Abstract
Functional modifications of neuronal P/Q-type voltage-dependent Ca2+ channels expressed in Xenopusoocytes by oxidation were examined electrophysiologically. Oxidation by external H2O2 enhanced the whole-oocyte currents through the Ca2+ channels composed of the α1A, α2/δ, and β3 subunits at negative voltages (<0 mV) without markedly affecting the currents at more positive voltages. Single-channel analysis showed that oxidation accelerates the overall channel opening process. The effect of H2O2 to enhance the Ca2+channel activity did not require heterologous expression of the α2/δ subunit, and it was not mimicked by a cysteine-specific oxidizing agent. The results suggest that oxidative stress may regulate the activity of neuronal Ca2+ channels and that regulation by oxidation may be important in some clinical situations, such as in reperfusion injury after ischemic episodes.
Keywords: Ca2+ channel, voltage-dependent gating, oxidation, hydrogen peroxide, voltage clamp, oocyte
Voltage-dependent Ca2+ channels are involved in many cellular functions, including neurotransmitter release and excitation–contraction coupling. Molecular and biochemical studies indicate that these Ca2+ channels are composed of at least three major polypeptides: α1, α2/δ, and β subunits (Hofmann et al., 1994; De Waard et al., 1996). The α1 subunit contains four homologous domains, each containing six putative transmembrane segments, to form a central ion conduction pore. The α2/δ subunit is a glycosylated transmembrane protein with disulfide links (Ellis et al., 1988). The β subunit interacts with the domain I–II linker of the α1 subunit (Pragnell et al., 1994) and influences the expression level and some electrophysiological properties of the Ca2+ channel complex (Mori et al., 1991; Hullin et al., 1992; De Waard and Campbell, 1995). Multiple genes for the α1 and β subunits have been isolated, and different combinations of these subunits are thought to account for the diverse Ca2+ channels in native cells (Hofmann et al., 1994). For example, neuronal voltage-dependent P/Q-type Ca2+ channels implicated in neurotransmitter release in the brain (Llinás et al., 1992; Uchitel et al., 1992) are thought to be formed of one α1A, one α2/δ, and one of the β2, β3, or β4 subunit (Mori et al., 1991).
Functional properties of voltage-dependent Ca2+channels are regulated post-translationally by many factors. Phosphorylation by protein kinase A (PKA) or protein kinase C (PKC) has been shown to increase Ca2+ channel currents in a variety of cells (Sculptoreanu et al., 1993; Werz et al., 1993; Yang and Tsien, 1993; Zamponi et al., 1997). In heterologous expression systems, PKA phosphorylation of the α1 subunit increases the Ca2+ channel activity (Sculptoreanu et al., 1993). G-proteins also are known to influence the electrophysiological properties of voltage-gated Ca2+ channels (Hescheler and Schultz, 1993), and direct binding of G-proteins to the Ca2+ channel α1 subunits has been documented (Zhang et al., 1996; De Waard et al., 1997).
Oxidation of amino acids is another important regulatory mechanism of protein function. Amino acid oxidation can be induced by various free radicals, which play important roles in numerous physiological and pathological conditions, such as neurodegenerative diseases (Olanow and Arendash, 1994; Gorman et al., 1996; Hensley et al., 1996) and reperfusion injury after ischemic episodes (Babbs, 1988; Chan, 1996). Ion channel properties are regulated by oxidation in both native cells and heterologous expression systems (Ruppersberg et al., 1991;Chiamvimonvat et al., 1995; Duprat et al., 1995; Park et al., 1995;Stephens et al., 1996; Tokube et al., 1996; Ciorba et al., 1997;Taglialatela et al., 1997). Some second messenger cascades also could influence the effectors via oxidation. Cellular nitric oxide leads to the generation of several free radicals, such as superoxide, which in turn could induce amino acid oxidation (Dawson and Dawson, 1996; Xia et al., 1996). Despite the physiological importance of the Ca2+ channels, oxidation sensitivity of the cloned voltage-dependent Ca2+ channels has not been explored fully. Chiamvimonvat et al. (1995) showed that extracellular cysteine oxidation of the cardiac (α1C) Ca2+channel decreased the channel activity by decreasing the mean open time. Here we examined how oxidation affects properties of P/Q-type (α1A) neuronal Ca2+ channels expressed inXenopus oocytes. We found that oxidation by hydrogen peroxide (H2O2) enhances the Ca2+ channel current by accelerating the channel opening process and that heterologous expression of the α2/δ subunit is not essential for oxidation to increase the channel activity. The oxidation sensitivity of the P/Q-type Ca2+ channel may play important roles in how the nervous system responds to oxidative stress episodes.
MATERIALS AND METHODS
Channel expression in oocytes. The Ca2+ channels were expressed in Xenopusoocytes by RNA injection, using the protocol already described (Hoshi, 1995). The α1A cDNA (GenBank accession number X57477) was obtained from Dr. Y. Mori (National Institute of Physiological Sciences, Okazaki, Japan), the α2/δ cDNA (GenBank accession numberM86621) was obtained from Dr. T. Snutch (University of British Columbia, Vancouver, BC, Canada), the β2a subunit cDNA (GenBank accession number X622497) was obtained from Dr. V. Flockerzi (Universität Heidelberg, Heidelberg, Germany), and the β3 subunit cDNA (GenBank accession number M88751) was obtained from Dr. K. Campbell (The University of Iowa, Iowa City, IA). The α1A, α2/δ, β2a, and β3 cDNAs were linearized with XbaI,EcoRI, NotI, and XbaI, respectively, and the RNAs were synthesized by using SP6, T7, T7, and T7 RNA polymerases, respectively, using commercially available kits (Ambion, Austin, TX). The ShBΔ6-46:T449V (López-Barneo et al., 1993) cDNA was linearized with NdeI, and the RNA was synthesized with T7 RNA polymerase. Each oocyte was injected with 40 nl of the RNA mixture, as described in the figure legends. The oocytes were kept at 17°C in ND96 solution [containing (in mm) 96 NaCl, 15 KCl, 1 MgCl2, 1.8 CaCl2, 2.5 Na-pyruvate, and 5 HEPES (NaOH), pH 7.6, plus 1% penicillin/streptomycin]. Electrophysiological data typically were obtained 3–4 d after injection.
