Abstract
GABA neurons in the substantia nigra pars reticulata receive input from GABAergic fibers originating in the forebrain. The role of dopaminergic D1 receptors located on these fibers was investigated using tight-seal whole-cell recordings from visually identified pars reticulata neurons of rat substantia nigra slices. Nondopaminergic pars reticulata neurons were characterized by their electrophysiological properties. Postsynaptic currents evoked by minimal stimulation in the presence of ionotropic glutamate receptor antagonists were blocked by bicuculline, indicating that they were GABAA IPSCs. Evoked GABAA IPSCs were potentiated by D1 receptor agonists. After application of D1 receptor agonists, miniature IPSCs [recorded in the presence of tetrodotoxin (TTX) and the Ca2+ channel blocker Cd2+] increased in frequency but not in amplitude. Effects of D1 receptor stimulation were mimicked by forskolin, as expected, if a cAMP-dependent mechanism was involved. The D1 antagonist SCH23390 blocked the effects of the agonists, and perfusion with SCH23390 resulted in a reduction of evoked IPSCs. In TTX and Cd2+, which prevented dopamine release, the D1 antagonist had no effect on miniature IPSCs. Blocking of monoamine uptake by imipramine increased the amplitude of evoked IPSCs. We conclude that dopamine released from dendrites of dopaminergic neurons enhances GABA release in the pars reticulata of the substantia nigra through D1 receptors presumably located on striatonigral afferents. These D1receptors, thereby, can reinforce D1 receptor-mediated activation of striatal projection neurons that inhibit the inhibitory output neurons of the basal ganglia in substantia nigra.
Keywords: dopamine, D1 receptors, GABAAIPSCs, GABA release, miniature IPSCs, forskolin, substantia nigra, direct pathway
Dopamine (DA)-containing neurons in the substantia nigra pars compacta (SNC) appear to play an important role in a number of neuronal functions. For instance, dysfunction of DA output from these neurons has been implicated in the etiology of motor disorders of the basal ganglia, e.g., Parkinson’s disease. DA exerts a modulatory effect on inhibitory basal ganglia output. The primary output structures of the basal ganglia are the substantia nigra pars reticulata (SNR) and the internal segment of the globus pallidus. Inhibitory output from the basal ganglia is controlled by two opposing but parallel pathways termed direct pathway and indirect pathway (Alexander and Crutcher, 1990). The direct pathway comprises GABAergic projections from neostriatal medium spiny neurons to SNR neurons and neurons of the internal segment of the globus pallidus, which in turn send GABAergic projections to the thalamus (Somogyi et al., 1979). The indirect pathway consists of GABAergic projections from neostriatal medium spiny neurons to the external segment of the globus pallidus (Graybiel and Ragsdale, 1983) and from the globus pallidus to the subthalamus (Rouzaire-Dubois et al., 1980). The subthalamus, in turn, sends excitatory glutamatergic (Smith and Parent, 1988) fibers to the SNR (Nakanishi et al., 1987; Parent, 1990; Robledo and Feger, 1990) and the internal segment of the globus pallidus (Kita and Kitai, 1987;Parent, 1990). Activation of the direct pathway results in a disinhibition of thalamic neurons; activation of the indirect pathway results in an inhibition of thalamic neurons.
DA interacts with both pathways. Dopaminergic neurons in the SNC (Dahlström and Fuxe, 1964; Moore et al., 1971; Ungerstedt, 1971) target neostriatal medium spiny neurons (Freund et al., 1984). DA interacts with the direct pathway via D1 receptors and with the indirect pathway via D2 receptors. The overall effect of DA is the attenuation of basal ganglia output, i.e., a reduction of the activity of GABAergic neurons in SNR and the internal segment of the globus pallidus.
Besides acting at the level of the neostriatum, DA may modulate functioning of the basal ganglia output via a direct influence in substantia nigra. DA release occurs not only from the nerve terminals in the neostriatum but also from the dendrites of DA neurons that extend their dendrites into the SNR (Geffen et al., 1976; Korf et al., 1976; Cheramy et al., 1981). DA released from dendrites of dopaminergic neurons is in a position to modulate the activity of those neurons in substantia nigra that convey basal ganglia output. In substantia nigra, D1 receptors are supposed to be localized selectively on terminals of GABAergic afferents from the forebrain (Barone et al., 1987; Harrison et al., 1990; Mansour et al., 1991; Cameron and Williams, 1993; Jaber et al., 1996). A possible mechanism is that D1 receptors on terminals of striatonigral fibers tune the efficiency of inhibition of SNR neurons.
Nondopaminergic, presumably GABAergic, neurons represent the majority of neurons within the SNR (Oertel et al., 1982) and can be separated from dopaminergic neurons by their electrical membrane properties (Nakanishi et al., 1987; Häusser et al., 1995; Richards et al., 1997). The aim of the present study was to elucidate the possible influence that DA in substantia nigra may have on inhibition of GABAergic neurons in the SNR. We suggest that through activation of D1 receptors on inhibitory afferents from the forebrain, endogenous DA can increase inhibition of inhibitory neurons in the SNR.
MATERIALS AND METHODS
Brain slice preparation. Wistar rats (12- to 16-d-old) were decapitated under ether anesthesia, and their brains were removed. Cross sections were made through the forebrain at the level of the optic chiasm and through the rostral side of the cerebellum. Right and left hemispheres were separated and trimmed by removing the cortex. Coronal slices (250-μm-thick) were cut from the caudal surface using a Vibratome (tpi; Balzers Union, Balzen, Liechtenstein). Slices were allowed to equilibrate for 1 hr at room temperature before commencement of recording. In the cross-sectioned slice, the SNR is located ventrolaterally and the SNC is located dorsolaterally (see Fig. 1A).
