Abstract
To assess the roles of two integrin α subunits (α6 and α8) in the developing chicken optic tectum, progenitors were infected with retroviral vectors that contained the marker gene lacZ plus antisense sequences from either the α6 or α8 integrin subunit cDNAs. On embryonic day 3 (E3), the vector was injected into tectal ventricles of chicken embryos. On E6, E7.5, E9, or later, chicken embryos were killed, and optic tecta were dissected and processed for histochemical detection of lacZ-positive cells. The antisense-bearing cell clones (descendants of a single infected progenitor) were analyzed for proliferation and migration patterns and were compared with lacZ-only vector-infected control clones. At E6, both α6 and α8 integrin antisense-containing cell clones were similar to controls. At E7.5, integrin α8 antisense-containing clones exhibited a cell number reduction in upper laminae (intermediate zone and tectal plate), and at E9, they exhibited a reduction in the ventricular zone as well. Integrin α6 antisense-containing cell clones exhibited no difference in total cell number at E9 but had a net laminar redistribution of more cells in the ventricular zone and less cells in the tectal plate. Our data show that different integrins play different roles during brain development: α6 integrin is essential for migration of tectal cells into specific laminae, and α8 integrin is essential for the survival of optic tectum cells. Also α8 integrin–substrate interactions may suppress early programmed cell death in premigratory and migratory neuroblasts.
Keywords: integrin, antisense, neuronal migration, apoptosis, retroviral vector, chicken embryo
Neurons born in the mammalian brain migrate long distances along radial glia to new locations, differentiate into specific cell types, develop elaborate morphologies, and form highly specific connections (Rakic, 1985, 1990). This is also true for neurons in the avian optic tectum (Gray et al., 1988; Gray and Sanes, 1991). Many adhesion molecule families participate in the processes mentioned above. Here, we have focused on the integrins. This family contains many α and β subunits that heterodimerize to produce more than 20 different receptors (for review, see Hynes, 1992). Functions of integrins include cell adhesion, organization of the actin-based cytoskeleton, and facilitation of cell migration. Integrins can also activate signal transduction pathways, regulate changes in gene expression, and ultimately affect cell survival by regulating apoptosis (Ruoslahti and Reed, 1994; Clark and Brugge, 1995; Meredith and Schwartz, 1997).
We showed previously that β1 integrins are essential for migration and survival of developing chick optic tectum cells by using a β1 antisense-containing retroviral vector (Galileo et al., 1992). Several possible α subunits could have been involved in mediating the β1 antisense effect. Two α integrin subunits that are potentially involved are α8 and α6.
The α6 integrin subunit is expressed in developing chick embryo brain (Bronner-Fraser et al., 1992) and retina (de Curtis et al., 1991; de Curtis and Reichardt, 1993; de Curtis and Gatti, 1994). Ligands for α6β1 integrin include laminins (de Curtis et al., 1991; de Curtis and Reichardt, 1993; de Curtis and Gatti, 1994; Delwel et al., 1994) and other molecules (Cheresh and Mecham, 1994). Interactions of α6β1 integrin with laminin-1 may mediate growth of avian ciliary ganglion neurons during pathfinding (Weaver et al., 1995). α6 integrin is necessary for early Xenopus nervous system development (Lallier et al., 1996), but a role has not been proposed for later stages of CNS development, such as brain cell migration. α6 integrins may regulate apoptosis, because they are upregulated in some tumor cells (Varner and Cheresh, 1996).
The chicken α8 subunit is expressed in optic tectum and retina (Bossy et al., 1991). The α8β1 integrin receptor can bind to extracellular matrix proteins tenascin-C (tenascin/cytotactin), vitronectin, and fibronectin (Schnapp et al., 1995). α8β1 integrin receptors mediate interactions of embryonic chick motor and sensory neurons with tenascin-C (Varnum-Finney et al., 1995) and promote attachment, cell spreading, and neurite outgrowth on fibronectin in vitro(Müller et al., 1995). α8 integrin is also likely to be involved in the regulation of axonal and dendritic growth of some neurons in the developing rat CNS (Einheber et al., 1996).
Because α8 and α6 integrins are found in developing tectum, promote neurite outgrowth in vitro, and bind to extracellular molecules found in developing tectum, we hypothesized that they are important during tectal development. Our results suggest that these two integrins may be used simultaneously during brain development in both general and specific manners.
MATERIALS AND METHODS
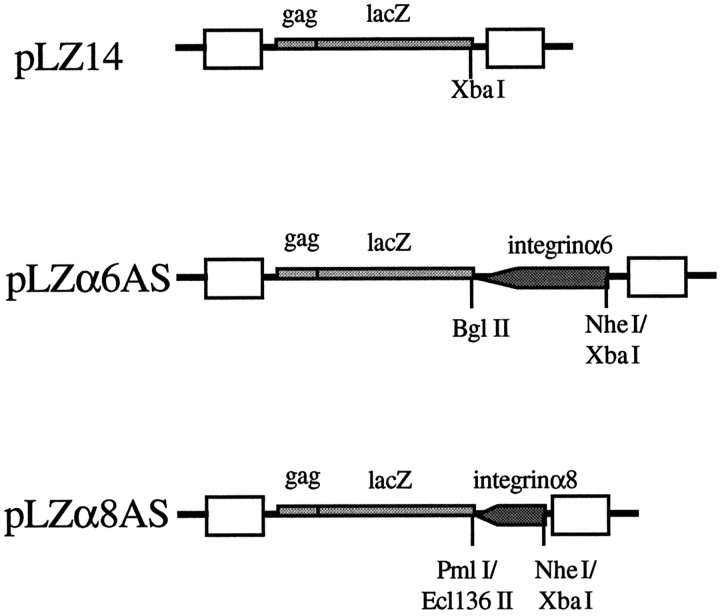
Virus production. The retroviral vectors used in this study are shown in Figure 2. pLZx denotes the plasmid encoding the viral genome, whereas LZx denotes the virus itself. pLZ14 is a lacZ-only vector that has been described previously (Galileo et al., 1992). pLZα6AS was made by ligating a 1.4 kbXbaI-BglII cDNA fragment of the chicken α6 integrin subunit (de Curtis et al., 1991) (obtained from Dr. Louis F. Reichardt, Howard Hughes Medical Institute, University of California at San Francisco) in the antisense orientation into unique NheI and BglII sites after lacZ in a derivative of pLZ14 (called pLZ14-TCS; data not shown) previously made for the purpose of cloning antisense sequences after the lacZ gene. The restriction enzymeNheI was added to the ligation mixture to reduce background ligation of pLZ14-TCS. The antisense sequences used for pLZα6AS are targeted against the common 5′ end of both α6A and α6B splicing variants. pLZα8AS was made similarly to pLZα6AS by placing a 0.3 kbXbaI-Ecl 136II fragment of the chicken α8 integrin cDNA (Bossy et al., 1991) (obtained from Dr. Louis F. Reichardt) in the antisense orientation into the unique NheI and Pml I sites after lacZ in pLZ14-TCS. The restriction enzymes Pml I andNheI were added to the ligation mixture to reduce background. Here, XbaI was compatible with and ligated toNheI, and Ecl 136 II was compatible with and ligated to Pml I. Constructs were confirmed by extensive restriction enzyme digestion analysis (data not shown).
Fig. 2.
Retroviral vectors used in this study. pLZ14, pLZα6AS, and pLZα8AS encode gag-lacZ fusion proteins. pLZα6AS and pLZα8AS are similar to pLZ14 with the addition of an antisense sequence (filled arrow) against either the α6 or the α8 integrin subunit. Boxes indicate viral long terminal repeats. Key restriction sites used in construction of the antisense vectors are marked. See Materials and Methods and Results for details.
Plasmid DNA to be used for virus production by transfection was purified by either Qiagen (Qiagen Inc., Chatworth, CA) or Promega Wizard Maxiprep (Promega, Madison, WI) DNA purification columns according to the recommended procedures. All viral vectors were produced by transient transfection of vector and helper plasmids into the QT6 quail fibrosarcoma cell line (Moscovici et al., 1977). These cells were used because they are highly transfectable by the calcium phosphate precipitation method and contain no endogenous Rous sarcoma virus sequences. To produce virus, 10 cm dishes of subconfluent QT6 cells were transfected with a mixture of 10 μg of vector plasmid plus 10 μg of helper plasmid (pBH1210) (Galileo et al., 1992) per plate using the method of Chen and Okayama (1987). Media containing shed virus particles was collected 2 and 3 d after transfection and concentrated by centrifugation at 15,000 rpm (∼30,000 ×g) in a Beckman SW28 rotor for 2.5 hr, or at 13,000 rpm for 12 hr. Both conditions resulted in good recovery of concentrated virus. Titers were determined by infection of QT6 cells in the presence of 10 μg/ml polybrene and subsequent staining for lacZ (see below) 2 d later.
