Abstract
Neuronal nitric oxide synthase (nNOS) neurons kill adjacent neurons through the action of NMDA-glutamate receptor activation, although they remain relatively resistant to the toxic effects of NMDA and NO. The molecular basis of the resistance of nNOS neurons to toxic insults is unknown. To begin to understand the molecular mechanisms of the resistance of nNOS neurons, we developed a pheochromacytoma-derived cell line (PC12) that is resistant to the toxic effects of NO. We found through serial analysis of gene expression (SAGE) that manganese superoxide dismutase (MnSOD) is enriched in the NO-resistant PC12 cell-derived line (PC12-R). Antisense MnSOD renders PC12-R cells sensitive to NO toxicity and increases the sensitivity to NO in the parental, NO-sensitive PC12 line (PC12-S). Adenoviral transfer of MnSOD protects PC12-S cells against NO toxicity. We extended these studies to cortical cultures and showed that MnSOD is enriched in nNOS neurons and that antisense MnSOD renders nNOS neurons susceptible to NMDA neurotoxicity, although it has little effect on the overall susceptibility of cortical neurons to NMDA toxicity. Overexpression of MnSOD provides dramatic protection against NMDA and NO toxicity in cortical cultures, but not against kainate or AMPA neurotoxicity. Furthermore, nNOS neurons from MnSOD −/− mice are markedly sensitive to NMDA toxicity. Adenoviral transfer of MnSOD to MnSOD−/− cultures restores resistance of nNOS neurons to NMDA toxicity. Thus, MnSOD is a major protective protein that appears to be essential for the resistance of nNOS neurons in cortical cultures to NMDA mediated neurotoxicity.
Keywords: nitric oxide, nNOS neuron, MnSOD, NMDA toxicity, resistance to nitric oxide, neurodegenerative diseases
Nitric oxide (NO) is a unique messenger molecule that serves diverse physiological functions throughout the body (Nathan, 1992; Schmidt and Walter, 1994; Garthwaite and Boulton, 1995; Yun et al., 1996). NO is synthesized froml-arginine by NO synthase (NOS). Three isoforms of NOS have been identified and are the products of three distinct genes: neuronal NOS (nNOS, Type I), immunological NOS (iNOS, Type II), and endothelial NOS (eNOS, Type III) (Bredt and Snyder, 1994; Marletta, 1994; Nathan and Xie, 1994). In the nervous system, nNOS is localized to discrete populations of neurons in the cerebellum, cortex, striatum, olfactory bulb, hippocampus, basal forebrain, and brain stem (Bredt et al., 1991;Vincent and Kimura, 1992). Excess production of NO via nNOS has been implicated in various neurotoxic paradigms (Dawson and Snyder, 1994;Dawson and Dawson, 1996; Iadecola, 1997). Excess glutamate acting via NMDA receptors may mediate cell death in focal cerebral ischemia (Choi, 1988; Choi and Rothman, 1990), trauma, and epilepsy, and in neurodegenerative diseases such as Huntington’s disease and Alzheimer’s disease (Meldrum and Garthwaite, 1990; Lipton and Rosenberg, 1994). In primary cerebral cortical cultures NMDA neurotoxicity is prevented by various NOS inhibitors (for review, seeDawson and Snyder, 1994; Dawson and Dawson, 1996). Evaluation of nNOS inhibitors in various stroke models has shown that selective inhibitors provide dramatic reductions in infarct volume in focal cerebral ischemia (Dalkara and Moskowitz, 1994; Iadecola, 1997; Samdani et al., 1997). In addition, selective nNOS inhibitors provide protection against the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in an animal model of Parkinson’s disease (Schulz et al., 1995b; Przedborski et al., 1996) and also provide protection against various mitochondrial neurotoxins (Schulz et al., 1995a).
Because NO is a reactive free radical, it has many potential targets to initiate neurotoxic cascades. A predominant mechanism by which NO may kill neurons is through the diffusion-limited reaction of NO with superoxide anion (O2•−) to generate peroxynitrite (ONOO−) (Beckman et al., 1990;Beckman, 1994), which is directly cytotoxic (Radi et al., 1991; Beckman and Crow, 1993; Xia et al., 1996). The toxic effects of NO and peroxynitrite may occur through multiple pathways. An important pathway may be NO/ONOO−-damaged DNA and subsequent activation of the enzyme poly(ADP-ribose) polymerase (PARP) (Zhang et al., 1994; Dawson and Dawson, 1996; Eliasson et al., 1997), a nuclear enzyme involved in DNA repair (Lautier et al., 1993). Excessive activation of PARP can rapidly deplete cellular energy stores, leading to cell death. Additionally, NO may elicit neurotoxicity through inhibition of mitochondrial respiration, nitrosylation of proteins, and lipid peroxidation (for review, see Yun et al., 1996).
nNOS neurons are remarkably spared from cell death in NMDA neurotoxicity, Huntington’s disease, Alzheimer’s disease, and vascular stroke (Thomas and Pearse, 1964; Ferrante et al., 1985; Beal et al., 1986; Koh et al., 1986; Koh and Choi, 1988; Uemura et al., 1990; Hyman et al., 1992; V. L. Dawson et al., 1993). Thus, nNOS neurons must possess protective mechanisms that render them resistant to the toxic NO environment they create. However, the molecular mechanisms that account for the selective resistance of nNOS neurons to neurotoxic insults remain unknown.
NO physiology has been clarified through the study of mice lacking the gene for nNOS (nNOS−/− mice) (Huang et al., 1993). nNOS−/− mice are dramatically resistant to permanent focal ischemia (Huang et al., 1994), MPTP neurotoxicity (Przedborski et al., 1996), and mitochondrial toxins (Schulz et al., 1996). Furthermore, cortical cultures from nNOS−/−mice are resistant to neurotoxicity (Dawson et al., 1996). In the cerebral cortex, all nNOS neurons stain for somatostatin, and almost all somatostatin neurons are nNOS positive (T. M. Dawson et al., 1991). The density of somatostatin-staining neurons is normal in the cerebral cortex of nNOS−/− mice, indicating that although nNOS has been disrupted, the neurons remain intact. In the mutant mice, somatostatin neurons are spared from NMDA neurotoxicity, indicating that the factors responsible for the selective resistance of nNOS neurons remain intact and probably are not nNOS itself (Dawson et al., 1996). This prompted us to explore further the molecular mechanisms that render nNOS neurons selectively resistant to neurotoxicity.
We elected to study the selective resistance of nNOS neurons to neurotoxicity by developing a PC12 cell-derived line that is resistant to NO-mediated toxicity. We reasoned that NO-resistant and NO-sensitive PC12 cell lines should express different sets of genes, some of which might account for the resistance of the PC-12 cells to NO. To identify these genes, we performed serial analysis of gene expression (SAGE) in NO-resistant and NO-sensitive PC12 cells. We found that manganese superoxide dismutase (MnSOD) is the predominant gene in NO-resistant PC12 cells, and that it is also selectively expressed in cortical nNOS neurons. Strikingly, MnSOD is required for the resistance of nNOS neurons to NMDA and NO-mediated neurotoxicity in cortical cultures.
MATERIALS AND METHODS
Cell culture. The rat PC12 cell line was maintained in DMEM supplemented with 5% fetal bovine serum, 10% horse serum, 2 mml-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were cultured in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Primary cortical cell cultures were prepared from gestational day 14 fetal rats or day 16 fetal mice as described previously (V. L. Dawson et al., 1993, 1996). The cortex was dissected under a microscope, incubated for 20 min in 0.0027% trypsin and saline solution [5% PBS (in mm) 40 sucrose, 30 glucose, 10 HEPES, pH 7.4]. Rat cortex was transferred to modified Eagle’s medium (MEM), 10% horse serum, 10% fetal bovine serum, and 2 mm glutamine, and cells were dissociated by trituration. Mouse cortex was dissected and the cells were dissociated by trituration in MEM, 20% horse serum, 25 mm glucose, and 2 mml-glutamine after a 30 min digestion in 0.027% trypsin and saline solution (Life Technologies, Gaithersburg, MD). Cells were plated in 15 mm multiwell (Nunc, Roskilde, Denmark) plates coated with polyornithine at a density of 3–4 × 105 cells per well. Four days after they were plated, the cells were treated with 10 μg/ml of 5-fluoro-2′-deoxyuridine for 3 d to inhibit proliferation of non-neuronal cells. Rat cultures were maintained in MEM, 5% horse serum, and 2 mm glutamine in 8% CO2, humidified, 37°C atmosphere. Murine cultures were maintained in MEM, 10% horse serum, 25 mm glucose, and 2 mml-glutamine in a 5% O2, 8% CO2, humidified, 37°C incubator. The medium was changed twice a week. Mature neurons (14 d in culture) were used for all experiments. In mature cultures, neurons represent 70–90% of the total number of cells (V. L. Dawson et al., 1993, 1996).
Cytotoxicity. Cells were exposed to test solutions as described previously (V. L. Dawson et al., 1991). Cells were washed with control salt solution (CSS) containing (in mm) 120 NaCl, 5.4 KCl, 1.8 CaCl2, 25 Tris-HCl, 15 glucose, pH 7.4. Except for kainate, all other drugs were applied in CSS for 5 min. Kainate was applied in MEM, 21 mm glucose for 24 hr in the incubator. Toxicity was assayed 20–24 hr after exposure to drug solutions by trypan blue exclusion as described (V. L. Dawson et al., 1993). Three to five photoprints at 10–20× were made of each well. Live cells (cells that exclude trypan blue) and dead cells (cells that take up trypan blue) were counted, and the percentage of cell death was determined. Approximately 500–1200 cells were counted per well. At least two separate experiments using three different wells were performed so that ∼3000–7200 neurons were counted for each data point. To assess rater reliability, some of the photomicrographs were counted by an additional observer blinded to the study design and treatment protocol. An inter-rater consistency >95% was observed for the cell counting.
In some experiments toxicity was assayed 20–24 hr after exposure to cytotoxic conditions by microscopic examination with computer-assisted cell counting after staining of all nuclei with 1 μg/ml Hoescht 33342 and staining of dead cell nuclei with 7 μm propidium iodide. Total and dead cells were counted. Glial nuclei fluoresce at a different intensity then neuronal nuclei and were gated out. The percentage of cell death was determined as the ratio of live to dead cells as compared with the percentage of cell death in control wells to account for cell death attributed to mechanical stimulation of the cultures. At least two separate experiments using four separate wells were performed with a minimum of 15,000–20,000 neurons counted per data point. All reagents were purchased from Sigma (St. Louis, MO).