Fig. 1.
Visual and electrophysiological characterization of SNR neurons. A, Photomicrograph of the ventrolateral region of a coronal slice of rat mesencephalon. A nondopaminergic neuron from the SNR was filled with Lucifer yellow. The ventral surface of the midbrain is oriented toward the lower edge of the photograph. B, Same neuron as in A at higher magnification. C, IR-DIC photograph showing an SNR neuron from which a whole-cell recording was performed.D1, Electrophysiological characteristics of non-DA;D2, DA neurons in SNR. Recordings (K+-filled electrodes) showing the responses of the neurons to current pulses (amplitudes as shown) of 200 msec duration. Both neurons recorded at their resting membrane potentials (−65 mV). Note the characteristic differences in the hyperpolarizing response shown by the two different types of neurons. Scale bars: A, B, 100 μm; C, 10 μm.
Recording from visualized neurons. Slices were placed in a small (∼400 μl), submerged glass-bottomed recording chamber. Neurons were visualized with infrared light and differential interference (IR-DIC) optics (Stuart et al., 1993). A fixed stage upright compound microscope, Zeiss Axioscope FS (Zeiss, Oberkochen, Germany), equipped with a high numerical aperture (NA) water immersion lens (40 × 0.75 NA, working distance 1.9 mm) with corresponding DIC optics and a 0.9 NA condenser, was used. Slices were illuminated with infrared light by the placement of an infrared filter in the light path. The image was detected with an infrared-sensitive video camera (Newvicon C2400, Hamamatsu, Hamamatsu City, Japan) and displayed on a standard black and white video monitor (Sony GmbH, Köln, Germany).
Whole-cell patch clamp-recordings were made from nondopaminergic and dopaminergic neurons of the SNR at room temperature (20–24°C). Slices were superfused continuously at a rate of ∼1.5 ml/min with an oxygenated solution containing (in mm): 130 NaCl, 2 KCl, 1.3 MgSO4, 1.25 KH2PO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose, pH 7.35. Recordings were obtained in the presence of 10 μm AMPA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) (RBI, Köln, Germany) and 1 μm NMDA receptor antagonistdl-2-amino-4-methyl-5-phosphono-3-pentenoic acid (4-methyl-APPA) (Sigma, Deisenhofen, Germany). All drugs were bath-applied with the complete exchange of the external solution not exceeding 30 sec (Jarolimek and Misgeld, 1997). DA, forskolin, imipramine, and bicuculline were from Sigma; SKF38393, SCH23390, quinpirole, and tetrodotoxin (TTX) were from RBI; and sulpiride was from Serva Feinbiochemica (Heidelberg, Germany).
For pharmacological characterization of nondopaminergic and dopaminergic neurons, a pipette solution was used containing (in mm): 130 KCl, 10 NaCl, 0.25 CaCl2, 2 MgCl2, 10 EGTA, 5 HEPES, 5 glucose, 5 Mg-ATP, pH 7.3; electrode resistance was 4–6 MΩ before seal formation. For characterization of the cells, Cs+-free pipettes were used to avoid blocking the action of the D2 receptor agonist quinpirole. For all other measurements, recording pipettes were filled with (in mm): 130 CsCl, 10 NaCl, 0.25 CaCl2, 2 MgCl2, 5 EGTA, 10 HEPES, 10 glucose, 2 Mg-ATP, pH 7.3 (4–6 MΩ). The discrimination of dopaminergic from nondopaminergic neurons on the basis of the sag in the membrane potential (see below) was still possible with CsCl-filled electrodes.
Whole-cell patch-clamp recordings were performed with a discontinuous single-electrode voltage-clamp amplifier (npi; Tamm, Germany). Neurons were approached in the bridge mode (pipette resistance compensated) with patch pipettes under visual control with positive pressure (Stuart et al., 1993). The holding current was set to −45 nA before negative pressure was applied until a tight seal was formed (>1 GΩ). After the holding current was reduced, negative pressure was applied until the whole-cell configuration was achieved (holding current set to zero). Immediately after breaking through, resting potentials of the neurons were in the range of −60 to −65 mV. After characterization, we voltage-clamped the neurons at −80 mV to minimize membrane depolarization by Cs+, which could have induced excessive Ca2+ influx through action potential firing. Holding currents were usually ∼100 pA, and cells requiring >150 pA were discarded. No correction for liquid junction potential changes was made (2 mV for K+ and 3 mV for Cs+ internal solution). Input resistance (>130 MΩ) and access resistance (<15 MΩ) were determined by analyzing the steady-state and transient responses to voltage commands, respectively. To ascertain that no major changes in the access resistance had occurred during the recordings, a 3 mV, 10 msec pulse was used before a minimal IPSC was evoked. Cells in which the capacitive transient was reduced by >10% were discarded. Synaptic currents were filtered at 1.3 kHz with a four-pole Bessel filter, sampled between 2 and 10 kHz using pClamp software (Axon Instruments, Foster City, CA), and stored on a DAT recorder.
