Abstract
Nicotine has many effects on CNS functions, presumably through its action on neuronal nicotinic acetylcholine receptors (AChRs). One subclass of AChRs that binds the snake venom toxin α-bungarotoxin (α-Bgt-AChRs) has been shown to modulate neurotransmission in the brain. We now show that α-Bgt-AChR activation by low doses of nicotine results in apoptotic cell death of both primary and immortalized hippocampal progenitor cells. In HC2S2-immortalized hippocampal progenitors, nicotine is cytotoxic to undifferentiated cells, whereas it spares the same cells once differentiation has been induced. The activation of α-Bgt-AChRs by nicotine results in the induction of the tumor suppressor protein p53 and the cdk inhibitor p21. The cytotoxic effect of nicotine is dependent on extracellular calcium levels and is probably attributable to the poor ability of undifferentiated progenitors to buffer calcium loads. The major calcium buffer in these cells, calbindin D28K, is present only after differentiation has been induced. Furthermore transfection of undifferentiated cells with calbindin results in dramatic protection against the cytotoxic effects of nicotine. These results show that nicotine abuse could have significant effects on the survival of progenitor populations in the developing and adult brain and also suggest an endogenous role for α-Bgt-AChRs in neuronal development and differentiation.
Keywords: nicotine, acetylcholine receptors, α-bungarotoxin, apoptosis, hippocampal progenitors, p53, cell cycle
Neuronal nicotinic acetylcholine receptors (AChRs) are widely distributed in the CNS, but very few instances of synaptic transmission have been reported, suggesting that these receptors might have a different, nontraditional role to play in CNS functions (for review, see Sargent, 1993; Clarke, 1993; Role and Berg, 1996). The belief that activation of AChRs can have profound physiological consequences, however, has received widespread acceptance because of the behavioral and physiological effects of nicotine in smokers. In addition to the addictive and neuroprotective properties of nicotine (Arneric et al., 1995; James and Nordberg, 1995), studies have also indicated significant cognitive, intellectual, and behavioral impairments in the offsprings of mothers who smoke during pregnancy (Sexton et al., 1990; Olds et al., 1994). Nicotine efficiently reaches the amniotic fluid and fetal blood achieving concentrations similar to, if not more than, that in the maternal blood (Lambers and Clark, 1996). Changes in the development of cholinergic markers have been reported in the brains of rat pups exposed to nicotine in utero (Navarro et al., 1989). These studies on the teratogenic effects of nicotine add an additional concern for the consequences of smoking among the general population. Although the mechanisms have not been established, it is possible that nicotine, through its actions on AChRs, can affect early developmental events in the CNS.
A class of AChRs that binds, and is functionally blocked by, the snake venom toxin α-bungarotoxin (α-Bgt-AChRs) is widely distributed in the mammalian CNS. One area with high levels of the receptor is the hippocampus (Freedman et al., 1993; Barrantes et al., 1995) in which they have been shown to be functional on the soma of CA1 interneurons in the stratum radiatum (Frazier et al., 1998) as well as on presynaptic terminals of CA3 mossy fibers (Gray et al., 1996). α-Bgt-AChRs have also acquired prominence recently because of their ability to modulate glutamatergic transmission in chick medial habenula (McGhee et al., 1995) and rat hippocampus (Gray et al., 1996), by virtue of their location at or near presynaptic terminals in these neurons. The ability of α-Bgt-AChRs to modulate synaptic events is probably attributable to their high calcium permeability (Seguela et al., 1993; Castro and Albuquerque, 1995; Rogers and Dani, 1995) and the efficient way in which they can raise intracellular free calcium levels ([Ca]i; Vijayaraghavan et al., 1992). These results have focused attention on calcium signaling by α-Bgt-AChRs and its consequences for neuronal function.
Many different lines of evidence suggest that α-Bgt-AChRs might have a role to play in neuronal development (for review, see Role and Berg, 1996). Expression patterns during development in the rat thalamocortical terminals suggest that α-Bgt-AChRs may affect events during a critical period of cortical synaptogenesis (Bina et al., 1995;Broide et al., 1996). The AChR α7 subunit, the main component of these receptors, appears early in the development of chick ciliary ganglion neurons (Corriveau and Berg, 1993) and also in presumptive myoblasts and related cell types (Corriveau et al., 1995). The significance of this regulation in the expression of α-Bgt-AChRs during neuronal development remains to be elucidated, but their high calcium permeability supports a role for these receptors in early gene expression (Greenberg et al., 1986). In addition, recent studies implicate α-Bgt-AChRs in cell proliferation in a neuroendocrine cell line (Quik et al., 1994). Exposure to α-Bgt saves chick motoneurons from naturally occurring cell death (Hory-Lee and Frank, 1995). InCaenorhabditis elegans, a mutation in the channel domain of an AChR homolog showing sequence homology to the vertebrate α7 gene, causes degeneration of specific early and late onset neurons (Treinin and Chalfie, 1995).
One problem with examining signal transduction events during development and differentiation of CNS neurons has been the paucity of homogeneous cell populations. At the same time, effects observed in such selected homogeneous populations of cells have to be validated in more physiological systems. Our approach, therefore, has been to identify a specific process triggered by AChR activation in primary systems and then to perform a detailed analysis of the signal transduction pathways involved, using a more homogeneous population of cells.
In recent years, immortalized progenitor cells from fetal and adult CNS have become systems of choice for the examination of developmental events (for review, see Cepko, 1989; Gage et al., 1995a). These cells are derived from primary cell cultures and exhibit their native characteristics (Eves et al., 1992; Hoshimaru et al., 1996). Despite numerous studies, there is no evidence for uncontrolled growth (Brustle and McKay, 1996), and progenitors grown in culture for 2 years show normal differentiation when grafted to neurogenic sites in the rat brain (Suhonen et al., 1996). It is because of this fidelity that progenitor cells grown in vitro are now considered as possible tools for cell replacement therapy in the nervous system (Brustle and McKay, 1996).
The hippocampal progenitors, HC2S2 cells, are advantageous for studies on cellular aspects of signaling during differentiation because they allow comparisons between a developmental and a differentiated setting. HC2S2 cells are committed to a neuronal fate, derived from adult rat hippocampus, and immortalized by the stable expression of v-myc driven by a tetracycline-controlled transactivator (Hoshimaru et al., 1996). These cells, therefore, form ideal systems to examine temporal events during early differentiation and development.
Here we show that primary progenitors derived from rat hippocampus show significant cell death when treated with nicotine at the concentrations to which both adult smokers, as well as fetuses of pregnant mothers who smoke, are exposed. This effect is mimicked in HC2S2 cells, in which identical treatment with nicotine causes a dramatic apoptotic cell death in undifferentiated cells but spares the same cells when differentiation has been induced. This effect of nicotine is attributable to the activation of α-Bgt-AChRs and the consequent expression of p53, a cell cycle-related protein. The susceptibility of undifferentiated HC2S2 cells to the toxic effects of nicotine probably results from their poor ability to buffer intracellular calcium caused by the lack of calbindin, a major mobile calcium buffer in these cells.
MATERIALS AND METHODS
Primary progenitor cells. Hippocampal progenitor cells were isolated from Fisher rats (passages 5–10) as previously described (Gage et al., 1995b). Briefly, hippocampi were isolated, and the cells were dissociated enzymatically and plated onto uncoated culture wells in DMEM/F-12 high-glucose medium containing 10% fetal bovine serum. After 24 hr, the medium was replaced with serum-free medium containing DMEM/F-12, N2 supplement (Life Technologies, Gaithersburg, MD), and 20 ng/ml FGF-2 (Boehringer Mannheim, Indianapolis, IN). Cells were passaged every 3–4 d. After two or three passages, the cells were plated onto 100 mm dishes coated with polyornithine (10 μg/ml) and laminin (5 μg/ml). Cell numbers were determined by staining with trypan blue and iodopropidium.
Detection of apoptosis. Apoptosis was detected by the terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end-labeling (TUNEL) method using the Apoptag kit (Oncor). Briefly, Apoptag is an in situ apoptosis detection kit used to detect the multitude of new 3′-OH DNA ends generated by fragmentation. Digoxygenin-dUTP is used to label DNA fragments that are then visualized by a peroxidase-conjugated anti-digoxygenin antibody. Intact cells that showed the reddish brown reaction product in the nucleus were taken as Apoptag-positive. Nuclear fragmentation was also visualized using 10 ng/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO). DNA laddering was examined by running total DNA from nicotine-treated cells, control cells, and cells treated with nicotine in the presence of α-Bgt on agarose gels according to the protocol described by Arends et al. (1990).