Electrophysiology. Whole-oocyte currents were recorded with a Warner 725C amplifier (Warner, Hamden, CT) at room temperature as described (Ciorba et al., 1997). The electrodes typically had an initial resistance of 0.5–1.0 MΩ when filled with 3 mKCl. The recording solution contained (in mm) 10 BaOH, 2 KCl, 0.1 EGTA, 80 NaOH, 1 niflumic acid, and 10 HEPES (MES), pH 7.2. The recording chamber (300 μl volume) was perfused continuously with the recording solution at ∼0.4 ml/min when the data were not digitized. Other solutions that were used are described in the legends. Fresh Ag/AgCl-treated ground electrodes and new glass micropipettes were used for each oocyte. The electrodes were immersed in the continuously perfusing bath for 5–10 min before insertion into oocytes to allow the junction potentials to equilibrate. Voltage drifts of both the voltage and current electrodes were recorded for each experiment. The results from oocytes with voltage drifts ≥ 5 mV were not included in the analysis. The magnitude or the direction of the voltage drift was not correlated with the size of the Ca2+channel current enhancement by oxidation (see Results).
The electrophysiological data were digitized with ITC-16 interfaces (Instrutech, Great Neck, NY), and the results were analyzed with Pulse/PulseFit (HEKA, Lambrecht, Germany), Igor Pro (WaveMetrics, Lake Oswego, OR), and DataDesk (DataDescriptions, Ithaca, NY) running on Apple Power Macintosh computers. Macroscopic linear capacitative and leakage currents were subtracted by using a modified P/nprotocol, as implemented in Pulse. Unless otherwise indicated, the holding voltage was −90 mV and the leak holding voltage was −105 mV. To construct macroscopic current–voltage (I–V) curves, we fit the current time courses with polynomials and estimated the peak current amplitudes from the polynomial fits. Statistical comparisons were made by using the data collected from the same batches of oocytes; they are represented as the mean ± SEM. Statistical significance was assumed at p = 0.05.
Single-channel data were obtained in the cell-attached configuration, using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). The patch-clamp output signal typically was low-pass-filtered through an eight-pole Bessel filter at 1400 Hz (Frequency Devices, Haverhill, MA). The patch pipette was filled with (in mm) 90 BaOH, 2 KCl, 0.1 EGTA, 10 NaOH, 1 niflumic acid, and 10 HEPES (MES), pH 7.2. The bath solution contained (in mm) 140 KCl, 11 EGTA, 2 MgCl2, and 10 HEPES (N-methyl-d-glucamine, NMG), pH 7.2. Capacitative and leak currents in the single-channel data were subtracted by using the data epochs without any opening as the templates. The single-channel records were idealized by using a customized routine implemented in Igor Pro that essentially emulates TAC (Bruxton, Seattle, WA).
Oxidizing solutions. Oxidizing conditions were established with H2O2 (Mallinckrodt, Phillipsburg, NJ) or 2,2′-dithio-bis(5-nitropyridine) (DTBNP; Sigma, St. Louis, MO) at the concentrations indicated in the legends. These solutions were prepared freshly immediately before use. Typically, the oocytes were treated with the Ba2+recording solution with an oxidizing agent for ∼2 min, and then the recording chamber was washed with the oxidant-free solution. The results presented are based on the data recorded before and after the oxidation treatment.
All procedures conformed to an animal use protocol approved by The University of Iowa Animal Care and Use Committee.
RESULTS
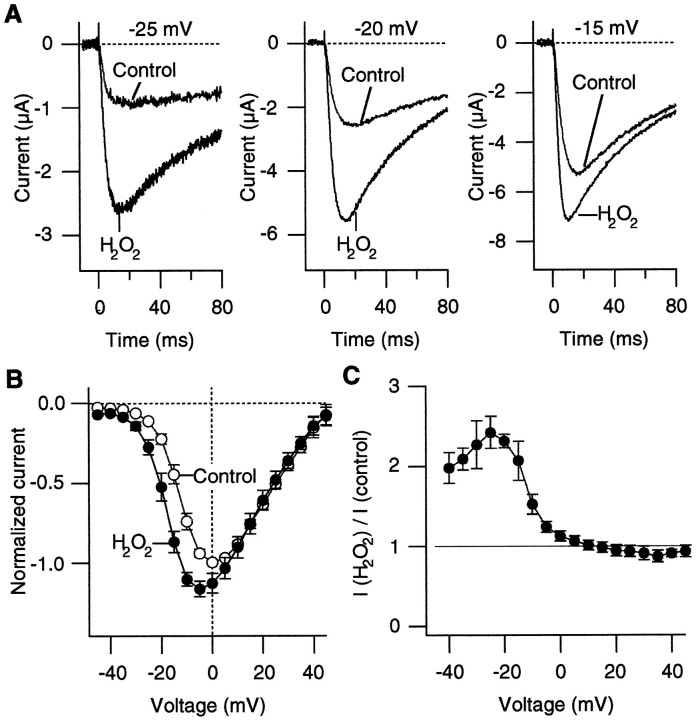
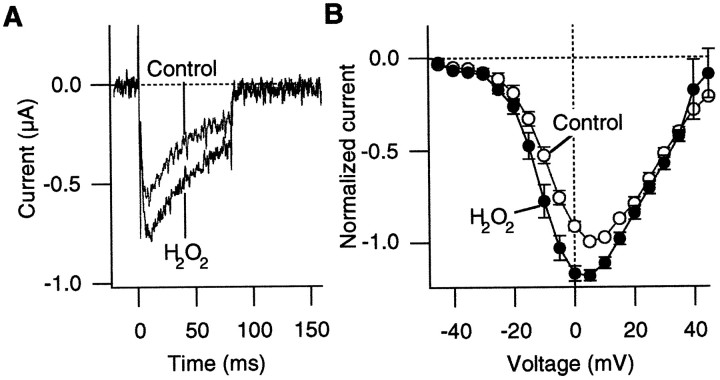
In response to depolarizing pulses, inward Ca2+channel currents were recorded from the oocytes injected with the α1A:α2/δ:β3 subunit RNAs (Fig.1A). The Ca2+ channel currents activated and inactivated rapidly on depolarization, as reported earlier (De Waard and Campbell, 1995). H2O2, which readily crosses the cell membrane, was used to examine the effects of oxidation on the Ca2+ channel properties. H2O2 has been shown to alter functional properties of a variety of ion channels (Ruppersberg et al., 1991;Duprat et al., 1995). Figure 1A compares the representative Ca2+ channel currents recorded from the α1A:α2/δ:β3 channels before and after oxidation by H2O2 (0.03%) at three different voltages. Oxidation by H2O2 markedly increased the Ca2+ channel current amplitudes. In many of the cells that were examined, the apparent inactivation time courses of the H2O2-enhanced currents at very negative voltages (from −30 to −20 mV) were often faster than those of the control currents (Fig. 1A; see below).
Fig. 1.