Embryos. Fertilized White Leghorn chicken eggs were obtained from SPAFAS (Roanoke, IL) and incubated at 37.5°C until the desiredHamburger and Hamilton (1951) stage was reached. Embryos were injected with viral concentrate at stages 16–18 [embryonic day 3 (E3)]. Viral concentrates (1–2 μl) were injected into the right tectal ventricle (Gray et al., 1988) using a pulled glass micropipette and a picopump (World Precision Instruments, Sarasota, FL). Before injection, 20–25 μl of concentrate was mixed with 1 μl of 1 mg/ml polybrene and 2 μl of 1% fast green dye. After injection, a few drops of sterile-filtered ampicillin (50 μg/ml) were placed on the embryo before the egg window was sealed with transparent tape, and the eggs were returned to the incubator. At appropriate times thereafter, embryos were removed from the eggshells, and tecta were dissected in calcium- and magnesium-free Tyrode’s solution and fixed in 2% formaldehyde (ACS grade, Sigma, St. Louis, MO) in PBS (150 mm NaCl, 15 mm sodium phosphate, pH 7.3) for 1–2 hr. Tecta were then rinsed in PBS several times and incubated overnight in a solution containing 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal; 1 mg/ml), 60 mm potassium ferricyanide, 60 mmpotassium ferrocyanide, and 2 mm MgCl2 in PBS. The next day, tecta were rinsed several times in PBS, post-fixed in 2% formaldehyde/2% glutaraldehyde in PBS, rinsed in PBS, and cleared in 70% glycerol. LacZ-positive cells were visualized with a dissecting microscope. Gathering of data concerning cell number and distribution of cells within clones was performed using a compound microscope. Sections containing clones of lacZ-positive cells were hand-cut and mounted on glass slides in glycerol. Every injection experiment involved injecting some embryos with an antisense vector and some embryos with the lacZ-only vector for direct comparison. Student’st test was used to compare mean numbers of cells/clone between lacZ-only and antisense-infected tecta.
Immunohistochemistry. Tecta were fixed by immersion in 2% formaldehyde (ACS grade) in PBS for ∼2 hr, rinsed in PBS, and sunk overnight in 30% sucrose in PBS at 4°C. Tecta were submerged in Tissue-Tek O.C.T. compound (Miles, Inc., Elkhart, IN) and frozen on dry ice. Cryostat sections were cut at ∼10 μm thickness and air-dried onto glass Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). To detect the low levels of α6 and α8 integrins present in the optic tectum, we used an indirect triple-layer immunofluorescent technique. For this, sections were incubated in primary antibody against either the α6 (P2C62C4 monoclonal antibody obtained from Dr. A. F. Horwitz, University of Illinois) or α8 (polyclonal anti-human α8 obtained from Dr. Lynn Schnapp, Mount Sinai Medical Center; polyclonal anti-chicken α8 obtained from Dr. Louis F. Reichardt) subunit of integrin in PBS containing 5% fetal bovine bovine serum (FBS) and 0.03% Triton X-100 for 0.5–1 hr at room temperature. Sections were then rinsed in PBS/FBS, incubated in a biotinylated secondary antibody raised against the species of the primary antibody (mouse or rabbit) in PBS/FBS/Triton X-100 for 0.5–1 hr, rinsed, and incubated in fluochrome-conjugated streptavidin in PBS/FBS/Triton X-100 for 0.5–1 hr. Immunofluorescent staining was visualized on a Nikon Microphot microscope equipped for epifluorescence.
Tests of antisense suppression. To test our antisense-containing vectors for their ability to attenuate their target integrin subunit, tecta were infected in vivo and then optic tectum (OT) cells were recovered several days later and analyzed for integrin expression by flow cytometry. Tectal ventricles were injected with virus at E3 as above for clonal analysis. On E7, tecta were dissected, the meninges were removed, and tecta were minced and incubated in 0.05% trypsin/0.02% EDTA for 10 min. A solution containing soybean trypsin-inhibitor and DNase I was then added, and the tissue was dissociated into single cells by trituration as described previously (Galileo et al., 1992). Single cells were pelleted gently by centrifugation and resuspended in 1% formaldehyde (ACS grade) for 30 min for fixation. Cells were gently pelleted, rinsed in PBS, and then rinsed in PBS containing 5% FBS (PBS/FBS). Cells were immunostained for lacZ and either α6 or α8 integrin by incubating cells in PBS/FBS containing anti-lacZ antibodies (Galileo et al., 1992), anti-integrin antibodies (see above method for immunostaining of tissue sections), and 0.03% Triton X-100 for 0.5 hr at room temperature. Cells were pelleted, rinsed in PBS/FBS, and resuspended in secondary donkey anti-primary species conjugated to R-phycoerythrin (Jackson Immunochemicals, West Grove, PA) to visualize lacZ-positive cells and biotinylated antibodies against the species in which the anti-integrin primary antibody was raised in PBS/FBS/Triton X-100 for 0.5 hr. After cells were rinsed in PBS/FBS, they were incubated in streptavidin–fluorescein in PBS/FBS/Triton X-100 for 0.5 hr to visualize integrin staining. Cells were rinsed in PBS/FBS and then subjected to two-color flow cytometry analysis on a Becton Dickinson (Mountain View, CA) FACS/Calibur. The level of integrin subunit immunostaining was analyzed on infected lacZ-positive cells as well as on uninfected lacZ-negative cells.
The vector LZα6AS was also tested for its ability to reduce α6 integrin expression in infected QT6 cells in vitro, which express this subunit. For this, QT6 cell cultures were infected with either LZ14 or LZα6AS. Approximately 1 week later, cells were removed from the dish by incubation in 0.05% trypsin/0.02% EDTA solution for 1–2 min and resuspended as single cells. Cells were gently pelleted and resuspended in 1% formaldehyde (ACS grade) for fixation for 30 min. After cells were rinsed in PBS/FBS, they were incubated in fluorescein–anti-mouse and either Texas Red-anti-rabbit or R-phycoerythrin-anti-rabbit in PBS/FBS/Triton X-100 for 30 min. Cells were rinsed and subjected to two-color flow cytometry analysis on a FACS/Calibur (Becton Dickinson) or an EPICS ELITE (Coulter Electronics, Hialeah, FL) flow cytometer. Means and SDs of plots were calculated using Becton Dickinson or Coulter software.
Flow cytometry analysis of end labeling. To demonstrate that the α8 integrin subunit is important for the survival of OT cells, tecta were infected in vivo, and single cells from E7–E8 tecta were analyzed for their pattern of DNA end labeling by flow cytometry. The end-labeling method used is an extremely sensitive fluorescent method that we developed to demonstrate widespread apoptosis in tissue sections during normal early OT development (Zhang and Galileo, 1998). For this, tectal ventricles were injected with virus on E3 as above for clonal analysis. On E8, tecta were dissociated into single cells as above except that no DNase I was used. Cells were fixed in suspension as above, rinsed with PBS, and then resuspended in 0.1% Triton X-100 in PBS for 10 min. After cells were rinsed with PBS twice, they were resuspended in a terminal transferase reaction mixture (15 U TdT/100 μl, 0.05 nmol digoxigenin-dUTP/100 μl, and 1× TdT buffer) for 1 hr at 37°C. Cells were then rinsed twice in PBS/FBS and resuspended in monoclonal anti-digoxigenin (Boehringer Mannheim, Indianapolis, IN) and polyclonal anti-lacZ antibody in PBS/FBS for 30 min at room temperature. After cells were rinsed in PBS/FBS, they were resuspended in biotinylated goat anti-mouse in PBS/FBS for 30 min at room temperature. After cells were rinsed, they were resuspended in R-phycoerytherin–anti-rabbit and streptavidin–fluorescein for 30 min at room temperature. After cells were rinsed, they were resuspended and subjected to analysis on a FACS/Calibur (Becton Dickinson) flow cytometer. Fluorescent end labeling was analyzed on infected lacZ-positive cells as well as on uninfected lacZ-negative cells. Means and SDs of histogram plots were calculated using Becton Dickinson Cell Quest software.
RESULTS
Integrin α8 and α6 expression in developing tectum
To demonstrate that α6 and α8 integrins and some of their known substrates are expressed in the developing chicken optic tectum specifically during periods of cell migration, we immunostained frozen sections of developing tecta. A sensitive triple-layer immunofluorescent staining method was used to visualize the low levels of α6 and α8 integrin subunit present in the optic tectum. Others have reported α6 integrin expression in the developing brain and spinal cord (Bronner-Fraser et al., 1992). Bossy et al. (1991) reported α8 integrin expression in E6 tectum, before neuronal migration occurs. We wished to extend these results to tectum specifically when cell migration occurs. E7 tectum sections were immunostained with two different polyclonal antibodies specific for integrin α8: one against chicken α8 and the other against human α8. Use of both of these antibodies resulted in a low-level widespread immunostaining pattern where cell surface outlines were visible. Figure1A shows immunostaining using the anti-chicken antibody where immunoreactivity appeared to be elevated in the axon-rich intermediate zone (IZ). For α6 integrin, a monoclonal antibody specific for chicken α6 integrin was used (Bronner-Fraser et al., 1992). Immunostaining was widespread such that cell surface outlines were weakly visible (Fig. 1B). Staining appeared to be higher in the germinative ventricular zone (VZ). Control sections in which the primary antibodies were omitted showed no visible staining. Contrast enhancement was used in these photomicrographs to emphasize the uneven distribution of the different subunits.
Fig. 1.
