Abstract
A new orexigenic peptide called hypocretin (orexin) has recently been described in neurons of the lateral hypothalamus and perifornical area. The medial and lateral hypothalamus have been loosely called satiety and feeding centers of the brain, respectively. Approximately one-third of all medial and lateral hypothalamic neurons tested, but not hippocampal neurons, show a striking nanomolar sensitivity to hypocretin. As studied with calcium digital imaging with fura-2, hypocretin raises cytoplasmic calcium via a mechanism based on G-protein enhancement of calcium influx through plasma membrane channels. The peptide has a potent effect at both presynaptic and postsynaptic receptors. Most synaptic activity in hypothalamic circuits is attributable to axonal release of GABA or glutamate. With whole-cell patch-clamp recording, we show that hypocretin, acting directly at axon terminals, can increase the release of each of these amino acid transmitters. Two hypocretin peptides, hypocretin-1 and hypocretin-2, are coded by a single gene; neurons that respond to one peptide also respond to the other. In addition to its effect on feeding, we find that this peptide also regulates the synaptic activity of physiologically identified neuroendocrine neurons studied in hypothalamic slices containing the arcuate nucleus, suggesting a second function of hypocretin in hormone regulation. The widespread distribution of hypocretin axons, coupled with the strong response to the peptide at both presynaptic and postsynaptic sites, suggests that the peptide probably modulates a variety of hypothalamic regulatory systems and could regulate the axonal input to these regions presynaptically.
Keywords: hypothalamus, presynaptic, neuroendocrine, neuromodulation, neuropeptide, glutamate, GABA, feeding
The hypothalamus has been called the control center of the brain for homeostasis, and neurons here regulate a substantial part of the internal milieu. Although the medial hypothalamus has been a fertile ground for the discovery of many neuroactive peptides, the lateral hypothalamus (LH) has been relatively barren. We recently described a new peptide, hypocretin, which shows robust expression in a restricted subset of lateral hypothalamic and perifornical neurons, with little apparent expression in neurons outside the hypothalamus (de Lecea et al., 1998). Axons from these cells show a strong innervation of the hypothalamus and ramify widely throughout the brain, including in areas that also have wide axonal distributions, such as the septal nuclei, preoptic area, central gray, and locus ceruleus. These data suggest that the cells that synthesize the peptide may exert a strong modulatory action on many different brain functions.
Subsequent to our description of hypocretin, a different group independently described the same peptide, which they called orexin, and found that it increased feeding in rats and that the peptide mRNA was upregulated in hungry animals (Sakurai et al., 1998). The hypocretin gene maps to a presumptive locus at chromosome 17q21 (de Lecea et al., 1998; Sakurai et al., 1998), a site that has been implicated in severe human neurodegenerative disorders (Wilhelmsen et al., 1994; Wijker et al., 1996), raising the possibility that a hypocretin deficit may contribute to this problem. Despite the excitement this peptide is generating on many fronts, there previously has been no characterization of its physiological actions on neurons and no assessment of neuroendocrine involvement. These are the focus of the present paper.
MATERIALS AND METHODS
Whole-cell recording in culture. Whole-cell patch-clamp experiments were performed in synaptically coupled medial hypothalamic neurons cultured for 10–21 d in vitroas described previously (Gao et al., 1998). The bath solution contained (in mm): 150 NaCl, 2.5 KCl, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.3 with NaOH. Whole-cell voltage clamp was used to observe spontaneous and miniature postsynaptic currents at −60 mV with a List (HEKA Elektronik) EPC-7 amplifier. Patch pipettes had a tip resistance of 4–6 MΩ when filled with pipette solution, which contained (in mm): 145 KCl, 1 MgCl2, 10 HEPES, 1.1 EGTA, 4 Mg-ATP, and 0.5 Na2-GTP, pH 7.3 with KOH. Only cells with an input resistance >0.8 GΩ were used. The series resistance was <30 MΩ and was partially compensated by the amplifier. All data were sampled at 3–10 kHz and filtered at 1–3 kHZ with an Apple Macintosh computer using AxoData software (Axon Instruments). Data were analyzed with Axograph 3.5 (Axon Instruments), plotted with Igor Pro (WaveMetrics, Lake Oswego, OR), and reported as the mean ± SEM. Student’st test was used to compare two groups of data.
Two active peptides are cleaved from preprohypocretin, hypocretin-1 and hypocretin-2 (also called orexin A and orexin B, respectively). All whole-cell recordings and most of the digital imaging studies were done with the 27 amino acid peptide hypocretin-2, PGPPGLQGRLQRLLQANGNHAAGILTM-NH2, made in-house. Hypocretin-1, using the structure described for orexin A (Phoenix Pharmaceuticals) (Sakurai et al., 1998), was used in some digital imaging experiments.