Data were analyzed with the Student’s t test for independent means. Statistical analyses were performed by using StatView 4.0 software (Abacus Concepts, Calabasas, CA).
Antisense oligonucleotides. Phosphorothioate oligodeoxynucleotides (S-oligodeoxynucleotides) in which all phosphodiester linkages were modified were synthetized, lyophilized, diluted in sterile water, and stored at −20°C. Oligonucleotides were chosen, purified, and used according to standard procedures (Bito et al., 1996; Rothstein et al., 1996). Oligonucleotides were chosen to exhibit minimal self-complementarity according to analysis with the computer program OLIGO 4 (National Biosciences, Plymouth, MN). All sequences chosen were specific and unrelated to any other nucleotide sequence in GenBank. The sequence for the antisense oligonucleotide to rat MnSOD used is 5′-CCCGACACAACATTGCTGA-3′, and it spans from six bases 5′ to 10 bases 3′ of the start codon. Control cultures received either no oligonucleotide or sense or random oligonucleotide (in which the base composition and extent of phosphodiester linkages were identical to that of the antisense oligonucleotide but the sequence was randomly assigned). Oligonucleotides were reconstituted in serum-free medium and filtered before addition to the cultures.
Infection of cultured cells with recombinant adenovirus.Primary cortical cells were maintained in culture for 14 d before being exposed to the adenoviral vector. PC12 cells were cultured to 90% confluence in 24-well plates. Cultures were exposed to 1 × 108 pfu/ml (1 × 1010particles/ml) of adenoviral vector for 1 hr in 0.5 ml of serum-free medium, followed by overnight incubation in 0.5 ml of 2% serum-containing medium in the presence of 1 × 108 pfu/ml of adenoviral vector. In this system (Ad.MnSOD), MnSOD gene transcription is under the control of the strong cytomegalovirus (CMV) promoter (R. M. Zwacka and J. F. Engelhardt, unpublished data). After overnight incubation with Ad.MnSOD in 2% serum-containing medium, 0.5 ml of 10% serum-containing medium was added to each well. Cells were cultured for an additional 24 hr to allow protein expression before any additional experiment or analysis was performed. Noninfected cells and cells exposed to a β-galactosidase-containing adenoviral vector (Ad.βGal) were included as controls. Ad.βGal also contains the β-galactosidase gene under the CMV promoter and allowed us to monitor the infection efficiency after X-Gal staining of adenovirus-exposed cells. Briefly, 24 hr after exposure to 1 × 108 pfu/ml of Ad.βGal, cells were fixed for 10 min at room temperature with 0.5% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) solution for 2 hr in a 37°C, non-CO2 incubator.
Western blot analysis. For protein analysis, culture plates were rinsed twice with cold PBS, pH 7. Cells were scraped and harvested in cold PBS, centrifuged at 2500 rpm, and resuspended in PBS. Cells were then sonicated on ice for a total of 2 min at 60% duty cycle and output level 2, using a Branson sonifier. Cell extracts were stored at −70°C. Total protein was assayed using the Bradford method (Pierce, Rockford, IL). Proteins in cell lysates were size-separated through denaturing polyacrylamide gel electrophoresis (SDS-PAGE). An equal amount of protein for each sample was heated at 100°C for 5 min with an equivalent volume of 2× sample buffer (containing 4% SDS and 10% β-mercaptoethanol) and loaded onto 12% polyacrylamide gels. The proteins were electrotransferred to a nitrocellulose membrane in tris-glycine-methanol buffer. The membrane was blocked for 1 hr at room temperature in a blocking solution mixture of 3% nonfat dry milk, 0.05% Tween-20, and Tris-buffered saline (TBS), pH 8.0. The membrane was then incubated for 1 hr at room temperature with primary antibody in blocking solution (rabbit anti-MnSOD serum diluted 1:3000; rabbit anti-CuZnSOD serum diluted 1:3000; rabbit anti-nNOS serum diluted 1:2000; anti-β-tubulin monoclonal diluted 1:10,000). All primary antibodies are previously characterized antibodies that recognize a single band on Western blot analysis (Oberley et al., 1990; Roskams et al., 1991). The membrane was rinsed with blocking solution for five washes of 5 min and incubated for 1 hr at room temperature in a 1:10,000 dilution of goat anti-rabbit IgG peroxidase-labeled antibody (Boehringer Mannheim, Indianapolis, IN), or goat anti-mouse IgG peroxidase-labeled antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The blot was washed 5 × 5 min and then processed for analysis using an Enhanced ChemiLuminiscence (ECL) detection kit (Kirkegaard and Perry Laboratories, Gaithersburg, MD) as described by this manufacturer.
MnSOD activity assay. MnSOD activity was assayed as described (Spitz and Oberley, 1989). In brief, a competitive inhibition assay was performed that used xanthine–xanthine oxidase-generated O2•− to reduce nitroblue tetrazolium (NBT) to blue formazan. Reduction of NBT was monitored spectrophotometrically at 560 nm. The amount of protein that inhibits NBT reduction to 50% of maximum is defined as 1 U of MnSOD activity. In this assay CuZnSOD is inhibited by 5 mm sodium cyanide. Enzymatic activity was expressed in units per milligram of protein.
NADPH–diaphorase staining. Cells were washed three times with CSS and fixed for 30 min at 4°C in a 4% paraformaldehyde (PF), 0.1 m phosphate buffer (PB). Fixative solution was washed away with TBS, and reaction solution was applied for 1 hr at 37°C. The reaction solution contains 1 mm NADPH, 0.2 mm nitroblue tetrazolium, 0.2% Triton X-100, 1.2 mm sodium azide, and 0.1 m Tris-HCl, pH 7.2. The reaction was terminated by washing away the reaction solution with TBS. All diaphorase-positive cells in each well were counted by using an inverted microscope.
Immunofluorescence. Cultured cells were washed three times with CSS and fixed for 30 min at 4°C in a 4% PF, 0.1 mPB. The cells were then washed in TBS and permeabilized with 0.2% Triton X-100 in TBS for 5 min. Blocking solution containing 5% normal goat serum (NGS), 0.1% Triton X-100 in TBS was then applied for 1 hr at room temperature. Primary antibodies to nNOS (monoclonal), MnSOD (polyclonal), or CuZnSOD (polyclonal) were diluted in blocking solution and applied to the cells overnight at 4°C. After rinsing the cells three times with TBS, fluorescence-conjugated secondary antibodies (fluorescein, FITC-conjugated anti-mouse IgG, or lissamine rhodamine-conjugated anti-rabbit IgG; Jackson ImmunoResearch) were applied in 1.5% NGS, TBS, 0.1% Triton X-100 for 1 hr at room temperature. After an additional three washes in TBS, cells were examined under a fluorescence microscope. Control cells lacking primary or secondary antibody were stained in parallel and failed to exhibit any immunostaining.
Southern blot and PCR analysis. Genotyping was performed by Southern blot analysis of genomic DNA from 14 d embryos as described by Lebovitz et al. (1996). The PCR approach was also used to determine MnSOD genotype of embryos and cortical cultures. Oligonucleotide primers were designed for specific amplification of exon 2 of the mouse MnSOD gene, and the human hypoxanthine phosphoribosyltransferase (HPRT) minigene was used to target exons 1 and 2 of MnSOD. PCR amplification of MnSOD was performed in a final volume of 25 μl, containing 1 μm each oligonucleotide primer (5′-ACA AGC ACA GCC TCC CAG AC-3′ and 5′-AGC CTC GTG GTA CTT CTC CTC-3′), 200 μm deoxynucleotides, 10 mmTris-HCl, pH 8.3, 50 mm KCl, 1.5 mmMgCl2, 0.01% gelatin, 1 U of Taq DNA polymerase (Boehringer Mannheim), and 2.5% DMSO. Twenty-six cycles of 94°C for 1 min, 62°C for 45 sec, and 72°C for 30 sec were performed with an initial denaturation step of 94°C for 3 min and a final elongation step at 72°C for 1 min. PCR products were resolved on 3% agarose gels. Amplifications of human HPRT were performed as described for the MnSOD gene except for the primer sequences: 5′-GCT GAG GAT TTG GAA AGG G-3′ and 5′-TTG CAG CCT TGA CCA TCT T-3′, and an annealing temperature of 55°C.
RESULTS
Generation of a NO-resistant PC12 cell line
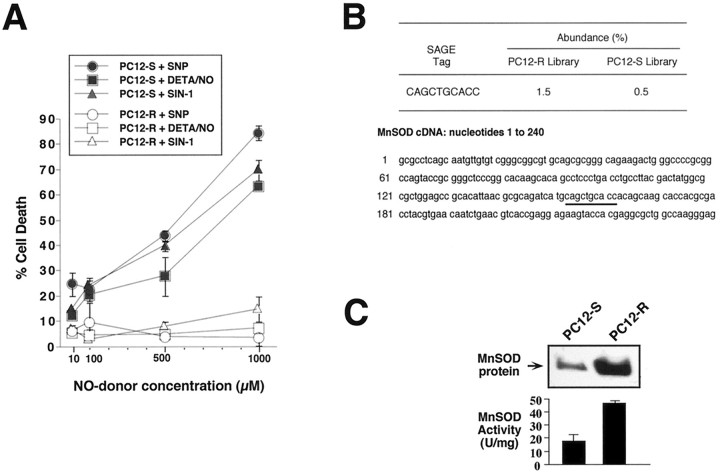
A brief (5 min) application of NO donors effectively causes death of cultured primary cortical neurons 20–24 hr later (V. L. Dawson et al., 1991). In an attempt to identify a cell line that would be reflective of NO-mediated cell death in cortical neuronal cultures, we screened a number of tumor cell lines for susceptibility to a 5 min exposure to NO donors as assessed 24 hr later (V. L. Dawson and T. M. Dawson, unpublished observations). Rat PC12 cells are remarkably sensitive to the toxic effects of NO donors (Fig.1A). A 5 min application of the NO donor sodium nitroprusside (SNP), elicits ∼45% cell killing at 500 μm, and almost 90% cell death at 1 mm as assessed 24 hr later. SNP that is depleted of NO by overnight incubation in buffer under light elicits no toxicity (data not shown).
Fig. 1.