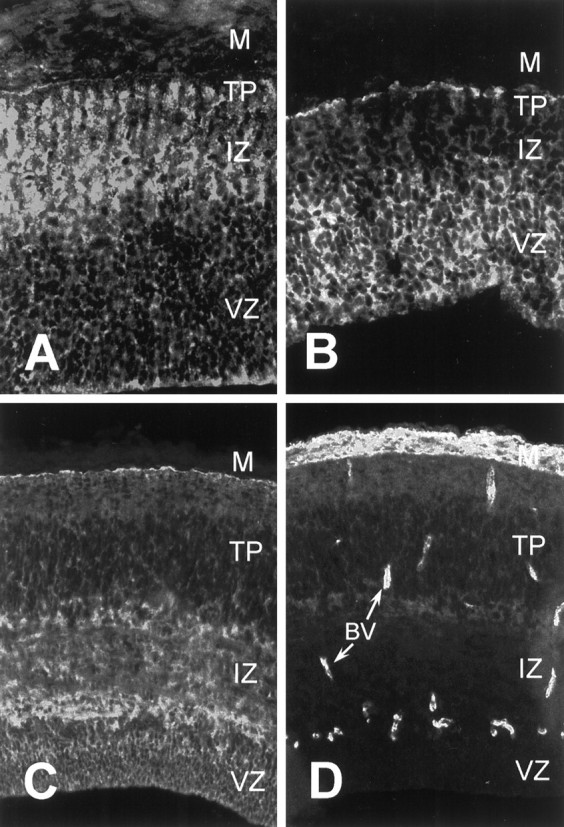
Expression of integrin subunits and substrates in the developing optic tectum. Cryosections of developing optic tectum were immunostained with antibodies against integrin α8 (A), integrin α6 (B), tenascin-C (C), and fibronectin (D). A, B, E7 tectum; C, D, E9 tectum. For all parts, the ventricular surface is down. Arrows inD denote blood vessels (BV).M, Meninges; VZ, ventricular zone;IZ, intermediate zone; TP, tectal plate. See Results for details.
We found two integrin α8β1 substrates, tenascin-C and fibronectin, expressed during neuronal migration. As shown in Figure 1C, moderate to high levels of anti-tenascin immunostaining were present at E9, primarily in the axonal layers, the IZ and stratum opticum (just deep to the meninges), as others have reported (Perez and Halfter, 1994; Yamagata et al., 1995). Anti-fibronectin immunostaining was widespread and present at low levels (Fig. 1D). The brightest staining was around blood vessels and in the meninges. Staining in the neural tissue was present at low levels in the tectal plate (TP) and tapered off to be undetectable in the VZ. Fibronectin has been found previously on mammalian cortical radial glia (Sheppard et al., 1991) but has not been reported in the developing tectum. Thus, we have found immunoreactivity for both α8 and α6 integrin subunits and two possible substrates for α8β1 integrin in developing tectum during periods of neuronal migration. We could not detect staining for the α6β1 integrin substrate laminin within the neural tissue of the optic tectum by triple-layer immunofluorescence [data not shown; 31–2 monoclonal antibody (Bayne et al., 1984)].
Construction and testing of antisense vectors
We constructed two recombinant retroviral vectors (LZα6AS and LZα8AS) that contained the lacZ marker gene plus sequences from the chicken α6 or α8 integrin subunits cloned in the antisense orientation (Fig. 2). These sequences both spanned the translational initiation site of their target messages and were cloned directly after lacZ in the vector constructs. LacZ served as a permanent marker to identify infected cell progeny to allow the distribution, cell number, and migration patterns to be followed. This strategy was used previously for the construction of the vector LZ16 for attenuating expression of the β1 integrin subunit (Galileo et al., 1992). This strategy was chosen also because there is only one possible transcript (from the viral LTR), and thus every cell containing the lacZ gene product contains the desired antisense sequence at the end of the lacZ message. Here, we provide evidence that α6 integrin is necessary for some tectal cells to migrate out of the ventricular zone and into the tectal plate. We also provide evidence that α8β1 integrin is involved in general survival and growth of optic tectum cells.
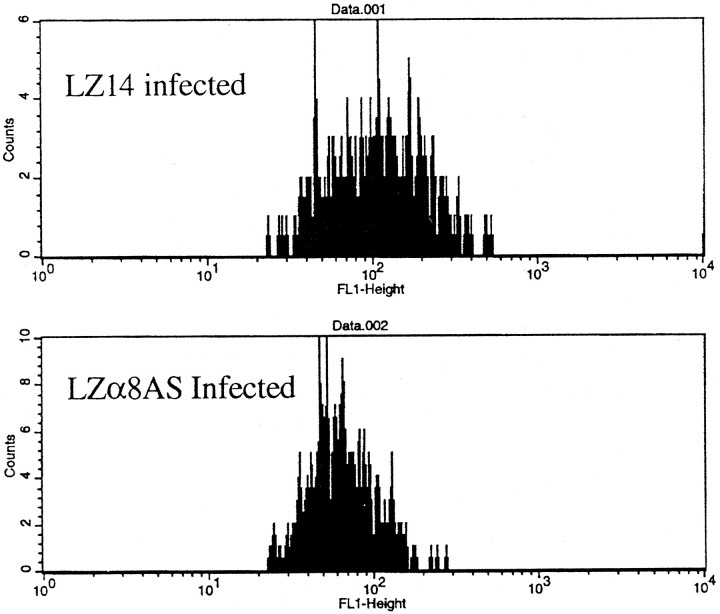
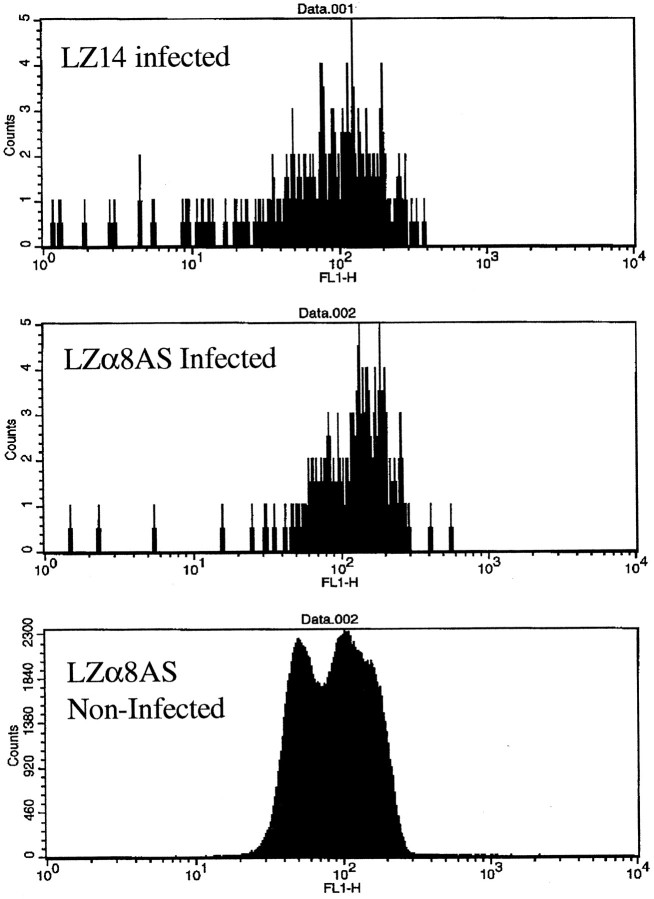
To test the ability of LZα8AS and LZα6AS to attenuate their respective integrins, we produced virions and infected E3 tectain vivo. Four days later, tecta were gently dissociated and cells were doubly immunostained with antibodies against lacZ and either α8 or α6 integrin. They then were subjected to two-color analysis by flow cytometry. We found that the mean level of the immunostaining peak for α8 integrin on OT cells infected with LZα8AS was reduced significantly compared with OT cells infected with LZ14 (Fig.3) (p < 0.0001) as well as compared with uninfected cells from the same tecta (p < 0.05; data not shown). We found that the mean level of the immunostaining peak for α6 integrin on OT cells infected with LZα6AS was also reduced (data not shown), although the results were less consistent.
Fig. 3.
Analysis of α8 integrin immunostaining of optic tectum cells by flow cytometry. Tecta were injected with either LZ14 or LZα8AS on E3, and dissociated tectal cells were analyzed for α8 integrin immunostaining on E7. The top panel shows α8 integrin levels in cells infected by LZ14. The bottom panel shows α8 integrin levels in cells infected by LZα8AS (p < 0.0001).
To further test LZα6AS, we analyzed by flow cytometry infected QT6 cells [a quail fibrosarcoma cell line (Moscovici et al., 1977)], which express a higher level of α6 integrin. QT6 cells were dissociated, doubly immunostained, and analyzed. Levels of α6 integrin immunostaining were markedly reduced on QT6 cells (p < 0.0001) (Fig.4) infected with LZα6AS compared with those infected with a lacZ-only control vector LZ10 [a functional equivalent of LZ14; see Galileo et al. (1990, 1992)]. Thus, the vectors LZα8AS and LZα6AS attenuated their targeted integrins significantly in OT cells infected in vivo and in QT6 cellsin vitro.
Fig. 4.
Analysis of α6 integrin immunostaining of QT6 cells by flow cytometry. QT6 cells were infected with either LZ10 or LZα6AS and analyzed for α6 integrin after dissociation and immunofluorescent staining. The filled graph represents levels of α6 integrin in control LZ10-infected cells. Theunfilled graph represents levels of α6 integrin in LZα6AS-infected cells (p < 0.0001).