H2O2 enhances Ba2+ currents through the α1A:α2/δ:β3 channels. A, Representative Ba2+currents recorded at −25, −20, and −15 mV before and after treatment with H2O2 (0.03%). B, PeakI–V curves from five cells before (open circles) and after (filled circles) treatment with H2O2 (0.03%). The currents through the α1A:α2/δ:β3 channels were elicited as inA. The depolarizing pulses were applied every 5 sec. In each cell the current amplitudes were normalized to the maximum control inward current amplitude that was recorded. C, Voltage dependence of the relative increase in the current amplitude induced by H2O2 (0.03%). The ratios of the peak current amplitudes after H2O2 treatment over the control amplitudes at different voltages from five cells are shown.
In most of the oocytes that were examined, H2O2increased the Ca2+ channel currents at the concentrations of 0.01–0.03%. When we used 0.03% H2O2, the Ca2+channel currents typically started to increase within 1 min of H2O2 application, although the effect latency varied considerably among the oocytes examined. Washing the bath with H2O2-free solution for up to 10 min did not reverse the effect of H2O2 to enhance the Ca2+ channel current amplitude.
The effect of H2O2 to enhance the Ca2+ channel current amplitude was voltage-dependent. Figure 1B compares the peakI–V curves obtained before and after H2O2 treatment (0.03%). H2O2 increased the Ca2+channel current amplitudes at negative voltages most markedly (from −40 to 0 mV), and the effect was negligible at more positive voltages (greater than or equal to +10 mV) (Fig. 1C). H2O2 (0.03%) typically increased the peak current amplitude at −20 mV by 100%.
The extrapolated apparent reversal voltage of the Ca2+ channel current was not markedly affected by oxidation, suggesting that voltage drifts or changes in the Ca2+ channel selectivity are not responsible for the observation. For the results compiled in Figure 1, B andC, the Pearson product–moment correlation coefficient between the voltage drift and the fractional increase in the current amplitude at −15 mV was only 0.059, further showing that simple voltage drifts do not account for the enhanced Ca2+channel current activity by H2O2. Similar enhancements of the Ca2+ channel currents were also observed without leak subtraction.
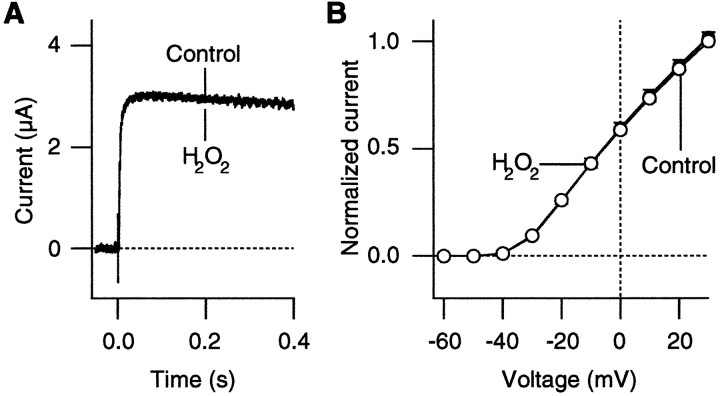
Because H2O2 might affect the surface potential in a nonspecific way, we performed control experiments with a voltage-dependent potassium channel. We selected the Shakerchannel mutant ShBΔ6-46:T449V (López-Barneo et al., 1993), because this mutant channel does not undergo rapid C-type inactivation and thus retains its function after the application of oxidizing agents (Schlief et al., 1996). Oxidation causes a rapid, irreversible destruction of the Shaker channels with strong C-type inactivation (Schlief et al., 1996). As shown in Figure2, H2O2 did not induce similar enhancements of the ionic currents through this voltage-dependent K+ channel. H2O2 (0.03%) affected neither the time course nor the I–V curve of the ShakerK+ currents. The results suggest that the enhancement of the Ba2+ currents by oxidation, shown in Figure 1, is specific to the expressed Ca2+channels and that voltage shifts, such as those induced by alterations in the membrane surface charge, are unlikely to be responsible for the observation.
Fig. 2.
H2O2 does not affect the K+ currents through Shaker channels.A, Representative ShBΔ6-46:T449V K+ currents recorded at −20 mV before and after treatment with H2O2 (0.03%). The bath solution contained (in mm) 130 NaCl, 10 KCl, 2 CaCl2, and 10 HEPES (NMG), pH 7.2. The currents were recorded 1 d after RNA injection. B, Normalized peak I–V curves from theShBΔ6-46:T449V channels from three cells recorded before (open circles) and after (filled circles) H2O2 treatment (0.03%). The currents were elicited as in A. In each cell the data were normalized to the control current value that was recorded at +30 mV.
Furthermore, the effect of H2O2 to enhance the inward currents in the oocytes injected with the Ca2+ channel subunit RNAs is not likely to be caused by the activation of endogenous inward currents or the reduction of the endogenous outward currents. We found that noninjected oocytes did not have any detectable time-dependent currents when we used the Ba2+ recording solution and that H2O2 (0.03%) did not affect their electrophysiological properties (data not shown). We also did not find any detectable inward currents in the oocytes injected with the α2/δ and β3 subunit RNAs without the α1A subunit RNA.
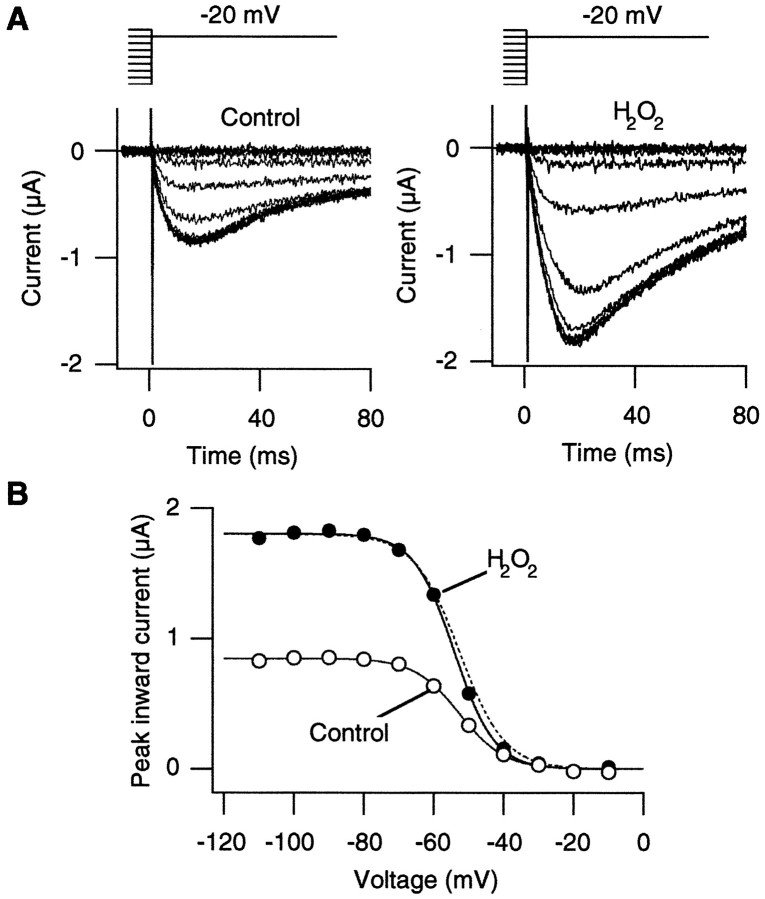
Several possible biophysical mechanisms exist to account for the observation that H2O2 enhances the Ca2+ channel currents. It is possible that H2O2 enhances the Ca2+channel current amplitudes by altering the voltage dependence of steady-state inactivation so that more channels are available to open on depolarization. We tested this hypothesis by comparing the prepulse voltage dependence of the Ca2+ channel currents before and after oxidation by H2O2. Oxidation by H2O2 enhanced the Ca2+channel currents recorded at −20 mV regardless of the prepulse voltage in the range from −100 to −50 mV (Fig.3A). Furthermore, the voltage dependence of prepulse inactivation was not markedly altered by H2O2 (Fig. 3B). The difference in the steepness of the prepulse inactivation curves before and after oxidation was not statistically significant in the oocytes that were examined (3.78 e0 and 3.71e0 in the control and H2O2 groups; paired t test;p = 0.40; n = 6). The midpoint of the prepulse inactivation curve was not affected statistically by H2O2 (−51 and −53 mV in the control and H2O2 groups; paired t test;p = 0.17; n = 6). These results show that H2O2 enhanced the Ca2+channel activity without altering the prepulse voltage dependence.
