Abstract
Glutamate release from nerve terminals is the consequence of Ca2+-triggered fusion of small synaptic vesicles with the presynaptic plasma membrane. ATP dependence of neurotransmitter release has been suggested to be founded, in part, on phosphorylation steps preceding membrane fusion. Here we present evidence for an essential role of phosphatidylinositol phosphorylation in stimulated release of neurotransmitter glutamate from isolated nerve terminals (synaptosomes). Specifically, we show that a phosphatidylinositol 4-kinase (PtdIns 4-kinase) activity resides on nerve terminal-derived small synaptic vesicles (SSVs) and that inhibition of the PtdIns 4-kinase activity in intact synaptosomes leads to attenuation of the evoked release of glutamate. The attenuation of transmitter release is reversible and correlates with respective changes in intrasynaptosomal PtdIns 4-kinase activity. Because only the Ca2+-dependent release of glutamate is affected, regulation appears to be at the level of exocytosis. Taken together, our data imply a mandatory role for PtdIns 4-kinase and phosphoinositide products in the regulated exocytosis of SSV in mammalian nerve terminals.
Keywords: phosphoinositides, glutamate exocytosis, synaptosomes, small synaptic vesicles, phospholipids, lipid kinase, phosphatidylinositol 4-kinase, priming, ATP dependent, calcium dependent, kinase inhibitors
Ca2+-stimulated secretion is now recognized to involve the docking of secretory vesicles with the plasma membrane before the fusion event itself (Scheller, 1995). Exocytotic membrane fusion is apparently preceded by a series of “priming” steps that render the vesicles fusion competent. Morphologically docked vesicles are thus thought to exist in functionally discrete pools (Parsons et al., 1995; Gillis et al., 1996). In neurons, two phases of stimulated transmitter release have been defined; the first is based on a readily releasable pool of vesicles that respond quickly to high [Ca2+]i, whereas the second, kinetically slower component depends on a supply from a pool of vesicles not yet competent for fusion. The priming of the latter represents a downstream Ca2+-target because [Ca2+]i decays after stimulation (Rosenmund and Stevens, 1996). Thus, these and other studies have presented the possibility that, apart from preparation for the immediate response to depolarization, the priming states of secretory vesicles may form the basis of short-term synaptic plasticity (Zucker, 1996).
Membrane fusion and priming of secretory vesicles are distinct in terms of their ATP dependence. Thus, whereas fusion can occur in the absence of ATP, priming requires the presence of ATP. The molecular basis of the ATP dependence of the latter has been suggested to be at two levels: first, in the ATPase activity of anN-ethylmaleimide-sensitive fusion (NSF) protein involved as a putative chaperone in the regulation of exocytotic membrane interactions [SNARE hypothesis (see Söllner and Rothman, 1994)], and second, in the formation of phosphoinositides from synaptic vesicle and plasma membrane resident phosphatidylinositol (PtdIns) (De Camilli et al., 1996). Although the details of the ATP dependence of secretion as suggested in the SNARE hypothesis are currently under debate (Bock and Scheller, 1997), recent studies have supported the original observations in chromaffin cells implicating phosphoinositides in regulated secretion (Eberhard et al., 1990). Thus with a PC12 cell model that allows separation of the priming process from the actual membrane fusion (Martin et al., 1995), the ATP dependence of priming originates, in part, from the phosphorylation of PtdIns to phosphoinositides. Two cytosolic factors, a phosphatidylinositol transfer protein (PITP) (Hay and Martin, 1993) and a phosphatidylinositol(4)phosphate 5-kinase [PtdIns(4)P 5-kinase] (Hay et al., 1995), mediate this phosphorylation. Filling the gap in this cascade, a chromaffin granule-associated phosphatidylinositol 4-kinase (PtdIns 4-kinase) is mandatory for regulated secretion of catecholamines from chromaffin cells (Wiedemann et al., 1996).
Investigations of the mechanism of transmitter release in nerve terminals by small synaptic vesicle (SSV) exocytosis have largely been focused on the elucidation of protein–protein interactions involved in vesicle docking (Kelly, 1993; Südhof, 1995). In contrast, the lipids and phospholipids of the membranes that undergo exocytosis have received relatively little attention with respect to their intimate structural and regulatory involvement in secretion. The putative involvement of phosphoinositides in neurotransmission has recently been addressed with the identification of a nerve terminal-associated inositol 5-phosphatase (McPherson et al., 1996), but the role of phosphoinositides in SSV-mediated neurotransmitter release has thus far not been investigated directly.
In current studies, we demonstrate, for the first time, the presence of a PtdIns 4-kinase activity in an isolated nerve terminal (synaptosome) preparation, an established model for the study of presynaptic modulation of SSV-mediated glutamate release at central synapses (Sihra and Nichols, 1993). We show that this PtdIns 4-kinase is localized to SSVs derived from synaptosomes and that the reversible inhibition of kinase activity by pharmacological manipulations correlates with the reversible attenuation of glutamate release. Our data therefore invoke a role for polyphoinositides in the ATP-dependent priming of SSVs in nerve terminals.
MATERIALS AND METHODS
SSV preparation. Cerebral cortices of 10 rats were homogenized, and a crude SSV fraction (LP2) was prepared according to Huttner et al. (1983). The P1fraction, which contained intact cells, nuclei, and cell debris, was discarded. The P2 crude synaptosomal fraction was washed and hypotonically lysed by a 1:10 dilution in ice-cold water. The lysate was centrifuged at 25,000 × g to form a pellet of mitochondria, dense plasma membrane vesicles, and postsynaptic densities, whereas the SSVs of the supernatant were collected by centrifugation at 165,000 × g(LP2). Fractionation of this crude SSV fraction was performed on continuous sucrose gradients (50–800 mm, 36 ml). Gradients were centrifuged at 65,000 × g for 5 hr at 4°C. Six gradient fractions, I–VI (from top to bottom), were collected and analyzed for (1) protein content (Bio-Rad DC protein assay, Bio-Rad, Hercules, CA), (2) membrane-bound PtdIns-kinase activity (mPtdIns-kinase), (3) solubilized PtdIns-kinase activity (sPtdIns-kinase), and (4) the presence of vesicle-associated membrane protein (VAMP)/synaptobrevin and Na+/K+-ATPase (α-subunit).
Immunoblot analysis. Samples of sucrose gradient fractions (30 μg of protein) and immunoprecipitated SSV were separated by SDS-PAGE and transferred to nitrocellulose. VAMP/synaptobrevin and the α-subunit of Na+/K+-ATPase were detected by polyclonal antibodies, and synaptophysin was detected by monoclonal antibodies (all at 1:1000) using an enhanced chemiluminescence reporter system (Amersham, Arlington Heights, IL) as described by Hodel et al. (1994).
Immunoprecipitation of SSVs. Monoclonal anti-synaptophysin antibodies (3 mg) (Sigma, St. Louis, MO) were adsorbed to protein G-Sepharose beads (0.5 ml) (Pharmacia, Piscataway, NJ). Antibodies were conjugated to the beads by addition of dimethylpimelinimidate (final concentration, 20 mm) for 2 hr at room temperature. After a 1 hr incubation in 0.2 m ethanolamine, the beads were washed several times with PBS (137 mm NaCl, 2.7 mm KCl, 1 mm Na2HPO4, 1.5 mmKH2PO4, 0.5 mmMgCl2, 0.9 mm CaCl2) and then once in PBS containing 10 mg/ml BSA. They were then resuspended and stored as a 50% slurry in PBS. Control protein G-beads were treated identically but in the absence of antibodies.