Characterization of PC12-R cells: resistance to NO toxicity and increased expression of MnSOD. A, Susceptibility of PC12-S and PC12-R cells to the NO donors sodium nitroprusside (SNP), diethylenetriamine nitric oxide adduct (DETA/NO), and 3-morpholino-sydnonimine hydrochloride (SIN-1). Cells were exposed to the NO donors for 5 min, and cytotoxicity was assayed 24 hr later by trypan blue exclusion. The wild-type PC12 line (PC12-S) is remarkably sensitive to the toxic effects of NO. An NO-resistant PC12 cell line (PC12-R) was generated by treating parental PC12-S cells with 100 μm SNP for 5 min, allowing the surviving cells to grow to confluence followed by successive retreatments with incremental doses of SNP until a cell line (PC12-R) was generated that was resistant to 1 mm SNP. B, Predominant differentially expressed SAGE tag in PC12-R compared with PC12-S cell populations corresponds to MnSOD. This specific tag sequence, its abundance in each tag library analyzed, and its location in the MnSOD cDNA sequence is indicated. C, Western blot and catalytic activity analyses of MnSOD in PC12-S and PC12-R cells indicate that MnSOD levels are increased in PC12-R when compared with PC12-S. For Western blot analysis, 10 μg total protein was loaded in each lane and electrophoresed under denaturing conditions on a 12% polyacrylamide gel. MnSOD was detected as an apparent 23 kDa protein band with a rabbit polyclonal antibody raised against MnSOD (Oberley et al., 1990). MnSOD activity was measured using the nitroblue tetrazolium method ofOberley and Spitz (1985) as later modified. Data represents the mean ± SEM of two to three independent experiments. Western blots are representative of two to three independent experiments.
In a manner analogous to that described by Edwards and colleagues (Liu et al., 1992) in which they identified the vesicular catecholamine transporter responsible for the resistance of a PC12 cell-derived line to MPP+, we reasoned that the development of a NO-resistant PC12 cell line would be useful in the identification of genes that confer resistance to NO-mediated toxicity. A NO-resistant PC12 cell line (PC12-R) was generated by treating parental PC12 cell cultures (PC12-S) with 100 μm SNP for 5 min and allowing the surviving cells to grow to confluence, followed by successive retreatments with incremental doses of SNP until a cell line (PC12-R) was generated that was resistant to 1 mm SNP (Fig. 1A). The PC12-R cells are also remarkably resistant to high doses of other NO donors such as diethylenetriamine nitric oxide adduct (DETA/NO) and 3-morpholino-sydnonimine hydrochloride (SIN-1) (Fig.1A). DETA/NO and SIN-1 that were depleted of NO by overnight incubation in buffer under light elicit no toxicity (data not shown).
MnSOD protein and activity levels are elevated in NO-resistant PC12 cells
Kinzler and colleagues (Velculescu et al., 1995) recently developed an elegant method called serial analysis of gene expression (SAGE) that allows the quantitative and simultaneous analysis of a large number of transcripts in a given cell population. SAGE provides a means for the quantitative cataloging and comparison of expressed genes in various normal, developmental, and diseased states (Velculescu et al., 1995). We used SAGE to identify genes that could account for the resistance of the PC12-R cell line to NO. More than 4000 transcripts were analyzed from PC12-S and PC12-R cell lines (M. Gonzalez-Zulueta, V. L. Dawson, and T. M. Dawson, unpublished observations). More than 100 transcripts were expressed at higher levels in the PC12-R cell population than in the PC12-S cell population. One of the most highly differentially expressed transcripts in PC12-R cells corresponded to the mRNA for manganese superoxide dismutase (MnSOD) (Fig. 1B). Because of the role of MnSOD in cellular antioxidant defense, we elected to focus our initial attention on the potential protective role of MnSOD against NO toxicity. We analyzed MnSOD protein levels in the PC12-S and PC12-R cell lines via Western blot analysis as well as MnSOD catalytic activity (Fig. 1C). Confirming the SAGE analysis, we show that MnSOD protein and activity levels are elevated dramatically in PC12-R cells compared with PC12-S cells.
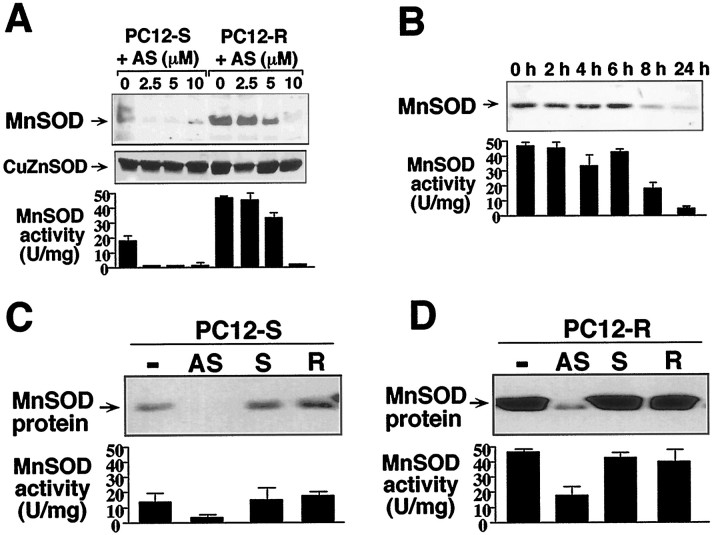
Antisense MnSOD increases susceptibility to NO-toxicity in PC12 cells
To investigate the potential role of MnSOD as a neuroprotective protein against NO-mediated toxicity in PC12 cells, we developed an antisense oligonucleotide approach to knock down MnSOD protein levels and catalytic activity (Fig. 2), following standard and well characterized procedures for neuronal cells (Bito et al., 1996; Rothstein et al., 1996). Consistent with the observations that PC12-S cells contain significantly less MnSOD than PC12-R cells, 2.5 μm antisense oligonucleotide to MnSOD completely eliminates MnSOD protein levels and catalytic activity in PC12-S cells, whereas 10 μm antisense oligonucleotide is required to abolish MnSOD protein levels and catalytic activity in the PC12-R cell line (Fig. 2A). Exposure of PC12-S and PC12-R cells to antisense oligonucleotide to MnSOD is not associated with a decrease in cell viability (data not shown). The superoxide anion is scavenged by a family of SOD enzymes (McCord, 1969; Fridovich, 1986; Bannister et al., 1987; Hassan, 1988; Fridovich, 1995). In eukaryotic cells, the copper-zinc (CuZn) SOD isoform is found mainly in the cytosol, whereas the MnSOD is located in the mitochondria. The amount of CuZnSOD in the PC12-R cell line is equivalent to that in the PC12-S cell line as assessed by Western blot analysis (Fig.2A), which indicates that CuZnSOD probably does not account for the resistance of the PC12-R cells to NO toxicity. Furthermore, the antisense oligonucleotide to MnSOD did not affect CuZnSOD protein levels (Fig. 2A), indicating that our antisense knockdown approach is specific for MnSOD and that it does not affect the expression of other constitutively expressed proteins. In the PC12-R cells, 10 μm antisense oligonucleotide significantly reduces MnSOD protein levels after 24 hr of exposure (Fig. 2B). Thus, in all future studies antisense oligonucleotides to MnSOD were applied for 24 hr at a concentration of 10 μm. Exposure to sense and random oligonucleotides did not show any effect on MnSOD protein and activity levels in PC12-S and PC12-R cells, confirming the specificity of our antisense knockdown experiments (Fig. 2C,D). Exposure of PC12-R cells to the antisense oligonucleotide resulted in reduction of both MnSOD protein and catalytic activity to levels similar to those present in control PC12-S cells.
Fig. 2.
Antisense oligonucleotide knockdown of MnSOD in PC12 cells. A, Dose–response of MnSOD protein and activity levels to antisense oligonucleotide to MnSOD. PC12-S and PC12-R cells were exposed to increasing concentrations of antisense oligonucleotides (AS) for 24 hr. After 24 hr in the presence of AS, cells were harvested for Western blot and activity analyses. For Western blot analysis, 10 μg of total protein was loaded in each lane. Antibodies against MnSOD detected an apparent 23 kDa protein band, and antibodies against CuZnSOD detected a 16 kDa protein band. B, MnSOD protein and activity levels in PC12-R cells over 24 hr in the presence of AS oligonucleotide to MnSOD. PC12-R cells were exposed to 10 μm AS oligonucleotide and harvested at the time points indicated after addition of the oligonucleotide. For Western blot analysis, 5 μg of total protein was loaded in each lane. C, D, Downregulation of MnSOD protein and activity levels in (C) PC12-S and (D) PC12-R cells is specific to AS oligonucleotide treatment. Cells were exposed for 24 hr to either no oligonucleotide (−), antisense oligonucleotide to MnSOD (AS), sense oligonucleotide (S), or random oligonucleotide (R); 5 μmeach oligonucleotide was used to treat PC12-S cells, and 10 μm each oligonucleotide was used for PC12-R. Ten micrograms of total protein were loaded in each lane for Western blot analysis. Western blots are representative of two to three independent experiments. Data represents the mean ± SEM of two to three independent experiments.