Effects of α6 integrin antisense in vivo
At E3, we infected progenitor cells in the developing chick optic tectum with retroviral vectors containing antisense sequences against the α6 or α8 integrin subunit to assess cell requirements for these molecules during proliferation and migration. Experiments contained embryos injected with each type of virus (some antisense and some lacZ-only control). Groups of tecta (both virus types) were then fixed at E6, E7.5, E9, and E12, histochemically stained for lacZ, and sectioned by hand. Clones of lacZ-positive cells were identified and analyzed as before (Galileo et al., 1992). Features of lacZ-only (LZ14) and lacZ-antisense (LZα6AS or LZα8AS) were compared.
E6
At E6, the tectum is composed of a thick VZ with a thin superficial marginal zone made up of axons from the large multipolar efferent neurons. Cells infected on E3 with the control vector (LZ14) have divided to form marked clones of ∼12 cells each. These clones appeared as radial arrays of cells that spanned the thickness of the ventricular zone.
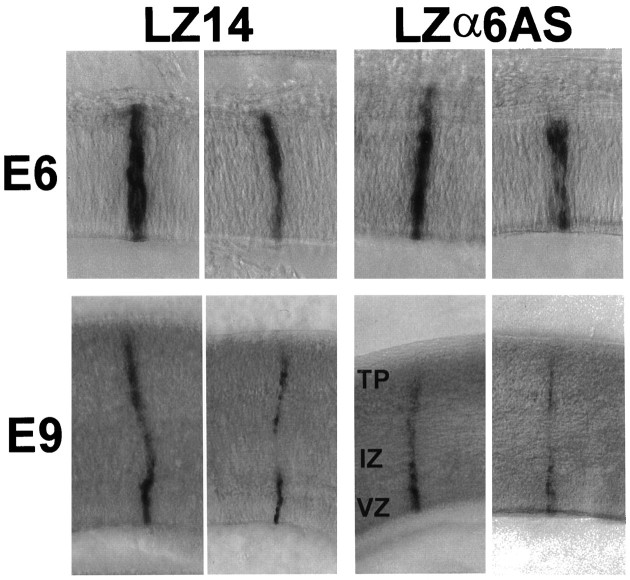
At E6, the appearance (Fig. 5,top) and average size of α6 integrin antisense-expressing clones was not different from that of control LacZ-only-expressing clones of ∼12 cells (p = 0.25) (Table1). Thus, the presence of α6 antisense sequences did not affect proliferation or radial stacking of cells within the ventricular zone.
Fig. 5.
Appearance of LZα6AS-infected cell clonesin vivo. Tecta were injected with LZ14 or LZα6AS on E3 and processed for X-gal histochemistry on E6 (top) and E9 (bottom). Shown are LZ14 control clones (left) or LZα6AS clones (right) in thick sections.
Table 1.
Effect of LZα6AS on E6 tectal cells
| Vector | Number of clones analyzed | Mean cell number/clone |
|---|---|---|
| LZα6AS | 218 | 11.4 ± 0.6 |
| LZ14 | 208 | 12.5 ± 0.7 |
Tectal ventricles were injected with LZα6AS or LZ14 on E3 (approximately stage 17), and tecta were analyzed at E6. The size of a clone of both control (LZ14) and experimental (LZα6AS) clones is given as mean cell number ± SEM. Significance of differences between mean numbers of cells was assessed by the two-tailedt test. p = 0.25.
E9
We did not analyze LZα6AS cell clones at E7.5. At E9, LZα6AS cell clones appeared to be normal by visual inspection (Fig. 5,bottom). The histochemical staining for lacZ in the LZα6AS clones at E9 appeared similar to LZ14 clones. After quantitation, there was no difference in the total cell number per clone between α6 integrin antisense-expressing and the control LZ14 clones. However, there was a significant difference in the distribution of the cells within two of the three laminae (Table2). Differences in cell numbers between LZα6AS and control LZ14 clones were found in the tectal plate (p < 0.0001) and ventricular zone (p < 0.01). There was no difference in the net cell number present in the intermediate zone (p= 0.86). There were more cells (2.3 cells more) in the ventricular zone and fewer cells (four cells less) in the tectal plate for LZα6AS clones compared with LZ14 control clones. Although the number of additional cells in the ventricular zone does not equal the number of fewer cells in the tectal plate, it is possible that the cells missing from the tectal plate are the same cells remaining in the ventricular zone. If this is correct, our results suggest that integrins containing an α6 subunit are necessary for the correct radial migration of a subpopulation of tectal cells destined for the tectal plate. Introduction of α6 integrin antisense appears to have delayed or prevented the migration of this subpopulation of tectal cells from the ventricular zone into the tectal plate.
Table 2.
Effects of LZα6AS on cell number and distribution at E9
| Clonal cell distribution | LZα6AS (n = 166) | LZ14 (n = 128) | p value |
|---|---|---|---|
| Total clone | 18.0 ± 0.8 (100%) | 19.8 ± 1.0 (100%) | 0.18 |
| Tectal plate | 5.0 ± 0.4 (28%) | 9.0 ± 0.6 (45%) | <0.0001 |
| Intermediate zone | 3.0 ± 0.2 (17%) | 3.0 ± 0.2 (15%) | 0.86 |
| Ventricular zone | 10.0 ± 0.4 (55%) | 7.7 ± 0.4 (39%) | <0.01 |
Tectal ventricles were injected with LZα6AS or LZ14 on E3 (approximately stage 17), and tecta were analyzed at E9. For the vector LZα6AS, the number of clones used for analysis was 166 (n = 166); for the vector LZ14, the number of clones used for analysis was 128 (n = 128). The cell distribution of mean cell number in each layer, ventricular zone, intermediate zone, and tectal plate, as well as mean total cells per clone, are expressed as the mean cell number ± SEM. Significance of differences between control and experimental mean numbers of cells was assessed by the two-tailed t test.
From the results in Table 2, if LZα6AS affected the majority of clones to a similar extent, then there would be corresponding shifts in the cells within the affected laminae among the majority of clones. To determine whether this was the case, relative frequency histograms were plotted for the VZ and TP of LZ14 and LZα6AS clones at E9 (Fig.6). These histograms show that within the VZ there was a corresponding, small shift in the population of LZα6AS clones toward containing more cells (Fig. 6, top). Also, for the TP there was a corresponding shift in the population of LZα6AS clones toward containing fewer cells (Fig. 6, bottom). These results suggest that most of the LZα6AS clones were affected similarly.
Fig. 6.
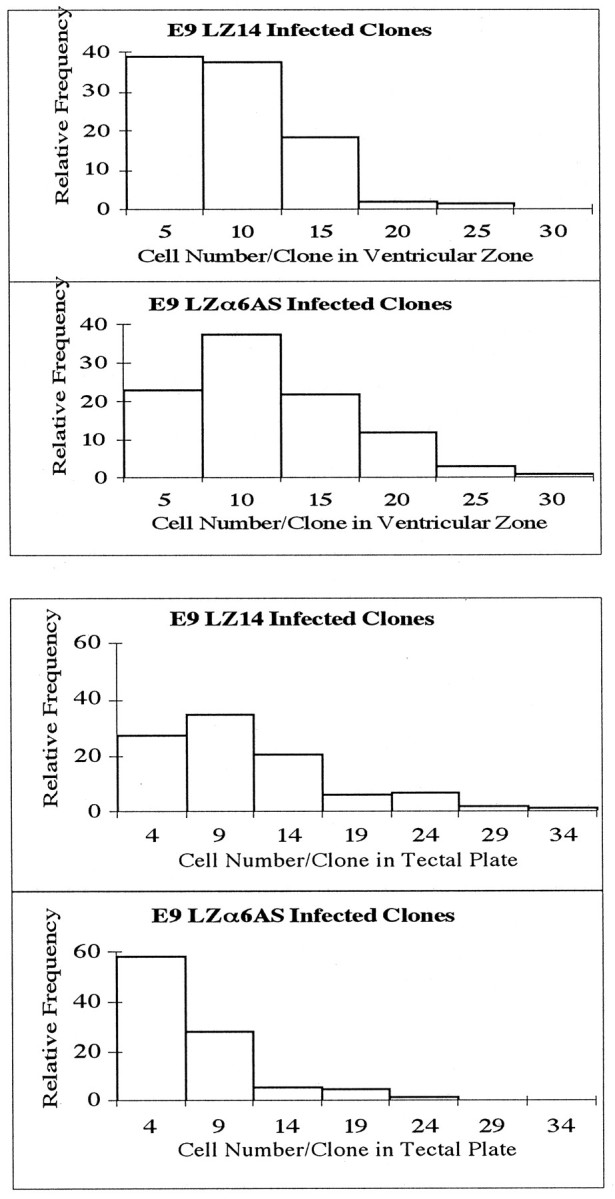
Relative frequency histogram of affected zones in LZα6AS-infected tecta at E9. The top panels show the relative frequency distribution of LZα6AS- and LZ14-infected cells within the ventricular zone. The bottom panels show the relative frequency distribution of LZα6AS- and LZ14-infected cells within the tectal plate.
Effects of α8 integrin antisense in vivo
E6
As shown in Figure 7 (top), typical E6 cell clones of both control (LZ14) and experimental (LZα8AS) groups were similar with respect to cell number, distribution, and the intensity of LacZ staining. Statistical analyses of E6 results are presented in Table 3. Cell numbers were similar for both groups (p = 0.49). Thus, the presence of α8 integrin antisense sequences did not have an effect on either the proliferation of infected progenitors or the radial stacking of clonal progeny at this stage.
Fig. 7.