For immunoprecipitation of SSVs, sucrose gradient fractions were diluted with PBS to obtain a protein concentration of 0.7 mg/ml. Aliquots (50 μl) were mixed with 100 μl PBS and precleared by incubation with 50 μl of control protein G-beads. After a 90 min incubation at room temperature, beads were removed by centrifugation at 500 × g, and the supernatant was used for (1) analysis of PtdIns-kinase activity, (2) immunoprecipitation with 25 μl of anti-synaptophysin beads, (3) control precipitation with 25 μl of protein G-beads, and (4) analysis for the presence of VAMP/synaptobrevin and Na+/K+-ATPase (α-subunit). After an overnight incubation at 4°C, beads were washed several times in PBS. They were either resuspended in 220 μl of buffer and used for assay of mPtdIns-kinase activity or boiled in SDS-gel sample buffer and analyzed by PAGE and immunoblotting as described above.
PtdIns-kinase assays. The mPtdIns-kinase assay was performed according to Husebye and Flatmark (1988) with some modifications. Briefly, samples from sucrose gradient fractions (35 μg protein/assay) or immunoprecipitated SSVs were assayed in kinase buffer (30 mm HEPES, pH 7.0, 0.1 mm EGTA, and 5 mm MgCl2) containing 0.5 mm[γ-32P]-ATP (10 μCi/assay). After 10 min at room temperature, phosphorylation of endogenous lipids by the membrane-bound kinases was stopped by addition of ice-cold chloroform/methanol/1m HCl (20:40:1). Lipids were extracted and analyzed on thin layer chromatograms and a Phosphorimager as described previously (Wiedemann et al., 1996). Results were standardized for the protein concentration of each sample.
The detergent-solubilized sPtdIns 4-kinase activity in sucrose gradient fractions or synaptosomes was assayed in vitro as described previously (Susa et al., 1992; Wiedemann et al., 1996). Synaptosomes were incubated under conditions parallel to those used in the release experiments. At the end of the release period, they were pelleted and proteins were extracted in 1% NP-40 in ice-cold buffer [25 mm Tris, pH 7.4, 10% glycerol, 1 mm4-(2-aminoethyl) benzenesulfonyl fluoride, 10 μmleupeptin, 5 μm aprotinin, 20 mm NaF, and 1 mm vanadate]. Sucrose gradient fraction proteins were similarly extracted. Kinase assays were performed in a final volume of 50 μl in assay buffer (25 mm3-[N-morpholino]propanesulfonic acid, pH 7.0, 5 mm MgCl2, 1 mm EGTA, and 0.1% NP-40) with 10 μl of a 1:10 dilution of the respective protein extracts, 1.7 μg/μl exogenous phospholipids (brain extract type I, Sigma), and 60 μm [γ-32P]-ATP (10 μCi/assay). After 10 min, the reaction was stopped by addition of 400 μl of 1 m HCl, and lipids were extracted and analyzed by thin-layer chromatography as described previously (Wiedemann et al., 1996). 32P-labeling of lipids on chromatograms was quantitated using a Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Synaptosome preparation and glutamate release. Rat cerebrocortical synaptosomes were purified on Percoll gradients as described previously (Sihra, 1997). Synaptosomal pellets were resuspended in incubation buffer (10 mm HEPES, pH 7.4, 140 mm NaCl, 5 mm KCl, 5 mmNaHCO3, 1.2 mmNaH2PO4, 1 mmMgCl2, 10 mm glucose) at a protein concentration of 0.25 mg/ml and incubated in a stirred cuvette at 37°C in a Perkin-Elmer LS3B spectrofluorometer (Perkin-Elmer, Emeryville, CA). CaCl2 was added after 3 min of incubation. To measure Ca2+-independent glutamate release, CaCl2 was excluded, and 0.2 mm EGTA was added 1 min before stimulation. Glutamate release stimulated by 30 mm KCl, 3 mm 4-aminopyridine (4AP), 1 mm/30 mm BaCl2/KCl, or 5 μm ionomycin, was assayed by on-line fluorometry as described previously (Nicholls and Sihra, 1986). Calibration and quantitation of release were performed using exogenous glutamate standards (5 nmol) (Sihra et al., 1992, 1993). Phenylarsine oxide (PAO) (Sigma) and 2,3-dimercaptopropanol or British Antilewisite (BAL) (Sigma) were added from 200-fold concentrated stock solutions in DMSO as indicated in the legends to the Figures.
RESULTS
To determine the importance of PtdIns phosphorylation in neurotransmitter release, we followed PtdIns 4-kinase activity in subcellular fractionation leading from homogenates of cerebral cortices through to purification of synaptosomes and subsequently to purified SSVs (Huttner et al., 1983). Measurement of the phosphorylation of endogenous PtdIns revealed a membrane-associated PtdIns 4-kinase activity enriched in the crude synaptosomal fraction (P2). This mPtdIns 4-kinase activity was found to co-purify with SSVs when synaptosomal lysates were resolved on sucrose gradients. The relative amount of SSVs present in gradient fractions I–VI was determined by immunoblot analysis using antibodies to VAMP/synaptobrevin and synaptophysin (not shown), whereas the relative contamination by plasma membrane was determined by antibodies directed to the α-subunit of Na+/K+-ATPase (Fig.1a). Quantitation of relative mPtdIns 4-kinase activities revealed a parallel distribution of this activity and the SSV-marker VAMP/synaptobrevin throughout the sucrose gradient, with the peak for the two parameters occurring in the same fraction V (Fig. 1a,b). The mPtdIns kinase assay was specific for measuring PtdIns 4-kinase activity, because PtdIns 3-kinase activity is inhibited in the presence of 0.1% NP-40 used in our assay (Susa et al., 1992). Notably, the PtdIns 3-kinase inhibitor wortmannin (Arcaro and Wymann, 1993; Okada et al., 1994) had no effect on glutamate release in our experiments (data not shown). When subcellular organelles in gradient fractions were solubilized and PtdIns kinase activities toward exogenously added substrates were measured, the sPtdIns 4-kinase activity was found to co-purify again with SSV (Fig. 1c). In addition, the presence of a sPtdIns(4)P 5-kinase activity could be detected in the fractions enriched in α-Na+/K+-ATPase (Fig. 1c). Although the sPtdIns 4-kinase and sPtdIns(4)P 5-kinase activities were not mutually exclusive in any one sucrose gradient fraction, the peaks of the two activities clearly coincided with the relative enrichment of SSV and plasma membranes, respectively.
Fig. 1.