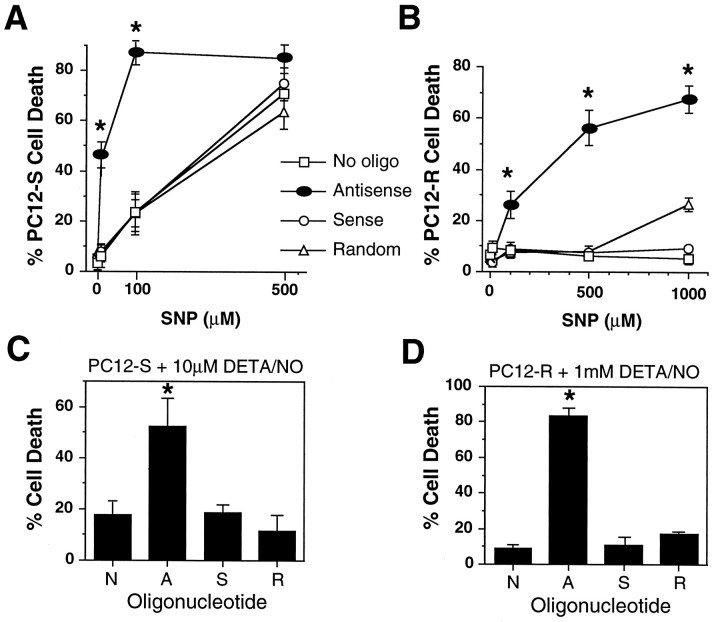
To test our hypothesis that MnSOD accounts for the resistance of the PC12-R cell line to NO mediated toxicity, we used antisense oligonucleotides to knock down MnSOD activity in PC12-S and PC12-R cells and exposed them to increasing concentrations of the NO donor SNP (Fig. 3A,B). Exposure of the PC12-S line to antisense oligonucleotide to MnSOD dramatically increases the sensitivity of these cells to SNP (Fig. 3A). SNP (10 μm) elicits minimal toxicity in PC12-S cells, but in the presence of antisense oligonucleotide to MnSOD ∼50% of the cells die. SNP (100 μm) causes ∼20% cell death in PC12-S cells and ∼90% cell death in antisense-treated cells (Fig.3A). In the PC12-R line, exposure to antisense oligonucleotide to MnSOD restores the sensitivity of these cells to SNP toxicity (Fig. 3B). Sense and random oligonucleotides with equivalent levels of sulfation have minimal effects on the susceptibility of both PC12-S and PC12-R lines to SNP. Knockdown of MnSOD via antisense oligonucleotides also correlates with an increased susceptibility of both PC12-S and PC12-R lines to another NO donor, DETA/NO. In PC12-S cells, 10 μm DETA/NO elicits ∼20% cell death, whereas in the presence of antisense MnSOD oligonucleotides DETA/NO-induced cell death is increased to ∼50% (Fig.3C). In the PC12-R line, 1 mm DETA/NO causes ∼10% cell death, and this proportion is increased to 80% when cells are exposed to antisense oligonucleotides (Fig. 3D).
Fig. 3.
Antisense MnSOD increases susceptibility to NO toxicity in PC12 cells. A, Effect of oligonucleotides to MnSOD on PC12-S sensitivity to SNP. Antisense, sense, or random oligonucleotide (5 μm each) were added to PC12-S. After 24 hr, cells were treated with 10, 100, and 500 μm SNP for 5 min, and the medium replaced with fresh oligonucleotide. Twenty-four hours later, toxicity was assessed by trypan blue exclusion. B, Effect of oligonucleotides to MnSOD on PC12-R susceptibility to SNP. Experiments were performed as described for PC12-S, with the exception that 10 μm each oligonucleotide was used and the highest SNP concentration tested was 1 mm. C, Effect of oligonucleotides to MnSOD on PC12-S sensitivity to 10 μm DETA/NO. The experiment was performed as in A, with the exception that only one dose (10 μm) of DETA/NO was tested. D, Effect of oligonucleotides to MnSOD on PC12-R susceptibility to 1 mm DETA/NO. The experiment was performed as inB, with the exception that only one dose (1 mm) of DETA/NO was tested. Data represents the mean ± SEM of two to three independent experiments. *p < 0.001.
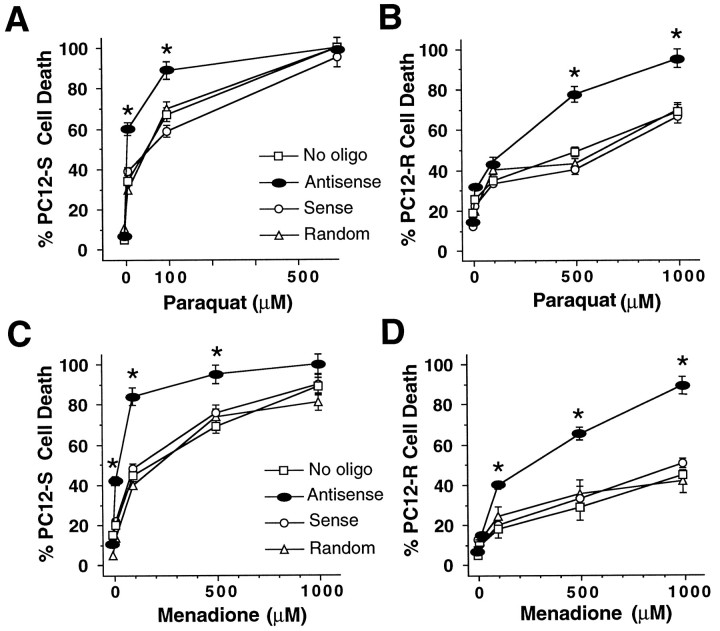
Because the toxic effects of NO are thought to occur mainly through an interaction with O2•−, we examined the effect of O2•−- generating compounds on cells exposed to antisense MnSOD oligonucleotide (Fig.4). PC12-R cells are twofold more resistant than PC12-S cells to the toxic effects of 500 μm paraquat and 500 μm menadione. The resistance of PC12-R cells to paraquat and menadione toxicity is not as profound as their resistance to NO generators (compare Fig.3B,D with Fig. 4B,D). Antisense knockdown of MnSOD increases the susceptibility of PC12-S cells and PC12-R cells to paraquat and menadione by 1.5- to twofold (Fig. 4). This increase is not as dramatic as the three- to eightfold increase in susceptibility to NO donors. Thus, although MnSOD may play a role in the cellular defense against excess O2•−, it appears to be critical in the protective pathways against NO-mediated cellular injury.
Fig. 4.
Effect of MnSOD knockdown on PC12 sensitivy to superoxide generators. A, Effect of oligonucleotides to MnSOD on PC12-S sensitivity to paraquat. Antisense, sense, or random oligonucleotides (5 μm each) were added to PC12-S. After 24 hr, cells were treated with 10, 100, and 500 μmparaquat for 5 min, and the medium was replaced with fresh oligonucleotide. Twenty-four hours later, toxicity was assessed by trypan blue exclusion. B, Effect of oligonucleotides to MnSOD on PC12-R susceptibility to paraquat. Experiments were performed as described for PC12-S, with the exception that 10 μmeach oligonucleotide was used, and the highest paraquat concentration tested was 1 mm. C, Effect of oligonucleotides to MnSOD on PC12-S sensitivity to menadione. Antisense, sense, or random oligonucleotides (5 μm each) were added to PC12-S. After 24 hr, cells were treated with 10, 100, and 500 μm and 1 mm menadione for 5 min, and the medium replaced with fresh oligonucleotide. Twenty-four hours later, toxicity was assessed by trypan blue exclusion. D, Effect of oligonucleotides to MnSOD on PC12-R susceptibility to menadione. Experiments were performed as described for PC12-S, with the exception that 10 μm each oligonucleotide was used. Data represents the mean ± SEM of two to three independent experiments. *p < 0.001.
Overexpression of MnSOD confers resistance to NO toxicity in PC12 cells
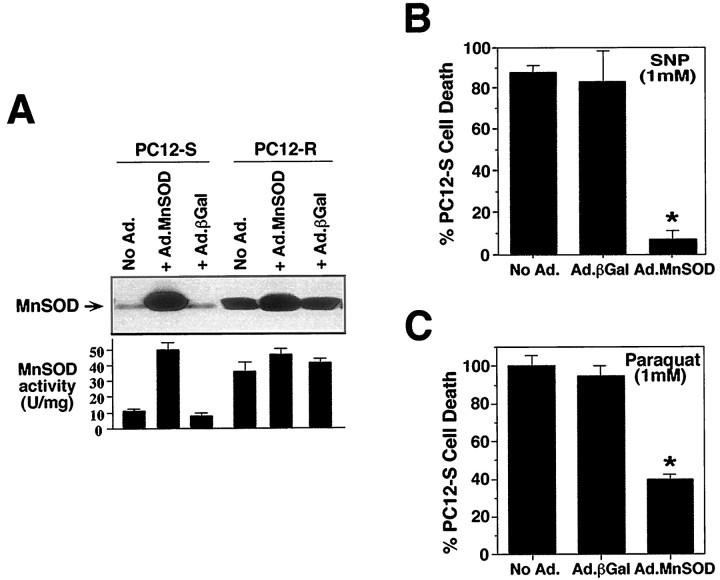
To further support our hypothesis that MnSOD accounts for the resistance of the PC12-R line to NO-mediated toxicity, we overexpressed MnSOD in PC12 cells via an adenovirus-derived vector containing the gene for MnSOD (Ad.MnSOD) (Fig. 5). PC12-S and PC12-R cells were exposed to 108 pfu/ml of Ad.MnSOD or to an adenovirus containing the reporter gene β-galactosidase (Ad.βGal). Infection with Ad.MnSOD dramatically increases MnSOD protein levels and MnSOD catalytic activity in PC12-S cells (Fig. 5A). PC12 cells transfected with Ad.βGal and Ad.MnSOD maintain a normal morphological appearance and remain viable, as demonstrated by the absence of trypan blue staining (data not shown). Ad.MnSOD has little effect on MnSOD protein and catalytic activity in PC12-R, which may be caused by the already high levels of MnSOD in this cell line (Fig. 5A). The control Ad.βGal virus has no effect on MnSOD levels in PC12-S or PC12-R cells.
Fig. 5.
Overexpression of MnSOD confers resistance to NO toxicity in PC12 cells. A, Western blot and activity analyses demonstrate overexpression of MnSOD after infection of PC12-S and PC12-R cells with an adenovirus-derived vector containing the MnSOD gene. Cells were exposed to 108 pfu/ml of either an adenoviral vector containing the MnSOD gene (Ad.MnSOD) or a control adenoviral vector containing the β-galactosidase gene (Ad.βGal). Cells were harvested 48 hr after exposure to adenovirus for Western blot and activity analyses. Total protein (10 μg) was loaded in each lane for Western blot analysis. Also at this time point, control cells infected with Ad.βGal were assayed for X-Gal staining to determine infection efficiency. The estimated infection efficiency was 90–100% with minimal cell loss. B, Effect of adenoviral-mediated overexpression of MnSOD on PC12-S susceptibility to SNP. Cells were exposed to 108 pfu/ml of either Ad.MnSOD or Ad.βGal, and 24 hr later they were treated with 1 mm SNP for 5 min. Toxicity was assayed 24 hr after treatment by trypan blue exclusion. C, Effect of adenoviral-mediated overexpression of MnSOD on PC12-S susceptibility to paraquat. Cells were exposed to 108 pfu/ml of either Ad.MnSOD or Ad.βGal and 24 hr later were treated with 1 mm paraquat for 5 min. Toxicity was assayed 24 hr after treatment by trypan blue exclusion. Data represents the mean ± SEM of two to three independent experiments. Western blots are representative of two to three independent experiments. *p < 0.001.