Measurements of IPSCs. Pharmacological effects on IPSCs evoked by minimal stimulation, on spontaneous IPSCs, and on IPSCs resistant to TTX and insensitive to Cd2+ (mIPSCs) were investigated. IPSCs were evoked by minimal stimulation through a patch pipette filled with extracellular saline (100–200 μsec, 0.1 Hz). The stimulation pipette was positioned within a 100 μm distance from the recorded cell within the SNR, laterally or medially to the recorded cell outside the cerebral peduncle. Stimulus intensities were selected to elicit all-or-none IPSCs (Edwards et al., 1990; Lambert and Wilson, 1993; Radnikow et al., 1997) (see Fig. 2A). A 20–50% proportion of failures was considered acceptable to classify evoked IPSCs as all-or-none events. Changes in amplitudes and latencies of stimulation-evoked IPSCs were analyzed. Averages of evoked IPSCs included events and failures unless stated otherwise. Latencies were measured from onset of stimulus to onset of evoked IPSCs. The paired Student’s t test was used to determine significant changes. Data from several cells are given as mean ± SEM. mIPSCs were recorded in the presence of TTX (0.3 μm) and Cd2+ (20–100 μm). For analysis, spontaneous IPSCs and mIPSCs were detected by a program written in our laboratory (Jarolimek and Misgeld, 1997). Drug effects were calculated as changes in the frequency of spontaneous and mIPSCs, respectively. The frequency was determined from the number of events within 60–120 sec epochs for control and different pharmacological conditions. Cumulative amplitude and frequency distributions were compared with the Kolmogoroff–Smirnoff test. Two distributions were considered to be significantly different when p < 0.01.
Fig. 2.
GABAA IPSCs evoked by minimal stimulation and spontaneously occurring IPSCs in the presence of the ionotropic glutamate receptor antagonists DNQX (10 μm) and 4-methyl-APPA (1 μm). A, Selected single traces of IPSCs evoked by minimal stimulation. Evoked IPSCs were all-or-none responses, with amplitude fluctuations at constant stimulus strength and events alternating with failures. B, Minimal IPSC amplitudes (mean ± SD; for nonfailures at the selected stimulus strength) plotted versus stimulus intensity. Stimulus intensities given in relative units (corresponding to a current range from 3.6 to 5.4 μA). An increase in the stimulus intensity decreased the number of failures but did not increase the amplitudes of evoked IPSCs. The number of failures are given in parentheses.C, GABAA receptor-mediated IPSCs evoked by minimal stimulation were blocked by the GABAA receptor antagonist bicuculline (BIC; 10 μm). Spontaneous IPSCs that persisted after blockade of the evoked IPSC disappeared later on. Twelve responses were averaged in each condition.D, Spontaneously occurring IPSCs were blocked by bicuculline (bottom trace).
Nondopaminergic neurons were separated from dopaminergic neurons electrophysiologically by injection of depolarizing and hyperpolarizing current in the bridge mode. For testing, all cells were hyperpolarized stepwise from −65 mV maximally to −110 to −120 mV. The degree of sag of the membrane potential back toward the resting potential during hyperpolarization was expressed as a ratio of the steady-state versus peak voltage during a hyperpolarizing current pulse.
For dye injections, internal solutions containing Lucifer yellow were prepared by dissolving 4 mg of powder (lithium salt; Sigma) in 40 μl of 100 mm LiCl and adding this to 1 ml of internal solution. At the end of the recording, the pipettes were withdrawn from the neurons to form outside-out patches, and the slices were immediately fixed in cold 4% paraformaldehyde in PBS (0.1m, pH 7.4) overnight. Slices were then dehydrated through graded alcohol, cleared in xylene, embedded, and photographed.
RESULTS
Identification of nondopaminergic neurons in the SNR
Recordings were made from visually identified neurons in the SNR (Fig. 1A–C). Nondopaminergic neurons were characterized by their electrophysiological properties. These included little or no sag of the membrane potential back toward the resting membrane potential during hyperpolarization (ratio of steady-state to peak sag, 0.98 ± 0.01; average ± SEM; n = 10) (Häusser et al., 1995) and brief action potentials (2.1 ± 0.2 msec as measured at their base; n = 4) (Fig.1D1, K+-filled electrodes). In contrast, another albeit small population of SNR neurons displayed a prominent sag in the membrane potential (Lacey et al., 1987, 1989;Häusser et al., 1995; Mercuri et al., 1995) (average sag ratio of 0.56 ± 0.03; n = 5) and broad action potentials (4.2 ± 0.8 msec; n = 3) (Fig.1D2, K+-filled electrodes). Neurons with the latter membrane properties were hyperpolarized by application of the D2 receptor agonist quinpirole (1 μm; n = 5), whereas the large group of neurons was not responsive to quinpirole or DA (n = 9). These characteristics identify the large group of neurons as nondopaminergic neurons (Häusser et al., 1995) and the small group as DA neurons of the SNR (Nakanishi et al., 1987). All further data reported in this study were obtained from nondopaminergic neurons recorded with Cs+-filled electrodes except where specifically indicated. Nondopaminergic neurons could then be separated from dopaminergic neurons only by the presence or absence of time-dependent hyperpolarization-activated inward rectification. With Cs+-filled electrodes, the measured ratio of steady-state to peak sag for nondopaminergic neurons was 0.98 ± 0.01 (n = 36); for dopaminergic neurons it was 0.6 ± 0.04 SEM (n = 5). These values correspond exactly to the values measured with K+-filled electrodes.