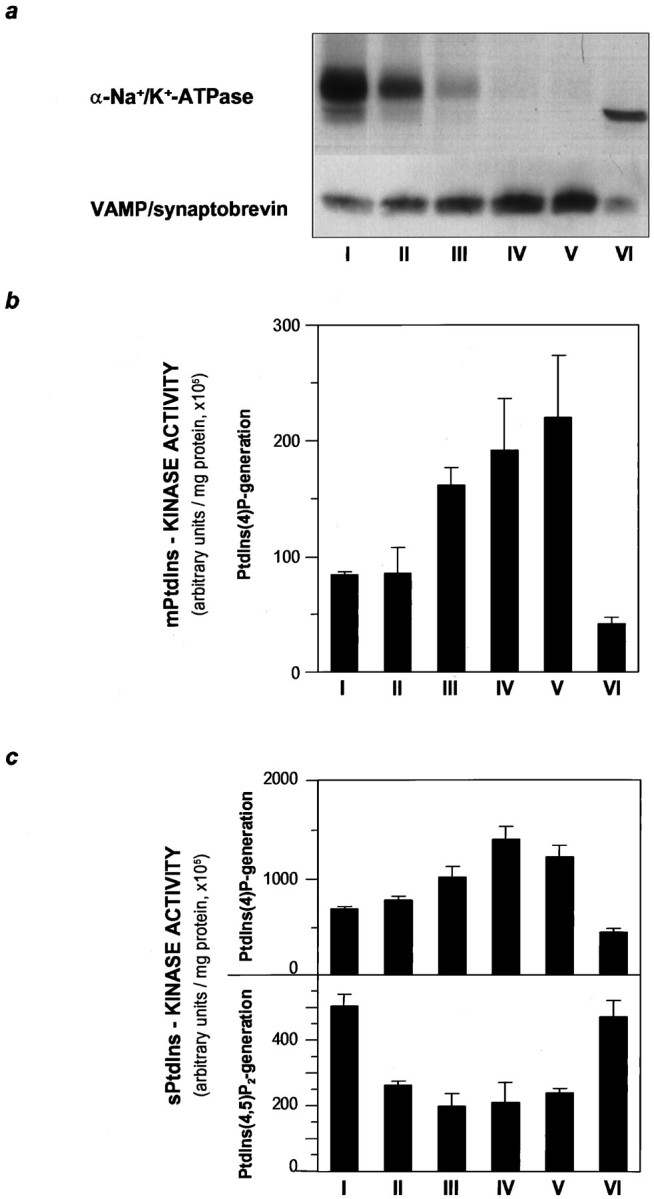
Localization of a membrane-associated PtdIns 4-kinase activity on SSVs. Crude SSV preparations were further purified on a continuous sucrose gradient. Six fractions (I–VI, from top to bottom of the gradient) were collected and analyzed.a, Separation of SSV from contaminating plasma membrane was demonstrated by immunoblot analysis of 30 μg of protein of each fraction. SSV and plasma membranes were detected by the use of antibodies to the marker proteins VAMP/synaptobrevin and the α-subunit of Na+/K+-ATPase, respectively. SSV accumulated in fractions IV and V, whereas most of the plasma membrane was recovered in the first fraction. The two bands at 140 and 100 kDa detected by the antibodies to Na+/K+-ATPase in fraction I represent the heterodimer of the α- and β-subunits, and the α-subunit monomer, respectively. b, Membrane-associated PtdIns 4-kinase activity within the sucrose gradient peaked in fractions III–V, enriched in SSVs and devoid of plasma membrane. Relative mPtdIns 4-kinase activity was measured by incubation of aliquots from fractions I–VI without addition of exogenous substrate. Phosphorylation of membrane-derived PtdIns was determined by separation of labeled phospholipids on thin layer chromatograms. PtdIns(4)32P was quantitated and expressed in arbitrary units. c, Solubilized PtdIns-kinase activity was determined in 1% NP-40 extracts of aliquots of fractions I–VI. When extracts were incubated with [γ-32P]ATP (10 μCi/assay) and exogenous phospholipids as substrate, PtdIns(4)32P (top panel) and PtdIns(4,5)32P2 (bottom panel) were formed. Relative amounts were quantitated and displayed as described for b. Distribution of sPtdIns 4-kinase and sPtdIns(4)P 5-kinase activity followed the distribution of the SSV-marker VAMP/synaptobrevin and of the plasma membrane marker Na+/K+-ATPase, respectively.
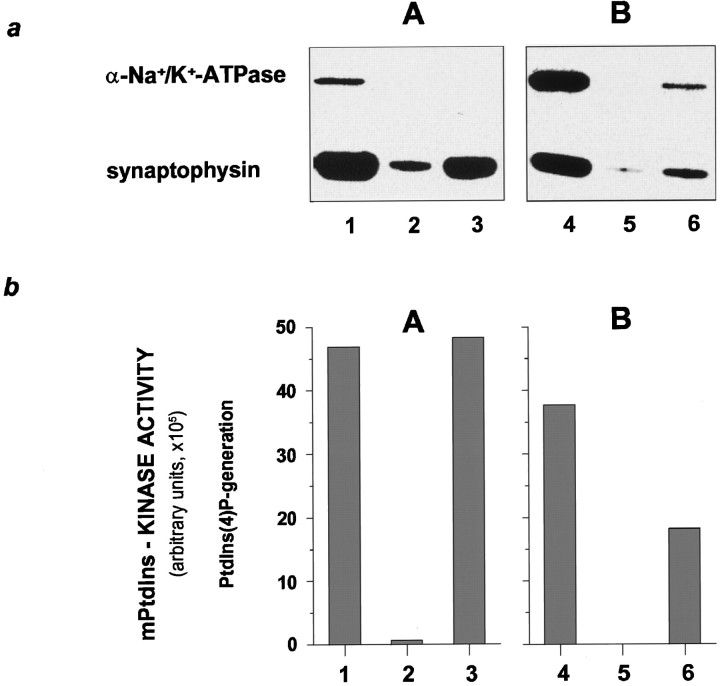
To confirm the specific localization of PtdIns 4-kinase activity to SSVs, we compared the PtdIns 4-kinase activity in two sucrose gradient fractions enriched in either VAMP/synaptobrevin or α-Na+/K+-ATPase. In these experiments, we immunopurified SSV using an antibody to synaptophysin, an intrinsic SSV membrane protein (Navone et al., 1986), and assayed the mPtdIns 4-kinase activity. Vesicles immunoprecipitated from the fraction enriched in VAMP/synaptobrevin contained no detectable α-Na+/K+-ATPase immunoreactivity (Fig.2a, A) but were significantly enriched in synaptophysin. Under the same conditions, relatively few synaptophysin-containing vesicles were immunoprecipitated from the sucrose fraction enriched in α-Na+/K+-ATPase (Fig.2a, B). Accordingly, the level of mPtdIns 4-kinase activity detectable in the immunoprecipitates paralleled the amount of synaptophysin precipitated rather than the contaminating α-Na+/K+-ATPase.
Fig. 2.
Immunoprecipitation of PtdIns 4-kinase activity with SSVs. a, Aliquots of sucrose gradient fractions enriched in SSVs (A) or contaminated with plasma membranes (B) were incubated with monoclonal antibodies to synaptophysin covalently linked to protein G-beads or with protein G-beads alone. The vesicles precipitated were analyzed by immunoblotting using antibodies to synaptophysin and the α-subunit of Na+/K+-ATPase as described for Figure 1a. Lanes 1 and 4display aliquots of the two fractions used for immunoprecipitation (1/10 of the starting material used for immunoprecipitation was loaded). Lanes 2 and 5 show minor unspecific binding of SSV to protein G-beads alone, whereaslanes 3 and 6 demonstrate complete and incomplete removal, respectively, of contaminating plasma membrane.b, The amount of mPtdIns 4-kinase activity, although rather small after precipitation, correlates with the amounts of SSV precipitated (lanes 3 and 6). Much of the mPtdIns 4-kinase activity present in the starting material (lanes 1 and 4) was lost during the precipitation protocol, but the experiment clearly demonstrates that the remaining activity is not derived from the plasma membrane.
