Abstract
There is considerable interest in the mechanisms by which systemic cytokines signal the CNS to elicit centrally controlled biological actions. This study determined the effects of intraperitoneal injections of interleukin-1β (IL-1β) on IL-1β mRNA and IL-1 receptor accessory protein (IL-1RAP) mRNA production in rat liver and brain using the reverse transcription-PCR. Saline or IL-1β (0.5 μg/kg) was injected intraperitoneally in subdiaphragmatically vagotomized and sham-operated (SHAM) rats. All injections were performed at dark onset, and rats were killed 2 hr after the injection. In SHAM rats, IL-1β increased IL-1β mRNA levels in the liver, hypothalamus, hippocampus, and brainstem. Subdiaphragmatic vagotomy blocked the IL-1β-induced increase in IL-1β mRNA in the brainstem and hippocampus and significantly attenuated the increase in the hypothalamus. Vagotomy did not affect IL-1β-induced IL-1β mRNA production in the liver. IL-1RAP mRNA was highly expressed in each region examined; however, no significant differences in IL-1RAP mRNA production were found in any region after IL-1β injection. The current results indicate that the vagus nerve is involved in transmitting cytokine signals to the brain and suggest that the induction of brain cytokines is a critical step in the pathway by which vagal-mediated signals result in centrally controlled symptoms of the acute phase response.
Keywords: interleukin-1, interleukin-1 receptor accessory protein, vagotomy, cytokine, vagus nerve, RT-PCR, mRNA, sleep, fever, acute phase response
Interleukin-1β (IL-1β) is a proinflammatory cytokine involved in the regulation of several physiological CNS processes (e.g., sleep and appetite regulation), and it plays a role in neural immune responses to tissue damage and infection (for review, see Krueger and Majde, 1994). The peripheral administration of IL-1β induces many of the symptoms accompanying the acute phase responses that are mediated by the CNS, including fever, excess sleep, and social withdrawal (Krueger et al., 1984; Watkins et al., 1995a; Bluthé et al., 1996; Hansen and Krueger; 1997). These symptoms also occur after the administration of substances that induce IL-1β production, such as bacterial lipopolysaccharide (LPS) or muramyl-dipeptide (Sehic and Blatteis, 1996; Goldbach et al., 1997;Kapás et al., 1997). Conversely, inhibition of IL-1β blocks many of the illness responses induced by these agents (Kent et al., 1992). Furthermore, fever, excess sleep, and behavioral effects of systemic IL-1β, LPS, or muramyl-dipeptide are blocked by the central inhibition of IL-1β (Kent et al., 1992; Klir et al., 1994; Takahashi et al., 1996). These data clearly indicate that both systemic and central pools of IL-1β are important in these responses.
The mechanisms by which peripheral cytokines signal the brain to elicit central manifestations of the acute phase response have not been conclusively identified. Cytokines are relatively large, hydrophilic peptides and are not expected to readily cross the blood–brain barrier. A saturable transport mechanism for several cytokines exists (Banks et al., 1991); however, it is uncertain whether the amounts shown to enter the brain are sufficient to activate central mechanisms. Furthermore, there is increasing evidence suggesting that vagal afferents transmit systemic cytokine information to the brain. Thus, in rats, mice, and guinea pigs, various measures of the acute phase response are inhibited by vagotomy (for review, see Watkins et al., 1995b; Blatteis and Sehic, 1997). IL-1β increases vagal afferent activity (Niijima, 1996), and IL-1 receptors are found on paraganglia in the hepatic vagus (Goehler et al., 1997). Furthermore, vagotomy blocks the induction of IL-1β mRNA in the brain of LPS-treated mice (Layé et al., 1995). Collectively, this evidence suggests that after an appropriate challenge, the production of IL-1β in brain may be a critical step in the pathway by which vagal-mediated signals result in centrally controlled symptoms of the acute phase response.
The aim of the present study was to determine (1) whether intraperitoneal injections of IL-1β induce IL-1β mRNA expression in the brain, and (2) whether this effect can be blocked by subdiaphragmatic vagotomy. The dose of IL-1β used in the current study, 0.5 μg/kg, was demonstrated previously to induce sleep and fever in rats; vagotomy blocked the fever and attenuated the sleep-inducing effects of this dose (Hansen and Krueger, 1997). The current experiments also sought to determine variations in interleukin-1 receptor accessory protein (IL-1RAP) mRNA levels in response to systemic IL-1β. The IL-1RAP has recently been cloned in mice (Greenfeder et al., 1995) and rats (Liu et al., 1996) and is involved in IL-1 binding and signal transduction.
MATERIALS AND METHODS
Animals. Adult male Sprague Dawley rats (250 gm at purchase; Harlan Sprague Dawley, Indianapolis, IN) were used in this study. The animals were housed individually and maintained on a 12 hr light/dark cycle (lights on at 5 a.m. and off at 5p.m.) and at 25 ± 1°C ambient temperature. Food and water were continuously available unless otherwise noted.
Surgeries. Bilateral subdiaphragmatic vagotomy (VX) and pyloroplasty were performed on rats as described previously (Hansen et al., 1997). Briefly, after an overnight fast, rats were anesthetized using ketamine and xylazine (87 and 13 mg/kg, respectively, i.p.). The stomach and lower esophagus were visualized from an upper midline laparotomy. The stomach was gently retracted down beneath the diaphragm to clearly expose both vagal trunks. At least 1 cm of the visible vagal nerve was dissected. In addition, all neural and connective tissue surrounding the esophagus immediately below the diaphragm was removed to transect all small vagal branches. The vagotomy was supplemented with pyloroplasty to prevent gastric stasis. An incision was made parallel to the axis of the pylorus, through the pyloric sphincter, and then the pylorus wall was reconstructed by sutures perpendicular to the pylorus axis. The stomach was returned to its normal position, and the incisions were closed. Sham-operated (SHAM) animals were prepared, subjected only to pyloroplasty. All animals gained weight during the recovery period and appeared healthy. Furthermore, there was no significant difference in body weight at the time of experimental testing.