Potentiation of evoked IPSCs by D1receptor stimulation
IPSCs were recorded in the presence of AMPA (DNQX; 10 μm) and NMDA (4-methyl-APPA; 1 μm) receptor antagonists to prevent fast synaptic excitation. We have used minimal stimulation, a method for activating only one or a few presynaptic fibers to evoke all-or-none IPSCs (Fig.2A,B). The responses were blocked on application of TTX (0.3 μm;n = 6). Also, evoked synaptic responses were abolished by bicuculline (10–20 μm; n = 3) (Fig.2C) as is characteristic for GABAAreceptor-mediated IPSCs. In the same cells, spontaneous synaptic currents were blocked by bicuculline (Fig.2D).
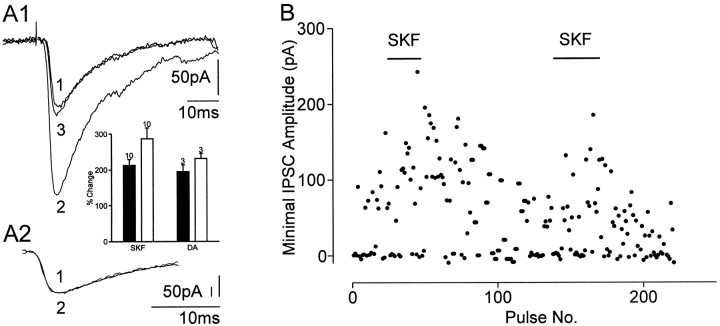
The D1 agonist SKF38393 (3 μm) (Fig.3) or DA (10 μm; in the presence of 1 μm D2 receptor antagonist sulpiride) increased the amplitudes of evoked IPSCs in all neurons (Table 1, Fig. 3A1) within a 3 min application time. Rise and decay time of the IPSCs, however, remained unchanged (Fig. 3A2). The potentiation of averaged IPSC amplitudes was associated with a shortening of the mean latency (Table 1), whereas the latency range (maximum–minimum) at which IPSCs were evoked in a single cell was not changed (control: maximum = 4.0 msec, minimum = 2.5 msec; D1 agonist: maximum = 3.5 msec, minimum = 1.9 msec). A reduction in failure rate that was observed in 12 of 13 cells contributed to the strong potentiation of the evoked IPSCs (Fig. 3, A, inset, andB). Wash-out of agonists partially reversed the potentiation. No change in holding currents or input resistances was observed. The D1 antagonist SCH23390 (0.1–1 μm; n = 3) blocked the agonist-induced potentiation of the IPSCs. Because Cameron and Williams (1993) reported that D1 agonists facilitated GABAB IPSPs but not GABAA IPSPs in dopaminergic neurons, we tested the effects of SKF38393 on IPSCs evoked by minimal stimulation in dopaminergic neurons in SNC. In four of five neurons there was no effect; in the remaining neuron SKF38393 increased the amplitude of the IPSC (from 41 to 159 pA) and reduced its latency (from 3.4 to 2.8 msec).
Fig. 3.
Effect of the D1 receptor agonist SKF38393 on GABAA IPSCs evoked by minimal stimulation.A, Superimposed IPSCs in control (trace 1), in the presence of SKF (3 μm; 5 min;trace 2) and after wash (15 min; trace 3). A1, SKF38393 increased the amplitude of the IPSC in a reversible manner, whereas latencies were reduced.A2, SKF38393 did not change rise time and decay of the IPSC; same traces as in A1 but with IPSCs superimposed and scaled to peak. Each trace represents the mean of 10–15 responses. Responses were averaged during the last 3 min in control or SKF38393, respectively. Inset, Bar graphs of the average amplitude changes of evoked IPSCs for SKF and DA when failures were excluded (black bars) or included (white bars) (mean ± SEM); the number of cells is indicated for each bar.B, Time course of change in the IPSC amplitude after application of SKF38393. In SKF38393, the IPSC amplitude increased and declined after returning to the control solution; SKF38393 was applied twice. Each point represents the amplitude of an IPSC evoked by minimal stimulation at a repetition rate of 0.1 Hz.
Table 1.
Modulation of evoked IPSCs (eIPSCs) and mIPSCs
| Agent | Amplitude (pA) | Latency (msec) | Frequency (Hz) | n | |||
|---|---|---|---|---|---|---|---|
| Control | Agent | Control | Agent | Control | Agent | ||
| eIPSCs | |||||||
| D1 agonists | 89.2 ± 12.3 | 193.9 ± 35.71-a | 3.2 ± 0.2 | 2.6 ± 0.11-a | 13 | ||
| Fors | 81.2 ± 32.1 | 273.6 ± 91.5 | 3.1 ± 0.2 | 2.6 ± 0.11-a | 4 | ||
| SCH | 113.5 ± 30.8 | 41.9 ± 10.9 | 3.2 ± 0.1* | 3.3 ± 0.2*1-a | 4 (3*) | ||
| mIPSCs | |||||||
| D1 agonists | 46.2 ± 10.6 | 45.7 ± 8.7 | 2.0 ± 2.0 | 6.0 ± 6.31-a | 9 | ||
| Fors | 39.7 ± 12.2 | 40.2 ± 8.6 | 1.4 ± 0.5 | 4.4 ± 1.41-a | 4 | ||
| SCH | 41.1 ± 11.4 | 45.9 ± 11.6 | 1.0 ± 0.8 | 1.0 ± 0.7 | 5 | ||
Data are given as mean ± SEM. mIPSCs were recorded in the presence of TTX (0.3 μm) and Cd2+ (20–100 μm). D1 agonists, SKF38393 (3–20 μm) and DA (10 μm + D2antagonist sulpiride 1 μm). Fors, Forskolin (5–20 μm). SCH, D1 antagonist SCH23390 (0.1–1 μm).
p < 0.05, two-tailed Student’s test.