We next determined whether the PtdIns 4-kinase present on SSV could be inhibited by PAO, an inhibitor of PtdIns 4-kinase activity and of catecholamine secretion (Schäfer et al., 1994; Wiedemann et al., 1996). Addition of 270 μm PAO to sucrose gradient-purified SSVs during the assay significantly inhibited the formation of PtdIns(4)P by the membrane-associated kinase activity (Fig. 3). When fractions enriched in sPtdIns(4)P 5-kinase were used (Fig. 1), the lack of effect on PtdIns(4,5)P2 production (data not shown) suggested a specificity of PAO-mediated inhibition to PtdIns 4-kinase activity.
Fig. 3.
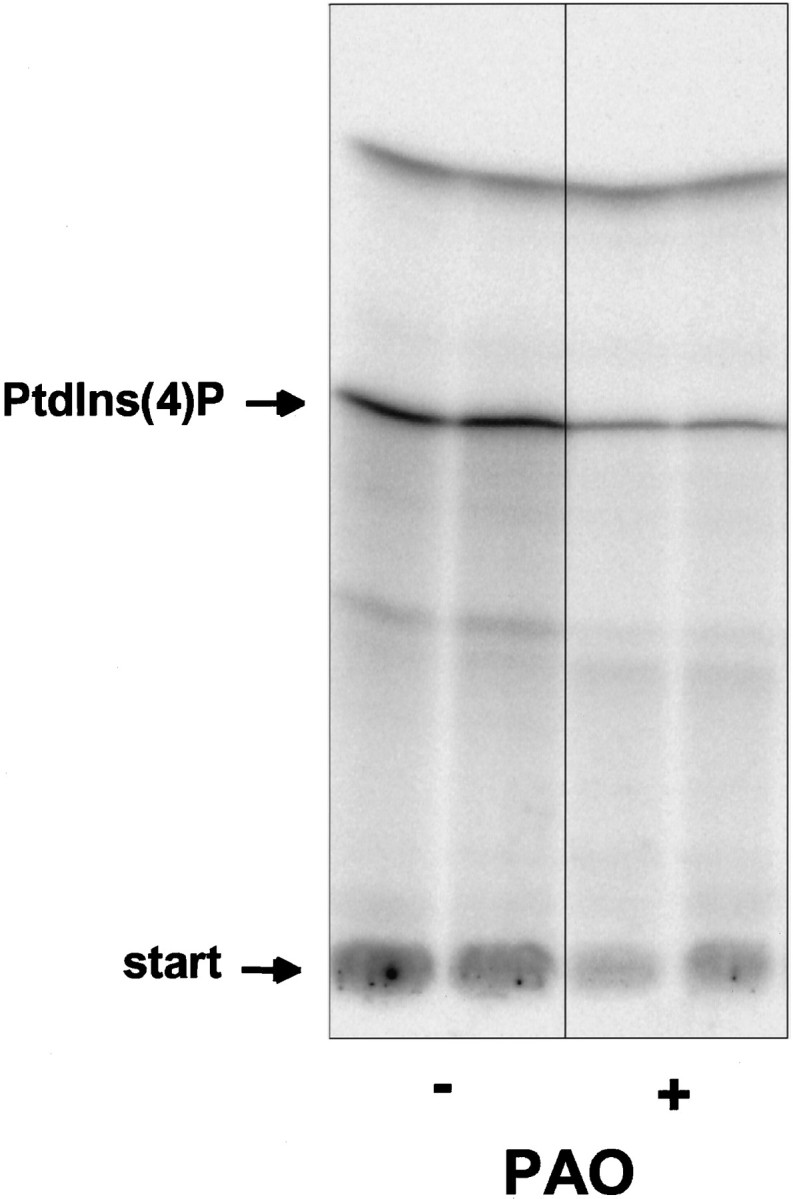
Phenylarsine oxide inhibits the PtdIns 4-kinase associated with SSV. Aliquots of sucrose gradient fractions enriched in SSVs (Fig. 1, IV and V) were incubated with 270 μm PAO (+) or with 0.5% DMSO (−), and the mPtdIns 4-kinase activity was determined as described for Figure 1b. Inhibition by PAO was demonstrated by the decreased production of PtdIns(4)32P in duplicates of treated versus control samples. Figure shows Phosphorimager results as used for quantitation in Figures 1, 2, and 6.
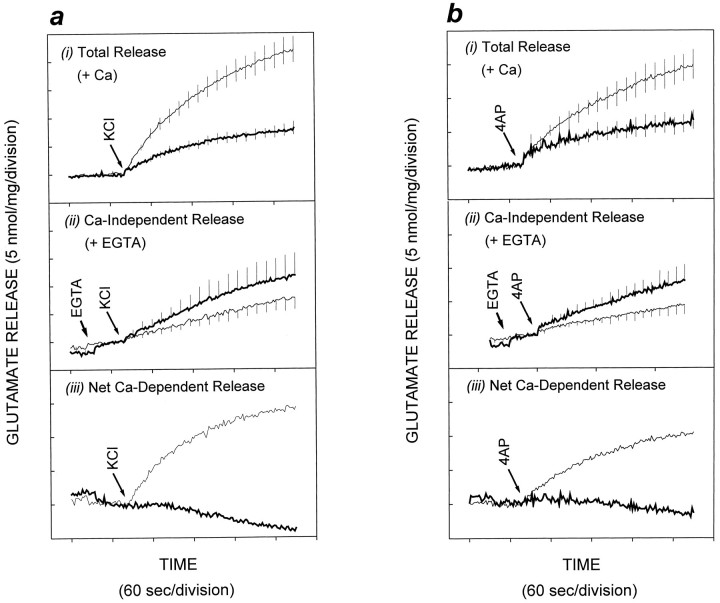
Having established an inhibitory effect of PAO on the SSV-associated PtdIns 4-kinase activity, in the following series of experiments we examined the role of PtdIns 4-kinase activity in neurotransmitter release from isolated nerve terminals. We stimulated glutamate release from cerebrocortical synaptosomes using a number of paradigms, in the absence and presence of pretreatment with PAO. Total glutamate release triggered by KCl-mediated depolarization was decreased by 70% after pretreatment of synaptosomes with 20 μm PAO for 10 min (Fig. 4a,i). When glutamate release was stimulated using 4AP (Tibbs et al., 1989; Sihra et al., 1992, 1993), 20 μm PAO caused a 60% decrease in total glutamate release (Fig. 4b,i). In contrast, PAO had no significant effect on the Ca2+-independent release (cytosolic efflux) of glutamate induced by either KCl or 4AP (Fig. 4a,ii, and b, ii, respectively); hence subtraction of Ca2+-independent release from the total release of glutamate revealed that PAO treatment effected a complete inhibition of the Ca2+-dependent component of glutamate release (Fig. 4a, iii,b, iii). Furthermore, when Ca2+ was substituted with Ba2+(Sihra et al., 1993), Ba2+/KCl-evoked glutamate release was decreased by 50% in the presence of 20 μmPAO (Fig. 5a). This not only confirms the specific effect of PAO on exocytotic release, it also rules out the possibility that PAO mediates its effect through the inhibition of a Ca2+/calmodulin-dependent enzyme, because it is known that Ba2+ binds poorly to calmodulin (Chao et al., 1984). Finally, the action of PAO on glutamate release was not simply caused by an effect of the inhibitor on an ion-channel activity upstream of exocytosis, because when direct entry of Ca2+ was mediated using ionomycin, glutamate release was once again attenuated in the presence of PAO (Fig.5b). Experiments with ionomycin and those in Figure 4 are not strictly comparable quantitatively. This is because depolarization-dependent release is mediated by localized Ca2+ entry, through voltage-dependent Ca2+ channel, and as such is efficiently coupled to glutamate release, whereas ionomycin effects delocalized Ca2+ entry and is thus a relatively weak secretagogue (Sihra et al., 1992). Nevertheless, the data with ionomycin do serve to suggest that PAO interferes with Ca2+-dependent release of neurotransmitter at a step between Ca2+ influx and exocytotic membrane fusion.