Fig. 3.
Oxidation by H2O2 does not change the prepulse voltage dependence of the α1A:α2/δ:β3 channels. A, Representative Ca2+channel currents recorded from the α1A:α2/δ:β3 channels at −20 mV before (left) and after (right) H2O2 (0.03%) treatment. The currents were recorded after 5 sec prepulses to the voltages from −110 to −10 mV in 10 mV increments every 30 sec. Voltage patterns are shown at thetop. B, The peak current amplitudes as elicited in A are plotted as a function of the prepulse voltage. The data were fit with Boltzmann functions. The equivalent charges and the half-maximum voltages for the data obtained before and after H2O2 (0.03%) treatment were 3.8e0, −53 mV and 4.3e0, −54 mV, respectively. Thedotted curve is a scaled Boltzmann fit for the control data to show that the H2O2 treatment did not markedly affect the voltage dependence. Similar results were obtained from five other cells.
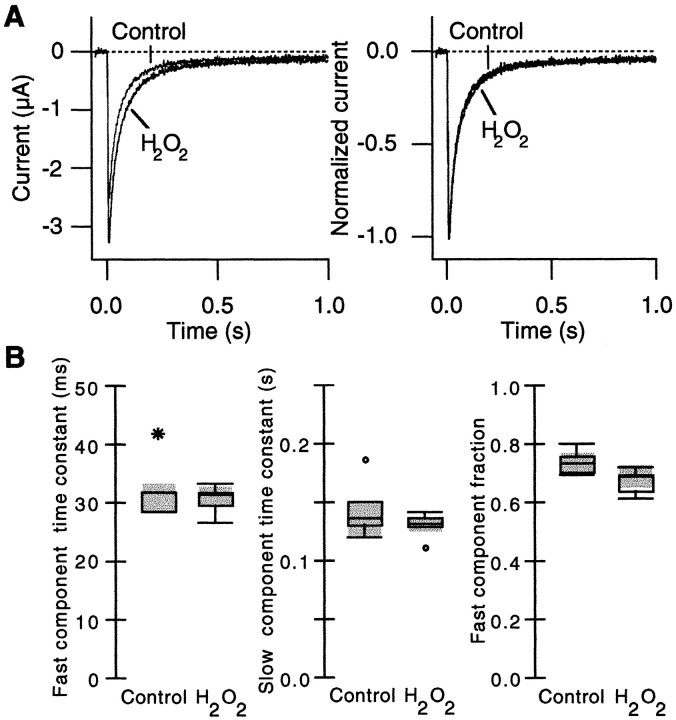
In many voltage-dependent ion channels, activation and inactivation are coupled at least partially so that slowed inactivation often enhances the peak current amplitude (Aldrich et al., 1983; Zagotta et al., 1989). For example, in A-type Shaker potassium channels the disruption of fast N-type inactivation by a deletion in the N terminus markedly increases the peak open channel probability (Hoshi et al., 1990). Kinetics of inactivation could be estimated from the time course of the macroscopic current decline only at the voltages for which the activation kinetics far exceeds the inactivation kinetics. We tested whether H2O2 enhances the Ca2+ channel current by slowing the inactivation time course. Inactivation time courses of the Ca2+channel currents were recorded at different voltages for which the activation process is expected to be faster than the inactivation process. The Ca2+ channel currents recorded at 0 mV in response to long pulses are shown in Figure4A. In the data that are shown, H2O2 enhanced the peak current amplitude by 30% at this voltage. The inactivation time course was well described by a sum of two exponentials at every voltage that was examined, and the inactivation parameters at 0 mV were compared by using box plots in Figure 4B. H2O2 did not affect the fast component time constant, the slow component time constant, or the relative fractions of the two inactivation components. Similar results were obtained by using the currents recorded at −15 and +15 mV. These results eliminate the possibility that slowed inactivation of the Ca2+channel is responsible for the enhanced peak current.
Fig. 4.
H2O2 does not alter the inactivation time course of the α1A:α2/δ:β3 channels.A, Representative Ca2+ channel currents recorded in response to 1 sec pulses to 0 mV before and after H2O2 (0.03%) treatment (left). The peak current increased by 30% after H2O2treatment at this voltage. The pulses were applied every 8 sec. The currents were scaled and are shown superimposed (right) to facilitate comparison. B, The inactivation time course at 0 mV was fit with a sum of two exponentials, and the inactivation parameters were compared by using box plots (Tukey, 1977).Left to Right, The fast component time constant, slow component time constant, and the relative fraction of the fast component are shown (n = 5).