HC2S2 cell culture. HC2S2 cells were grown in DMEM/F-12 medium along with N2 supplement (Life Technologies) and 2 ng/ml FGF-2 (Boehringer Mannheim) as described (Hoshimaru et al., 1996). Cells were plated on dishes coated with polyornithine (10 μg/ml; Sigma) and laminin (5 μg/ml, Collaborative Biomedical Products). Differentiation was initiated by the addition of 0.1 μg/ml doxycycline (Sigma). For imaging experiments, the cells were plated on coated glass coverslips.
For experiments looking at cell loss, cells were grown in 16 mm wells. At the end of the treatment period they were trypsinized (0.25 ml ATV trypsin containing 0.125% trypan blue for 3 min). An aliquot was then transferred to a hemocytometer for counting. Phase-bright cells that excluded the trypan blue were counted as survivors.
RT-PCR. Total mRNA was isolated from cells using the RNAzol method according to the manufacturer’s instructions (TelTest). The samples (50 ng of mRNA) were subjected to reverse transcription for 75 min at 42°C and then denatured at 95°C for 5 min. The reaction mixture contained (in μl): H2O, 7; 10× PCR buffer (500 mm KCl and 100 mm Tris-HCl, pH 8.4), 2; 10 mm dNTP, 2; random hexamers, 1; 25 mmMgCl2, 3; RNAsin, 0.25; and reverse transcriptase, 0.5. The samples then underwent PCR (23 cycles) consisting of the following steps: 2 min at 94°C, 2 min at 60°C, 2 min at 72°C, and, after 23 cycles of amplification, 10 min at 70°C. The reaction mix for the PCR was (in μl): 10× PCR buffer, 8; 25 mmMgCl2, 2; [32P]dCTP, 0.2;Taq polymerase (Perkin-Elmer, Emeryville, CA), 0.5; 5′ and 3′ primers, 2; and H2O, 65.3. For α7, the 5′ sense primer was AGA TAT CAC CAC CAT GAC and the 3′ primer was GCC TGC GTG GTG GAC. For the endogenous control, the RPL27 5′ primer was GAA CAT TGA TGA TGG CAC CTC and the 3′ primer was GGG GGA TAT CCA CAG AGT ACC. After PCR, samples were migrated in a 6% acrylamide gel. Quantitation was performed using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and the linearity of the reaction was controlled for 23 cycles of amplification.
Fluorescence labeling of cells. For fluorescence labeling of HC2S2 cells, the cells were grown in four- to eight-well Lab-Tek plates overnight. For α-Bgt labeling, cells were incubated with biotin-α-Bgt (Molecular Probes, Eugene, OR) at 1:500 dilution for 1 hr. They were then washed in PBS and fixed, followed by a 1 hr incubation with extravidin-FITC. To determine nonspecific labeling, the first incubation was done in the presence of 1 μmunlabeled α-Bgt on sister cultures.
For immunofluorescence experiments, cells were fixed with 4% paraformaldehyde for 10 min. After fixation they were washed three times with PBS and preincubated with PBS containing 4% preimmune donkey serum (PBS-DS) and 0.4% Triton X-100 for 30 min. Primary antibodies (Abs) were then added, and the incubation was continued overnight at 4°C. Antibodies used in this study were polyclonal rabbit (rb) antisera against p21, rb Ab-1 (PharMingen, San Diego, CA), and rb Ab-C-19 (Santa Cruz Biotechnology, Santa Cruz, CA); 2 rb Abs for calbindin (PharMingen); and one anti-p53 Ab (Do-1, polyclonal; antisera; PharMingen). After the overnight incubation, the cells were washed two times for 10 min each with PBS and one time with PBS-DS. The secondary Abs (donkey anti-rabbit and donkey anti-mouse; The Jackson Laboratory, Bar Harbor, ME) were conjugated with either FITC or Cy3 or with biotin and used at a dilution of 1:500 in PBS-DS. When biotin-conjugated Abs were used, cells were then incubated with extravidin–FITC conjugate diluted at 1:500 in PBS. In all cases, the last wash included DAPI to stain the nuclei (see above). Slides were then mounted in 100 mm Tris-HCl, pH 8.5, containing 25% glycerol, 10% polyvinyl alcohol (Air Products), and 2.5% 1,4-diazobicyclo-(2.2.2)-octane (Sigma).
Specificity was verified in each case by using controls in which the primary Ab was omitted and also, when possible, by using a different irrelevant primary Ab. Fluorescence imaging was performed using four-color confocal scanning laser microscopy [Zeiss Axiovert and Bio-Rad (Hercules, CA) MRC1000].
Western blotting. Cell extracts were run on SDS-PAGE. Immunoblotting was done using polyvinylidene difluoride membranes (Millipore, Bedford, MA). Ab-1 (PharMingen) was used for the detection of p21, and a mixture of polyclonal antibody (pAb) 240 and pAb 421 (a generous gift from J. Baudier, Institut National de la Santé et de la Recherche Médicale) was used for p53 immunodetection. Actin monoclonal antibody (mAb; Boehringer Mannheim) was used to evaluate relative amounts of sample loaded.
Calbindin transfection. Rat calbindin D28 cDNA (Hunziker and Schrickel, 1988), a generous gift from Dr. Hilmar Bading (Medical Research Council, Cambridge, UK) was cloned in pBK–cytomegalovirus (Stratagene) at SalII–Knp1 restriction sites. The Lipofectin method (DOTAP; Boehringer Mannheim) was used for transient transfections. Seventy microliters of the stock DOTAP were diluted in 100 μl of PBS and mixed with 10 μg of calbindin in 100 μl of PBS. The mixture was incubated for 10 min at room temperature and then added onto undifferentiated HC2S2 cells in a 10 cm dish. Nicotine was added 1 d after transfection, and calbindin immunochemistry was performed 12 hr later, as described above. Numbers of fragmented nuclei per 100 cells were determined by DAPI staining in calbindin-positive and calbindin-negative cells in the same dish.
For the immunochemistry, cells were fixed in 4% paraformaldehyde in PBS for 10 min, rinsed three times with PBS (5 min each wash), and preincubated in PBS containing 0.4% Triton X-100 and 4% PBS-DS for 30 min. The cells were then incubated with primary mouse anti-calbindin mAb (Sigma) at 1:1000 dilution overnight at 4°C. The next day the primary Ab was washed away with two washes of PBS and one with PBS-DS (each wash 10 min). Cells were then incubated with peroxidase-conjugated secondary Ab (donkey anti-mouse; The Jackson Laboratory) in PBS-DS and then washed three times in PBS. In the last wash, 10 ng/ml DAPI was included to visualize the nuclei. Peroxidase staining was visualized using the diaminobenzidine kit (Vector Laboratories, Burlingame, CA).
RESULTS
Nicotine causes DNA fragmentation in primary hippocampal progenitor cells
We first tested the effects of nicotine, at doses to which smokers and their offspring are exposed, on the survival of primary hippocampal progenitor cells. Primary progenitors were isolated from adult Fisher rats and grown in culture for 5–10 passages (see Materials and Methods; Gage et al., 1995b). Cells were then exposed to 0.5 μm nicotine overnight. Cell numbers were determined by trypan blue exclusion. The total number of cells in control dishes were not significantly different from those in nicotine-treated dishes. Because these cells are a heterogeneous, dividing population, and cell counting would clearly underestimate the effects of nicotine, the numbers of apoptotic cells were directly examined. Apoptosis was detected by the TUNEL staining method. Cells that retained their morphology but showed staining of their nuclei were taken as Apoptag-positive. Significantly more stained nuclei were detected in nicotine-treated dishes. Averaging nine fields from three separate experiments showed that the number of apoptotic cells in nicotine-treated dishes was ∼30-fold higher than in untreated controls (3.6 ± 0.04 vs 0.1 ± 0.02%; mean ± SEM;p < 0.005, Mann–Whitney U test; Fig.1). Pretreatment of the cells with 100 nm α-Bgt followed by its continuous presence during nicotine treatment reduced the number to 0.6 ± 0.1%, a reduction in cell death by 83%. The toxin, by itself, did not significantly affect cell numbers at this dose.