Fig. 4.
Attenuation of KCl- and 4AP-evoked glutamate release in synaptosomes by phenylarsine oxide. Synaptosomes were incubated in the presence of 0.5% DMSO or 20 μm PAO for 10 min, and glutamate release was evoked by (a) 30 mm KCl (KCl) or (b) 3 mm 4-aminopyridine (4AP), either in the presence of (i) 1 mmCa2+ (total release) or (ii) 0.2 mm EGTA (Ca2+-independent release), with (iii) being the Ca2+-dependent component of release (total release minus Ca2+-independent release). Release in the presence of PAO is depicted with a thicker line trace. Results are mean ± SEM of results obtained from at least three independent synaptosomal preparations per condition.
Fig. 5.
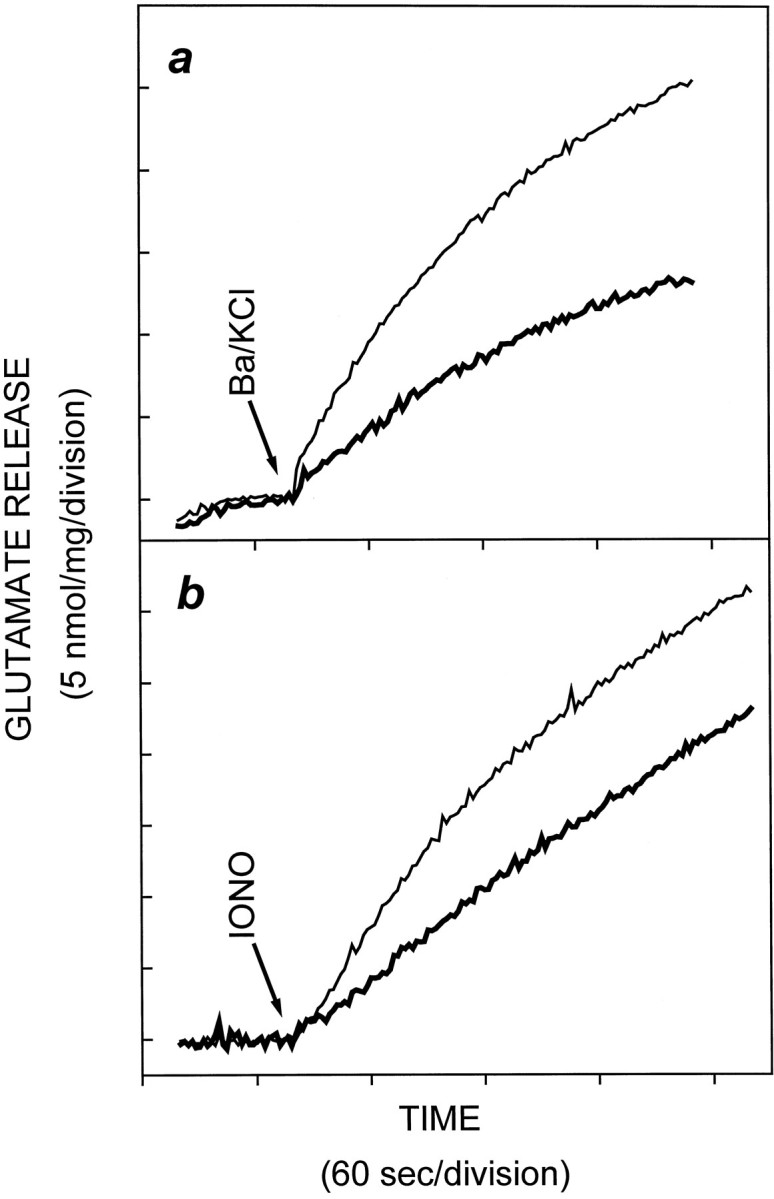
Phenylarsine oxide attenuation of glutamate release is independent of Ca2+/calmodulin-dependent activities and impinges at a step distal to Ca2+entry. Synaptosomes were incubated in the presence of 0.5% DMSO or 20 μm PAO for 10 min, and glutamate release was evoked by (a) 1 mm Ba2+ plus 30 mm KCl (in the absence of Ca2+ and presence of 0.2 mm EGTA) or (b) 5 μm ionomycin (in the presence of 1 mmCa2+). Release in the presence of PAO is depicted with a thicker line trace. Results are mean ± SEM of results obtained from at least three independent synaptosomal preparations per condition.
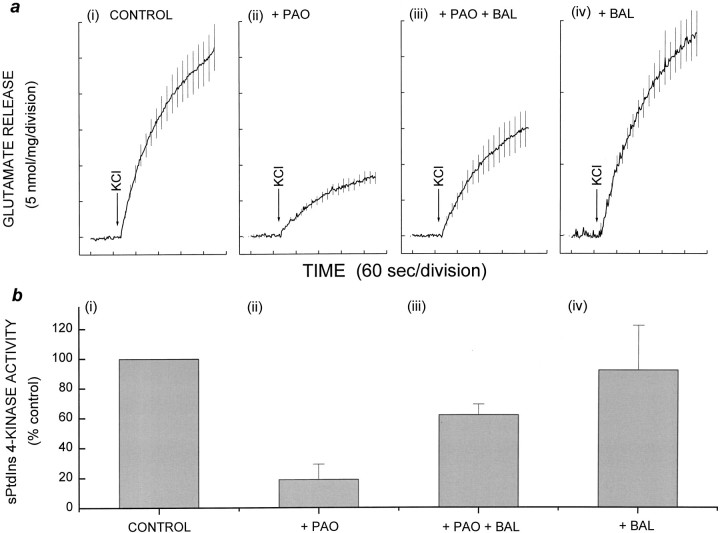
PAO can be removed from its targets using small dithiol compounds (Zahler and Cleland, 1968), thereby allowing reversal of its inhibitory effects. Accordingly, glutamate release from PAO-blocked synaptosomes was partially restored with 2,3-dimercaptopropanol (BAL) (Fig. 6). Treatment with 20 μm PAO for 20 min decreased the total KCl-evoked glutamate release to 30% (Fig. 6a, ii) of control release (Fig. 6a, i). Sequential treatment of synaptosomes with 20 μm PAO followed by 100 μm BAL resulted in the recovery of glutamate release to 65% of controls (Fig. 6a, iii), whereas treatment with BAL alone had no effect (Fig. 6a, iv).
Fig. 6.