The results presented so far suggest that H2O2enhances the α1A:α2/δ:β3 Ca2+ channel activity by accelerating the channel opening transition. We directly tested this possibility by examining the effects of H2O2 at the single-channel level. Representative openings of α1A:α2/δ:β3 Ca2+channels at −10 mV obtained before and after oxidation by H2O2 are shown in Figure5A. Consistent with the whole-oocyte results presented earlier, H2O2(0.03%) increased the peak open probability (Fig. 5C). Comparison of the first latency distributions (Fig. 5D) showed that oxidation by H2O2 markedly decreased the median first latency from 16 to 9 msec. The difference between the two first latency distributions was statistically significant (Kolmogorov–Smirnov two-sample test; p < 0.01). Oxidation by H2O2 decreased the median first latency by 42 ± 7% (n = 4). Neither the mean open time (Fig. 5D) nor the unitary current amplitude was markedly affected by oxidation by H2O2. The mean open times before and after the H2O2treatment were 0.46 and 0.50 msec, respectively (p = 0.103; paired t test;n = 4). The mean single-channel amplitudes before and after the H2O2 treatment were 0.99 and 0.98 pA, respectively (p = 0.319; paired ttest; n = 4). Thus, the single-channel results show that oxidation enhances the peak Ca2+ channel current amplitude in part by accelerating the overall channel opening transitions.
Fig. 5.
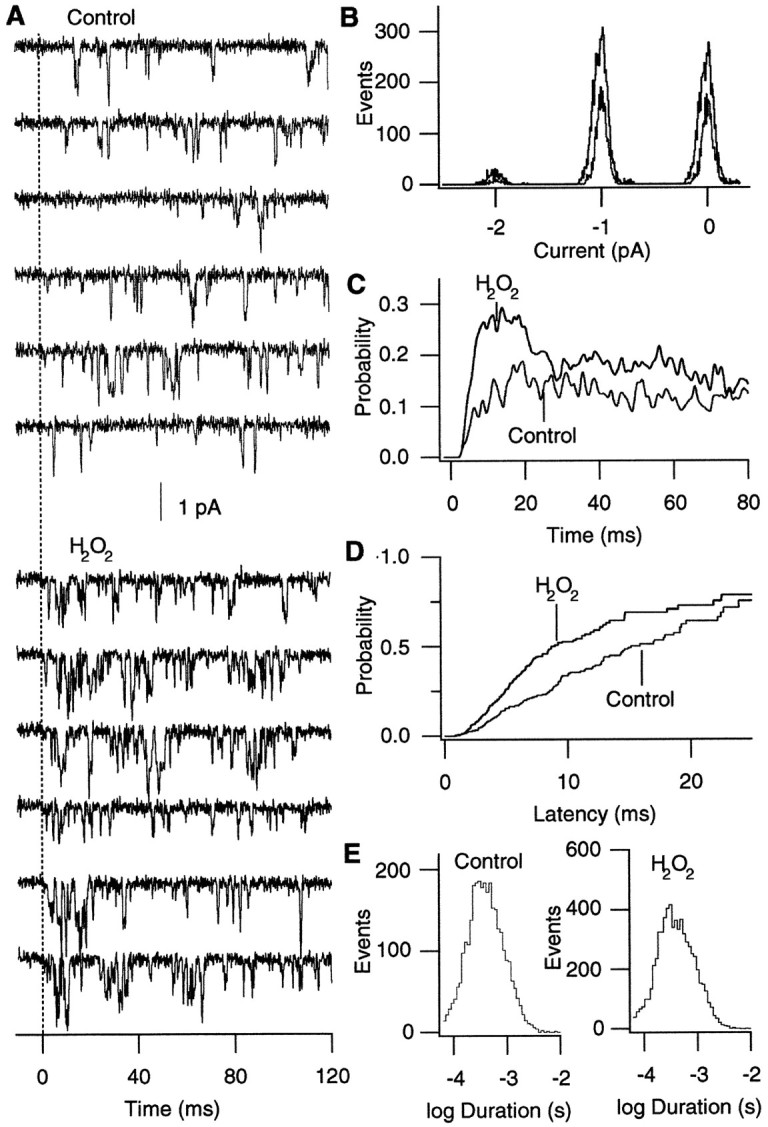
H2O2 accelerates the overall opening transition of the α1A:α2/δ:β3 channels.A, Representative openings of α1A:α2/δ:β3 channels before and after oxidation by H2O2(0.03%). The openings were elicited by pulses to −10 from −90 mV every 8 sec. H2O2 was applied to the bath. Consecutive data epochs are shown. B, Histograms of the amplitudes of the single-channel events before and after oxidation by H2O2. Amplitudes of the single-channel openings detected by the single-channel idealization program are plotted. The histograms generated from the openings recorded at −10 mV before and after H2O2 are shown superimposed. At each peak the smallest curve represents the control openings, and the other curve represents the data obtained after oxidation. C, Ensemble averages of the channel openings recorded as in A. The average sweeps were filtered further by using a Gaussian filter at 0.5 kHz. D, First latency distributions obtained before and after oxidation by H2O2 (0.03%). H2O2decreased the median first latency from 15 to 9 msec in the data that are shown. E, Open time histograms before and after oxidation by H2O2. The mean open times before and after H2O2 application were 0.50 and 0.51 msec, respectively. The distributions are corrected for the number of active channels. All of the data shown in this figure came from the same patch. Similar results were obtained in three other experiments.
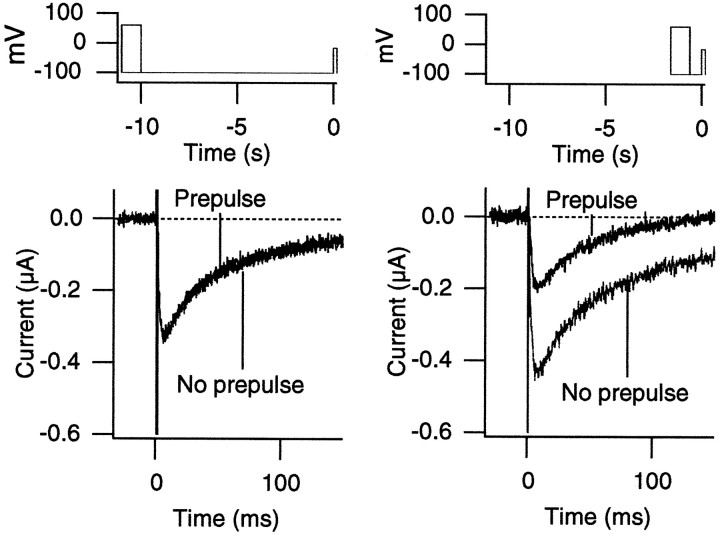
In many voltage-dependent Ca2+ channels, large conditioning depolarization enhances the current amplitude, and in some cases this voltage-dependent enhancement represents the voltage-dependent removal of the inhibitory effect of G-proteins (for review, see Dolphin, 1996). This voltage-dependent inhibition of the channels by G-proteins has been explained by using “willing” and “reluctant” Ca2+ channel types (Bean, 1989). We examined whether H2O2 enhances the Ca2+ channel activity by removing the interaction of the channel with the endogenous oocyte G-proteins. We recorded the α1A:α2/δ:β3 Ca2+ channel currents with a variety of voltage pulse protocols involving large conditioning prepulses. Representative Ca2+ channel currents recorded at −15 mV after 1 sec prepulses to +60 mV are shown in Figure6. In none of the protocols used were the Ca2+ channel currents after the conditioning pulses greater in amplitude than the currents recorded without the prepulses. Thus, these results suggest that oxidation by H2O2 does not mimic the effect of large conditioning depolarization prepulse.