Fig. 1.
Nicotine-induced cell death in primary hippocampal progenitors. Primary hippocampal progenitor cells were treated overnight with either control solution (Control) or 0.5 μm nicotine (Nicotine). Cell death was visualized using the Apoptag Kit (Oncor). Peroxidase-positive nuclei in cells that still retained their morphology were counted. Treatment with nicotine resulted in an increase in the number of apoptotic cells. Cell counts revealed a 30-fold increase in the number of Apoptag-positive cells after nicotine treatment (p < 0.005; Mann–Whitney Utest). This effect of nicotine was blocked by preincubation with 100 nm α-Bgt for 30 min, followed by its continued presence during nicotine treatment (Nic.+ αBgt). Five hundred cells per field, three fields per dish were counted. The values expressed in the y-axis are Apoptag-positive cells as a percentage of total cells counted from three separate experiments (mean ± SEM).
Nicotine induces cell death in undifferentiated HC2S2 cells
Primary progenitor cells are extremely heterogeneous, and putative neuronal progenitors make up only a small fraction of the total, thus making it very difficult to examine molecular transduction events in these cells. Therefore, we used HC2S2-immortalized hippocampal progenitors (Hoshimaru et al., 1996) to examine these events. In addition, the use of HC2S2 cells also enabled us to examine nicotinic effect in the context of neuronal development and differentiation.
Treatment of undifferentiated HC2S2 cells with a relevant concentration of nicotine (0.5 μm) for 36 hr caused a >50% reduction in live cell numbers (Fig.2A, a vsc, B; Table 1) compared with untreated controls, as evidenced by trypan blue exclusion and cell counting. This time point allowed us to consistently and reliably estimate cell loss. Remaining cells in the dish did not show any morphological changes and were indistinguishable from controls. Preincubating the cells with 50–100 nm α-Bgt for 45 min, followed by the continued presence of the toxin, completely protected the cells from nicotine-mediated toxicity (Fig. 2A,b, B). The toxin by itself had no effect on cell numbers. Similar treatment with 20–50 nmmethyllycaconitine (MLA), an antagonist known to target α-Bgt-AChRs (Ward et al., 1990), also protected the cells from the cytotoxic effects of nicotine. Culture wells treated with 0.5 μmnicotine in the presence of 20 nm MLA had 97 ± 6% of the cells in control wells treated with MLA alone (mean ± SEM, two experiments). Unlike with α-Bgt, the total number of cells in MLA-treated dishes was slightly lower (80 ± 3% of untreated controls). Whether this result is attributable to some nonspecific toxicity of the insecticide, a possible partial agonist function of MLA, or hydrolysis products of the norditerpenoid alkaloid that might have agonistic properties (Hardick et al., 1995) is not known. Nicotine had significant effects on cell survival at concentration ranges from 5 nm to 5 μm (Fig. 2C), spanning the plasma levels of nicotine in smokers. Whether the small but significant decrease in the efficacy at 5 μm nicotine is attributable to possible desensitization of AChRs remains to be seen.
Fig. 2.

Effect of nicotine on the survival of HC2S2 progenitor cells. HC2S2 cells plated in 24 mm culture wells were treated with nicotine for a 36 hr period after which cell survival was assessed by trypan blue exclusion and counting. Values represent the mean ± SEM from two to four experiments, each done in triplicate.A, Bright-field images of HC2S2 cells that were untreated (a, Con), treated with 0.5 μmnicotine in the presence of 100 nm α-Bgt (b, Nic + Bgt), or treated with 0.5 μmnicotine alone (c, Nic). Nicotine caused a dramatic reduction in the number of trypan blue excluding cells; that reduction was completely reversed in the presence of α-Bgt. Scale bar, 50 μm.B, Quantitation of the cytotoxic effects of nicotine by cell counts. Nicotine-treated wells showed a 50% decrease in live cell numbers that was reversible by pretreating the cells with 100 nm α-Bgt followed by the continued presence of the toxin. The toxin by itself did not significantly affect cell numbers. Cell numbers in untreated control = 411 ± 59 × 103 cells/24 mm well (mean ± SEM from 4 experiments). Values are expressed as percent live cells compared with untreated controls (% Con). C, Dose–response for the effect of nicotine on the survival of undifferentiated HC2S2 cells. Nicotine significantly decreased the survival of undifferentiated progenitors at all the concentrations tested between 5 nm and 5 μm. The lesser efficacy of the 5 μm concentration could be attributable to a more rapid desensitization of the receptors. Values are mean ± SEM from two experiments done in triplicate.
Table 1.
Time course of the effects of nicotine on the survival of HC2S2 cells
| Treatment | Cell numbers | |||
|---|---|---|---|---|
| Live (% Untreated) | Dead adherent (% Untreated) | Dead floaters (% Untreated) | Total dead (% Untreated) | |
| Nicotine (12 hr) | 82.9 ± 9.4 | 10.2 ± 1.6 | 2 ± 1.414 | 12 |
| Nicotine (24 hr) | 71.5 ± 2.2 | 18.5 ± 2.12 | 5.5 ± 0.71 | 24 |
| Nicotine (36 hr) | 47.5 ± 3.53 | 32.5 ± 3.56 | 23.5 ± 2.12 | 56 |
Cells were treated with 0.5 μm nicotine for varying periods. At each time point the numbers of living cells, dead adherent cells, and floating cells were counted and expressed as a percentage of total cells from untreated sister cultures at each time point. Dead adherent cells were determined using the TUNEL staining protocol, and the dead floaters were determined by trypan blue exclusion. The mean ± SEM values for total cell numbers in untreated cultures were (×103): time 0, 201 ± 21.5; 12 hr, 422 ± 10; 24 hr, 703 ± 42; and 36 hr, 1303 ± 56. The percentage of dead cells in untreated cultures (adherent + floaters) at these time points was 0.5%; 0.52%; 0.85%; and 0.5% of the total number of cells, respectively. All values are mean ± SEM from three experiments, done in triplicate.
Total cell counts were done for cells treated with nicotine for various time points. Live cells, dead adherent cells, and floating cells were counted. Cell numbers were compared with total cells in untreated sister cultures (Table 1). The total number of cells did not change between the controls and the nicotine-treated dishes, but the number of dead cells increased progressively with time from 13% at 12 hr after nicotine treatment to 56% of the total cell number by 36 hr (Table 1). These results rule out a significant contribution to nicotine-induced loss of live cell numbers by general cell cycle arrest and suggest that cell death is the major contributor to this effect. The surprising result was that the total number of cells did not change significantly. If the percentage of dividing cells at 24 hr is only 71.5% that of the untreated control (Table 1), then even if no additional cell death occurs in the next 12 hr period (the percentage of dead cells is actually more than double at 36 hr; Table 1), we should still expect at least a 30% decrease in total cell number. This implies that either the rate of cell division is much faster than the rate of cell death or there are compensatory mechanisms triggered directly or indirectly by nicotine treatment. We come back to this point below.
HC2S2 cells that are under the control of a tettransactivator can be induced to differentiate by the addition of 0.1 μg/ml doxycycline for 5–10 d, by which time they become terminally differentiated (Hoshimaru et al., 1996). Identical treatment of differentiated cells with 0.5 μm (Fig.3A, Con vsNic, B) or with 5 μm nicotine (data not shown) had no effect on cell survival. These results show that low concentrations of nicotine, such as those found in the serum of smokers, can induce cell death in undifferentiated, rapidly dividing progenitor cells but spare the same cells when they have differentiated.
Fig. 3.
Nicotine is not cytotoxic to differentiated HC2S2 cells. Differentiated HC2S2 cells were treated with 0.5 mmnicotine as described in Figure 2. Values represent the mean ± SEM from two experiments, each done in triplicate. A, Both control (Con) and nicotine-treated (Nic) cells showed robust neurons with well-defined processes. Scale bar, 50 μm. B, Cell counts from two independent determinations done in triplicate revealed no significant difference in cell numbers between the two conditions.