* h = 3 for latency measurement.
Potentiation of mIPSCs by D1 receptor stimulation
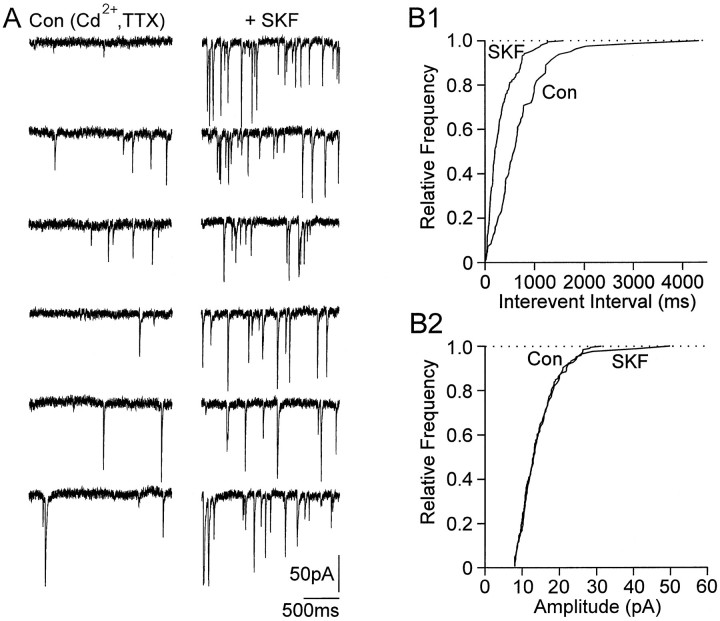
D1 receptor stimulation activates adenylyl cyclase in striatonigral neurons (Surmeier et al., 1995). A role for cAMP has been suggested as an intracellular messenger in the facilitation of mIPSCs (Capogna et al., 1995; Mitoma and Konishi, 1996; Trudeau et al., 1996;Jarolimek and Misgeld, 1997). We therefore investigated the effect of D1 receptor stimulation on constitutive release by measuring mIPSCs in the presence of TTX (0.3 μm) and the Ca2+ channel blocker Cd2+(20–100 μm). D1 receptor stimulation by bath application of SKF38393 (3 μm, n = 4; 20 μm, n = 2) or DA (10 μm, in the presence of 1μm sulpiride; n = 3) increased the frequency of mIPSCs in all nine cells tested, in seven of which the increase was significant (p < 0.01) (Fig. 4) (for mean values of frequency and amplitudes, see Table 1). The enhancement of the frequency was not accompanied by a change in amplitude, suggesting that the probability of release was enhanced.
Fig. 4.
Facilitating effect of SKF38393 on TTX-resistant and Cd2+-insensitive mIPSCs. A, mIPSCs were recorded in the presence of TTX (0.3 μm) and Cd2+ (100 μm). Six traces (2 sec each) are shown in Cd2+ and TTX and during application of SKF38393 (3 μm). B1, Cumulative frequency;B2, amplitude distributions (same cell as inA) before and after application of SKF38393. SKF38393 induced an increase in frequency of mIPSCs, whereas no change of the amplitude distribution was observed. The number of events used for cumulative distributions was 83 for control and 191 for SKF38393.
Spontaneous IPSCs were investigated in 15 cells, in 12 of which the amplitude of evoked IPSCs had been increased by D1 receptor stimulation (SKF, 3 μm, n = 9; DA, 10 μm, in the presence of 1 μm sulpiride,n = 3). In the three remaining cells, only spontaneous activity was recorded (SKF, 3 μm). In four of these 15 cells, an increase in the frequency of spontaneous IPSCs was observed. In two of these cells, statistical analysis revealed a significant increase (p < 0.01) accompanied by a small but insignificant increase in IPSC amplitudes. The inconsistency of D1 effect on spontaneous IPSCs in contrast to the consistent effect on mIPSCs may be explained by a variability in the contribution of action potential-dependent or -independent IPSCs to spontaneous IPSCs. TTX and Cd2+ reduced the frequency and amplitude of IPSCs (percentage change: −42.1 ± 9.9% for frequency, −28.2. ± 10.6% for amplitude; n= 6). The data suggest that a considerable number of D1receptor-insensitive GABA release sites contributed to spontaneous IPSCs.
To test whether stimulation of adenylyl cyclase indeed facilitates mIPSCs in substantia nigra pars reticulata neurons, we applied the adenylyl cyclase activator forskolin (5–20 μm). All effects observed after D1 receptor stimulation were also found after forskolin application. Amplitudes of evoked IPSCs were increased and latencies were reduced (Table 1). In all cells the frequency of spontaneous IPSCs was strongly increased. The frequency of mIPSCs was also increased (Table 1) in all cells, indicating an action of forskolin that is independent of Ca2+ influx.