Three weeks after either VX or SHAM surgery the completeness of vagotomy was assessed as described previously (Hansen and Krueger, 1997). This test is based on the satiety effect of cholecystokinin (CCK), which is known to be mediated by the vagus nerve (Smith et al., 1981). In brief, rats were injected intraperitoneally with saline or 4 μg/kg CCK (CCK-octapeptide; Peninsula Laboratories, Belmont, CA) after 20 hr of food deprivation; a minimum of 3 d was allowed between the saline and CCK injections. Food intake was then measured after 1 hr in both SHAM and VX rats.
Experimental protocol. The animals were then allowed at least another 1 week recovery period. During this time, rats received daily intraperitoneal injections of pyrogen-free saline at dark onset, the time when the experimental treatments were done. Rats were injected with saline or 0.5 μg/kg recombinant human IL-1β (R & D Systems, Minneapolis, MN). IL-1β was dissolved in saline, and all injections were given by the intraperitoneal route and delivered in an injection volume of 1 ml/kg. Three groups of rats were used. Group I (n = 6) were SHAM rats that received saline injections. In a preliminary study, it was found that there are no significant differences in IL-1β or IL-1RAP mRNA expression in the liver or brain of saline-injected SHAM and VX rats. Group II rats (n = 6) were SHAM, and group III (n = 6) were VX rats; groups II and III were injected with IL-1β. Rats were killed by decapitation 2 hr after either saline or IL-1β injection. The liver and brain were quickly removed, and the hypothalamus, hippocampus, and brainstem were rapidly dissected. Liver and brain samples were snap-frozen in liquid nitrogen and stored at −80°C until RNA extraction.
In a separate control experiment, rats (n = 4) were injected with 0.5 μg/kg IL-1β that had been heat-inactivated at 95°C for 30 min. Rats were killed 2 hr after the injection, and brain and liver samples were collected as above. Also, as a positive control, rats (n = 5 per group) were injected intraperitoneally with saline or 100 μg/kg Escherichia coli LPS (055:B5; Sigma, St. Louis, MO). For this study, rats were killed 90 min after the injection, and liver samples were collected.
RNA extraction. Total cellular RNA was isolated after homogenization of the tissue samples in guanidine thiocyanate–phenol solution (RNA STAT-60; Tel-Test, Friendswood, TX) according to the instructions provided. Each brain region was homogenized in 2 ml and ∼100 mg of liver tissue in 3 ml of RNA-STAT-60 using a microtissue grinder. Liver and brain samples from each rat were homogenized and processed individually. The integrity of the RNA was checked by denaturing agarose gel electrophoresis and ethidium bromide staining. The total amount was measured by spectrophotometry at an absorbance of 260 nm.
Preparation of internal standard cRNAs. A plasmid containing the coding region for rat IL-1β was constructed using PCR amplification of cDNA synthesized from rat brain total RNA. The 5′ oligonucleotide used was 5′-GAA GAGCTCATGGCAACTGTCCCTGAACTC-3′, which introduces anSacI site (underlined) upstream of the initiator methionine codon. The 3′ antisense oligonucleotide used was 5′-CAG CTCGAGTTAGGAAGACACGGGTTCCATGGT-3′, which introduces an XhoI site (underlined) after the termination codon. The PCR product was visualized by ethidium bromide staining after agarose gel electrophoresis. The DNA was extracted from the gel slices (Geneclean; BIO 101, La Jolla, CA), digested with the restriction enzymes SacI and XhoI, and ligated intoSacI- and XhoI-digested pBluescript (Stratagene, La Jolla, CA). The ligated plasmid was transformed into DH5α bacteria (Life Technologies, Gaithersburg, MD), and colonies containing the insert were selected and amplified overnight following standard procedures (Sambrook et al., 1989). Plasmid DNA was purified using the Qiagen (Chatsworth, CA) Plasmid Mini Kit. An IL-1β mutant plasmid containing a 217 bp deletion of the coding region was made using restriction digestion with PstI followed by ligation with T4 DNA ligase; there are two PstI sites within the IL-1β coding region that are 217 bp apart. The internal standard for reverse transcription (RT)-PCR was generated by in vitrotranscription of the mutated plasmid using T3 RNA polymerase. The DNA template was removed by extensive DNase I digestion.
To prepare IL-1RAP internal standard, we followed a modified procedure of that of Riedy et al. (1995). A fragment of the rat IL-1RAP gene was amplified by PCR using cDNA that was reverse-transcribed from rat brain total RNA. The 5′ oligonucleotide used was 5′-CACGACTTACTGCAGCAAAGTTGC-3′, which is identical in sequence to the mRNA strand. The 3′ antisense oligonucleotide was 5′- AGGGGTGACTTTCTTGATGCTCAAAGGGACGTCATCAGGCTTCTTTCCATC-3′. The first 24 bases starting from the 5′ end of this primer are 66 bases downstream on the message in relation to the next 27 bases at the 3′ end of the primer, thereby producing a 66 bp deletion in the final PCR product. This PCR product was then cloned using the TA cloning kit (Invitrogen, Carlsbad, CA) according to the instructions provided. The plasmid construct was amplified in DH5α bacteria as described above. The IL-1RAP internal standard cRNA was generated by in vitrotranscription of the mutated gene using SP6 RNA polymerase followed by DNase I digestion.