Presence of α-Bgt-AChRs on HC2S2 cells
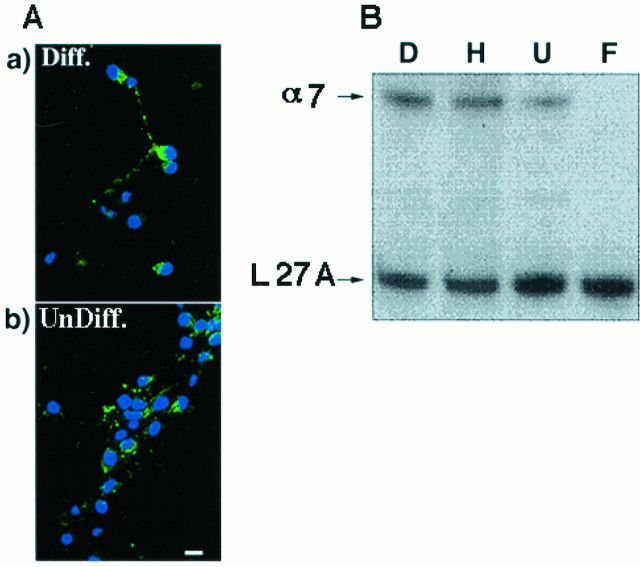
The ability of α-Bgt and MLA to reverse the effect of nicotine suggests that it is mediated by the activation of α-Bgt-AChRs. Surface-binding experiments (Halvorsen and Berg, 1989) using 10 nm125I-α-Bgt indicate that HC2S2 cells express low levels of toxin binding on their surface. Results show that undifferentiated cells had 3.9 ± 0.81 fmol/106cells. Surprisingly, differentiated HC2S2 cells had 12.5 ± 3.37 fmol/106 cells (mean ± SEM from two or three experiments, each done in triplicate), suggesting that the lack of nicotine-mediated toxicity in these cells was not attributable to lack of surface receptors. Assuming only 50% of the cells express surface toxin binding at a given time (see below), there would be ∼4800 α-Bgt-binding sites on the surface of an undifferentiated cell. Fluorescence experiments using biotinylated α-Bgt followed by extravidin-FITC showed specific detectable fluorescence on approximately half of the cells in both the undifferentiated and differentiated conditions (Fig.4A), indicating that there might be variations in the levels of surface expression of the receptor between cells.
Fig. 4.
Presence of α-Bgt-AChRs on HC2S2 cells. α-Bgt-AChRs on HC2S2 cells were detected by surface radiolabeled toxin binding (see Results), fluorescence labeling, and RT-PCR experiments. A, Fluorescence labeling of HC2S2 cells. Cells were labeled with biotinylated α-Bgt followed by Cy3-conjugated extravidin. Background label was assessed by labeling in the presence of 1 μm α-Bgt. Both the differentiated (Diff) and the undifferentiated (Undiff) cells showed detectable levels of toxin binding on their surface (position of cells is represented by the DAPI staining in blue). In the differentiated cells, punctate labeling was also observed on the processes. Scale bar, 25 μm.B, RT-PCR showing the presence of α7 message in HC2S2 cells. Using primers flanking a 460 bp region of the putative cytoplasmic domain of the rat α7 gene, RT-PCR was performed on RNA isolated from differentiated HC2S2 cells (lane D), adult rat hippocampus (lane H), undifferentiated HC2S2 cells (lane U), and rat fibroblasts (lane F). The level of α7 message was normalized to that of the control ribosomal protein L 27A message in the same PCR mix. The α7 message is expressed in differentiated HC2S2 cells in levels comparable to those in the adult hippocampus. Lesser, although significant, expression was seen in the undifferentiated cells. Fibroblasts do not show any detectable expression of the α7 message.
The α7 gene product is the only identified protein that forms a part of rat brain α-Bgt-AChRs, and all the α-Bgt binding sites in the rat hippocampus contain the α7 gene product. RT-PCR experiments showed the presence of α7 message in both undifferentiated and differentiated HC2S2 cells in levels comparable to those in the adult rat hippocampus (Fig. 4B). Quantitation using a PhosphorImager demonstrated that differentiated HC2S2 cells have ∼40% greater amounts of the message.
Nicotine-induced apoptosis and the cell cycle
We next examined the mechanism underlying nicotine-induced cell death. For this, we chose a time point (12 hr) when there was significant cell death, but 98% of the cells were still adherent. The first set of experiments was designed to determine whether nicotine-induced cell death was apoptotic in nature. Evidence for this comes from DNA laddering experiments. In apoptosis there is a characteristic pattern of DNA degradation in multiples of ∼200 bp (Arends et al., 1990). Undifferentiated HC2S2 cells were treated with 0.5 μm nicotine for 12 hr. DNA was then extracted and run on an agarose gel. As shown in Figure5A, nicotine-treated cells showed a characteristic laddering of DNA. The smallest band observed (Fig. 5A, arrowhead) was ∼200 bp in length. Increasing nicotine concentration to 50 μm did not appear to cause any more DNA fragmentation. On the other hand, 100 nm α-Bgt significantly protected the cells from nicotine-mediated endonuclease action (Fig. 5A).
Fig. 5.
Nicotine induces apoptosis in undifferentiated HC2S2 cells. The effect of treatment with 0.5 μm nicotine for 12 hr on the survival of undifferentiated HC2S2 cells was examined by the following methods. A, DNA laddering. DNA was extracted from cells 12 hr after no treatment (Con) or after treatment with 0.5 μmnicotine (Nic 0.5), 50 μmnicotine (Nic 50), or 0.5 μm nicotine in the presence of 100 nm α-Bgt (Nic +Bgt). Nicotine-induced DNA fragmentation characteristic of apoptosis was seen in undifferentiated HC2S2 cells and was prevented by pretreatment with α-Bgt. The arrow shows the position of the 247 bp DNA marker. B, Fluorescence and immunocytochemistry. Nicotine-induced cell death in undifferentiated HC2S2 cells is apoptotic as seen by nuclear fragmentation (arrow) visualized by staining undifferentiated cells with DAPI 12 hr after exposure to 0.5 μm nicotine (bottom panel). Immunofluorescence on the same field of cells indicates the induction of p53 (red) and p21 (green) or p53 and p21 (yellow) expression (top panel). In the presence of bungarotoxin, neither nuclear fragmentation nor the induction of p53/p21 is seen (insets). Scale bar, 25 μm. C, Western blotting. Western blots of untreated cells (lane 1,C), cells treated with 0.5 μm nicotine in the presence of 100 nm α-Bgt (lane 2,N + Bgt) or with nicotine alone at 0.5 μm (lane 3, N) revealed the induction of both p53 and p21 proteins by nicotine. No induction was observed in control cells or in cells treated with nicotine in the presence of α-Bgt. In differentiated HC2S2 cells (lanes 4, 5) no p53 signal was seen in either control (lane 4, C) or in cells treated with 0.5 μm nicotine (lane 5,N). Differentiated cells showed endogenous levels of p21 under both conditions and in a manner independent of the expression of p53. All lanes were probed with an actin antibody to estimate the approximate amounts loaded.
Further confirmation of the apoptotic nature of nicotine-mediated cell death comes from the following experiments. Cells were exposed to 0.5 μm nicotine for a 12 hr period, at the end of which they were fixed and stained with 10 ng/ml DAPI. Visualization of DAPI-stained cells indicated the presence of fragmented nuclei, characteristic of apoptosis (Fig. 5B, bottom panel). Such fragmentation was not detectable in cells treated with nicotine in the presence of α-Bgt (Fig.5B, bottom panel, inset; Table2). The number of fragmented nuclei on nicotine treatment (10 ± 1% total; Table 2) correlated well with the number of adherent cells that were Apoptag-positive at this time point (10.2 ± 1.6; Table 2). Another characteristic of apoptosis is the requirement for the induction of specific cell death-related proteins. Induction of expression of the tumor suppressor protein p53 followed by p21, a cdk inhibitor, has been shown to be one pathway for programmed cell death (for review, see Meikrantz and Schlegel, 1995). In the same population of cells stained for DAPI, we examined the induction of p53 and p21 expression by 0.5 μm nicotine. Robust expression of both p53 and p21 was observed using immunofluorescence measurements (Fig. 5B, top panel; Table 2). Once again, treatment with α-Bgt abolished this induction (Fig. 5B, a,inset). Fifty-three percent of nicotine-treated cells were p53-positive, whereas 61% were p21-positive (Table 2). No p53-positive cells were detected in cells treated with nicotine in the presence of α-Bgt, whereas ∼6% of the cells showed detectable immunoreactivity to p21 (Table 2). These experiments clearly demonstrate the ability of nicotine to induce the expression of two cell cycle-related proteins shown to play a role in the apoptotic pathway. The discrepancy between our DAPI and cell count data versus the expression of the two cell cycle proteins can be explained by the fact that induction of these genes precedes overt DNA fragmentation and cell death. The explanation might be that there is a time lag between the induction of programmed cell death and overt signs of cell death (namely, complete nuclear fragmentation and trypan blue inclusion). A recent study demonstrated the existence of at least 14 gene products that are induced by p53 expression and are probably involved in the apoptotic process, and that maximal cell death might occur as late as 36 hr after induction of p53 (Polyak et al., 1997).