Parallel inhibition and recovery of glutamate release and PtdIns 4-kinase activity. Synaptosomes were incubated with DMSO or PAO for 10 min followed by DMSO or BAL for a further 10 min. Glutamate release was measured as described for Figure 4.a, (i–iv), Total KCl-evoked glutamate release. b, (i–iv), sPtdIns 4-kinase activity in protein extracts of synaptosomes. Treatment with (i, iv) 0.5% DMSO or (ii, iii) 20 μmPAO for 10 min, as described for Figure 4, was followed by treatment with (i, ii) 0.5% DMSO or (iii, iv) 100 μm BAL for 10 min. Results are mean ± SEM of results obtained from at least three independent synaptosomal preparations per condition.
To demonstrate that the inhibition of release by PAO was mediated through its effect on PtdIns 4-kinase, we determined lipid kinase activity under the conditions of our release experiments. The lack of sufficient starting material and the likely loss of any inhibitory effects of PAO during purification precluded the direct determination of PtdIns 4-kinase activity associated with SSV derived from synaptosomes in release studies. Instead, immediately after release experiments, we detergent-treated synaptosomes and measured the total extracted PtdIns 4-kinase activity (Fig. 6b). These experiments revealed that control PtdIns 4-kinase activity (Fig.6b, i) was inhibited by 80% in the presence of PAO (Fig. 6b, ii). Significantly, it was evident from these experiments that PtdIns 4-kinase activity and glutamate release varied in concert. Thus, in correlation with the recovery of glutamate release, the PtdIns 4-kinase activity of PAO-inhibited synaptosomes was also partially rescued (to 62% of control activity) by subsequent treatment with 100 μm BAL (Fig.6b, iii); BAL alone had no effect on kinase activity (Fig. 6b, iv).
DISCUSSION
We examined the involvement of phosphoinositides in the exocytosis of SSVs from isolated nerve terminals (synaptosomes) purified from rat cerebrocortex. Kinase activities leading to the generation of phosphorylated species of PtdIns, namely PtdIns(4)P and PtdIns(4,5)P2, were apparent in synaptosomes. Using assays designed to detect phosphorylation of endogenous PtdIns by membrane-associated kinases, we have identified a major PtdIns 4-kinase activity localized to SSVs. Although synaptosomal PtdIns 4-kinase activity was evidently also detectable in plasma membrane fractions, where it is presumably used in phospholipase C-dependent pathways, we confirmed the presence of an SSV-associated PtdIns 4-kinase activity in immunoprecipitated SSVs devoid of plasma membrane contamination. In view of our previous observation that vesicle-associated PtdIns 4-kinase activity can be inhibited by PAO (Schäfer et al., 1994;Wiedemann et al., 1996), we studied, in parallel, the effect of this inhibitor on synaptosomal PtdIns 4-kinase activity and glutamate release. We demonstrate that PAO suppresses Ca2+-dependent glutamate release evoked with a number of stimulation paradigms and that suppression of transmitter release occurred concomitantly with the inhibition of PtdIns 4-kinase activity. The lack of effect of vanadate, a tyrosine phosphatase inhibitor, on glutamate release (data not shown) implies that the reported effect of PAO on tyrosine phosphorylation does not play a role in the modulation of secretion (Wiedemann et al., 1996). Taken together, our results suggest that an SSV-associated PtdIns 4-kinase may be essential for sustaining the capacity of nerve endings to secrete neurotransmitter during repetitive stimulation.
Although PtdIns 4-kinase activity has been demonstrated previously in chromaffin granules (Phillips, 1973; Wiedemann et al., 1996), coated vesicles (Campbell et al., 1985), and glucose transporter 4 (GLUT4)-containing transport vesicles (Del Vecchio and Pilch, 1991), our study is the first invoking the production of phosphoinositides on SSVs that mediate fast synaptic transmission. In examining the role of phosphoinositide metabolism in vesicle dynamics, the production of PtdIns(4,5)P2 might be considered to be a key event, in view of its relative abundance compared with PtdIns(3,4,5)P3 or PtdIns(3,4)P2 and observations that the asymmetric concentrations of PtdIns(4,5)P2 may be as high as 2 mol % in the cell membrane (Liscovitch et al., 1994). Furthermore, although PtdIns(3,4,5)P3 has also been implicated in a number of signaling cascades, wortmannin, a PtdIns 3-kinase inhibitor, had no effect on glutamate release from synaptosomes.
In the current model of priming of the release machinery, PtdIns(4,5)P2 is assigned a central role, although the details of this function have yet to be elucidated. It is thought that PtdIns(4,5)P2 is formed through the concerted activity of soluble PITP, membrane-bound PtdIns 4-kinase, and cytosolic PtdIns(4)P 5-kinase (Martin, 1997). Consistent with this model, we show that a SSV-associated PtdIns 4-kinase phosphorylates endogenous PtdIns in purified SSVs (Fig. 1b). In these mPtdIns-kinase assays, the lack of phosphorylation of PtdIns(4)P to PtdIns(4,5)P2implies either that there is no significant PtdIns(4)P 5-kinase activity tightly associated with SSV or that its activity depends on co-factors lost or destroyed during SSV purification. Interestingly, however, when SSVs from the same sucrose gradient fractions were solubilized and used in sPtdIns kinase assays with exogenous phospholipids added as substrate, PtdIns(4)P 5-kinase activity was found largely in parallel with the plasma membrane Na+/K+-ATPase immunoreactivity, but with some activity also associated with SSVs (Fig. 1c). Although this may be caused by an increase in sensitivity attributable to the supply of much more substrate to the assay, it may also suggest that detergent solubilization either releases a soluble PtdIns(4)P 5-kinase entrapped in plasma membrane vesicles or activates a kinase associated with both plasma membrane vesicles and SSVs.
Although PtdIns(4,5)P2 production in the plasma membrane (e.g., as a substrate for phospholipase C) is not in doubt, the question as to where PtdIns(4,5)P2 is essential for the priming of docked secretory vesicles is still the subject of debate. In the proximity of the docking site, localization of PtdIns(4)P 5-kinase on both plasma membrane and SSVs may well turn out to be essential for the concerted action of the enzymes involved in PtdIns(4,5)P2 production (Liscovitch et al., 1994). Measurement of intrasynaptosomal PIP2 production, with all the players in appropriate compartments, would be the most direct way of examining this hypothesis, but 32P-orthophosphate labeling of synaptosomes produces background levels of phospholipid labeling that are unworkably high. Notwithstanding this, the dependence of glutamate release on PtdIns 4-kinase activity (Fig. 4), taken together with demonstration of an SSV-associated PtdIns 4-kinase activity, clearly reveals the first step toward PtdIns(4,5)P2 production to be crucial for SSV exocytosis.
PtdIns 4-kinase and downstream PtdIns(4,5)P2 production could regulate glutamate release by mediating two different types of modulatory influences, the first involving the breakdown of PtdIns(4,5)P2 [by phospholipase C (PLC)] to second messengers (diacylglycerol and InsP3) and the second involving direct actions of PtdIns(4,5)P2 or higher phosphorylated intermediates. We have shown previously that modulation of glutamate release by diacylglycerol substitutes, phorbol esters, through protein kinase C (PKC) activation, occurs with 4AP stimulation but not with KCl-mediated depolarization (Barrie et al., 1991; Coffey et al., 1993). Our observation that KCl-evoked release is effectively inhibited by PAO therefore argues against the observed effect being caused by attenuation of PKC activity as a consequence of reduced levels of substrate PtdIns(4,5)P2 for PLC. In other experiments, we have shown that intrasynaptosomal Ca2+ stores do not support glutamate release (Nicholls et al., 1987); thus it is unlikely that an alteration in the levels of InsP3 is the basis of PAO-mediated inhibition of glutamate. In the absence of evidence that metabotropic influences of PtdIns(4,5)P2 breakdown affect release, our experiments invoke potential direct roles of PtdIns(4,5)P2 itself, or higher phosphorylated derivatives.