Slice recording. Arcuate nucleus (ARC) frontal slices, 350–400 μm thick, were obtained from 3-week-old Sprague Dawley rats. Slices were incubated in an interface chamber with constant flow (4 ml/min) of the oxygenated artificial CSF (ACSF) containing (in mm): 124 NaCl, 3.0 KCl, 2.0 CaCl2, 2.0 MgCl2, 1.23 NaH2PO4, 26 NaHCO3, 10 glucose, 0.1 D,L-2-amino-5-phosphonovalerate (AP-5), and 0.01 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), pH 7.4, at room temperature. Whole-cell patch-clamp recordings were made with an Axoclamp-2B amplifier (Axon Instruments). Glass pipettes were filled with: 145 mm potassium methylsulfate, 2 mmMgCl2, 0.1 mm CaCl2, 1.1 mm EGTA, 10 mm HEPES, 2 mmNa-ATP, 0.3 mm Na-GTP, pH 7.2, 290 mOsm, and 5–7 MΩ resistance. Hypocretin (30 μm) was dissolved in ACSF and applied to ARC slices using the microdrop technique (Christian and Dudek, 1988; Belousov and van den Pol, 1997). A small drop was pressure ejected from the hypocretin-containing pipette (7–10 μm tip diameter) onto the area of the recorded cell. To stimulate axons that innervated the recorded ARC neuron, a bipolar electrode was placed in the dorsal or ventrolateral part of the ARC. Electrical impulses (0.2 msec, 0.5 Hz, 40–250 μA) were delivered through an isolation unit from a Grass 44 stimulator. In some slices, a bipolar electrode was also placed on the median eminence to generate antidromic responses in ARC neurons, identifying them as neuroendocrine cells (stimulating current, 50–70 μA). In all slice experiments, data were monitored and stored on a Macintosh Quadra 800 computer using AxoData 1.2 with the subsequent off-line analysis by AxoGraph 3.0 (Axon Instruments) or Igor Pro (WaveMetrics). Recordings were made at 2000 Hz frequency acquisition.
Immunocytochemistry. Hypocretin-2 was conjugated with glutaraldehyde to keyhole limpet hemocyanin, dialyzed in phosphate buffer, mixed with complete Freund’s adjuvant, and injected subcutaneously and intradermally in three rabbits. Subsequent boosts were made from the hypocretin conjugate mixed with incomplete Freund’s adjuvant. Use of rabbits for this purpose was approved by the Yale University Committee on Animal Use. Each of the rabbits made antiserum against the peptide and stained the same cells in the LH. Rats were deeply anesthetized and perfused transcardially with 4% paraformaldehyde. Brains were removed, cut into 20–50-μm-thick sections, and stained with immunoperoxidase as described previously (van den Pol, 1985). Adsorption of the antigen with the antibody or elimination of the primary antiserum blocks immunostaining.
Fura-2 imaging. Neurons were loaded with fura-2 AM (5 μm; Molecular Probes, Eugene, OR) and studied on a Nikon inverted microscope fitted with a 40× Olympus UV objective with high 340 nm light transmittance (van den Pol et al., 1996a,b). Fluor software (Universal Imaging Corporation, West Chester, PA) was used to control a Lambda 10 Sutter filter wheel with 340 and 380 nm filters and a 150 W xenon light source. Calcium standards from Molecular Probes were used to calibrate the system according to the equation of Grynkiewicz et al. (1985). Cells were imaged while in a linear-flow chamber, allowing a complete change of solution in 5 sec (Forscher et al., 1987).
RESULTS
Hypocretin modulates glutamatergic and GABAergic signaling in cultured hypothalamic neurons
In our previous studies of hypothalamic neuromodulators, which included neuropeptide Y, adenosine, metabotropic glutamate receptor, and GABAB receptor agonists, we found primarily inhibitory actions (Obrietan et al., 1995; Chen and van den Pol, 1996, 1998; van den Pol et al., 1996b; Obrietan and van den Pol, 1997). In striking contrast, hypocretin increases synaptic activity in hypothalamic cultures. Almost all fast synaptic activity in the hypothalamus is generated by release of either the excitatory transmitter glutamate (van den Pol et al., 1990) or the inhibitory transmitter GABA (Randle and Renaud, 1987; Decavel and van den Pol, 1990; Renaud and Bourque, 1991). In some parts of the brain, peptide modulation is restricted to one or another of the amino acid transmitters, as in the hippocampus, in which neuropeptide Y decreases glutamate release but has little effect on GABA (Bleakman et al., 1992). For this reason, we studied GABA or glutamate activity selectively in neurons cultured from the medial hypothalamus, a brain region that has been suggested as a satiety center. To determine whether hypocretin responses would be found in GABAergic neurons, we blocked glutamate actions with AP-5 (100 μm) and CNQX (10 μm) and studied spontaneous IPSCs (sIPSCs) in voltage-clamped neurons. Hypocretin caused an increase in the frequency of IPSCs in three of five cells (mean increase, 56%; range, 36–79%; paired t test, p < 0.05;n = 5) (Fig.1A,D), measured 1 min after application of the peptide. Bicuculline (20 μm) blocked the IPSCs (data not shown). In parallel, we tested the effect on glutamatergic neurons in the presence of the GABAA antagonist bicuculline (20 μm) and found that hypocretin increased the frequency of spontaneous EPSCs (sEPSCs) in four of six neurons by 83% (range, 35–130%;p < 0.05; n = 5) (Fig.1B,C). Glutamate receptor antagonists AP-5 (100 μm) and CNQX (10 μm) blocked the EPSCs (data not shown). These data indicate that both glutamate- and GABA-releasing neurons, which account for almost all fast synaptic activity, express hypocretin receptors.
