent on prostaglandins, extend our previous results, which showed that this vagal response is partly mediated via cholecystokinin (CCK) (Kurosawa et al., 1997). Interestingly, gastric vagal afferents are known to convey satiation signals to the CNS in a CCK-dependent manner (Smith et al., 1985), IL-1-induced anorexia is partly mediated via type A CCK receptors in peripheral organs (Daun and McCarthy, 1993), and intravenous IL-1 sensitizes gastric afferents to the stimulatory actions of CCK on type A receptors (Bucinskaite et al., 1997; Kurosawa et al., 1997). Moreover, IL-1-induced anorexia is blocked after systemically, but not centrally, administered cyclooxygenase inhibitors (Hellerstein et al., 1989; Uehara et al., 1989; Shimizu et al., 1991;McCarthy and Daun, 1992). These findings strongly suggest the involvement of gastric vagal afferents, CCK, and prostaglandins in mediating anorexic behaviors after IL-1 administration.
Conclusions
It is increasingly evident that the immune system has the capacity to affect central neural functions via several independent pathways. Our present findings reveal that there is a local production of IL-1 and PGE2 receptors in vagal sensory neurons and that blood-borne IL-1 stimulates vagal viscerosensory pathways in a partially prostaglandin-dependent manner. This reinforces the growing awareness that the vagus nerve fundamentally participates in relaying information of peripheral inflammatory insults to central autonomic regulatory centers.
Footnotes
This work was supported by grants from The Swedish Medical Research Council, The Wenner-Gren Center Foundation for Scientific Research, The Swedish Society for Medicine, The Swedish Association of Rheumatology Research, The King Gustaf V 80th Year Foundation, and foundations of the Karolinska Institute, Nanna Swartz, Sven and Ebba-Christina Hagberg, Harald and Greta Jeansson, Sven and Dagmar Sahlén, Ulla and Gustaf af Uggla, Börje Dahlin, Lars Hierta, and Åke Wiberg. A.E. was supported by a Research Assistant Fellowship from the Swedish Medical Research Council. We thank Dr. S. Gillis (Immunex Research and Development Corp., Seattle, WA) for generously providing the preparation of interleukin-1β and Dr. R. Hart (Rutgers University, Newark, NJ) for providing the cDNA encoding the IL-1 receptor. We thank Paul Sawchenko, Gunnar Grant, and Elaine Brown for their critical evaluation of this manuscript.
Correspondence should be addressed to Anders Ericsson, Lab of Rheumatology, Center for Molecular Medicine, Building L8:04, The Karolinska Hospital, S-171 76, Stockholm, Sweden.
REFERENCES
- 1.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 2.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Bergren DR, Gustafson JM, Myers DL. Effect of prostaglandin F2 alpha on pulmonary rapidly-adapting-receptors in the guinea pig. Prostaglandins. 1984;27:391–405. doi: 10.1016/0090-6980(84)90198-9. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Kressel M, Neuhuber WL. Vagal afferent innervation of rat abdominal paraganglia as revealed by anterograde DiI-tracing and confocal microscopy. Acta Anat. 1995;152:127–132. doi: 10.1159/000147691. [DOI] [PubMed] [Google Scholar]
- 5.Besedovsky HO, del Rey A. Mechanism of virus-induced stimulation of the hypothalamus–pituitary–adrenal axis. J Steroid Biochem. 1989;34:235–239. doi: 10.1016/0022-4731(89)90087-3. [DOI] [PubMed] [Google Scholar]
- 6.Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. NeuroReport. 1996;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- 7.Bret-Dibat JL, Bluthe RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 8.Bucinskaite V, Kurosawa M, Miyasaka K, Funakoshi A, Lundeberg T. Interleukin-1-beta sensitizes the response of the gastric vagal afferent to cholecystokinin in rat. Neurosci Lett. 1997;229:33–36. doi: 10.1016/s0304-3940(97)00406-0. [DOI] [PubMed] [Google Scholar]
- 9.Cao C, Matsumura K, Yamagata K, Watanabe Y. Induction by lipopolysaccharide of cyclooxygenase-2 mRNA in rat brain; its possible role in the febrile response. Brain Res. 1995;697:187–196. doi: 10.1016/0006-8993(95)00839-i. [DOI] [PubMed] [Google Scholar]