Fig. 6.
H2O2 does not mimic the effects of conditioning depolarization. The currents through the α1A:α2/δ:β3 channels were recorded at −15 mV after 1 sec prepulses to +60 mV. The voltage protocols are shown at thetop. The interpulse intervals were 10 sec (left) and 600 msec (right). The voltage pulses were applied every 20 sec. Large conditioning prepulses failed to enhance the Ca2+ channel currents recorded at −15 mV. The holding voltage was −100 mV. With the 600 msec interpulse interval (right), the peak current is smaller because recovery from inactivation is slow when compared with the interpulse duration.
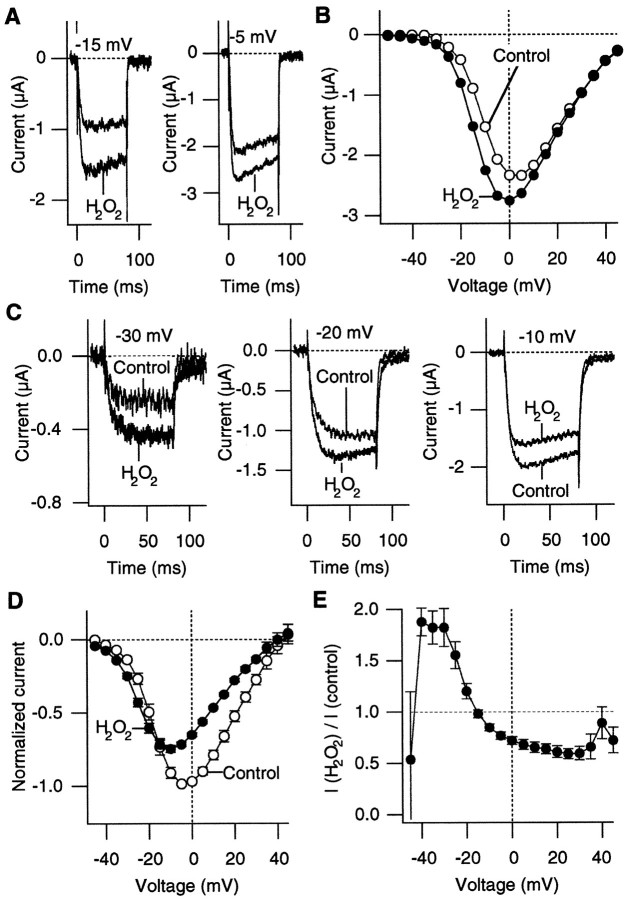
There are many potential targets of oxidation by H2O2 in the α1A:α2/δ:β3 Ca2+ channel complex. As the first step toward identifying the oxidation effector, the α1A and β3 subunit RNAs only, without the α2/δ subunit RNA, were injected into oocytes to test whether the α2/δ subunit is required for oxidation to enhance the Ca2+ channel currents. Representative Ca2+ channel currents recorded from the oocytes injected only with the α1A and β3 RNAs are shown in Figure7. The α2/δ subunit has been shown to increase the functional expression level of the α1A-containing channels (Mori et al., 1991; De Waard and Campbell, 1995). The inward currents recorded from oocytes injected with α1A and β3 RNAs were generally smaller than those from the oocytes injected with the α1A:α2/δ/β3 RNAs together, and the voltage for which the maximum inward current was observed was shifted slightly to more positive voltages. The application of H2O2increased the peak current amplitude of the Ca2+channel current recorded at −10 mV in a statistically significant manner (average increase, 46%; one-tailed paired t test;p = 0.021; statistical significance also was observed at −15, −10, −5, 0, +5, +10, and +15 mV; n = 4). As with the α1A:α2/δ:β3 channels, the effect of H2O2 to enhance the peak current amplitude was voltage-dependent (Fig. 7B). H2O2enhanced the Ca2+ channel activity at negative voltages (−15 to +15 mV), and it did not markedly affect the channel activity at more positive voltages (from +20 to +45 mV). These results suggest that heterologous expression of the α2/δ subunit may not be required for oxidation to enhance the Ca2+ channel current, although the effects of endogenous α2/δ subunits in oocytes cannot be ruled out. The α2/δ subunit may modulate the oxidation sensitivity of the Ca2+ channel complex, because the average increase in the current amplitude was smaller than that for the α1A:α2/δ:β3 channel.
Fig. 7.
H2O2 enhances the Ca2+ channel currents through the α1A:β3 channels without α2:δ. A, Representative Ca2+ channel currents through the α1A:β3 channels recorded at −10 mV before and after H2O2 (0.03%) treatment. B, PeakI–V curves from four cells before (open circles) and after (filled circles) H2O2 (0.03%) treatment. The currents through the α1A:β3 channels were recorded in response to 80 msec pulses every 8 sec.
Multiple genes for the β subunit exist, and coexpression of the α1A and α2/δ subunits with different β subunits results in Ca2+ channels with different activation and inactivation properties (Birnbaumer et al., 1994; De Waard and Campbell, 1995). We examined whether the effects of oxidation by H2O2 to enhance the Ca2+channel activity depended on which β subunit was coexpressed by recording from the α1A:α2/δ:β2a channels. As reported previously (Hullin et al., 1992; De Waard and Campbell, 1995), the inactivation time course of the α1A:α2/δ:β2a channel was slower than that of the α1A:α2/δ:β3 channel. At least in some of the cells that were examined (Fig.8A,B), H2O2 application clearly enhanced the currents through the α1A:α2/δ:β2a channels at negative voltages (<10 mV) without affecting the currents at more positive voltages (>10 mV), as observed with the α1A:α2/δ:β3 channels. In other cells, however, H2O2 had mixed effects on the α1A:α2/δ:β2a channels (Fig. 8C–E). H2O2 increased the Ca2+channel currents at negative voltages (−40 to −20 mV), but it decreased the currents at more positive voltages. The observation that at least some cells with the α1A:α2/δ:β2a channels respond to H2O2 in the same way as do the α1A:α2/δ:β3 cells suggests that the α1A subunit and/or the structural components shared by the β2a and β3 subunits are involved in the action of oxidation to enhance the Ca2+ channel current. It is also likely that there are multiple oxidation targets in the α1A:α2/δ:β2a channel, because H2O2 can increase or decrease the Ca2+ channel currents, depending on the voltage.
Fig. 8.