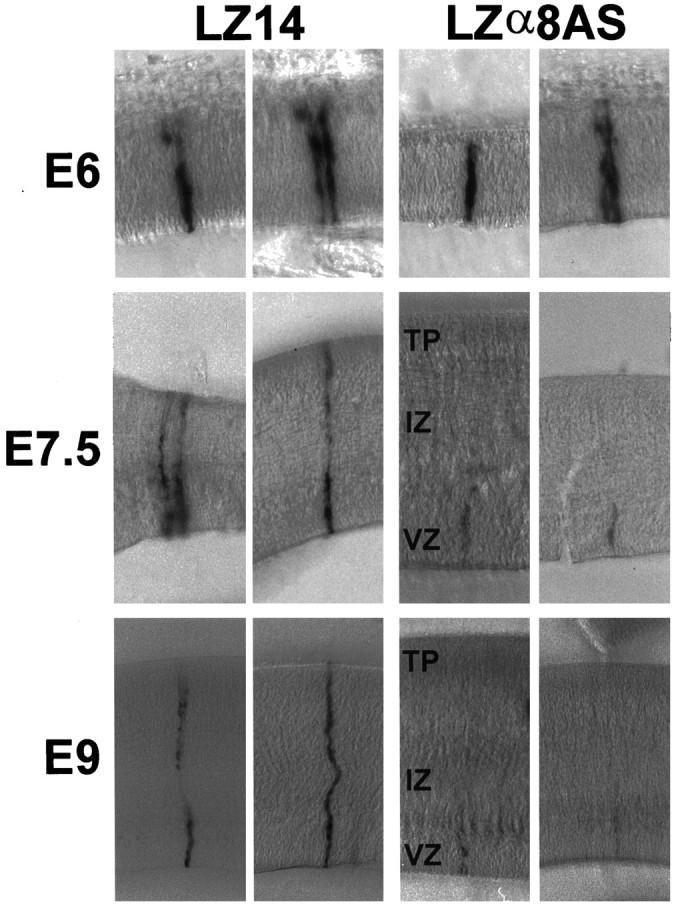
Appearance of LZα8AS-infected cell clonesin vivo. Tecta were injected with LZ14 or LZα8AS on E3 and processed for X-gal histochemistry on E6 (top), E7.5 (middle), or E9 (bottom). Shown are LZ14 control clones (left) or LZα8AS clones (right) in thick sections.
Table 3.
Effect of LZα8AS on E6 tectal cells
| Vector | Number of clones analyzed | Mean cell number/clone |
|---|---|---|
| LZα8AS | 163 | 11.6 ± 0.6 |
| LZ14 | 104 | 10.9 ± 0.7 |
Tectal ventricles were injected with LZα8AS or LZ14 on E3 (approximately stage 17), and tecta were analyzed at E6. The average size of a clone of both control (LZ14) and experimental (LZα8AS) clones is given as mean cell number ± SEM. Significance of differences between mean numbers of cells was assessed by the two-tailed t test. p = 0.49.
E7.5
After E6, cells begin to migrate out of the ventricular zone along radial glial cells so that by E7.5 the tectum consists of a ventricular zone, an intermediate zone (the former marginal zone), and a newly forming tectal plate. Typical cell clones are shown in Figure 7(middle), and it can be seen that less staining is evident in upper laminae of the antisense α8-expressing cell clones. The average total cell number per clone as well as cell number in the tectal plate and the intermediate zone was reduced (p < 0.0001) for α8 integrin antisense-expressing cell clones (Table4). However, the cell number in the ventricular zone of α8 integrin antisense-expressing cell clones was similar to that of control clones (p = 0.65). In the control (LZ14) clones, there was a substantial net increase in the total cell number at E7.5 compared with E6 (approximately 10 cells), as well as a substantial redistribution of cells out of the ventricular zone into upper laminae (approximately 10 cells). In α8 antisense-containing clones, there was only a slight net increase in either total cell number at E7.5 compared with E6 (approximately two cells) or redistribution into upper laminae (approximately one cell).
Table 4.
Effects of LZα8AS on number and distribution of tectal cells at E7.5
| Clonal cell distribution | LZα8AS (n = 134) | LZ14 (n = 55) | p value |
|---|---|---|---|
| Total clone | 13.7 ± 0.8 (100%) | 21.2 ± 2.0 (100%) | <0.0001 |
| Tectal plate | 0.4 ± 0.1 (3%) | 5.0 ± 0.6 (24%) | <0.0001 |
| Intermediate zone | 1.0 ± 0.2 (7%) | 4.5 ± 0.6 (21%) | <0.0001 |
| Ventricular zone | 12.3 ± 0.4 (90%) | 11.7 ± 1.1 (55%) | 0.65 |
Tectal ventricles were injected with LZα8AS or LZ14 on E3 (approximately stage 17), and tecta were analyzed at E7.5. For the vector LZα8AS, the number of clones used for analysis was 134 (n = 134); for the vector LZ14, the number of clones used for analysis was 55 (n = 55). The cell distribution of a mean cell number in each layer, ventricular zone, intermediate zone, and tectal plate, as well as mean total cells per clone, are expressed as the mean cell number ± SEM. Significance of differences between control and experimental mean numbers of cells was assessed by the two-tailed t test.
The significant difference between mean numbers of cells in the intermediate zone and tectal plate in LZ14 and LZα8AS clones could be attributable to either a large effect on a subpopulation of LZα8AS clones or a lesser effect on the majority of clones. To distinguish between these two possibilities, results from these two laminae (IZ and TP) are shown as relative frequency histograms in Figure8. It can be seen for both laminae that most of the population of clones were drastically reduced in cell number in LZα8AS-infected clones. Thus, LZα8AS appears to have affected most, if not all, clones.
Fig. 8.

Relative frequency histogram of tectal clones infected with LZα8AS and LZ14 at E7.5. The top panelsshow the relative frequency distribution of LZα8AS- and LZ14-infected cells within the tectal plate. The bottom panels show the relative frequency distribution of LZα8AS- and LZ14-infected cells within the intermediate zone.
E9
Between E7.5 and E9, some proliferation continues and much radial migration occurs, resulting in a substantial thickening of the tectal plate. Shown in Figure 7, bottom, are typical control and α8 antisense-infected cell clones at E9. There was a net increase in total cell number in control clones between E7.5 and E9 of approximately seven cells (Tables 4, 5). However, there was a net decrease in α8 antisense-containing clones by approximately five cells. The mean total cell number in α8 antisense-containing clones and the number in each of the three layers (ventricular zone, intermediate zone, and tectal plate) were dramatically reduced compared with those of the control clones (p < 0.0001 for all comparisons). These decreases suggest that most of the α8 antisense-bearing cells died. Also, in general, α8 antisense-bearing cells that were still present were fainter in staining for lacZ, also suggesting that these remaining cells may be dying.
Table 5.
Effects of LZα8AS on number and cell distribution at E9
| Clonal cell distribution | LZα8AS (n = 103) | LZ14 (n = 65) | p value |
|---|---|---|---|
| Total clone | 8.8 ± 1.0 (100%) | 28.6 ± 2.4 (100%) | <0.0001 |
| Tectal plate | 1.8 ± 0.5 (20%) | 14.8 ± 1.2 (52%) | <0.0001 |
| Intermediate zone | 1.1 ± 0.2 (12%) | 3.6 ± 0.4 (13%) | <0.0001 |
| Ventricular zone | 6.0 ± 0.4 (68%) | 10.1 ± 1.0 (35%) | <0.0001 |
Tectal ventricles were injected with LZα8AS or LZ14 on E3 (approximately stage 17), and tecta were analyzed at E9. For the vector LZα8AS, the number of clones used for analysis was 103 (n = 103); for the vector LZ14, the number of clones used for analysis was 65 (n = 65). The cell distribution of a mean cell number in each layer, ventricular zone, intermediate zone, and tectal plate, as well as mean total cells per clone are expressed as the mean cell number ± SEM. Significance of differences between control and experimental mean numbers of cells was assessed by the two-tailed t test.
To gain a better understanding of the effects of LZα8AS on the depletion of cells over time, a relative frequency histogram of the total cell number/clone for LZα8AS and LZ14 cell clones on E7.5 and E9 is shown in Figure 9. For control LZ14 clones, there was a shift in the population toward slightly larger clones between E7.5 and E9. LZα8AS cell clones as a population, however, were shifted relative to controls toward being smaller in cell number at E7.5. This shift toward smaller clones was even more pronounced at E9. Thus, over time, LZα8AS cell clones became smaller as a population. These data also are consistent with the notion that this antisense vector caused cells to die.
Fig. 9.