Overexpression of MnSOD in PC12-S cells renders them almost completely resistant to the toxic effects of 1 mm SNP, which is equivalent to the resistance of PC12-R cells to 1 mm SNP (Fig. 5B). The Ad.βGal virus has no significant effects on the susceptibility of PC12-S or PC12-R to SNP. Similar results were obtained with DETA/NO (data not shown). Overexpression of MnSOD provides some protection against O2•−- generating compounds. Paraquat (1 mm) causes 100% cell death in uninfected PC12-S cells, but only 40% cell death in cells overexpressing MnSOD (Fig.5C). Similar results were obtained with menadione (data not shown). This contrasts with the nearly complete protection provided by Ad.MnSOD against NO donor toxicity. Ad.MnSOD had no effect on the susceptibility of PC12-R cells to NO donors or O2•−-generating compounds (data not shown), which correlates with the high levels of MnSOD protein and remarkable resistance to NO and O2•−of these cells before infection.
MnSOD is selectively enriched in nNOS neurons
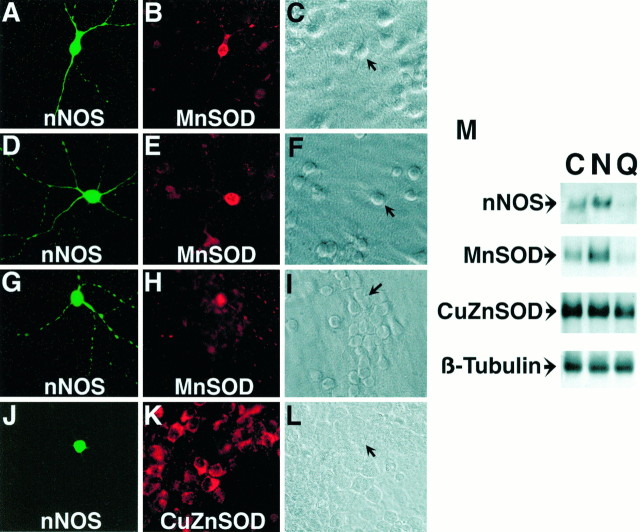
Because increased levels of MnSOD may account for the resistance of the PC12-R line to NO-mediated toxicity, this prompted us to investigate the potential protective role of MnSOD in nNOS neurons. nNOS neurons in primary cortical cultures comprise ∼1–2% of the total neuronal population (Bredt et al., 1991). Strikingly, MnSOD is enriched in nNOS neurons, as indicated by immunohistochemical colocalization studies (Fig.6A–I). In contrast, CuZnSOD is expressed ubiquitously in all neurons with no enrichment in nNOS neurons (Fig. 6J–L). nNOS neurons represent ∼2% of the total neuronal population in cortical cultures, and immunohistochemical analysis of cortical neurons in culture indicates that every neuron that expresses nNOS also expresses MnSOD at high levels. We do not detect any nNOS-staining neuron that does not show intense positive staining for MnSOD. On the other hand, 2–5% of the total neuronal population in cortical cultures expresses high levels of MnSOD, and some of these neurons do not stain for nNOS. MnSOD-positive/nNOS-negative neurons show lower levels of MnSOD than MnSOD-positive/nNOS-positive neurons (data not shown).
Fig. 6.
MnSOD is selectively enriched in nNOS neurons. Immunofluorescence staining of 14 d cultured rat cortical neurons indicates that nNOS neurons (A, D, G) are enriched in MnSOD (B, E, H). nNOS and MnSOD are both extranuclear proteins concentrated mostly in the neuronal cell body and processes. In contrast, nNOS (J) neurons are not enriched in CuZnSOD (K), which is expressed ubiquitously in cortical neurons. Hoffman modulation images of cells are depicted to the right of the corresponding immunofluorescent images (C, F, I, L), andarrows indicate nNOS neurons. M, MnSOD levels parallel nNOS levels after NMDA or quisqualate treatment. Western blot analysis of nNOS, MnSOD, CuZnSOD, and β-tubulin levels in primary cortical cultures after treatment with control salt solution (C), NMDA (N), or quisqualate (Q). Rat neuronal cultures were exposed for 5 min to either 500 μm NMDA or 20 μm quisqualate. Twenty-four hours later, cells were harvested for Western blot analysis. Total protein (50 μg) was loaded in each lane and electrophoresed in a denaturing 10% polyacrylamide gel. Immunofluorescent images and Western blots are representative of two to three independent experiments.
Treatment of cortical cultures with 300–500 μm NMDA enriches for nNOS neurons, whereas treatment with 20 μmquisqualate depletes nNOS neurons from cortical cultures (V. L.Dawson et al., 1993). Using this approach we confirm the enrichment of nNOS neurons by NMDA treatment and the depletion of nNOS neurons after quisqualate treatment by Western blot analysis (Fig.6M). Accompanying the enrichment in nNOS levels in NMDA-treated cultures is an enrichment in MnSOD (Fig.6M). Quisqualate depletes both nNOS and MnSOD protein levels (Fig. 6M). NMDA and quisqualate treatments do not show any detectable effect on CuZnSOD levels or β-tubulin levels, thus confirming the specific enrichment of MnSOD in nNOS neurons.
Antisense MnSOD renders nNOS neurons susceptible to NMDA toxicity
Forty-eight hour exposure to 10 μm antisense oligonucleotide to MnSOD effectively reduces MnSOD protein levels and catalytic activity in primary cortical neuronal cultures, as indicated by Western blot analysis and MnSOD catalytic activity (Fig.7A). We then assessed the susceptibility of nNOS neurons and the total neuronal population to NMDA neurotoxicity after knockdown of MnSOD by antisense oligonucleotide (Fig. 7B,C). NMDA (300 μm) kills ∼20–30% of the nNOS neurons. In the presence of 10 μm antisense oligonucleotide to MnSOD, ∼85% of the nNOS neurons die (Fig. 7B). After 500 μm NMDA treatment, ∼50% of the nNOS neurons die, and exposure to antisense oligonucleotide to MnSOD leads to almost complete loss of nNOS neurons (Fig. 7B). In contrast, the increased susceptibility of the total neuronal population to either 300 or 500 μm NMDA is not influenced by exposure to antisense oligonucleotide to MnSOD (Fig.7C). Sense and random oligonucleotides with equivalent levels of sulfation do not have any detectable effect. Although phosphorothioate derivatives of oligos may be toxic, especially to neurons, we did not encounter any intrinsic neurotoxicity of our phosphorothioate derivatives. Indeed, our antisense, sense, and random oligos, which contain an equivalent amount of sulfation, have no intrinsic neurotoxicity in our neuronal cultures. Furthermore, the antisense MnSOD oligo only affected the susceptibility of nNOS neurons to NMDA neurotoxicity. To illustrate the differential resistance and susceptibility of nNOS neurons to NMDA neurotoxicity in the absence and presence of antisense oligonucleotide to MnSOD, respectively, we plotted the percentage of nNOS neuron survival versus the percentage of total neuronal survival (Fig. 7D). Consistent with the notion that nNOS neurons are markedly resistant to NMDA neurotoxicity is the observation that nNOS neurons preferentially survive NMDA treatment compared with the total neuronal population (Fig.7B–D). In contrast, nNOS neurons are markedly susceptible to reductions in MnSOD, whereas the susceptibility of the total neuronal cell population to NMDA toxicity does not change after reductions in MnSOD.
Fig. 7.
Antisense MnSOD renders nNOS neurons susceptible to NMDA toxicity. A, Western blot and activity analyses of MnSOD in primary cortical neurons after exposure to oligonucleotides. Cultures were exposed for 24 hr to either no oligonucleotide (−), antisense oligonucleotide to MnSOD (AS), sense oligonucleotide (S), or random oligonucleotide (R). All oligonucleotides were used at 10 μm concentration. Thirty micrograms of total protein were loaded in each lane. Catalytic activity data from three independent experiments were analyzed with the Student’s t test for independent means. Statistical analysis was performed by using StatView 4.0 software. MnSOD catalytic activity after antisense oligonucleotide knockdown was significantly different from MnSOD activity in untreated, sense, and random oligonucleotide-treated cells (p < 0.001).B, Effect of antisense knockdown of MnSOD on susceptibility of nNOS neurons to NMDA toxicity. Cultures were exposed to either no oligonucleotide, 10 μm antisense oligonucleotide, or 10 μm random oligonucleotide. Twenty-four hours later, cells were treated for 5 min with 0, 300, or 500 μm NMDA, and fresh oligonucleotides were added to the medium. After 24 hr, cultures were stained for nNOS neurons by NADPH–diaphorase staining. C, Susceptibility of cultures to NMDA toxicity. Treatment was performed as inA, and total cell death was estimated by trypan blue staining 24 hr after exposure to NMDA. D, The differential resistance and susceptibility of nNOS neurons to NMDA neurotoxicity in the absence and presence of antisense oligonucleotide to MnSOD, respectively, is illustrated by plotting the ratio between the percentage of nNOS neuron survival and the percentage of total neuronal survival. Data represents the mean ± SEM of two to three independent experiments. Western blots are representative of two to three independent experiments. *p < 0.001.