Modulation of inhibition by endogenous DA
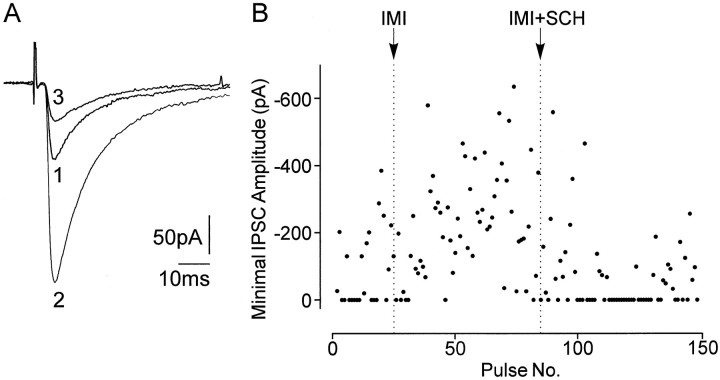
As shown above, measurements of evoked IPSCs provide a sensitive indicator for an enhancement of inhibition in SNR neurons by D1 receptor stimulation. Thus, we investigated whether inhibition of SNR neurons is potentiated by endogenous DA. DA may be released from the dendrites of DA neurons that are in close apposition to SNR neurons (Björklund and Lindvall, 1975; Fallon and Moore, 1978). The D1 receptor antagonist SCH23390 (0.1–1 μm) reduced the amplitude of evoked IPSCs (Table 1, Fig.5A) within 10 min of application (Fig. 5B) and increased the failure rate in three of four cells. SKF38393 or DA applied in the presence of SCH23390 was without effect in three cells (Fig. 5B). The reduction of IPSCs by SCH23390 might indicate a tonic stimulation of D1 receptors by endogenous DA. To exclude a nonspecific effect of the D1 receptor antagonist on IPSCs, we tested its effect on mIPSCs. In the presence of TTX and Cd2+, DA should not be tonically released (Rice et al., 1997). Indeed there was no effect of the D1 receptor antagonist on mIPSCs (n = 5), neither on their frequency nor on their amplitudes (Table 1). Further support for the idea of a tonic stimulation of D1 receptors in slices came from experiments in which DA reuptake was inhibited by the monoamine uptake inhibitor imipramine. Within 10 min of application, imipramine (1 μm) caused an increase in the amplitude of evoked IPSCs (from 72.3 ± 12.3 pA to 161.2 ± 27.8 pA;n = 4) (Fig.6A), accompanied by a decrease in number of failures in all cells and a reduction of the mean latency (from 3.1 ± 0.3 msec to 2.7 ± 0.3 msec). In all cells, the potentiation of evoked IPSCs was antagonized by SCH23390 (0.1 μm), which could even reduce the amplitude of evoked IPSCs below control (Fig. 6B).
Fig. 5.
Effect of the D1 receptor antagonist SCH23390 on GABAA IPSCs evoked by minimal stimulation.A, Superimposed IPSCs in control (trace 1) and after application of SCH23390 (1 μm;SCH, 15 min; trace 2). The D1antagonist strongly reduced the amplitude of the GABAAIPSCs. Each trace represents the average of 10–15 traces.B, Time course of the change in the amplitude of IPSCs on application of the D1 antagonist SCH23390 and after coapplication of SCH23390 with the D1 agonist SKF38393 (3 μm; SCH+SKF). The D1antagonist SCH23390 strongly reduced the IPSC amplitudes and completely blocked the facilitating effects of the D1 agonist SKF38393. Each point represents the amplitude of an IPSC evoked by minimal stimulation at a repetition rate of 0.1 Hz.
Fig. 6.
Effect of the monoamine uptake inhibitor imipramine on IPSCs evoked by minimal stimulation. A, Superimposed IPSCs in control (trace 1), in the presence of imipramine (1 μm; trace 2), and in the combined presence of imipramine (1 μm) and SCH23390 (0.1 μm; trace 3). Each trace represents the average of 10–15 traces. B, Time course of change in the IPSC amplitude during perfusion with imipramine (IMI) and imipramine together with SCH23390 (IMI+SCH). The amplitude of evoked IPSCs was increased by imipramine associated with a decrease in failure number. Subsequent application of SCH23390 decreased the amplitude of the IPSCs below control level, whereas the failure rate increased, indicating a tonic effect of endogenous DA on GABA release. Each point represents the amplitude of an IPSC evoked by minimal stimulation at a repetition rate of 0.1 Hz.
DISCUSSION
D1 receptors enhance GABAA synaptic currents in nondopaminergic midbrain neurons
Activation of D1 receptors stimulates the release of GABA from slices of the basal ganglia, including the SNR (Floran et al., 1990). For DA neurons in the ventral tegmental area it has been suggested that GABAB receptor-mediated neurotransmission is selectively facilitated by D1 receptor-mediated enhancement of release in the midbrain (Cameron and Williams, 1993). Here, we report that facilitation of GABA release by D1 receptor activation enhances GABAA IPSCs in nondopaminergic, presumably GABAergic (Oertel et al., 1982; Richards et al., 1997), neurons of the SNR. IPSCs evoked by minimal stimulation were potentiated and their latencies decreased. The frequency of mIPSCs recorded in the presence of TTX and Cd2+ was increased. In substantia nigra, D1 receptors are selectively localized on terminals of GABA-containing afferents (Barone et al., 1987; Harrison et al., 1990; Mansour et al., 1991; Cameron and Williams, 1993; Jaber et al., 1996). These afferents innervate both dopaminergic and nondopaminergic neurons in the midbrain (Nakanishi et al., 1987; Stanford and Lacey, 1996). The selective distribution of D1 receptors and the enhancement of GABA release through D1 receptors can explain an enhanced GABAergic inhibition in dopaminergic and nondopaminergic neurons. However, it is not clear why only GABAB inhibition was found to be enhanced in dopaminergic neurons (Cameron and Williams, 1993). It is possible that GABAB receptors are selectively present at synapses formed by forebrain GABAergic afferents with dopaminergic neurons (Johnson et al., 1992; Cameron and Williams, 1993;Stanford and Lacey, 1996). That GABAA IPSCs are not facilitated in dopaminergic neurons in SNC is supported by our preliminary findings, but requires further study.