Table 2.
Comparison of the effects of nicotine and nicotine + α-Bgt on apoptotic parameters
| Parameter | Cell numbers (% Total) | |
|---|---|---|
| Nicotine | Nicotine + α-Bgt | |
| Cell death | 10.2 ± 1.6 | 1.75 ± 0.81 |
| Fragmentation (DAPI staining) | 10 ± 1 | ND |
| p53-positive | 53 ± 4 | ND |
| p21-positive | 61 ± 3 | 6 ± 4 |
Undifferentiated HC2S2 cells were treated with nicotine alone (0.5 μm) or nicotine in the continued presence of 100 nm α-Bgt for 12 hr. Cell death among adherent cells was determined by the Apoptag method. The results are expressed as a percentage of total number of adherent cells in the dish. The values for DAPI staining, p53, and p21 represent the mean ± SEM from two experiments done in triplicate. ND, Not detected.
To test this possibility, we withdrew nicotine at 12 hr, thoroughly washed the cells, and counted cells for cell death after a 12 or 24 hr drug-free period. Cell death increased after nicotine withdrawal, and the numbers of apoptotic cells were 22 and 28% less than those in the continued presence of nicotine at the 24 and 36 hr time points, respectively. This finding suggests that the initial 12 hr period of nicotine exposure is enough to trigger most of cell death-initiating events that last over the next 24 hr. The percentage of p53-positive cells at 24 hr remained more or less the same after nicotine withdrawal (at hour 12) and increased by 50% in the chronic presence of nicotine (Table 3). This must mean that a larger number of cells express p53 at 24 and 36 hr than at 12 hr. In the case of nicotine withdrawal experiments, it implies that more cells are being recruited into the p53 pathway in the absence of the agonist, or the p53 expressing cells continue to divide. One way to explain this finding and the cell number data (Table 1) is that nicotine might induce a long-lasting cell proliferation as seen in small lung carcinoma cells (Codignola et al., 1994), and a considerable proportion of these cells undergo apoptosis. The upper threshold for this proliferation might be set by other factors (e.g., FGF) in the medium. Our data suggest that although apoptosis is the main mechanism underlying nicotine-induced cell death, the actions of nicotine must be complex involving downstream factors other than the ones measured in this study (see Discussion).
Table 3.
Effects of nicotine withdrawal on cell death
| Time | Chronic Nicotine | Nicotine (12 hr followed by washout) |
|---|---|---|
| 24 hr cell death | 28 ± 1 | 22 ± 0.8 |
| p53 | 75 ± 4 | 60 ± 7.7 |
| 36 hr cell death | 58 ± 3 | 42 ± 1.6 |
| p53 | 80 ± 3 | 55 ± 1.73 |
Cells were incubated with 0.5 μm nicotine chronically, or they were incubated for 12 hr with nicotine, washed three times with 1 ml of medium, and allowed to grow for another 12 or 24 hr. The number of adherent cells was calculated and for each category and expressed as percentage of total adherent cells in the dish. Cell death was calculated by the Apoptag method. Mean total numbers (×103) at 24 hr were 600 ± 24 and 576 ± 28 and for 36 hr were 900 ± 40 and 872 ± 11, for chronic nicotine and the nicotine withdrawal experiments, respectively. Values represent mean ± SEM from two experiments. Nicotine continued to cause additional cell death even 24 hr after its removal.
Western blots of extracts from both undifferentiated and differentiated HC2S2 cells were performed to determine whether a similar induction of p53 occurs in differentiated cells. Although expression of p53 in response to nicotine was seen in undifferentiated HC2S2 cells, no such expression of the protein was detected in differentiated neurons (Fig.5C). The cdk inhibitor p21, which is induced early in the differentiation of HC2S2 cells (F. Berger and F. H. Gage, unpublished results) was present in differentiated cells in the absence and presence of nicotine. In undifferentiated HC2S2 cells, p21 expression was only observed in conjunction with p53 on treatment with nicotine (Fig. 5C). These results suggest that the induction of p53 in response to nicotine exposure is only seen in undifferentiated HC2S2 cells and that the presence of p21 in differentiated cells appears independent of both nicotine exposure and p53 induction. p53-independent induction of p21 is known and is thought to have a role in neuronal survival (Poluha et al., 1996).
Role of calcium in nicotine-mediated apoptosis
An obvious mechanism by which α-Bgt-AChRs could mediate the effects of nicotine is by increasing [Ca]i (MacNicol and Schulman, 1992; Vijayaraghavan et al., 1992, 1995). We examined the role of calcium in nicotine-mediated apoptosis of undifferentiated HC2S2 cells.
Calcium-imaging studies using cells loaded with fura-2 AM and fluo-3 AM were done on undifferentiated progenitor cells. As expected, no calcium transients were observed on treatment with 0.5 μmnicotine (Vijayaraghavan et al., 1992; also see Discussion). The effect of nicotine on cell death was, however, dependent on extracellular calcium. A 12-fold reduction of calcium in the media (from 1.2 mm to 100 μm) protected undifferentiated HC2S2 cells from nicotine-induced cell death, although at this calcium concentration cell numbers were lower than in controls with normal extracellular calcium (Fig. 6). Furthermore, incubation of HC2S2 cells with 10 μmKN-62, an inhibitor of calcium/calmodulin-dependent kinase (Cam kinase), showed significant protection against the toxic effects of nicotine (Fig. 6), lending additional evidence for the calcium dependence of this effect.
Fig. 6.
Nicotine-induced apoptosis is calcium-dependent. The effect of 0.5 μm nicotine on the survival of undifferentiated HC2S2 cells was examined at two different calcium concentrations (1.2 mm vs 100 μm). Lowering external calcium 10-fold to 100 μm reversed the ability of nicotine to mediate cell death in these cells. Cells were tested for nicotine-induced cytotoxicity in the presence or absence of 10 μm KN-62 (Calbiochem, La Jolla, CA), an inhibitor of CAM kinase II. In the presence of KN-62, the ability of nicotine to kill undifferentiated HC2S2 cells was reduced. The inhibitor by itself does not significantly affect cell survival. Results show that the effect of nicotine is calcium-dependent.
Involvement of calbindin D28K in nicotine-mediated apoptosis
One reason for the calcium-dependent susceptibility of undifferentiated HC2S2 cells could be the lack of adequate endogenous calcium-buffering mechanisms. A major calcium buffer in hippocampal neurons is calbindin D28K. Studies show that hippocampal cells that express calbindin are much more resistant to calcium cytotoxicity than those that do not (Mattson et al., 1991), suggesting that expression of this protein is important in protecting neurons from external calcium insults.