Overexpression of MnSOD confers resistance to NMDA and NO toxicity in primary cortical neurons
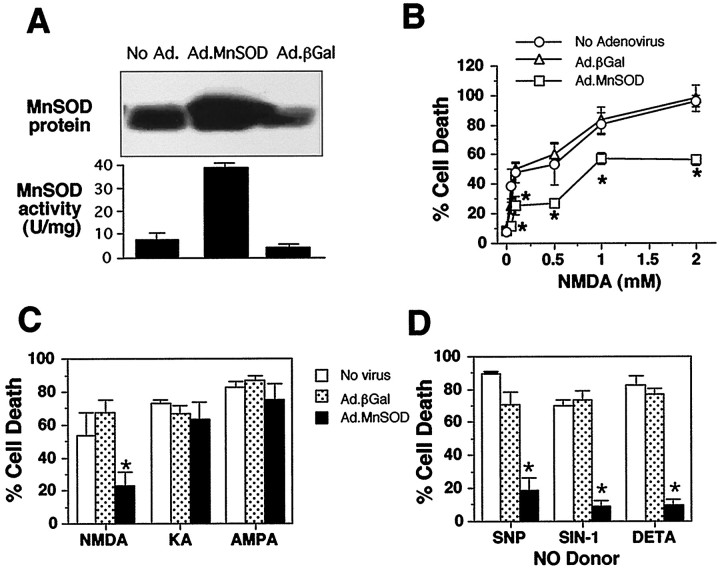
A number of approaches have been used to transfect genes of interest into primary neurons (Werner et al., 1990). These approaches yield ∼1–2% transfection efficiency sometimes in the setting of marked toxicity. Various studies suggest that modified adenovirus is an efficient vector for successful gene transfer into neurons (Akli et al., 1993; Davidson et al., 1993; Le Gal La Salle et al., 1993). Despite the initial experiments indicating the feasibility of this approach, most investigators have not been able to achieve significant gene transfer efficiency in the setting of minimal neurotoxicity (Kozarsky and Wilson, 1993). The purification and storage of the virus are critical to the success of efficient infection of primary neurons with minimal toxicity. We now show 90–100% infection efficiency in primary neuronal cultures, with essentially no loss of cell viability, as indicated by transfection of primary cortical cultures with Ad.βGal adenovirus (Fig.8A). Although we routinely achieve 90–100% infection of neurons with adenovirus, there is some heterogeneity in the expression patterns. In a similar manner, we are able to infect 90–100% of cortical neurons with the Ad.MnSOD virus (Fig. 8B–F). Uninfected control cultures or cultures infected with Ad.βGal show minimal immunostaining for MnSOD, with 2–5% of the total neuronal population showing intense staining (data not shown). Cortical neurons transfected with Ad.βGal and Ad.MnSOD maintain a normal morphological appearance and remain viable, as demonstrated by a normal morphological appearance under Hoffman modulation optics and the absence of trypan blue staining (data not shown). Confirming that the Ad.MnSOD virus overexpresses MnSOD is our observation that MnSOD protein levels and catalytic activity are increased dramatically after infection with Ad.MnSOD, whereas Ad.βGal has no effect on MnSOD levels or catalytic activity (Fig. 9A).
Fig. 8.

Efficient gene transfer into primary neurons using an adenovirus vector. A, Neurons (90–100%) were infected with 108 pfu/ml of an adenovirus containing the β-galactosidase reporter gene, as assessed by X-Gal staining 24 hr after exposure of cultures to the virus. B–F, The rat MnSOD gene was efficiently transferred (90–100% infection efficiency) into primary neurons via an adenovirus (Ad.MnSOD), as assessed by immunofluorescence staining of cultures 24 hr after exposure to 108 pfu/ml of Ad.MnSOD. D, F, Hoffman modulation images corresponding to panels C and E, respectively. Images are representative of three to four independent experiments.
Fig. 9.
MnSOD overexpression confers resistance to NMDA and NO toxicity in primary cortical neurons. A, Western blot and activity analyses of MnSOD in rat cortical neurons afterin vitro exposure to either control salt solution (No Ad.), 108 pfu/ml of MnSOD-containing adenovirus (Ad.MnSOD), or 108 pfu/ml of β-galactosidase-containing adenovirus (Ad.βGal). Cells were harvested for Western blot and activity analyses 48 hr after exposure to the adenovirus. B, Susceptibility of cortical neurons to NMDA toxicity in cultures that were not exposed to adenovirus and in cultures infected with Ad.MnSOD or Ad.βGal. C, Susceptibility of cortical neurons to NMDA, kainate (KA), and AMPA after exposure to control salt solution (no virus), Ad.MnSOD, or Ad.βGal. Cultures were exposed to 108 pfu/ml Ad.MnSOD or Ad.βGal, and 24 hr later treated with 0.5 mm NMDA, 0.1 mm kainate, or 0.1 mm AMPA. NMDA was applied for 5 min and then washed off. KA and AMPA were applied for 14 hr. Toxicity was assessed 24 hr after exposure to the toxic agent by trypan blue exclusion.D, Susceptibility of cortical neurons to NO donors after exposure to control salt solution (no virus), Ad.MnSOD, or Ad.βGal. Cultures were exposed to 108 pfu/ml Ad.MnSOD, or Ad.βGal, and 24 hr later they were treated for 5 min with 1 mm SNP, 2 mm SIN-1, or 2 mmDETA/NO. Toxicity was assessed 24 hr after exposure to the toxic agent by trypan blue exclusion. Data represents the mean ± SEM of two to three independent experiments. Western blots are representative of two to three independent experiments. *p < 0.001.
Infection of primary cortical neurons with Ad.MnSOD confers resistance to NMDA toxicity at all doses examined, whereas Ad.βGal has no effect (Fig. 9B). We also compared the effects of adenoviral-mediated overexpression of MnSOD on kainate and AMPA toxicity with the effects on NMDA toxicity (Fig. 9C). Our results show that overexpression of MnSOD has no effect on kainate or AMPA toxicity but is protective against NMDA-mediated neurotoxicity. This is consistent with the notion that NO neurotoxicity is mediated by NMDA receptor activation, but not by kainate or AMPA receptor activation (V. L. Dawson et al., 1993), and it further illustrates the selective protective effect of MnSOD on NMDA and NO-mediated toxicity. Adenoviral-mediated overexpression of β-galactosidase has no effect on NMDA, kainate, or AMPA toxicity.
To assess the potential role of MnSOD in protecting cortical neurons from NO-mediated toxicity, we applied various NO donors and assessed neurotoxicity in primary cortical neuronal cultures after adenoviral-mediated overexpression of MnSOD (Fig. 9D). Overexpression of MnSOD provides dramatic protection against SNP-, SIN-1-, and DETA/NO-mediated neurotoxicity, whereas overexpression of β-galactosidase has no detectable effect on NO toxicity (Fig.9D).
MnSOD is required for nNOS neuron resistance to NMDA-induced toxicity
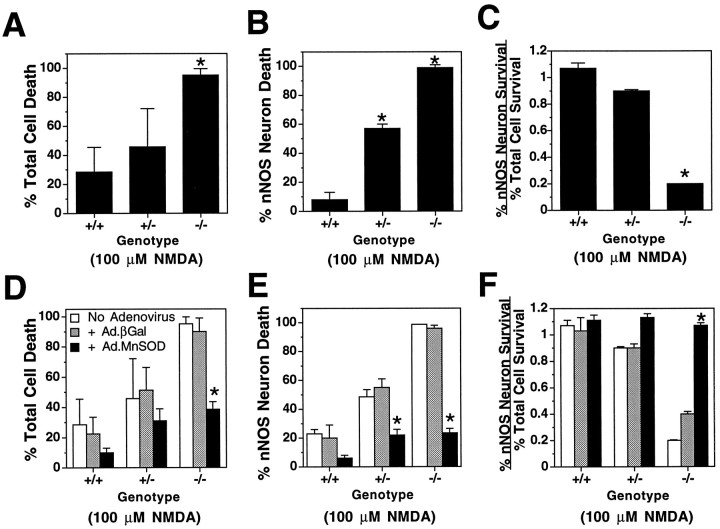
To test further whether MnSOD is the endogenous gene that confers resistance of nNOS neurons to NMDA-induced toxicity, we evaluated the susceptibility of nNOS neurons to NMDA-induced toxicity in mice in which the gene coding for MnSOD had been disrupted by homologous recombination (Li et al., 1995; Lebovitz et al., 1996). Sixty-one individual embryos from MnSOD heterozygous matings were screened for the MnSOD gene via Southern blot analysis and PCR. Ten (16%) wild-type (+/+), 34 (56%) heterozygous (+/−), and 17 (28%) null (−/−) independent cortical neuronal cultures were derived from each embryo and maintained in vitro in low (5%) O2 for 10 d before experiments were performed. Each culture was exposed to 100 μm NMDA for 5 min. Twenty hours later, cell death and NADPH–diaphorase staining for nNOS-containing neurons were assessed. The total neuronal population in MnSOD−/− cortical cultures is significantly more susceptible to the toxic effects of a relatively low dose (100 μm) of NMDA, with a threefold increase in the proportion of total cell death (Fig.10A). MnSOD+/− cultures also tend to be more susceptible than wild-type cultures to NMDA-induced death. The nNOS neuronal population is significantly reduced in MnSOD+/−cultures and is almost completely lost in MnSOD−/−mice after NMDA treatment (Fig. 10B). The dramatically increased susceptibility of nNOS neurons to NMDA toxicity in MnSOD-deficient cortical cultures is also evident when we calculate the ratio between the percentage of surviving nNOS neurons and the percentage of total cell survival (Fig. 10C). Estimation of this ratio is important in the analysis of our data because it demonstrates the dramatic effect of complete elimination of MnSOD on susceptibility to NMDA toxicity in nNOS neurons, and that the sensitivity to NMDA-induced death is selectively increased in nNOS neurons.
Fig. 10.
MnSOD is required for nNOS neuron survival.A, Effect of targeted disruption of MnSOD on susceptibility of cortical neurons to NMDA toxicity. Wild-type (+/+), MnSOD+/−, and MnSOD−/−cultures were treated for 5 min with 100 μm NMDA, and total cell death was estimated by trypan blue staining or computer-assisted cell counting 24 hr after exposure to NMDA.B, Effect of targeted disruption of MnSOD on susceptibility of nNOS neurons to NMDA toxicity. Cultures were treated for 5 min with 100 μm NMDA, and after 24 hr cells were stained for nNOS neurons by NADPH–diaphorase staining.C, The differential resistance and susceptibility of nNOS neurons to NMDA neurotoxicity in wildtype (+/+), MnSOD+/−, and MnSOD−/−cultures is illustrated by plotting the ratio between the percentage of nNOS neuronal survival and the percentage of total neuronal survival.D, Susceptibility of cortical neurons to 100 μm NMDA toxicity in wild-type (+/+), MnSOD+/−, and MnSOD−/−cultures that were not exposed to adenovirus and in cultures infected with Ad.MnSOD or Ad.βGal. E, Susceptibility of nNOS neurons to 100 μm NMDA toxicity in wild-type (+/+), MnSOD+/−, and MnSOD−/−cultures that were not exposed to adenovirus and in cultures infected with Ad.MnSOD or Ad.βGal. F, The differential resistance and susceptibility of nNOS neurons to NMDA neurotoxicity in wild-type (+/+), MnSOD+/−, and MnSOD−/− cultures that were not exposed to adenovirus and in cultures infected with Ad.MnSOD or Ad.βGal is illustrated by plotting the ratio between the percentage of nNOS neuronal survival and the percentage of total neuronal survival. Data represent the mean ± SEM of five independent experiments. *p < 0.001.