An enhancement of the frequency of mIPSCs was to be expected from the likely mechanism of action of D1 receptors. In all cell lines tested to date, D1 receptors stimulate the formation of cAMP in response to agonists (Jaber et al., 1996). Pharmacological tools used in our experiments, e.g., application of a selective D1 agonist, application of DA in the presence of a D2 receptor antagonist, and the inhibition of their effects by a selective D1 antagonist, unanimously point to the involvement of a D1 receptor in the effects observed in our study. All effects of D1 receptor stimulation on evoked and mIPSCs in the midbrain could be mimicked by forskolin, which is known to activate adenylyl cyclase. The enhancement of inhibitory synaptic transmission is in line with previous studies at hippocampal (Capogna et al., 1995; Trudeau et al., 1996; Jarolimek and Misgeld, 1997) and cerebellar (Llano and Gerschenfeld, 1993; Mitoma and Konishi, 1996) synapses but contrasts with a study on magnocellular neurons in rat basal forebrain nuclei in which forskolin and D1 receptor agonists were found to reduce GABAA IPSCs (Momiyama and Sim, 1996).
A consistent finding was the decrease in latencies of evoked IPSCs after D1 receptor stimulation as well as the application of forskolin. Unlike our other findings, this finding is not easily explained by an increase in the probability of transmitter release. The variability of latencies with which IPSCs were evoked in a single cell may arise from different lengths of branches originating from the stimulated axon that form synapses with the target neuron (Mody et al., 1994). A reduction in mean latency could result if synapses of short branches were activated more reliably in the presence of D1receptor agonists. The fact that the range of latencies also was reduced in comparison to controls renders this explanation unlikely. An alternative explanation is the involvement of another mechanism in addition to the increase in probability of GABA release such as the activation of L-type Ca2+ currents that has been described for the somata of neostriatal medium spiny neurons (Hernández-López et al., 1997). If present at GABAergic terminals, activation of L-type Ca2+ channels could promote spike propagation in axonal terminals and Ca2+ influx.
D1 receptors stimulate GABA release from forebrain afferents
D1 receptor activation strongly enhanced stimulation-evoked IPSCs and the frequency of mIPSCs. In contrast, there was no consistent effect on spontaneous action potential-dependent IPSCs. A likely explanation is that spontaneous IPSCs involve a significant number of D1-insensitive GABA release sites. Indeed, spontaneous action potential-dependent IPSCs are thought to originate from local neurons (Nakanishi et al., 1987;Stanford and Lacey, 1996) that do not carry D1 receptors (see introductory remarks). To our knowledge, mIPSCs have not been recorded from SNR neurons to date. Stanford and Lacey (1996) report that only 30% of SNR neurons display spontaneous IPSCs and that these IPSCs are all blocked by TTX. In contrast, we found spontaneous IPSCs in all cells and a proportion of IPSCs persisted in TTX and Cd2+. The difference is likely to be attributable to the fact that we recorded IPSCs under conditions of a large driving force for chloride ions. There is no obvious reason to assume that mIPSCs originate only from collaterals of local inhibitory neurons. For spontaneous action potential-dependent IPSCs, but not for mIPSCs, it may be necessary to preserve GABA-containing cells intact in the slice preparation.
Dendritically released DA tonically modulates inhibition of output neurons
Besides acting in the striatum, DA may modulate the striatonigral pathway via direct influences in substantia nigra, because release of DA occurs not only from nerve terminals but also from the dendrites of nigrostriatal DA neurons (Geffen et al., 1976; Korf et al., 1976;Cheramy et al., 1981). Our data indicate that inhibitory synaptic transmission is indeed potentiated by endogenous DA acting at D1 receptors in substantia nigra. IPSCs evoked by minimal stimulation were reduced by the D1 receptor antagonist SCH23390, possibly indicating a tonic stimulation of D1receptors by endogenous DA. Nonspecific effects of the D1antagonist on GABAA IPSCs can be excluded because no effect on mIPSCs was observed. mIPSCs were recorded under conditions (TTX and Cd2+) under which DA release should not occur (Rice et al., 1997). Further evidence for endogenous DA acting tonically at D1 receptors on GABAergic afferents to SNR neurons was obtained by blocking the uptake of DA by imipramine. In the concentration used here, it is likely to affect the norepinephrine (Cragg et al., 1997) and the dopamine transporter. In the presence of the monoamine uptake inhibitor imipramine, the amplitude of evoked IPSCs was increased and the failure rate was decreased. Subsequent application of the D1 antagonist SCH23390 reduced IPSC amplitudes even below control values.