Examining HC2S2 cells for calbindin expression using immunofluorescence showed very little to no expression of the protein in undifferentiated HC2S2 cells (Fig. 7A). Expression of calbindin seems to be initiated only after the induction of differentiation in these cells (Fig. 7B), suggesting that the lack of this buffer might make undifferentiated HC2S2 cells more susceptible to calcium-mediated cytotoxicity. To directly determine whether calbindin expression is responsible for the susceptibility of undifferentiated HC2S2 cells to nicotine, we examined nicotine-induced apoptosis in cells transiently transfected with calbindin D28. Undifferentiated cells were transiently transfected with rat calbindin cDNA using DOTAP. The cells were then exposed to nicotine for 12 hr, after which they were tested for both calbindin expression, using an anti-calbindin mAb (Sigma) followed by a peroxidase-conjugated secondary Ab, and for fragmented nuclei using DAPI staining. The degree of DNA damage in calbindin-positive cells was 38-fold less than that seen in HC2S2 cells that did not express the buffer (Fig.7C). These results suggest that lack of adequate calcium-buffering mechanisms might underlie the susceptibility of undifferentiated HC2S2 cells to nicotine-mediated toxicity and further confirm the role of changes in [Ca]i in mediating the effects of α-Bgt-AChRs. However, ∼50% of calbindin-positive cells showed changes in morphology that resemble changes in early differentiation (Fig. 7B). This difference in morphology was a consequence of calbindin expression and was unaltered by nicotine treatment or by mock transfection. Although there was no difference in the extent of protection against the cytotoxicity of nicotine among the two morphological populations (data not shown), this finding raises the possibility that protection by calbindin might be attributable to induction of early differentiation events, and not attributable to a direct buffering of α-Bgt-AChR-mediated calcium increases.
Fig. 7.
The apoptotic effects of nicotine are dependent on the expression of calbindin D28K. A, Expression of calbindin-D28K. Undifferentiated and differentiated HC2S2 cells were incubated overnight with Abs against calbindin D28K followed by an FITC-conjugated secondary. Little to no expression of calbindin was seen in undifferentiated HC2S2 cells, whereas robust signals were obtained from differentiated cells. These results suggest that calbindin is expressed only after the induction of differentiation in these cells. Scale bar, 25 μm. B, Rescue of undifferentiated HC2S2 cells by transient transfection of calbindin. Undifferentiated HC2S2 cells were transiently transfected with calbindin D28K. Cells were then treated with 0.5 μmnicotine and stained for nuclear fragmentation with DAPI (top panel) and with an anti-calbindin mAb (bottom panel). Calbindin-positive cells (top andbottom panels, long arrows) showed very little nuclear fragmentation, whereas cells that do not express the gene had large numbers of fragmented nuclei (top panel,short arrows). Scale bar, 25 μm. C, Quantitation of calbindin-mediated rescue. The numbers of fragmented nuclei were counted in undifferentiated cells treated with 0.5 μm nicotine that were calbindin-positive (D28 +ve) or did not express the gene (D28 −ve). Cells that did not express calbindin showed a 38-fold greater number of fragmented nuclei than calbindin-positive cells (p < 0.003, Mann–Whitney U test). Results are mean ± SEM from three independent determinations.
DISCUSSION
The results presented here demonstrate that nicotine can induce apoptotic cell death in undifferentiated hippocampal progenitor cells but spares the same cells when they are differentiated. This effect of nicotine is antagonized by α-Bgt and results in the induction of the tumor suppressor protein p53. The apoptotic effect is dependent on the ability of the cells to tolerate changes in [Ca]i. Sensitivity to calcium insults, in turn, is probably dependent on the levels of calbindin expression in these cells.
A mechanistic explanation for the various actions of nicotine on the CNS has only recently begun to emerge. Major players for mediating some of these effects have turned out to be the α-Bgt-AChRs. Recent studies have shown that these receptors can play an important role as modulators of synaptic strength in the CNS (McGhee et al., 1995; Gray et al., 1996; Alkondon et al., 1996) and could also have roles in the pathophysiology of diseases such as Alzheimer’s disease (Gray et al., 1996) and schizophrenia (Freedman et al., 1993, 1997).
The role that α-Bgt-AChRs play during neuronal development has previously been inferred from their expression profiles in both CNS and peripheral neurons, as well as from studies on their effects on neurite outgrowth and survival (see introductory remarks). These findings indicate that the receptors might be expressed early and could play a role in early development and differentiation events (Role and Berg, 1996). Our study on cell death in primary hippocampal progenitor cells shows a 30-fold increase in the number of apoptotic cells after nicotine treatment. The small numbers of apoptotic cells are expected because the action of nicotine depends on the expression of α-Bgt-AChRs. If the receptors are expressed only in a subpopulation of neuronally committed progenitors, then one would expect only a tiny percentage of the total progenitor population to be affected. This fact makes the examination of transduction mechanisms quite difficult and motivated us to examine more homogeneous populations.
In our study on HC2S2 cells, the expression of α-Bgt-AChRs in undifferentiated neuronal progenitors precedes most neuronal markers examined (Hoshimaru et al., 1996). The level of expression of surface α-Bgt binding in undifferentiated HC2S2 cells is very low (∼2400–4800 binding sites per cell). This level is approximately three orders of magnitude less than that on chick ciliary ganglion neurons (Halvorsen and Berg, 1989) and ∼50-fold lower than that obtained from primary rat hippocampal neurons in culture (S. Vijayaraghavan, unpublished data). The presence of these sites in other neuronally committed hippocampal progenitors (Komourian and Quik, 1996) suggests that low levels of toxin binding might be prevalent among immature hippocampal neurogenic cells. Nevertheless, this low number is clearly sufficient to trigger nicotine-mediated effects.
The cause of nicotine-induced loss of live cell numbers is mainly attributable to cell death. It does not appear, from our data, that general arrest of cell division or induction of differentiation plays a significant role in these effects. The apoptotic nature of nicotine-induced cell death has been confirmed in multiple ways in this study. Cell counts, DNA laddering, TUNEL reactivity, DAPI staining, and the induction of p53 and p21 all indicate that cells undergo apoptotic cell death when treated with nicotine and also implicate at least one of the two cell cycle-dependent proteins in the mediation of this effect. Nicotine seems to have the ability to trigger the expression of p53 only in undifferentiated cells. One possibility is that, in undifferentiated HC2S2 cells, influx of calcium on α-Bgt-AChR activation triggers the expression of p53. Excitotoxicity in neurons has been shown to involve the induction of p53 (Jordan et al., 1997;Sakhi et al., 1997). Differentiated HC2S2 cells might be better equipped to handle calcium loads and thus might be in a position to resist the nicotine-dependent induction of p53 expression. The role of p53 in calcium-mediated toxicity is evident from studies on p53 null mutants. Knock-out mice lacking p53 are resistant to excitotoxic insults (Xiang et al., 1996).
Quantitation of nicotine-mediated changes in the various parameters measured (Tables 1-3) suggests that there might actually be a long-lasting proliferative effect of nicotine on HC2S2 cells, despite the rapid expression of p53 and p21. This finding implies that p53 can overcome the inhibitory effects of p21 on cell cycle as has been shown in other systems (Kagawa et al., 1997). The simplest explanation would be that nicotine induces proliferation of undifferentiated HC2S2 cells, as seen in lung small cell carcinoma cells (Codignola et al., 1994), a large proportion of which undergo p53-mediated apoptotic cell death. This explanation would seem more consistent with current thinking in the field that changes in the dynamic balance between cell division and apoptosis triggered by the same stimulus would ultimately decide the fate of that cell (Xia et al., 1995).
Calcium flux caused by the activation of α-Bgt-AChRs seems to play a major role in the mediation of many of the effects of the receptors. An indication of the developmental consequence of this receptor activation comes from studies of an AChR homolog in C. elegans (Treinin and Chalfie, 1995). In the nematode, a naturally occurring mutation in the channel domain of this homolog results in cell death, probably caused by calcium influx through a slowly desensitizing receptor. A similar calcium-dependent mechanism is indicated for the effects of low nicotine doses on primary progenitors and HC2S2 cells. The cytotoxic effect of low levels of nicotine on HC2S2 cells appears to be dependent on calcium flux from the outside. Reducing extracellular calcium reduces the susceptibility of the cells to the toxic effects of nicotine, as does incubating the cells with nicotine in the presence of KN-62, a Cam kinase inhibitor. Induction of Cam kinase on AChR activation has been demonstrated in PC12 cells (MacNicol and Schulman, 1992). Although these studies show the requirement of calcium for the cytotoxic effects of nicotine, the steps in the pathway in which calcium is required have yet to be worked out. No calcium transients were induced by nicotine, which was as expected. Most of the bulk [Ca]i increases observed with nicotine in neurons come from the activation of voltage-gated calcium channels (Vijayaraghavan et al., 1992). Very little to no functional expression of calcium channels is seen in undifferentiated HC2S2 cells (Hoshimaru et al., 1996). Thus, one can infer that, if calcium flux is an initial step in the pathway, then localized increases in concentrations of the ion passing through α-Bgt-AChR channels must be important. Such localized differences in ligand-gated ion channel-mediated changes in [Ca]i unaccompanied by alterations in bulk [Ca]i underlie changes in gene expression during synaptic transmission in the hippocampus (Deisseroth et al., 1996).