Fig. 1.
Both excitatory and inhibitory neurons express hypocretin receptors. To determine the transmitter identity of neurons that respond to hypocretin, different transmitter antagonists were used, and responses to hypocretin were studied with whole-cell recording in voltage-clamped cells held at −60 mV. A, In the presence of glutamate receptor antagonists AP-5 (100 μm) and CNQX (10 μm), only sIPSPs were found, and these were completely blocked with the GABAAantagonist bicuculline (20 μm) (data not shown). When hypocretin (1 μm) was bath-applied, the frequency of sIPSCs increased dramatically, and after peptide washout, the frequency of sIPSCs decreased to its normal baseline. B, In the presence of the GABAA antagonist bicuculline (20 μm), sEPSCs were found, and these were completely blocked by AP-5 (100 μm) and CNQX (10 μm) (data not shown). Hypocretin (1 μm) increased the frequency of sEPSCs, and these returned to normal after peptide washout. Both GABA and glutamate evoked inward currents (downward deflection) attributable to the composition of the pipette solution. C,D, Mean increase in sEPSCs and sIPSCs evoked by hypocretin and the return toward control levels after peptide washout.
Hypocretin enhances evoked IPSP in ARC slices
A high density of immunoreactive axons was found in the lateral and medial hypothalamus (Fig.2A–D); many of the fibers had large endings suggestive of presynaptic boutons. Many terminals were found in the ARC (Fig. 2C,D) but not in the median eminence (Fig. 2E). We therefore tested the hypothesis that hypocretin regulates the activity of neuroendocrine neurons in hypothalamic slices containing the ARC. A bipolar electrode was used to stimulate the lateral edge of the ARC (Fig. 3A). Hypocretin was applied by microdrop as described in detail elsewhere (Christian and Dudek,1988; Belousov and van den Pol, 1997). Six of seven current-clamped neurons showed an increase in the amplitude of the evoked IPSP in response to microdrop application of hypocretin, with a mean increase of 48% from 7.1 ± 0.9 mV to 10.6 ± 1.5 mV (range, 40–61%) based on the responding cells (Fig. 3B). This was a significant increase in the evoked IPSP (paired ttest, p < 0.003). No cells showed a decrease in the IPSP.
Fig. 2.
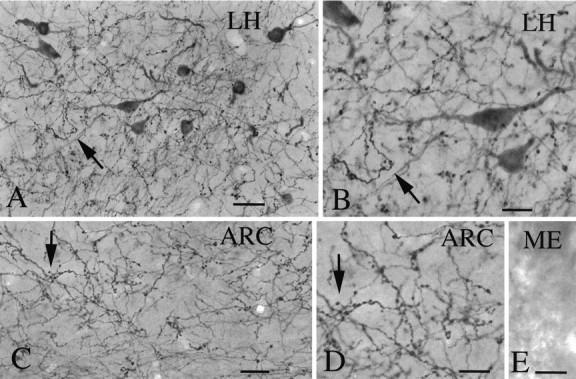
Hypocretin immunoreactivity. A, Immunocytochemical staining revealed high densities of axons in both the lateral hypothalamus (A, B,LH) and arcuate nucleus (C,D, ARC) of the hypothalamus. Around immunoreactive cells in the LH, a high density of immunoreactive axons was found. E, Immunoreactive axons were not found in the external zone of the median eminence (ME), in which neurosecretory axons release pituitary tropins that are carried to the anterior pituitary by the portal blood system. Scale bars:A, 25 μm; B, 12 μm; C, 25 μm; D, E, 12 μm.
Fig. 3.
Hypothalamic slice electrophysiology.A, Experimental paradigm for recording hypothalamic slices that included the arcuate nucleus (ARC).B, In the presence of AP-5 (100 μm) and CNQX (10 μm), IPSPs were evoked by orthodromic electrical stimulation (asterisk), as shown in A. The IPSP was blocked in the presence of bicuculline (20 μm), indicating that it was attributable to GABA release. Hypocretin (HCRT) applied by microdrop (30 μm) caused a substantial increase in the amplitude of the IPSP. This recovered to baseline levels after washout of the peptide. Each trace is the mean of eight sweeps. C, After testing hypocretin in B, the axon of the same cell was antidromically stimulated from the median eminence. That median eminence stimulation evoked an action potential even in the presence of control application of Co2+ (1 mm) indicated that the action potential response was not attributable to recurrent collaterals releasing transmitters on the recorded cell. A fixed latency of 3–5 mS between stimulus artifact and antidromic spike was routinely found. These data indicate that hypocretin enhances the action of transmitters released onto identified neuroendocrine neurons.