H2O2 has multiple effects on the α1A:α2/δ:β2a channels. A, Ca2+ channel currents from the α1A:α2/δ:β2a channels recorded at −15 and −5 mV before and after H2O2 (0.03%) treatment from one cell.B, Peak I–V curves before (open circles) and after (filled circles) H2O2 (0.03%) treatment from the α1A:α2/δ:β2a channels. The pulses were applied every 10 sec. The data are from the same cell as shown in A.C, Ca2+ channel currents from the α1A:α2/δ:β2a channels recorded at −30, −20, and −10 mV before and after H2O2 (0.03%) treatment, illustrating that H2O2 can increase or decrease the current amplitude depending on the membrane voltage. The pulses were applied every 8 sec. D, Peak I–Vcurves of the α1A:α2/δ:β2a channels from five cells before (open circles) and after (filled circles) H2O2 (0.03%) treatment.E, Voltage dependence of the H2O2 action on the α1A:α2/δ:β2a channels. The ratios of the peak inward current amplitudes after the H2O2 treatment over the control amplitudes from five cells are plotted.
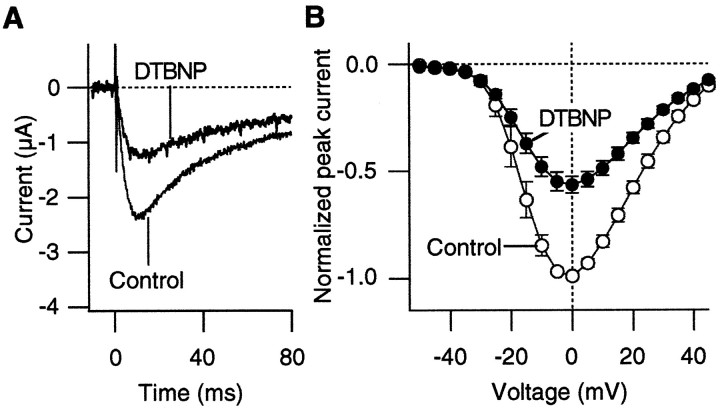
Unlike H2O2, which is a general oxidizing agent, DTBNP preferentially oxidizes cysteine (Islam et al., 1993; Stephens et al., 1996). DTBNP is a lipophilic agent that is expected to be membrane-permeable (Islam et al., 1993). We examined whether DTBNP mimics the effect of H2O2 to enhance the Ca2+ channel current amplitudes at negative voltages. Representative Ca2+ channel currents that were recorded before and after DTBNP (50 μm) application are shown in Figure9. DTBNP decreased the Ca2+ channel current amplitudes at all of the voltages that were examined. The results suggest that the effects of H2O2 and DTBNP may be mediated by the oxidation of different amino acid residues.
Fig. 9.
DTBNP, a lipophilic cysteine-specific oxidizing agent, decreases the Ca2+ channel currents through the α1A:α2/δ:β3 channels. A, Representative Ca2+ channel currents recorded at +15 mV before and after DTBNP (50 μm) treatment. The pulses were applied every 8 sec. B, Peak I–V curves of the α1A:α2/δ:β3 channels from five cells before (open circles) and after (filled circles) DTBNP (50 μm) treatment.
DISCUSSION
Oxidation is a fundamentally important physiological regulatory mechanism. The results presented in this study show that the P/Q-type Ca2+ channel activity is enhanced by oxidation, especially at negative voltages. The results suggest that this effect of oxidation to enhance the Ca2+ channel activity does not involve changes in the inactivation property or prepulse voltage dependence. Furthermore, it is not likely that the effect of oxidation requires the heterologously expressed α2/δ subunit.
Oxidation increases the peak Ca2+ channel current amplitudes through the α1A:α2/δ:β3 channels at the negative voltages where the peak open probability is not saturated (<+10 mV), suggesting that oxidation alters the gating transitions of the channel. The single-channel analysis shows that the faster channel opening induced by oxidation contributes to the enhanced peak open probability (see Fig. 5). The accelerated inactivation time course seen in some cells expressing the α1A:α2/δ:β3 channels (see Fig.1A) is consistent with this conclusion, because activation and inactivation are likely to be coupled.
Oxidizing agents such as H2O2 could alter properties of the Ca2+ channels expressed in oocytes in a variety of ways. Because H2O2 did not alter the Shaker K+ channels noticeably (see Fig. 2), it is unlikely that voltage drifts or shifts, which are expected to affect different voltage-dependent ion channels similarly, are responsible for the effect of oxidation to enhance the Ca2+ channel current. The oxidizing agents could act indirectly by lipid oxidation (Porter et al., 1995) or by oxidizing amino acids in the endogenous nonchannel proteins, which in turn could affect the expressed channels. Alternatively, the oxidizing agents could oxidize amino acid residues directly in the Ca2+ channel complex. The α1A:α2/δ:β3 Ca2+ channel is a large protein complex composed of ∼4000 amino acid residues. This large size makes it difficult to identify the amino acid residues that are oxidized to enhance the Ca2+ channel activity. Our experiments, however, indicate that the α2/δ subunit may not be necessary for oxidation to enhance the Ca2+ channel activity, suggesting that the disulfide links in the α2/δ subunit may not be involved. Because oxidation by H2O2 enhanced the currents through the α1A:α2/δ:β3 and α1A:α2/δ:β2a channels at the negative voltages, the structural elements shared by these two β subunits and/or the α1A subunit are likely to be involved. The results using the α1A:α2/δ:β2a channels also indicate that the β subunit may modulate the effect of H2O2(see Fig. 8).
Chiamvimonvat et al. (1995) showed that oxidation of the cardiac α1C Ca2+ channel by a cysteine-specific oxidation reagent decreased the macroscopic Ca2+ channel currents without affecting their kinetics or voltage dependence. They further showed that oxidation reduced the single-channel mean open time. The effect of oxidation to inhibit the Ca2+channel activity in the α1C channel observed by Chiamvimonvat et al. (1995) is similar to the results obtained in this study with the α1A:α2/δ:β3 channels with DTBNP (see Fig. 9), suggesting that the effect of oxidation to decrease the α1A:α2/δ:β3 Ca2+ channel current may be mediated by extracellular cysteine oxidation. It will be interesting to see whether the α1C-containing channels are enhanced by H2O2 as observed here with the α1A channels. These results, taken together, indicate that oxidizing agents have multiple effects on the Ca2+ channel activity, depending on the channel subunit composition and the membrane voltage. Considering that in vivo cell membrane voltage is not likely to be in a positive range for any extended periods of time, the current enhancing effect of oxidation at negative voltages (from −40 to 0 mV; see Fig. 1) may be more physiologically relevant.