A role for PtdIns(4,5)P2 in an ATP-dependent priming step preceding exocytosis was invoked from studies showing that the breakdown of this phospholipid with phospholipase C (Eberhard et al., 1990; Hay et al., 1995) or its occlusion with antibodies (Hay et al., 1995) led to inhibition of secretion. The mechanism by which PtdIns(4,5)P2 acts remains the subject of debate. It is known that PtdIns(4,5)P2 is a positive regulator of phospholipase D (Brown et al., 1993; Liscovitch et al., 1994), which produces phosphatidic acid during stimulation by the small GTP-binding protein ADP-ribosylation factor (ARF) (Cockcroft et al., 1994). These observations, taken together with others showing that phosphatidic acid in turn stimulates PtdIns(4)P 5-kinase (Moritz et al., 1992; Jenkins et al., 1994), have led to the hypothesis that membrane microdomains enriched in PtdIns(4,5)P2 and phosphatidic acid are produced at the expense of phosphatidylcholine and PtdIns, when PtdIns(4,5)P2 and ARF-containing vesicles interact with plasmalemmal phospholipase D (Liscovitch et al., 1994). In support of this scenario, a recent report suggests that the ARF protein involved in the secretion of catecholamines in adrenal chromaffin cells (Morgan and Burgoyne, 1993) might be ARF6, shown to be present on the secretory granules of these cells (Galas et al., 1997).
Our experiments describing the presence of an SSV-associated PtdIns 4-kinase in synaptosomes provides evidence for a phosphoinositide cascade leading to the production of PIP2 being involved in transmitter release. Numerous studies have indicated that although exocytosis is an extremely rapid event, judging by the observed vesicle recycle times of 90–110 sec (Ryan et al., 1993; Reid and Bewick, 1997), endocytosis is a slower process, particularly under conditions of intense stimulation (Smith and Betz, 1996). In the current study, because the release of glutamate immediately after stimulation is most affected by PAO, we believe that this reflects an effect of PtdIns 4-kinase inhibition on the exocytotic limb of the synaptic vesicle cycle. Our experiments, however, do not exclude the possibility that PtdIns 4-kinase activity persisting in endosomal compartments also plays a role in SSV endocytosis.
Presuming that PtdIns(4,5)P2 and phosphatidic acid production represents the basis of ATP dependence of priming, the question remains as to the precise mechanism(s) involved. The presence of phosphatidic acid would make vesicles fusogenic (Koter et al., 1978), and negatively charged phospholipids, by virtue of their radical physical effects on the membrane structure, may well induce vesicle-fusion competence (Sheetz and Singer, 1974). On the other hand, the negative charges of PtdIns(4,5)P2 head groups would tend to tighten the curvature of the vesicles and thus by destabilizing stalk intermediates between fusing membranes may rather inhibit fusion (Chernomordik, 1996; Martin, 1997). It is not clear which, if either, of these mechanisms is operational physiologically, but if the latter situation were to prevail, it would necessarily require proteins that bind PtdIns(4,5)P2 to relieve the inhibition and allow fusion to proceed. In this respect, the prime candidate for such a role is the vesicular membrane protein synaptotagmin, which not only binds PtdIns(4,5)P2 through its C2B domain but notably shifts its avidity from PtdIns(3,4,5)P3 to PtdIns(4,5)P2as Ca2+ concentrations are raised to those that would be achieved during cell stimulation (Schiavo et al., 1996). Although the aforementioned forms a working hypothesis for the priming actions of PtdIns(4,5)P2, the picture is undoubtedly more complex in view of the known interactions of this phospholipid with proteins containing pleckstrin homology (Harlan et al., 1994) and C2 (Bazzi and Nelsestuen, 1991) domains, as well as others (Martin, 1997; Toker and Cantley, 1997). In this context, interactions with dynamin, a protein intimately involved in endocytosis (De Camilli et al., 1996) and a number of components of the cytoskeleton (see Martin, 1997), may prove to be very significant.
The requirement of phosphoinositides in priming originally suggested from results obtained in neuroendocrine cells (Eberhard et al., 1990;Hay et al., 1995) has now been supported by the data presented above with respect to SSV exocytosis. Moreover, recent genetic studies attributing secretory mutations to lesions in genes for PITP (Hamilton et al., 1997), PtdIns 4-kinase (Carvajal et al., 1996), and PtdIns(4)P 5-kinase (Zhang et al., 1995) have confirmed the importance of phosphoinositides in the ATP-dependent priming in exocytic vesicles. Finally, protein–lipid interactions mediated by phosphoinositides may be involved additionally in the recruitment of cytoskeletal (Janmey, 1994), cytosolic (Bazzi and Nelsestuen, 1991; Harlan et al., 1994), or membrane-associated proteins (Fukuda et al., 1994) to participate in the transport, stabilization of docking, or actual fusion of synaptic vesicles. Determination of the precise details of phosphoinositide involvement in membrane fusion processes presents the next major challenge in the elucidation of the mechanism of neurotransmitter release.
Footnotes
This work was partially supported by a grant from the Peter Samuel Royal Free Fund. T.S.S. is supported by a Wellcome Trust University Award. We thank Professor Fred Gorelick (Yale University) for the loan of his spectrofluorometer. Antibodies to VAMP/synaptobrevin and the α-subunit of Na+/K+-ATPase were generously provided by T. Rapoport (Harvard University) and K. Geering (Lausanne University), respectively.
Correspondence should be addressed to Dr. Talvinder S. Sihra at his present address: Department of Pharmacology, Medawar Building, University College London, Gower Street, London WC1E 6BT, UK. E-mail: t.sihra@ucl.ac.uk.