- 10.Carrithers JA, Liu F, Shirer HW, Orr JA. Mechanisms for the tachypneic response to the thromboxane A2 mimetic U-46,619 in rabbits. Am J Physiol. 1994;266:R321–R327. doi: 10.1152/ajpregu.1994.266.2.R321. [DOI] [PubMed] [Google Scholar]
- 11.Chuluyan HE, Saphier D, Rohn WM, Dunn AJ. Noradrenergic innervation of the hypothalamus participates in adrenocortical responses to interleukin-1. Neuroendocrinology. 1992;56:106–111. doi: 10.1159/000126215. [DOI] [PubMed] [Google Scholar]
- 12.Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- 13.Curran T, Gordon MB, Rubino KL, Sambucetti LC. Isolation and characterization of the c-fos(rat) cDNA and the analysis of post-translational modification in vitro. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- 14.Dantzer R. How do cytokines say hello to the brain? Neural versus humoral mediation. Eur Cytokine Netw. 1994;5:271–273. [PubMed] [Google Scholar]
- 15.Daun JM, McCarthy DO. The role of cholecystokinin in interleukin-1-induced anorexia. Physiol Behav. 1993;54:237–241. doi: 10.1016/0031-9384(93)90105-o. [DOI] [PubMed] [Google Scholar]
- 16.Dunn AJ, Chuluyan HE. The role of cyclooxygenase and lipoxygenase in the interleukin-1-induced activation of the HPA axis: dependence on the route of injection. Life Sci. 1992;51:219–225. doi: 10.1016/0024-3205(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 17.Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Ericsson A, Sawchenko PE. c-fos-Based functional mapping of central pathways subserving the effects of interleukin-1 on the hypothalamo–pituitary–adrenal axis. In: DeSouza EB, editor. The neurobiology of cytokines. Academic; New York: 1993. pp. 155–171. [Google Scholar]
- 19.Ericsson A, Kovács K, Sawchenko P. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ericsson A, Liu C, Hart R, Sawchenko PE. Distribution of the type 1 interleukin-1 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 21.Ericsson A, Ek M, Wahlström I, Kovács K, Liu C-L, Hart R, Sawchenko PE. Pathways and mechanisms for interleukin-1 mediated regulation of the hypothalamic–pituitary–adrenal axis. In: McCarty R, Aguilera G, Sabban EL, Kvetnansky R, editors. Stress: molecular genetic and neurobiological advances. Gordon and Breach; New York: 1996. pp. 101–120. [Google Scholar]
- 22.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleshner M, Goehler LE, Hermann J, Relton JK, Maier SF, Watkins LR. Interleukin-1 beta induced corticosterone elevation and hypothalamic NE depletion is vagally mediated. Brain Res Bull. 1995;37:605–610. doi: 10.1016/0361-9230(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 24.Gaykema RP, Dijkstra I, Tilders FJ. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136:4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]
- 25.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 26.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract. Neurosci Lett. 1987;76:151–156. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 27.Gwyn DG, Leslie RA, Hopkins DA. Observation of afferent and efferent organization of vagus nerve and the innervation of the stomach in the squirrel monkey. J Comp Neurol. 1985;239:163–175. doi: 10.1002/cne.902390204. [DOI] [PubMed] [Google Scholar]
- 28.Hart RP, Liu C, Shadiack AM, McCormack RJ, Jonakait GM. An mRNA homologous to interleukin-1 receptor type I is expressed in cultured rat sympathetic ganglia. J Neuroimmunol. 1993;44:49–56. doi: 10.1016/0165-5728(93)90267-3. [DOI] [PubMed] [Google Scholar]
- 29.Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello DA. Interleukin-1-induced anorexia in the rat. J Clin Invest. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollingsworth SA, Deayton JM, Thorburn GD. Prostaglandin E2 administered to fetal sheep increases the plasma concentration of adrenocorticotropin (ACTH) and the proportion of ACTH in low molecular weight forms. Endocrinology. 1995;136:1233–1240. doi: 10.1210/endo.136.3.7867577. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins DA, Armour JA. Ganglionic distribution of afferent neurons innervating the canine heart and cardiopulmonary nerves. J Auton Nerv Syst. 1989;26:213–222. doi: 10.1016/0165-1838(89)90170-7. [DOI] [PubMed] [Google Scholar]