The effect of localized calcium flux through α-Bgt-AChR channels is proving to be very significant, judging from our results and from the results of recent studies. In the absence of observable differences in bulk [Ca]i in the presence or absence of α-Bgt, nicotine activates the production of arachidonic acid in chick ciliary ganglion neurons in culture in a manner sensitive to the toxin (Vijayaraghavan et al., 1992, 1995). In rat hippocampal neurons, activation of α-Bgt-AChRs causes a calcium-dependent increase in the efficacy of glutamatergic transmission in the absence of contributions from voltage-gated calcium channels and at very low concentrations of nicotine. These results suggest that α-Bgt-AChRs are localized in close proximity to release sites in presynaptic terminals of hippocampal neurons (Gray et al., 1996). Our data from HC2S2 cells support a similar spatial relationship between α-Bgt-AChRs and their effector sites.
The acute sensitivity of HC2S2 cells to changes in local calcium levels suggests a poor ability to maintain calcium homeostasis. This might be important for these cells developmentally, making them very responsive to calcium-dependent cues. One reason for the inability of undifferentiated HC2S2 cells to tolerate calcium insults is the lack of adequate calbindin expression. The expression of this protein seems to be differentiation-dependent in these cells. Robust expression of calbindin is observed in differentiated HC2S2 cells. The idea that lack of calbindin expression makes HC2S2 cells susceptible to calcium-mediated toxicity is consistent with studies on cell death in hippocampal neurons (Mattson et al., 1991; Goodman et al., 1993;Beck et al., 1994). Strong evidence comes from our finding that undifferentiated HC2S2 cells transfected with calbindin are resistant to nicotine-mediated apoptosis. However, although calbindin-protected cells that were morphologically indistinct from control untransfected cells, we cannot rule out the possibility that the effects of expression of this calcium buffer are attributable to its ability to induce early differentiation. If true, the results would imply that calbindin expression triggers early differentiation events and also that protection against the toxic effects of nicotine must be conferred to the cells very early on in the differentiation process before morphological changes are observed.
Nicotine appears to have contradictory effects on cell survival in different systems. The drug protects cultured striatal neurons against NMDA receptor-mediated neurotoxicity (Marin et al., 1994). In PC12 cells, nicotine can rescue differentiated cells from NGF deprivation-induced cell death but cannot prevent cell death under the same conditions in undifferentiated cells (Yamashita and Nakamura, 1996). In spinal motoneurons, nicotine promotes survival (Messi et al., 1997). On the other hand, α-Bgt, by its action on neuronal AChRs, also protects motoneurons from naturally occurring cell death (Hory-Lee and Frank, 1995). Our results strongly suggest that nicotinic effects in the brain are not simple consequences of expression levels of AChRs. Phenomena such as nicotine-induced upregulation of receptors might not be primary determinants of the action of this drug. Rather, the expression and functional interaction between components of various signal transduction pathways triggered by nicotine would ultimately determine the effect for a given cell. The effect of nicotine on cell survival probably depends on a number of factors such as specific gene expression, cell cycle stage, developmental stage, levels of trophic factors, and calcium-buffering capabilities. These could determine both the cytotoxic and protective effects of nicotine. At the same time, the complex requirements would enable the drug to selectively target defined population of cells, resulting in elimination or protection. For example, expression of p21 is essential for the survival of differentiating neurons (Poluha et al., 1996), thus possibly making nicotine a candidate for neuroprotection under conditions that might downregulate the expression of the gene (e.g., aging and changes in growth factor levels).
Finally, our results show that nicotine abuse could have significant consequences to the developing CNS in fetuses of mothers who smoke and could alter development of α-Bgt-AChR-containing hippocampal progenitors in the brains of teens and adults using nicotine. Our results suggest that untimely loss or increase of specific progenitor populations could underlie some of the cognitive and behavioral effects of nicotine. Further in vivo experiments, currently under way, will address this issue in greater detail.
Footnotes
This work was supported by grants from the National Institute on Aging and the National Institute of Neurological Diseases and Stroke (F.H.G.), the National Institute on Drug Abuse (S.V.), and a grant-in-aid from the American Heart Association, California Affiliate (S.V.). Part of this work was performed at University of California San Diego, Department of Biology. We thank Profs. Darwin Berg, William Betz, and Nicholas Spitzer and Dr. Angie Ribera for comments on this manuscript. We also thank Dr. Hilmar Bading (Medical Research Council, Cambridge, UK) for the calbindin cDNA and Dr. J. Baudier (Institut National de la Santé et de la Recherche Médicale) for p53 antibodies.
Correspondence should be addressed to Sukumar Vijayaraghavan, Department of Physiology and Biophysics, C-240, University of Colorado Health Sciences Center, 4200 East Ninth Avenue, Denver, CO 80262.
Dr. Berger’s present address: U.318 Institut National de la Santé et de la Recherche Médicale–Université Joseph Fourier De Grenoble, Pavillon B, Centre Hospitalier Universitaire, BP 217, F 38043, Grenoble Cedex 9, France.
REFERENCES
- 1.Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. V: Alpha-bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. [PubMed] [Google Scholar]
- 2.Arends MJ, Harmon BV, Kerr JFR. Apoptosis: the role of the endonuclease. In: Potten CS, editor. Perspectives on mammalian cell death. Oxford UP; New York: 1990. pp. 229–258. [Google Scholar]
- 3.Arneric SP, Sullivan JP, Decker MW, Brioni JD, Bannon AW, Briggs CA, Donnelly-Roberts D, Radek RJ, Marsh KC, Kyncl J, Williams M, Buccafusco JJ. Alzheimer’s disease and associated disorders, Vol 9, pp 50–61. Lippincott–Raven; Philadelphia: 1995. Potential treatment of Alzheimer’s disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolyric-like and cytoprotective properties. [DOI] [PubMed] [Google Scholar]
- 4.Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S. α-Bungarotoxin binding sites in rat hippocampal and cortical cultures: colocalization with α7 subunits and up-regulation by chronic nicotine treatment. Brain Res. 1995;672:228–236. doi: 10.1016/0006-8993(94)01386-v. [DOI] [PubMed] [Google Scholar]
- 5.Beck KD, Hefti F, Widmer HR. Deafferentation removes calretinin immunopositive terminals, but does not induce degeneration of calbindin D-28k and parvalbumin expressing neurons in the hippocampus of adult rats. J Neurosci Res. 1994;39:298–304. doi: 10.1002/jnr.490390307. [DOI] [PubMed] [Google Scholar]
- 6.Bina KG, Guzman P, Broide RS, Leslie FM, Smith MA, O’Dowd DK. Localization of alpha 7 nicotinic receptor subunit mRNA and alpha-bungarotoxin binding sites in developing mouse somatosensory thalamocortical system. J Comp Neurol. 1995;363:321–332. doi: 10.1002/cne.903630212. [DOI] [PubMed] [Google Scholar]
- 7.Broide RS, Robertson RT, Leslie FM. Regulation of alpha7 nicotinic acetylcholine receptors in the developing rat somatosensory cortex by thalamocortical afferents. J Neurosci. 1996;16:2956–2971. doi: 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brustle O, McKay RDG. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996;6:688–695. doi: 10.1016/s0959-4388(96)80104-8. [DOI] [PubMed] [Google Scholar]
- 9.Castro AG, Albuquerque EX. α-Bungarotoxin sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cepko CL. Immortalization of neural cells via a retrovirus-mediated oncogene transduction. Annu Rev Neurosci. 1989;12:47–65. doi: 10.1146/annurev.ne.12.030189.000403. [DOI] [PubMed] [Google Scholar]
- 11.Clarke PBS. Nicotinic receptors in mammalian brain: localization and relation to cholinergic function. Progr Brain Res. 1993;98:77–83. doi: 10.1016/s0079-6123(08)62383-3. [DOI] [PubMed] [Google Scholar]