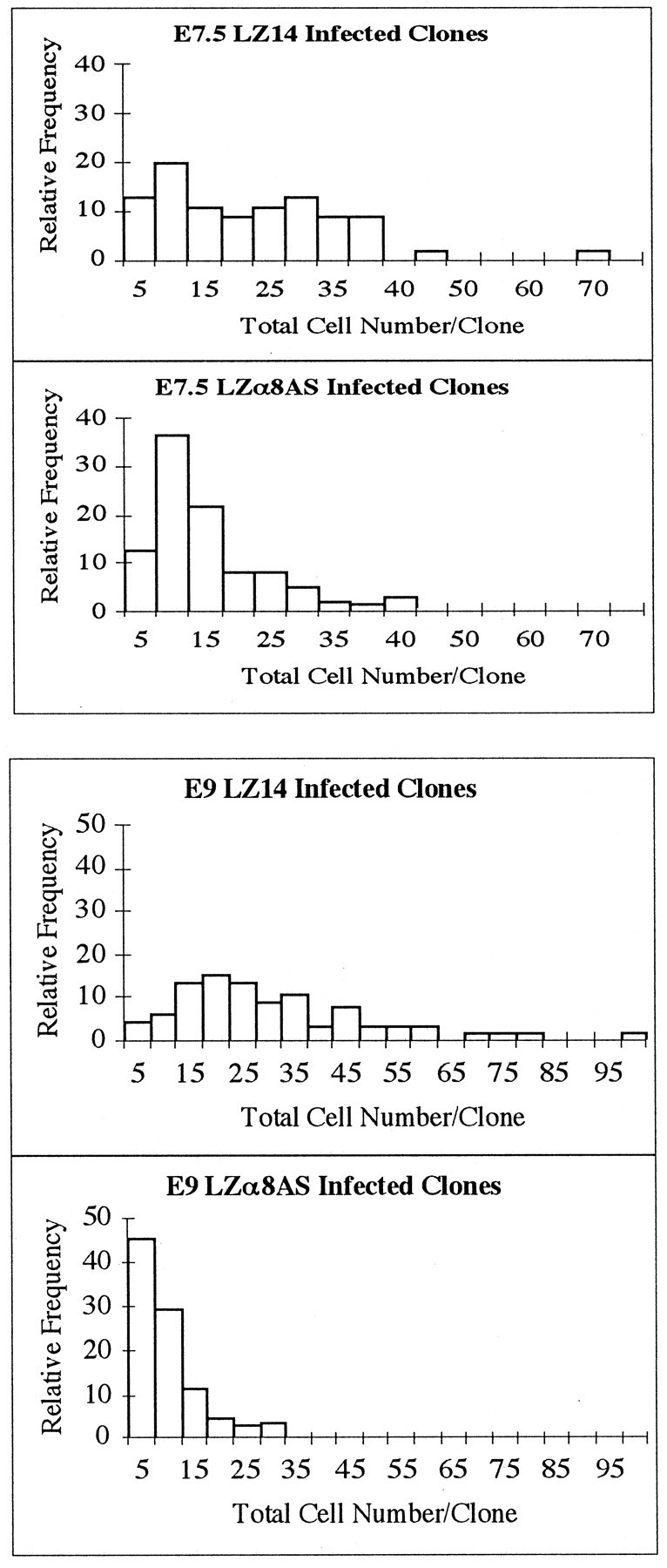
Relative frequency histogram of LZα8AS- and LZ14-infected clones at E7.5 and E9. The top two histograms display the relative frequency distributions of total cell number within clones at E7.5. The bottom two histograms display the relative frequency distributions of total cell number within clones at E9.
LZα8AS virus-infected clones did not appear different from control LZ14 clones until E7.5. It may be that α8β1 first affected the survival of cells that migrated into the intermediate zone and tectal plate or that after E6 fewer cells were produced, so that fewer cells migrated out of the ventricular zone. Our E6 results do not support an effect on cell production, so we favor the first explanation. Integrin α8 antisense-bearing cells in the ventricular zone at E9 were fewer in number than in control clones, indicating that these cells also were affected and eventually died. It is currently unknown whether the earlier-dying cells in the upper laminae and/or the relatively later-dying ventricular zone cells died because of a direct effect or an indirect effect. It is hypothesized that cells died as a direct result of the interruption of integrin signaling events involved in the suppression of apoptosis.
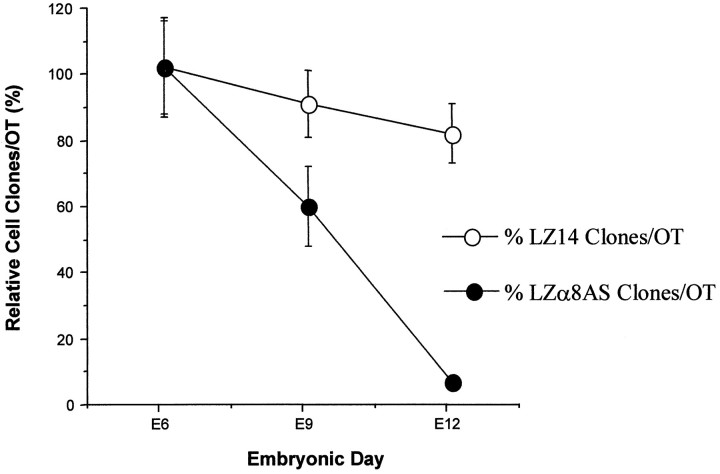
To further explore the possibility of cell death of α8 antisense-expressing cells, previously injected tecta (on E3) of different ages (E6, E9, and E12) were analyzed for the total number of radial clones per embryo. It seemed reasonable that if α8 antisense-containing cells were dying, then there should be a marked decrease in the number of radial clones per embryo by E12. We found this to be the case (Fig. 10). For this experiment, one group of embryos was injected on E3 with control virus LZ14 and another group with antisense virus LZα8AS from the same batch of eggs. At E6, E9, and E12, the tecta of several (four to six) of each type of embryo were processed histochemically for lacZ-positive clones and analyzed. The mean clone number for each virus type at E6 was set as 100%, and the means for later days were displayed as a percentage of this number. Controls showed a slight decrease in the number of radial clones per embryo between E6 and E12. At E12, however, LZα8AS-infected embryos showed a dramatic decrease in clones per embryo to <5% of the number present at E6 (Fig. 10). These results indicate that α8 antisense-expressing cells and clones died.
Fig. 10.
Disappearance of LZα8AS infected clones over time. A group of embryos were injected with either LZα8AS or LZ14 at E3, and some were killed at E6, some at E9, and some at E12. The mean cell clones per tectum at E6 are expressed as 100%, and the mean cell clones per tectum at both E9 and E12 are expressed as the mean percentage (relative to that at E6) ± SEM (error bars).
To investigate the alternative possibility that α8 antisense-containing cells were not dying but were migrating away from radial arrays, E9 tecta were qualitatively analyzed for the presence of cells outside radial arrays. Tectal pieces were analyzed using a dissecting microscope at a magnification at which single, blue cells could be discerned in the tissue that was cleared previously in glycerol. No evidence was found of marked cells outside the radial arrays in α8 antisense-infected tecta. Taken together, our results indicate that α8 antisense-containing cells died and did not migrate from the radial arrays.
Although the α8 antisense-containing cells likely died by apoptosis, we attempted to gain evidence for this by using a very sensitive fluorescent in situ end-labeling technique (FISEL+) (Zhang and Galileo, 1998) and flow cytometry analysis. During normal tectal development between E7 and E9 there exists a widespread naturally occurring peak of FISEL+ labeling (Zhang and Galileo, 1998) similar to ISEL+ labeling seen in developing mammalian cerebral cortex (Blaschke et al., 1996). The sensitive ISEL+ labeling has been regarded as a means to identify apoptotic cells, although the work of Takahashi et al. (1996) suggests otherwise. The peak of labeling in tectum appears to be centered around E7.5–E8. Flow cytometry analysis of dissociated tectum cells labeled by FISEL+ revealed that a considerable number of the total cells were labeled and therefore may be undergoing apoptosis. We performed FISEL+ on LZ14- and LZα8AS-infected OT cells dissociated on E8 to determine whether there was an increase in the mean level of FISEL+ labeling in α8 antisense-containing cells compared with control cells. We found that there was a significant increase in the mean level of FISEL+ labeling in LZα8AS-infected cells compared with LZ14 control-infected cells (Fig. 11) (p < 0.0001). Similarly, there was a significant increase in the mean level of labeling for LZα8AS-infected cells over uninfected cells within the same tecta (p < 0.0005), and there was no significant difference between the mean labeling level of uninfected cells from LZα8AS-infected tecta and LZ14-infected cells (p > 0.10). If FISEL+ labeling does reflect apoptotic cells, as we believe, then the observed increase in mean FISEL+ labeling in α8 antisense-containing cells provides evidence that these cells died by apoptosis.
Fig. 11.
Flow cytometry analysis of LZα8AS-infected cells after FISEL+. The bottom panel shows the FISEL+ labeling pattern of normal uninfected cells from tecta that were injected with LZα8AS at E3 and processed at E8. At least two populations appear to be present, although overlapping. The top panel shows the FISEL+ labeling pattern of LZ14-infected cells (p > 0.10 between means of top and bottom panels). The middle panel shows the FISEL+ labeling pattern of LZα8AS-infected cells in the same population as thebottom panel. These cells showed increased FISEL+ labeling compared with the LZ14-infected controls (p < 0.0001) as well as compared with uninfected cells (p < 0.0005). Many fewer cells are shown in the top two panels than in the bottom panel because FISEL+ labeling was measured only in the small fraction of total dissociated cells that were infected and lacZ+.
Taken together with the observations that α8 antisense-containing cells disappeared from radial arrays almost completely by E12 and did not go elsewhere in the tectum, we conclude that OT cells required α8 integrin for their survival and that OT cells died by apoptosis when α8 integrin was attenuated. Because integrin signaling is known to suppress apoptosis (see Discussion), we believe that the neuronal death was a direct effect of interfering with α8 integrin signaling events. However, we cannot rule out that death was caused by an indirect or secondary effect after inhibition of migration. Perhaps death was a result of the inability of the migration-inhibited cells to enter the correct microenvironment for survival (e.g., neurotrophins).