Adenoviral overexpression of MnSOD in MnSOD−/− cultures rescues nNOS neurons from NMDA-induced death
If the loss of nNOS neurons after NMDA treatment in cultures from MnSOD-deficient mice is caused by the lack of MnSOD, overexpression of the protein would be predicted to protect nNOS neurons from NMDA-induced death. Wild-type, MnSOD+/−, and MnSOD−/− cortical neuronal cultures from individual embryos were infected with Ad.MnSOD or Ad.βGal, and 48 hr later they were treated with 100 μm NMDA. Although no significant change in total cell death after NMDA treatment is observed in wild-type and MnSOD+/− cultures, overexpression of MnSOD significantly rescues MnSOD−/− cultures from NMDA-induced death (Fig. 10D). Overexpression of MnSOD also has a dramatic protective effect on the nNOS neuronal population in MnSOD−/− cultures, and to a lesser extent in the MnSOD+/− cultures (Fig.10E). The proportion of surviving nNOS neurons after NMDA treatment in Ad.MnSOD-infected MnSOD−/−cultures is comparable to that observed in wild-type cultures. The ratio of surviving nNOS neurons to total cell survival in the MnSOD−/− cultures after overexpression of MnSOD is also dramatically increased to wild-type levels (Fig. 10F). Thus, these data suggest that MnSOD is a major endogenous determinant of NMDA resistance in nNOS neurons.
DISCUSSION
The molecular mechanisms underlying the remarkable resistance of nNOS neurons to NMDA-glutamate receptor toxicity have been under intensive investigation. In this study, we provide several independent and complementary lines of evidence that MnSOD may be necessary for the selective resistance of nNOS neurons to NMDA neurotoxicity: (1) the NO-resistant PC12-R cell line contains elevated levels of MnSOD as determined by SAGE, Western blot, and catalytic activity analyses; (2) antisense oligonucleotide knockdown of MnSOD renders PC12-R cells susceptible to NO toxicity; (3) adenoviral overexpression of MnSOD confers resistance to NO toxicity in the NO-sensitive, PC12-S cell line; (4) nNOS neurons in rat cortical neuronal cultures are enriched in MnSOD; (5) antisense MnSOD causes nNOS neurons to become susceptible to NMDA toxicity, although it has little effect on overall neuronal toxicity; (6) adenovirus-mediated overexpression of MnSOD in cortical cultures confers resistance to NMDA and NO-mediated neurotoxicity but has no effect on kainate and AMPA neurotoxicity; (7) nNOS neurons from MnSOD−/− mice are markedly susceptible to NMDA-induced toxicity; and finally, (8) nNOS neurons in MnSOD−/− mice are rescued from death by overexpressing MnSOD. Together these observations provide strong evidence that MnSOD may be the principal endogenous protective protein against NMDA and NO-mediated neurotoxicity in nNOS neurons.
MnSOD is critical for survival against NO toxicity
The majority of NO induced toxicity may occur through an interaction with O2•− to form the highly reactive and toxic free radical peroxynitrite (Radi et al., 1991; Beckman and Crow, 1993; Beckman, 1994; Xia et al., 1996). Thus, excess production of either NO or O2•− could lead to deleterious generation of peroxynitrite. Peroxynitrite may inactivate MnSOD through tyrosine nitration and contribute to further cytotoxicity by diminishing mitochondrial O2•−scavenger capacity (MacMillan-Crow et al., 1996). Thus, it may be critical to prevent mitochondrial peroxynitrite formation by effective scavenging of O2•−. Although MnSOD is important in the cellular defense against excess O2•−, it appears to play a more significant role against NO toxicity. Consistent with this notion are our observations that alterations in MnSOD levels have more pronounced effects on NO toxicity than on O2•−toxicity. Mammalian cells have several enzymes that scavenge O2•−, including the cytosolic CuZnSOD and the mitochondrial MnSOD (McCord, 1969; Fridovich, 1986; Bannister et al., 1987; Hassan, 1988; Fridovich, 1995). Previous studies suggest that CuZnSOD may play an important role in NO/peroxynitrite-mediated cell death (Troy et al., 1996). However, our observations that (1) CuZnSOD levels remain unchanged in the PC12-R NO-resistant cell line, (2) CuZnSOD is ubiquitously expressed in cultured cortical neurons, and (3) CuZnSOD levels are not altered by NMDA or quisqualate treatments that enrich and deplete cultures of nNOS neurons, respectively, indicate that under normal conditions CuZnSOD probably plays a minimally protective role against NMDA and NO neurotoxicity in nNOS neurons. On the other hand, MnSOD, which is enriched in nNOS neurons and NO-resistant PC12-R cells, seems to play a major role in protection against NMDA and NO toxicity. The importance of mitochondrial scavenging of O2•− is further illustrated by the recent demonstration that only overexpression of MnSOD, but not CuZnSOD or mutant MnSOD lacking the mitochondrial matrix signal, protects against free radical toxicity (Wong, 1995). Furthermore, insertion of the mitochondrial signal sequence into CuZnSOD results in protection against free radical attack (Wong, 1995).
MnSOD is essential and sufficient for nNOS neuron resistance to NMDA toxicity
Since the original description of NADPH–diaphorase neurons surviving stroke damage by Thomas and Pearse (1964), and the remarkable survival of NADPH–diaphorase neurons in the setting of severe neuronal loss in the striatum of Huntington’s disease (Ferrante et al., 1985), the molecular mechanisms underlying the resistance of nNOS neurons to these insults as well as NMDA neurotoxicity have been a mystery. The discovery that nNOS catalytic activity accounts for NADPH–diaphorase staining provided clues but no obvious explanation (T. M. Dawson et al., 1991; Hope et al., 1991). Various theories on the remarkable resistance of nNOS neurons to toxic insults have ranged from the expression of nNOS as the responsible mechanism, the expression of unknown protective proteins, to the relative lack of glutamate receptor expression (Dawson et al., 1992). Recent colocalization studies of nNOS with glutamate receptor subunits suggest that nNOS neurons do contain glutamate receptors (Catania et al., 1995; Landwehrmeyer et al., 1995;Standaert et al., 1996). Experiments in nNOS−/−mice indicate that nNOS itself does not account for the selective resistance of nNOS neurons to NMDA neurotoxicity (Dawson et al., 1996). However, nNOS−/− mice were later shown to express enzymatically active splice variants of nNOS (Brenman et al., 1996). Although these splice variants fail to produce NO upon NMDA receptor stimulation (Dawson et al., 1996), it is still possible that nNOS activity may account, at least in part, for the selective resistance of nNOS neurons to damage.
It is quite surprising that MnSOD is selectively enriched in cultured cortical nNOS neurons and that it plays a critical role in the selective resistance of nNOS neurons to NMDA-induced neurotoxicity. We would have expected an enzyme such as MnSOD to be expressed ubiquitously and to play a major protective role against most, if not all, toxic insults. However, MnSOD is dramatically enriched in cultured nNOS neurons, because no nNOS neuron was detected that did not show intense positive staining for MnSOD. On the other hand, some neurons expressed relatively high levels of MnSOD and did not stain for nNOS. Detailed and comprehensive immunohistochemical studies in rodent brain on the localization of MnSOD have not been reported. However, limited studies indicate that MnSOD has a heterogeneous distribution. In the striatum, MnSOD is enriched in cholinergic neurons and somatostatin neurons, which contain nNOS (Inagaki et al., 1991a). It is also highly enriched in cholinergic neurons of the basal forebrain (Inagaki et al., 1991b). In the hippocampus, MnSOD is enriched mainly in parvalbumin-containing neurons and is rarely in nNOS neurons (Matsui et al., 1996). Thus, in some regions of the brain the selective resistance of nNOS neurons may be attributed to other factors. Future studies will be required to determine the amount of coexpression of nNOS and MnSOD in rodent brain.
Our findings that MnSOD is required for nNOS neuron survival after NMDA-induced toxicity in cultures from MnSOD-deficient mice are critical in supporting and complementing the data obtained in PC12 cells and rat cortical cultures after antisense oligonucleotide knockdown of MnSOD mRNA and adenovirus-mediated overexpression of MnSOD. Previous studies that have suggested that MnSOD is important for the resistance to toxic cellular insults have relied on overexpression or antisense knockdown of MnSOD (Jones, 1986; Wong et al., 1989; Wong, 1995). These methods do not insure that MnSOD is the endogenous mechanism of protection. Our findings in MnSOD−/− mice provide a direct demonstration that endogenous MnSOD is essential and sufficient for the cellular survival against toxic insults. The complete absence of MnSOD renders nNOS neurons dramatically sensitive to the toxic effects of NMDA, and adenoviral replacement of MnSOD preferentially enhances the survival of nNOS neurons to NMDA-induced toxicity. Thus, MnSOD plays a key protective role against NMDA-induced toxicity in neurons containing nNOS.
Glutamate neurotoxicity and mitochondrial function
Recent studies indicate that mitochondrial dysfunction is a primary event in glutamate neurotoxicity (Schinder et al., 1996; White and Reynolds, 1996). A perfect balance between mitochondrial function and intracellular calcium homeostasis is essential for cell survival. Overstimulation of NMDA receptors causes a massive Ca2+ influx that leads to an imbalance in mitochondrial homeostasis and mitochondrial dysfunction that in turn triggers subsequent neuronal death cascades (Schinder et al., 1996;White and Reynolds, 1996). Given the high metabolic requirements of the brain, it is reasonable to hypothesize that mitochondria are principal targets of calcium-dependent effectors of excitotoxicity (White and Reynolds, 1996). The mitochondrial electron transport chain is sensitive to NO and free radical generation (Zhang et al., 1990;Schweizer and Richter, 1994). Recent evidence suggests that oxidizing agents increase the likelihood of activation of the permeability transition pore in the mitochondrial membrane, which results in mitochondrial depolarization (Connern and Halestrap, 1994). Mitochondrial depolarization and alteration of the electron transport chain decrease ATP synthesis. The reduction of cellular energy together with the generation of oxygen free radicals subsequent to excitotoxic stimulation and high ATP consumption lead to cell collapse. Furthermore, the mitochondrial electron transport chain has been shown to be an important source of glutamate-induced reactive oxygen species (Dugan et al., 1995). Thus, free radical formation as indicated by oxidation of nonfluorescent dihydrorhodamine 123 to fluorescent rhodamine 123 is dramatically and specifically enhanced by NMDA receptor activation but not by kainate receptor activation (Dugan et al., 1995). Our observation that MnSOD protects cortical cultures against NMDA-mediated neurotoxicity, but not against AMPA or kainate toxicity, further substantiates the importance of mitochondrial dysfunction in NMDA neurotoxicity as well as the selective protective effects of MnSOD against NMDA and NO-mediated toxicity. Because the rate of reaction of NO with O2•− to form peroxynitrite is extremely rapid, peroxynitrite production is particularly limited by the diffusion constants of NO and O2•−. Because mitochondria are an important source of free radical formation after NMDA neurotoxicity, the selective enrichment of MnSOD in nNOS neurons effectively scavenges excess O2•− and protects nNOS neurons against the toxic effects of NO. On the other hand, NO diffuses to adjacent non-nNOS neurons and reacts with NMDA-induced O2•−, which is not effectively scavenged in mitochondria. Thus, peroxynitrite is preferentially produced in non-nNOS neurons, ultimately setting in motion irreversible processes leading to cell death.