Neuroendocrine neurons that secrete factors that control the anterior pituitary gland send efferent axons to the median eminence in which the factors are released from axon terminals. Antidromic stimulation of axons in the median eminence was used to detect neurosecretory neurons of the ARC. Neurons (n = 3) that were antidromically identified showed an increase in the amplitude of the IPSP in the presence of hypocretin. To ensure that the response to stimulation of the median eminence was attributable to antidromic activation of ARC neurons, at the end of the experiments, control bath application of Co2+ was used to block orthodromic activation of recurrent collaterals (Fig. 3C). These data indicate that hypocretin modulates the afferent input that controls putative neuroendocrine neurons.
Calcium response to hypocretin
Electrical stimulation of the LH leads to increased feeding (Miller, 1960); hypocretin, made in LH cell bodies, also increases feeding (Sakurai et al., 1998). Lesions of the LH cause a long-lasting reduction in body weight and food intake in both male and female rodents (Powley and Keesey, 1970; van den Pol, 1982). In addition to the cell bodies (Gautvik et al., 1996; de Lecea et al., 1998; Sakurai et al., 1998) (Fig. 2A,B), large numbers of hypocretin immunoreactive axons and their terminals are found in the LH (Fig. 2A,B), suggesting that hypocretin could have a direct effect on neurons in the LH. In our past work on hypothalamic cultures, calcium digital imaging with fura-2 proved to be sensitive in detecting responses to both transmitters and a number of peptide and nonpeptide neuromodulators (Obrietan et al., 1995; van den Pol et al., 1996a,b). Because hypocretin immunoreactive axons terminate in both lateral (Fig.2B) and medial (Fig. 2D) hypothalamus, we tested the hypothesis that each of these areas would express hypocretin receptors that alter cytoplasmic calcium. Both lateral (23% of 180 neurons) (Fig.4A) and medial (Fig.4B) hypothalamic neurons showed substantial Ca2+ rises in response to hypocretin (1 μm) stimulation. In 1260 medial hypothalamic neurons from 21 experiments, a mean of 29.7% of the neurons showed a response to 1 μm hypocretin, using a criterion Ca2+rise of at least 20 nm. The mean (± SE) Ca2+ rise was 106 ± 7 nm, with a range of 20–930 nm.
Fig. 4.
Calcium responses to hypocretin. A, Based on fura-2 calcium recordings, neurons from the lateral hypothalamus (LH), the site of the hypocretin immunoreactive cell bodies, showed strong responses to hypocretin (HCRT) (1 μm) and clear recovery after two bath applications of the peptide. Horizontal lines above Ca2+ trace indicate time of drug application. Except for Figure 3C, all other experiments used hypocretin-2. B, Medial hypothalamus cells also showed strong Ca2+ elevations in response to hypocretin but no response to the C-terminal 1–17 (1–17) peptide of preprohypocretin (1 μm). C, Hypocretin-1 (HCRT: 1), using the 33 amino acid structure with the double disulfide bonds found for orexin A, and hypocretin-2 (HCRT: 2) were compared. Each evoked a Ca2+ rise in this typical medial hypothalamic neuron. D, Hypothalamic neuron that showed an increasing peak response to increasing concentrations of the peptide, with no response to 1 nm hypocretin, a response to 10 nm hypocretin, and a bigger response to 100 nmhypocretin.
As a control, a 17 amino acid peptide based on amino acids 1–17 of the C terminal of preprohypocretin was also tested. No response to the 1–17 amino acid sequence was found in 194 cells, and no response was found in 29 neurons that responded to hypocretin (Fig.4B). Preprohypocretin is cleaved to form two peptides, hypocretin-1 and hypocretin-2. The actions of hypocretin-1 have not been examined previously in single neurons. In three experiments, all 28 neurons that showed a response to one peptide also responded to the other with similar amplitude Ca2+rises (Fig. 4C). Thus, most hypothalamic neurons express receptors that are sensitive to both hypocretin-1 and hypocretin-2. Neurons responded in a dose-dependent manner (Fig.4D) to ≥5 nm hypocretin.
Hypocretin could generate a Ca2+ rise by increasing the release of an excitatory transmitter or by increasing the frequency of action potentials. To test these hypotheses, we examined the effect of hypocretin in the presence of tetrodotoxin (TTX) (1 μm). Even in the presence of TTX, hypocretin evoked a Ca2+ rise of at least 30 nm in 27% of 248 neurons (Fig. 5A). In the responding cells, hypocretin evoked a mean rise of 120 ± 16 nm Ca2+. These results support the concept that functional hypocretin receptors are found on or near the cell body of approximately one-third of hypothalamic neurons and that the Ca2+ rise is not dependent on synaptic modulation or action potentials.
Fig. 5.