CONCLUSIONS
The direct and indirect striatonigral pathways are the primary substrates through which DA exerts effects on basal ganglia outflow. The GABAergic striatonigral terminals of the direct pathway inhibit GABAergic output neurons in SNR and in the internal segment of the globus pallidus. Dopaminergic neurons of SNC excite GABAergic neurons in the striatum via D1 receptors (Hernández-López et al., 1997), resulting in an increase of GABAergic output to both nuclei. Potentiation of GABAergic transmission by activation of presynaptic D1 receptors in substantia nigra intensifies the inhibition of SNR output neurons and provides functional significance to the close apposition of these neurons to DA-containing dendrites.
Footnotes
This study was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie BMBF (01 KI 9001/26-2e). The participation of Dr. J. F. X. O’Callaghan in the initial experiments of this study is acknowledged. We thank Dr. D. Feldmeyer for helpful discussions. The technical support of A. Lewen and C. Heuser is highly appreciated.
Correspondence should be addressed to Dr. Ulrich Misgeld, I. Physiologisches Institut der Universität Heidelberg, Im Neuenheimer Feld 326, D-69120 Heidelberg, Germany.
REFERENCES
- 1.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 2.Barone P, Tucci I, Parashos SA, Chase TN. D-1 dopamine receptor changes after striatal quinolinic acid lesion. Eur J Pharmacol. 1987;138:141–145. doi: 10.1016/0014-2999(87)90351-7. [DOI] [PubMed] [Google Scholar]
- 3.Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- 4.Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- 5.Capogna M, Gähwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 7.Cragg SJ, Rice ME, Greenfield SA. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:863–873. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- 8.Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand [Suppl] 1964;232:1–55. [PubMed] [Google Scholar]
- 9.Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol (Lond) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 11.Floran B, Aceves J, Sierra A, Martinez-Fong D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett. 1990;116:136–140. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- 12.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 13.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 14.Graybiel AM, Ragsdale CW. Biochemical anatomy of the striatum. In: Emson PC, editor. Chemical neuroanatomy. Raven; New York: 1983. pp. 427–504. [Google Scholar]
- 15.Harrison MB, Wiley RG, Wooten GF. Selective localization of striatal D1 receptors to striatonigral neurons. Brain Res. 1990;528:317–322. doi: 10.1016/0006-8993(90)91674-6. [DOI] [PubMed] [Google Scholar]
- 16.Häusser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 19.Jarolimek W, Misgeld U. GABAB receptor-mediated inhibition of tetrodotoxin-resistant GABA release in rodent hippocampal CA1 pyramidal cells. J Neurosci. 1997;17:1025–1032. doi: 10.1523/JNEUROSCI.17-03-01025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SW, Mercuri NB, North RA. 5-Hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- 22.Korf J, Zieleman M, Westerink BHC. Dopamine release in substantia nigra? Nature. 1976;260:257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- 23.Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol (Lond) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert NA, Wilson WA. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993;11:1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- 26.Llano I, Gerschenfeld HM. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol (Lond) 1993;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour A, Meador-Woodruff JH, Zhou Q-Y, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1991;45:359–371. doi: 10.1016/0306-4522(91)90233-e. [DOI] [PubMed] [Google Scholar]
- 28.Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 29.Mitoma H, Konishi S. Long-lasting facilitation of inhibitory transmission by monoaminergic and cAMP-dependent mechanism in rat cerebellar GABAergic synapses. Neurosci Lett. 1996;217:141–144. [PubMed] [Google Scholar]
- 30.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 31.Momiyama T, Sim JA. Modulation of inhibitory transmission by dopamine in rat basal forebrain nuclei: activation of presynaptic D1-like dopaminergic receptors. J Neurosci. 1996;16:7505–7512. doi: 10.1523/JNEUROSCI.16-23-07505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore RY, Bhatnagar RK, Heller A. Anatomical and chemical studies of a nigro-neostriatal projection in the cat. Brain Res. 1971;30:119–135. doi: 10.1016/0006-8993(71)90009-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- 34.Oertel WH, Tappaz ML, Berod A, Mugnaini E. Two-color immunohistochmistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res Bull. 1982;9:463–474. doi: 10.1016/0361-9230(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 35.Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 36.Radnikow G, Rohrbacher J, Misgeld U. Heterogeneity in use-dependent depression of inhibitory postsynaptic potentials in the rat neostriatum in vitro. J Neurophysiol. 1997;77:427–434. doi: 10.1152/jn.1997.77.1.427. [DOI] [PubMed] [Google Scholar]
- 37.Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- 38.Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 39.Robledo P, Feger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Res. 1990;518:47–54. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- 40.Rouzaire-Dubois B, Hammond C, Hamon B, Feger J. Pharmacological blockade of the globus pallidus-induced inhibitory response of subthalamic cells in the rat. Brain Res. 1980;200:321–329. doi: 10.1016/0006-8993(80)90923-3. [DOI] [PubMed] [Google Scholar]
- 41.Smith Y, Parent A. Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res. 1988;453:353–356. doi: 10.1016/0006-8993(88)90177-1. [DOI] [PubMed] [Google Scholar]
- 42.Somogyi P, Hodgson AJ, Smith AD. An approach to tracing neuron networks in the cerebral cortex and the basal ganglia. Combination of Golgi staining, retrograde transport of horseradish peroxidase and anterograde degeneration of synaptic boutons in the same material. Neuroscience. 1979;4:1805–1852. doi: 10.1016/0306-4522(79)90059-9. [DOI] [PubMed] [Google Scholar]
- 43.Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- 45.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 46.Trudeau L-E, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 47.Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol Scand [Suppl] 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]