Although MnSOD seems to be a major protective protein that confers resistance to nNOS neurons to NMDA and NO mediated-neurotoxicity, we cannot exclude the possibility that other protective proteins exist and are preferentially expressed in nNOS neurons. Consistent with this notion is our observation that almost complete elimination of MnSOD catalytic activity in the PC12-R NO-resistant cell line does not lead to complete susceptibility to NO-mediated toxicity. Furthermore, by using differential display analysis of NMDA-treated cortical cultures versus quisqualate-treated cortical cultures, we have identified novel transcripts that are enriched in nNOS neurons (V. Christov, V. L. Dawson, and T. M. Dawson, unpublished observations). Thus, nNOS neurons may preferentially express multiple protective genes, within which MnSOD plays a major protective role.
Footnotes
M.G.-Z. is supported by a postdoctoral research award from the Boehringer Ingelheim Fonds (Stuttgart, Germany). V.L.D. is supported by United States Public Health Service Grant NS33142, the American Heart Association, and the Muscular Dystrophy Association. T.M.D. is an established investigator of the American Heart Association and is supported by United States Public Health Service Grants NS01578 and NS33277, and the Paul Beeson Faculty Scholar Award in Aging Research. We thank Dr. V. Velculescu and Dr. K. Kinzler for providing the SAGE procedure and very helpful advice on SAGE, Dr. Allen Mandir for his assistance with the SAGE software, R. Anderson and Dr. B. L. Davidson for providing adenoviral vectors, and Ann Schmidt for typing assistance.
Under an agreement between Johns Hopkins University and Guilford Pharmaceuticals, T.M.D. and V.L.D. are entitled to a share of sales royalty received by the University from Guilford. T.M.D. and the University also own Guilford stock, and the University stock is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the University in accordance with its conflict of interest policies.
Correspondence should be addressed to Ted M. Dawson, Departments of Neurology and Neuroscience, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 2-210, Baltimore, MD 21287.
REFERENCES
- 1.Akli S, Caillaud C, Vigne E, Stratford-Pericaudet LD, Poenaru L, Perricaudet M, Kahn A. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- 2.Bannister JA, Bannister WH, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. Crit Rev Biochem. 1987;22:111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF, Kowall NW, Ellison DW. Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS. Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. Ann NY Acad Sci. 1994;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 8.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 9.Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 10.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 11.Catania MV, Tolle TR, Monyer H. Differential expression of AMPA receptor subunits in NOS-positive neurons of cortex, striatum, and hippocampus. J Neurosci. 1995;15:7046–7061. doi: 10.1523/JNEUROSCI.15-11-07046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 13.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 14.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302:321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkara T, Moskowitz MA. The complex role of nitric oxide in the pathophysiology of focal cerebral ischemia. Brain Pathol. 1994;4:49–57. doi: 10.1111/j.1750-3639.1994.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 16.Davidson BL, Allen DE, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 17.Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and periphral tissues. Proc Natl Acad Sci USA. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger in the brain: the free radical, nitric oxide. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- 20.Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Uhl GR, Snyder SH. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci USA. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson VL, Dawson TM. Free radicals and neuronal cell death. Cell Death Differ. 1996;3:71–78. [PubMed] [Google Scholar]
- 22.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson VL, Dawson TM, Bartley DA, Uhl GR, Snyder SH. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson VL, Kizushi VK, Huang PL, Snyder SH, Dawson TM. Resistance to neurotoxicity in cortical neuronal cultures from neuronal nitric oxide synthase deficient mice. J Neurosci. 1996;16:2479–2487. doi: 10.1523/JNEUROSCI.16-08-02479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugan LL, Sensi SL, Canzoniero LMT, Handran SD, Rothman SM, Lin T-S, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliasson MJL, Sampei D, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang Z-Q, Dawson TM, Snyder SH, Dawson VL. Poly (ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 27.Ferrante RJ, Kowall NW, Beal MF, Richardson EP, Bird ED, Martin JB. Selective sparing of a class of striatal neurons in Huntington’s disease. Science. 1985;230:561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- 28.Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 29.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 30.Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- 31.Hassan HM. Biosynthesis and regulation of superoxide dismutases. Free Radical Biol Med. 1988;5:377–385. doi: 10.1016/0891-5849(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 32.Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 35.Hyman BT, Marzloff K, Wenniger JJ, Dawson TM, Bredt DS, Snyder SH. Relative sparing of nitric oxide-containing neurons in the hippocampal formation in Alzheimer disease. Ann Neurol. 1992;32:818–824. doi: 10.1002/ana.410320618. [DOI] [PubMed] [Google Scholar]
- 36.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki S, Suzuki K, Taniguchi N, Takagi H. Localization of Mn-superoxide dismutase (Mn-SOD) in cholinergic and somatostatin-containing neurons in the rat neostriatum. Brain Res. 1991a;549:174–177. doi: 10.1016/0006-8993(91)90618-6. [DOI] [PubMed] [Google Scholar]
- 38.Inagaki S, Takagi H, Suzuki K, Akai F, Taniguchi N. Intense immunoreactivity for Mn-superoxide dismutase (Mn-SOD) in cholinergic and non-cholinergic neurons in the rat basal forebrain. Brain Res. 1991b;541:354–357. doi: 10.1016/0006-8993(91)91038-3. [DOI] [PubMed] [Google Scholar]
- 39.Jones GRN. Free radicals and immunological killing: the case of tumor necrosis factor. Med Hypotheses. 1986;21:267–271. doi: 10.1016/0306-9877(86)90019-8. [DOI] [PubMed] [Google Scholar]
- 40.Koh J-Y, Peters S, Choi DW. Neurons containing NADPH-diaphorase are selectively resistant to quinolinate toxicity. Science. 1986;234:73–76. doi: 10.1126/science.2875522. [DOI] [PubMed] [Google Scholar]
- 41.Koh J-Y, Choi DW. Vulneability of cultures cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988;8:2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozarsky KF, Wilson JM. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 43.Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB, Young AB. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 1995;15:5297–5307. doi: 10.1523/JNEUROSCI.15-07-05297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lautier D, Lagueux J, Thibodeau J, Menard L, Poirier GG. Molecular and biochemical features of poly(ADP-ribose) metabolism. Mol Cell Biochem. 1993;122:171–193. doi: 10.1007/BF01076101. [DOI] [PubMed] [Google Scholar]
- 45.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Gal La Salle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Med. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 48.Lipton SA, Rosenberg PA. Excitatory aminoacids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:615–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 50.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 52.Matsui T, Ohta A, Takagi H. Morphological studies on Mn-SOD, NOS and calcium binding proteins in the rat hippocampus. Osaka City Med J. 1996;42:1–13. [PubMed] [Google Scholar]
- 53.McCord JM. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 54.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 55.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 56.Nathan CF. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 57.Oberley LW, Spitz DR. Nitroblue tetrazolium. In: Greenwald R, editor. CRC handbook of methods for oxygen radical research. CRC; Boca Raton, FL: 1985. pp. 217–220. [Google Scholar]
- 58.Oberley TD, Oberley LW, Slattery AF, Lauchner LJ, Elwell JH. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol. 1990;137:199–214. [PMC free article] [PubMed] [Google Scholar]
- 59.Przedborski S, Jackon-Lewis V, Yokohama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 61.Roskams AJ, Bredt DS, Dawson TM, Ronnett GV. Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron. 1991;13:289–299. doi: 10.1016/0896-6273(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 62.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Welty DF. Knockout of glutamate transporter reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 63.Samdani A, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 64.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfuction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 66.Schulz JB, Matthews RT, Jenkins BG, Ferrante RJ, Siwek D, Henshaw DR, Cipolloni PB, Mecocci P, Kowall NW, Rosen BR, Beal MF. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J Neurosci. 1995a;15:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulz JB, Matthews RT, Muqit MMK, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995b;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 68.Schulz JB, Huang PL, Matthews RT, Passov D, Fishman MC, Beal MF. Striatal malonate lesions are attenuated in neuronal nitric oxide synthase knockout mice. J Neurochem. 1996;67:430–433. doi: 10.1046/j.1471-4159.1996.67010430.x. [DOI] [PubMed] [Google Scholar]
- 69.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 70.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 71.Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex, and hippocampus. Mol Brain Res. 1996;42:89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 72.Thomas E, Pearse AGE. The solitary active cells. Histochemical demonstration of damage-resistant nerve cells with a TPN-diaphorase reaction. Acta Neuropathol (Berl) 1964;3:238–249. doi: 10.1007/BF00684399. [DOI] [PubMed] [Google Scholar]
- 73.Troy CM, Derossi D, Prochiantz A, Greene LA, Shelanski ML. Downregulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxide-peroxynitrite pathway. J Neurosci. 1996;16:253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uemura Y, Kowall NW, Beal MF. Selective sparing of NADPH- diaphorase-somatostatin-neuropeptide Y neurons in ischemic gerbil striatum. Ann Neurol. 1990;27:620–5. doi: 10.1002/ana.410270606. [DOI] [PubMed] [Google Scholar]
- 75.Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 76.Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 77.Werner M, Madreperla S, Lieberman P, Adler R. Expression of transfected genes by differentiated, post-mitotic neurons and photoreceptors in primary cell cultures. J Neurosci Res. 1990;25:50–57. doi: 10.1002/jnr.490250107. [DOI] [PubMed] [Google Scholar]
- 78.White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong GHW. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochem Biophys Acta. 1995;1271:205–209. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]
- 80.Wong GHW, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 81.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yun H-Y, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]