The above mutant cRNAs were used as internal controls for RT-PCR of the respective molecule of interest. Because of the exponential nature of PCR, small differences in either RT or PCR efficiencies may result in large errors, which make quantitation of the wild-type mRNA difficult. In the current experiment, mutant cRNA is added before the RT reaction, which controls for differences in RT efficiencies. In addition, the same pair of primers amplifies both wild-type and mutant cDNA, thereby allowing normalization of differences in PCR amplification efficiency among the samples. Finally, because the wild-type and mutant RNAs are different sizes, they can be separated by gel electrophoresis and semiquantified by the ratio of densitometric measurements of the RT-PCR products visualized on ethidium bromide-stained gels.
RT-PCR. First-strand cDNA was synthesized by random-priming using 2 μg (liver samples) and 2.5 μg (brain samples) of total RNA, internal standard RNAs, 50 ng of DNA random hexanucleotides, and 200 U of Superscript II RNase H− reverse transcriptase (Life Technologies) according to the manufacturer’s instructions. Briefly, the amount of internal standard RNA used was determined by RT-PCR so that its level approximated that of the target RNA. RT was performed at 42°C for 70 min, and the reaction was terminated by heating at 95°C for 10 min. Aliquots (4 μl for brain samples and 1 μl for liver samples) of the RT reaction were amplified by PCR usingTaq DNA polymerase (Promega, Madison, WI) in a reaction volume of 50 μl. The primers for IL-1β were 5′-GACCTGTTCTTTGAGGCTGAC-3′ (sense) and 5′-TCCATCTTCTTCTTTGGGTATTGTT-3′ (antisense), which amplify a 578 bp product corresponding to wild-type IL-1β and a 361 bp product corresponding to the mutant IL-1β. The primers for the IL-1RAP were 5′-CACGACTTACTGCAGCAAAGTTGC-3′ (sense) and 5′-AGGGGTGACTTTCTTGATGCTCAA-3′ (antisense), which amplify a 616 bp product corresponding to wild-type IL-1RAP and a 550 bp product corresponding to the mutant IL-1RAP. For the brain and liver samples, respectively, cDNA for IL-1β was amplified for 33 and 34 cycles, whereas cDNA for IL-1RAP was amplified for 26 and 27 cycles. These cycle numbers were chosen based on a preliminary study determining the linear range of amplification for each respective molecule (Fig.1). This also confirmed that both the wild-type and mutant RNAs were amplified uniformly. In each PCR, denaturation was at 95°C for 45 sec, annealing was at 60°C for 45 sec, and extension was at 72°C for 2 min (for the final cycle, extension was 7 min). Furthermore, for each cDNA, PCR was performed in duplicate. DNA sequencing, performed at the University of Tennessee Molecular Resource Center, was used to confirm sequence specificity.
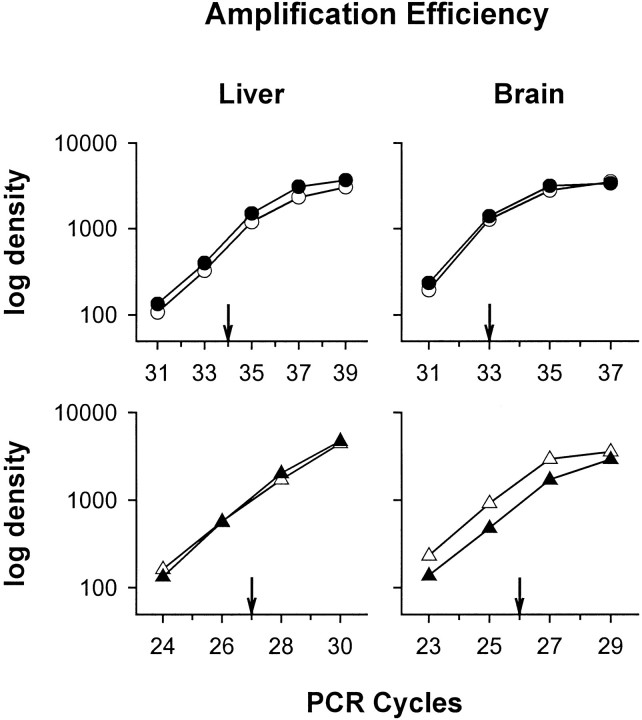
Fig. 1.
Optimized linearity regions for PCR amplification of various cycles. RT-PCR was performed on liver and brain samples, which included total RNA and corresponding amounts of IL-1β or IL-1RAP internal standard cRNA. The amplification courses for IL-1β (top) wild-type (open circles) and mutant (closed circles) and for IL-1RAP (bottom) wild-type (open triangles) and mutant (closed triangles) were obtained by densitometric measurements of the ethidium bromide-stained agarose gel and plotted in a semilogarithmic scale against the cycle number. Arrows indicate the number of PCR cycles subsequently used for IL-1β mRNA and IL-1RAP mRNA in liver and brain samples.
After amplification, aliquots of the PCR products (10 μl for IL-1β and 5 μl for IL-1RAP) were electrophoresed on horizontal gels containing 2% (w/v) agarose using 0.2× Tris-acetate/EDTA buffer. Gels were run at 100 V for 1 hr for IL-1β and 80 V for 3 hr for IL-1RAP. The gels were stained with ethidium bromide (0.5 μg/ml) for 5–10 min and then washed for 1–2 hr in water. The gels were then photographed under ultraviolet light using a charge-coupled device camera. Band densities were obtained by densitometric measurements of the RT-PCR products using public domain software NIH Image 1.54 for one-dimensional gels according to the protocol provided. The amounts of IL-1β mRNA and IL-1RAP mRNA were expressed as a ratio of densitometric measurements derived from the target message and the internal standard.
Statistical analysis. All data are expressed as mean ± SE and were analyzed by one-way ANOVA. When appropriate, post hoc analysis was done using the Student–Newman–Keuls (SNK) multiple comparison test. In all tests, an α level ofp < 0.05 was taken as an indication of statistical significance.