DISCUSSION
We decided to study the role of two particular integrins during brain development for two reasons. First, our previous results (Galileo et al., 1992) directly implicated β1 integrins in controlling cell migration and survival. Second, immunolocalization of fibronectin, laminin, and tenascin (Liesi, 1990; Sheppard et al., 1991; Hunter et al., 1992; Perez and Halfter, 1994; Pearlman and Sheppard, 1996; Yuasa, 1996; Yuasa et al., 1996) has indirectly implicated involvement of integrins in brain development. Here we used our previous antisense/retroviral strategy to directly implicate α6 integrin in cell migration and α8 integrin in cell survival during brain development.
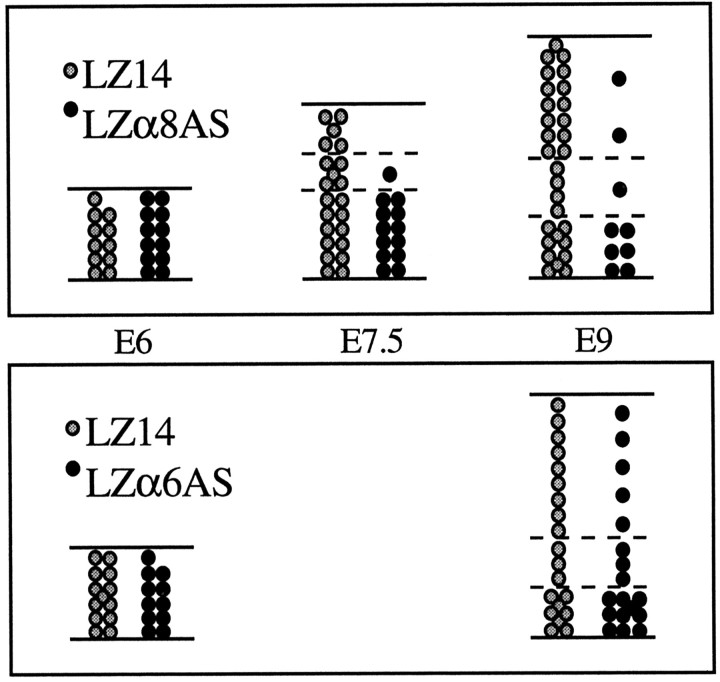
Attenuation of integrin subunits α6 and α8 had different effects on the development of optic tectum cells. Our results are summarized in a quantitative diagram in Figure 12. These results show that different integrins play different roles during brain development. Our α6 antisense results show that an α6 integrin (either α6β1 or α6β4) influences specifically the radial migration of a small subpopulation of tectal cells. Our α8 antisense results here and our previous β1 antisense results (Galileo et al., 1992) show that α8β1 integrin influences cell survival in developing brain. These are the first demonstrations of roles for α6 and α8 integrins during brain development. Our work also demonstrates that multiple integrin receptors are used concurrently to perform different functions within developing brain tissue and cells.
Fig. 12.
Summary data. The effects of antisense α8 and α6 sequences on the clonal development of tectal cells are shown in this quantitative summary diagram. Each circle,solid or stipled, represents a cell, and the number of cells shown are the mean numbers of cells at each age. Data are taken from Tables 1-5.
LZα8AS reduced the level of α8 integrin in dissociated OT cells, and LZα6AS reduced α6 integrin in a cell line in vitro. These results were similar. Therefore, we believe that the in vivo behaviors of infected OT cells were caused by the attenuation of α6 and α8 integrin subunits. In addition, two drastically different phenotypes occurred with vectors LZα6AS and LZα8ASin vivo, which act as controls for each other against nonspecific effects.
Transgenic mouse knock-outs of integrin α6 or α8 subunits have not yielded defects in brain development (Georges-Labouesse et al., 1996;Müller et al., 1997). Our limited α6 antisense results may be consistent with knock-out results, because redistribution of cells within our clones was not obvious before quantitation. The loss of this subpopulation of cells may not cause any dramatic abnormalities, regardless of whether they die, survive and differentiate into another cell type, or survive and differentiate into ectopic cells of normal phenotype. Knock-out integrin α8 mice exhibited deficits in kidney morphogenesis but no adverse effects on nervous system development. Detailed analyses of the brain may not have been preformed in these cases. In light of our results, it would be interesting to examine the colliculi of these mice in detail at appropriate developmental stages to determine whether effects similar to ours occurred.
Alternatively, our antisense results may be attributable to either species differences or other factors such as the timing of perturbation. Integrin α6 expression was required for early nervous system development in Xenopus laevis in vivo when attenuated by antisense α6 mRNA expression (Lallier et al., 1996). Knock-out mice for integrin α1 showed no phenotype (Gardner et al., 1996); however, experiments using injection of α1 antisense oligonucleotides into chick embryos resulted in neural crest and neural tube abnormalities (Kil et al., 1996). These examples suggest that in vivo results obtained from deletion or attenuation of an integrin subunit may depend on the stage of attenuation. Early attenuation in knock-out mice may result in compensation for the deletion, but this ability may be inactivated or lost at some later stage during development. Mechanisms of compensation both at the molecular and cellular levels have been postulated for integrin knock-out mice (for review, see Gullberg and Ekblom, 1997).
If α6 integrin was attenuated significantly in vivo as in QT6 cells, our results add more evidence that integrins control cell migration in developing brain (Galileo et al., 1992). This subunit appears to be involved in migration of a specific cell subpopulation that may use α6 integrin receptors to follow a migratory substrate to its proper destination and/or for signaling to initiate such a process. Our results suggest that neuronal subpopulations are guided to their proper laminae, at least in part, by using different integrins. Presently, very few other molecules have been implicated in controlling laminar distribution of cells in the developing brain. One system may be interactions between mouse disabled-1 (Howell et al., 1997) and a receptor for the reeler gene product (D’Arcangelo et al., 1995; Hirotsune et al., 1995; Ogawa et al., 1995), or a parallel pathway. In tectum, the α6 integrin-dependent migration process may parallel that of incoming retinal axons that use receptors to follow lamina-specific cues in the optic tectum (Yamagata and Sanes, 1995; Inoue and Sanes, 1997).
It is not known whether the α6 integrin-dependent cell subpopulation can eventually migrate out of the ventricular zone, can differentiate in its new location, or ultimately can survive. We speculate that these displaced neuroblasts might survive initially because of intact α8 integrin–substrate interactions. Death may occur later because of deprivation of neurotrophic factors or connectivity. Potential substrate molecules are also unknown. Distinct forms of laminin, other known substrates, or unknown substrates for α6 integrins may be present in optic tectum and on radial glia. For instance, a specific chain of laminin (B2) has been found on radial glia in mammals (Liesi, 1990, 1992), and its unknown receptor has been implicated in promoting neuronal migration (Liesi et al., 1992, 1995). Hunter et al. (1992)also reported the presence of laminin β2 (s-laminin) in developing rat brain and on glia in culture.
Our LZα8AS results resemble previous β1 integrin antisense results (Galileo et al., 1992), although LZα8AS affected cell survival more dramatically. The α8 antisense may reduce more efficiently the expression level of α8β1 than would the β1 antisense, because there are many possible β1 integrin heterodimers yet only one known α8 heterodimer (α8β1).
We believe that death of LZα8AS-infected cells is a direct result of interfering with an integrin signaling pathway. Various reviews have discussed the role of integrin adhesion systems in the regulation of apoptosis (Dedhar and Hannigan, 1996; LaFlamme and Auer, 1996; Varner and Cheresh, 1996; Meredith and Schwartz, 1997). Their anti-apoptosis function may be mediated through signaling cascades involving focal adhesion kinase in some cases (Richardson and Parsons, 1995; Meredith and Schwartz, 1997). β1 integrins (Scott et al., 1997) and α5β1 integrin (Zhang et al., 1995) support survival of cells on fibronectin, possibly by upregulating Bcl-2 expression. α8β1 is also a fibronectin receptor, so it is possible that α8β1 inhibits apoptosis of optic tectum cells similarly. We think that α8β1 may inhibit apoptosis of optic tectum cells by upregulating Bcl-2 and/or other Bcl-2-like molecules or by downregulating apoptosis-promoting factors such as Bax (Deckwerth et al., 1995), changing the Bcl-2/Bax ratio in favor of cell death (Knudson and Korsmeyer, 1997;Meredith and Schwartz, 1997). Immature neurons in the developing brain migrate along radial glial fibers (Rakic, 1985, 1990). Fibronectin has been found on radial glia and is thought to be involved in radial migration of neurons in the developing mammalian cortex (Sheppard et al., 1991; Pearlman and Sheppard, 1996). Tenascin also has been found on radial glia in developing mammalian cerebellum (Yuasa, 1996; Yuasa et al., 1996). Prevention of apoptosis by the extracellular matrix is dependent on the expression and function of particular integrin heterodimers (LaFlamme and Auer, 1996), which in turn interact with particular matrix substrates. LZα8AS may have caused the loss of anchorage of tectal neurons to a substrate on radial glia, and this triggered apoptosis. It is not known whether fibronectin and tenascin are involved in the suppression of apoptosis in the optic tectum. Either or both of these molecules may be interacting with α8β1 integrins on developing tectal cells to facilitate cell survival or migration or both.