- 32.Kalix P. Prostaglandins cause cyclic AMP accumulation in peripheral nerve. Brain Res. 1979;162:159–163. doi: 10.1016/0006-8993(79)90766-2. [DOI] [PubMed] [Google Scholar]
- 33.Kandasamy SB, Thiagarajan AB, Harris AH. Possible involvement of prostaglandins in increases in rat plasma adrenocorticotropic hormone and corticosterone levels induced by radiation and interleukin-1 alpha alone or combined. Fundam Appl Toxicol. 1995;25:196–200. doi: 10.1006/faat.1995.1055. [DOI] [PubMed] [Google Scholar]
- 34.Kapcala LP, He JR, Gao Y, Pieper JO, DeTolla LJ. Subdiaphragmatic vagotomy inhibits intra-abdominal interleukin-1 beta stimulation of adrenocorticotropin secretion. Brain Res. 1996;728:247–254. doi: 10.1016/0006-8993(96)00511-2. [DOI] [PubMed] [Google Scholar]
- 35.Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol. 1992;87:383–396. doi: 10.1016/0034-5687(92)90019-s. [DOI] [PubMed] [Google Scholar]
- 36.Katsuura G, Gottschall PE, Dahl RR, Arimura A. Adrenocorticotropin release induced by intracerebroventricular injection of recombinant human interleukin-1 in rats: possible involvement of prostaglandin. Endocrinology. 1988;122:1773–1779. doi: 10.1210/endo-122-5-1773. [DOI] [PubMed] [Google Scholar]
- 37.Kurosawa M, Uvnäs-Moberg K, Miyasaka K, Lundeberg T. Interleukin-1 increases activity of the gastric vagal afferent nerve partly via stimulation of type A CCK receptor in anesthetized rats. J Auton Nerv Syst. 1997;62:72–78. doi: 10.1016/s0165-1838(96)00111-7. [DOI] [PubMed] [Google Scholar]
- 38.Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol. 1995;79:1679–1686. doi: 10.1152/jappl.1995.79.5.1679. [DOI] [PubMed] [Google Scholar]
- 39.Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central regulations of autonomic functions. Oxford UP; New York: 1990. pp. 88–103. [Google Scholar]
- 40.Matsumura K, Watanabe Y, Onoe H, Watanabe Y. Prostacyclin receptor in the brain and central terminals of the primary sensory neurons—an autoradiographic study using a stable prostacyclin analogue [3H]iloprost. Neuroscience. 1995;65:493–503. doi: 10.1016/0306-4522(94)00505-y. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy DO, Daun JM. The role of prostaglandins in interleukin-1 induced gastroparesis. Physiol Behav. 1992;52:351–353. doi: 10.1016/0031-9384(92)90283-8. [DOI] [PubMed] [Google Scholar]
- 42.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto A, Murakami N, Nakamori T, Sakata Y, Watanabe T. Possible involvement of prostaglandin E in development of ACTH response in rats induced by human recombinant interleukin-1. J Physiol (Lond) 1989;411:245–256. doi: 10.1113/jphysiol.1989.sp017571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakano K, Okugawa K, Hayashi H, Abe S, Sohmura Y, Tsuboi T. Establishment of dye-uptake method (A375 assay) for quantitative measurement of IL-1: correlation with LAF assay. Dev Biol Stand. 1988;69:93–101. [PubMed] [Google Scholar]
- 45.Niijima A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 46.Panzenbeck MJ, Hintze TH, Kaley G. 6-Keto-prostaglandin E1 is a potent coronary vasodilator and stimulates a vagal reflex in dogs. J Pharmacol Exp Ther. 1988;244:814–819. [PubMed] [Google Scholar]
- 47.Rivier C, Chizzonite R, Vale W. In the mouse, the activation of the hypothalamic–pituitary–adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology. 1989a;125:2800–2805. doi: 10.1210/endo-125-6-2800. [DOI] [PubMed] [Google Scholar]
- 48.Rivier C, Vale W, Brown M. In the rat, interleukin-1a and -β stimulate adrenocorticotropin and catecholamine release. Endocrinology. 1989b;125:3096–3102. doi: 10.1210/endo-125-6-3096. [DOI] [PubMed] [Google Scholar]
- 49.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- 50.Rotondo D, Abul HT, Milton AS, Davidson J. Pyrogenic immunomodulators increase the levels of prostaglandin E2 in the blood simultaneously with the onset of fever. Eur J Pharmacol. 1988;154:145–152. doi: 10.1016/0014-2999(88)90091-x. [DOI] [PubMed] [Google Scholar]
- 51.Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726:160–166. [PubMed] [Google Scholar]