RESULTS
Controls
In this study, food intake analysis was used to assess the completeness of vagotomy. CCK significantly inhibited food intake in SHAM (groups I and II) [ANOVA, F(1,16) = 12.13;p < 0.005; SNK test, q(4,16) = 10.31; p < 0.01] but not in VX rats. Food intake was decreased by 57% in CCK-injected SHAM rats compared with the saline injection (3.33 ± 0.51 vs 7.77 ± 0.19 gm, respectively). In contrast, CCK did not significantly decrease food intake in VX rats compared with the saline injection (6.33 ± 0.64 vs 7.10 ± 0.32 gm, respectively).
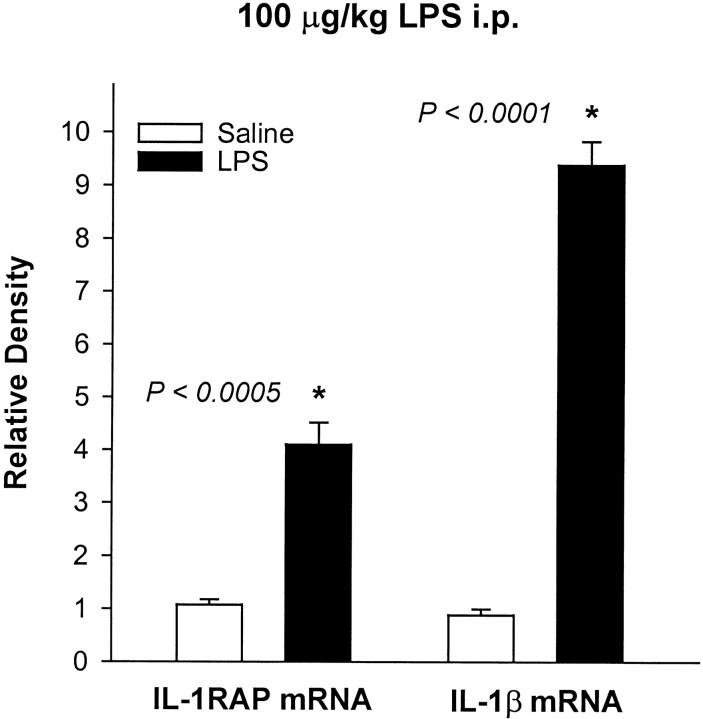
Heat-inactivated IL-1β had no effect on IL-1β mRNA or IL-1RAP mRNA levels in any brain region or in the liver (data not shown). In contrast, the intraperitoneal injection of 100 μg/kg LPS caused large increases in IL-1β mRNA and IL-1RAP mRNA levels in the liver (Fig.2). Taken together, these data indicate that the effects of IL-1β were not caused by contaminating endotoxin. In addition, the sequences of the IL-1β and IL-1RAP PCR products, as well as their respective internal controls, corresponded to the appropriate mRNA, as determined by DNA sequence analysis. Furthermore, each had the expected electrophoretic mobility (Fig.3). Two additional controls were included in the PCR experiments to rule out possible genomic DNA contamination and general DNA contamination (Kwok and Higuchi, 1989). In the first control, rat genomic DNA was amplified with appropriate sense and antisense primers. It was found that either no product or a larger product was amplified, indicating that the primers either spanned exons or covered introns. This control was necessary because the genomic structures of rat IL-1β and rat IL-1RAP are unknown. The second control was performed by PCR amplification in the absence of RT to rule out possible DNA contamination; no bands were observed.
Fig. 2.
Effects of intraperitoneal injections of saline or 100 μg/kg LPS on IL-1β mRNA and IL-1RAP mRNA levels in rat liver 90 min after the injection. The amounts of IL-1β mRNA and IL-1RAP mRNA are expressed as ratios of densitometric measurements of the samples to the corresponding internal standard. Data are presented as mean ± SE (error bars). *Significant difference compared with the saline injection (t test).
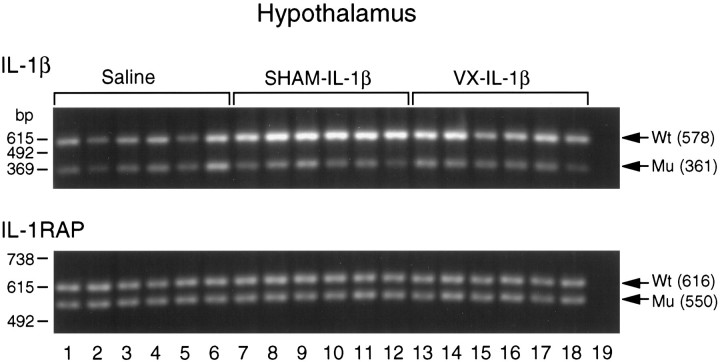
Fig. 3.
Gel electrophoresis and ethidium bromide staining of RT-PCR-amplified IL-1β and IL-1RAP mRNAs and corresponding internal standard cRNAs in the hypothalamus of rats in response to an intraperitoneal injection of saline (lanes 1–6) or 0.5 μg/kg IL-1β in sham-operated (SHAM,lanes 7–12) and subdiaphragmatically vagotomized rats (VX, lanes 13–18). Lane 19 is a no-RT control run in each gel to verify the lack of DNA contamination. The PCR products for IL-1β are 578 and 361 bp for the wild-type (Wt) and mutant (Mu), respectively. The PCR products for IL-1RAP are 616 and 550 bp for theWt and Mu, respectively. On theleft axis is a 123 bp DNA marker (Life Technologies). See Materials and Methods for a detailed description of the RT-PCR procedure.