- 12.Codignola A, Tarroni P, Cattaneo MG, Vicentini LM, Clementi F, Sher E. Serotonin release and cell proliferation are under the control of alpha-bungarotoxin-sensitive nicotinic receptors in small-cell lung carcinoma cell lines. FEBS Lett. 1994;342:286–290. doi: 10.1016/0014-5793(94)80518-0. [DOI] [PubMed] [Google Scholar]
- 13.Corriveau RA, Berg DK. Coexpression of multiple acetylcholine receptor genes in neurons: quantitation of transcripts during development. J Neurosci. 1993;15:2662–2671. doi: 10.1523/JNEUROSCI.13-06-02662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corriveau RA, Romano SJ, Conroy WG, Oliva L, Berg DK. Expression of neuronal acetylcholine receptor genes in vertebrate skeletal muscle during development. J Neurosci. 1995;15:1372–1383. doi: 10.1523/JNEUROSCI.15-02-01372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 16.Eves EM, Tucker S, Downen M, Rosner MR, Wainer BH. Immortal rat hippocampal cell lines exhibit neuronal and glial lineages and neurotrophin gene expression. Proc Natl Acad Sci USA. 1992;89:4373–4377. doi: 10.1073/pnas.89.10.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman R, Wetmore C, Stromberg I, Leonard S, Olsen L. α-Bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulous M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995a;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 21.Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci. 1995b;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman JH, Wasterlain CG, Massarweh WF, Dean E, Sollas AL, Sloviter RS. Calbindin-D28K immunoreactivity and selective vulnerability to ischemia in the dentate gyrus of the developing rat. Brain Res. 1993;606:309–314. doi: 10.1016/0006-8993(93)90999-4. [DOI] [PubMed] [Google Scholar]
- 23.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani J. Hippocampal synaptic transmission is enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- 25.Halvorsen SW, Berg DK. Specific down regulation of the α-bungarotoxin binding component on chick autonomic neurons by ciliary neurotrophic factor. J Neurosci. 1989;9:3673–3680. doi: 10.1523/JNEUROSCI.09-10-03673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardick DJ, Cooper G, Scott-Ward T, Blagbrough IS, Potter BV, Wonnacott S. Conversion of the sodium channel activator aconitine into a potent alpha-7 nicotinic ligand. FEBS Lett. 1995;365:79–82. doi: 10.1016/0014-5793(95)00426-a. [DOI] [PubMed] [Google Scholar]
- 27.Hory-Lee F, Frank E. The nicotinic blocking agents dtubocurarine and α-bungarotoxin save motoneurons from naturally occurring cell death in the absence of neuromuscular blockade. J Neurosci. 1995;15:6453–6460. doi: 10.1523/JNEUROSCI.15-10-06453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshimaru M, Ray J, Sah DWY, Gage FH. Differentiation of the immortalized adult neuronal progenitor c ell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proc Natl Acad Sci USA. 1996;93:1518–1523. doi: 10.1073/pnas.93.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunziker W, Schrickel S. Rat brain calbindin D28: six domain structure and extensive amino acid homology with chicken calbindin D28. Mol Endocrinol. 1988;2:465–473. doi: 10.1210/mend-2-5-465. [DOI] [PubMed] [Google Scholar]
- 30.James JR, Nordberg A. Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: emphasis on Alzheimer’s disease and Parkinson’s disease. Behav Genet. 1995;25:149–159. doi: 10.1007/BF02196924. [DOI] [PubMed] [Google Scholar]
- 31.Jordan J, Galindo MF, Prehn JH, Weichselbaum RR, Beckett M, Ghadge GD, Roos RP, Leiden JM, Miller RJ. p53 expression induces apoptosis in hippocampal pyramidal neuron cultures. J Neurosci. 1997;17:1397–1405. doi: 10.1523/JNEUROSCI.17-04-01397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagawa S, Fujiwara T, Hizuta A, Yasuda T, Zhang WW, Roth JA, Tanaka N. p53 expression overcomes p21WAF1/CIP1-mediated G1 arrest and induces apoptosis in human cancer cells. Oncogene. 1997;15:1903–1909. doi: 10.1038/sj.onc.1201362. [DOI] [PubMed] [Google Scholar]
- 33.Komourian J, Quik M. Characterization of nicotinic receptors in immortalized hippocampal neurons. Brain Res. 1996;718:37–45. doi: 10.1016/0006-8993(96)00024-8. [DOI] [PubMed] [Google Scholar]
- 34.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 35.Landmesser L, Pilar G. Interactions between neurons and their targets during in vivo synaptogenesis. Fed Proc. 1978;37:2016–2022. [PubMed] [Google Scholar]
- 36.MacNicol M, Schulman H. Multiple calcium signaling pathways converge on CaM kinase in PC12 cells. FEBS Lett. 1992;304:237–240. doi: 10.1016/0014-5793(92)80627-s. [DOI] [PubMed] [Google Scholar]
- 37.Marin P, Maus M, Desagher S, Glowinsky J, Premont J. Nicotine protects striatal neurons against N-methyl-d-aspartate receptor-mediated neurotoxicity. NeuroReport. 1994;5:1977–1980. doi: 10.1097/00001756-199410000-00035. [DOI] [PubMed] [Google Scholar]
- 38.Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excitoprotective roles for the calcium-binding protein calbindin D28K in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 39.McGhee DS, Heath MJS, Gelber S, Devay P, Role LW. Nicotinic enhancement of fast excitatory transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 40.Meikrantz W, Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995;58:160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- 41.Messi ML, Renganathan M, Grigorenko E, Delbono O. Activation of α7-nicotinic receptor promotes survival of spinal cord motoneurons. FEBS Lett. 1997;411:32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- 42.Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in the developing brain. J Pharmacol Exp Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- 43.Olds DL, Henderson CR, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- 44.Poluha W, Poluha DK, Chang B, Crosbie NE, Schnoff CM, Kilpatrick DL, Ross AH. The cyclin-dependent kinase inhibitor p21 (WAF) is required for the survival of differentiating neuroblastoma cells. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 46.Quik M, Chan J, Patrick J. α-Bungarotoxin blocks the nicotinic receptor mediated increase in cell number in a neuroendocrine cell line. Brain Res. 1994;655:161–167. doi: 10.1016/0006-8993(94)91610-1. [DOI] [PubMed] [Google Scholar]
- 47.Rogers M, Dani J. Comparison of quantitative calcium flux through NMDA, ATP, and ACh-receptor channels. Biophys J. 1995;68:501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 49.Sakhi S, Bruce A, Sun N, Tocco G, Baudry M, Schreiber SS. Induction of tumor suppressor p53 and DNA fragmentation in organotypic hippocampal cultures following excitotoxin treatment. Exp Neurol. 1997;145:81–87. doi: 10.1006/exnr.1997.6451. [DOI] [PubMed] [Google Scholar]
- 50.Sargent PB. The diversity of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;10:403–457. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 51.Seguela P, Wadiche J, Dinely Moore K, Dani J, Patrick J. Molecular cloning, functional properties and distribution of rat brain α7: a nicotinic channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sexton M, Fox NL, Hebel R. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. Int J Epidemiol. 1990;19:72–77. doi: 10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- 53.Suhonen JA, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 54.Treinin M, Chalfie M. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron. 1995;14:871–877. doi: 10.1016/0896-6273(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 55.Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind α-bungarotoxin raise intracellular free calcium. Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 56.Vijayaraghavan S, Huang B, Blumenthal EM, Berg DK. Arachidonic acid as a possible negative feedback regulator of nicotinic acetylcholine receptors on neurons. J Neurosci. 1995;15:3679–3687. doi: 10.1523/JNEUROSCI.15-05-03679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward JM, Cockroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990;12:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- 58.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 59.Xiang H, Hochman DW, Saya H, Fujiwara T, Schwartzkroin PA, Morrison RS. Evidence for p53-mediated modulation of neuronal viability. J Neurosci. 1996;16:6753–6765. doi: 10.1523/JNEUROSCI.16-21-06753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamashita H, Nakamura S. Nicotine rescues PC 12 cells from death induced by nerve growth factor deprivation. Neurosci Lett. 1996;213:145–147. doi: 10.1016/0304-3940(96)12829-9. [DOI] [PubMed] [Google Scholar]