Mechanisms of hypocretin elevation of calcium.A, To demonstrate that hypocretin can evoke Ca2+ elevations directly at the cell body rather than by altering synaptic interactions, hypocretin (1 μm) was added in the presence of tetrodotoxin (TTX) (1 μm), used to block action potential-mediated release of transmitters. B, Depleting intracellular stores of Ca2+ with thapsigargin (2 μm) pretreatment did not affect the action of hypocretin in elevating Ca2+ in the presence of 1 μm TTX.C, Eliminating extracellular Ca2+ and adding the Ca2+ chelator EGTA (1 mm) completely blocked the Ca2+ rise evoked by 1 μm hypocretin (HCRT). When bath Ca2+ levels were returned to normal, the action of hypocretin returned. D, Cd2+ (100 μm) blocked the Ca2+ rise, indicating an extracellular origin of Ca2+. E, Pretreatment of hypothalamic neurons with bisindolylmaleide (1 μm for 12 hr), a PKC inhibitor, completely blocked the actions of hypocretin (1 μm). Glutamate (GLU) (10 μm), applied as a control, evoked a large Ca2+ rise. F, Most cortical cells showed no response to hypocretin, as shown by the typical cell in f-1. A small percent of cortical cells did show a small Ca2+ rise in response to hypocretin, as shown in f-2. A control application of glutamate (GLU) (10 μm) evoked a Ca2+ rise.
A hypocretin-evoked increase in cytoplasmic Ca2+could result from release from intracellular stores or could come from outside the cell via Ca2+ channels in the plasma membrane. To test whether the hypocretin-mediated increase in Ca2+ is dependent on intracellular stores, we depleted the intracellular stores with thapsigargin (2 μm). In 24 neurons that responded to the peptide, hypocretin induced a 113 ± 15 nmCa2+ rise; 45 min after thapsigargin treatment, hypocretin evoked virtually the same amplitude Ca2+rise in these cells (110 ± 12 nm). This absence of a thapsigargin effect (Fig. 5B) suggests that hypocretin does not act primarily by a mechanism dependent on release of Ca2+ from intracellular stores.
The thapsigargin experiments suggest that hypocretin acts by increasing Ca2+ influx from outside the cell. To test this hypothesis further, we used a zero Ca2+ buffer with 1 mm EGTA. In normal Ca2+- containing HEPES buffer, hypocretin evoked a 130 ± 12 nmCa2+ rise in 38 neurons. In contrast, in the Ca2+-free buffer, hypocretin evoked a negligible Ca2+ rise of 7 ± 3 nm in the same neurons (Fig. 5C). In parallel, adding Cd2+ to the buffer blocked any hypocretin-evoked Ca2+ rise (Fig. 5D). These data support the view that hypocretin raises intracellular Ca2+by opening plasma membrane Ca2+ channels that allow Ca2+ entry from extracellular space.
Intracellular signaling
Next, we assessed possible intracellular signaling pathways that mediate the excitatory effects of hypocretin. cAMP levels in [3H]adenine-labeled cells were measured by determining the ratio of [3H]ATP, [3H]ADP and [3H]AMP to [3H]cAMP on cultures done in triplicate (Wong et al., 1991). Hypocretin (10 nm to 10 μm) did not increase cAMP levels in primary cultures of hypothalamic neurons. In sister cultures, a 12 ± 0.82 (mean ± SE) and 25.4 ± 3.6-fold increase was triggered by vasoactive intestinal peptide (VIP) (1 μm) and forskolin (1 μm), respectively. The VIP-evoked cAMP rise was not affected by the coadministration of hypocretin (data not shown). These results suggest that the hypocretin receptor does not act via heteromeric Gi or Gs proteins.
Another possibility is that the hypocretin receptor couples to a Gq protein, as suggested in transfected non-neuronal cells (Sakurai et al., 1998). Activation of Gq triggers the stimulation of phospholipase C, which, via the hydrolysis of phosphatidylinositol and the generation of inositol 1,4,5-trisphosphate and diacylglycerol, can activate protein kinase C (PKC) (Exton, 1994). To test whether hypocretin triggers PKC activation, hypothalamic neurons were pretreated with the PKC-specific inhibitor bisindolylmaleide (1 μm). Bisindolylmaleide completely blocked hypocretin responses (Fig. 5E), and no hypocretin-mediated Ca2+ rise was found in 128 neurons. These results suggest that hypocretin may work via Gq-mediated PKC, resulting in phosphorylation of Ca2+ channels that has been reported to increase Ca2+ conductance (Yang and Tsien, 1993; Stea et al., 1995).
Small cortical response
Hypocretin immunoreactive axons are found in the cortex but in small numbers, suggesting that a minor response to the peptide might be found in cortical neurons. Consistent with this hypothesis, only 5% of 122 cultured cortical neurons showed a hypocretin-evoked (1 μm) Ca2+ rise >30 nm(Fig. 5F). In the six responding cells, the mean Ca2+ rise was 65 ± 6 nmCa2+, approximately half the amplitude of that found in hypothalamic neurons. No Ca2+ responses were found in 128 hippocampal neurons, and in parallel, no effect of hypocretin (1 μm) on synaptic activity was found in synaptically coupled hippocampal neurons (n = 7).