Vagotomy blocks the increases in IL-1β mRNA in brain
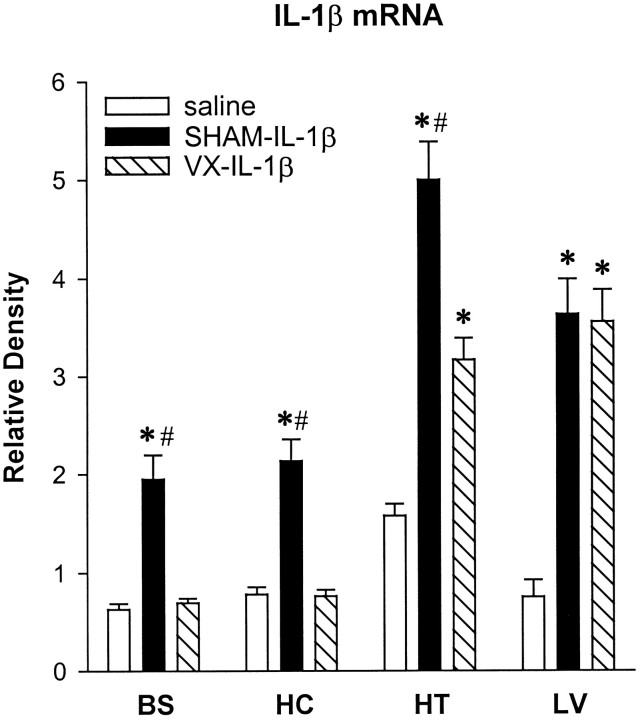
An example of the RT-PCR-amplified IL-1β mRNA and corresponding internal standard cRNA is shown in Figure 3 for the hypothalamus. The averaged values for IL-1β mRNA in the liver, brainstem, hippocampus, and hypothalamus are shown in Figure 4. The intraperitoneal injection of IL-1β increased IL-1β mRNA levels in the liver of both SHAM and VX rats (Fig. 4) [F(2,15) = 30.3; p < 0.0001]. In IL-1β-treated rats, there was a significant increase in liver IL-1β mRNA compared with the saline treatment in both SHAM [SNK,q(3,15) = 9.659; p < 0.01] and VX rats [SNK, q(2,15) = 9.399;p < 0.01]. In contrast, vagotomy blocked IL-1β induction of IL-1β mRNA in the brainstem [F(2,15) = 25.0; p < 0.0001] and hippocampus [F(2,15) = 30.7;p < 0.0001]. In the brainstem, IL-1β mRNA was increased significantly after IL-1β treatment in the SHAM rats compared with the saline injection [SNK,q(3,15) = 8.878; p < 0.01] and compared with the VX rats [SNK, q(2,15) = 8.436; p < 0.01]. Similarly, in the hippocampus IL-1β significantly increased IL-1β mRNA in SHAM rats compared with the saline injection [SNK, q(2,15) = 9.525;p < 0.01] and compared with the IL-1β-treated VX rats [SNK, q(3,15) = 9.661; p< 0.01]. In the hypothalamus, IL-1β increased IL-1β mRNA levels in both SHAM and VX rats [F(2,15) = 41.7;p < 0.0001]. In IL-1β-treated rats, there was a significant increase in hypothalamic IL-1β mRNA compared with the saline injection in SHAM [SNK, q(3,15) = 12.91;p < 0.01] and VX rats [SNK,q(2,15) = 6.0; p < 0.01]; however, this effect was significantly attenuated in the VX rats compared with the SHAM rats [SNK, q(2,15) = 6.91; p < 0.01].
Fig. 4.
Effects of intraperitoneal injections of saline or IL-1β (0.5 μg/kg) on IL-1β mRNA expression in the brainstem (BS), hippocampus (HC), hypothalamus (HT), and liver (LV) of sham-operated (SHAM) and vagotomized (VX) rats 2 hr after the injection. The amounts of IL-1β mRNA are expressed as ratios of densitometric measurements of the samples to the corresponding cRNA internal standard. Data are presented as mean ± SE (error bars) of the values obtained after two PCRs of the appropriate cDNA. *p < 0.01 compared with saline treatment (SNK test);#p < 0.01 compared with VX-IL-1β (SNK test).
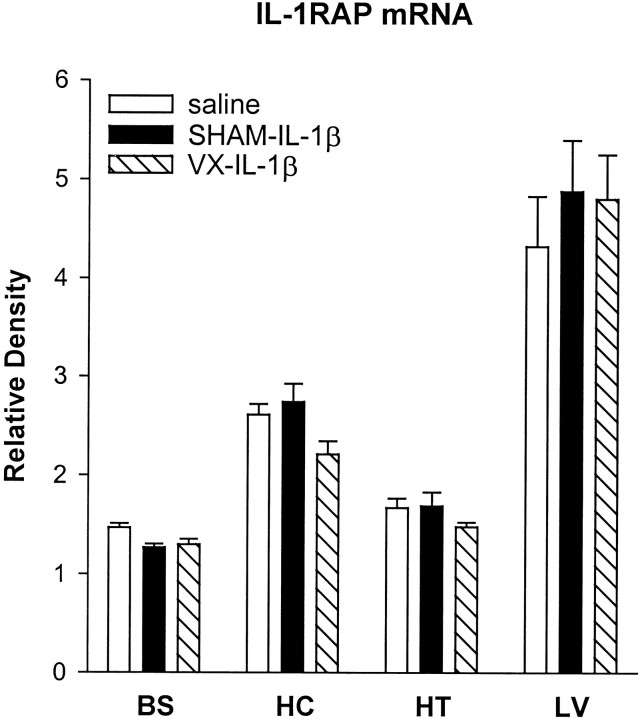
Intraperitoneal injection of IL-1β has no effect on IL-1RAP mRNA production
An example of the RT-PCR-amplified IL-1RAP mRNA and corresponding internal standard cRNA is shown in Figure 3 for the hypothalamus. The averaged values for IL-1RAP mRNA in the liver, brainstem, hippocampus, and hypothalamus are shown in Figure 5. Consistent with a previous report (Liu et al., 1996), IL-1RAP mRNA was highly expressed in rat liver and brain; this study adds the brainstem to the list of brain regions that express IL-1RAP mRNA. The intraperitoneal injection of IL-1β, however, had no significant effects on IL-1RAP mRNA production in any region examined in SHAM or VX rats (Fig. 5), although there was a slight tendency toward increased IL-1RAP mRNA levels in the liver of the SHAM and VX rats.