- 52.Sharkey KA, Williams RG, Dockray GJ. Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology. 1984;87:914–921. [PubMed] [Google Scholar]
- 53.Shimizu H, Uehara Y, Shimomura Y, Kobayashi I. Central administration of ibuprofen failed to block the anorexia induced by interleukin-1. Eur J Pharmacol. 1991;195:281–284. doi: 10.1016/0014-2999(91)90547-4. [DOI] [PubMed] [Google Scholar]
- 54.Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 55.Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Székely M, Romanovsky AA (1998) Signalling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am J Physiol, in press. [DOI] [PubMed]
- 56.Simrose RL, Fewell JE. Body temperature response to IL-1 beta in pregnant rats. Am J Physiol. 1995;269:R1179–R1182. doi: 10.1152/ajpregu.1995.269.5.R1179. [DOI] [PubMed] [Google Scholar]
- 57.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 58.Staszewska-Barczak J. Prostanoids and cardiac reflexes of sympathetic and vagal origin. Am J Cardiol. 1983;52:36A–45A. doi: 10.1016/0002-9149(83)90175-3. [DOI] [PubMed] [Google Scholar]
- 59.Taguchi O, Kikuchi Y, Hida W, Iwase N, Okabe S, Chonan T, Takishima T. Prostaglandin E2 inhalation increases the sensation of dyspnea during exercise. Am Rev Respir Dis. 1992;145:1346–1349. doi: 10.1164/ajrccm/145.6.1346. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi K, Abe T, Takahashi N, Abe K. Molecular cloning and intrarenal localization of rat prostaglandin E2 receptor EP3 subtype. Biochem Biophys Res Commun. 1993;194:885–891. doi: 10.1006/bbrc.1993.1904. [DOI] [PubMed] [Google Scholar]
- 61.Tilders FJ, De Rijk RH, Van Dam AM, Vincent VA, Schotanus K, Persoons JH. Activation of the hypothalamus–pituitary–adrenal axis by bacterial endotoxins: routes and intermediate signals. Psychoneuroendocrinology. 1994;19:209–232. doi: 10.1016/0306-4530(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 62.Uehara A, Ishikawa Y, Okumura T, Okamura K, Sekiya C, Takasugi Y, Namiki M. Indomethacin blocks the anorexic action of interleukin-1. Eur J Pharmacol. 1989;170:257–260. doi: 10.1016/0014-2999(89)90546-3. [DOI] [PubMed] [Google Scholar]
- 63.Uno T, Hisa Y, Tadaki N, Okamura H, Ibata Y. Tyrosine hydroxylase-immunoreactive cells in the nodose ganglion for the canine larynx. NeuroReport. 1996;7:1373–1376. doi: 10.1097/00001756-199605310-00008. [DOI] [PubMed] [Google Scholar]
- 64.Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe T, Morimoto A, Sakata Y, Murakami N. ACTH response induced by interleukin-1 is mediated by CRF secretion stimulated by hypothalamic PGE. Experimentia. 1990;46:481–484. doi: 10.1007/BF01954238. [DOI] [PubMed] [Google Scholar]
- 66.Watanobe H, Nasushita R, Takebe K. A study on the role of circulating prostaglandin E2 in the adrenocorticotropin response to intravenous administration of interleukin-1beta in the rat. Neuroendocrinology. 1995;62:596–600. doi: 10.1159/000127055. [DOI] [PubMed] [Google Scholar]
- 67.Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994a;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 68.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994b;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 69.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995a;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 70.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995b;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 71.Weidenfeld J, Abramsky O, Ovadia H. Evidence for the involvement of the central adrenergic system in interleukin 1-induced adrenocortical response. Neuropharmacology. 1989;28:1411–1414. doi: 10.1016/0028-3908(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 72.Young IR, Loose JM, Kleftogiannis F, Canny BJ. Prostaglandin E2 acts via the hypothalamus to stimulate ACTH secretion in the fetal sheep. J Neuroendocrinol. 1996;8:713–720. [PubMed] [Google Scholar]
- 73.Zucker IH, Panzenbeck MJ, Barker S, Tan W, Hajdu MA. PGI2 attenuates baroreflex control of renal nerve activity by a vagal mechanism. Am J Physiol. 1988;254:R424–R430. doi: 10.1152/ajpregu.1988.254.3.R424. [DOI] [PubMed] [Google Scholar]