Presynaptic actions of hypocretin
Although hypocretin evoked a substantial increase in synaptic activity, little direct effect on membrane potential was found in 28 neurons tested, even in cells in which increases in synaptic activity were detected. To test the hypothesis that hypocretin exerted an effect directly on presynaptic axons to alter release of neurotransmitter, we examined the effect of the peptide on miniature postsynaptic currents (mPSCs) in the presence of TTX in vitro. To study modulation of GABA-mediated mIPSCs, glutamate receptors were blocked with AP-5 (100 μm) and CNQX (10 μm). The miniature events that were found in this buffer were caused by the release of GABA and were blocked by bicuculline (20 μm) (data not shown). In three of five neurons, hypocretin generated a substantial increase in the frequency of mIPSCs, with a mean increase of 54.7 ± 12.3% (range, 30.2–67.4%; paired t test,p < 0.05; n = 5) (Fig.6A,D). To determine whether hypocretin also might increase the release of glutamate-secreting cells, we did parallel experiments in TTX (1 μm) and a GABA receptor antagonist. Hypocretin caused an increase in the frequency of glutamate-mediated mEPSCs by 48.2 ± 9.7% (range, 40.6– 65.1%) in three of five synaptically coupled neurons (p < 0.05; n = 5) (Fig. 6B,C); AP-5 and CNQX blocked the mEPSCs (data not shown). We found no change in the amplitude of the mEPSC in cells that showed an increase in frequency (Fig. 6E), suggesting that the increase in frequency of mPSCs was mediated at a presynaptic site of hypocretin action.
Fig. 6.
Presynaptic mechanism for hypocretin to enhance GABA or glutamate secretion. In the presence of TTX (1 μm), mPSCs were studied with whole-cell voltage-clamp recording. A, In the presence of the glutamate receptor antagonists AP-5 (100 μm) and CNQX (10 μm), hypocretin (1 μm) increased the frequency of mIPSCs. After washout of the peptide, the frequency of mIPSCs returned to baseline levels. B, An example of hypocretin (1 μm) increasing the frequency of mEPSCs in the presence of bicuculline (20 μm). C,D, Mean increase in mEPSCs and mIPSCs and the return toward baseline levels after peptide washout. E, Cumulative probability for mEPSC amplitude in the presence of hypocretin (1 μm) or in its absence (control). Superimposition of the two lines indicates that although hypocretin increased the frequency of mEPSCs, it did not change the amplitude, suggesting that the peptide acted at a presynaptic site to enhance transmitter release.
DISCUSSION
In the present set of experiments, we demonstrate that hypocretin increases synaptic activity of both glutamatergic and GABAergic neurons, and in part, this is attributable to actions at presynaptic receptors on GABA- and glutamate-secreting axons. In addition, we find that the afferent input to physiologically identified neuroendocrine neurons is modulated by hypocretin. Mechanistically, hypocretin elevates cytosolic calcium levels via plasmalemmal calcium channels. These actions are consistent with activation of a Gq protein acting via PKC to phosphorylate voltage-activated calcium channels.
Neuroendocrine modulation
Most, if not all, fast synaptic activity in the hypothalamus appears to be mediated by GABA and glutamate release (van den Pol et al., 1990; Randle and Renaud, 1987; Renaud and Bourque, 1991; van den Pol and Trombley, 1993), and this is also true of the ARC (Belousov and van den Pol, 1997), an area of the hypothalamus that regulates the endocrine system. Immunocytochemical analysis of hypocretin axons indicates a strong innervation of the ARC. Our finding that hypocretin modulates the afferent input to physiologically identified neuroendocrine neurons in the hypothalamus suggests that hypocretin could modulate the final common neuronal pathway regulating the hormone system. The antidromically identified neuroendocrine neurons probably maintain axon terminals in the external zone of the median eminence but may also send axons to the neurohypophysis. Pituitary tropins are released into the pituitary portal system in the median eminence with a dependence on the electrical activity of the neuroendocrine neurons. That hypocretin-containing axons innervate the ARC and hypocretin enhances signaling to these neurons suggests that by influencing the electrical activity of ARC neurons, hypocretin could play a pivotal role in hormone regulation. A number of different pituitary tropins are contained in neurons of the ARC, and the identity of the specific neuroendocrine neurons that respond to hypocretin remains to be determined. Although many different transmitters and modulators are found in axon terminals in the external zone of the median eminence, the striking lack of hypocretin fibers here suggests that hypocretin-containing cells probably do not directly control pituitary secretions but may indirectly participate in neuroendocrine regulation by modulating the activity of neurons that do directly control the pituitary.
Hypocretin immunoreactive axons are found not only in the ARC but also in other medial regions of the hypothalamus that may play a role in endocrine regulation and in other hypothalamic functions. Innervation of the LH is also found. In parallel, that both lateral and medial hypothalamic neurons respond to hypocretin suggests that hypocretin may play a role in the general modulation of a number of hypothalamic functions. This is consistent with the effects of hypocretin on hypothalamic neurons that contain either GABA or glutamate.