Fig. 5.
Effects of intraperitoneal injections of saline or IL-1β (0.5 μg/kg) on IL-1RAP mRNA expression in the brainstem (BS), hippocampus (HC), hypothalamus (HT), and liver (LV) of sham-operated (SHAM) and vagotomized (VX) rats 2 hr after the injection. The amounts of IL-1RAP mRNA are expressed as ratios of densitometric measurements of the samples to the corresponding cRNA internal standard. Data are presented as mean ± SE (error bars) of the values obtained after two PCRs of the appropriate cDNA.
DISCUSSION
In the present study, the intraperitoneal injection of IL-1β increased IL-1β mRNA levels in the liver, hypothalamus, hippocampus, and brainstem of control rats. This finding is consistent with other studies showing that IL-1β is capable of inducing its own production (Dinarello, 1996; Ilyin and Plata-Salamán, 1996). Furthermore, it is agreeable with studies that found that the systemic injection of substances that induce IL-1β (e.g., LPS) induce IL-1β in the brain (Ban et al., 1992; Layé et al., 1995). A second major finding of this study was that vagotomy blocked the induction of IL-1β mRNA in the hippocampus and brainstem of IL-1-treated rats and significantly attenuated the effect in the hypothalamus. These results are consistent with the study of Layé et al. (1995); they found that vagotomy blocked systemic LPS-induced increases in IL-1β mRNA in the hippocampus and hypothalamus of mice. In that study, they also found that increases in pituitary IL-1β mRNA as well as the increase in plasma levels of IL-1β were not affected by vagotomy. Similarly, in this study, the IL-1β-induced increase in IL-1β mRNA in the liver was not affected by vagotomy.
The finding that vagotomy blocked the increase in IL-1β mRNA expression in the hippocampus and brainstem and significantly attenuated this effect in the hypothalamus indicates that the vagus nerve plays a role in transmitting peripheral cytokine signals to the brain. It is consistent with the reports that IL-1β induces dose-dependent and long-lasting increases in the afferent activity of the vagus nerve (Niijima, 1996), and LPS sensitizes vagal afferent terminals by a cytokine-dependent mechanism (Hua et al., 1996). IL-1 receptors are found in liver paraganglia (Goehler et al., 1997), as indicated by IL-1 receptor antagonist binding. Furthermore, various behavioral and central actions of both IL-1β and LPS are inhibited by vagotomy. Subdiaphragmatic vagotomy inhibits sleep (Hansen and Krueger, 1997; Kapás et al., 1997), fever (Watkins et al., 1995a; Sehic and Blatteis, 1996; Goldbach et al., 1997; Opp and Toth, 1997;Romanovsky et al., 1997), hyperalgesia (Watkins et al., 1994), decreased food intake (Bret-Dibat et al., 1995), and decreased social interaction (Bluthé et al., 1996) in response to peripheral LPS or IL-1β. In addition, vagotomy inhibits increases in ACTH secretion (Gaykema et al., 1995), c-Fos expression in brain (Wan et al., 1994;Gaykema et al., 1995), hypothalamic norepinephrine depletion (Fleshner et al., 1995), and elevated plasma corticosteriod levels (Fleshner et al., 1995) produced by various peripheral immune stimuli. Finally, localized cytokine production and interactions with receptors (e.g., on liver, thoracic, and laryngeal paraganglia; Goehler et al., 1997) could explain the findings that many CNS manifestations of the acute phase response occur in the absence of measurable circulating cytokines (Kluger, 1991). Collectively, these data clearly suggest the existence of sensors for IL-1β that send information to the CNS via vagal afferents.
The fact that IL-1β mRNA production in the hypothalamus was not totally blocked by vagotomy suggests that alternative pathways exist, in addition to the subdiaphragmatic vagus, which communicate peripheral cytokine signals to the brain. This is consistent with a previous study on sleep and fever in rats using the same dose of IL-1β, in which it was found that vagotomy blocks the fever but only attenuates the sleep-inducing effects of systemic IL-1β (Hansen and Krueger, 1997). It is possible that IL-1β crosses the blood–brain barrier (Banks et al., 1991) and induces its own production in the hypothalamus. The systemic production of other readily diffusable messengers such as nitric oxide, which is known to play a role in sleep (Kapás and Krueger, 1996) and thermoregulation (Scammell et al., 1996), may also affect these responses. Furthermore, it is possible that IL-1β activates additional afferent sensory nerves in addition to the subdiaphragmatic vagus, e.g., thoracic branches of the vagus (Goehler et al., 1997). Hence, the failure of vagotomy to completely block systemic IL-1β or LPS-induced centrally mediated responses likely involves both the dose (e.g., Hansen and Krueger, 1997; Romanovsky et al., 1997) and route of administration (e.g., Bluthé et al., 1996; Goldbach et al., 1997). The relative contribution of the vagus nerve in communicating information from the periphery to the hypothalamus, versus the hippocampus and brainstem, remains to be determined.