Recently, we found in the optic tectum an early period of apparent normal widespread programmed cell death on E7.5–E8 when extensive radial migration of neurons is taking place (Zhang and Galileo, 1998). This is when much of our experimentally induced death also occurs as a result of the antisense α8 vector. Therefore, a hypothesis is proposed for the involvement of α8β1 integrin–substrate interactions in the regulation of early neural cell survival in the optic tectum: excess cells are generated. Only a certain percentage of the newly generated neurons make and keep stable contact interactions with the radial glial scaffolds via α8β1 integrins. These interactions would be made in the ventricular zone and maintained into the tectal plate. Those that do not maintain contacts die. Such interactions would provide a mechanism for keeping cells alive and would control the cell number and distribution patterns in the optic tectum for later neuronal connectivity. If this hypothesis endures subsequent experimentation, then a new type of interaction (integrin–substrate) may be added to those that have been found previously to influence neuronal survival, such as neurotrophic factors and connectivity (for review, see Henderson, 1996a,b).
Footnotes
This work was supported by a grant from the National Institute for Neurological Disorders and Stroke to D.S.G. We thank Dr. Lou Reichardt for cDNA clones and antibodies, Dr. Rick Horwitz and Dr. Lynn Schnapp for antibodies, Dr. Josh Sanes for helpful comments, and Dr. Richard Cameron for critical discussions.
Correspondence should be addressed to Dr. Deni S. Galileo, Department of Cellular Biology and Anatomy, Medical College of Georgia, Augusta, GA 30912-2000.
REFERENCES
- 1.Bayne EK, Anderson MJ, Fambrough DM. Extracellular matrix organization in developing muscle: correlation with acetylcholine receptor aggregates. J Cell Biol. 1984;99:1486–1501. doi: 10.1083/jcb.99.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 3.Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin α8 subunit: a new integrin β1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronner-Fraser M, Artinger M, Muschler J, Horwitz AF. Developmentally regulated expression of α6 integrin in avian embryos. Development. 1992;115:197–211. doi: 10.1242/dev.115.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheresh DA, Mecham RP. Integrins: molecular and biological responses to the extracellular matrix. Academic; San Diego: 1994. [Google Scholar]
- 7.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 8.D’Arcangelo G, Miao GG, Chen S-C, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 9.Deckwerth TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friendman B, McClain J, Pan L, Stahl N, Ip NY, Kato A, Yancopolous GD. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron. 1995;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 10.de Curtis I, Gatti G. Identification of a large complex containing the integrin α6β1 laminin receptor in neural retinal cells. J Cell Sci. 1994;107:3165–3172. doi: 10.1242/jcs.107.11.3165. [DOI] [PubMed] [Google Scholar]
- 11.de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor α6β1 and its isoforms. Development. 1993;118:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Curtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: sequence analysis of the chick integrin α6 subunit-evidence for transcriptional and posttranslational regulation. J Cell Biol. 1991;133:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 14.Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DLA, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the α3Aβ1 and α6Aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the α8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Galileo DS, Gray GE, Owens GC, Majors J, Sanes JR. Neurons and glia arise from a common progenitor in chick optic tectum: demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci USA. 1990;87:458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galileo DS, Majors J, Horwitz AF, Sanes JR. Retrovirally introduced antisense integrin RNA inhibits neuroblast migration in vivo. Neuron. 1992;9:1117–1131. doi: 10.1016/0896-6273(92)90070-t. [DOI] [PubMed] [Google Scholar]
- 18.Gardner H, Kreidberg J, Koteliansky V, Jaenish R. Deletion of α1 integrin by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;301:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 19.Georges-Labouesse E, Messadeq N, Yehia G, Cadelbert L, Dierich A, Le Meur M. Absence of the α6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 20.Gray GE, Sanes JR. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991;6:211–225. doi: 10.1016/0896-6273(91)90357-6. [DOI] [PubMed] [Google Scholar]
- 21.Gray GE, Glover JC, Majors J, Sanes JR. Radial arrangement of clonally related cells in the chicken optic tectum: lineage analysis with a recombinant retrovirus. Proc Natl Acad Sci USA. 1988;85:7356–7360. doi: 10.1073/pnas.85.19.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gullberg D, Ekblom P (1997) Integrins during development. In: Integrin-ligand interaction (Eble JA, Kühn K, eds), pp 253–267. Austin: R. G. Landes Company.
- 23.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 24.Henderson CE. Programmed cell death in the developing nervous system. Neuron. 1996a;17:579–585. doi: 10.1016/s0896-6273(00)80191-9. [DOI] [PubMed] [Google Scholar]
- 25.Henderson CE. Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol. 1996b;6:64–70. doi: 10.1016/s0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- 26.Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, Nakao K, Katsuki M, Hayashizaki Y. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 27.Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DD, Llinas R, Ard M, Merlie JP, Sanes JR. Expression of s-laminin and laminin in the developing rat central nervous system. J Comp Neurol. 1992;323:238–251. doi: 10.1002/cne.903230208. [DOI] [PubMed] [Google Scholar]
- 29.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 30.Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 31.Kil SH, Lallier T, Bronner-Fraser M. Inhibition of cranial neural crest adhesion in vitro and migration in vivo using integrin antisense oligonucleotides. Dev Biol. 1996;179:91–101. doi: 10.1006/dbio.1996.0243. [DOI] [PubMed] [Google Scholar]
- 32.Knudson CM, Korsmeyer SJ. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 33.LaFlamme SE, Auer KL. Integrin signaling. Semin Cancer Biol. 1996;7:111–118. doi: 10.1006/scbi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 34.Lallier TE, Whittaker CA, DeSimone DW. Integrin α6 expression is required for early nervous system development in Xenopus laevis. Development. 1996;122:2539–2554. doi: 10.1242/dev.122.8.2539. [DOI] [PubMed] [Google Scholar]
- 35.Liesi P. Extracellular matrix and neuronal movement. Experientia. 1990;46:900–907. doi: 10.1007/BF01939382. [DOI] [PubMed] [Google Scholar]
- 36.Liesi P. Neuronal migration on laminin involves neuronal contact formation followed by nuclear movement inside a preformed process. Exp Neurol. 1992;117:103–133. doi: 10.1016/0014-4886(92)90119-b. [DOI] [PubMed] [Google Scholar]
- 37.Liesi P, Seppala, Trenkner E. Neuronal migration in cerebellar microcultures is inhibited by antibodies against a neurite outgrowth domain of laminin. J Neurosci Res. 1992;33:170–176. doi: 10.1002/jnr.490330122. [DOI] [PubMed] [Google Scholar]
- 38.Liesi P, Hager G, Dodt H-U, Seppala I, Zieglgansberger W. Domain-specific antibodies against the B2 chain of laminin inhibit neuronal migration in the neonatal rat cerebellum. J Neurosci Res. 1995;40:199–206. doi: 10.1002/jnr.490400208. [DOI] [PubMed] [Google Scholar]
- 39.Meredith JE, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 40.Moscovici C, Moscovici MG, Jemenez H. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977;11:95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- 41.Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 44.Pearlman AL, Sheppard M. Extracellular matrix in early cortical development. Prog Brain Res. 1996;108:119–134. [PubMed] [Google Scholar]
- 45.Perez RG, Halfter W. Tenascin protein and mRNA in the avian visual system: distribution and potential contribution to retinotectal development. Perspect Dev Neurosci. 1994;2:75–87. [PubMed] [Google Scholar]
- 46.Rakic P. Contact regulation of neuronal migration. In: Edelman GM, Thiery JP, editors. The cell in contact. Wiley; New York: 1985. pp. 67–91. [Google Scholar]
- 47.Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 48.Richardson A, Parsons JT. Signal transduction through integrins: a central role for focal adhesion kinase?. BioEssays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 49.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 50.Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin α8β1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- 51.Scott G, Casidy L, Busacco A. Fibronectin suppresses apoptosis in normal human melanocytes through an integrin-dependent mechanism. J Invest Dermatol. 1997;108:147–153. doi: 10.1111/1523-1747.ep12332650. [DOI] [PubMed] [Google Scholar]
- 52.Sheppard AM, Hamilton SK, Pearlman AL. Changes in the extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J Neurosci. 1991;11:3928–3942. doi: 10.1523/JNEUROSCI.11-12-03928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Nowakowski RS, Caviness VS. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neurogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 55.Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weaver CD, Yoshida CK, de Curtis I, Reichardt LF. Expression and in vitro function of β1-integrin laminin receptors in the developing avian ciliary ganglion. J Neurosci. 1995;15:5275–5285. doi: 10.1523/JNEUROSCI.15-07-05275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamagata M, Sanes JR. Lamina-specific cues guide outgrowth and arborization of retinal axons in the optic tectum. Development. 1995;121:189–200. doi: 10.1242/dev.121.1.189. [DOI] [PubMed] [Google Scholar]
- 58.Yamagata M, Herman J-P, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuasa S. Bergmann glial development in the mouse cerebellum as revealed by tenascin expression. Anat Embryol. 1996;194:223–234. doi: 10.1007/BF00187133. [DOI] [PubMed] [Google Scholar]
- 60.Yuasa S, Kawamura K, Kuwano R, Ono K. Neuron-glia interrelations during migration of purkinje cells in the mouse embryonic cerebellum. Int J Dev Neurosci. 1996;14:429–438. [PubMed] [Google Scholar]
- 61.Zhang Z, Galileo D (1998) Widespread programmed cell death in early developing chick optic tectum. NeuroReport, in press. [DOI] [PubMed]
- 62.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The α5 β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]