H2O2 is a membrane-permeable oxidizing agent, and it is a precursor of many reactive free radicals (Halliwell, 1992). H2O2 has been implicated in neurodegenerative diseases such as Parkinson’s (Jenner and Olanow, 1996) and Alzheimer’s (Multhaup et al., 1997). Various oxidants are known to increase the cytoplasmic free Ca2+ concentration, although the exact mechanism for the increase is yet to be established (Suzuki et al., 1997). H2O2 has been shown to increase the intracellular Ca2+ level in rat neurons (Oyama et al., 1996). The results presented here indicate that the enhanced Ca2+ flux through the neuronal voltage-dependent Ca2+ channels could contribute to the oxidant-induced increase in the cytosolic Ca2+level.
The involvement of nitric oxide in the generation of free radicals has received increasing attention. Nitric oxide can react with superoxide to form peroxynitrite, which is a powerful oxidant implicated in cellular injury (Beckman et al., 1990). A recent study has shown that nitric oxide synthase also can generate superoxide directly at lowl-arginine concentrations, contributing to the formation of peroxynitrite (Xia et al., 1996). Peroxynitrite is capable of oxidizing methionine to regulate the Shaker channel inactivation (M. Ciorba, S. Heinemann, H. Weissbach, N. Brot, T. Hoshi, unpublished data). Thus, these free radicals, possibly involving nitric oxide, could oxidize the P/Q-type Ca2+ channels to enhance the Ca2+ influx. Excess free radicals are generated during reperfusion injury after ischemic attacks (Gress, 1994; Chan, 1996) and also during the course of neurodegeneration (Gorman et al., 1996; Hensley et al., 1996). Selective enhancement of P/Q-type Ca2+ channels, thought to be involved in neurotransmitter release in neurons of the CNS (Llinás et al., 1992), may serve important physiological functions in how the nervous system responds to oxidative stress.
Footnotes
This work was supported in part by the Human Frontier Science Program, McKnight Foundation, and National Institutes of Health Grant GM57654. We thank Dr. J. Thommandru and Ms. M. Masropour for technical assistance and S. Lover for noise cancellation ideas.
Correspondence should be addressed to Dr. Toshinori Hoshi, Department of Physiology and Biophysics, Bowen 5660, The University of Iowa, Iowa City, IA 52242.
REFERENCES
- 1.Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983;306:436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- 2.Babbs CF. Reperfusion injury of postischemic tissues. Ann Emerg Med. 1988;17:1148–1157. doi: 10.1016/s0196-0644(88)80060-x. [DOI] [PubMed] [Google Scholar]
- 3.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Chiamvimonvat N, O’Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res. 1995;76:325–334. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- 8.Ciorba M, Heinemann SH, Brot N, Weissbach H, Hoshi T. Regulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci USA. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 10.De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol (Lond) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Waard M, Gurnett CA, Campbell K. Structural and functional diversity of voltage-activated calcium channels. Ion Channels. 1996;4:41–87. doi: 10.1007/978-1-4899-1775-1_2. [DOI] [PubMed] [Google Scholar]
- 12.De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 13.Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends Neurosci. 1996;19:35–43. doi: 10.1016/0166-2236(96)81865-0. [DOI] [PubMed] [Google Scholar]
- 14.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci USA. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MH. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 16.Gorman AM, McGowan A, O’Neill C, Cotter T. Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci. 1996;139:45–52. doi: 10.1016/0022-510x(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 17.Gress DR. Stroke. Revolution in therapy. West J Med. 1994;161:288–291. [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 19.Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, Aksenova M, Wu JF, Carney JM. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid β peptide. Ann NY Acad Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 20.Hescheler J, Schultz G. G-proteins involved in the calcium channel signaling system. Curr Opin Neurobiol. 1993;3:360–367. doi: 10.1016/0959-4388(93)90129-m. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 22.Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 24.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, Flockerzi V. Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta, and brain. EMBO J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam MS, Berggren PO, Larsson O. Sulfhydryl oxidation induces rapid and reversible closure of the ATP-regulated K+ channel in the pancreatic β-cell. FEBS Lett. 1993;319:128–132. doi: 10.1016/0014-5793(93)80051-u. [DOI] [PubMed] [Google Scholar]
- 26.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 27.Llinás R, Sugimori M, Hillman DE, Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 28.López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- 29.Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 30.Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Beher D, Masters CL, Beyreuther K. Reactive oxygen species and Alzheimer’s disease. Biochem Pharmacol. 1997;54:533–539. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 31.Olanow CW, Arendash GW. Metals and free radicals in neurodegeneration. Curr Opin Neurol. 1994;7:548–558. doi: 10.1097/00019052-199412000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Oyama Y, Okazaki E, Chikahisa L, Nagano T, Sadakata C. Oxidative stress-induced increase in intracellular Ca2+ and Ca2+-induced increase in oxidative stress: an experimental model using dissociated rat brain neurons. Jpn J Pharmacol. 1996;72:381–385. doi: 10.1254/jjp.72.381. [DOI] [PubMed] [Google Scholar]
- 33.Park MK, Lee SH, Lee SJ, Ho WK, Earm YE. Different modulation of Ca-activated K channels by the intracellular redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflügers Arch. 1995;430:308–314. doi: 10.1007/BF00373904. [DOI] [PubMed] [Google Scholar]
- 34.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 35.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 36.Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991;352:711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- 37.Schlief T, Schonherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflügers Arch. 1996;431:483–493. doi: 10.1007/BF02191894. [DOI] [PubMed] [Google Scholar]
- 38.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 39.Stephens GJ, Owen DG, Robertson B. Cysteine-modifying reagents alter the gating of the rat cloned potassium channel Kv1.4. Pflügers Arch. 1996;431:435–442. doi: 10.1007/BF02207283. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 41.Taglialatela M, Castaldo P, Iossa S, Pannaccione A, Fresi A, Ficker E, Annunziato L. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc Natl Acad Sci USA. 1997;94:11698–11703. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokube K, Kiyosue T, Arita M. Openings of cardiac K-ATP channel by oxygen free radicals produced by xanthine oxidase reaction. Am J Physiol. 1996;40:H478–H489. doi: 10.1152/ajpheart.1996.271.2.H478. [DOI] [PubMed] [Google Scholar]
- 43.Tukey JW. Exploratory data analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- 44.Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werz MA, Elmslie KS, Jones SW. Phosphorylation enhances inactivation of N-type calcium channel current in bullfrog sympathetic neurons. Pflügers Arch. 1993;424:538–545. doi: 10.1007/BF00374919. [DOI] [PubMed] [Google Scholar]
- 46.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 48.Zagotta WN, Hoshi T, Aldrich RW. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with Shaker mRNA. Proc Natl Acad Sci USA. 1989;86:7243–7247. doi: 10.1073/pnas.86.18.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G-proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G-proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]