Current and past data suggest that locally produced cytokines stimulate cytokine receptors on, or functionally connected to, vagal afferents, which send signals to the brain to induce brain production of cytokines. Brain IL-1β is involved in the regulation of several physiological processes, as well as being a crucial mediator of many illness responses. There is a diurnal rhythm of IL-1β mRNA levels in the brain of rats, with the highest levels corresponding to peak sleep periods (Taishi et al., 1997). IL-1 levels in CSF of cats vary with the sleep–wake cycle (Lue et al., 1988). IL-1β mRNA levels in the hypothalamus and brainstem increase during sleep deprivation (Mackiewicz et al., 1996). Furthermore, the central administration of anti-IL-1β antibodies, the IL-1 receptor antagonist, or an IL-1 receptor fragment inhibits increases in sleep (Takahashi et al., 1996), behavioral responses (Kent et al., 1992), and fevers (Klir et al., 1994) that are induced by peripherally injected immune stimuli. Inhibition of brain IL-1β also inhibits spontaneous sleep (Opp and Krueger, 1991; Takahashi et al., 1996) and IL-1β-induced anorexia (Plata-Salamán, 1994). A critical role for IL-1β in the brain is also indicated by recent findings in IL-1β knock-out mice; these mice develop lower fevers in response to LPS and display a higher mortality rate attributable to influenza infection (Kozak et al., 1995). IL-1 type I receptor knock-out mice sleep less than their strain controls and are unresponsive to systemic IL-1β injections (Fang et al., 1997). These studies suggest that brain cytokines are likely the critical mediators of centrally mediated illness responses as well as important mediators in normal physiological processes.
IL-1β is one member of a family of molecules currently containing at least nine members. These include three ligands, IL-1α and β and the IL-1 receptor antagonist, two receptors, a soluble receptor, an IL-1 receptor-associated kinase, the IL-1-converting enzyme, and the IL-1RAP (for review, see Dinarello, 1996). The IL-1RAP has been cloned in mice (Greenfeder et al., 1995) and rats (Liu et al., 1996) and appears to be necessary for IL-1 binding and signal transduction. The IL-1RAP forms a complex with the IL-1 type I receptor and either IL-1β or IL-1α, but not with the IL-1 receptor antagonist, and increases the binding affinity for IL-1β (Greenfeder et al., 1995). Furthermore, cells lacking the IL-1RAP do not respond to IL-1 (Wesche et al., 1996); however, when the IL-1RAP is expressed in these cells, IL-1 responsiveness is restored (Wesche et al., 1997). In principle, the upregulation or downregulation of any one component of the IL-1 family could influence the level of activation of the entire IL-1 system. It was, therefore, of interest to determine whether IL-1RAP mRNA could also be influenced by systemic IL-1β.
In the present study, we found IL-1RAP mRNA to be highly expressed in all regions examined; to obtain a discernable signal, cDNA for IL-1RAP was amplified either 26 or 27 cycles, whereas the same cDNA required 33 or 34 cycles of amplification for IL-1β mRNA in the brain and liver samples, respectively. There were no significant differences in IL-1RAP mRNA in response to the intraperitoneal administration of IL-1β in any region in control or vagotomized rats. This finding is consistent with another study that also found no significant differences in IL-1RAP mRNA levels after intracerebroventricular microinfusions of IL-1β (Ilyin and Plata-Salamán, 1996). Greenfeder et al. (1995)initially reported increases in IL-1RAP mRNA in the lung and spleen, decreases in the liver, and no change in the brain in response to IL-1α. Using an RNase protection assay, Liu et al. (1996) showed decreases in IL-1RAP mRNA in the liver and no changes in the hippocampus 24 hr after two doses of intraperitoneal LPS. In contrast, in this study we observed increased IL-1RAP mRNA expression in the liver of rats 90 min after an intraperitoneal injection of LPS. Thus, the regulation of this molecule appears to be complex and may be similar to the IL-1 type I receptor; IL-1 type I receptor mRNA is increased 3 hr after the intraperitoneal administration of LPS and then diminished after 20 hr (Reinisch et al., 1994). Regardless of these considerations, the regulation of IL-1β and its role in centrally mediated illness responses likely involves the entire IL-1 system, as well as that of other cytokines, such as tumor necrosis factor-α.
In conclusion, this study provides additional evidence for the involvement of the vagus nerve as a cytokine-to-brain communication pathway. It further indicates that cytokine signals arising from the periphery are capable of inducing cytokine messages in the brain. The subsequent release of IL-1β in the brain is likely a critical step in the pathway by which vagally mediated signals result in centrally controlled physiological functions and symptoms of the acute phase response.
Footnotes
This work was supported in part by National Institutes of Health Grants NS25378, NS31453, and NS27250, Office of Naval Research Grant N00014-90-J-1069, and National Research Service Award MH11688.
Correspondence should be addressed to Dr. James M. Krueger, Department of Veterinary and Comparative Anatomy, Pharmacology and Physiology, College of Veterinary Medicine, Washington State University, Wegner Hall, Room 205, Pullman, WA 99164-6520.