REFERENCES
- 1.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrie AP, Nicholls DG, Sanchez-Prieto J, Sihra TS. An ion channel locus for the protein kinase C potentiation of transmitter glutamate release from guinea-pig cerebrocortical synaptosomes. J Neurochem. 1991;57:1398–1404. doi: 10.1111/j.1471-4159.1991.tb08306.x. [DOI] [PubMed] [Google Scholar]
- 3.Bazzi MD, Nelsestuen GL. Proteins that bind calcium in a phospholipid-dependent manner. Biochemistry. 1991;30:971–979. doi: 10.1021/bi00218a013. [DOI] [PubMed] [Google Scholar]
- 4.Bock JB, Scheller RH. Protein transport. A fusion of new ideas. Nature. 1997;387:133–135. doi: 10.1038/387133a0. [DOI] [PubMed] [Google Scholar]
- 5.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 6.Campbell CR, Fishman JB, Fine RE. Coated vesicles contain a phosphatidylinositol kinase. J Biol Chem. 1985;260:10948–10951. [PubMed] [Google Scholar]
- 7.Carvajal JJ, Pook MA, dos Santos M, Doudney K, Hillermann R, Minogue S, Williamson R, Hsuan JJ, Chamberlain S. The Friedreich’s ataxia gene encodes a novel phosphatidylinositol-4-phosphate 5-kinase. Nat Genet. 1996;14:157–162. doi: 10.1038/ng1096-157. [DOI] [PubMed] [Google Scholar]
- 8.Chao SH, Suzuki Y, Zysk JR, Cheung WY. Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol. 1984;26:75–82. [PubMed] [Google Scholar]
- 9.Chernomordik L. Non-bilayer lipids and biological fusion intermediates. Chem Phys Lipids. 1996;81:203–213. doi: 10.1016/0009-3084(96)02583-2. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 11.Coffey ET, Sihra TS, Nicholls DG. Protein kinase C and the regulation of glutamate exocytosis from cerebrocortical synaptosomes. J Biol Chem. 1993;268:21060–21065. [PubMed] [Google Scholar]
- 12.De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 13.Del Vecchio RL, Pilch PF. Phosphatidylinositol 4-kinase is a component of glucose transporter (GLUT 4)-containing vesicles. J Biol Chem. 1991;266:13278–13283. [PubMed] [Google Scholar]
- 14.Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K. Inositol-1,3,4,5-tetrakisphosphate binding to C2B domain of IP4BP/synaptotagmin II. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 16.Galas MC, Helms JB, Vitale N, Thierse D, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. A potential role for a secretory granule-associated ARF6 protein. J Biol Chem. 1997;272:2788–2793. doi: 10.1074/jbc.272.5.2788. [DOI] [PubMed] [Google Scholar]
- 17.Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton BA, Smith DJ, Mueller KL, Kerrebrock AW, Bronson RT, van Berkel V, Daly MJ, Kruglyak L, Reeve MP, Nemhauser JL, Hawkins TL, Rubin EM, Lander ES. The vibrator mutation causes neurodegeneration via reduced expression of PITP alpha: positional complementation cloning and extragenic suppression. Neuron. 1997;18:711–722. doi: 10.1016/s0896-6273(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 19.Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 20.Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 21.Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 22.Hodel A, Schafer T, Gerosa D, Burger MM. In chromaffin cells, the mammalian Sec1p homologue is a syntaxin 1A-binding protein associated with chromaffin granules. J Biol Chem. 1994;269:8623–8626. [PubMed] [Google Scholar]
- 23.Husebye ES, Flatmark T. Phosphatidylinositol kinase of bovine adrenal chromaffin granules: kinetic properties and inhibition by low concentrations of Ca2+. Biochim Biophys Acta. 1988;968:261–265. doi: 10.1016/0167-4889(88)90015-8. [DOI] [PubMed] [Google Scholar]
- 24.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janmey PA. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- 27.Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72[Suppl]:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 28.Koter M, de Kruijff B, van Deenen LL. Calcium-induced aggregation and fusion of mixed phosphatidylcholine-phosphatidic acid vesicles as studied by 31P NMR. Biochim Biophys Acta. 1978;514:255–263. doi: 10.1016/0005-2736(78)90296-1. [DOI] [PubMed] [Google Scholar]
- 29.Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994;269:21403–21406. [PubMed] [Google Scholar]
- 30.Martin TF. Phosphoinositides as spatial regulators of membrane traffic. Curr Opin Neurobiol. 1997;7:331–338. doi: 10.1016/s0959-4388(97)80060-8. [DOI] [PubMed] [Google Scholar]
- 31.Martin TF, Hay JC, Banerjee A, Barry VA, Ann K, Yom HC, Porter BW, Kowalchyk JA. Late ATP-dependent and Ca++-activated steps of dense core granule exocytosis. Cold Spring Harb Symp Quant Biol. 1995;60:197–204. doi: 10.1101/sqb.1995.060.01.022. [DOI] [PubMed] [Google Scholar]
- 32.McPherson PS, Garcia E, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 33.Morgan A, Burgoyne RD. A synthetic peptide of the N-terminus of ADP-ribosylation factor (ARF) inhibits regulated exocytosis in adrenal chromaffin cells. FEBS Lett. 1993;329:121–124. doi: 10.1016/0014-5793(93)80206-a. [DOI] [PubMed] [Google Scholar]
- 34.Moritz A, De Graan PN, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–7210. [PubMed] [Google Scholar]
- 35.Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholls DG, Sihra TS. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls DG, Sanchez-Prieto J, Sihra TS. The role of the plasma membrane and intracellular organelles in synaptosomal calcium regulation. Soc Gen Physiol Series. 1987;42:31–43. [PubMed] [Google Scholar]
- 38.Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- 39.Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JH. Phosphatidylinositol kinase. A component of the chromaffin-granule membrane. Biochem J. 1973;136:579–587. doi: 10.1042/bj1360579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid B, Bewick GS. Synaptic vesicle recycle time and releasable pool size in motor nerve terminals of rat fast- and slow-twitch muscles. J Physiol (Lond) 1997;499:28P. [Google Scholar]
- 42.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 43.Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- 44.Schäfer T, Wiedemann C, Gitler C, Burger MM. Effects of arsenicals on the secretory process in chromaffin cells. Ann NY Acad Sci. 1994;710:356–367. doi: 10.1111/j.1749-6632.1994.tb26642.x. [DOI] [PubMed] [Google Scholar]
- 45.Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 46.Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sihra TS. Protein phosphorylation and dephosphorylation in isolated nerve terminals (synaptosomes). In: Hemmings HC Jr, editor. Regulatory protein modification: techniques and protocols. Humana; Totowa, NJ: 1997. pp. 67–119. [Google Scholar]
- 49.Sihra TS, Nichols RA. Mechanisms in the regulation of neurotransmitter release from brain nerve terminals: current hypotheses. Neurochem Res. 1993;18:47–58. doi: 10.1007/BF00966922. [DOI] [PubMed] [Google Scholar]
- 50.Sihra TS, Bogonez E, Nicholls DG. Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J Biol Chem. 1992;267:1983–1989. [PubMed] [Google Scholar]
- 51.Sihra TS, Piomelli D, Nichols RA. Barium evokes glutamate release from rat brain synaptosomes by membrane depolarization: involvement of K+, Na+, and Ca2+ channels. J Neurochem. 1993;61:1220–1230. doi: 10.1111/j.1471-4159.1993.tb13612.x. [DOI] [PubMed] [Google Scholar]
- 52.Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and endocytosis. Nature. 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- 53.Söllner T, Rothman JE. Neurotransmission: harnessing fusion machinery at the synapse. Trends Neurosci. 1994;17:344–348. doi: 10.1016/0166-2236(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 54.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 55.Susa M, Keeler M, Varticovski L. Platelet-derived growth factor activates membrane-associated phosphatidylinositol 3-kinase and mediates its translocation from the cytosol. Detection of enzyme activity in detergent-solubilized cell extracts. J Biol Chem. 1992;267:22951–22956. [PubMed] [Google Scholar]
- 56.Tibbs GR, Barrie AP, Van Mieghem FJ, McMahon HT, Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- 57.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 58.Wiedemann C, Schäfer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 59.Zahler WL, Cleland WW. A specific and sensitive assay for disulfides. J Biol Chem. 1968;243:716–719. [PubMed] [Google Scholar]
- 60.Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1995;92:4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zucker RS. Exocytosis: a molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]