Presynaptic actions and nonhypothalamic responses
That functional hypocretin receptors appear to exist on cell bodies and on presynaptic axons suggests that hypocretin could have a direct effect on cytosolic calcium at the cell body, and in addition, could modulate incoming axonal input from a wide variety of sources. It remains to be determined if extrahypothalamic projections to the hypothalamus express presynaptic hypocretin receptors on their axons. Because hypocretin can increase cytosolic calcium levels in neurons independent of action potentials, this suggests that hypocretin may enhance presynaptic transmitter release by a parallel mechanism of increasing calcium levels of the presynaptic bouton, which would enhance calcium-dependent transmitter release. Calcium increases in response to hypocretin have been described in non-neuronal cells transfected with hypocretin receptor cDNA (Sakurai et al., 1998). The lack of a direct effect of hypocretin on membrane potential is consistent with a neuromodulator function of the peptide.
Although the focus in the present study is on hypothalamic actions of hypocretin, there are a small number of fibers that innervate the cerebral cortex, and consistent with that, we find that a small percentage of cortical cells tested show small responses to hypocretin. Because hypocretin axons are found in many brain regions, these data suggest that hypocretin receptors may also have a widespread distribution and that the receptor density may parallel the level of innervation. This remains to be substantiated in other brain regions and with other approaches, including in situ hybridization and immunostaining with hypocretin receptor antisera. That cells in some areas of the rat brain, for instance the hippocampus, appear unresponsive to hypocretin, suggests that functional hypocretin receptors are expressed in a limited population of CNS neurons.
Hypocretin and energy homeostasis
In light of our data, the role of hypocretin in physiological homeostasis is not restricted to the enhancement of feeding (Sakurai et al., 1998) but is also involved in the regulation of the neuroendocrine system in the ARC, perhaps related to endocrine regulation of energy balances. ARC neurons not only synthesize and release neuropeptide Y, involved in feeding (Leibowitz, 1991; Stanley, 1993) and endocrine (McDonald and Koenig, 1993) regulation, but also express receptors for leptin (Hakansson et al., 1998), the signal that adipose tissue releases into the vascular system that reduces food intake and plays a key role in some forms of obesity (Zhang et al., 1994); falling leptin levels may initiate mechanisms for energy conservation (Flier and Maratos-Flier, 1998). Our finding that hypocretin regulates the synaptic input to these arcuate cells and that hypocretin-containing axons strongly innervate this area suggests the hypocretin is in a key position to influence neurons that are the hub for vascular signals from the periphery and afferent and efferent axonal signals involved in feeding. Feeding studies have demonstrated that both GABA and glutamate can regulate hypothalamic control of food intake (Maldonado-Irizarry et al., 1995; Stanley et al., 1996), suggesting that one mode of hypocretin action based on the present paper is to modulate presynaptically GABA and glutamate circuits involved in energy regulation. The fact that hypocretin can modulate neurons containing either the primary excitatory or primary inhibitory transmitter in the hypothalamus suggests that the local microcircuitry, particularly the proximity of hypocretin axons to other axons, may be critical in hypocretin function. This raises the general question of how to best interpret animal responses to mass injections of hypocretin into the hypothalamus or ventricular system that may activate circuits other than those that would be activated by discrete axonal release of the peptide.
That approximately one-third of all hypothalamic neurons express hypocretin receptors and that hypocretin axon terminals are found throughout the hypothalamus suggests that hypocretin could influence the general level of activity in many hypothalamic systems. Included are other recently identified medial and lateral hypothalamic systems involved in regulating energy balances, including those related to melanin-concentrating hormone, melanocyte-stimulating hormone, Agouti-related protein, (Mountjoy et al., 1994; Qu et al., 1996; Huszar et al., 1997; Ollmann et al., 1997), and neurons that respond to glucose (Oomura, 1983). These are found in the same hypothalamic regions as high densities of hypocretin axons, suggesting that many opportunities exist for hypocretin cells to not only directly affect postsynaptic neurons, but also, based on data presented here, to modulate the synaptic input to these areas at the axonal terminal. Thus, hypocretin may be an important link in the chain of regulatory cells that orchestrate behavioral, metabolic, and endocrine systems to maintain energy homeostasis.
Footnotes
This work was supported by National Institutes of Health Grants NS31573 and NS34887, the National Science Foundation, the United States Army Research Office, and the Air Force Office of Scientific Research. We thank Y. Yang and J. Belousov for excellent technical assistance and Dr. C. Heller for help with the facilities.
Correspondence should be addressed to Anthony van den Pol, Department of Neurosurgery, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520.