REFERENCES
- 1.Ban E, Haour F, Lenstra R. Brain interleukin-1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4:48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1α, murine IL-1α and murine IL-1β are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259:988–996. [PubMed] [Google Scholar]
- 3.Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997;12:1–9. [Google Scholar]
- 4.Bluthé R-M, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. NeuroReport. 1996;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- 5.Bret-Dibat JL, Bluthé R-M, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Biological basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 7.Fang J, Wang Y, Krueger JM (1997) The effects of interleukin-1β on sleep are mediated by the type I receptor. Am J Physiol, in press. [DOI] [PubMed]
- 8.Fleshner M, Goehler LE, Hermann J, Relton JK, Maier SF, Watkins LR. Interleukin-1β induced corticosterone elevation and hypothalamic NE depletion is vagally mediated. Brain Res Bull. 1995;37:605–610. doi: 10.1016/0361-9230(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 9.Gaykema RPA, Dijkstra I, Tilders FJH. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136:4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]
- 10.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldbach J-M, Roth J, Zeisberger E. Fever suppression by subdiaphragmatic vagotomy in guinea pigs depends on the route of pyrogen administration. Am J Physiol. 1997;272:675–681. doi: 10.1152/ajpregu.1997.272.2.R675. [DOI] [PubMed] [Google Scholar]
- 12.Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin-1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1β. Am J Physiol. 1997;273:1246–1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 14.Hansen MK, Kapás L, Fang J, Krueger JM (1997) Cafeteria diet-induced sleep is blocked by subdiaphragmatic vagotomy in rats. Am J Physiol, in press. [DOI] [PubMed]
- 15.Hua X-Y, Chen P, Fox A, Myers RR. Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea: studies with interleukin-1β and tumor necrosis factor-α. J Neurosci. 1996;16:4742–4748. doi: 10.1523/JNEUROSCI.16-15-04742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilyin SE, Plata-Salamán CR. In vivo regulation of the IL-1β system (ligand, receptors I and II, receptor accessory protein, and receptor antagonist) and TNF-α mRNAs in specific brain regions. Biochem Biophys Res Commun. 1996;227:861–867. doi: 10.1006/bbrc.1996.1597. [DOI] [PubMed] [Google Scholar]
- 17.Kapás L, Krueger JM. Nitric oxide donors SIN-1 and SNAP promote non-rapid eye movement sleep in rats. Brain Res Bull. 1996;41:293–298. doi: 10.1016/s0361-9230(96)00227-4. [DOI] [PubMed] [Google Scholar]
- 18.Kapás L, Hansen MK, Chang H-Y, Krueger JM (1997) Vagotomy attenuates but does not prevent the somnogenic and febrile effects of lipopolysaccharide in rats. Am J Physiol, in press. [DOI] [PubMed]
- 19.Kent S, Bluthé R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 20.Klir JJ, McClellan JL, Kluger MJ. Interleukin-1β causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. Am J Physiol. 1994;266:1845–1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- 21.Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak W, Zheng H, Conn CA, Soszynski D, Van der Ploeg LH, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1β-deficient mice. Am J Physiol. 1995;269:969–977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 23.Krueger JM, Majde JA. Microbial products and cytokines in sleep and fever regulation. Crit Rev Immunol. 1994;14:355–379. doi: 10.1615/critrevimmunol.v14.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 24.Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984;246:994–999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 25.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 26.Layé S, Bluthé R-M, Kent S, Combe C, Médina C, Parnet P, Kelley K, Dantzer R. Subdiaphragmatic vagotomy blocks the induction of interleukin-1β mRNA in the brain of mice in response to peripherally administered lipopolysaccharide. Am J Physiol. 1995;268:1327–1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Chalmers D, Maki R, De Souza EB. Rat homolog of mouse interleukin-1 receptor accessory protein: cloning, localization and modulation studies. J Neuroimmunol. 1996;66:41–48. doi: 10.1016/0165-5728(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 28.Lue FA, Bail M, Jephthah-Ocholo J, Carayanniotis K, Gorczynski R, Moldofsky H. Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. Int J Neurosci. 1988;42:179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- 29.Mackiewicz M, Sollars PJ, Ogilvie MD, Pack AI. Modulation of IL-1β gene expression in the rat CNS during sleep deprivation. NeuroReport. 1996;7:529–533. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- 30.Niijima A. The afferent discharges from sensors for interleukin-1β in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 31.Opp MR, Krueger JM. Interleukin-1 receptor antagonist blocks interleukin-1-induced sleep and fever. Am J Physiol. 1991;260:453–457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 32.Opp MR, Toth LA. Circadian modulation of interleukin-1-induced fever in intact and vagotomized rats. Ann NY Acad Sci. 1997;813:435–436. doi: 10.1111/j.1749-6632.1997.tb51729.x. [DOI] [PubMed] [Google Scholar]
- 33.Plata-Salamán CR. Meal patterns in response to the intracerebroventricular administration of interleukin-1β in rats. Physiol Behav. 1994;55:727–733. doi: 10.1016/0031-9384(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 34.Reinisch N, Wolkersdorfer M, Kähler CM, Ye K, Dinarello CA, Wiedermann CJ. Interleukin-1 receptor type I mRNA in mouse brain as affected by peripheral administration of bacterial lipopolysaccharide. Neurosci Lett. 1994;166:165–167. doi: 10.1016/0304-3940(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 35.Riedy MC, Timm EA, Jr, Stewart CC. Quantitative RT-PCR for measuring gene expression. Biotechniques. 1995;18:70–76. [PubMed] [Google Scholar]
- 36.Romanovsky AA, Simons CT, Székely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:407–414. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, Ed 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 38.Scammell TE, Elmquist JK, Saper CB. Inhibition of nitric oxide synthase produces hypothermia and depresses lipopolysaccharide fever. Am J Physiol. 1996;271:333–338. doi: 10.1152/ajpregu.1996.271.2.R333. [DOI] [PubMed] [Google Scholar]
- 39.Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726:160–166. [PubMed] [Google Scholar]
- 40.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 41.Taishi P, Bredow S, Guha-Thakurta N, Obál F, Jr, Krueger JM. Diurnal variations of interleukin-1β mRNA and β-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S, Kapás L, Fang J, Seyer JM, Wang Y, Krueger JM. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol. 1996;271:101–108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- 43.Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 45.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Gilbert L, Martin D, Maier SF. Blockade of interleukin-1-induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995a;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995b;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 47.Wesche H, Neumann D, Resch K, Martin MU. Co-expression of mRNA for type I and type II interleukin-1 receptors and the IL-1 receptor accessory protein correlates to IL-1 responsiveness. FEBS Lett. 1996;391:104–108. doi: 10.1016/0014-5793(96)00713-2. [DOI] [PubMed] [Google Scholar]
- 48.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin MU. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1 induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases). J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]