REFERENCES
- 1.Belousov AB, van den Pol AN. Local synaptic release of glutamate from neurons in the rat hypothalamic arcuate nucleus. J Physiol (Lond) 1997;499:747–761. doi: 10.1113/jphysiol.1997.sp021966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleakman D, Harrison NL, Colmers WF, Miller RJ. Investigations into neuropeptide Y-mediated presynaptic inhibition in cultured hippocampal neurones of the rat. Br J Pharmacol. 1992;107:334–340. doi: 10.1111/j.1476-5381.1992.tb12747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, van den Pol AN. NPY Y1- and Y2-like receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, van den Pol AN. Presynaptic GABA-B autoreceptor modulation of P/Q type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J Neurosci. 1998;18:1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian EP, Dudek F. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988;59:90–109. doi: 10.1152/jn.1988.59.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Decavel C, van den Pol A. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Kilduff T, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 9.Flier JS, Maratos-Flier E. Obesity and the hypothalamus—novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 10.Forscher P, Kaczmarek L, Buchanan J, Smith SJ. Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J Neurosci. 1987;7:3600–3611. doi: 10.1523/JNEUROSCI.07-11-03600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X-B, Chen G, van den Pol AN. GABA-dependent firing of glutamate-evoked action potentials at AMPA/kainate receptors in developing hypothalamic neurons. J Neurophysiol. 1998;79:716–726. doi: 10.1152/jn.1998.79.2.716. [DOI] [PubMed] [Google Scholar]
- 12.Gautvik KM, de Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, Bloom FE, Sutcliffe JG. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Poenie M, Tsien R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 14.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister BM. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfied LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz SF. Brain neuropeptide Y: an integrator of endocrine, metabolic and behavioral processes. Brain Res Bull. 1991;27:333–337. doi: 10.1016/0361-9230(91)90121-y. [DOI] [PubMed] [Google Scholar]
- 17.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald JK, Koenig J. Neuropeptide Y actions on reproductive and endocrine functions. In: Colmers WF, Wahlestedt C, editors. The biology of neuropeptide Y and related peptides. Humana; Totowa, NJ: 1993. pp. 419–456. [Google Scholar]
- 19.Miller NE. Motivational effects of brain stimulation and drugs. Fed Proc. 1960;19:846–853. [PubMed] [Google Scholar]
- 20.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 21.Obrietan K, van den Pol AN. GABA-B receptor mediated inhibition of GABA-A receptor calcium elevations in developing hypothalamic neurons. J Neurophysiol. 1998;79:1360–1370. doi: 10.1152/jn.1998.79.3.1360. [DOI] [PubMed] [Google Scholar]
- 22.Obrietan K, Belousov A, Heller HC, van den Pol AN. Adenosine pre- and postsynaptic modulation of glutamate-dependent calcium activity in hypothalamic neurons. J Neurophysiol. 1995;74:2150–2162. doi: 10.1152/jn.1995.74.5.2150. [DOI] [PubMed] [Google Scholar]
- 23.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 24.Oomura Y. Glucose as a regulator of neuronal activity. Adv Metab Disord. 1983;10:31–65. doi: 10.1016/b978-0-12-027310-2.50008-6. [DOI] [PubMed] [Google Scholar]
- 25.Powley TL, Keesey RE. Relationship of body weight to the lateral hypothalamic feeding syndrome. J Comp Physiol Psychol. 1970;70:25–36. doi: 10.1037/h0028390. [DOI] [PubMed] [Google Scholar]
- 26.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek J, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 27.Randle JCR, Renaud LP. Actions of gamma-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol (Lond) 1987;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 30.Stanley BG. Neuropeptide Y in multiple hypothalamic sites controls eating behavior, endocrine, and autonomic systems for body energy balance. In: Colmers WF, Wahlestedt C, editors. The biology of neuropeptide Y and related peptides. Humana; Totowa, NJ: 1993. pp. 457–509. [Google Scholar]
- 31.Stanley BG, Willett VL, Donias HW, Dee MG, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 32.Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 33.van den Pol AN. Lateral hypothalamic damage and body weight regulation: role of gender, diet, and lesion placement. Am J Physiol. 1982;242:R265–R274. doi: 10.1152/ajpregu.1982.242.3.R265. [DOI] [PubMed] [Google Scholar]
- 34.van den Pol AN. Silver-intensified gold and peroxidase as dual ultrastructural immunolabels for pre- and postsynaptic neurotransmitters. Science. 1985;228:332–335. doi: 10.1126/science.2858916. [DOI] [PubMed] [Google Scholar]
- 35.van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- 37.van den Pol AN, Cao V, Belousov AB. Dopamine enhancement and depression of glutamate-regulated calcium and electrical activity in hypothalamic neurons. J Neurophysiol. 1996a;76:3934–3948. doi: 10.1152/jn.1996.76.6.3934. [DOI] [PubMed] [Google Scholar]
- 38.van den Pol AN, Obrietan K, Chen G, Belousov AB. Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci. 1996b;16:5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wijker M, Wszolek ZK, Wolters ECH, Rooimans MA, Pals G, Pfeiffer RF, Lynch T, Rodnitzky RL, Wilhelmsen KC, Arwert F. Lo-calization of the gene for rapidly progressive autosomal dominantpar-kinsonism and dementia with pallido-ponto-nigral degeneration to chromosome 17q21. Hum Mol Genet. 1996;5:151–154. doi: 10.1093/hmg/5.1.151. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelmsen KC, Lynch T, Pavlou E, Higgens M, Hygaard TG. Localization of disinhibition-dementia-parkinsonism-amyotropy complex to 17q21–22. Am J Hum Genet. 1994;55:1159–1165. [PMC free article] [PubMed] [Google Scholar]
- 41.Wong YH, Federman A, Pace AM, Zachary I, Evans T, Pouyssegur J, Bourne HR. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional clonging of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]







